Abstract
Children with autism spectrum disorder (ASD) are 4 times as likely to experience gastrointestinal symptoms as children without ASD. The gut microbiota has increasingly been the subject of investigation as a contributing factor to these symptoms in this population because there is evidence to suggest that alterations in the intestinal microflora are correlated with gastrointestinal and ASD symptom severity. Probiotic therapy has been proposed as a treatment for augmented gastrointestinal symptom severity in children with ASD. This narrative review systematically searched the literature to provide an update for practitioners on the state of the evidence surrounding probiotic therapy in children with ASD as a treatment option for reducing gastrointestinal symptoms. A total of 186 articles were screened and 5 articles met the inclusion criteria. A collective sample of 117 children with ASD is represented and outcomes addressed include improvement in gastrointestinal symptoms as well as influence of probiotic supplementation on the gut microbiota and ASD symptoms and behavior. There is promising evidence to suggest that probiotic therapy may improve gastrointestinal dysfunction, beneficially alter fecal microbiota, and reduce the severity of ASD symptoms in children with ASD. Future research is still warranted in this area because there are methodologic flaws in the available literature and optimal species, strains, dosages, and duration of treatment have not been identified.
Keywords: autism spectrum disorder, autism, probiotics, gastrointestinal symptoms, children, behavior
Introduction
Autism spectrum disorder (ASD) is characterized by deficits in 2 major domains, the social communication and interaction domain as well as the restrictive, repetitive patterns of behavior domain (1). In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, pervasive developmental disorder not otherwise specified, Asperger disorder, and autistic disorder are included under the umbrella term of ASD (1). Estimates from 2012 indicate that 1 in 68 children across the United States has ASD, with the prevalence being ∼4.5 times greater among boys than girls (2).
Symptoms associated with ASD include impairments in social-emotional reciprocity, nonverbal communication behaviors, and the development and maintenance of relationships, as well as stereotypical repetitive behaviors (1). In addition to these neurodevelopmental concerns, gastrointestinal symptoms are also commonly reported among those with ASD, with many experiencing diarrhea, constipation, or both (3–5). The true prevalence of gastrointestinal symptoms among children with ASD has been difficult to estimate due to methodologic variability among studies and potentially inadequate recognition in this population (6). Although these limitations have led to a large range of cited occurrences, a recent meta-analysis by McElhanon et al. (3) determined that children with ASD were 4 times as likely to experience gastrointestinal symptoms as children without ASD. This adds strength to the previously reported prevalence of noninflammatory bowel disease disorders being ∼2.5 times greater among individuals with ASD (total individuals with ASD = 14,381) aged ≤35 y compared with a general hospital population (total population = 2,393,778) (7).
Gastrointestinal dysfunction in children with ASD can affect many facets of their life. Although more recognized presentations of symptoms (e.g., diarrhea) can easily be translated to affect well-being, the less-understood, and perhaps more prevalent, nontypical presentations can also greatly affect a child's quality of life. For example, maladaptive behaviors, such as sleep disturbances, aggressive behavior, irritability, or self-injury, may be outlets of symptoms for some children with ASD who are experiencing gastrointestinal dysfunction (3, 5, 8–10). Although immediately affecting the quality of life of the child, these conditions can also affect parental and family stress levels (11).
The cause of gastrointestinal dysfunction in children with ASD has not been definitively determined, but there is some evidence that the composition of the gut microbiota may play a role (12, 13). Additional factors may also include increased intestinal inflammation and permeability (14) and mitochondrial dysfunction (15). The gut microbiota, which consists of bacteria, fungi, and viruses (16), has been found to be influential in maintaining normal gastrointestinal tract function, immune homeostasis, and endocrine regulation (16), as well as participating in bidirectional signaling between the gastrointestinal tract and the brain (17).
Metabolomics, which is the analysis of cellular metabolites, uses a variety of sensitive analytical techniques that allows for the identification and quantification of metabolites in organisms, cells, or tissues and provides insight into the physiologic state of the host (18). Metabolomics can help establish potential biomarkers for conditions, as seen in the works of Wang et al. (19) and West et al. (20). When analyzing serum metabolic changes in individuals with and without ASD in the Chinese Han population, Wang et al. (19) found that 2 metabolites were consistently associated with ASD, which may prove useful in the diagnosis and evaluation of ASD. Similarly, in a US cohort, West et al. (20) were also able to identify biomarkers through the analysis of serum metabolites in an effort to determine a metabolic signature that could help identify those at high risk of developing ASD. Likewise, Gevi et al. (21) found that when a cohort of Italian children with ASD were compared with neurotypical controls, there were significant differences between the groups in select urinary metabolites, which may help elucidate some of the comorbidities associated with ASD, including dysbiosis of the gut microbiome. Utilizing metabolomic profiles can also prove useful in comparing pre- and post-treatment interventions in understanding individuals (18). Studies examining the alteration of the gut microbiome on the basis of targeted interventions (e.g., probiotics) have analyzed metabolites in urine and feces, as well as abundance of bacteria.
Studies have shown altered composition of the gut microbiota in children with ASD compared with neurotypical controls (12, 13, 22–25). These differences consist of a lower relative abundance of several bacteria including the genera Bifidobacterium, Prevotella, Streptococcus, Enterococcus, Lactococcus, Lactobacillus, Ruminococcus, Coprococcus, and Staphyloccus (24, 26, 27) and higher Desulfovibrio abundance and higher Firmicutes-to-Bacteroidetes ratios (12, 28, 29), the latter of which has preliminarily been associated with the severity of ASD (13, 29). In addition to alterations in the gut microbiota, bacterial metabolites that are present in the urine and feces of children with ASD also appear to be different than what is found in children without ASD (26, 30, 31). As noted in a comprehensive review by Mussap et al. (32), gut dysbiosis in children with ASD has been attributed to the high concentrations of the metabolites tartaric acid, d-mannitol, hippurate, phenyacetyl-glutamine, 3-hydroxybenzoic acid, and p-hydroxyphenylacetic acid found in urine of individuals with ASD. Similarly, gut dysbiosis has also been associated with the increase in metabolites associated with nicotine acid metabolism, mitochondrial dysfunction, and the disruption of antioxidant status and amino acid metabolism in those with ASD (32).
With a lack of evidence-based recommendations, a consensus report on the evaluation, diagnosis, and treatment of gastrointestinal dysfunction in individuals with ASD was published in 2010 by Buie et al. (33). This committee of experts recommended that algorithms for the assessment of gastrointestinal dysfunction be established. Although guidelines for evaluation of gastrointestinal symptoms in children exist (e.g., gastroesophageal reflux, chronic abdominal pain, and constipation), there needs to be evidence-based adaptations to evaluate these symptoms in children with ASD (33). Current opinion suggests that providers should consider the possibility that gastrointestinal symptoms, and particularly pain, be considered as a possible “setting event” that can increase the likelihood of problem behaviors. Clinicians should review gastrointestinal symptoms as well as behaviors that are associated with gastrointestinal dysfunction, such as irritability, social withdrawal, stereotypy, and hyperactivity (5), in addition to other sources of pain or discomfort, when clinically evaluating individuals with ASD (33).
Recommendations for nutritional management of gastrointestinal symptoms in children with ASD are also at the level of expert opinion, due to the lack of evidence-based guidelines that account for the complex overlay of impairments seen in this population. In 2015, an expert panel of Registered Dietitian Nutritionists with clinical expertise in working with children with ASD put forth an 11-step algorithm to assist clinicians in navigating barriers to nutritional management of gastrointestinal symptoms in this population (34). Within this framework, clinicians working with children with ASD who have constipation, for example, were prompted to evaluate the feasibility of adding a supplement (e.g., prebiotic or probiotic or bulk-forming agent) to the child's regimen. To further expand on this concept, the purpose of this review is to examine the current literature to address if common gastrointestinal symptoms (abdominal pain, gastroesophageal reflux, diarrhea, and constipation) in children with ASD improve with the provision of probiotics. Evaluating the current state of the evidence will provide clinicians with a clearer picture of whether or not probiotics may benefit their clients with ASD who are experiencing gastrointestinal symptoms and can guide them in addressing professional and parental queries on this practice.
Methods
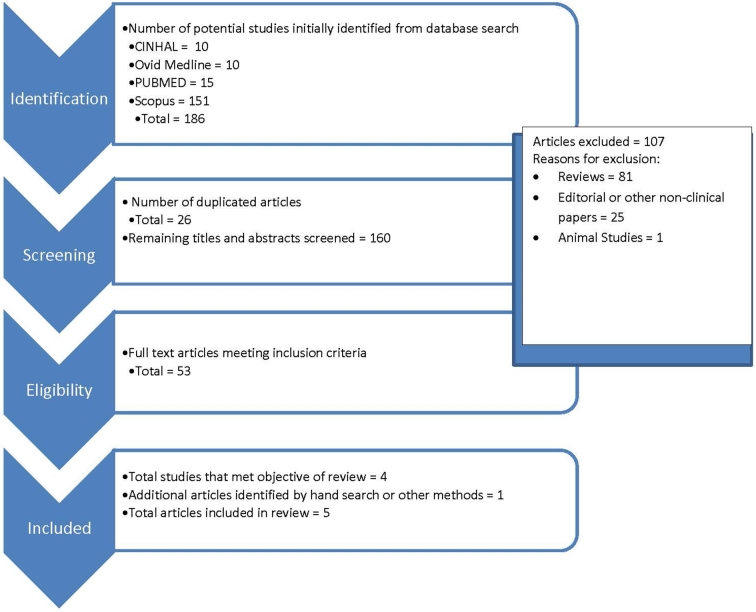
The literature was searched by using PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), CINAHL (https://www.ebscohost.com/nursing/products/cinahl-databases/cinahl-complete), SCOPUS (https://www.elsevier.com/solutions/scopus), and OVID Medline (http://www.ovid.com/site/catalog/databases/901.jsp) with the use of the keywords and MeSH terms “probiotics,” “prebiotics,” “synbiotics,” “lactobacillus,” “autism,” “pervasive developmental disorder,” and “Asperger's”. Figure 1 provides details on the steps of the search strategy. In summary, search limitations included articles that were primary research, published in the English language between 1 January 2007 and 1 November 2017, and studies that included individuals with ASD aged ≤18 y. Duplicate articles from the initial search results were removed first at which point the search limitations were applied by the primary author to the remaining titles and abstracts. Articles were excluded by the primary author if they were reviews, included participants ≥18 y of age, were published outside the desirable date range, or were not published in the English language. All of the remaining article titles and abstracts were examined by the primary author for relevance to the purpose of this narrative review.
FIGURE 1.
Process of study selection for inclusion.
Reference lists of the relevant articles as well as pertinent reviews were screened to identify additional articles for inclusion. A summary of the available evidence has been provided in Table 1. The strength of the evidence was analyzed by using the Quality Criteria Checklist, a risk-of-bias tool created by the Academy of Nutrition and Dietetics that facilitates critical appraisal of the research, as part of the evidence analysis process (35). Ratings for each study are provided in Table 1.
TABLE 1.
Evidence summary on the effectiveness of probiotic therapy in modulation of gastrointestinal dysfunction in children with ASD1
| Study | |||||
|---|---|---|---|---|---|
| Shaaban et al. (2017) (37) | Tomova et al. (2015) (29) | West (2013) (38) | Kaluzna-Czaplinska and Blaszczyk (2012) (31) | Parracho et al. (2010) (36) | |
| Study design | Prospective, open-label noncontrolled trial | Noncontrolled trial | Noncontrolled trial | Noncontrolled trial | Double-blind, placebo-controlled, crossover trial |
| Location | Egypt | Slovakia | USA | Poland | United Kingdom |
| Participants, n | 60 Children: 30 ASD, 30 age-/sex-matched controls (relatives) | 29 Children: 10 ASD, 9 non-ASD siblings, 10 non-ASD controls | 33 Children with ASD | 22 Children with ASD | 22 Children with ASD |
| Age | 5–9 y (mean age: 84.77 ± 16.37 mo) | ASD = 2–9 y: non-ASD siblings = 5–17 y; non-ASD controls = 2–11 y | 3–16 y (mean age = 7.92 y) | 4–10 y (mean age: 5.6 ± 1.6 y) | Males = 9.2 ± 2.4 y; females = 8.5 ± 2.1 y (5 withdrew before second feeding arm) |
| Sex | 19 boys (63.3%); 11 girls (36.7%) | ASD = 90% males; non-ASD siblings = 77.7% males; non-ASD controls = 100% males | Not reported | 91% males | 91% males |
| Diagnosis/classification of participants | ASD (DSM-V) | ASD (ICD-10 criteria); non-ASD siblings; non-ASD controls | ASD (no reported criteria for ASD diagnosis) | ASD (DSM-IV criteria) | ASD (no reported criteria for ASD diagnosis) |
| Strain of probiotic | 3 strains: Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacteria longum | “Children Dophilus”: Blend of: 3 strains of Lactobacillus (60%), 2 strains of Bifidumbacteria (25%), 1 strain of Streptococcus (15%) (exact strain information not provided) | Delpro capsule: 2 billion CFUs of each of the following: Lactobacillus delbrueckii, L. acidophilus, Lactobacillus casei, B. longum, Bifidobacteria bifidum; with an additional 8 mg Del-immune V powder | L. acidophilus | Lactobacillus plantarum WCFS1 |
| Dose of probiotic | 5 g of powder/d (each gram contained 100 × 106 CFUs of each strain) | Not provided | 10 billion CFUs total | 5 × 109 CFUs | 4.5 × 1010 CFUs |
| Duration of probiotic therapy | 1 time/d for 3 mo | 3 times/d for 4 mo | 1 capsule, 3 times/d for 21 d | 2 times/d for 2 mo | Daily for 3 wk |
| Compliance | Not reported, but parents were contacted weekly regarding compliance | Not measured | Not measured; also instructed to discontinue any other concomitant probiotics | Not measured | Not measured |
| GI symptoms at baseline | GI symptoms assessed include constipation, diarrhea, stool consistency and smell, flatulence, and abdominal pain | Parent questionnaire at baseline only | 84% had moderate or severe constipation at baseline (based on ATEC); 56% had moderate or severe diarrhea at baseline (based on ATEC) | No objective reporting provided; authors indicated all children had “severe” GI problems (abdominal pain, constipation, diarrhea) | Parents recorded GI function and symptoms in a diary throughout the study |
| Measurement of GI symptoms | 6-GSI used to assess GI symptoms (lower score = fewer GI symptoms) | Parent questionnaire at baseline only | 21-d stool frequency diary before and after intervention; fourth domain in ATEC includes 2 questions measuring severity of diarrhea and constipation | No | Parents recorded GI function and symptoms in a diary throughout the study |
| Measurement of gut microbiota | Yes—pre- and post-treatment fecal specimens were collected to assess fecal microbial composition | Yes—pre- and post-treatment fecal specimens were collected to detect gut microflora and cytokine levels | No | Measured pre- and post- treatment urinary DA, which is a metabolite of Candida, and DA-to-LA ratio, which is a marker of invasive candidiasis; fecal specimens were not studied | Yes—fecal specimens were used to detect gut microflora; collected before and after each feeding arm and the end of each washout period |
| Description of baseline gut microflora and comparison to control | Yes—baseline fecal microflora was assessed for presence of Lactobacilli and Bifidobacteria | Yes—baseline gut microflora for participants with and without ASD was described | No | Described baseline and post–probiotic DA and DA-to-LA ratio levels | Yes—baseline, feeding arm, and washout bacterial population levels were described; unable to compare to individuals without ASD because they were not included in this study |
| Food intake | Not recorded | Not recorded | Not recorded | 45% of participants had a “restricted diet” and the authors noted that all of the children were on a “sugar-free diet”; no further clarification provided | Not recorded |
| Additional psychological testing | ADOS/ADI-R | CARS, ADI | None | None | DBC-P/TBPS |
| Behavior analysis | ATEC was administered pre- and postintervention | CARS and ADI administered only at baseline | ATEC was administered before and after Delpro intervention; higher ATEC scores suggest greater severity of ASD symptoms | None | Yes—change in behavior with placebo and probiotic treatment was evaluated using DBC-P tool and TBPS score |
| Blood samples or additional samples/measurements | 6-GSI; anthropometric measurements | Plasma—oxytocin, testosterone and dehydroepiandrosterone-sulfate concentrations; fecal—TNF-α | No | No | No |
| Attrition | Not reported | Not reported | 75.7% for ATEC scores; 63.6% for stool diaries | Not reported | 27.4% |
| Results | Children with ASD had lower Bifidobacteria levels than did age- and sex-matched controls at baseline and fecal Lactobacilli and Bifidobacteria concentrations increased as a result of supplementation (P < 0.0001) | Children with ASD and their siblings had more GI dysfunction than did controls (P < 0.05) with GI symptom severity and autism severity being strongly, positively correlated (R = 0.78, P = 0.01) | 52% of the total respondents reported severe constipation at baseline with a decline to 20% reporting severe constipation after treatment; 20% of the total respondents reported severe diarrhea at baseline with a decline to none after treatment | Urine DA (a metabolite of Candida species) level was higher in children before probiotic supplementation (160.04 ± 22.88 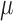 mol/mmol creatinine; P < 0.05) mol/mmol creatinine; P < 0.05) |
Probiotic therapy significantly increased the amount of Lactobacilli/Enterococci (P < 0.05) and decreased the Clostridium coccoides (P < 0.05) found in the stool samples of children with ASD as compared with placebo |
| 6-GSI scores increased/improved (P < 0.0001) in children with ASD after 3 mo of probiotic supplementation, specifically in the symptoms of constipation (P < 0.01), abdominal pain (P < 0.002), flatulence (P < 0.037), and stool consistency (P < 0.023); reduced constipation scores were significantly negatively correlated with increased fecal Bifidobacteria (r = –0.441, P < 0.015) | Bacteroidetes-to-Firmicutes ratio was lower (P < 0.05) and Lactobacillus was higher (P < 0.05) in children with ASD as compared with controls | Of the 25 ATEC responders, there was a significant decrease across all ATEC domain scores after finishing 21 d of Delpro intervention (P < 0.05 for all domains: speech/language/communication, sociability, sensory/cognitive awareness, health/physical/behavior). Mean ATEC scores decreased from 72.8 before treatment to 58.3 after treatment (P < 0.05) | Urine DA-to-LA ratio (a marker of invasive candidiasis) decreased from a mean of 3.15 ± 0.41 before probiotic treatment to a mean of 2.77 ± 0.28 after probiotic treatment | 94% (n = 16) of the diaries recording bowel function and GI symptoms during the feeding arms of the trial were returned while only 17% (n = 3) of the diaries recording the same symptoms during the washout period were returned | |
| ATEC scores significantly improved after 3 mo of probiotic supplementation (P < 0.0001), specifically, speech/language/communications subscale (P < 0.017), health/physical/behavioral (P < 0.0001), sensory/cognitive awareness (P < 0.026), and sociability (P < 0.001) all improved after supplementation; GI symptom improvement (as measured by 6-GSI) was strongly correlated to improved autism severity (as measured by ATEC) (r = 0.674, P = 0.0001) | Children with ASD had higher, but not significant, amounts of Clostridia cluster 1 and Desulfovibrio than controls or siblings | Of those who experienced a decrease in the fourth domain after Delpro intervention, 57% and 52% reported a decrease in constipation and diarrhea, respectively. One participate reported an increase in constipation and 2 reported an increase in diarrhea | There was no difference in the number of daily bowel movements noted between the probiotic or placebo arms | ||
| In children with greater severity of GI symptoms, there were lower amounts of Clostridia cluster 1 and Desulfovibrio and a lower Bacteroidetes-to-Firmicutes ratio than in those children with mild GI symptoms, albeit not significantly. Conversely, children with more severe autism (CARS ≥50) had greater Clostridia cluster 1 and Desulfovibrio and a lower Bacteroidetes-to-Firmicutes ratio than in those children with mild autism (not significant) | Of the 21 pre- and 18 post-stool log responders, stool frequency increased by 0.2 d, which was not significant | There were significantly fewer “hard” stools reported during the probiotic feeding arm (8.1%) vs. placebo (15.9%) (P < 0.01) | |||
| There was a very strong correlation between Desulfovibrio and the ADI restricted/repetitive behavior score (R = 0.83, P < 0.05) | Stools were significantly more “formed” during the probiotic feeding arm (73.3%) vs. placebo (64.8%) (P < 0.01) | ||||
| Probiotic therapy elicited a decrease in Firmicutes, which increased the Bacteroidetes-to-Firmicutes ratio to levels seen in the children without ASD. A similar effect was seen with regard to decreasing Bifidobacterium in children with ASD to levels found in controls | No significant difference noted for abdominal pain, bloating, or flatulence between either treatment | ||||
| Fecal TNF-α concentrations were higher in children with ASD and their siblings compared with children without ASD, although not significantly, and there was a strong correlation between TNF-α concentrations and GI symptoms (R = 0.78, P < 0.05). There was also a trend toward a correlation between TNF-α concentrations and severity of ASD, as measured by ADI (R = 0.7, P = 0.06). Probiotic therapy significantly decreased TNF-α concentrations (2-tailed P < 0.05) | 88% (n = 15) of TBPSs were completed with no significant differences noted between the treatments; however, baseline scores were significantly higher (P < 0.05) than scores measured during the probiotic and placebo periods | ||||
| Limitations | Children with ASD with dual diagnoses or with GI disorders were not included (concurrent neurodevelopmental or psychiatric conditions were excluded) | GI measurement was conducted at baseline only with parent questionnaire, which would have introduced bias. | Unclear if similar results would be found with just probiotic supplementation (without immune modulator) | Objective measurement of GI symptoms was not collected pre- or postprobiotic supplementation | The probe set used in this study obtained <50% coverage of the total bacterial count |
| This was a small, nonblinded, convenience sample, and there was no control for diet or additional therapy, even though the authors noted that the children in this study were receiving ongoing behavioral therapy | Behavior analysis was only done at baseline; therefore, we are unable to determine if probiotics influenced alteration in ASD symptoms | Potential risk of bias due to industry affiliation | Change in ASD symptoms were reported; however, no description of how behaviors were measured pre- and postprobiotic supplementation | Three adverse events were reported and included a 3-d skin rash after starting the probiotic, diarrhea during the probiotic feeding arm, and weight loss during the probiotic feeding arm of the trial | |
| No control for diet, medication, or additional therapy | Convenience sample without control group or control of confounding variables; group may be skewed as heavily represented by those with severe constipation or diarrhea | Design of the trial may have been too ambitious for this population; the significant drop-out rate affected the statistical power of the trial | |||
| No analysis of change in gut microbiome or intestinal permeability after treatment | High interindividual variability found in this trial suggests that future research may need to focus the intervention in a more defined subgroup of ASD (e.g., similar baseline fecal microbiota or similar GI symptoms) to elucidate more meaningful results | ||||
| Compliance not measured and duration of treatment may not be ideal | Lack of change in behavior may have been hindered by the short-term treatment period. Exploration of change in behavior using a single-subject design may be warranted | ||||
| A more objective tool for measuring GI symptoms and severity may have improved reporting | |||||
| Quality of evidence (27) | Neutral | Neutral | Negative | Negative | Neutral |
ADI, Autism Diagnostic Interview; ADI-R, Autism Diagnostic Interview – Revised; ADOS, Autism Diagnostic Observational Schedule; ASD, autism spectrum disorder; ATEC, Autism Treatment Evaluation Checklist; CARS, Childhood Autism Rating Scale; DA, d-arabinitol; DBC-P, Development Behavior Checklist—Primary Carer Version; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM-V, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; GI, gastrointestinal; ICD-10, International Classification of Diseases, 10th Revision; LA, l-arabinitol; TBPS, Total Behavior Problem Score; 6-GSI, modified GI Severity Index.
Although there is a growing body of literature describing the diversity of the gut microbiota and augmented gut permeability in individuals with ASD, within the past 10 y there have only been 5 published trials to our knowledge that have collectively examined the effect of probiotic therapy on gut microbiota/gastrointestinal symptoms in 117 children with ASD. Three trials [Parracho et al. (36), Tomova et al. (29), and Shaaban et al. (37)] examined changes in the gut microbiota before and after probiotic therapy by examining stool samples, whereas Kaluzna-Czaplinska and Blaszczyk (31) analyzed urine for metabolites as a marker for the presence of Candida. West et al. (38) collected Autism Treatment Evaluation Checklist (ATEC) scores and 21-d stool-frequency diaries before and after a 3-wk probiotic supplementation intervention. Three studies were conducted in Europe (29, 31, 36), one in Egypt (37), and one study was conducted in the United States (38). Study designs consisted of one double-blind, placebo-controlled crossover trial (36) and 4 noncontrolled clinical trials (29, 31, 37, 38).
Current Status of Knowledge
Demographic characteristics and clinical information
Children were aged from 2 to 16 y across all 5 studies, with >80% of them being males when sex was reported. Children were recruited on the basis of a diagnosis of ASD by using International Classification of Diseases, 10th Revision (29), or Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (31, 37), criteria. No criteria for ASD diagnosis by Parracho et al. (36) or West et al. (38) were provided. Additional study-required psychological assessments included the following: the Autism Diagnostic Interview (ADI) (29, 37); the Childhood Autism Rating Scale (CARS) (29); the Development Behavior Checklist—Primary Carer Version with a corresponding Total Behavior Problem Score (TBPS) (36); the Autism Diagnostic Observational Schedule (37); and the ATEC (38). Kaluzna-Czaplinska and Blaszczyk (31) and West et al. (38) did not require any additional psychological assessments to assess the severity of ASD or change in ASD symptoms with probiotic therapy.
Probiotic supplementation
Probiotic interventions varied across all of the trials; however, the majority of the probiotic supplements provided consisted of Lactobacillus species. Table 1 provides a comparison of probiotic supplementation across all of the studies. In summary, 2 studies provided single-strain probiotic therapy, which consisted of Lactobacillus plantarum WCFS1, provided at a dose of 4.5 × 1010 CFUs once daily for 3 wk (36) and Lactobacillus acidophilus 5 × 109 CFUs twice daily for 2 mo (31). The remaining 3 studies provided blended probiotic formulations. Tomova et al. (29) provided a blend of Lactobacillus, Bifidobacterium, and Streptococcus strains, with the majority of the strains being from the Lactobacillus genus (exact strains and strength were not reported), given 3 times/d for 4 mo. West et al. (38) provided 2 billion CFUs of each of the following strains per capsule (Delpro) with a suggested dosing of 1 capsule 3 times/d for 21 d: Lactobacillus delbruecki, L. acidophilus, Lactobacillus casei, Bifidobacteria longum, and Bifidobacteria bifidum with an additional 8 mg Del-immune V powder. Del-immune V powder is “a lysed, lyophilized powder, which contains peptidoglycan, muramyl peptides, and nucleotide-containing components or DNA motifs that is derived from L rhamnosus V strain”. Del-immune V has been associated with stimulation serum IFNs and a host of cytokines (38). Finally, Shaaban et al. (37) provided a blend of L. acidophilus, Lactobacillus rhamnosus, and B. longum once daily for 3 mo.
Gastrointestinal symptoms
The documentation of gastrointestinal symptoms varied among all 5 trials, with a comparison provided in Table 1. Kaluzna-Czaplinska and Blaszczyk (31) simply reported that all of the children with ASD in their study suffered from significant gastrointestinal issues without any objective measurement, whereas Parracho et al. (36) utilized parent/guardian reported diaries that collected information on gastrointestinal function and symptoms during the entire trial. Tomova et al. (29) had parents complete a scored gastrointestinal questionnaire at baseline but not at the end of the probiotic intervention, whereas Shabaan et al. (37) had pre- and postprobiotic gastrointestinal symptom questionnaire results available. West et al. (38) used a combination of ATEC scores (specifically the fourth domain, which included 2 questions that elicited constipation and diarrhea severity) and a 21-d pre- and post-treatment stool-frequency diary for evaluation of change in gastrointestinal symptoms after probiotic therapy.
Given the lack of gastrointestinal symptom and severity recording, Kaluzna-Czaplinska and Blaszczyk (31) could not evaluate the improvement in gastrointestinal symptoms among their cohort with probiotic supplementation. The study design by Parracho et al. (36) allowed the participants to serve as their own control with baseline and washout periods included in their protocol. In this trial in 22 children with ASD, there was no difference in the number of daily stools during 3 wk of probiotic therapy compared with 3 wk of placebo, but there were significantly fewer “hard” (P < 0.01) and more “formed” (P < 0.01) stools reported with probiotic therapy compared with placebo. No other differences in bowel function or symptoms, including abdominal pain, bloating, or flatulence, were noted between the placebo and probiotic intervention groups (36).
Tomova et al. (29) found that 90% of the children with ASD in their study (ASD participants = 10) had some form of gastrointestinal symptom and the gastrointestinal symptom severity score was higher among the children with ASD (P < 0.05) and their siblings (P < 0.05) than in the control group at baseline. Gastrointestinal symptom intensity had a strong, positive correlation with severity of ASD, as measured by the ADI scale (R = 0.78, P = 0.01); however, the same correlation was not found between the CARS evaluation and gastrointestinal symptoms (29). In addition, gastrointestinal symptoms were strongly correlated with fecal TNF-α concentrations (R = 0.78, P < 0.05), which were used as a marker for intestinal inflammation (29).
Similarly, Shaaban et al. (37) found that there was significant improvement in gastrointestinal symptoms after 3 mo of probiotic supplementation (P < 0.0001) when measured by using a modified version of the Gastrointestinal Severity Index (6-GSI) in 30 children with ASD. More specifically, there was significant improvement in constipation (P < 0.01), stool consistency (P < 0.023), flatulence (P < 0.037), and abdominal pain (P < 0.002).
West et al. (38) indicated that 52% and 20% of their study population (n = 33) had severe constipation and diarrhea, respectively, at baseline before probiotic treatment, with noticeable decreases in each symptom after probiotic therapy. Although statistical significance was not reported, the prevalence of severe constipation decreased from 52% before treatment to 20% after treatment. Similarly, the prevalence of severe diarrhea decreased from 20% before treatment to none after treatment.
Gut microbiota in children with ASD
To complete the investigation of probiotic supplementation on gastrointestinal function in children with ASD, we must explore not only the change in reported gastrointestinal symptoms (which can be inconsistent and underrecognized in this population) but also the change in the gut microbiota as a result of this intervention. As mentioned previously, the diversity or dysbiosis of the gut microbiome can influence the prevalence and severity of gastrointestinal symptoms in individuals with ASD (12, 13). Several of the studies examined within this review described the gut microbiota landscape of children with ASD by analyzing the fecal microbiota (29, 36, 37) and objectively measured change in the gut microbiome in some fashion (29, 31, 36, 37).
Landscape of the gut microbiome
Tomova et al. (29) reported that the fecal microbiota in children with ASD indicated dysbacteriosis at both the phylum and species levels. When compared with controls without ASD, children with ASD had significantly lower Bacteroidetes-to-Firmicutes ratios (P < 0.05) and significantly higher amounts of Lactobacillus (P < 0.05). Although not significant, children who had more severe gastrointestinal symptoms had a lower abundance of Clostridia and Desulfovibrio and a lower Bacteroidetes-to-Firmicutes ratio than did children with mild gastrointestinal symptoms (8.1E+ 07 compared with 9.9E+ 08; 8.3E+ 07 compared with 1.3E+ 08 and 0.33 compared with 0.64, respectively; NS) (29). Alternatively, children with more severe ASD, as indicated by a CARS score of ≥50, had higher Clostridia and Desulfovibrio abundance and a lower Bacteroidetes-to-Firmicutes ratio than children with mild ASD; however, this too did not reach significance (2.6E+ 08 compared with 1.2E+ 08; 1.1E+ 08 compared with 9.9E+ 07 and 0.42 compared with 0.48, respectively; NS) (29). Despite the trend of lower Bacteroidetes-to-Firmicutes ratios in children with more severe ASD and more severe gastrointestinal symptoms, there was only a weak, negative trend toward correlation between Bacteroidetes-to-Firmicutes ratio and severity of ASD (using the ADI score) (29). However, there was a trend toward a positive correlation between the ADI score and abundance of Desulfovibrio (R = 0.61, P = 0.01), which largely stemmed from the very strong correlation between Desulfovibrio and the restricted or repetitive behavior subscale score from the ADI (R = 0.83, P < 0.05) (29).
Shaaban et al. (37) also noted differences in the abundance of beneficial bacteria present in children with ASD compared with neurotypical controls (children with ASD had significantly lower levels of Bifidobacteria than the control group; P = 0.0001); however, they did not explore correlations between bacteria abundance and ASD-related or gastrointestinal-related symptoms. Kaluzna-Czaplinska and Blaszczyk (31) did not study fecal specimens to describe or measure changes in the gut microflora of children with ASD; however, they did measure urine d-arabinitol (DA), which is a metabolite of the Candida species, and DA-to–l-arabinitol (LA) ratios, which is a surrogate marker for invasive candidiasis. At baseline, they found that children with ASD had DA levels of 160.04 ± 22.88 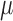 mol/mmol creatinine and a DA-to-LA ratio of 3.15 ± 0.41, which is higher than levels reported in healthy children (39). Parracho et al. (36) examined bacterial populations in fecal samples of 17 children with ASD with the use of fluorescence in situ hybridization. They found no significant differences in the fecal microbiota between the baseline, washout, or placebo samples.
mol/mmol creatinine and a DA-to-LA ratio of 3.15 ± 0.41, which is higher than levels reported in healthy children (39). Parracho et al. (36) examined bacterial populations in fecal samples of 17 children with ASD with the use of fluorescence in situ hybridization. They found no significant differences in the fecal microbiota between the baseline, washout, or placebo samples.
Influence of probiotic supplementation on the gut microbiota
Tomova et al. (29), who utilized real-time PCR to analyze fecal microbiota, reported that probiotic therapy in 10 children with ASD significantly decreased the amount of Bifidobacterium (P < 0.05) and brought the abundance down to levels similar to those found in controls without ASD. Similarly, Desulfovibrio significantly decreased in children with ASD after 4 mo of probiotic therapy (P < 0.05) (29). Fecal TNF-α was found to be higher (although not significantly) among children with ASD and their siblings than in controls and probiotic therapy significantly decreased fecal TNF-α concentrations in children with ASD from baseline (2-tailed P < 0.05). Likewise, although not reaching significance, fecal TNF-α concentrations showed a trend toward correlation with autism severity as measured by the ADI (R = 0.7, P = 0.06) and gastrointestinal symptoms were strongly correlated with fecal TNF-α concentrations (R = 0.78, P < 0.05) (29).
In the trial completed by Parracho et al. (36), when L. plantarum WCFS1 was provided at a dose of 4.5 × 1010 CFUs once daily for 3 wk to 17 children with ASD, there was a significant alteration in the gut microbiota as compared with placebo when assessed utilizing fluorescence in situ hybridization analysis. Specifically, the amount of Lactobacillus and Enterococcus increased (P < 0.05) and Clostridium coccoides decreased (P < 0.05) (36). Of note, there was a significant drop-out rate in this trial, which affected the statistical power. Likewise, after 3 mo of probiotic supplementation, Shaaban et al. (37) found that children with ASD experienced a significant increase in Bifidobacteria and Lactobacillus (both P < 0.0001) in their fecal stool samples. When Kaluzna-Czaplinska and Blaszczyk (31) provided 22 children with ASD who had baseline gastrointestinal issues (constipation, diarrhea, and abdominal pain) with L. acidophilus 5 × 109 CFUs twice daily for 2 mo, probiotic therapy significantly reduced urine DA (P < 0.05) and urine DA-to-LA ratio.
Influence of probiotic supplementation on behavior
As described previously, some maladaptive behaviors may be associated with gastrointestinal dysfunction in individuals with ASD. As such, 3 of the studies included within this review examined change in behavior as a result of probiotic intervention (36–38). Fifteen of the 17 children with ASD who completed both feeding arms (placebo and probiotic) in the trial by Parracho et al. (36) had TBPSs. These scores were derived from parent/guardian completion of the Development Behavior Checklist, a 96-item questionnaire that elicits responses on behavioral/emotional disturbances across the following 5 domains: social-relating problems, disruptive/antisocial behavior, communication, self-absorbed behavior, and anxiety problems. TBPSs in both feeding arms of the trial (probiotic and placebo) were significantly decreased from baseline (P < 0.05); however, there were no significant intergroup differences (36). Of note, the median baseline score of 58 and the median placebo score of 47 were values that suggested clinically relevant behavior and emotional problems (36). Although no differences were noted in median scores for the 5 subdomains among the placebo or probiotic feeding arms, the probiotic feeding arm experienced a significant decrease in the median TBPSs for disruptive/antisocial behavior, communication, anxiety problems, and self-absorbed behavior from baseline (P < 0.05) (36).
West et al. (38) used the ATEC to assess change in ASD symptoms from baseline to post–probiotic intervention (40). The ATEC evaluates autism severity across 4 domains: speech/language/communication, sociability, sensory/cognitive awareness, and health/physical/behavior (40). Higher ATEC scores indicate more severe ASD symptoms. In this trial, ATEC scores were available for 25 out of 33 children and the authors found that ATEC scores significantly decreased after 21 d of Delpro (P < 0.05 for all domains) (38). As previously described, Delpro is a probiotic supplement with a blend of Lactobacillus and Bifidobacterium species with the addition of Del-immune V powder. The mean ATEC score decreased from 72.8 to 58.3 pre- to post-treatment, respectively (–19.9%; P < 0.05), with 88% (n = 22) of respondents reporting a decrease in total ATEC score (38).
When evaluating change in ATEC scores by domain, the domain that showed the largest change in score (–25.5%) among the most participants (92%; n = 23) was the health/physical/behavior domain (P < 0.05), which includes 2 questions assessing severity of constipation and diarrhea (38). The next largest change in score (–22.9%) among 75% (n = 19) of the respondents was in the sociability domain (P < 0.05) (38). The speech and sensory/cognitive awareness domains each improved by ∼11% among 56% (n = 14) and 72% (n = 18) of participants, respectively (each P < 0.05) (38). Similar to West et al., Shaaban et al. (37) also found that 3 mo of probiotic supplementation significantly decreased total ATEC scores (P = 0.0001) and all 4 subdomain scores (all P < 0.05). The largest score improvement before and after probiotic supplementation was noted in the health/physical/behavior domain (36.83 ± 8.32 compared with 27.10 ± 5.83; P = 0.0001) (37). The Autism Research Institute provides normative data for comparison of ATEC scores (41); however, individual improvements in ASD symptoms, regardless of their relation to normative data, may be clinical meaningful to children with ASD and their families.
Limitations
There is limited primary research examining the role of probiotic therapy in alteration of gastrointestinal symptoms in children with ASD. The literature consists of only a few small, poorly controlled studies that lacked consistent measurement of gastrointestinal symptoms. Ingestion of food sources of probiotics or change in dietary habits/food selectivity was not controlled for or evaluated in any of the trials, and therapies that included prebiotics or synbiotics were not studied. In addition, concurrent therapies and medications, including dietary supplements and additional probiotics, were not controlled for. There were inconsistent strains, dosages, and duration of probiotic intervention provided to participants across all 4 studies and no reports on compliance, which limits the ability to analyze the results. Concurrent strains and additives used (e.g., the immunomodulator Del-immune V) limit the ability to identify which species, strain, or therapy may have contributed the most benefit to the host. In addition, the heterogeneity of ASD and the variability in the psychological testing tools (e.g., CARS compared with ADI compared with ATEC) limit the ability to establish relations. There was a lack of control or placebo groups in many of the studies, and there was risk of selection bias. One study also did not disclose any conflicts of interest; however, there appeared to be a risk of bias due the presence of industry investigators (38). The small sample sizes, especially among the studies examining the gut microbiota, may have resulted in studies that were inadequately powered to detect significant changes among the participants. More rigorous study designs, such as the double-blind, placebo-controlled, crossover trial by Parracho et al. (36), appeared to be more difficult to conduct in this population, as evidenced by the high attrition rate.
Conclusions
Although there are several methodologic weaknesses in the literature examining the influence of probiotic supplementation on modulating gastrointestinal dysfunction, there are some promising results. As highlighted by Tomova et al. (29) and in other literature (12, 13, 22–25, 37), there does appear to be intestinal dysbiosis in children with ASD as compared with neurotypical controls, as well as increased inflammation and permeability (29), which may be contributing to the severity of gastrointestinal and ASD symptoms in children with ASD.
Of the 3 studies that measured change in gastrointestinal function after probiotic supplementation, all reported improvement in stools (fewer hard stools, constipation, and diarrhea and more formed stools) (36–38). Although not all changes met significance, this still may have practical application because the study samples may have been inadequately powered to detect significant changes. Furthermore, as the research continues to explore modulating the gut microbiome to improve ASD symptoms, we may uncover more precision with probiotic therapy, which could yield more consistent, significant results.
Probiotic therapy, despite the variability in species, strains, dosages, and duration utilized, consistently altered the fecal microbiota or urine metabolites in a beneficial way. Parracho et al. (36) showed improvements in Lactobacillus and Enterococcus and a reduction in Clostridium abundance in the fecal microbiota of children with ASD, whereas Tomova et al. (29) showed a normalization of the Bacteroidetes-to-Firmicutes ratio in children with ASD to near that of children without ASD and a decrease in intestinal inflammation and permeability. Shabaan et al. (37) noted improvements in Bifidobacteria and Lactobacillus after probiotic supplementation, and Kaluzna-Czaplinska and Blaszczyk (31) showed that probiotic therapy reduced total urine DA concentrations as well as DA-to-LA ratios, indicating a reduction in Candida. Gaining a more complete understanding of the bacterial and fungal microbiota of individuals with ASD will allow future research to tailor probiotic therapy to species that may be contributing to ASD symptom severity, including gastrointestinal symptoms.
In addition to the alteration in gastrointestinal dysfunction and microbiota, more than half of the studies also included assessment of change in behavior measured by different tools after probiotic therapy (22, 37, 38). All of these studies noted a reduction in the severity of ASD symptoms after the probiotic intervention and, although not all reached significance, there still may be clinical applications for this. It is also unclear if the time frame of probiotic therapy was long enough in each study to be able to establish impact on symptoms, especially for behavioral changes, and the trials did not control for concurrent therapies or interventions that may have affected the outcomes.
Overall, there were few reported side effects as a result of probiotic therapy. Nearly 14% (n = 3) of the children in the trial by West et al. (38) reported either worsening constipation or diarrhea severity with probiotic supplementation. Three adverse advents were reported by Parracho et al. (36) during the probiotic intervention, which included a 3-d episode of a skin rash, diarrhea, and weight loss (36). Confounding variables were not accounted for in either study and no other side effects were reported by the remaining investigators.
In summary, the evidence presented here supports the continued investigation of probiotic therapy in modulating gastrointestinal symptoms in children with ASD. In a survey of pediatric or family-medicine physicians, 19% encouraged the use of probiotic therapy in patients with ASD (42), whereas in a more recent, albeit smaller, study in 22 children with ASD, nearly 17% used probiotics in addition to traditional approaches to assist in the management of their condition (43). The documented use and recommendation of probiotic use in the management of ASD symptoms, in combination with the scientific advancement in understanding and appreciating the relation that the gut microbiome may have with ASD symptoms, certainly highlights the need for continued research in this field. Questions that still remain include what are the optimal species, strains, strength, and duration of probiotic therapy and how can we move forward with more precise interventions based on individual needs.
Implications for Practice
Medical and behavioral clinicians should work together to develop integrated care plans that address the complex needs of individuals with ASD (33). Recognition of gastrointestinal dysfunction will allow for more appropriate treatment plans. For example, addressing problem behaviors with behavioral treatment when the underlying setting event (i.e., gastrointestinal discomfort) is not being addressed or investigated is not an effective treatment strategy.
The health effects of probiotics vary on the basis of the species and strain of the bacteria and the nature of the host environment. Precision care, where interventions such as probiotic supplementation are chosen on the basis of the individual, is needed if we are to experience the full potential benefits of this treatment. At this time, practitioners may consider probiotic therapy in children with ASD who have severe gastrointestinal dysfunction (e.g., constipation or diarrhea) because they may experience some reduction in symptoms; however, the optimal species, strain, and dose has yet to be identified and will likely be individualized based on the heterogeneity of ASD and the host microbiome. The reported side effects have been minimal but would warrant discontinuation of treatment if experienced. Caregivers and health care providers should be aware of the developing scientific findings that seem to indicate that altered gut microbiota and intestinal inflammation and permeability may be present in children with ASD and that more research is needed to determine the most effective interventions to mitigate these factors.
Although the focus of this review was to examine the current literature to address if common gastrointestinal symptoms in children with ASD improve with the provision of probiotics, the nutritional impact on the gut microbiome and new potential therapeutic interventions should not be overlooked. Changes in diet can significantly influence the landscape of the gut microbiome and should be considered in attempts to mitigate gastrointestinal disturbances (44, 45). For example, the intake of animal protein has been positively correlated with diversity of gut microbiota, whereas a diet high in saturated fats increases the relative abundance of Bacteroides and Bilophila and increases anaerobic flora (46). Although there have been studies that have found positive outcomes for additional dietary interventions (e.g. omega-3 FAs, gluten-free or casein-free diet, and ketogenic diet), further research is still necessary in order to support these therapeutic options (45).
Potential therapeutic interventions may also include fecal microbiota transplantation (FMT) and microbiota transfer therapy (MTT), which may help reverse gut dysbiosis as well as alleviate gastrointestinal and possibly even some ASD symptoms (45, 47). FMT, in which individuals with gut dysbiosis receive fecal microbiota from healthy individuals, has shown positive outcomes in individuals with irritable bowel syndrome and inflammatory bowel disease (45). Although the safety of FMT needs to be investigated further (48), there may be potential for this to be a therapeutic intervention for children with ASD who suffer from gastrointestinal disturbances (45).
MTT is a modified protocol of FMT that includes 2 wk of antibiotic treatment, a bowel cleanse, and a high dose of standardized human gut microbiota for ∼2 mo (45). For example, Kang et al. (47) followed 18 children with ASD who had moderate to severe gastrointestinal dysfunction in an open-label trial for 18 wk, in which they received 10 wk of MTT treatment. Significant improvements in gastrointestinal and ASD symptoms, as well as microbiota diversity, were found at the end of the MTT treatment and were maintained for 8 wk after the treatment commenced (47).
Future Research
Future research should bear in mind the specific needs of children with ASD and their families when creating study designs and protocols. Alleviating some of the burdens that may hinder this population from completing participation in research trials should be taken into account to optimize attrition rates. Consistent and standardized methods for measuring ASD and gastrointestinal symptoms are paramount and confounding variables, such as therapies, medications, and diet, should be controlled for. In addition, focusing on a more defined subset of ASD may improve homogeneity of the study groups and improve the analysis and application of the findings. Future research may need to consider each subject as an individual case study to establish any correlation between gastrointestinal symptoms and behavior and what effects probiotics may have on each (36). As the gut microbiota of individuals with ASD continues to be explored, studies that aim to measure alteration in gastrointestinal dysfunction with probiotic therapy should include baseline and post-treatment analysis of the fecal microbiota to help establish relations between bacterial abundance, ASD symptoms, and effect of probiotic therapy.
Acknowledgments
All authors read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: RP and JZ, no conflicts of interest.
Abbreviations used:
- ADI
Autism Diagnostic Interview
- ASD
autism spectrum disorder
- ATEC
Autism Treatment Evaluation Checklist
- CARS
Childhood Autism Rating Scale
- DA
d-arabinitol
- FMT
fecal microbiota transplantation
- LA
l-arabinitol
- MTT
microbiota transfer therapy
- TBPS
Total Behavior Problem Score
References
- 1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed Washington (DC): American Psychiatric Association; 2013. [Google Scholar]
- 2. CDC. Autism spectrum disorder data & statistics. 2016. [Internet]. [updated 2016 Jul 11; cited 2017 Dec 1]. Available from: https://www.cdc.gov/ncbddd/autism/data.html. [Google Scholar]
- 3. McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics 2014;133(5):872–83. [DOI] [PubMed] [Google Scholar]
- 4. Kang V, Wagner GC, Ming X. Gastrointestinal dysfunction in children with autism spectrum disorders. Autism 2014;7(4):501–6. [DOI] [PubMed] [Google Scholar]
- 5. Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord 2014;44(5):1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Furuta GT, Williams K, Kooros K, Kaul A, Panzer R, Coury DL, Fuchs G. Management of constipation in children and adolescents with autism spectrum disorders. Pediatrics 2012;130(Suppl 2):S98–105. [DOI] [PubMed] [Google Scholar]
- 7. Kohane IS, McMurry A, Weber G, MacFadden D, Rappaport L, Kunkel L, Bickel J, Wattanasin N, Spence S, Murphy S, et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PloS One 2012;7(4):e33224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pusponegoro HD, Ismael S, Sastroasmoro S, Firmansyah A, Vandenplas Y. Maladaptive behavior and gastrointestinal disorders in children with autism spectrum disorder. Pediatr Gastroenterol Hepatol Nutr 2015;18(4):230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nikolov RN, Bearss KE, Lettinga J, Erickson C, Rodowski M, Aman MG, McCracken JT, McDougle CJ, Tierney E, Vitiello B, et al. Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J Autism Dev Disord 2009;39(3):405–13. [DOI] [PubMed] [Google Scholar]
- 10. Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism—comparisons to typical children and correlation with autism severity. BMC Gastroenterol 2011;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delahaye J KE, Sikora D, Hall TA, Orlich F, Clemons TE, van der Weerd E, Glick L, Kuhlthau K. The relationship between health-related quality of life and sleep problems in children with autism spectrum disorders. Res Autism Spectr Disord 2014;8(3):292–303. [Google Scholar]
- 12. Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, Jousson O, Leoncini S, Renzi D, Calabrò A, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017;5(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010;16(4):444–53. [DOI] [PubMed] [Google Scholar]
- 14. Russo AJ. Decreased plasma myeloperoxidase associated with probiotic therapy in autistic children. Clin Med Insights Pediatr 2015;9:13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frye RE, Rose S, Slattery J, MacFabe DF. Gastrointestinal dysfunction in autism spectrum disorder: the role of the mitochondria and the enteric microbiome. Microbial Ecology Health Dis 2015;26:27458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol 2014;28(8):1221–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009;6(5):306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roessner U, Bowne J. What is metabolomics all about? BioTechniques 2009;46(5):363–5. [DOI] [PubMed] [Google Scholar]
- 19. Wang H, Liang S, Wang M, Gao J, Sun C, Wang J, Xia W, Wu S, Sumner SJ, Zhang F, et al. Potential serum biomarkers from a metabolomics study of autism. J Psychiatry Neurosci 2016;41(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. West PR, Amaral DG, Bais P, Smith AM, Egnash LA, Ross ME, Palmer JA, Fontaine BR, Conard KR, Corbett BA, et al. Metabolomics as a tool for discovery of biomarkers of autism spectrum disorder in the blood plasma of children. PloS One 2014;9(11):e112445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gevi F, Zolla L, Gabriele S, Persico AM. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol Autism 2016;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Medical Microbiol 2005;54(10):987–91. [DOI] [PubMed] [Google Scholar]
- 23. Mayer EA, Padua D, Tillisch K. Altered brain-gut axis in autism: comorbidity or causative mechanisms? BioEssays 2014;36(10):933–9. [DOI] [PubMed] [Google Scholar]
- 24. Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PloS One 2013;8(7):e68322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013;155(7):1451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PloS One 2013;8(10):e76993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol 2011;77(18):6718–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, Bennett A, Jabado O, Hirschberg DL, Lipkin WI. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PloS One 2011;6(9):e24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav 2015;138:179–87. [DOI] [PubMed] [Google Scholar]
- 30. Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci 2012;57(8):2096–102. [DOI] [PubMed] [Google Scholar]
- 31. Kaluzna-Czaplinska J, Blaszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrition 2012;28(2):124–6. [DOI] [PubMed] [Google Scholar]
- 32. Mussap M, Noto A, Fanos V. Metabolomics of autism spectrum disorders: early insights regarding mammalian-microbial cometabolites. Expert Rev Mol Diagn 2016;16(8):869–81. [DOI] [PubMed] [Google Scholar]
- 33. Buie T, Campbell DB, Fuchs GJ III, Furuta GT, Levy J, Vandewater J, Whitaker AH, Atkins D, Bauman ML, Beaudet AL, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics 2010;125(Suppl 1):S1–18. [DOI] [PubMed] [Google Scholar]
- 34. Berry RC, Novak P, Withrow N, Schmidt B, Rarback S, Feucht S, Criado KK, Sharp WG. Nutrition management of gastrointestinal symptoms in children with autism spectrum disorder: guideline from an expert panel. J Acad Nutr Diet 2015;115(12):1919–27. [DOI] [PubMed] [Google Scholar]
- 35. Academy of Nutrition and Dietetics Chicago: evidence analysis manual: steps in the Academy evidence analysis process [Internet]. [updated 2016; cited 2017 Dec 1]. Available from: https://www.andeal.org/files/Docs/2012_Jan_EA_Manual.pdf. [Google Scholar]
- 36. Parracho HM, Gibson GR, Knott F, Bosscher D, Kleerebezem M, McCartney AL. A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int J Probiotics Prebiotics 2010;5(2):69–74. [Google Scholar]
- 37. Shaaban SY, El Gendy YG, Mehanna NS, El-Senousy WM, El-Feki HSA, Saad K, El-Asheer OM. The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr Neurosci 2017Jul 7:1–6. doi: 10.1080/1028415X.2017.1347746. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38. West R, Roberts E, Sichel LS, Sichel J. Improvements in gastrointestinal symptoms among children with autism spectrum disorder receiving the Delpro® probiotic and immunomodulator formulation. J Prob Health 2013;1(1):102. [Google Scholar]
- 39. Stradomska TJ, Sobielarska D, Mielniczuk Z, Jagiellowicz D, Syczewska M, Dzierzanowska D. Determination of urinary D-/L-arabinitol ratios as a biomarker for invasive candidiasis in children with cardiac diseases. J Med Microbiol 2010;59(Part 12):1490–6. [DOI] [PubMed] [Google Scholar]
- 40. Autism Research Institute Autism Treatment Evaluation Checklist (ATEC) [Internet]. [cited 2017 Aug 16]. Available from: https://www.autism.com/ind_atec. [Google Scholar]
- 41. Autism Research Institute Studies confirm validity of ATEC report [Internet]. [cited 2017 Dec 1]. Available from: https://www.autism.com/ind_atec_report. [Google Scholar]
- 42. Golnik AE, Ireland M. Complementary alternative medicine for children with autism: a physician survey. J Autism Dev Disord 2009;39(7):996–1005. [DOI] [PubMed] [Google Scholar]
- 43. Huang A, Seshadri K, Matthews TA, Ostfeld BM. Parental perspectives on use, benefits, and physician knowledge of complementary and alternative medicine in children with autistic disorder and attention-deficit/hyperactivity disorder. J Altern Complement Med 2013;19(9):746–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu Z, Knight R. Dietary effects on human gut microbiome diversity. Bri J Nutr 2015;113(Suppl):S1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Q, Han Y, Dy ABC, Hagerman RJ. The Gut microbiota and autism spectrum disorders. Front Cell Neurosci 2017;11:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017;15(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 2017;5(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Navarro F, Liu Y, Rhoads JM. Can probiotics benefit children with autism spectrum disorders? World J Gastroenterol 2016;22(46):10093–102. [DOI] [PMC free article] [PubMed] [Google Scholar]