Abstract
Inflammatory bowel disease (IBD) describes chronic relapsing remitting inflammation of the gastrointestinal tract including ulcerative colitis and Crohn’s disease. The prevalence of IBD is rising across the globe. Despite a growing therapeutic arsenal, current medical treatments are not universally effective, do not induce lasting remission in all, or are accompanied by short- and long-term adverse effects. Therefore, there is a clinical need for novel therapeutic strategies for IBD. Current treatments for IBD mainly manipulate the immune system for therapeutic gain by inhibiting pro-inflammatory activity. There is a robust endogenous immunoregulatory capacity within the repertoire of both innate and adaptive immune responses. An alternative treatment strategy for IBD is to hijack and bolster this endogenous capability for therapeutic gain. This review explores this hypothesis and presents current evidence for this therapeutic direction in immune cell function, cytokine biology, and alternative mechanisms of immunoregulation such as microRNA, oligonucleotides, and the endocannabinoid system.
Keywords: inflammatory bowel disease, immunoregulation, cytokine, ulcerative colitis, Crohn’s disease, treatment
INTRODUCTION
Inflammatory bowel disease (IBD) describes relapsing, remitting chronic inflammation of the gastrointestinal (GI) tract. The main phenotypes are ulcerative colitis and Crohn’s disease. The prevalence of IBD remains highest in Europe and the United States and continues to rise across the globe.1 The etiology of IBD is complex and involves dynamic interplay between genetics, the immune system, and environmental exposures including intestinal microbial dysbiosis.1 Advances in IBD research has widened therapeutic opportunities and impact on disease treatment and natural progression.
Current treatments for IBD aim to inhibit pro-inflammatory immune responses, targeting both inflammatory cells and cytokines. There are several agents available to achieve this including steroids, thiopurines, and biologic antitumor necrosis factor (TNF) and anti-integrin therapies. However, not all patients are responsive to these drugs.2 There are emerging novel biologics for IBD: for example, anti-p40 biologic targeting interleukin (IL)-12 and IL-23 offering multiple cytokine inhibition. Trials have suggested that these new biologics are not globally effective across all IBD patient cohorts.3 Furthermore, currently available drugs are associated with serious adverse side effects including susceptibility to infection, bone marrow suppression, liver dysfunction, and increased risk of malignancy.2 Therefore, there is a clinical need for newer, safer treatments for IBD.
There is a robust endogenous immunoregulatory capacity within the repertoire of both innate and adaptive immune responses. An alternative treatment strategy for IBD could be to hijack and bolster this endogenous capability for therapeutic gain. This review explores this hypothesis and presents current evidence for this therapeutic direction.
IMMUNE CELL POPULATIONS
Regulatory T Cells
Regulatory T cells (Tregs) are vital contributors to immune homeostasis. There are various subsets of Tregs, including IL-10 producing Tr1 cells, CD4+CD25+Foxp3+ or CD4+CD25-Foxp3+ Tregs, and IL-35 producing T cells.4 Foxp3+ Tregs include thymic-derived Tregs (for central tolerance) and peripheral induced CD4+ T cells (for local tolerance). Thymic-derived Tregs naturally express Foxp3, and peripheral CD4+ T cells can upregulate Foxp3 under certain circumstances, such as in response to pro-inflammatory cytokines.4
In the intestine, Tregs secrete anti-inflammatory cytokines and modulate T-cell function to exert immunosuppressive activity. Tregs also downregulate costimulatory molecules on antigen-presenting cells to maintain immune tolerance through expression of T-cell receptors specific for enteric flora and dietary antigens. Imbalance between Treg and effector T-cell populations is an important mechanism underlying IBD pathogenesis.4,5
Patients in IBD remission express more CD4+CD25high and Foxp3+ Tregs in their peripheral bloodstream, compared with patients with active disease.6 These cells maintain their suppressive role, determined by proliferative suppression of CD4+C25- T-cells, correlated with disease activity and CRP levels.6,7 In the gastrointestinal mucosa, patients with IBD express more CD4+CD25high and Foxp3+ Tregs compared with noninflamed mucosa.6 Compared with normal mucosa, patients with ulcerative colitis express more Foxp3+ Tregs in their inflamed and noninflamed mucosa. Expression appears predominantly in the lamina propria.8 In mesenteric lymph nodes of patients with ulcerative colitis, CD4+CD25+ cells express Foxp3 and suppress the proliferation of autologous CR4+CD25- cells.8 Overall, IBD is associated with reduced peripheral and modestly increased Tregs in the colonic mucosa. Whilst many questions remain unanswered in Treg biology, the question relevant to this review is potential promotion of the potent anti-inflammatory activity of Tregs as a therapeutic strategy for IBD.
Promotion of the anti-inflammatory functions of Tregs can be achieved indirectly through multiple diverse actions, and there is a broad base of investigative work in the literature to this effect. Intestinal dysbiosis plays a pivotal role in IBD pathogenesis.1 Indeed, bacterial components are important for Treg function, for example, spore-forming clusters IV and XIVa of the Clostridium genus promote Treg accumulation in the colons of mice.9 Hence, manipulation of intestinal bacteria may bolster the mucosal Treg population and promote a tolerogenic microenvironment. Butyrate, an energy source for colonocytes, can promote Treg activity and suppress serum and colonic mucosal levels of IL-17, leading to amelioration of TBNS-induced colitis in rats.10 Lactoferrin, an endogenous pleiotropic protein secreted at mucosal sites, can modulate inflammation by shifting the phenotype of CD4+ T cells away from Th17 and towards Tregs.11
Oral immune therapies introduce low-dose antigens to the gut to manipulate the local host immune system, and this strategy can preferentially expand mucosal Treg populations to promote oral antigenic tolerance. There have been numerous promising preclinical studies and a few clinical trials that have suggested benefit in IBD, as comprehensively reviewed elsewhere.12 An example of this strategy is the oral administration of nonabsorbable, autologous colonic protein-derived antigens (Alequel). This ameliorated immune-mediated colitis in mice and induced remission in human Crohn’s disease in phase I and II trials.13–16 The mechanism is not fully understood but likely involves promotion of Treg cells, upregulation of IL-10, and an altered CD4+/CD8+ ratio.
Another example of enhancing Treg responses as part of a wider immunosuppressive repertoire is the use of anti-CD3 monoclonal antibody biologics, such as otelixizumab. The mechanism of action is multifactorial, including inhibition of antigenic stimulation by internalization of the CD3/TCR complex inhibiting antigenic stimulation, promotion of effector T cell apoptosis, and amplification of TGFβ signalling to create a tolerogenic microenvironment via promotion of Treg and tolerogenic dendritic cell populations.17 These novel biologics have been assessed in different autoimmune conditions, with particular focus in type 1 diabetes mellitus. In a murine model of IBD, the use of an anti-CD3 antibody attenuated T cell transfer-induced enterocolitis.18 These agents have been trialled in humans in both acute-severe ulcerative colitis19–21 and Crohn’s disease.22 Although initial small studies were optimistic, larger studies showed this agent was not effective in acute-severe UC and was associated with an unacceptable toxicity profile. Different biologics to the CD3 receptor are in clinical development and emerging, and this is likely to continue as a future area of therapeutic development in IBD.
Can we use regulatory T cells directly as a therapy? Adoptive transfer of autologous Tregs has emerged as another promising therapeutic avenue in IBD. This strategy of enhancing endogenous cell-based immunoregulation has been shown to be effective in preclinical models.23–28 For example, adoptive transfer of CD4+CD25+ Tregs in a T cell transfer model of murine IBD significantly reversed clinical and histological markers of colitis by migrating to the intestine, proliferating and directly interacting with effector T cells.26 Challenges in application of this treatment to humans include in vitro cell expansion to achieve an effective dose, potential plasticity of the expanded cells toward a pro-inflammatory phenotype, and ensuring an effective and consistent homing to the gastrointestinal tract on infusion. Canavan et al published data to show successful isolation, characterization, and expansion of a specific Treg population deemed suitable for this application.29 A clinical trial (TRIBUTE) to assess these cells in human Crohn’s disease is in the early stages of recruitment (UK Trials Gateway; ISRCTN97547683).
Another group reported data from a 12-week, open-label, multicenter phase 1/2a clinical trial to investigate the safety and efficacy of adoptive transfer of a different Treg subset, namely intravenous ovalbumin-specific Tr1 Tregs in humans, expanded from peripheral mononuclear blood cells in refractory Crohn’s disease.30 This treatment was efficacious in 40% of patients, dose-dependent (lower dose was more efficacious), and well tolerated with few adverse effects. A larger multicenter, international, randomized, placebo controlled study of this antigen specific Treg therapy (Ovasave) began but terminated early (NCT02327221). The early termination of this study was reportedly due to manufacturing difficulties, and the trial is to recommence recruitment (https://www.biopharma-reporter.com/Article/2016/05/27).
The therapeutic potential of this cell population may extend beyond quashing inflammation to hindering colitis-associated colon cancer, making further study of these populations paramount.31
Immunoregulatory B Cells
B cells are an integral part of the adaptive immune system; they produce antibodies, secrete cytokines, and can also communicate with other cells including Tregs to maintain gut homeostasis.32 In humans, therapies that target B cells (eg, rituximab) are not effective in IBD and can clinically exacerbate or even induce colitis.33 This observation affirms an important, protective role of B cells in maintaining intestinal homeostasis.
In recent years, interest has grown around a “regulatory” phenotype that can secrete IL-10, IL-35, and transforming growth factor (TGF)-β to maintain peripheral tolerance.34 In this review, IL-33 and IL-35 are discussed later as anti-inflammatory cytokines with a therapeutic potential worth exploiting for therapeutic gain. The IL-10-producing B cells are present in the gastrointestinal mucosa of patients with active Crohn’s disease and ulcerative colitis compared with healthy controls.35
Sattler et al reported that intraperitoneal IL-33 injection into IL10-/- mice exacerbated colitis, whereas IL-33-treated wild type mice (with intact IL-10 receptors) did not develop colitis.36 Upon investigating the underlying mechanism, there was no change in the frequency of CD25+ or Foxp3+ Tregs but an increase in IL-10 producing B cells (BregIL-33) in wild type mice—but not IL10-/- mice. Adoptive transfer of these BIL-33 cells transferred into IL-10-/- mice led to histological and clinical improvements in colitis. Taken together, this important study suggests that IL-10 deficiency reduces the function of this immunoregulatory cell population.
In support of these findings, intravenous adoptive transfer of peritoneal cells into mice with DSS-induced colitis improved disease activity scores, mucosal recovery, and survival rates.37 The mechanism is likely through dual activity of IL-10 and TGF-β secreted by natural regulatory B cells (and also macrophages).37 Adoptive transfer of these immunoregulatory B cells may therefore be an attractive treatment in the future, but this needs further assessment in preclinical models of IBD alongside a greater understanding of the biological function of the cells in the human gastrointestinal tract.
Tolerogenic Dendritic Cells
Dendritic cells are professional antigen-presenting cells that induce immune tolerance within the gastrointestinal mucosa to luminal contents.38
The mechanisms that underlie the anti-inflammatory role of dendritic cells is not fully defined. Their influence over pro-inflammatory T cells and Treg development is likely vital to protective functions. For example, CD103+ dendritic cells isolated from mesenteric lymph nodes can induce the differentiation of naïve CD4+ T cells into Foxp3+ Tregs.39 The CD103+ dendritic cells are downregulated during experimental colitis and produce thymic stromal lymphopoietin in response to TLR activation, which suppresses the secretion of IL-17 from T cells and promotes Foxp3+ Treg development.40 In human IBD, patients with Crohn’s disease have more CD103+ lymphocytes in their lamina propria compared with patients with ulcerative colitis or healthy controls.41
The anti-inflammatory capabilities of CD103+ dendritic cells are reduced in murine colitis models.42 The ability of CD103+ dendritic cells to promote the development of Foxp3+ Tregs is significantly impaired in T cell–induced colitis in RAG-/- mice, and instead, CD103+ dendritic cells appear to favor the development of interferon-γ-producing CD4+ T cells in the inflammatory microenvironment.42 This is discouraging when considering the application of dendritic cells as cell therapy for IBD. Nevertheless, there have been some encouraging data. Vasoactive intestinal peptide-induced dendritic cells can induce Tregs, which show a protective and therapeutic value in trinitrobenzene sulfonic acid (TNBS)-induced murine colitis.43 Granulocyte-colony stimulating factor (G-CSF) induces IL-10-secreting T cells in the peripheral blood, as well as plasmacytoid dendritic cells in the lamina propria in human Crohn’s disease patients who clinically respond to G-CSF therapy.44
Colonic microbiota play a role in the pathogenesis of IBD.1 These bacteria impact mucosal dendritic cell function, and there is evidence that this interaction can lead to promotion of immunoregulatory mechanisms including oral tolerance.45 The CD103+CD11b+ dendritic cells can recognize flagellin in the lamina propria and upregulate IL-23, which induces epithelial cells to express RegIII-γ, a bactericidal c-type lectin, in response to TLR-5 activation.46 Mice treated with cecal bacterial antigen-pulsed regulatory dendritic cells secrete high levels of IL-10 and ameliorate colitis in T-cell transfer-induced murine colitis.47 In the same study, regulatory dendritic cells also induced the differentiation of naïve CD4+ T cells into CD4+CD25+Foxp3+ T cells in vitro and in vivo. These findings support the growing hypothesis that the gut microbiota can shape dendritic cell populations. The question is whether this can be targeted for therapeutic gain in IBD.
In keeping with this hypothesis, Bifidobacterium infantis increases CD103+ and retinoic acid–metabolizing dendritic cells that induce Foxp3+ Tregs and suppress Th17 cells.48 This partially explains why Bifidobacterium infantis can suppress IL-17 production in DSS-induced murine colitis and induce IL-10 in vitro.49 Taken together, it would appear that adoptively transferred tolerogenic dendritic cells could prove therapeutically beneficial for IBD, and this continues to be explored in preclinical models. However, it is clear that dendritic cell plasticity and function in the gastrointestinal mucosa are complex, with conflicting data, and further work will be required to translate to human disease. In a single-center phase 1 trial, 9 patients with treatment-refractory Crohn’s disease were treated with autologous transfer of tolerogenic dendritic cells. These cells were derived from blood monocytes in vitro and delivered by ultrasound-guided intraperitoneal injection. The treatment was well-tolerated, induced remission in 1 patient, and showed good clinical response in 2 patients.50 Additional clinical trials of this therapy are recruiting (Intralesional Tolerogenic Dendritic cells in Crohn’s Disease Treatment [TolDecCDintra], NCT02622763).
Further mechanistic studies are required to better understand dendritic cell biology in the gastrointestinal tract and identify the target patient population most likely to benefit from this cell therapy.
Innate Lymphoid Cells
Innate lymphoid cells (ILC) are a recently discovered population of immune cells.51 These ILCs are subclassified into type 1, type 2 and type 3, based on transcription factor association, cell surface marker expression, and cytokine response.51 Unlike T cells and B cells, ILCs do not express recombined antigen-specific receptors.52 The biology of ILCs has been summarized elsewhere.51–53 In summary, type 1 ILCs constitutively express transcription factor T-bet and are activated in response to IL-12 and IL-18. They secrete type 1 cytokines including interferon-γ. Their key role is to promote immunity to intracellular bacteria, viruses, and parasites. Type 2 ILCs express GATA3 and ROR-α and are activated in response to alarmins such as IL-25, IL-33, and thymic stromal lymphopoietin. They secrete type 2 cytokines including IL-4, IL-5, and IL-13. Their key role is to promote immunity to helminths. Type 3 ILCs constitutively express ROR-γt and are activated in response to IL-23, IL-1α, and IL-1β. They secrete IL-17 and IL-22. Their key role is to promote immunity to extracellular bacteria, especially to maintain tolerance to commensal gut microbiota.
In IBD, patients with Crohn’s disease have an increased expression of intraepithelial type 1 ILCs within the colonic mucosa.54 These cells produce interferon-γ and worsen colitis in anti-CD40–induced murine colitis, so they may contribute to disease pathogenesis.54 Suppressing the expression and function of such cells may be therapeutically advantageous. Type 3 ILCs play a role in maintaining intestinal homeostasis and could be a promising cellular target. Specifically, a subset termed IL-22 producing NCR+ (CD56NKp44) type 3 ILCs make up around 5% of normal colonic lymphocytes, resist mucosal infection, and promote epithelial barrier integrity.55 Although present in small numbers relative to other lymphocytes, ILCs exert a potent effect on immune responses.56 The NCR+ subtype confers protection in DSS-induced and T cell transfer murine colitis, through increased secretion of IL-22 resulting from retinoic acid signaling57 or expression of aryl hydrocarbon receptor (essential for the maintenance and function of type 3 innate lymphoid cells).58
The emergence of this new cell type with a key role in the arena of gastrointestinal mucosal immunity offers a potential new therapeutic target in IBD. However, a significant amount of preclinical investigation and a better characterization of their role in the human GI tract in health and disease is required before translational studies can be considered and developed. Caution is warranted as ILCs are central to the development of inflammation-associated colonic cancer through IL-22 stimulation of epithelial cell proliferation.59
ANTI-INFLAMMATORY CYTOKINES
IL-10
Therapeutically enhancing interleukin (IL)-10—the quintessential anti-inflammatory cytokine—showed promising efficacy in preclinical models of IBD, but unfortunately human trials to date have failed to show a direct translational benefit in human patients.60 This is likely to reflect the differences in biology from murine models to human disease alongside considerations in patient recruitment, disease characterization, and cytokine delivery. As such, there still is interest at progressing this therapeutic strategy forward. For example, a phase 1, double-blind, randomized control trial to assess safety, tolerability, and pharmacology of IL-10 was commenced but prematurely terminated in 2016 due to potential risk of further dosing in healthy subjects (NCT02711462). Currently, a phase 2a, double-blind, randomized control trial is underway to assess IL-10 as an add-on therapy to infliximab in patients with active ulcerative colitis (NCT03269695).
Back in the laboratory, success of preclinical studies have fuelled the race to identify novel anti-inflammatory cytokines with translational capability to bolster the immunoregulatory capacity of the immune system to sequester inflammation, often by indirectly upregulating IL-10.
IL-27
The known biology of interleukin-27 (IL-27) has recently been reviewed.61 In brief, interleukin-27 is a heterodimeric, type-1 cytokine expressed by many cell types including dendritic cells, macrophages, plasma cells, epithelial cells, and endothelial cells. Interleukin-27 is composed of Epstein-Barr virus–induced gene 3 and p28 subunits, and the IL-27 receptor is composed of 2 subunits: gp130 and IL27Rα. Upon receptor binding, Janus Kinase/STAT, PI3K/Akt, or MAPK signalling pathways are activated. This results in phosphorylation of STAT1, STAT3, STAT5, and STAT6. The best known downstream effects of IL-27 involve regulating T cell responses (eg, inhibition of the lineage commitment of Th17 cells by blocking retinoic acid receptor–related orphan receptor (ROR)-γt, preventing Fas-mediated activation-induced cell death, and inhibiting Th2 differentiation and cytokine expression through increasing T-bet and suppressing GATA-3 expression) and inducing IL-10 production from Tr1 T regulatory cells.
An observational, cross-sectional study of 54 IBD patients reported that IL-27 mRNA is increased in patients with active Crohn’s disease compared with inactive disease, with higher mucosal IL-27 protein expression in patients with active ulcerative colitis, compared with tissue controls or active Crohn’s disease.62 The biological consequences of this expression profile in the inflamed mucosa is unclear, although a protective effect is suspected through extrapolation of IL-27-mediated immunosuppressive capabilities.61
There have been several murine studies investigating the role of IL-27 in different colitis models. In T cell transfer models, IL-27 is essential for enhancing Treg function and enabling IL-10 secreting B cells to attenuate colitis.63, 64 In DSS models, colitis is more severe and survival reduced in IL-27-R-/- mice.65 IL27-R-/- in RAG-/- mice also suffer more severe colitis compared with RAG-/- mice with intact IL-27 receptors.65 Hanson et al demonstrated that mucosal delivery of IL-27, by a Lactococcus lactis bacterial vector, is therapeutically beneficial in both T cell transfer and DSS models of murine colitis and is dependent upon increased IL-10 production by T cells in the gastrointestinal mucosa.66 Mucosally delivered IL-27 attenuated innate cell-driven acute murine colitis through IL-10 dependent, T cell independent mechanisms including inhibition of chemokine gradient and neutrophil infiltration.67 These data suggest that IL-27 promotes immunosuppression through IL-10 amplification. However, neutralization of IL-27 worsens colitis in IL-10-/- mice, indicating other mechanisms are also important.68 Furthermore, IL-27 can suppress Th17 and Th1 differentiation independent of IL-10.69 Treating murine colitis (induced by both TNBS and DSS) with single-chain human IL-27, delivered by subcutaneous osmotic pumps, clinically improves colitis through suppression of IL-17 producing T-helper cells.69 Therefore, IL-27 appears capable of attenuating colitis by IL-10 dependent and independent mechanisms.
However, there is conflicting murine data in the literature that require further consideration. It has been reported that IL-27 promotes colitis, enhancing IL-6 and IL-1β production and promoting Th17 differentiation; loss of IL27Rα has been associated with clinical improvements in murine colitis models.70
Most of this data arises from preclinical models. The role of IL-27 in human IBD requires characterization before IL-27-directed therapy for IBD can be further explored in the clinical arena.
IL-33
Interleukin-33 (IL-33) is a member of the IL-1 family of cytokines and is constitutively expressed in the nucleus of most epithelial, endothelial, and stromal cells where it regulates gene expression through stability of chromatin structure.71 Interleukin-33 can sequester NF-κB to dampen pro-inflammatory responses and can be released into the extracellular environment to function as an “alarmin” or damage associated molecular pattern.71 Immune cells can actively secrete IL-33 in response to pro-inflammatory cytokines such as TNF, IL-1β, IL-3, and IL-4.71 Given the complexities of pro- inflammatory vs anti-inflammatory effects, the role of IL-33 in IBD remains unclear. Again, most data have been derived from preclinical models of IBD, and the translational impact of this to the human disease is not yet defined.
In the gastrointestinal tract from an anti-inflammatory perspective, IL-33 and its receptor ST2 are expressed on colonic Tregs.72 IL-33 promotes TGF-β1-medited Treg differentiation and the accumulation of Tregs in an inflammatory environment.72 Interleukin-33 promotes IgA production (important for maintaining gut-microbial homeostasis) and prevents IL-1α-dependent colitis and colitis-associated colon cancer.73 Intraperitoneal-delivered recombinant IL-33 in DSS-induced murine colitis clinically, macroscopically, and histologically improves inflammation through reducing Th17 and Th1 cell populations in the lamina propria, effectively switching from a Th1-mediated to Th2-mediated response.74 Conversely, IL-33 can drive eosinophilic infiltration and potent mucosal adaptive immune responses, which lead to chronic ileitis.75 Genetic ablation of or monoclonal antibodies against ST2 can significantly improve colitis in murine models. Administration of IL-33 has also been shown to increase intestinal permeability.76
Is there any information from human studies? In a large pediatric and adult patient cohort, Latiano et al reported that single nucleotide polymorphisms in the IL-33 gene (such as rs3939286) are associated with IBD susceptibility.77 Pediatric carriers of the IL-33 polymorphism were 44% more likely to respond to steroids compared with those who did not carry the risk allele. Adult patients carrying the risk allele were more likely to have extensive, severe disease. Patients with IBD also had increased IL-33 mRNA expression in inflamed mucosa.77 Studies have confirmed a significant relationship between increased IL-33 expression, increased severity of disease and ulcerative colitis disease phenotype, and similar to IL-33, ST2 expression is increased in the intestinal mucosa in patients with UC and is positively associated with disease activity: ST2 has been proposed as an activity biomarker for predicting clinical outcomes in UC.78, 79
Although we know IL-33 is increased in human IBD, the biological consequence of this is unclear. It would appear that IL-33 plays an important role in IBD pathogenesis, although the underlying mechanisms are not yet characterised in vivo.
IL-35 and IL-37
Interleukin 35 (IL-35) and interleukin 37 (IL-37) are 2 recently discovered cytokines whose anti-inflammatory properties have attracted interest in IBD. Interleukin 35 is a member of the IL-12 family and is produced by CD4+Foxp3+ Tregs, activated B cells, and likely tolerogenic dendritic cells. Interleukin 35 regulates IL-10-producing B cells and suppresses Th17 responses in vitro.80 Interleukin 37 is a member of the IL-1 cytokine family, and although cytokines in this family tend to be pro-inflammatory, IL-37 can function as part of a negative feedback mechanism to limit inflammation in an IL-10-independent fashion.81
Gene and protein expression of IL-35 is higher in colonic mucosa from adult patients with IBD compared with healthy controls.82 Interleukin 37 expression in infiltrating immune cells and intestinal epithelial cells is also increased.83 Although reduced levels of the cytokines themselves have been reported in the peripheral blood of IBD patients compared with healthy controls,82 other studies reveal an increased presence of circulating IL-35-expressing CD4+ and CD8+ T cells and increased IL-37-producing CD14+ monocytes, -CD56+ natural killer cells, and -CD19+ B cells in patients with IBD, compared with healthy controls or patients with inactive disease.35
Gene expression of major splice variant IL-37b is increased in the inflamed mucosa in patients with ulcerative colitis and Crohn’s disease and is undetectable in normal colonic mucosa.84 Expression is predominantly in the epithelia and infiltrating immune cells of the inflamed mucosa.85 The consequence of this is not clear, but a clue may come from a study where IL-37b gene transfer enhanced the therapeutic effects of mesenchymal stromal cells in the resolution of DSS-induced murine colitis.85 In DSS-induced colitis in a transgenic mouse expressing human IL-37 under a CMV promoter, IL-37 expression was only detectable in the colon when the epithelial barrier had been disrupted.86 Although IL-37 increases IL-10, the protective effects of IL-37 do not disappear upon blocking IL-10R in IL-37 transgenic mice—the protective effects are not dependent upon IL-10 production and are yet uncharacterised.
There is limited data on the biological function of IL-35 in the gastrointestinal tract. Collison et al reported that IL-35 can induce the generation of a unique subpopulation of Tregs—IL-35-producing Tregs—in vitro and in vivo, that can function independently of IL-10 and TGF-β.87 Both IL-35 and IL-27 belong to the IL-12 family of heterodimeric cytokines, share the Epstein-Barr virus induced gene protein 3 subunit, and are upregulated in IBD.61,80 In this regard, Wirtz et al investigated the differential role of these 2 cytokines through DSS-induced colitis, TNBS-induced colitis, T cell transfer, and spontaneous colitis models in IL-27p28-/- and EB13-/- mice.88 The study found IL-27p28 (the unique subunit of IL-27)-deficient mice were similar to wild type mice, whereas Epstein-Barr virus induced gene protein 3 (shared subunit)-deficient mice developed severe or worsened colitis, with decreased survival. In addition, the administration of single-chain IL-35 prevented the development of colitis through suppressing Th1 and Th17 responses.88
Interleukin 37 and interleukin 35 thus offer promising therapeutic potential in modulating inflammation in IBD, independent of IL-10. Again, the role of these cytokines in human IBD requires characterization before the therapeutic potential can be fully understood. Perhaps these cytokines will offer the greatest efficacy in patients with an IL-10 high expression phenotype.
ENDOGENOUS MOLECULAR TARGETS TO REGULATE IMMUNE RESPONSES
miRNA
A micro RNA (miRNA) is a small, single-stranded, noncoding, hairpin ribonucleic acid (RNA) approximately 22 nucleotides long. Transcribed miRNA binds to a specific mRNA and induces its degradation. One of the first microarray-based studies in this field found that patients with ulcerative colitis had a differential expression of miRNAs, distinct from those expressed in patients with irritable bowel syndrome, Crohn’s disease, microscopic colitis, or infectious colitis.89 There was also differential expression of miRNAs in patients with active ulcerative colitis compared with latent disease. Specifically, miRNA-192 was reduced during active ulcerative colitis. Subsequently, a differential expression of miRNA between ileal and colonic Crohn’s disease has been reported.90 This endogenous method of post-transcriptional gene regulation is therefore of interest in IBD pathogenesis and possibly highlights an opportunity to finely tune inflammatory activity.
The miR-192 targets the chemokine macrophage-inflammatory peptide 2α, which increases the migration of neutrophils and lymphocytes to the intestinal mucosa.89 The miR-141 can suppress expression of the chemokine CXCL12β and, thus, CXCL12β-mediated T cell, neutrophil, and macrophage migration to the inflamed gut.91 Therapeutic upregulation of miRNA-141 in TNBS-induced murine colitis reduces leukocyte recruitment to inflamed intestinal mucosa.91 The miR-146 is upregulated upon TLR activation and targets the IL-1 receptor–associated kinase and TNF receptor–associated factor 6, upstream of NF-κB, to limit inflammation in a negative feedback fashion.92
Upregulation of certain miRNAs may limit inflammation. For example, miR-155 is thought essential for Treg development and, although insufficient to independently control Treg function, it can augment other miRNAs in doing so.93
The miR-10a suppresses Th17 and Th1 responses and downregulates the expression of IL-12/IL-23p40 and nucleotide-binding oligomerization domain-containing protein 2 (NOD2) on dendritic cells. This reduces the secretion of IL-12 and IL-23.94 Interestingly, miRNA-10a is decreased in the inflamed colonic mucosa and peripheral blood mononuclear cells in patients with IBD and is later upregulated following anti-TNF-α treatment.94 This observation may partly explain the therapeutic mechanism of anti-TNF biologic therapy.
NOD2 plays a critical role in modulating a protective inflammatory response and in maintaining intestinal homeostasis.95 NOD2 polymorphisms in Crohn’s disease commonly lead to epithelial barrier dysfunction and chronic inflammation and likely impact microbial dysbiosis.95 The miR-146a is a nitrous oxide–triggered, downstream messenger of NOD2 signalling that promotes pro-inflammatory responses through sonic hedgehog signalling and expression of the transcription factor and cell fate protein NUMB.96 The miRNA-192, 495, 512, and 671 were found to decrease NOD2, downregulate NF-κB, and inhibit IL-8 and CXCL3 mRNA in colonic epithelial HCT116 cells.97 However, miR-192 was not associated with NOD2 regulation in a pediatric cohort.98 Nonetheless, further characterization is required, and miRNA may be a therapeutic target for patients with NOD2 mutation phenotypes of IBD.
MicroRNAs also offer an opportunity to therapeutically target the intestinal epithelium directly. In human ulcerative colitis and DSS-induced murine colitis, miR-150 targets the transcription factor c-Myb, which leads to decreased apoptosis,99 suggesting that upregulation of miR-150 may be therapeutic to protect the epithelial barrier.
MicroRNAs with a pro-inflammatory role also exist, including miRNA-301a, which increases TNF-α production and Th17 cell differentiation.100 In these circumstances, an antisense miRNA may have therapeutic benefit. Proposed RNA interference targets are summarized in Table 1.
Table 1:
miRNA MANIPULATION FOR THERAPEUTIC CONSIDERATION
| miRNA | Proposed Direction of modulation | Potential Therapeutic Effect |
|---|---|---|
| miR-192 | Increase | Decreases migration of neutrophils and lymphocytes to the intestinal mucosa |
| miR-141 | Increase | Reduce CXCL12β-mediated T-cell, neutrophil and macrophage migration to the inflamed bowel |
| miR-146 | Increase | Reduce inflammation, downstream of IL-1 receptor, through blocking NF-kB pathway |
| miR-146a | Decrease | Decrease inflammation downstream of NOD2 |
| miR-155 | Increase | Promote Treg function |
| miR-10a | Increase | Reduced inflammation by suppressing Th17 and Th1 responses and secretion of IL-12 and IL-23 |
| miR-192 | Increase | Downregulate NF-κB and inhibit IL-8 and CXCL3 |
| miR-495 | ||
| miR-512 | ||
| miR-671 | ||
| miR-150 | Increase | Decreases apoptosis |
| miR-301a | Decrease | Reduce TNF-a production and Th17 cell differentiation |
Clinical studies exploring this treatment strategy are lacking for now. A phase 1 trial examining miRNA-RX34 in liver cancer was prematurely terminated after 5 immune-related serious events were reported (NCT01829971). A better understanding of the clinical safety and utility, along with identification of miRNAs that upregulate immunosuppressive host responses, will be of future interest as a novel future therapy in IBD.
Oligonucleotides
An oligonucleotide describes a short, synthetic DNA or RNA molecule. The therapeutic rationale is similar to miRNA—mucosal delivery binds to target nucleotide sequences and stimulates or inhibits effector pathway gene expression. The most successful studies to date using this strategy in IBD have exploited the TGFβ pathway. TGFβ is a multifunctional cytokine with immunosuppressive effects, produced by many immune and nonimmune cells.101 TGFβ is important in controlling many cellular functions, including proliferation, differentiation, cell-cycle regulation, wound healing, and angiogenesis. Loss of the TGFβ-signalling pathway is associated with IBD.102, 103 The endogenous regulatory protein SMAD7 blocks TGFβ-mediated inhibition of NF-κB, through activating NF-κB inhibitor-α.104, 105 In Crohn’s disease, TGFβ signalling is inhibited by high levels of SMAD7 protein, resulting in unregulated production of pro-inflammatory cytokines.105 Antisense SMAD7 oligonucleotide (Mongersen) has been developed as a therapeutic agent for use in IBD, blocking mRNA of SMAD7, inhibiting protein production, and removing mucosal presence of this endogenous regulator of TGFβ signalling. Mongersen appeared safe and capable of improving endoscopic and clinical markers of colitis, as well as inducing remission in patients with active Crohn’s disease in phase 2 trials.105–107 Following this, a phase 3, double-blind, randomized control trial commenced, investigating the efficacy of Mongersen as a maintenance therapy in Crohn’s disease (NCT02641392). However, this was prematurely discontinued based on lack of emerging benefit, and further developments are awaited.
Toll-like receptor 9 (TLR9) is expressed in numerous antigen-presenting cells including dendritic cells, B cells, and macrophages.108 Activation of TLR9 occurs upon binding of cytosine-guanine nucleotide sequences present on microbial DNA fragments and synthetic oligonucleotides. In intestinal epithelial cells, if the apical surface is stimulated, the noncanonical NF-κB pathway is activated, resulting in release of anti-inflammatory molecules such as IL-10.109–111 In the laboratory, the antimicrobial peptide cathelicidin and synthetic oligonucleotides are capable of inducing this pathway.112 Although both show translational potential, human studies concentrate on synthetic oligonucleotides. A double-blind, placebo-controlled, randomized control trial of a TLR9 agonist in 131 patients with moderate to severe ulcerative colitis was performed.110 Although not sufficient to induce clinical remission compared with placebo, TLR9 oligonucleotide DIMS0150 induced symptomatic remission, mucosal healing, and histological improvement in colitis at 4 weeks. A previous open-label phase 2a trial showed that, of the 22 patients enrolled, oral administration of TLR9 oligonucleotide BL-7040 induced remission in 2 patients and mucosal healing in 8 patients. Mucosal neutrophil and IL-6 levels were also reduced.111 Because these findings are modest, additional trials are required.
Therefore, oligonucleotides can inhibit (SMAD7) or stimulate (TLR9) critical pathways to bolster immunoregulatory capability for therapeutic gain in IBD.
The Endocannabinoid System
This system involves multiple endogenous ligands, enzymes, and molecules responsible for cannabinoid biosynthesis and cellular uptake.113 The cannabinoid Δ9-tetrahydro cannabinol binds to the G-protein coupled receptors termed CB1 and CB2.
The CB1 and CB2 signalling pathways reduce intestinal inflammation.113 Inflammation can upregulate endogenous cannabinoid levels that impact intestinal motility. Although both receptors seem to be important, they are differentially expressed in both normal and inflamed intestinal mucosa.114,115 In healthy colon, CB1 receptors are expressed on the epithelium, smooth muscle, and submucosal myenteric plexus where they contribute to wound healing. Both CB1 and CB2 receptors are expressed on plasma cells in the lamina propria. In IBD, patients have increased expression of colonic epithelial CB2 receptors.114 The CB2 receptors are expressed on macrophages and the immune cell infiltrate during colitis, whereas CB1 receptors are upregulated in enteric neurons and endothelium.114–116 Clearly, regulating intestinal motility is not the only important function of this system; there is also immunoregulatory capability.
The CB2 agonists can ameliorate cytokine-mediated colonic inflammation in explant tissue, without affecting epithelial permeability.117 They can also reduce reactive oxidative species production within intestinal epithelial cells and ameliorate murine experimental colitis.118 Central cannabinoid receptors have a greater intestinal anti-inflammatory effect compared with peripheral receptors.119
The CB2 agonism could be a new therapeutic direction for IBD. Activation of the CB2 receptor does not trigger the psychotropic side effects associated with cannabinoids, making this a clinically attractive therapeutic target.120
There are several ways to bolster the endogenous endocannabinoid system, including promoting biosynthetic enzymes and inhibiting CB1 and CB2 ligand degradation pathways. For example, blocking cellular uptake and intracellular degradation pathways through VDM11 (an endocannabinoid membrane transport inhibitor), a fatty acid amide hydrolase inhibitor, or a combination of these reduces intestinal inflammation through increasing the concentration of anandamide, a fatty acid neurotransmitter degraded by fatty acid amide hydrolase inhibitors.121,122
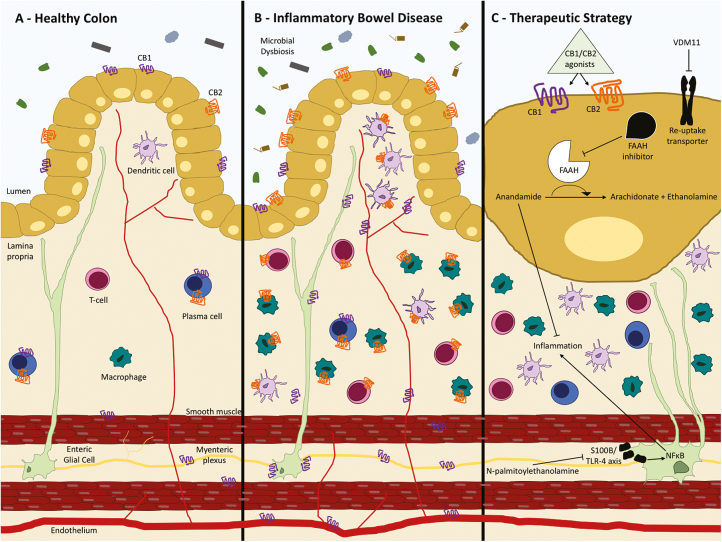
N-acylethanolamine-hydrolysing acid amidase inhibitors increase an endogenous anti-inflammatory fatty acid known as N-palmitoylethanolamine. N-palmitoylethanolamine has a high affinity for the endocannabinoid receptors and can subsequently reduce colonic inflammation and improve colitis through targeting the S100B/TLR-4 axis on enteric glial cells, reducing the downstream inflammatory effects of NF- κB.123 Enteric glial cells are astrocyte-like cells found in enteric ganglia of the submucosal and myenteric plexus that promote epithelial barrier repair.124 Glial regulation of epithelial barrier function can become disrupted in IBD, and cannabidiol has been shown to restore glial-immune homeostasis to ameliorate inflammation.125 This therapeutic strategy is summarised in Fig. 1.
FIGURE 1.
Targeting the endocannabinoid system in IBD. A, In the noninflamed colon, CB1 is expressed on colonic epithelial cells, smooth muscle cells, and the submucosal myenteric plexus. CB1 and CB2 are expressed on plasma cells in the lamina propria. B, In IBD, CB1 is upregulated on enteric neurons and endothelium, whereas CB2 is expressed on the inflammatory cell infiltrate and is upregulated on colonic epithelial cells. C, CB1 and CB2 ligand degradation pathways can be inhibit by VDM11 or a fatty acid amide hydrolase inhibitor that increases the concentration of anandamide, which helps ameliorate inflammation in the gut. N-palmitoylethanolamine can also inhibit inflammation by sequestering NF- kB by targeting the S100B/TLR-4 axis on enteric glial cells.
Translating endocannabinoid in vitro and in vivo studies into clinical practice is controversial given associations with cannabis misuse. The efficacy of cannabis cigarettes on patients with treatment-refractory Crohn’s disease has been assessed in a small, randomized trial and did not induce remission.126 A more recent randomized control trial of oral canabidiol was safe in Crohn’s disease but with no statistical efficacy demonstrated.127 A double-blind, randomized control trial of 36 patients with Crohn’s disease is planned for July 2018 to determine whether oral cannabidiol is a safe and effective adjunct therapy for symptomatic relief in Crohn’s disease (NCT03467620). The intestinal endocannabinoid system offers promise as a therapeutic target in IBD.
WHERE DO WE GO FROM HERE?
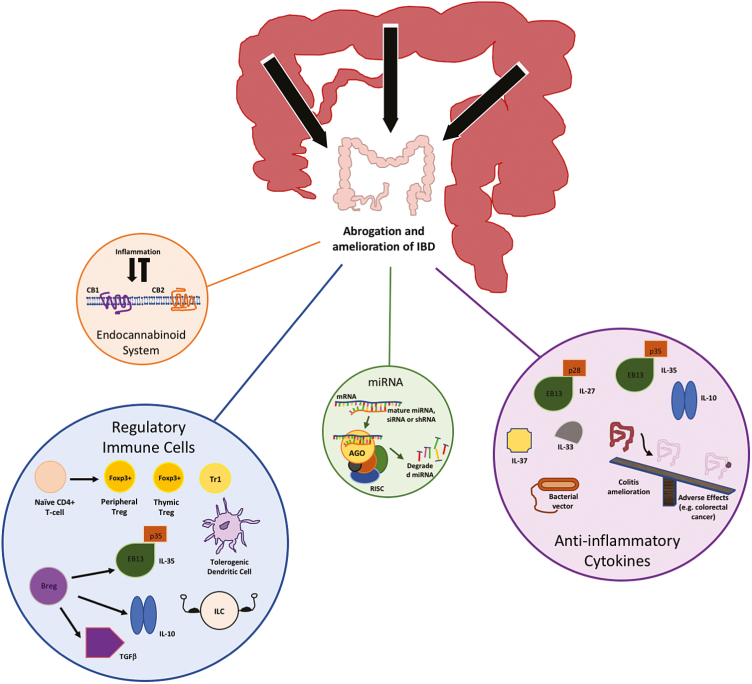
Our understanding of the immune system has advanced considerably over the past few years, providing insight into ways we may be able to harness its endogenous checks and balances for therapeutic gain (Fig. 2). However, these mechanisms are complex and potential adverse effects must be considered.
FIGURE 2.
Strategies to promote endogenous immune-mediated, anti-inflammatory activity in IBD.
There are several novel treatments to bolster the endogenous anti-inflammatory activity close to or recruiting to clinical trials currently, and these agents may enter the future therapeutic arena for IBD if shown to be efficacious with a satisfactory side effect profile.
In addition, there are other novel strategies to treat IBD emerging that may exert effect through partial enhancement of the endogenous, immunosuppressive mechanisms and not discussed here. An example of this is mesenchymal stem cell therapy that is mainly immunosuppressive through inhibition of many pro-inflammatory cells types, such as dendritic cells, B cells, effector T cells, and NK cells. They also act however to promote Treg and immunoregulatory macrophage subsets, enhancing immunosuppressive capability.128
There is significant variability at an individual patient level in IBD disease activity, progression over time, and response to treatment. Better characterization of disease phenotypes will better inform research and treatment decisions in the era of personalized medicine.
Conflicts of interest: The authors have no conflicts of interest to disclose.
Author contributions: All authors made substantial contributions to the manuscript. MM and SD identified the review topic. RP and CA prepared the first draft of the manuscript. RP prepared the figures. All authors reviewed the literature and critically revised the manuscript. All authors approved the final version for submission.
Supported by: Drs. Andrews and Durum are supported by the Intramural Research Program of the National Institutes of Health and National Cancer Institute, USA.
REFERENCES
- 1. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–217. [DOI] [PubMed] [Google Scholar]
- 2. Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. [DOI] [PubMed] [Google Scholar]
- 3. Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6–19. [DOI] [PubMed] [Google Scholar]
- 4. Geem D, Harusato A, Flannigan K, et al. Harnessing regulatory T cells for the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamada A, Arakaki R, Saito M, et al. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2016;22:2195–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. [DOI] [PubMed] [Google Scholar]
- 7. Holmén N, Lundgren A, Lundin S, et al. Functional CD4+CD25HIGH regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflamm Bowel Dis. 2006;12:447–456. [DOI] [PubMed] [Google Scholar]
- 8. Yu QT, Saruta M, Avanesyan A, et al. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191–199. [DOI] [PubMed] [Google Scholar]
- 9. Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous clostridium species. Science. 2011;331:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang M, Zhou Q, Dorfman RG, et al. Butyrate inhibits interleukin-17 and generates tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 2016;16:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacManus CF, Collins CB, Nguyen TT, et al. VEN-120, a recombinant human lactoferrin, promotes a regulatory T cell [treg] phenotype and drives resolution of inflammation in distinct murine models of inflammatory bowel disease. J Crohns Colitis. 2017;11:1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ilan Y. Oral immune therapy: targeting the systemic immune system via the gut immune system for the treatment of inflammatory bowel disease. Clin Transl Immunology. 2016;5:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Israeli E, Goldin E, Shibolet O, et al. Oral immune regulation using colitis extracted proteins for treatment of Crohn’s disease: results of a phase I clinical trial. World J Gastroenterol. 2005;11:3105–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Margalit M, Israeli E, Shibolet O, et al. A double-blind clinical trial for treatment of Crohn’s disease by oral administration of alequel, a mixture of autologous colon-extracted proteins: a patient-tailored approach. Am J Gastroenterol. 2006;101:561–568. [DOI] [PubMed] [Google Scholar]
- 15. Israeli E, Zigmond E, Lalazar G, et al. Oral mixture of autologous colon-extracted proteins for the Crohn’s disease: a double-blind trial. World J Gastroenterol. 2015;21:5685–5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Israeli E, Drori A, Ilan Y. Long-term efect of short-term oral administration of a mixture of autologous proteins extracted from the colon of patients with Crohn’s disease: a memory effect of oral tolerance induction. Jacobs J Gastroenterol Hepatol. 2015;2:012. [Google Scholar]
- 17. Kuhn C, Weiner HL. Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy. 2016;8:889–906. [DOI] [PubMed] [Google Scholar]
- 18. Forster K, Goethel A, Chan CW, et al. An oral CD3-specific antibody suppresses T-cell-induced colitis and alters cytokine responses to T-cell activation in mice. Gastroenterology. 2012;143:1298–1307. [DOI] [PubMed] [Google Scholar]
- 19. Plevy S, Salzberg B, Van Assche G, et al. A phase I study of visilizumab, a humanized anti-CD3 monoclonal antibody, in severe steroid-refractory ulcerative colitis. Gastroenterology. 2007;133:1414–1422. [DOI] [PubMed] [Google Scholar]
- 20. Baumgart DC, Targan SR, Dignass AU, et al. Prospective randomized open-label multicenter phase I/II dose escalation trial of visilizumab (hum291) in severe steroid-refractory ulcerative colitis. Inflamm Bowel Dis. 2010;16:620–629. [DOI] [PubMed] [Google Scholar]
- 21. Sandborn WJ, Colombel JF, Frankel M, et al. Anti-CD3 antibody visilizumab is not effective in patients with intravenous corticosteroid-refractory ulcerative colitis. Gut. 2010;59:1485–1492. [DOI] [PubMed] [Google Scholar]
- 22. Van Der Woude CJ, Stokkers P, Van Bodegraven AA, et al. Phase I, double-blind, randomized, placebo-controlled, dose-escalation study of NI-0401 (a fully human anti-CD3 monoclonal antibody) in patients with moderate to severe active Crohn’s disease. Inflamm Bowel Dis. 2010;16:1708–1716. [DOI] [PubMed] [Google Scholar]
- 23. Powrie F, Leach MW, Mauze S, et al. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. [DOI] [PubMed] [Google Scholar]
- 24. Morrissey PJ, Charrier K, Braddy S, et al. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maloy KJ, Salaun L, Cahill R, et al. CD4+CD25+ T® cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. [DOI] [PubMed] [Google Scholar]
- 27. Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watanabe K, Rao VP, Poutahidis T, et al. Cytotoxic-T-lymphocyte-associated antigen 4 blockade abrogates protection by regulatory T cells in a mouse model of microbially induced innate immune-driven colitis. Infect Immun. 2008;76:5834–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Canavan JB, Scottà C, Vossenkämper A, et al. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut. 2016;65:584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Desreumaux P, Foussat A, Allez M, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology. 2012;143:1207–1217.e1. [DOI] [PubMed] [Google Scholar]
- 31. Blat D, Zigmond E, Alteber Z, et al. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol Ther. 2014;22:1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang L, Ray A, Jiang X, et al. T regulatory cells and B cells cooperate to form a regulatory loop that maintains gut homeostasis and suppresses dextran sulfate sodium-induced colitis. Mucosal Immunol. 2015;8:1297–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goetz M, Atreya R, Ghalibafian M, et al. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm Bowel Dis. 2007;13:1365–1368. [DOI] [PubMed] [Google Scholar]
- 34. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. [DOI] [PubMed] [Google Scholar]
- 35. Fonseca-Camarillo G, Furuzawa-Carballeda J, Yamamoto-Furusho JK. Interleukin 35 (IL-35) and IL-37: intestinal and peripheral expression by T and B regulatory cells in patients with inflammatory bowel disease. Cytokine. 2015;75:389–402. [DOI] [PubMed] [Google Scholar]
- 36. Sattler S, Ling GS, Xu D, et al. IL-10-producing regulatory B cells induced by IL-33 (breg(IL-33)) effectively attenuate mucosal inflammatory responses in the gut. J Autoimmun. 2014;50:107–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu T, Ren J, Wang W, et al. Treatment of dextran sodium sulfate-induced experimental colitis by adoptive transfer of peritoneal cells. Sci Rep. 2015;5:16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Osorio F, Fuentes C, López MN, et al. Role of dendritic cells in the induction of lymphocyte tolerance. Front Immunol. 2015;6:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ dcs induces foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spadoni I, Iliev ID, Rossi G, et al. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 2012;5:184–193. [DOI] [PubMed] [Google Scholar]
- 41. Oshitani N, Watanabe K, Maeda K, et al. Differential expression of homing receptor CD103 on lamina propria lymphocytes and association of CD103 with epithelial adhesion molecules in inflammatory bowel disease. Int J Mol Med. 2003;12:715–719. [PubMed] [Google Scholar]
- 42. Laffont S, Siddiqui KR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol. 2010;40:1877–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gonzalez-Rey E, Delgado M. Therapeutic treatment of experimental colitis with regulatory dendritic cells generated with vasoactive intestinal peptide. Gastroenterology. 2006;131:1799–1811. [DOI] [PubMed] [Google Scholar]
- 44. Mannon PJ, Leon F, Fuss IJ, et al. Successful granulocyte-colony stimulating factor treatment of Crohn’s disease is associated with the appearance of circulating interleukin-10-producing T cells and increased lamina propria plasmacytoid dendritic cells. Clin Exp Immunol. 2009;155:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Niess JH. Role of mucosal dendritic cells in inflammatory bowel disease. World J Gastroenterol. 2008;14:5138–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kinnebrew MA, Buffie CG, Diehl GE, et al. Interleukin 23 production by intestinal CD103(+)CD11B(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamanishi H, Murakami H, Ikeda Y, et al. Regulatory dendritic cells pulsed with carbonic anhydrase I protect mice from colitis induced by CD4+CD25- T cells. J Immunol. 2012;188:2164–2172. [DOI] [PubMed] [Google Scholar]
- 48. Konieczna P, Ferstl R, Ziegler M, et al. Immunomodulation by bifidobacterium infantis 35624 in the murine lamina propria requires retinoic acid-dependent and independent mechanisms. Plos One. 2013;8:e62617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tanabe S, Kinuta Y, Saito Y. Bifidobacterium infantis suppresses proinflammatory interleukin-17 production in murine splenocytes and dextran sodium sulfate-induced intestinal inflammation. Int J Mol Med. 2008;22:181–185. [PubMed] [Google Scholar]
- 50. Jauregui-Amezaga A, Cabezón R, Ramírez-Morros A, et al. Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory Crohn’s disease: a phase I study. J Crohns Colitis. 2015;9:1071–1078. [DOI] [PubMed] [Google Scholar]
- 51. Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. [DOI] [PubMed] [Google Scholar]
- 52. Spits H, Artis D, Colonna M, et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. [DOI] [PubMed] [Google Scholar]
- 53. Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765–774. [DOI] [PubMed] [Google Scholar]
- 54. Fuchs A, Vermi W, Lee JS, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. 2013;38:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goldberg R, Prescott N, Lord GM, et al. The unusual suspects–innate lymphoid cells as novel therapeutic targets in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:271–283. [DOI] [PubMed] [Google Scholar]
- 56. Pearson C, Uhlig HH, Powrie F. Lymphoid microenvironments and innate lymphoid cells in the gut. Trends Immunol. 2012;33:289–296. [DOI] [PubMed] [Google Scholar]
- 57. Mielke LA, Jones SA, Raverdeau M, et al. Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. J Exp Med. 2013;210:1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qiu J, Guo X, Chen ZM, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kirchberger S, Royston DJ, Boulard O, et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med. 2013;210:917–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marlow GJ, van Gent D, Ferguson LR. Why interleukin-10 supplementation does not work in Crohn’s disease patients. World J Gastroenterol. 2013;19:3931–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Andrews C, McLean MH, Durum SK. Interleukin-27 as a novel therapy for inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:2255–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Furuzawa Carballeda J, Fonseca Camarillo G, Yamamoto-Furusho JK. Interleukin 27 is up-regulated in patients with active inflammatory bowel disease. Immunol Res. 2016;64:901–907. [DOI] [PubMed] [Google Scholar]
- 63. Do JS, Visperas A, Sanogo YO, et al. An IL-27/lag3 axis enhances foxp3+ regulatory T cell-suppressive function and therapeutic efficacy. Mucosal Immunol. 2016;9:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mishima Y, Liu B, Hansen JJ, et al. Resident bacteria-stimulated interleukin-10-secreting B cells ameliorate T-cell-mediated colitis by inducing T-regulatory-1 cells that require interleukin-27 signaling. C Cell Mol Gastroenterol Hepatol. 2015;1:295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Troy AE, Zaph C, Du Y, et al. IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. J Immunol. 2009;183:2037–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hanson ML, Hixon JA, Li W, et al. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology. 2014;146:210–221.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McLean MH, Andrews C, Hanson ML, et al. Interleukin-27 is a potential rescue therapy for acute severe colitis through interleukin-10-dependent, T-cell-independent attenuation of colonic mucosal innate immune responses. Inflamm Bowel Dis. 2017;23:1983–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dann SM, Le C, Choudhury BK, et al. Attenuation of intestinal inflammation in interleukin-10-deficient mice infected with citrobacter rodentium. Infect Immun. 2014;82:1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sasaoka T, Ito M, Yamashita J, et al. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G568–G576. [DOI] [PubMed] [Google Scholar]
- 70. Visperas A, Do JS, Bulek K, et al. IL-27, targeting antigen-presenting cells, promotes th17 differentiation and colitis in mice. Mucosal Immunol. 2014;7:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17:122–131. [DOI] [PubMed] [Google Scholar]
- 72. Schiering C, Krausgruber T, Chomka A, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Malik A, Sharma D, Zhu Q, et al. IL-33 regulates the iga-microbiota axis to restrain IL-1α-dependent colitis and tumorigenesis. J Clin Invest. 2016;126:4469–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhu J, Wang Y, Yang F, et al. IL-33 alleviates DSS-induced chronic colitis in C57BL/6 mice colon lamina propria by suppressing th17 cell response as well as th1 cell response. Int Immunopharmacol. 2015;29:846–853. [DOI] [PubMed] [Google Scholar]
- 75. De Salvo C, Wang XM, Pastorelli L, et al. IL-33 drives eosinophil infiltration and pathogenic type 2 helper T-cell immune responses leading to chronic experimental ileitis. Am J Pathol. 2016;186:885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sedhom MA, Pichery M, Murdoch JR, et al. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut. 2013;62:1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Latiano A, Palmieri O, Pastorelli L, et al. Associations between genetic polymorphisms in IL-33, IL1R1 and risk for inflammatory bowel disease. Plos One. 2013;8:e62144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kobori A, Yagi Y, Imaeda H, et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45:999–1007. [DOI] [PubMed] [Google Scholar]
- 79. Díaz-Jiménez D, De la Fuente M, Dubois-Camacho K, et al. Soluble ST2 is a sensitive clinical marker of ulcerative colitis evolution. BMC Gastroenterol. 2016;16:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sawant DV, Hamilton K, Vignali DAA. Interleukin-35: expanding its job profile. J Interf Cytokine Res. 2015;35:499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nold MF, Nold-Petry CA, Zepp JA, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li Y, Wang Y, Liu Y, et al. The possible role of the novel cytokines il-35 and il-37 in inflammatory bowel disease. Mediators Inflamm. 2014;2014:136329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Weidlich S, Bulau AM, Schwerd T, et al. Intestinal expression of the anti-inflammatory interleukin-1 homologue IL-37 in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;59:e18–e26. [DOI] [PubMed] [Google Scholar]
- 84. Imaeda H, Takahashi K, Fujimoto T, et al. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin Exp Immunol. 2013;172:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang WQ, Dong K, Zhou L, et al. IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice. Acta Pharmacol Sin. 2015;36:1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McNamee EN, Masterson JC, Jedlicka P, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A. 2011;108:16711–16716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Collison LW, Chaturvedi V, Henderson AL, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wirtz S, Billmeier U, Mchedlidze T, et al. Interleukin-35 mediates mucosal immune responses that protect against T-cell-dependent colitis. Gastroenterology. 2011;141:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wu F, Zikusoka M, Trindade A, et al. Micrornas are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635.e24. [DOI] [PubMed] [Google Scholar]
- 90. Wu F, Zhang S, Dassopoulos T, et al. Identification of micrornas associated with ileal and colonic Crohn’s disease. Inflamm Bowel Dis. 2010;16:1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Huang Z, Shi T, Zhou Q, et al. Mir-141 regulates colonic leukocytic trafficking by targeting CXCL12Β during murine colitis and human Crohn’s disease. Gut. 2014;63:1247–1257. [DOI] [PubMed] [Google Scholar]
- 92. Taganov KD, Boldin MP, Chang K-J, et al. NF- B-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci. 2006;103:12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kohlhaas S, Garden OA, Scudamore C, et al. Cutting edge: the foxp3 target mir-155 contributes to the development of regulatory T cells. J Immunol. 2009;182:2578–2582. [DOI] [PubMed] [Google Scholar]
- 94. Wu W, He C, Liu C, et al. Mir-10a inhibits dendritic cell activation and th1/th17 cell immune responses in IBD. Gut. 2015;64:1755–1764. [DOI] [PubMed] [Google Scholar]
- 95. Philpott DJ, Sorbara MT, Robertson SJ, et al. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14:9–23. [DOI] [PubMed] [Google Scholar]
- 96. Ghorpade DS, Sinha AY, Holla S, et al. NOD2-nitric oxide-responsive microrna-146a activates sonic hedgehog signaling to orchestrate inflammatory responses in murine model of inflammatory bowel disease. J Biol Chem. 2013;288:33037–33048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chuang AY, Chuang JC, Zhai Z, et al. NOD2 expression is regulated by micrornas in colonic epithelial HCT116 cells. Inflamm Bowel Dis. 2014;20:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zahm AM, Hand NJ, Tsoucas DM, et al. Rectal micrornas are perturbed in pediatric inflammatory bowel disease of the colon. J Crohns Colitis. 2014;8:1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bian Z, Li L, Cui J, et al. Role of mir-150-targeting c-myb in colonic epithelial disruption during dextran sulphate sodium-induced murine experimental colitis and human ulcerative colitis. J Pathol. 2011;225:544–553. [DOI] [PubMed] [Google Scholar]
- 100. He C, Shi Y, Wu R, et al. Mir-301a promotes intestinal mucosal inflammation through induction of IL-17A and TNF-α in IBD. Gut. 2016;65:1938–1950. [DOI] [PubMed] [Google Scholar]
- 101. Travis MA, Sheppard D. TGF-β activation and function in immunity. Annu Rev Immunol. 2014;32:51–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hahm KB, Im YH, Parks TW, et al. Loss of transforming growth factor beta signalling in the intestine contributes to tissue injury in inflammatory bowel disease. Gut. 2001;49:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jarry A, Bossard C, Sarrabayrouse G, et al. Loss of interleukin-10 or transforming growth factor β signaling in the human colon initiates a T-helper 1 response via distinct pathways. Gastroenterology. 2011;141:1887–96.e1. [DOI] [PubMed] [Google Scholar]
- 104. Monteleone G, Mann J, Monteleone I, et al. A failure of transforming growth factor-β1 negative regulation maintains sustained NF-κB activation in gut inflammation. J Biol Chem. 2004;279:3925–3932. [DOI] [PubMed] [Google Scholar]
- 105. Monteleone G, Fantini MC, Onali S, et al. Phase I clinical trial of smad7 knockdown using antisense oligonucleotide in patients with active Crohn’s disease. Mol Ther. 2012;20:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Feagan BG, Sands BE, Rossiter G, et al. Effects of mongersen (GED-0301) on endoscopic and clinical outcomes in patients with active Crohn’s disease. Gastroenterology. 2018;154:61–64.e6. [DOI] [PubMed] [Google Scholar]
- 107. Monteleone G, Neurath MF, Ardizzone S, et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med. 2015;372:1104–1113. [DOI] [PubMed] [Google Scholar]
- 108. Frosali S, Pagliari D, Gambassi G, et al. How the intricate interaction among toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J Immunol Res. 2015;2015:489821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lee J, Mo JH, Katakura K, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. [DOI] [PubMed] [Google Scholar]
- 110. Atreya R, Bloom S, Scaldaferri F, et al. Clinical effects of a topically applied toll-like receptor 9 agonist in active moderate-to-severe ulcerative colitis. J Crohns Colitis. 2016;10:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dotan I, Levy-Nissenbaum E, Chowers Y, et al. Ameliorating active ulcerative colitis via an orally available toll-like receptor-9 modifier: a prospective open-label, multicenter phase II trial. Dig Dis Sci. 2016;61:3246–3254. [DOI] [PubMed] [Google Scholar]
- 112. Marin M, Holani R, Shah CB, et al. Cathelicidin modulates synthesis of toll-like receptors (tlrs) 4 and 9 in colonic epithelium. Mol Immunol. 2017;91:249–258. [DOI] [PubMed] [Google Scholar]
- 113. Di Sabatino A, Battista N, Biancheri P, et al. The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal Immunol. 2011;4:574–583. [DOI] [PubMed] [Google Scholar]
- 114. Wright K, Rooney N, Feeney M, et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437–453. [DOI] [PubMed] [Google Scholar]
- 115. Kimball ES, Schneider CR, Wallace NH, et al. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Liver Physiol. 2006;0776:364–371. [DOI] [PubMed] [Google Scholar]
- 116. Croci T, Landi M, Galzin A, et al. Role of cannabinoid CB 1 receptors and tumor necrosis factor- a in the gut and systemic anti-inflammatory activity of SR 141716 (Rimonabant ) in rodents. Br J Pharmacol. 2003;141716:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Harvey BS, Nicotra LL, Vu M, et al. Cannabinoid CB2 receptor activation attenuates cytokine-evoked mucosal damage in a human colonic explant model without changing epithelial permeability. Cytokine. 2013;63:209–217. [DOI] [PubMed] [Google Scholar]
- 118. Borrelli F, Fasolino I, Romano B, et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol. 2013;85:1306–1316. [DOI] [PubMed] [Google Scholar]
- 119. Fichna J, Bawa M, Thakur GA, et al. Cannabinoids alleviate experimentally induced intestinal inflammation by acting at central and peripheral receptors. Plos One. 2014;9:e109115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Van Sickle MD. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. [DOI] [PubMed] [Google Scholar]
- 121. Storr MA, Keenan CM, Emmerdinger D, et al. Targeting endocannabinoid degradation protects against experimental colitis in mice: involvement of CB1 and CB2 receptors. J Mol Med (Berl). 2008;86:925–936. [DOI] [PubMed] [Google Scholar]
- 122. D’Argenio G, Valenti M, Scaglione G, et al. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. Faseb J. 2006;20:568–570. [DOI] [PubMed] [Google Scholar]
- 123. Esposito G, Capoccia E, Turco F, et al. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut. 2014;63:1300–1312. [DOI] [PubMed] [Google Scholar]
- 124. Yu YB, Li YQ. Enteric glial cells and their role in the intestinal epithelial barrier. World J Gastroenterol. 2014;20:11273–11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. de Filippis D, Esposito G, Cirillo C, et al. Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PLoS One. 2011;6:e28159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Naftali T, Schleider LB, Dotan I, et al. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11:1276–1280.e1. [DOI] [PubMed] [Google Scholar]
- 127. Naftali T, Mechulam R, Marii A, et al. Low-dose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a randomized controlled trial. Dig Dis Sci. 2017;62:1615–1620. [DOI] [PubMed] [Google Scholar]
- 128. Dave M, Mehta K, Luther J, et al. Mesenchymal stem cell therapy for inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:2696–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]