Abstract
The ability of nutrition scientists to measure the status, bioavailability, and bioefficacy of micronutrients is affected by lack of access to the parts of the body through which a nutrient may travel before appearing in accessible body compartments (typically blood or urine). Stable isotope–labeled tracers function as safe, nonradioactive tools to follow micronutrients in a quantitative manner because the absorption, distribution, metabolism, and excretion of the tracer are assumed to be similar to the unlabeled vitamin or mineral. The International Atomic Energy Agency (IAEA) supports research on the safe use of stable isotopes in global health and nutrition. This review focuses on IAEA's contributions to vitamin A, iron, and zinc research. These micronutrients are specifically targeted by the WHO because of their importance in health and worldwide prevalence of deficiency. These 3 micronutrients are included in food fortification and biofortification efforts in low- and middle-income regions of the world. Vitamin A isotopic techniques can be used to evaluate the efficacy and effectiveness of interventions. For example, total body retinol stores were estimated by using 13C2-retinol isotope dilution before and after feeding Zambian children maize biofortified with β-carotene to determine if vitamin A reserves were improved by the intervention. Stable isotopes of iron and zinc have been used to determine mineral bioavailability. In Thailand, ferrous sulfate was better absorbed from fish sauce than was ferrous lactate or ferric ammonium citrate, determined with the use of different iron isotopes in each compound. Comparisons of one zinc isotope injected intravenously with another isotope taken orally from a micronutrient powder proved that the powder increased total absorbed zinc from a meal in Pakistani infants. Capacity building by the IAEA with appropriate collaborations in low- and middle-income countries to use stable isotopes has resulted in many advancements in human nutrition.
Keywords: bioconversion, biofortification, compartmental modeling, fortification, micronutrients, retinol isotope dilution, stable isotopes
Introduction
The WHO advises that many micronutrients are limited in human diets and may need public health interventions to address deficiency (1). Of particular concern, 3 essential micronutrients specifically targeted for improvement are vitamin A, iron, and zinc. Deficiencies in these micronutrients are largely due to a large proportion of the diet being composed of high-carbohydrate staple foods that are micronutrient poor. For example, in many African countries, >50% of available calories are from staple grains, roots, and tubers (2). In order to diminish deficiencies of these key nutrients, they are often added to foods that have high-reaching coverage in a process called fortification (3). In addition, biofortification is a plant-breeding technique that naturally enhances micronutrient content of plant foods (4, 5). Both of these long-term, sustainable processes are aimed at lowering the need for direct supplementation to vulnerable groups. Supplementation is relatively expensive and requires continuous human resource inputs (6).
Measuring the micronutrient value of foods and the status of individuals and target groups is not always straightforward. Three terms associated with micronutrients are as follows: 1) bioaccessibility, defined as the quantity of a nutrient that is available for absorption after digestion; 2) bioavailability, defined as the proportion of a given nutrient that is absorbed and available for physiologic function (7); and 3) bioefficacy, defined as the proportion of a nutrient that is ultimately converted to the active form (e.g., retinol produced from dietary provitamin A carotenoids). Determination of these parameters is not trivial (8†). Stable isotope methods are the most powerful techniques available to scientists for evaluating vitamin A status and provitamin A bioefficacy (9†, 10, 11†, 12) and iron and zinc bioavailability from meals (13†). The International Atomic Energy Agency (IAEA) has invested in capacity building of laboratories worldwide, and one of their main objectives is to foster the safe use of stable isotopes in human nutrition and health. The purpose of this review is to inform the reader about stable isotope methods and their application to nutrition programming. It also serves to highlight some of the work that the IAEA has supported over the past 2 decades on these 3 micronutrients, denoted throughout this article by “†” after a citation number.
Current Status of Knowledge
Safety and natural abundance of stable isotopes
A common misconception when the term “isotope” is used when explaining nutrition research is that it refers to compounds that are radioactive. In fact, radioisotopes are far less abundant in nature than other stable isotopic forms. The difference in isotopic forms of an element relates to the number of neutrons in the nucleus; all isotopic forms have the same number of protons (e.g., 6 for carbon) (Figure 1). The distribution of an element among each form is called the natural abundance. For example, carbon is predominantly 12C (98.9%), but occurs in a stable form as 13C (1.1%), which is a common isotope used in nutrition studies (14). Radioactive carbon, 14C, is found only in trace amounts (0.0000000001%), and 11,12-14C2-retinyl acetate was used in vitamin A nutrition studies in conjunction with accelerator MS, because 14C has a relatively long half-life, allowing it to be followed for months (15–17). The radioactive form of hydrogen [tritium, 3H; trace amounts in nature (10−16%)] was introduced into 11,12-3H2-retinyl acetate and used in the seminal work in humans for the determination of vitamin A requirements and half-life (18). In addition to 13C, the stable isotope of hydrogen, 2H [deuterium; 0.015% (14)], is used to label organic molecules, such as vitamin A, for nutrition studies (Figure 2). Researchers have used 2H-labeled water to measure breast-milk intake volume in infants (19–21†). Vitamin A (20†) and zinc (21†) intakes were estimated in Mexican and Indian infants, respectively, by combining breast-milk intake with routine analysis of micronutrient content of the milk.
FIGURE 1.
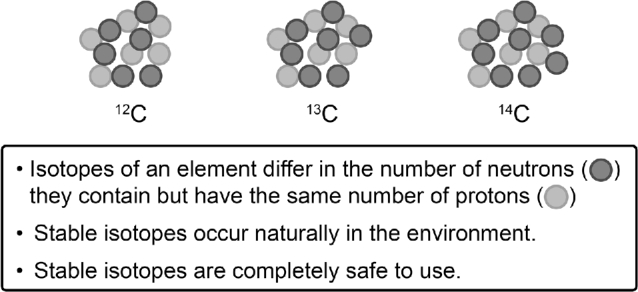
The 3 isotopic forms of carbon. 12C is the most common of the isotopic forms (98.9%) and 13C is the stable form (1.1%); 14C is the radioactive form and is only found in trace amounts in nature.
FIGURE 2.
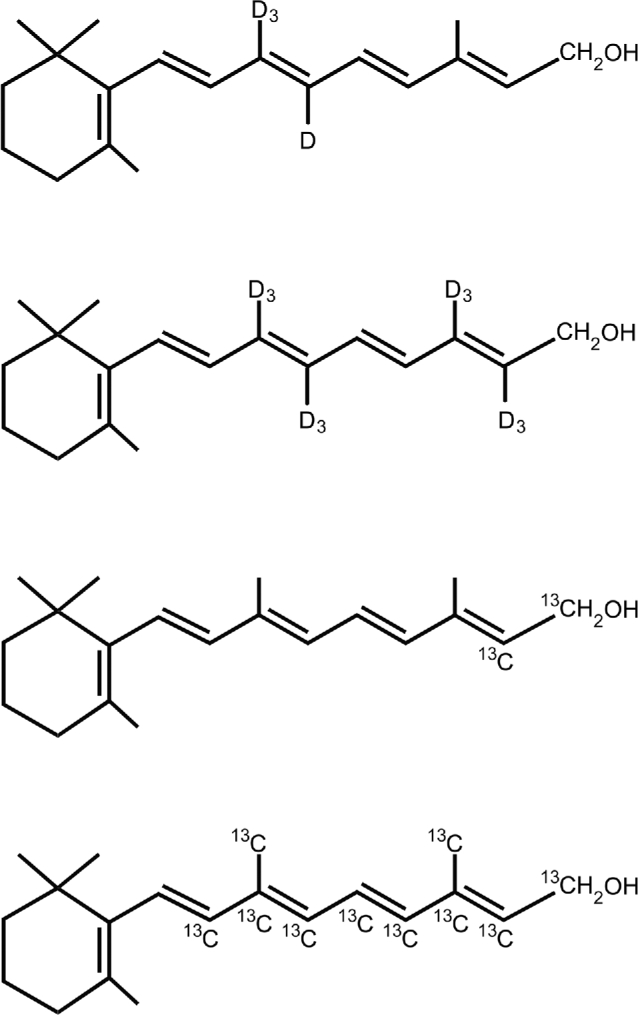
The 4 most common stable isotope–labeled vitamin A structures. These molecules are usually administered as the retinyl acetate compounds in retinol isotope dilution and compartmental modeling studies. Top to bottom: 10, 19, 19, 19-D4-retinol; 10, 19, 19, 19, 14, 20, 20, 20-D8-retinol; 14,15–13C2-retinol; and 8, 9, 10, 11, 12, 13, 14, 15, 19, 20-13C10-retinol. D, deuterium.
The stable isotopes of elemental iron and zinc can be used to study these essential minerals. The most common iron form is 56Fe (91.75%), and isotopes used for bioavailability studies include 57Fe (2.12%) and 58Fe (0.28%) (14). The most common zinc forms are 64Zn (49.17%) and 66Zn (27.73%). The stable isotopes used for nutrition research include 67Zn (4.04%), 68Zn (18.45%), and 70Zn (0.61%) (14). To date, there are no isotopic methods used to evaluate iron status. The utility of monitoring the size of the exchangeable zinc pool (EZP) to reflect changes in zinc intake or absorption has been investigated (22).
Vitamin A status assessment and provitamin A carotenoid bioavailability studies
IAEA-funded nonisotopic work with vitamin A analogs
Dose-response tests are a simpler alternative or complementary technique to stable isotope approaches, but these tests only qualitatively define liver retinol stores, whereas isotope methods are quantitative. The dose response is based on the accumulation of apo-retinol-binding protein in the liver during times of vitamin A depletion and rapid release of the holo-retinol-binding protein after a challenge dose (23). The modified relative dose response (MRDR) test, which determines a serum ratio of orally consumed 3,4-didehydroretinol (vitamin A2) to retinol, similar to tracer dilution, can detect the degree of vitamin A deficiency in a population (10) but does not quantify total body retinol stores in an individual or group (9†). For example, the MRDR test was used to evaluate high-dose supplementation (24†) and a green leafy vegetable intervention (25†) in Ghanaian lactating women. Furthermore, the MRDR test was used in Senegal to determine a high prevalence (73.5%) of low liver stores of vitamin A (MRDR ≥0.06) among 6-mo-old breastfed infants, whereas plasma retinol concentrations only identified 15% as deficient (<0.7 μmol/L) (26†). The MRDR test was used to determine baseline vitamin A liver status of 7- to 9-mo-old infants in Ghana before an intervention (27†), whereas after the intervention, retinol isotope dilution (RID; discussed below) was used to determine that body stores did not differ between infants receiving a vitamin A supplement or placebo (mean ± SD: 436 ± 303 and 434 ± 186 μmol, respectively). Both MRDR and RID analyses diagnosed the cohort as having adequate vitamin A status.
Stable isotope methods
RID can be applied to evaluate the vitamin A status of groups with the use of 2H- or 13C-labeled retinyl acetate (Figure 2). After the subject consumes the labeled dose of retinyl ester, the retinol is distributed and diluted into the vitamin A pool after absorption (Figure 3). The enrichment in the blood with the labeled compound is determined after a mixing period, which is typically >10 d. Studies in rats suggest that mixing has not fully occurred before 10 d, especially when the RID test is applied to individuals with adequate vitamin A status (30). The measured ratio of labeled tracer to endogenous vitamin A is used to estimate total body stores and total liver reserves of vitamin A after correcting for natural abundance, absorption, and catabolism of the dose (29). A baseline blood sample measured for isotopic natural abundance is not necessarily required in all subjects because it is often considered to be nonenriched in the case of 2H or a mean baseline 13C natural abundance of a subsample is used (27†, 31†) to account for interindividual variation. It is recommended to measure infection or inflammation status by analyzing serum concentrations of the acute-phase reactant C-reactive protein and convalescence reactant α1-acid glycoprotein (32, 33). Participants with fever should not be enrolled because active infection decreases the amount of dose absorbed (34), causes enhanced retinol losses in urine (35), and may influence the ratio of isotopic enrichment of retinol in serum to that in liver (36), variables that affect estimated retinol stores. RID has been applied in a number of countries including Cameroon, China, Ghana, Mexico, Thailand, and Zambia to determine vitamin A status or changes in status during interventions (37†). More studies are currently underway in other African countries.
FIGURE 3.
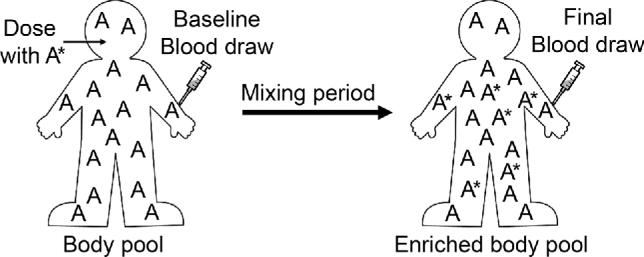
For vitamin A status assessment, a dose of vitamin A ester labeled with stable isotope is administered after a baseline blood sample. A period of the dose mixing with the vitamin A body pool is necessary before the follow-up blood sample is taken for analysis by MS (adapted from reference 28). Prediction equations use the data from the mass spectrometer, along with key assumptions about absorption, storage, and catabolism of dose, to estimate total body retinol stores (29).
A variety of methods to analyze the isotopic enrichment of samples are available, and the tracer used depends upon the type of mass spectrometer available to the researcher. It is essential that collaborating partners know the type of mass spectrometer that will be used in the final analysis in order to familiarize themselves and understand the advantages and limitations of the system, to determine the number of atoms needed to be labeled with stable isotope in the molecule for appropriate detection postdosing, and to evaluate the amount of serum needed for the analysis. Moreover, the amount of stable isotope dose to be administered needs to be calculated on the basis of the sensitivity of the instrument, the anticipated pool size, and the amount of retinol estimated in the sample for analysis. For example, if the mass spectrometer available is a GC-combustion-isotope-ratio mass spectrometer, the number of labeled atoms needed is fewer (2 carbons needed as 13C) than that used by GC-MS, which requires ≥4 atoms labeled as 2H or 13C to provide detectable enrichment in serum (38†). The mass of dose also differs, with recent doses as low as 1.0 μmol 13C2-retinyl acetate given to children for vitamin A assessment using the GC-combustion-isotope-ratio MS analysis (31†); 1.75 or 2.95 μmol 13C10-retinyl acetate for stable isotope reference methods using LC–atmospheric pressure MS (39), or LC–tandem MS (40), respectively; and 28 μmol to women for vitamin A assessment with the use of GC-MS analysis (41†). Researchers reduced the dose amount to 6.0 μmol 2H4- or 2H8-retinyl acetate in studies underway in African children supported in part by the IAEA with the use of GC-MS with electron capture detection. These unique features among isotope methods, mass spectrometers, and labeling underscore the importance of knowing the research question asked and the methodology available to the research team for the final analysis.
The paired RID technique in which total body retinol pool size is estimated before and after an intervention can be used to determine the efficacy or bioefficacy of provitamin A carotenoids consumed with careful study design (42†). An efficacy study in Chinese children showed that green and yellow vegetables maintained total body retinol stores (43†). Another efficacy study in Filipino children showed that vitamin A status inversely affected bioconversion of provitamin A carotenoids to retinol (44†). To calculate bioefficacy factors, appropriate control groups are required (Figure 4). Bioefficacy is estimated as the ratio of the change in stores in the study group to that in the positive control group, adjusted for the difference between the total reference dose consumed and the total carotenoid consumed during the intervention, corrected for loss or maintenance estimated in the negative control group. When the paired RID test is used, it is necessary to determine a baseline serum retinol isotopic natural abundance postintervention because some of the baseline isotopic tracer may still be measurable and the intervention itself could change isotope enrichment depending on foods consumed. Specifically, provitamin A–biofortified C4 plants, such as maize, sorghum, and millet, are enriched in 13C, and therefore vitamin A produced from these provitamin A sources could change the serum 13C-enrichment of retinol in the subjects when consumed (45). This effect has been proposed as a biomarker for biofortified C4 crop intervention effectiveness (45), discussed later in this review.
FIGURE 4.
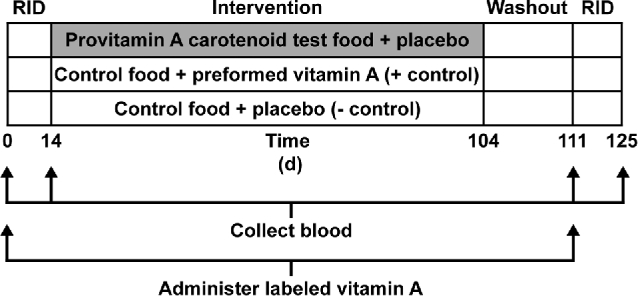
Study design for the paired-isotope dilution method in a highly controlled, 90-d feeding intervention with a high provitamin A–containing food, such as vegetables, a fortified food, or a biofortified staple crop. Negative (placebo) and positive (supplemental preformed vitamin A) control groups are recommended. If both control groups are included, a bioefficacy factor can be estimated for provitamin A–containing foods. RID, retinol isotope dilution.
To gain further information about vitamin A body kinetics, researchers have used stable isotope–labeled vitamin A data to perform compartmental modeling (46). Similar to the RID methods, subjects consume an isotopically labeled dose of retinyl acetate, and then blood is ideally collected several times on the first day and at specific time points thereafter. The changing fraction of the dose in the serum is mathematically modeled by constructing several theoretical compartments within the body by using kinetic constants that describe the movement of retinol throughout the body and its irreversible degradation. Attempts have been made to use this model to develop RID equations that could be applied as early as 3–5 d postdose by estimating the ratio of isotopic enrichment of retinol in plasma and stores reached in the body at any given time (47†, 48†). Some studies make use of a composite model (i.e., “super child” or “super woman”) in which data points from multiple subjects, who each contribute to a fraction of the total number of time points, are combined to form a population-level compartmental model (49†, 50). These super models are used to estimate mean total body retinol stores and kinetic parameters of the associated groups. Recently, a “super child” approach was used in 15 Mexican preschool children to evaluate the bioefficacy of provitamin A carotenoids from intrinsically labeled β-carotene in plants grown with 2H2O; the estimated bioefficacy factor was 3.3 μg β-carotene to 1 μg retinol (51†).
RID has been modified since its inception and will likely continue to change as analytical techniques advance, and a focus is placed on adapting the methods for accessibility and ease of use in low-income regions performing large-scale population surveys. To this end, research recommendations were suggested to improve future RID applications (52†, 53†), including making the method more field-friendly and confirming its applicability in diverse populations.
Evaluation of vitamin A fortification efforts with the use of stable isotopes
The first known evaluation of a vitamin A–fortification program with the use of stable isotopes used the paired RID technique to evaluate changes in vitamin A status 1 y after the introduction of vitamin A–fortified sugar in Nicaragua (54). The children had adequate liver retinol concentrations at baseline (0.52 μmol/g liver), and 1 y later liver concentrations had significantly increased and 43% of the children were above the cutoff considered hypervitaminotic [i.e., 1 μmol/g liver (10)]. The paired RID method was also used to evaluate the change in vitamin A status of Mexican children fed fortified milk (55†). Liver vitamin A concentrations of the children fed the fortified milk were ∼2 times higher than the control group after 3 mo of feeding (55†). The method has similarly been used to evaluate total body retinol stores of Thai children fed fortified rice for an average of 54 d (31†). Total body stores almost doubled in this short time frame despite not showing any significant change in serum retinol concentrations (31†). These findings underscore the importance of continued monitoring in populations who have adopted fortification of staple foods with preformed retinol. When stores are adequate, excess preformed vitamin A will accumulate in the liver (56) and may have detrimental effects on bone health (57).
Biofortification efforts to improve population vitamin A status
The bioefficacy of provitamin A carotenoids to retinol, referred to as a retinol activity equivalent, is currently defined by the Institute of Medicine (IOM) as 12 μg β-carotene (or 24 μg α-carotene and β-cryptoxanthin) to 1 μg retinol in healthy humans, by assigning a static bioconversion factor to pure β-carotene in oil of 2 μg:1 μg retinol, multiplied by an average absorption factor of 6 due to plant matrix effects (58). Biofortification initiatives use the IOM's bioefficacy factor to establish staple-crop target levels for provitamin A carotenoids, usually based on β-carotene (59). However, a static bioefficacy factor may not reflect what actually happens in vivo when provitamin A carotenoids are fed to different groups of humans with different vitamin A statuses and carotenoid-processing genotypes (60). Despite the IOM's recommendation of 12:1 and the maximum theoretical value of 0.94:1, bioefficacy values ranging from 2:1 to 28:1 have been reported (39, 61), showing how this value changes with current vitamin A status, due to feedback inhibition of absorption and processing of carotenoids (62), as well as genotype and plant matrix effects. Inaccuracies are also possible when using nonisotopic methods (12), such as changes in serum retinol concentration (61). The IOM's bioefficacy factor is used for predictions; however, researchers can calculate an actual bioefficacy factor on the basis of experimental outcomes when applying stable isotope techniques. The vitamin A value of a given plant can be determined by growing the plant on a stable isotope substrate, such as heavy water (2H2O), feeding the labeled plant, and taking appropriate blood samples (63†). The bioefficacy of the provitamin A in the labeled plant source is calculated by comparing the carotenoid-derived retinol in a subject consuming the plant with that from a reference dose of stable isotope–labeled retinyl acetate.
The success of provitamin A–biofortification programs may require target populations to adopt different-colored staples than what are traditionally and culturally acceptable (64). For example, Zambians typically consume white maize and most varieties of biofortified maize are orange. Nutritionists have demanded that biofortified crops be evaluated for their impact on the nutritional status of target groups (65). Stable isotope methods are among the most powerful to evaluate bioefficacy (10, 11†, 12) and could support effectiveness studies if applied to subgroups of populations in areas where the crops have been disseminated.
Sweet potato
The first biofortified crop targeted for dissemination was orange-fleshed sweet potato because of its widespread availability and high achievable β-carotene concentrations (66). Stable isotope techniques have been used to estimate β-carotene bioefficacy from these crops. The vitamin A value of β-carotene from sweet potato determined in Bangladeshi men was ∼13 μg β-carotene to 1 μg retinol with the use of paired RID with 2H-vitamin A before and after a 60-d intervention, which was based on the increase in total body retinol stores (67). More recent work in Bangladeshi women who were fed orange-fleshed sweet potato, however, did not result in a net gain of total body retinol stores but did contribute to higher circulating serum β-carotene concentrations (41†).
Effectiveness studies are used to follow improvement in status after the crop of interest has been broadly released for an extended period. A 2-y integrated effectiveness study was undertaken in Mozambique that included the introduction of sweet potato vines into households and monitoring for 2 agricultural cycles. Children in intervention households ate more orange-fleshed sweet potato and had higher blood-spot retinol concentrations than controls (68). However, it should be noted that, after this study, many of the children still had very low serum retinol concentrations, defined as <0.7 μmol/L by the WHO (69). In an effectiveness study of orange sweet potato in Uganda, vitamin A status was improved in children but only after making corrections and shifting the deficiency cutoff value from 0.7 to 1.05 μmol retinol/L (70†). Thus, more quantitative measures of vitamin A status in populations of interest are needed to better predict actual vitamin A status (10), especially when evaluating agriculture-based interventions.
Provitamin A–biofortified maize
Studies with biofortified maize began with evaluation in animal models (71–73), because traditionally bred maize did not have high levels of provitamin A when the research first began (74). Favorable bioefficacy factors for provitamin A carotenoids (i.e., more efficient than the 12:1 value recommended by the IOM) in the maize were obtained in animal models, and human studies followed. A conversion factor of 6.5 ± 3.5 μg β-carotene to 1 μg retinol was obtained in 6 women in the United States who consumed biofortified maize porridge, which was determined by HPLC with electrochemical detection in reference to retinyl palmitate (75). A bioefficacy factor of 3.0 ± 1.5:1 was determined in 9 healthy Zimbabwean men by feeding 2H2O-labeled maize (76). Although similar to the values in the animal studies, these values are estimates of bioefficacy factors with a single-exposure meal in healthy adults and may not reflect what would occur with longer feeding trials in target populations. For example, in a study feeding biofortified maize to Zambian children for 3 mo, the estimated bioefficacy factor was 10.4:1 with the use of paired RID (56).
Long-term maize intake studies in children have been completed to determine if these favorable bioefficacy factors can translate into improved liver concentrations of vitamin A (56, 77). In the first of these controlled feeding studies in Zambia, 3- to 5-y-old children adapted very quickly to eating the orange maize and enjoyed freshly harvested maize much more than maize that was held in storage (77); however, the β-carotene content of the maize was not adequate to maintain vitamin A stores after a high-dose vitamin A supplement, resulting in a higher MRDR value after the trial (78). The need for quantitative evaluation of orange maize interventions led to a second trial 2 y later, which evaluated the impact of 90 d of feeding orange maize to 5- to 7-y-old children with the use of 13C2-RID to estimate the total body retinol pool before and after the intervention (56). The total body retinol stores were similarly increased in the children consuming the orange maize or receiving a preformed vitamin A supplement (an increase of 84 and 98 μmol, respectively), which was much more than the 13-μmol increase in children consuming their usual low-carotenoid white maize during the same time frame. These results showed that consuming orange maize could be efficacious in these settings.
A change in the natural enrichment of 13C in serum retinol may be a way to evaluate interventions with provitamin A carotenoids (79). This phenomenon was studied in overweight individuals when a negative shift was measured in the ratio of 13C to 12C in serum retinol after a 3-mo vegetable intervention (79). In the first Zambian study mentioned above, the change in natural abundance of 13C in serum retinol was positively shifted in the 3- to 5-y-old children eating orange maize for 46 d (45), showing that the β-carotene from this maize is efficacious and contributed to the vitamin A requirements of these children, even though liver stores did not increase. Similarly, gerbils fed an orange maize feed showed an increase in 13C enrichment in serum retinol compared with controls (80).
Effectiveness studies are needed to measure the generational effects of feeding β-carotene–enhanced staple crops to population groups (7). Feeding orange maize to sows during gestation and lactation improved HPLC-determined liver concentrations of vitamin A in offspring compared with sows fed white maize and administered a high-dose vitamin A supplement (81). Sensitive techniques, such as RID, applied to subgroups of populations who adopt biofortified crops will provide useful data to public health administrators. Nutrition education campaigns will also likely improve acceptance and willingness to pay in target populations for biofortified crops (82).
Provitamin A–biofortified cassava
The progression of studies with biofortified cassava mirrored those with maize. In vitro (83) and animal (84) studies were conducted in parallel. In studies conducted in animals with yellow biofortified cassava, the bioefficacy factor was 3.7 μg β-carotene to 1 μg retinol (84). Two human studies that used the retinyl palmitate response in the plasma TG-rich lipoprotein fractions were completed in women with the use of biofortified cassava in porridge (85, 86). The bioefficacy factors for β-carotene in these studies were 2.80 ± 1.77 and 4.5 ± 3.1 μg β-carotene to 1 μg retinol, indicating readily bioaccessible β-carotene. Larger human studies should consider stable isotope methods, especially during effectiveness evaluations (45, 56).
Golden Rice
Transgenic approaches to enhance provitamin A concentrations in the rice endosperm were undertaken, producing high-β-carotene (1.6 μg/g) “Golden Rice” (87) and the improved (26 ± 5 μg/g) “Golden Rice 2” (88). In 5 healthy adults, the conversion factor for Golden Rice β-carotene (with the use of a particular genotype containing 20 μg β-carotene/g) to retinol was 3.8 ± 1.7 μg to 1 μg, with a range of 1.9–6.4:1, and was determined by using rice grown in heavy water (2H2O) and 13C10-retinyl acetate as a reference dose (89). In Chinese children fed pure β-carotene, Golden Rice, or spinach, the β-carotene to retinol bioefficacy factors were 2.0, 2.1, and 7.3 μg to 1 μg, respectively, which were evaluated by using a combination of 2H- and 13C-labeled compounds (39). This efficient bioefficacy factor for Golden Rice, similar to pure β-carotene in oil, likely reflects the low level of β-carotene in the rice as well as favorable bioaccessibility from this matrix.
Iron and zinc stable isotope studies
To date, iron and zinc isotopes are not routinely used for assessing the status of individuals. Iron has many biomarkers of status, and typically, population surveys use hemoglobin, hematocrit, soluble transferrin receptor, and ferritin (90). Interventions to improve iron status include low-dose supplementation, fortification, and promotion of heme iron sources or enhancers of iron absorption (91, 92).
The recommended method to assess population zinc status is the prevalence of plasma zinc concentration less than the age-, sex- and time of day–specified cutoffs (93). A prevalence >20% of the group studied below the specified cutoff should call for an intervention to improve zinc status (94†). Zinc interventions include supplements as an adjunctive therapy for selected infections, fortification, dietary diversification, biofortification, and behavior modification strategies, such as the promotion of breastfeeding (95†). Low zinc intakes occur in the absence of these programs and in populations with low rates of breastfeeding, even when breast-milk zinc concentrations are adequate. Adequate zinc concentrations were measured in Indian women, but low breast-milk consumption resulted in low zinc intakes by their infants (21†).
Iron stable isotope bioavailability tests
Studies with iron stable isotopes are focused on determining differences in bioavailability due to dietary factors by using test foods that have been fortified, biofortified, or contain a potential inhibitor [e.g., phytic acid (8†)] or enhancer [e.g., ascorbic acid (96)] of absorption. Isotopes used for labeling test meals in bioavailability studies include 57Fe (2.12%) and 58Fe (0.28%) (14). Typically, the tests and controls are performed within the same individual for optimal quality control, because iron status tightly regulates iron absorption. Thus, it is important to measure relative bioavailability of iron sources within the same individual. The basic study design for stable iron incorporation into erythrocytes (RBCs) for bioavailability studies is detailed in an IAEA manual [Figure 5 (97†)]. Applications of this isotopic technique in women include the evaluation of bioavailability of different iron fortificants from fish sauce in Thailand (98†) and the influence of ascorbic acid on iron absorption in Switzerland (99).
FIGURE 5.
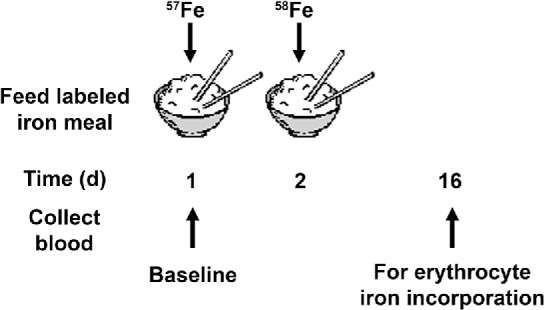
Design used for iron bioavailability studies to compare the quantities of iron absorbed and used between 2 test meals (based on references 97†, 98†, and 99). Two blood samples are required to determine the incorporation of the isotopes into the erythrocytes. After sample preparation, the iron is analyzed with an appropriate mass spectrometer and ratios of the stable isotopes are compared before and after dosing to determine the amount of iron incorporated into the RBCs. † following a citation number indicates a work that the International Atomic Energy Agency has supported on stable isotopes.
Foods to be evaluated for iron bioavailability can either be extrinsically labeled by adding the isotope to the test meal or intrinsically labeled by growing the food in an isotopically enriched environment. As with intrinsically labeled provitamin A carotenoid studies, the advantage of this approach is that the isotope is located in the same components as the predominant naturally occurring 56Fe. Iron isotope enrichment is determined by 2 MS methods: inductively coupled plasma–MS (ICP-MS) and the more sensitive, but costlier, thermal ionization MS (TIMS). ICP-MS of iron isotopes, however, suffers from interferences from other molecules, such as nickel and argon compounds. Therefore, these interferences must be accounted for by standardization with TIMS (100). Thus, similar to vitamin A MS, familiarity with instrumentation and methodology is important.
Zinc stable isotope tests
Unlike iron and vitamin A, zinc does not have a well-defined marker of body stores. Most studies interested in evaluating zinc status measure plasma zinc, which responds quickly to dietary intake or supplement usage (101). Zinc has many naturally occurring stable isotope forms including 64Zn (most abundant), 66Zn, 67Zn, 68Zn, and 70Zn, with the less abundant forms (i.e., 67Zn, 68Zn, and 70Zn) preferred as tracers because they result in a lower background signal. The same techniques used to measure iron are used for zinc (i.e., ICP-MS and TIMS), along with the same limitations due to interfering compounds (102).
Similar to iron, zinc stable isotopes can be used to evaluate absorption efficiency. However, the regulation of zinc absorption by zinc status is much less strong, but still occurs (103), and absorption is affected by the dose size administered or the amount consumed. Therefore, the difference in total zinc absorbed from 2 different dietary sources is most relevant to determining the superiority of one source or another, whereas the fractional absorbed zinc is most relevant when comparing a standard dose of 2 fortificants or supplements. To determine bioavailability, a subject consumes a test meal (with the same considerations as iron) containing one zinc stable isotope while a second, different zinc stable isotope is injected intravenously and assumed to be equivalent to 100% absorption. The ratio of the 2 isotopes in urine (or plasma can be used) is examined after an appropriate mixing time (Figure 6). After correcting for dose size and enrichment, fractional absorbed zinc and total zinc absorption from the test meal can be determined (22). For example, a zinc-absorption study provided women with typical wheat and zinc-biofortified wheat tortillas containing different zinc isotopes (67Zn and 70Zn), and compared the enrichment in urine with an intravenous dose of 68Zn [Figure 6 (104†)]. More zinc was absorbed from the biofortified wheat tortillas (104†).
FIGURE 6.
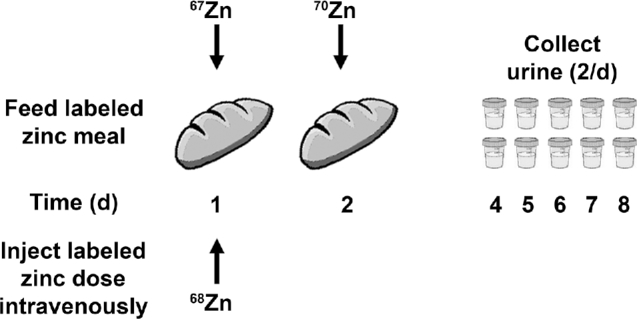
Design for a zinc-bioavailability study to determine the difference in absorption of zinc from typical wheat and a zinc-enhanced variety [based on a study evaluating tortillas (104†)]. Zinc isotopes are administered orally with the test food on days 1 and 2 and intravenously on the first day. Urine samples are collected on days 4 through 8 (2/d) and analyzed with an appropriate mass spectrometer. † following a citation number indicates a work that the International Atomic Energy Agency has supported on stable isotopes.
Although much of zinc is not accessible to measurement in the body due to its incorporation into proteins and function in cellular processes, ∼20% of body zinc exchanges rapidly with plasma and is called the exchangeable zinc pool or EZP, which has been suggested as a biomarker of zinc status (22). One study determined the change in EZP after supplementation, where it was responsive (105†). Further research on the topic is warranted because EZP could prove to be a more robust estimate of zinc stores than plasma concentrations alone. Stable isotope compartmental modeling can also be used to examine the kinetics of the zinc pool (22), similar to the type of modeling described for vitamin A research earlier in this review.
Fortification of staple foods with iron and zinc
In the processing of grains, minerals are often discarded with the hull and the endosperm. Therefore, iron is often one of the components added back during the fortification process. However, this may not be feasible in some countries where the crops are locally grown and consumed and not processed at centralized mills. Countries that want to improve mineral nutrition need to choose a widely consumed food that is centrally processed and inexpensive for target groups. In Thailand, this vehicle was identified as fish sauce. To evaluate bioavailability, iron absorption from the intrinsically labeled compounds was determined via erythrocyte incorporation of 57Fe and 58Fe. Different fortificants were explored, and absorption from the fish sauce was significantly lower with the use of ferrous lactate and ferric ammonium citrate than from ferrous sulfate (98†). The same method was used to compare absorption of the iron fortificants ferric ammonium citrate or a 2:1 mixture of ferrous sulfate (FeSO4) and ferric sodium EDTA (FeNaEDTA) in a rice dessert in Thai children aged 8–24 mo (106†). The study showed that, although either approach was sufficient to provide adequate iron, absorption was significantly higher in the FeSO4+FeNaEDTA group. Similarly, FeNaEDTA alone showed ∼40% better absorption than ferrous fumarate in Haitian women and children, and a mixture of the 2 iron sources was not better than ferrous fumarate alone (107†). In both the Thai and Haitian studies, it was shown that, although not as bioavailable as FeNaEDTA, cheaper iron sources, such as ferric ammonium citrate or ferrous fumarate, were effective at improving iron status.
Another fortification approach was undertaken in Brazil with the use of drinking water as a vehicle after a study identified a significant prevalence of anemia assessed by hemoglobin concentration (29.3%), and iron deficiency assessed by mean corpuscular volume, transferrin saturation, and RBC distribution width (75%) among preschool children who attended full-time daycare centers (108†). The intervention showed that by fortifying the childcare center's drinking water with both iron and ascorbic acid, functional iron improved [defined as mean corpuscular volume (109)] and iron deposits increased [defined as ferritin concentrations (109)] compared with an unfortified control water in Brazilian children (110†).
An example of zinc fortification is point-of-use fortification by including zinc in micronutrient powders that are added to traditional foods shortly before consumption. In a trial in Pakistani infants, total absorbed zinc from a zinc-containing micronutrient powder sprinkled on rice and lentils increased when compared with a control powder (1.2 ± 0.5 and 0.1 ± 0.1 mg, respectively) and the EZP was enhanced (4.5 ± 1.0 and 3.7 ± 0.6 mg/kg, respectively) (105†).
Iron and zinc in staple crops
Many studies have been conducted with in vitro methodology evaluating the bioaccessibility of minerals from staple crops. However, due to whole-body regulatory systems involved in mineral metabolism, the consensus was that human studies will ultimately be needed to assess the impact of biofortified crops on zinc and iron nutrition (111†).
Iron-biofortification efforts
Rice and beans are among several crops that have been biofortified with iron and tested in humans. Considering the global prevalence of iron deficiency anemia and the billions of people who consume rice and other staples, iron-biofortified crops could make an impact. For example, due to inadequate intake of dietary iron and poor bioavailability, prevalences of anemia and iron deficiency in India were 39% and 62%, respectively (112†). Biofortified rice could improve iron intakes among consumers who consume rice as a staple, such as in India. The first study, to our knowledge, to show the potential of iron-biofortified rice, which contained 3.21 mg Fe/kg, to improve iron body stores in humans was orchestrated in Filipino women, where it was compared with a local rice variety that only contained 0.57 mg/kg (113). The study used hemoglobin and serum ferritin, which are traditional biomarkers of iron status. After controlling for baseline iron stores in these women, calculated body iron was higher in the biofortified rice group than in the control group (113).
High-iron beans were studied in Rwandese women by determining the differences in bioavailability on the basis of phytic acid and polyphenol content (114†). When 57Fe and 58Fe incorporations into RBCs were determined after the consumption of mixed meals, high-iron beans did not seem to overcome the inhibitory effects of phytic acid and polyphenols on bioavailability. Therefore, the recommendation was to select beans that had high iron, low phytate, and low polyphenols for future studies (114†).
Zinc-biofortification efforts
Most research studies on zinc-biofortified crops have used zinc isotopic bioavailability techniques. In the zinc-biofortification study described earlier (Figure 6), it was determined that meals that included wheat tortillas made with zinc-biofortified wheat resulted in 0.5 mg/d higher absorption than control wheat (104†). The bioavailability of zinc from rice-based diets that provided 4.8 mg Zn/d did not have any more absorbed zinc than a conventional rice-based diet at 3.8 mg Zn/d, but the quantity of zinc absorbed increased from fortified rice containing 6.03 mg Zn/d (115†). Thus, either zinc needs to be increased further in biofortified varieties to match fortified levels or phytate levels need to be decreased (115†). Since then, a biofortified maize variety with 34 μg Zn/g grain was able to provide amounts of zinc similar to a fortified maize with 60 μg Zn/g grain (1.1 ± 0.5 and 1.2 ± 0.4 mg Zn/d, respectively), significantly more than the control maize with 21 μg Zn/g grain (0.6 ± 0.2 mg Zn/d) in young rural Zambian children (116†). The biofortified variety was able to meet the zinc requirements for this vulnerable population (116†).
As biofortification efforts move forward, developing cultivars that have multiple micronutrients should be pursued (64). Pearl millet that was biofortified with iron and zinc was shown to provide significantly increased quantities of these nutrients compared with a test meal (117†). Quality-protein maize often has increased levels of zinc (118), and it is known that the synergistic effects between vitamin A and zinc lead to enhanced overall nutrient metabolism (7) and may reduce malaria morbidity when supplemented together (119†). Therefore, maize bred with quality protein, enhanced zinc, and increased provitamin A carotenoids may supply better nutrition than any single-nutrient approach for populations that have high staple-crop intakes.
Simulations have shown that biofortification of rice with zinc could readily affect zinc intakes of women and children in Bangladesh (120) and adults following traditional eating patterns in China (121). Manipulating both iron and zinc in rice may be feasible (122). Furthermore, depressed nutrient intakes occur in children who have repeated infections, such as endemic malaria, and therefore reevaluating micronutrient target levels may be important in regions that adopt biofortified staple crops (123).
Conclusions
Worldwide efforts to improve micronutrient status include fortification and biofortification. These food-based approaches have the potential to reach nearly all sectors of the population, including, but not limited to, low-income groups who are vulnerable to micronutrient deficiency. In this regard, population nutrient assessment needs to move beyond rural populations and include urban and affluent groups. This is particularly true for preformed vitamin A–fortification programs that have populationwide coverage where excessive intakes could lead to hypervitaminosis A. Mineral absorption is more highly regulated than preformed vitamin A, and therefore the risk of mineral toxicity is much less from high intakes of mineral-fortified or biofortified foods.
In summary, the RID methods discussed here are powerful tools to examine provitamin A carotenoid bioefficacy and vitamin A status from deficiency to toxicity in a diverse range of populations. Iron isotopes have been valuable in recommending appropriate fortificants for foods and defining the interaction with inhibitors and enhancers of absorption. Zinc isotopes are also valuable for absorption studies, and developments that use EZP may yield new insights in evaluating zinc status. Further refinement of these techniques to increase their accuracy, accessibility, and cost-effectiveness will help guide fortification efforts in the future. The use of these techniques to evaluate food-based interventions will inform nutrition scientists, public health professionals, and policy makers, especially with regard to biofortification.
Acknowledgments
The authors’ responsibilities were as follows—JS and SAT: collaborated to produce the manuscript; CL: provided guidance on studies funded by the IAEA; NM: worked with SAT to develop the concept and scope of the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by Global Health Funds at the University of Wisconsin–Madison and the International Atomic Energy Agency.
Author disclosures: JS, CL, NM, and SAT, no conflicts of interest.
Abbreviations used:
- EZP
exchangeable zinc pool
- FeNaEDTA
ferric sodium EDTA
- IAEA
International Atomic Energy Agency
- ICP-MS
inductively coupled plasma–MS
- IOM
Institute of Medicine
- MRDR
modified relative dose response
- RID
retinol isotope dilution
- TIMS
thermal ionization MS
References
- 1. WHO. Essential nutrition actions: improving maternal, newborn, infant and young child health and nutrition. Geneva (Switzerland): WHO; 2013. [PubMed] [Google Scholar]
- 2. Benson TD. Africa's food and nutrition security situation: where are we and how did we get here? Washington (DC): International Food Policy Research Institute; 2004. [Google Scholar]
- 3. WHO The role of food fortification in the control of micronutrient malnutrition. In: Allen LH, De Benoist B, Dary O, Hurrell R, editors. Guidelines on food fortification with micronutrients. Geneva (Switzerland): WHO; 2006. p. 1–38. [Google Scholar]
- 4. Tanumihardjo SA, Bouis H, Hotz C, Meenakshi JV, McClafferty B. Biofortification of staple crops: an emerging strategy to combat hidden hunger. Compr Rev Food Sci Food Saf 2008;7:329–34. [Google Scholar]
- 5. Tanumihardjo SA. Food-based approaches for ensuring adequate vitamin A nutrition. Compr Rev Food Sci Food Saf 2008;7:373–81. [Google Scholar]
- 6. Neidecker-Gonzales O, Nestel P, Bouis H. Estimating the global costs of vitamin A capsule supplementation: a review of the literature. Food Nutr Bull 2007;28:307–16. [DOI] [PubMed] [Google Scholar]
- 7. Tanumihardjo SA, Palacios N, Pixley KV. Provitamin A carotenoid bioavailability: what really matters? Int J Vitam Nutr Res 2010;80:336–50. [DOI] [PubMed] [Google Scholar]
- 8. Davidsson L, Tanumihardjo SA. Bioavailability. In: Caballero B, Allen LH, Prentice A, editors. Encyclopedia of human nutrition. 3rd ed Waltham (MA): Academic Press; 2013. p. 149–55. [Google Scholar]
- 9. Tanumihardjo SA. Usefulness of vitamin A isotope methods for status assessment: from deficiency through excess. Int J Vitam Nutr Res 2014;84(Suppl 1):16–24. [DOI] [PubMed] [Google Scholar]
- 10. Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of Nutrition for Development (BOND)—vitamin A review. J Nutr 2016;146(Suppl):1816S–48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Furr HC, Green MH, Haskell M, Mokhtar N, Nestel P, Newton S, Ribaya-Mercado JD, Tang G, Tanumihardjo S, Wasantwisut E. Stable isotope dilution techniques for assessing vitamin A status and bioefficacy of provitamin A carotenoids in humans. Public Health Nutr 2005;8:596–607. [DOI] [PubMed] [Google Scholar]
- 12. Tang G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am J Clin Nutr 2010;91(Suppl):1468S–73S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davidsson L, Tanumihardjo SA. New frontiers in science and technology: nuclear techniques in nutrition. Am J Clin Nutr 2011;94(Suppl):691S–5S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meija J, Coplen TB, Berglund M, Brand WA, De Bièvre P, Gröning M, Holden NE, Irrgeher J, Loss RD, Walczyk T et al. Isotopic compositions of the elements 2013 (IUPAC technical report). Pure Appl Chem 2016;88:293–306. [Google Scholar]
- 15. Aklamati EK, Mulenga M, Dueker SR, Buchholz BA, Peerson JM, Kafwembe E, Brown KH, Haskell MJ. Accelerator mass spectrometry can be used to assess vitamin A metabolism quantitatively in boys in a community setting. J Nutr 2010;140:1588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho CC, de Moura FF, Kim S-H, Burri BJ, Clifford AJ. A minute dose of 14C-β-carotene is absorbed and converted to retinoids in humans. J Nutr 2009;139:1480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dueker SR, Lin Y, Buchholz BA, Schneider PD, Lamé MW, Segall HJ, Vogel JS, Clifford AJ. Long-term kinetic study of β-carotene, using accelerator mass spectrometry in an adult volunteer. J Lipid Res 2000;41:1790–800. [PubMed] [Google Scholar]
- 18. Bausch J, Rietz P. Method for the assessment of vitamin A liver stores. Acta Vitaminol Enzymol 1977;31:99–112. [PubMed] [Google Scholar]
- 19. Medoua GN, Sajo Nana EC, Ndzana ACA, Makamto CS, Etame LS, Rikong HA, Oyono JLE. Breastfeeding practices of Cameroonian mothers determined by dietary recall since birth and the dose-to-the-mother deuterium-oxide turnover technique. Matern Child Nutr 2012;8:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopez-Teros V, Limon-Miro AT, Astiazaran-Garcia H, Tanumihardjo SA, Tortoledo-Ortiz O, Valencia ME “Dose-to-mother” deuterium oxide dilution technique: an accurate strategy to measure vitamin A intake in breastfed infants. Nutrients 2017;9:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Samuel TM, Thomas T, Thankachan P, Bhat S, Virtanen SM, Kurpad AV. Breast milk zinc transfer and early post-natal growth among urban South Indian term infants using measures of breast milk volume and breast milk zinc concentrations. Matern Child Nutr 2014;10:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tran CD, Gopalsamy GL, Mortimer EK, Young GP. The potential for zinc stable isotope techniques and modelling to determine optimal zinc supplementation. Nutrients 2015;7:4271–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muto Y, Smith JE, Milch PO, Goodman DS. Regulation of retinol-binding protein metabolism by vitamin A status in the rat. J Biol Chem 1972;247:2542–50. [PubMed] [Google Scholar]
- 24. Tchum SK, Tanumihardjo SA, Newton S, de Benoist B, Owusu-Agyei S, Arthur FK, Tetteh A. Evaluation of vitamin A supplementation regimens in Ghanaian postpartum mothers with the use of the modified-relative-dose-response test. Am J Clin Nutr 2006;84:1344–9. [DOI] [PubMed] [Google Scholar]
- 25. Tchum SK, Newton S, Tanumihardjo SA, Benoist B, Owusu-Agyei S, Arthur FK. Evaluation of a green leafy vegetable intervention in Ghanaian postpartum mothers. Afr J Food Agric Nutr Dev 2009;9:1294–308. [Google Scholar]
- 26. Agne-Djigo A, Idohou-Dossou N, Kwadjode KM, Tanumihardjo SA, Wade S. High prevalence of vitamin A deficiency is detected by the modified relative dose-response test in six-month-old Senegalese breast-fed infants. J Nutr 2012;142:1991–6. [DOI] [PubMed] [Google Scholar]
- 27. Newton S, Owusu-Agyei S, Asante KP, Amoaful E, Mahama E, Tchum SK, Ali M, Adjei K, Davis CR, Tanumihardjo SA. Vitamin A status and body pool size of infants before and after consuming fortified home-based complementary foods. Arch Public Health 2016;74:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tanumihardjo SA. Vitamin A fortification efforts require accurate monitoring of population vitamin A status to prevent excessive intakes. Procedia Chem 2015;14:398–407. [Google Scholar]
- 29. Gannon BM, Tanumihardjo SA. Comparisons among equations used for retinol isotope dilution in the assessment of total body stores and total liver reserves. J Nutr 2015;145:847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanumihardjo SA. Vitamin A status assessment in rats with 13C4-retinyl acetate and gas chromatography/combustion/isotope ratio mass spectrometry. J Nutr 2000;130:2844–9. [DOI] [PubMed] [Google Scholar]
- 31. Pinkaew S, Wegmuller R, Wasantwisut E, Winichagoon P, Hurrell RF, Tanumihardjo SA. Triple-fortified rice containing vitamin A reduced marginal vitamin A deficiency and increased vitamin A liver stores in school-aged Thai children. J Nutr 2014;144:519–24. [DOI] [PubMed] [Google Scholar]
- 32. Thurnham D, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. In: Report: priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15–17 September 2010. Geneva (Switzerland): WHO; 2012. p. 63–80. [Google Scholar]
- 33. Suri DJ, Tanumihardjo JP, Gannon BM, Pinkaew S, Kaliwile C, Chileshe J, Tanumihardjo SA. Serum retinol concentrations demonstrate high specificity after correcting for inflammation but questionable sensitivity compared with liver stores calculated from isotope dilution in determining vitamin A deficiency in Thai and Zambian children. Am J Clin Nutr 2015;102:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sivakumar B, Reddy V. Absorption of labelled vitamin A in children during infection. Br J Nutr 1972;27:299–304. [DOI] [PubMed] [Google Scholar]
- 35. Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr 2001;21:167–92. [DOI] [PubMed] [Google Scholar]
- 36. Gieng SH, Green MH, Green JB, Rosales FJ. Model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. J Lipid Res 2007;48:904–13. [DOI] [PubMed] [Google Scholar]
- 37. Lopez-Teros V, Chileshe J, Idohou-Dossou N, Fajarwati T, Medoua Nama G, Newton S, Vinod Kumar M, Wang Z, Wasantwisut E, Hunt JR. International experiences in assessing vitamin A status and applying the vitamin A-labeled isotope dilution method. Int J Vitam Nutr Res 2014;84(Suppl 1):40–51. [DOI] [PubMed] [Google Scholar]
- 38. Preston T. Existing and emerging technologies for measuring stable isotope labelled retinol in biological samples: isotope dilution analysis of body retinol stores. Int J Vitam Nutr Res 2014;84(Suppl 1):30–9. [DOI] [PubMed] [Google Scholar]
- 39. Tang G, Hu Y, Yin S-a, Wang Y, Dallal GE, Grusak MA, Russell RM. β-Carotene in Golden Rice is as good as β-carotene in oil at providing vitamin A to children. Am J Clin Nutr 2012;96:658–64. Retraction in: Tang G, Hu Y, Yin S-a, Wang Y, Dallal GE, Grusak MA, Russell RM. Am J Clin Nutr 2015;102:715. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. Oxley A, Berry P, Taylor GA, Cowell J, Hall MJ, Hesketh J, Lietz G, Boddy AV. An LC/MS/MS method for stable isotope dilution studies of β-carotene bioavailability, bioconversion, and vitamin A status in humans. J Lipid Res 2013;55:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jamil KM, Brown KH, Jamil M, Peerson JM, Keenan AH, Newman JW, Haskell MJ. Daily consumption of orange-fleshed sweet potato for 60 days increased plasma β-carotene concentration but did not increase total body vitamin A pool size in Bangladeshi women. J Nutr 2012;142:1896–902. [DOI] [PubMed] [Google Scholar]
- 42. Haskell MJ, Ribaya-Mercado JD. Handbook on vitamin A tracer dilution methods to assess status and evaluate intervention programs. Washington (DC): HarvestPlus; 2005. [Google Scholar]
- 43. Tang G, Gu X, Hu S, Xu Q, Qin J, Dolnikowski GG, Fjeld CR, Gao X, Russell RM, Yin S. Green and yellow vegetables can maintain body stores of vitamin A in Chinese children. Am J Clin Nutr 1999;70:1069–76. [DOI] [PubMed] [Google Scholar]
- 44. Ribaya-Mercado JD, Solon FS, Solon MA, Cabal-Barza MA, Perfecto CS, Tang G, Solon JAA, Fjeld CR, Russell RM. Bioconversion of plant carotenoids to vitamin A in Filipino school-aged children varies inversely with vitamin A status. Am J Clin Nutr 2000;72:455–65. [DOI] [PubMed] [Google Scholar]
- 45. Sheftel J, Gannon BM, Davis CR, Tanumihardjo SA. Provitamin A-biofortified maize consumption increases serum xanthophylls and 13C-natural abundance of retinol in Zambian children. Exp Biol Med 2017;242:1508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cifelli CJ, Green JB, Green MH. Use of model-based compartmental analysis to study vitamin A kinetics and metabolism. In: Litwack G, editor. Vitamins and hormones. Amsterdam: Elsevier; 2007. p. 161–95. [DOI] [PubMed] [Google Scholar]
- 47. Green MH, Ford JL, Green JB, Berry P, Boddy AV, Oxley A, Lietz G. A retinol isotope dilution equation predicts both group and individual total body vitamin A stores in adults based on data from an early postdosing blood sample. J Nutr 2016;146:2137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Green MH. Evaluation of the “Olson equation,” an isotope dilution method for estimating vitamin A stores. Int J Vitam Nutr Res 2014;84(Suppl 1):9–15. [DOI] [PubMed] [Google Scholar]
- 49. Haskell MJ, Lembcke JL, Salazar M, Green MH, Peerson JM, Brown KH. Population-based plasma kinetics of an oral dose of [2H4] retinyl acetate among preschool-aged, Peruvian children. Am J Clin Nutr 2003;77:681–6. [DOI] [PubMed] [Google Scholar]
- 50. Gannon BM, Valentine AR, Davis CR, Howe JA, Tanumihardjo SA. Duration of retinol isotope dilution studies with compartmental modeling affects model co mplexity, kinetic parameters, and calculated vitamin A stores in US women. J Nutr. 2018;148: (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lopez-Teros V, Ford JL, Green MH, Tang G, Grusak MA, Quihui-Cota L, Muzhingi T, Paz-Cassini M, Astiazaran-Garcia H. Use of a “super-child” approach to assess the vitamin A equivalence of Moringa oleifera leaves, develop a compartmental model for vitamin A kinetics, and estimate vitamin A total body stores in young Mexican children. J Nutr 2017;147:2356–63. [DOI] [PubMed] [Google Scholar]
- 52. Tanumihardjo SA, Kurpad AV, Hunt JR. Research recommendations for applying vitamin A-labelled isotope dilution techniques to improve human vitamin A nutrition. Int J Vitam Nutr Res 2014;84(Suppl 1):52–9. [DOI] [PubMed] [Google Scholar]
- 53. Lietz G, Furr HC, Gannon BM, Green MH, Haskell M, Lopez-Teros V, Novotny JA, Palmer AC, Russell RM, Tanumihardjo SA et al. Current capabilities and limitations of stable isotope techniques and applied mathematical equations in determining whole-body vitamin A status. Food Nutr Bull 2016;37(Suppl 2):87–103. [DOI] [PubMed] [Google Scholar]
- 54. Ribaya-Mercado JY, Solomons NW, Medrano Y, Bulux J, Dolnikowski GG, Russell RM, Wallace CB. Use of the deuterated-retinol-dilution technique to monitor the vitamin A status of Nicaraguan schoolchildren 1 y after initiation of the Nicaraguan national program of sugar fortification with vitamin A. Am J Clin Nutr 2004;80:1291–8. [DOI] [PubMed] [Google Scholar]
- 55. Lopez-Teros V, Quihui-Cota L, Mendez-Estrada RO, Grijalva-Haro MI, Esparza-Romero J, Valencia ME, Green MH, Tang G, Pacheco-Moreno BI, Tortoledo-Ortiz O et al. Vitamin A-fortified milk increases total body vitamin A stores in Mexican preschoolers. J Nutr 2013;143:221–6. [DOI] [PubMed] [Google Scholar]
- 56. Gannon BM, Kaliwile C, Arscott SA, Schmaelzle S, Chileshe J, Kalungwana N, Mosonda M, Pixley K, Masi C, Tanumihardjo SA. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: a community-based, randomized placebo-controlled trial. Am J Clin Nutr 2014;100:1541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tanumihardjo SA. Vitamin A and bone health: the balancing act. J Clin Densitom 2013;16:414–9. [DOI] [PubMed] [Google Scholar]
- 58. Institute of Medicine Food and Nutrition Board Vitamin A. In: Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2001. p. 82–161. [PubMed] [Google Scholar]
- 59. Hotz C, McClafferty B. From harvest to health: challenges for developing biofortified staple foods and determining their impact on micronutrient status. Food Nutr Bull 2007;28:271–9. [DOI] [PubMed] [Google Scholar]
- 60. Borel P, Desmarchelier C. Genetic variations associated with vitamin A status and vitamin A bioavailability. Nutrients 2017;9:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Pee S, West CE, Permaesih D, Martuti S, Muhilal Hautvast JG. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and beta-carotene in schoolchildren in Indonesia. Am J Clin Nutr 1998;68:1058–67. [DOI] [PubMed] [Google Scholar]
- 62. Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, von Lintig J. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem 2013;288:9017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tang G. Vitamin A value of plant food provitamin A—evaluated by the stable isotope technologies. Int J Vitam Nutr Res 2014;84(Suppl 1):25–9. [DOI] [PubMed] [Google Scholar]
- 64. Nuss ET, Tanumihardjo SA. Maize: a paramount staple crop in the context of global nutrition. Compr Rev Food Sci Food Saf 2010;9:417–36. [DOI] [PubMed] [Google Scholar]
- 65. King JC. Evaluating the impact of plant biofortification on human nutrition. J Nutr 2002;132(Suppl):511S–3S. [DOI] [PubMed] [Google Scholar]
- 66. van Jaarsveld P, Faber M. Orange sweetpotato as a staple or complementary food. In: Tanumihardjo SA, editor. Carotenoids and human health. Totowa (NJ): Humana Press; 2013. p. 303–15. [Google Scholar]
- 67. Haskell MJ, Jamil KM, Hassan F, Peerson JM, Hossain MI, Fuchs GJ, Brown KH. Daily consumption of Indian spinach (Basella alba) or sweet potatoes has a positive effect on total-body vitamin A stores in Bangladeshi men. Am J Clin Nutr 2004;80:705–14. [DOI] [PubMed] [Google Scholar]
- 68. Low JW, Arimond M, Osman N, Cunguara B, Zano F, Tschirley D. A food-based approach introducing orange-fleshed sweet potatoes increased vitamin A intake and serum retinol concentrations in young children in rural Mozambique. J Nutr 2007;137:1320–7. [DOI] [PubMed] [Google Scholar]
- 69. WHO Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Geneva (Switzerland): WHO; 2011. [Google Scholar]
- 70. Hotz C, Loechl C, Lubowa A, Tumwine JK, Ndeezi G, Nandutu Masawi A, Baingana R, Carriquiry A, de Brauw A, Meenakshi JV et al. Introduction of β-carotene-rich orange sweet potato in rural Uganda resulted in increased vitamin A intakes among children and women and improved vitamin A status among children. J Nutr 2012;142:1871–80. [DOI] [PubMed] [Google Scholar]
- 71. Howe JA, Tanumihardjo SA. Carotenoid-biofortified maize maintains adequate vitamin A status in Mongolian gerbils. J Nutr 2006;136:2562–7. [DOI] [PubMed] [Google Scholar]
- 72. Davis CR, Howe JA, Rocheford TR, Tanumihardjo SA. The xanthophyll composition of biofortified maize (Zea mays sp.) does not influence the bioefficacy of provitamin A carotenoids in Mongolian gerbils (Meriones unguiculatus). J Agr Food Chem 2008;56:6745–50. [DOI] [PubMed] [Google Scholar]
- 73. Davis C, Jing H, Howe JA, Rocheford T, Tanumihardjo SA. β-Cryptoxanthin from supplements or carotenoid-enhanced maize maintains liver vitamin A in Mongolian gerbils (Meriones unguiculatus) better than or equal to β-carotene supplements. Br J Nutr 2008;100:786–93. [DOI] [PubMed] [Google Scholar]
- 74. Pixley K, Palacios-Rojas N, Babu R, Mutale R, Surles R, Simpungwe E. Biofortification of maize with provitamin A carotenoids. In: Tanumihardjo SA, editor. Carotenoids and human health. Totowa (NJ): Humana Press; 2013. p. 271–92. [Google Scholar]
- 75. Li S, Nugroho A, Rocheford T, White WS. Vitamin A equivalence of the β-carotene in β-carotene-biofortified maize porridge consumed by women. Am J Clin Nutr 2010;92:1105–12. [DOI] [PubMed] [Google Scholar]
- 76. Muzhingi T, Gadaga TH, Siwela AH, Grusak MA, Russell RM, Tang G. Yellow maize with high β-carotene is an effective source of vitamin A in healthy Zimbabwean men. Am J Clin Nutr 2011;94:510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nuss ET, Arscott SA, Bresnahan K, Pixley KV, Rocheford T, Hotz C, Siamusantu W, Chileshe J, Tanumihardjo SA. Comparative intake of white- versus orange-colored maize by Zambian children in the context of promotion of biofortified maize. Food Nutr Bull 2012;33:63–71. [DOI] [PubMed] [Google Scholar]
- 78. Bresnahan KA, Chileshe J, Arscott S, Nuss E, Surles R, Masi C, Kafwembe E, Tanumihardjo SA. The acute phase response affected traditional measures of micronutrient status in rural Zambian children during a randomized, controlled feeding trial. J Nutr 2014;144:972–8. [DOI] [PubMed] [Google Scholar]
- 79. Howe JA, Valentine AR, Hull AK, Tanumihardjo SA. 13C Natural abundance in serum retinol acts as a biomarker for increases in dietary provitamin A. Exp Biol Med 2009;234:140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gannon BM, Pungarcher I, Mourao L, Davis CR, Simon P, Pixley KV, Tanumihardjo SA. 13C Natural abundance of serum retinol is a novel biomarker for evaluating provitamin A carotenoid-biofortified maize consumption in male Mongolian gerbils. J Nutr 2016;146:1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Heying EK, Grahn M, Pixley KV, Rocheford T, Tanumihardjo SA. High-provitamin A carotenoid (orange) maize increases hepatic vitamin A reserves of offspring in a vitamin A-depleted sow-piglet model during lactation. J Nutr 2013;143:1141–6. [DOI] [PubMed] [Google Scholar]
- 82. Meenakshi JV, Banerji A, Manyong V, Tomlins K, Mittal N, Hamukwala P. Using a discrete choice experiment to elicit the demand for a nutritious food: willingness-to-pay for orange maize in rural Zambia. J Health Econ 2012;31:62–71. [DOI] [PubMed] [Google Scholar]
- 83. Thakkar SK, Maziya-Dixon B, Dixon AG, Failla ML. β-carotene micellarization during in vitro digestion and uptake by Caco-2 cells is directly proportional to β-carotene content in different genotypes of cassava. J Nutr 2007;137:2229–33. [DOI] [PubMed] [Google Scholar]
- 84. Howe JA, Maziya-Dixon B, Tanumihardjo SA. Cassava with enhanced β-carotene maintains adequate vitamin A status in Mongolian gerbils (Meriones unguiculatus) despite substantial cis-isomer content. Br J Nutr 2009;102:342–9. [DOI] [PubMed] [Google Scholar]
- 85. La Frano MR, Woodhouse LR, Burnett DJ, Burri BJ. Biofortified cassava increases β-carotene and vitamin A concentrations in the TAG-rich plasma layer of American women. Br J Nutr 2013;110:310–20. [DOI] [PubMed] [Google Scholar]
- 86. Liu W. Vitamin A equivalence of the β-carotene in biofortified cassava in women. Ames (IA): Iowa State University, 2009. [Google Scholar]
- 87. Ye X, Al-Babili S, Klöti A, Zhang J, Lucca P, Beyer P, Potrykus I. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 2000;287:303–5. [DOI] [PubMed] [Google Scholar]
- 88. Paine JA, Shipton CA, Chaggar S, Howells RM, Kennedy MJ, Vernon G, Wright SY, Hinchliffe E, Adams JL, Silverstone AL et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotechnol 2005;23:482–7. [DOI] [PubMed] [Google Scholar]
- 89. Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA. Golden Rice is an effective source of vitamin A. Am J Clin Nutr 2009;89:1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Suchdev PS, Namaste SM, Aaron GJ, Raiten DJ, Brown KH, Flores-Ayala R. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Adv Nutr 2016;7:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stoltzfus RJ, Dreyfuss ML. Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. Washington (DC): ILSI Press; 1998. [Google Scholar]
- 92. Lynch SR, Stoltzfus RJ. Iron and ascorbic acid: proposed fortification levels and recommended iron compounds. J Nutr 2003;133(Suppl):2978S–84S. [DOI] [PubMed] [Google Scholar]
- 93. King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, Raiten DJ. Biomarkers of Nutrition for Development (BOND)—zinc review. J Nutr 2016;146(Suppl):858S–85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. de Benoist B, Darnton-Hill I, Davidsson L, Fontaine O, Hotz C. Conclusions of the Joint WHO/UNICEF/IAEA/IZiNCG Interagency Meeting on Zinc Status Indicators. Food Nutr Bull 2007;28:480S–4S. [DOI] [PubMed] [Google Scholar]
- 95. Hess SY, Lonnerdal B, Hotz C, Rivera JA, Brown KH. Recent advances in knowledge of zinc nutrition and human health. Food Nutr Bull 2009;30(Suppl 1):5–11. [DOI] [PubMed] [Google Scholar]
- 96. Diaz M, Rosado JL, Allen LH, Abrams S, Garcia OP. The efficacy of a local ascorbic acid-rich food in improving iron absorption from Mexican diets: a field study using stable isotopes. Am J Clin Nutr 2003;78:436–40. [DOI] [PubMed] [Google Scholar]
- 97. Assessment of iron bioavailability in humans using stable iron isotope techniques. Vienna (Austria): International Atomic Energy Agency; 2012. [Google Scholar]
- 98. Walczyk T, Tuntipopipat S, Zeder C, Sirichakwal P, Wasantwisut E, Hurrell RF. Iron absorption by human subjects from different iron fortification compounds added to Thai fish sauce. Eur J Clin Nutr 2005;59:668–74. [DOI] [PubMed] [Google Scholar]
- 99. Fidler MC, Davidsson L, Zeder C, Walczyk T, Marti I, Hurrell RF. Effect of ascorbic acid and particle size on iron absorption from ferric pyrophosphate in adult women. Int J Vitam Nutr Res 2004;74:294–300. [DOI] [PubMed] [Google Scholar]
- 100. Chen Z, Griffin IJ, Plumlee LM, Abrams SA. High resolution inductively coupled plasma mass spectrometry allows rapid assessment of iron absorption in infants and children. J Nutr 2005;135:1790–5. [DOI] [PubMed] [Google Scholar]
- 101. Wessells KR, Jorgensen JM, Hess SY, Woodhouse LR, Peerson JM, Brown KH. Plasma zinc concentration responds rapidly to the initiation and discontinuation of short-term zinc supplementation in healthy men. J Nutr 2010;140:2128–33. [DOI] [PubMed] [Google Scholar]
- 102. Ramanujam VMS, Yokoi K, Egger NG, Dayal HH, Alcock NW, Sandstead HH. Polyatomics in zinc isotope ratio analysis of plasma samples by inductively coupled plasma-mass spectrometry and applicability of nonextracted samples for zinc kinetics. Biol Trace Elem Res 1999;68:143–58. [DOI] [PubMed] [Google Scholar]
- 103. Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr 2000;130:1374S–7S. [DOI] [PubMed] [Google Scholar]
- 104. Rosado JL, Hambidge KM, Miller LV, Garcia OP, Westcott J, Gonzalez K, Conde J, Hotz C, Pfeiffer W, Ortiz-Monasterio I et al. The quantity of zinc absorbed from wheat in adult women is enhanced by biofortification. J Nutr 2009;139:1920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ariff S, Krebs NF, Soofi S, Westcott J, Bhatti Z, Tabassum F, Bhutta ZA. Absorbed zinc and exchangeable zinc pool size are greater in Pakistani infants receiving traditional complementary foods with zinc-fortified micronutrient powder. J Nutr 2014;144:20–6. [DOI] [PubMed] [Google Scholar]
- 106. Chavasit V, Porasuphatana S, Suthutvoravut U, Zeder C, Hurrell R. Iron bioavailability in 8-24-month-old Thai children from a micronutrient-fortified quick-cooking rice containing ferric ammonium citrate or a mixture of ferrous sulphate and ferric sodium ethylenediaminetetraacetic acid. Matern Child Nutr 2015;11(Suppl 4):179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Herter-Aeberli I, Eliancy K, Rathon Y, Loechl CU, Marhône Pierre J, Zimmermann MB. In Haitian women and preschool children, iron absorption from wheat flour-based meals fortified with sodium iron EDTA is higher than that from meals fortified with ferrous fumarate, and is not affected by Helicobacter pylori infection in children. Br J Nutr 2017;118:273–9. [DOI] [PubMed] [Google Scholar]
- 108. de Almeida CAN, Ramos APP, João CA, João CR, Ricco RG, Dutra-de-Oliveira JE. Jardinópolis without anemia, first stage: anthropometric and iron nutrition status evaluation (Portuguese). Rev Paul Pediatr 2007;25:254–7. [Google Scholar]
- 109. Hambidge M. Biomarkers of trace mineral intake and status. J Nutr 2003;133(Suppl 3):948–55. [DOI] [PubMed] [Google Scholar]
- 110. de Almeida CAN, De Mello ED, Ramos AP, João CA, João CR, Dutra-de-Oliveira JE. Assessment of drinking water fortification with iron plus ascorbic acid or ascorbic acid alone in daycare centers as a strategy to control iron-deficiency anemia and iron deficiency: a randomized blind clinical study. J Trop Pediatr 2014;60:40–6. [DOI] [PubMed] [Google Scholar]
- 111. Fairweather-Tait S, Lynch S, Hotz C, Hurrell R, Abrahamse L, Beebe S, Bering S, Bukhave K, Glahn R, Hambidge M et al. The usefulness of in vitro models to predict the bioavailability of iron and zinc: a consensus statement from the HarvestPlus expert consultation. Int J Vitam Nutr Res 2005;75:371–4. [DOI] [PubMed] [Google Scholar]
- 112. Thankachan P, Muthayya S, Walczyk T, Kurpad AV, Hurrell RF. An analysis of the etiology of anemia and iron deficiency in young women of low socioeconomic status in Bangalore, India. Food Nutr Bull 2007;28:328–36. [DOI] [PubMed] [Google Scholar]
- 113. Haas JD, Beard JL, Murray-Kolb LE, del Mundo AM, Felix A, Gregorio GB. Iron-biofortified rice improves the iron stores of nonanemic Filipino women. J Nutr 2005;135:2823–30. [DOI] [PubMed] [Google Scholar]
- 114. Petry N, Egli I, Gahutu JB, Tugirimana PL, Boy E, Hurrell R. Stable iron isotope studies in Rwandese women indicate that the common bean has limited potential as a vehicle for iron biofortification. J Nutr 2012;142:492–7. [DOI] [PubMed] [Google Scholar]
- 115. Islam MM, Woodhouse LR, Hossain MB, Ahmed T, Huda MN, Ahmed T, Peerson JM, Hotz C, Brown KH. Total zinc absorption from a diet containing either conventional rice or higher-zinc rice does not differ among Bangladeshi preschool children. J Nutr 2013;143:519–25. [DOI] [PubMed] [Google Scholar]
- 116. Chomba E, Westcott CM, Westcott JE, Mpabalwani EM, Krebs NF, Patinkin ZW, Palacios N, Hambidge KM. Zinc absorption from biofortified maize meets the requirements of young rural Zambian children. J Nutr 2015;145:514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kodkany BS, Bellad RM, Mahantshetti NS, Westcott JE, Krebs NF, Kemp JF, Hambidge KM. Biofortification of pearl millet with iron and zinc in a randomized controlled trial increases absorption of these minerals above physiologic requirements in young children. J Nutr 2013;143:1489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nuss ET, Tanumihardjo SA. Quality protein maize for Africa: closing the protein inadequacy gap in vulnerable populations. Adv Nutr 2011;2:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Owusu-Agyei S, Newton S, Mahama E, Febir LG, Ali M, Adjei K, Tchum K, Alhassan L, Moleah T, Tanumihardjo SA. Impact of vitamin A with zinc supplementation on malaria morbidity in Ghana. Nutr J 2013;12:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Arsenault JE, Yakes EA, Hossain MB, Islam MM, Ahmed T, Hotz C, Lewis B, Rahman AS, Jamil KM, Brown KH. The current high prevalence of dietary zinc inadequacy among children and women in rural Bangladesh could be substantially ameliorated by zinc biofortification of rice. J Nutr 2010;140:1683–90. [DOI] [PubMed] [Google Scholar]
- 121. Qin Y, Melse-Boonstra A, Yuan B, Pan X, Dai Y, Zhou M, Wegmueller R, Zhao J, Kok FJ, Shi Z. Zinc biofortification of rice in China: a simulation of zinc intake with different dietary patterns. Nutrients 2012;4:517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sperotto RA, Boff T, Duarte GL, Santos LS, Grusak MA, Fett JP. Identification of putative target genes to manipulate Fe and Zn concentrations in rice grains. J Plant Physiol 2010;167:1500–6. [DOI] [PubMed] [Google Scholar]
- 123. Bresnahan KA, Chileshe J, Tanumihardjo SA. Quantification of food and nutrient intakes in Zambian children with and without malaria under controlled feeding conditions. Exp Biol Med 2014;239:45–51. [DOI] [PubMed] [Google Scholar]


