Abstract
Listeria monocytogenes meningitis is the third most common cause of bacterial meningitis in adults and has high mortality and morbidity rates. We describe the clinical course and score brain pathology of 5 patients who died of listeria meningitis. All patients were immunocompromised and ages ranged between 48 and 76 years. Three cases were confirmed by cerebrospinal fluid culture; one was confirmed by brain culture; and one diagnosis was based on a positive blood culture and neuropathological findings. Mild inflammation of meningeal arteries was found in 3 of 5 cases (60%). Moderate/severe ventriculitis was seen in 4 of 4 cases (100%), abscesses in 3 of 4 cases (75%), mild vascular inflammation in 4 of 5 cases (80%), mild/moderate hemorrhage in 2 of 4 cases (50%), mild/moderate thrombosis of meningeal artery in 3 of 5 cases (60%), and 1 case (25%) showed a moderate infarct. The inflammatory cells present in the meninges were characterized by a mix of monocytes, macrophages, and neutrophils and removal of apoptotic inflammatory cells by macrophages (efferocytosis). Gram stain showed intra- and extracellular presence of rod-shaped bacteria in 3 cases. Pathological examination was characterized by moderate to severe ventriculitis, abscesses and abundant efferocytosis which has been suggested to be exploited by L. monocytogenes for cell-to-cell spread.
Keywords: Autopsy, Bacterial meningitis, Brain histopathology, Efferocytosis, Listeria monocytogenes
INTRODUCTION
Bacterial meningitis is a severe infection of the meninges and the brain and is associated with a high mortality and morbidity rates (1–3). The most common causative pathogens are Streptococcus pneumoniae and Neisseria meningitidis, which are spread by human-to-human transmission (4). Histopathological studies in humans with pneumococcal or meningococcal meningitis have documented a wide range of brain injuries, including infarction, hemorrhages, abscesses and ventriculitis, vascular inflammation, and reactive vasculopathy (5).
Listeria monocytogenes is the third most common cause of bacterial meningitis and is identified in 5%–6% of adult cases (6, 7). It is one of the most severe causes of meningitis, with a reported mortality between 30% and 36% and rates of unfavorable outcome up to 61% (8, 9). L. monocytogenes is an intra-cellular pathogen that is transmitted through contaminated food (10). It primarily affects elderly or immunocompromised patients, such as cancer patients or those using immunosuppressive drugs (8, 11, 12). Diagnosis of L. monocytogenes meningitis can be challenging due to slow onset of symptoms (7), atypical cerebrospinal fluid (CSF) findings (13) consistent with a relatively low grade of infection and high percentage of negative CSF culture (14). A subset of patients presents with brainstem encephalitis, which typically occurs in young adults who are often immunocompromised (15).
Little is known on the brain pathology caused by L. monocytogenes meningitis in adult patients as only a small number of case reports and small case series have described histopathological findings of brain tissue in listeria infection (15–20). In this study, we present the brain pathology results of 5 patients who died of L. monocytogenes meningitis.
MATERIALS AND METHODS
Patients
Autopsy cases of community-acquired listeria meningitis between 1985 and 2016 were identified from 2 nation-wide prospective cohort studies (2, 21) and the neuropathology database of the Academic Medical Center, Amsterdam. Two authors (M.K., M.B.) identified cases from the databases and retrieved available clinical and laboratorial information from a chart review or study databases. The pathology specimens and clinical data of these patients were collected in the MeninGene-Path biobank as previously described (5). Informed consent was obtained from next of kin for brain autopsy at the time of death. Tissue was obtained and used in accordance with the Declaration of Helsinki and the Academic Medical Center (AMC) Research Code.
Five autopsy cases could be identified from the 2 nationwide cohort studies and the AMC neuropathology database. For the 3 brain autopsies that were performed in a hospital other than the AMC, histology slides, tissue blocks, and autopsy reports were requested. For brain autopsy, 10 slides per case were available on average, with a range of 2–23. Four cases included at least 1 slide of cortex and brainstem (mesencephalon, pons, and medulla oblongata). Basal ganglia and hippocampus regions were sampled in 3 cases and the cerebellum in 4 cases. The case from which only 2 slides were available consisted of a sample of meninges located near the cerebellum and of the spinal cord.
Histopathology
The macroscopic examination and sampling of the brain were carried out by the local pathologists. The formalin-fixed, paraffin-embedded brain tissue samples were cut and stained with hematoxylin and eosin at the local institutes. All available paraffin blocks and slides were sent to the AMC. The sampled brain regions of individual cases were documented. If it had not yet been done, Gram staining was performed on the available paraffin blocks. A neuropathologist (J.Y.E.L.) scored the slides using a pathology scale as described previously (5). On this scale severity of meningeal inflammation, parenchymal damage, vascular inflammation, thrombosis and ventriculitis was scored on a 0–3 scale. Histological evaluation was performed with a Zeiss Axiopskop light microscope (Munich, Germany) with 6 object lenses of magnification of ×2, ×4, ×10, ×20, ×40, and ×100 and LED light source was used.
Selection of Patients
Clinical data are shown in Table 1 and additional information on body autopsies is shown in Table 2.
TABLE 1.
Patient Baseline Characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Year of admission | 1992 | 1993 | 2001 | 2009 | 2012 |
| Age (years) | 48 | 71 | 56 | 76 | 73 |
| Sex | M | F | M | F | M |
| Medical history | Hemophilia type A, hepatitis C, and AIDS | Breast cancer with liver metastases | Arthritis | Renal failure, polymyalgia rheumatica | Alcohol abuse, renal failure, diabetes type 2, arthritis, metastasized lung carcinoma |
| Immunosuppressive drugs | Unknown | Chemotherapy | Unknown | Prednisone | Prednisone and methotrexate |
| Laboratory results | |||||
| Leucocytes (cells/mL) | NA | 13.2 | 22.4 | 15.7 | 9.9 |
| C-reactive protein (mg/L) | NA | NA | 297 | 271 | 172 |
| Indexes of CSF inflammation | |||||
| Leucocytes (cells/mL) | NA | NA | 7200 | 298 | 1614 |
| CSF-blood-glucose ratio | NA | NA | 0.14 | 0.49 | 0.23 |
| Protein level (g/L) | NA | NA | 1.9 | 2.6 | 3.8 |
| CSF culture positive | NA | NA | Yes | Yes | Yes |
| Radiological imaging | No abnormalities | NA | Oedema right hemisphere | Abscesses in right fronto-parietal lobe and left parietal lobe | No abnormalities |
| Time to death (days) | 2 | 2 | 2 | 3 | 4 |
| Serotype (sequence type) | NA | NA | 4b (2) | 1/2a (155) | 1/2c (9) |
CSF, cerebrospinal fluid; NA, data not available.
TABLE 2.
Available Data on Body Autopsy of All 5 Patients
| Patient | Cause of Death | Heart and Blood Vessels | Thoracic Cavity | Lungs | Abdomen | Liver | Brain |
|---|---|---|---|---|---|---|---|
| 1 | Acute circulatory shock and fulminant bacterial meningitis | NA | NA | Pulmonary congestion, edema, fibrosis, and emphysema, infestation of fungi, bloody fluid | Gastro-intestinal bleeding with bloody fluid in stomach, small intestine, and colon | Liver cirrhosis (post hepatitis B infection) | Meningitis |
| 2 | Septic shock based on abscess in the douglas cavity | Infarction of left ventricle), atherosclerosis aorta | Pleural and mediastinal metastases | Bilateral basal pneumonia, pulmonary hypertension, lung metastases | Metastases right adrenal gland and in the douglas cavity with perforation of the rectum | Metastases | Meningitis |
| 3 | Brain herniation | Left ventricle hypertrophy | NA | Lung edema, pulmonary congestion, atelectasis | No abnormalities | Cirrhosis and hepatomegaly | Meningoencephalitis |
| 4 | Respiratory insufficiency, sepsis and meningitis | Hypertrophy of both ventricles | NA | Emphysema | No abnormalities | Normal | Purulent meningo-encephalitis with microbleedings and fibrinoid necrosis |
| 5 | Septic shock and meningitis | Hypertrophic ventricles | Pleural adhesions | Pulmonary congestion | No abnormalities | Metastases of non-small cell carcinoma | Fulminant meningitis, ventriculitis, and edema |
NA, data not available.
RESULTS
Case Series and Pathology
Patient 1
A 48-year-old man with a history of hemophilia type A, hepatitis C, and AIDS was admitted to the hospital with fever, diarrhea, and headache in 1992 (Table 1). Data on use of medication, physical examination, and laboratory results could not be retrieved. A cranial CT scan on admission was normal. On day 2, the patient became hypotensive with active gastro-intestinal bleeding, after which a cardiac arrest occurred. Resuscitation was initiated but the patient died. L. monocytogenes meningitis was diagnosed from postmortem leptomeningeal cultures. Pathology showed moderate multifocal meningeal inflammation without cortical infiltration (Table 2). There was focal mild inflammation of meningeal arteries and parenchymal small vessels. A moderate degree of ventriculitis was seen with inflammation of the choroid plexus and ependyma of the lateral ventricle, aqueduct, and fourth ventricle with focal destruction of ependyma and extension into the periventricular tissue. Focal thrombosis was seen in the meningeal artery and vein with partial obstruction. Infarction, bleeding, or abscesses were not identified.
Patient 2
A 71-year-old woman with breast cancer, metastasized to the liver for which she received palliative chemotherapy (megestrol) and local radiotherapy, was admitted to the hospital because of fever, nausea, dyspnea, and diarrhea in 1993. Because of suspected recurrent urosepsis or sepsis, empiric treatment with cefotaxime and gentamicin was started. After 1 day she developed neck stiffness and blood culture grew L. monocytogenes. Listeria meningitis was suspected and antibiotic therapy was switched to intravenous (i.v.) amoxicillin. The same day she developed septic shock and died. Pathology showed metastasis in the Douglas cavity with infiltration in the rectum with abscess formation. Brain autopsy showed mild multifocal meningeal inflammation with focal cortical infiltration. Meningeal arteries showed no inflammation and parenchymal small vessels showed focal mild inflammation. There was a small perivascular bleeding in mesencephalon. Several small abscesses of approximately 1 mm were present in the corpus callosum and sub-ependymal tissue of the lateral ventricle. Moderate to severe ventriculitis was seen in the lateral ventricle, aqueduct, and fourth ventricle with focal deep infiltration into the periventricular tissue. Thrombosis was present in a meningeal artery with partial obstruction. No infarctions were found.
Patient 3
A 56-year-old man with hypertension and arthritis was admitted to the intensive care unit in 2001 with a 2-day history of headache, vomiting, and fever (39.8°C). He did not open his eyes, he bent his arms and made incomprehensible sounds with a painful stimulus (Glasgow Coma Scale [GCS] score, E1M4V2). Further neurologic examination showed neck stiffness, a deviation of head and eyes to the right, and a left-sided hemiparesis. Cranial CT-scan showed hypodensity and swelling of the right hemisphere consistent with edema. Lumbar puncture revealed CSF leukocytes of 7200 cells/mL, protein of 1.9 g/L, and CSF-blood-glucose ratio of 0.14. Empirical treatment was started consisting of i.v. amoxicillin, ceftriaxone, and dexamethasone. CSF and blood cultures grew L. monocytogenes (serotype 4b, sequence type 2). Repeated cranial CT showed progressive edema in the right hemisphere. After day 2 his level of consciousness worsened and he died. Pathology showed dense inflammation of the meninges with focal cortical infiltration. The meningeal arteries showed focal sub-endothelial inflammation with extension in the media layer. Inflammation of small parenchymal vessels was seen in a moderate degree and was accompanied by thrombosis and bleeding. Infarction was not observed. An abscess (6 mm diameter) was observed in the amygdala. Moderate ventriculitis was found in the lateral and fourth ventricles with focal ependymal destruction. Thrombosis with partial obstruction was frequently observed in the meningeal arteries and veins.
Patient 4
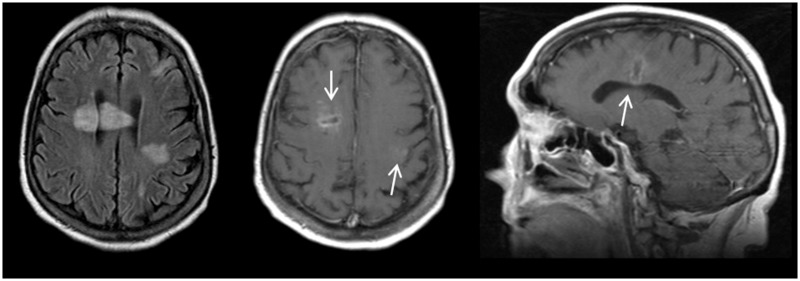
A 76-year-old woman with renal failure and polymyalgia rheumatica for which she was treated with prednisone, presented with headache, disorientation, and vomiting in 2009. She had a temperature of 38.2°C. On painful stimulus she opened her eyes and localized symmetrically and made incomprehensible sounds (GCS score, E3M5V2). Neurological examination showed neck stiffness and right central facial nerve palsy. Lumbar puncture revealed CSF leukocytes of 298 cells/mL, protein of 3.8 g/L, and CSF-blood-glucose ratio of 0.49. Empirical treatment with i.v. amoxicillin was started. Cranial MRI-scan showed multiple abscesses in the right fronto-parietal lobe and left parietal lobe (Fig. 1). CSF culture grew L. monocytogenes (serotype 1/2a, sequence type 155). On day 1, her level of consciousness suddenly worsened with respiratory insufficiency. She was intubated and ventilated but died on day 3. Pathology showed a moderate degree of inflammation of the meninges and multifocal cortical infiltration. The meningeal arteries showed no inflammation. Abscesses were extensively present in subcortical white matter, along with multifocal infarction and bleeding. A small abscess of approximately 1 mm was observed in the mesencephalon, between the red nuclei. Small arteries in between the abscesses were relatively well preserved with focal mild endothelial infiltration. In infarcted areas, small parenchymal arteries showed thrombosis with inflammation and hyalinization of the vascular wall. Severe ventriculitis was seen in the lateral ventricle, aqueduct, and fourth ventricle with multifocal deep infiltration into the periventricular tissue. The meningeal arteries and veins showed no thrombosis.
FIGURE 1.
Abnormalities on brain MRI in patient 4. Abscess in right fronto-parietal lobe crossing the corpus callosum and an abscess in the left fronto-parietal lobe. Axial T2-weighted image (A), axial (B), and sagittal (C) T1-weighted image with contrast enhancement.
Patient 5
A 73-year-old man with a history of alcohol abuse, renal failure, diabetes type 2, arthritis, hypertension, and lung carcinoma with bone metastasis presented with epileptic seizures in 2012. He had been treated with prednisone and methotrexate. He had a temperature of 38°C. He opened his eyes, abnormally flexed to painful stimuli and made incomprehensible sounds (GCS score, E2M3V2). Neurologic examination showed neck stiffness. Lumbar puncture revealed CSF leukocytes of 1614 cells/mL (82% neutrophils), protein of 3.8 g/L, and CSF-blood-glucose ratio of 0.23. Empirical treatment with i.v. ceftriaxone, amoxicillin, and gentamicin was started. Cranial CT showed some atrophy but was otherwise normal. CSF cultures grew L. monocytogenes (serotype 1/2c, sequence type 9). At day 1, he developed respiratory and heart failure. Supportive care was withdrawn at day 4 and he died. Pathology showed severe meningeal inflammation with only focal mild inflammation of meningeal arteries.
Histopathology of Listeria Meningitis
Results of body and brain autopsy are summarized in Tables 2 and 3. In 3 patients the cause of death was determined as a combination of systemic and central nervous system infection; one patient died of fulminant systemic infection (patient 2) and one of brain herniation (patient 3). Autopsy showed co-existing disease indicating an immunocompromised state in all patients. Infection foci in other organs than the brain were found in 4 of 5 patients, although the causative organism of these foci remained unclear in 3 of these patients.
TABLE 3.
Pathological Scoring per Case
| Patient Number | Total Pathology Score | Meningeal Infiltration | Brain Parenchymal Damage |
Vascular Inflammation |
Thrombosis |
Ventriculitis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infarction | Bleeding | Parenchymal Infiltration | Abscess | Meningeal Medium-Large Artery | Small Parenchymal Vessels | Artery | Venous | Capillary | ||||
| 1 | 8 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 2 |
| 2 | 8 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 3 |
| 3* | 16 | 3 | 0 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 2 |
| 4* | 22 | 2 | 2 | 2 | 2 | 3 | 0 | 3 | 0 | 0 | 3 | 3 |
| 5* | – | 3 | IM | IM | IM | IM | 1 | IM | IM | IM | IM | IM |
Score: 0 = absence of abnormality, 1 = focal mild abnormality, 2 = focal severe or multifocal mild abnormalities, 3 = multifocal severe abnormalities.
IM, insufficient material to rate.
Positive Gram staining.
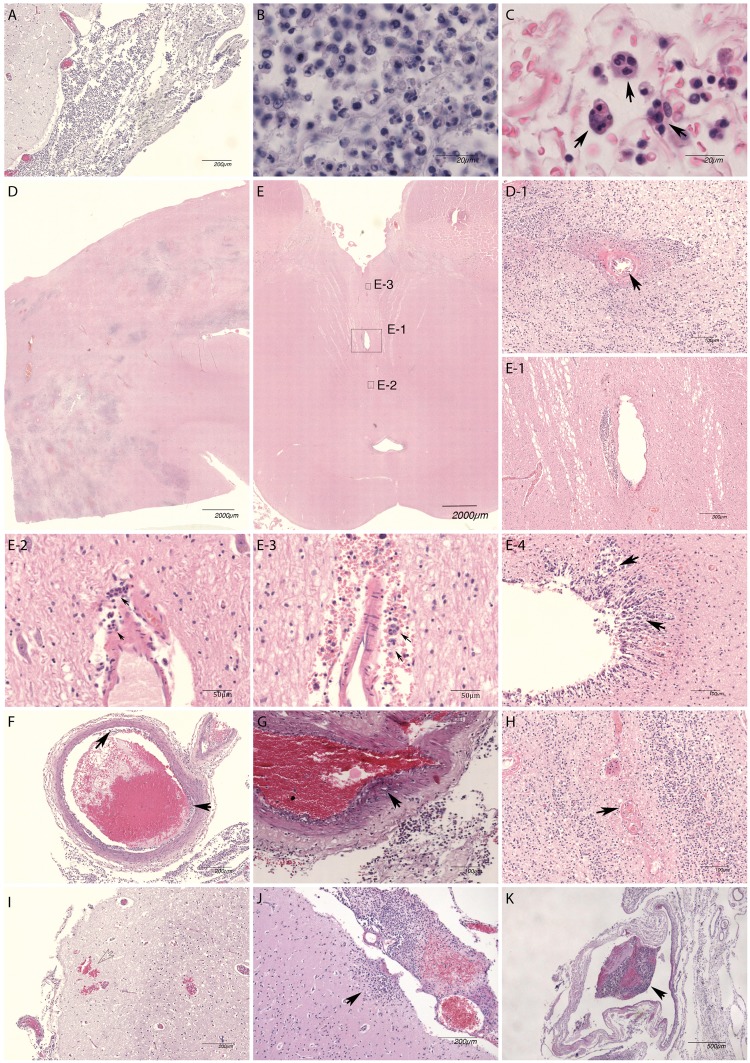
Brain pathology showed severe meningeal inflammation (Fig. 2A, patient 3), a mixture of monocytes/macrophages and neutrophils with relatively high proportion of monocytes/macrophages in meninges (Fig. 2B, patient 3), and frequent presence of phagocytosis of neutrophils or macrophages by macrophages (efferocytosis, arrows) in the meninges in all patients (Fig. 2C, patient 2). Monocytes/macrophages formed about half of the population in the meningeal inflammatory cells in all cases.
FIGURE 2.
Histopathological features of listeria meningitis. Brain pathology images of L. monocytogenes meningitis patients showing severe meningeal inflammation (A, patient 3), a mixture of monocytes/macrophages and neutrophils with relatively high proportion of monocytes/macrophages in meninges (B, patient 3) and frequent presence of phagocytosis of neutrophils or macrophages by macrophages (efferocytosis, arrows) in the meninges (C, patient 2). Extensive abscess formation in deep subcortical white matter (D, patient 4) was seen with only mild endotheliitis in centrally located vessel (D-1, arrow). Brainstem (mesencephalon) showed no prominent abnormalities on low magnification (E, patient 4), but higher magnification showed a small abscess (E-1), which probably developed from perivascular inflammation and remotely spread perivascular inflammation (E-2 and E-3) with efferocytosis (arrows). Ventriculitis (E-4) was a frequent finding, with infiltration in periventricular tissue (arrows). Meningeal artery (F, patient 1) usually only showed mild sub-endothelial inflammation (arrows). The meningeal artery inflammation rarely extended into the media layer (G, patient 5). Thrombosis of small parenchymal vessel (H, arrow) and parenchymal bleeding (I) were seen in a patient with extensive abscess formation (patient 4). Cortical extension of meningeal inflammation was a frequent finding (J, patient 2). Thrombosis of meningeal arteries and veins (K, patient 1) was often seen, mostly without obstruction.
Brain abscesses were identified in 3 of 4 cases (75%), which were located in vicinity of ventricles or originated from parenchymal perivascular space. Extensive abscess formation in deep subcortical white matter (Fig. 2D, patient 4) was seen with mild endotheliitis in centrally located vessel (Fig. 2D-1, arrow). Brainstem (mesencephalon) showed no prominent abnormalities in low magnification (Fig. 2E, patient 4), but higher magnification showed a small abscess (Fig. 2E-1), which probably developed from perivascular inflammation, and multiple locations of remotely spread perivascular inflammation (Fig. 2E-2, E-3) with efferocytosis (Fig. 2E-3, arrows). Ventriculitis was present in 4 of 4 patients (100%), with infiltration in periventricular tissue (Fig. 2E-4, arrows).
Meningeal artery usually showed mild sub-endothelial inflammation (Fig. 2F, arrows, patient 1). The meningeal artery inflammation rarely extended into the media layer (Fig. 2G, patient 5). Thrombosis of small parenchymal vessel (Fig. 2H) and parenchymal bleeding (Fig. 2I) were seen in a patient with extensive abscess formation (patient 4). Cortical extension of meningeal inflammation was a frequent finding (Fig. 2J, patient 2). Inflammation of parenchymal small vessels was observed mainly in regions with abscesses. Thrombosis of meningeal arteries and veins (Fig. 2K, patient 1) was often seen, mostly without obstruction.
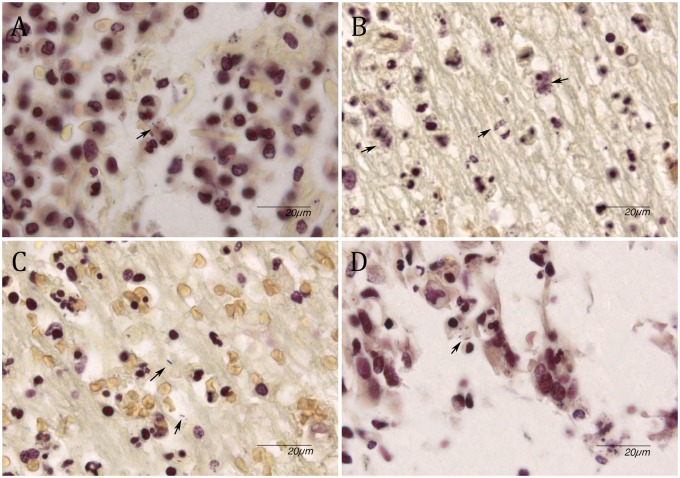
Gram stain showed occasional intra- and extracellular presence of Gram-positive rods in the meninges of three cases (Fig. 3A;Table 3). One case (patient 4) with diffuse deep parenchymal abscesses showed abundant bacteria which were present intra- and extracellularly (Fig. 3B, C). These bacteria were also visible intracellularly in the ependymal cells of the ventricle and showed perivascular inflammation (Fig. 3D).
FIGURE 3.
Gram stain. (A) Occasional presence of rod-shaped bacteria in meningeal inflammatory cells (arrow). (B) Presence of multiple rod-shaped bacteria in cytoplasm of cells in subcortical abscess (arrows). (C) Extracellular location of rod-shaped bacteria (arrows). (D) Ventricular inflammation with presence of intracellular rod-shaped bacteria (arrow).
DISCUSSION
Our study shows brain abscesses and ventriculitis are observed as neuropathological features of listeria meningitis. Brain abscesses were present in all but one patient and were located in vicinity of ventricles or originated from parenchymal perivascular space. One case with diffuse deep parenchymal abscesses showed abundant bacteria which were present intra- and extracellularly. Once entered via the ventricle, perivascular extension of bacteria and inflammation seems to be the mechanism of formation of abscesses. A similar route of infection could explain micro-abscesses present in our cases and previously studied human cases (16, 22). Abscesses have been described in listeria meningitis in the cerebral white matter, basal ganglia, or brainstem (15, 16, 19, 20). Many bacteria were found in these lesions, within phagocytes or free in the brain parenchyma around necrotic areas (19, 23). The rate of brain abscesses is substantially higher than the frequency of abscess observed in a pneumococcal meningitis autopsy study that identified brain abscesses in 6 of 31 cases (19%, p = 0.04) (5). The choroid plexus is hypothesized to be one of the first places for bacterial invasion in listeria meningitis thereby resulting in ventricular inflammation (24, 25). Ventriculitis was seen in all 4 cases of which ventricular slides were available and showed moderate (2 cases) to severe (2 cases) inflammation with invasion in the periventricular tissue of the lateral ventricle, cerebrum, and brainstem. Extension of inflammation into the ventricular wall with ependymal and subependymal perivascular infiltration of leucocytes has been previously described in listeria meningitis cases (20). In pneumococcal meningitis cases, ventriculitis was found in 68% (19 of 28 cases) of autopsies of which 6 cases with mild (32%), 9 cases moderate (47%), and 4 cases severe inflammation (5). Pathological changes of the ventricle structures can cause hydrocephalus. A relatively high percentage (15%) of hydrocephalus cases in bacterial meningitis is caused by L. monocytogenes (26). This may have a relation with the inflammatory structural damage during infection. In our 5 autopsy cases there were no patients with hydrocephalus.
Our study shows abundant efferocytosis in all 5 cases. Phagocytosis of apoptotic or non-apoptotic neutrophils by macrophages is an important macrophage-neutrophil cooperation in infection that enhancing the macrophage antimicrobial activity and the resolution of the infection (27). In addition to the innate antimicrobial mechanism, efferocytosis is a process to engulf apoptotic cells, forming a vesicle around the dead cell (an efferosome) thereby removing the dying cell. In that way, efferocytosis leads to removal of the infected cell before the membrane breaches and toxins and oxidants leak into the tissue, enhancing the inflammatory response. L. monocytogenes exploits efferocytosis for cell-to-cell spreading by pore-forming toxin listeriolysin O and 2 phospholipase C enzymes (28). During this process, listeria triggers host membrane repair mechanisms promoting release of exofacial phosphatidylserine that accelerates efferocytosis, and further cell-to-cell spread of listeria. Interestingly, efferocytosis also induces secretion of vascular endothelial growth factor (VEGF) (29, 30). We previously described high VEGF cerebrospinal fluid concentration to be associated with poor disease outcome in patients with listeria meningitis (31). Survival within phagocytotic cells by promoting cell-to-cell spread or evading pyroptosis has been observed in other bacteria such as Shigella (32) and Mycobacterium tuberculosis (33). Chlamydia pneumonia and Yersinia pestis are able to infect neutrophil that subsequently are uptaken by macrophages and therefore protect these bacteria from further human immune reaction and facilitates distant dissemination (34, 35).
Monocytes and macrophages were the most prominent inflammatory cells in listeria meningitis. Monocytes recruitment is a feature of the inflammatory response during the infection (36). This is an important difference as compared to pneumococcal meningitis (5). Monocytes play a crucial role for bacterial clearance, through internalization of L. monocytogenes, recruiting and activation of other monocytes and promotion of bacteria killing by tumor necrosis factor (TNF)- and inducible nitric-oxide (NO) synthase (iNOS)-producing dendritic cells (TipDCs) (37). Although perivascular spread of listeria is suggested, we found relatively mild meningeal arterial inflammation compared to pneumococcal meningitis (5), and a corresponding low proportion of patients with infarction (25%), as compared to pneumococcal meningitis (61%) (5).
Brainstem encephalitis has been described as a specific sub-entity of listeria meningitis (16, 22). Brainstem encephalitis was histopathologically diagnosed and defined by “foci of subacute inflammation with micro-abscesses and immunohistochemically identified listeria bacteria present in brain stem tissue (definite)” or presence of “microglial nodules, diffuse microglial activation or scattered lymphocytes (probable)” (22). Reviewing our study with extensive ventriculitis and presence of periventricular abscesses, the frequently observed abscesses in the brainstem during listeria meningitis could be the consequence of ventricular invasion and extension of bacteria and inflammation through blood vessels. In a listeria rhombencephalitis study in sheep, different stages of neuropathological change were observed after natural course of disease (23). In ruminants, rhombencephalitis is more common than in humans and the route of infection might be different but inflammation and tissue damage are similar. Intraparenchymal lesions first develop close to small vessels in which a progressive increase in bacteria, vascular damage, inflammation with neutrophils, and micro-abscesses are seen. These lesions in sheep were most frequently found in the brainstem and did not have a relationship to meninges, ependymal cells, or cranial nerve tracts (23). However, contradicting studies in animals show intra-axonal entry and transmission of L. monocytogenes has been found with presence of L. monocytogenes in axons, Schwann cells, satellite cells, and ganglionic neurons (38, 39). Intra-axonal migration of L. monocytogenes is in ruminants is possible due to distribution of E-cad expression in satellite cells and myelinating Schwann cells (40). A previous autopsy study in L. monocytogenes brainstem encephalitis patients supported this hypothesis as it showed inflammation in the cranial nerves in 5 of 9 patients, suggesting the cranial nerves may be involved in the route of infection in L. monocytogenes brainstem encephalitis (16). Our study did not focus on the potential intra-axonal route of infection through cranial nerves as these were not systematically sampled, which is a limitation of study. Our data suggest that in patients with listeria meningitis the bacteria spread through blood vessels, cerebrospinal fluid, and immune cells—efferocytosis—into the brain. A limitation of our study is the number of described patients; it is small and therefore broad statistical analyses cannot be performed.
Our neuropathologic study of 5 listeria meningitis cases was characterized by the presence of moderate to severe ventriculitis and abscesses. Monocytes and macrophages were the most prominent inflammatory cells and we have shown (for first time in humans) abundant efferocytosis exploited by L. monocytogenes for cell-to-cell spread. Listeria spreads through the CSF and blood vessels into the brain, although the disease is less likely to cause vasculitis and cerebrovascular complication as compared to pneumococcal meningitis.
This study was supported by the Netherlands Organization for Health Research and Development (ZonMw; NWO-Veni-Grant [916.13.078] to Matthijs C. Brouwer, NWO-Vidi-Grant [016.116.358] to DB), the Academic Medical Center (AMC Fellowship to Diederik van de Beek), and the European Research Council (ERC Starting Grant to Diederik van de Beek).
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. van de Beek D, de Gans J, Tunkel AR, et al. Community-acquired bacterial meningitis in adults. N Engl J Med 2006;354:44–53 [DOI] [PubMed] [Google Scholar]
- 2. Bijlsma MW, Brouwer MC, Kasanmoentalib ES, et al. Community-acquired bacterial meningitis in adults in the Netherlands, 2006–14: A prospective cohort study. Lancet Infect Dis 2016;16:339–47 [DOI] [PubMed] [Google Scholar]
- 3. Lucas MJ, Brouwer MC, van der Ende A, et al. Outcome in patients with bacterial meningitis presenting with a minimal Glasgow Coma Scale score. Neurol Neuroimmunol Neuroinflamm 2014;1:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van de Beek D, Brouwer M, Hasbun R, et al. Community-acquired bacterial meningitis. Nat Rev Dis Primers 2016;2:16074. [DOI] [PubMed] [Google Scholar]
- 5. Engelen-Lee JY, Brouwer MC, Aronica E, et al. Pneumococcal meningitis: Clinical-pathological correlations (meningene-path). Acta Neuropathol Commun 2016;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thigpen MC, Whitney CG, Messonnier NE, et al. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011;364:2016–25 [DOI] [PubMed] [Google Scholar]
- 7. Koopmans MM, Brouwer MC, Bijlsma MW, et al. Listeria monocytogenes sequence type 6 and increased rate of unfavorable outcome in meningitis: Epidemiologic cohort study. Clin Infect Dis 2013;57:247–53 [DOI] [PubMed] [Google Scholar]
- 8. Charlier C, Perrodeau E, Leclercq A, et al. Clinical features and prognostic factors of listeriosis: The MONALISA national prospective cohort study. Lancet Infect Dis 2017;17:510–9 [DOI] [PubMed] [Google Scholar]
- 9. Koopmans MM, Bijlsma MW, Brouwer MC, et al. Listeria monocytogenes meningitis in the Netherlands, 1985–2014: A nationwide surveillance study. J Infect 2017;75:12–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramaswamy V, Cresence VM, Rejitha JS, et al. Listeria—review of epidemiology and pathogenesis. J Microbiol Immunol Infect 2007;40:4–13 [PubMed] [Google Scholar]
- 11. Costerus JM, Brouwer MC, van der Ende A, et al. Community-acquired bacterial meningitis in adults with cancer or a history of cancer. Neurology 2016;86:860–6 [DOI] [PubMed] [Google Scholar]
- 12. van Veen KEB, Brouwer MC, van der Ende A, et al. Bacterial meningitis in patients using immunosuppressive medication: A population-based prospective nationwide study. J Neuroimmune Pharmacol 2017;12:213–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brouwer MC, van de Beek D, Heckenberg SG, et al. Community-acquired Listeria monocytogenes meningitis in adults. Clin Infect Dis 2006;43:1233–8 [DOI] [PubMed] [Google Scholar]
- 14. Gellin BG, Broome CV.. Listeriosis. JAMA 1989;261:1313–20 [PubMed] [Google Scholar]
- 15. Armstrong RW, Fung PC.. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: Case report and review. Clin Infect Dis 1993;16:689–702 [DOI] [PubMed] [Google Scholar]
- 16. Antal EA, Loberg EM, Dietrichs E, et al. Neuropathological findings in 9 cases of listeria monocytogenes brain stem encephalitis. Brain Pathol 2005;15:187–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sepp AH, Roy TE.. Listeria monocytogenes infections in Metropolitan Toronto. A clinicopathological study. Can Med Assoc J 1963;88:549–61 [PMC free article] [PubMed] [Google Scholar]
- 18. Niklasson PM, Hambraeus A, Lundgren G, et al. Listeria encephalitis in five renal transplant recipients. Acta Med Scand 1978;203:181–5 [DOI] [PubMed] [Google Scholar]
- 19. Kirk J. Diagnostic ultrastructure of Listeria monocytogenes in human central nervous tissue. Ultrastruct Pathol 1993;17:583–92 [DOI] [PubMed] [Google Scholar]
- 20. Uldry PA, Kuntzer T, Bogousslavsky J, et al. Early symptoms and outcome of Listeria monocytogenes rhombencephalitis: 14 Adult cases. J Neurol 1993;240:235–42 [DOI] [PubMed] [Google Scholar]
- 21. van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004;351:1849–59 [DOI] [PubMed] [Google Scholar]
- 22. Antal EA, Dietrichs E, Loberg EM, et al. Brain stem encephalitis in listeriosis. Scand J Infect Dis 2005;37:190–4 [DOI] [PubMed] [Google Scholar]
- 23. Cordy DR, Osebold JW.. The neuropathogenesis of listeria encephalomyelitis in sheep and mice. J Infect Dis 1959;104:164–73 [DOI] [PubMed] [Google Scholar]
- 24. Berche P. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb Pathog 1995;18:323–36 [DOI] [PubMed] [Google Scholar]
- 25. Lopez S, Prats N, Marco AJ.. Expression of E-selectin, P-selectin, and intercellular adhesion molecule-1 during experimental murine listeriosis. Am J Pathol 1999;155:1391–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasanmoentalib ES, Brouwer MC, van der Ende A, et al. Hydrocephalus in adults with community-acquired bacterial meningitis. Neurology 2010;75:918–23 [DOI] [PubMed] [Google Scholar]
- 27. Silva MT. Macrophage phagocytosis of neutrophils at inflammatory/infectious foci: A cooperative mechanism in the control of infection and infectious inflammation. J Leukoc Biol 2011;89:675–83 [DOI] [PubMed] [Google Scholar]
- 28. Czuczman MA, Fattouh R, van Rijn JM, et al. Listeria monocytogenes exploits efferocytosis to promote cell-to-cell spread. Nature 2014;509:230–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Golpon HA, Fadok VA, Taraseviciene-Stewart L, et al. Life after corpse engulfment: Phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J 2004;18:1716–8 [DOI] [PubMed] [Google Scholar]
- 30. Vandivier RW, Henson PM, Douglas IS.. Burying the dead: The impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 2006;129:1673–82 [DOI] [PubMed] [Google Scholar]
- 31. Koopmans MM, Brouwer MC, Geldhoff M, et al. Cerebrospinal fluid inflammatory markers in patients with Listeria monocytogenes meningitis. BBA Clin 2014;1:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kobayashi T, Ogawa M, Sanada T, et al. The Shigella OspC3 effector inhibits caspase-4, antagonizes inflammatory cell death, and promotes epithelial infection. Cell Host Microbe 2013;13:570–83 [DOI] [PubMed] [Google Scholar]
- 33. Martin CJ, Booty MG, Rosebrock TR, et al. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe 2012;12:289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rupp J, Pfleiderer L, Jugert C, et al. Chlamydia pneumoniae hides inside apoptotic neutrophils to silently infect and propagate in macrophages. PLoS One 2009;4:e6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spinner JL, Winfree S, Starr T, et al. Yersinia pestis survival and replication within human neutrophil phagosomes and uptake of infected neutrophils by macrophages. J Leukoc Biol 2014;95:389–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. North RJ. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med 1970;132:521–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol 2004;4:812–23 [DOI] [PubMed] [Google Scholar]
- 38. Disson O, Lecuit M.. Targeting of the central nervous system by Listeria monocytogenes. Virulence 2012;3:213–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Henke D, Rupp S, Gaschen V, et al. Listeria monocytogenes spreads within the brain by actin-based intra-axonal migration. Infect Immun 2015;83:2409–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Madarame H, Seuberlich T, Abril C, et al. The distribution of E-cadherin expression in listeric rhombencephalitis of ruminants indicates its involvement in Listeria monocytogenes neuroinvasion. Neuropathol Appl Neurobiol 2011;37:753–67 [DOI] [PubMed] [Google Scholar]