Abstract
Aims
Cardiac magnetic resonance (CMR) is recommended as a second-line method to diagnose ventricular arrhythmia (VA) substrate. We assessed the diagnostic yield of CMR including high-resolution late gadolinium-enhanced (LGE) imaging.
Methods and results
Consecutive patients with sustained ventricular tachycardia (VT), non-sustained VT (NSVT), or ventricular fibrillation/aborted sudden death (VF/SCD) underwent a non-CMR diagnostic workup according to current guidelines, and CMR including LGE imaging with both a conventional breath-held and a free-breathing method enabling higher spatial resolution (HR-LGE). The diagnostic yield of CMR was compared with the non-CMR workup, including the incremental value of HR-LGE. A total of 157 patients were enrolled [age 54 ± 17 years; 75% males; 88 (56%) sustained VT, 52 (33%) NSVT, 17 (11%) VF/SCD]. Of these, 112 (71%) patients had no history of structural heart disease (SHD). All patients underwent electrocardiography and echocardiography, 72% coronary angiography, and 51% exercise testing. Pre-CMR diagnoses were 84 (54%) no SHD, 39 (25%) ischaemic cardiomyopathy (ICM), 11 (7%) non-ischaemic cardiomyopathy (NICM), 3 (2%) arrhythmogenic right ventricular cardiomyopathy (ARVC), 2 (1%) hypertrophic cardiomyopathy (HCM), and 18 (11%) other. CMR modified these diagnoses in 48 patients (31% of all and 43% of those with no SHD history). New diagnoses were 9 ICM, 28 NICM, 8 ARVC, 1 HCM, and 2 other. CMR modified therapy in 19 (12%) patients. In patients with no SHD after non-CMR tests, SHD was found in 32 of 84 (38%) patients. Eighteen of these patients showed positive HR-LGE and negative conventional LGE. Thus, HR-LGE significantly increased the CMR detection of SHD (17–38%, P < 0.001).
Conclusion
CMR including HR-LGE imaging has high diagnostic value in patients with VAs. This has major prognostic and therapeutic implications, particularly in patients with negative pre-CMR workup.
Keywords: Cardiac magnetic resonance, Late gadolinium enhancement, Ventricular arrhythmia, Structural heart disease, Diagnosis
What’s new?
In patients presenting with ventricular arrhythmia, free-breathing late gadolinium-enhanced (LGE) CMR enables the detection of arrhythmogenic substrates with higher spatial resolution.
The addition of such high-resolution LGE imaging to the CMR protocol significantly improves the performance of CMR in detecting LV or RV substrates, particularly in patients with no SHD as per pre-CMR assessment.
Results from CMR in patients with ventricular arrhythmias significantly impact patient management.
Introduction
Ventricular arrhythmias (VAs) are major determinants of human morbidity and mortality, potentially leading to sudden cardiac death (SCD). The vast majority of malignant VAs, namely ventricular tachycardia (VT) and ventricular fibrillation (VF), occur on structurally diseased hearts.1 The presence or absence of an underlying structural heart disease is a major determinant of prognosis and has an impact on treatment management.2 According to recent guidelines, transthoracic echocardiography (TTE) and coronary angiography remain the first-line imaging methods for the diagnostic management of patients with VAs, while cardiac magnetic resonance (CMR) should be considered when echocardiography does not provide accurate assessment of ventricular function and/or evaluation of structural changes (Class IIa, level of evidence B).3 However, a broader use of CMR in the diagnostic management of patients with VAs might be desirable. When systematically performing CMR in 82 consecutive patients with malignant ventricular arrhythmias, White et al.4 showed that CMR alters up to 50% of diagnoses as compared to the conventional diagnostic workup including TTE and coronary angiography. Furthermore, relevant myocardial disease was found in 24% of patients categorized as having normal hearts based on non-CMR imaging. These findings suggest that CMR may more accurately depict ischaemic and non-ischaemic substrates, thanks to a higher ability in characterizing myocardial tissue and detecting wall motion abnormalities. The recent development of respiratory-navigated late gadolinium-enhanced (LGE) CMR methods now gives access to myocardial scar assessment with much higher spatial resolution.5 Although the method was initially developed for atrial imaging, it has also been shown valuable in providing a detailed three-dimensional architecture of ventricular scars to guide catheter ablation procedures for VAs.6–8 We hypothesized that it may also prove relevant in detecting focal substrates and identifying the underlying disease in patients with VAs. The aim of this study was to document the diagnostic yield of CMR including high-resolution LGE imaging in the diagnostic workup of patients with VAs.
Methods
Population
Consecutive patients from January 2013 to October 2015 who referred to our centre for the management of VAs were prospectively enrolled. Inclusion criteria were a first known episode of VT [sustained or non-sustained (NS)], VF, or aborted SCD. NSVT was defined as VT lasting more than three complexes and less than 30 s, while sustained VT was defined as VT lasting more than 30 s. Asymptomatic VTs detected on systematic Holter or telemetry recordings during the diagnostic workup of a known cardiomyopathy were considered for inclusion. Criteria for non-inclusion were contraindications to gadolinium-enhanced CMR, history of catheter ablation, and recent acute coronary syndrome <3 months. In the population referred for VF/SCD, troponin values up to 10 times the norm were considered as potentially related to the no/low flow episode and not considered as acute coronary syndromes. All patients underwent a standardized non-CMR diagnostic workup according to the most recent guidelines,3 followed by a CMR study including high-resolution LGE imaging. The study was approved by the local Institutional Ethics Committee, and all patients gave informed consent.
Non-cardiac magnetic resonance diagnostic workup
Except for the systematic use of CMR, the diagnostic workup conformed to recent guidelines.3 The aetiological diagnosis was based on patient history, clinical symptoms, electrocardiography (ECG), TTE, as well as biochemical testing, Holter ECG, signal average ECG, exercise stress testing, coronary angiography, or coronary computed tomography angiography, as appropriate. A cardiologist with 10 years of experience in managing patients with ventricular arrhythmias reviewed all results, blinded to CMR findings, and assigned each patient to one of the six following categories: (i) no structural heart disease (No SHD), (ii) ischaemic cardiomyopathy (ICM), (iii) non-ischaemic cardiomyopathy (NICM), (iv) arrhythmogenic right ventricular cardiomyopathy (ARVC), (v) hypertrophic cardiomyopathy (HCM), and (vi) other. A negative diagnostic workup was defined on the basis of the absence of known SHD, normal 12-lead ECG, normal two-dimensional echocardiography and absence of obstructive coronary artery disease after non-invasive or invasive test. ICM was defined by the following conditions: prior history of myocardial infarction or obstructive coronary artery disease (CAD) on invasive coronary angiography. Non-ischaemic cardiomyopathy was defined as altered left ventricular (LV) ejection fraction (LVEF) and LV dilatation on echocardiography or prior history of troponin rise in the absence of obstructive CAD. Arrhythmogenic right ventricular cardiomyopathy was diagnosed according to the modified Task Force Criteria.9 Hypertrophic cardiomyopathy was diagnosed according to recent echocardiography guidelines.10 All cases that could not be attributed to any of the five first diagnostic categories were categorized as other. Ventricular dilatation and systolic dysfunction on TTE were defined based on previously reported normal values in men and women on the left ventricle and righr ventricle.11 Of note, patients with mild LV or RV dilatation and/or EF impairment and an otherwise negative workup were categorized as other, because these findings were considered to be possibly arrhythmia induced and not definitely related to SHD.12
Cardiac magnetic resonance
Cardiac magnetic resonance imaging was performed on a 1.5-T scanner (Avanto, Siemens Medical Systems, Erlangen, Germany), equipped with a 32-channel cardiac coil. The imaging protocol comprised cine imaging, conventional breath-held LGE imaging and free-breathing LGE imaging at higher spatial resolution in all patients. Conventional cine and LGE imaging sequence parameters conformed to recent recommendations.13 Cine steady-state free precession sequences were acquired in two-chamber, three-chamber, four-chamber views, a stack of contiguous 6-mm-thick short-axis slices encompassing the whole LV, and a stack of similar transaxial slices encompassing the whole RV, with the following parameters: TR/TE, 20/1.4 ms; flip angle, 60°; slice thickness, 6 mm; pixel size, 1.6 × 1.4 mm; and 30 frames per cardiac cycle. Conventional LGE imaging was performed 10 min after the injection of 0.2 mmol/kg gadoterate meglumine (Guerbet, Aulnay-sous-Bois, France) using a breath-held and inversion recovery-prepared turbo Fast Low Angle Shot sequence in three stacks of contiguous 6-mm-thick slices encompassing the whole ventricles in short axis, two-chamber, and four-chamber orientations, with the following parameters: TR/TE, 700/1.4 ms; flip angle, 10°; voxel size, 1.8 × 1.4 × 6 mm; inversion time, 260–320 ms depending on the results of a TI scout scan performed immediately before acquisition. High-resolution LGE imaging was performed immediately after, hence initiated 15-17 min after contrast injection, using a three-dimensional, inversion-recovery-prepared, ECG-gated, respiration-navigated gradient echo pulse sequence with fat saturation.5 Typical imaging parameters were the following: TR/TE, 6.1/2.4 ms; flip angle, 22°; voxel size, 1.25 × 1.25 × 2.5 mm; inversion time, 260–320 ms depending on the results of a TI scout scan performed immediately before acquisition; parallel imaging using GRAPPA technique with R = 2; 42 reference lines; acquisition time, 5–10 min depending on patient’s heart and breath rate.
Cardiac magnetic resonance analysis and diagnostic yield
Two readers with 5 and 15 years of experience in CMR reviewed all CMR studies. RV and LV volumes and EF were quantified using Argus software (Siemens Medical Systems, Erlangen, Germany). Ventricular dilatation and systolic dysfunction were defined based on previously reported normal values in men and women on the left ventricle and right ventricle.14 Cine images were visually assessed to look for LV or RV wall motion abnormalities. End-diastolic LV myocardial thickness was measured on cine short-axis images. LGE was categorized as subendocardial, midwall, subepicardial, or transmural. Subendocardial LGE with coronary distribution was considered to be of ischaemic origin, whereas midwall and subepicardial LGE were considered to be of non-ischaemic origin.15 Non-ischaemic LGE patterns were further characterized as either striae-like or focal/patchy. LGE extent was quantified in numbers of segments, using the 17-segment American Heart Association (AHA) model on the left ventricle, and a 9-segment model on the right ventricle (anterior, lateral, inferior, septal at basal and mid levels, plus apical). Conventional LGE imaging was first reviewed blinded from the results of high-resolution LGE imaging, and the two readers established a first diagnosis in consensus, based on the non-CMR diagnostic workup and the conventional cine and LGE CMR methods. Subsequently, the same readers reviewed the high-resolution LGE images and established a final diagnostic decision, attributing each patient to one of the six diagnostic categories. Absence of SHD was defined as normal LVEF and LV end-diastolic volume (LVEDV), absence of wall motion abnormality, and negative LGE. ICM was defined as subendocardial or transmural LGE with vascular distribution. The criteria for NICM were positive LGE of non-ischaemic distribution (midwall or subepicardial) or altered LVEF and/or elevated LVEDV in the absence of ischaemic-like LGE. Arrhythmogenic right ventricular cardiomyopathy was diagnosed according to the Task Force Criteria.9 Hypertrophic cardiomyopathy was diagnosed according to recent guidelines.10 All cases that could not be attributed to any of the five previous diagnostic categories were categorized as other. Of note, patients with mild LV or RV dilatation and/or EF impairment and an otherwise negative workup were categorized as other, because these findings were considered to be possibly arrhythmia induced and not definitely related to SHD.11 In patients with no prior history of SHD, the diagnostic yield of CMR with and without the adjunction of high-resolution LGE images was assessed in comparison with the prior diagnosis based on non-CMR data. In all cases with a diagnostic change motivated by CMR, the substrate location was compared with the VT morphology, whenever available. The electrocardiographic localization of ventricular tachycardia was based on previously reported criteria.16 In addition, these patients were followed up for a minimum of 6 months to document the impact of the diagnostic change on therapy management. Clinical outcome was analysed, arrhythmia recurrence being documented based on clinical symptoms, as well as ECG recordings or implantable cardioverter defibrillator (ICD) logs whenever available (Holter ECG was not systematically performed during follow-up). In patients who underwent catheter ablation during follow-up, image integration was performed and mapping studies were reviewed. The relationship between imaging substrate and ablation targets at electroanatomical mapping was analysed, targets being defined either on substrate maps during sinus rhythm (low voltage or local abnormal ventricular activity) or with the use of pace mapping or VT entrainment mapping.
Statistical analysis
The Shapiro–Wilk test of normality and D’Agostino tests for skewness and kurtosis were used to assess whether quantitative data conformed to the normal distribution. Continuous variables are expressed as mean ± SD. Categorical variables are expressed as fraction (%). Continuous variables were compared using independent-sample parametric or non-parametric tests depending on data normality (unpaired Student’s t-test, analysis of variance or Mann–Whitney). Categorical variables were compared using the Fisher’s exact test or the χ2 test, as appropriate. All statistical tests were two tailed. A P-value of <0.05 was considered to indicate statistical significance. Analyses were performed using NCSS 8 (NCSS Statistical Software, Kaysville, UT, USA).
Results
Population
A total of 157 consecutive patients were enrolled. Of these, 52 (33%) patients presented with NSVT, 88 (56%) patients with sustained VT, and 17 (11%) patients with VF or aborted sudden death. Patient clinical characteristics are shown in Table 1. The majority of patients were male (75%). Mean age at presentation was 54 ± 17 years. A prior diagnosis of SHD was present in 45 of 157 (29%) patients. This comprised 32 (20%) ICM, 6 (4%) NICM, 2 (1%) ARVC, and 5 (3%) other (all with history of surgically repaired tetralogy of Fallot). There was no history of SHD in 112 of 157 (71%) patients. A history of myocardial infarction and of revascularisation was significantly more present in patients with VF/SCD and sustained VT than in patients with NSVT. Of the 157 patients, 50 (32%) patients presented with asymptomatic arrhythmia, including 35/52 (67%) of the patients with NSVT and 15/88 (17%) of the patients with sustained VT. Asymptomatic sustained VTs were detected on systematic Holter or telemetry recordings during the diagnostic workup of a known cardiomyopathy. In the sustained VT group, the VT morphology was monomorphic with LV origin in 38 of 88 patients, monomorphic with RV origin in 25 of 88 patients (including 14 patients with RV outflow tract origin), polymorphic in 9 of 88 patients, and unknown in 16 of 88 (18%) patients.
Table 1.
Clinical characteristics
| Total population (n = 157) | NSVT (n = 52) | Sustained VT (n = 88) | VF/SCD (n = 17) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 54 ± 17 | 52 ± 15 | 56 ± 16 | 45 ± 25 | 0.02b |
| Female gender | 40 (25%) | 19 (37%) | 18 (20%) | 3 (18%) | 0.04a |
| Cardiovascular risk factors | |||||
| Active smoking | 65 (41%) | 24 (46%) | 31 (35%) | 10 (59%) | 0.07 |
| Diabetes | 15 (10%) | 3 (6%) | 10 (11%) | 2 (12%) | 0.27 |
| Hypertension | 53 (34%) | 16 (31%) | 35 (40%) | 2 (12%) | 0.03b |
| Hyperlipidaemia | 43 (27%) | 14 (27%) | 26 (30%) | 3 (18%) | 0.32 |
| Obesity | 78 (50%) | 32 (62%) | 41 (47%) | 5 (29%) | 0.02c |
| BMI (kg/m2) | 26 ± 5 | 27 ± 5 | 26 ± 4 | 23 ± 4 | 0.01b,c |
| History | |||||
| No history of SHD | 112 (71%) | 45 (87%) | 56 (64%) | 11 (65%) | 0.04a,c |
| Myocardial infarction | 32 (20%) | 3 (6%) | 25 (28%) | 4 (24%) | 0.03a,c |
| Revascularization | 27 (17%) | 3 (6%) | 20 (23%) | 4 (24%) | 0.03a,c |
| NICM | 6 (4%) | 2 (4%) | 3 (3%) | 1 (6%) | 0.63 |
| ARVC | 2 (1%) | 0 (0%) | 2 (2%) | 0 (0%) | 0.28 |
| HCM | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | – |
| Other | 5 (3%) | 2 (4%) | 2 (2%) | 1 (6%) | 0.41 |
| Clinical symptoms | |||||
| Chest pain | 22 (14%) | 4 (8%) | 16 (18%) | 2 (12%) | 0.09 |
| Palpitation | 60 (38%) | 16 (31%) | 43 (49%) | 1 (6%) | 0.04a,b,c |
| Dyspnoea | 13 (8%) | 2 (4%) | 10 (11%) | 1 (6%) | 0.13 |
| Lipothymia | 26 (17%) | 6 (12%) | 20 (23%) | 0 (0%) | 0.03b |
| Syncope | 30 (19%) | 1 (2%) | 12 (14%) | 17 (100%) | 0.02a,b,c |
| None | 50 (32%) | 35 (67%) | 15 (17%) | 0 (0%) | 0.001a,b,c |
ARVC, arrhythmogenic right ventricular cardiomyopathy; BMI, body mass index; HCM, hypertrophic cardiomyopathy; NICM, non-ischaemic cardiomyopathy; NSVT, non-sustained ventricular tachycardia; SHD, structural heart disease; VF/SCD, ventricular fibrillation/sudden cardiac death; VT, ventricular tachycardia.
Significant difference between NSVT and sustained VT groups.
Significant difference between sustained VT and VF/SCD groups.
Significant difference between NSVT and VF/SCD groups.
Non-cardiac magnetic resonance diagnostic tests
The list of non-CMR imaging tests performed in the studied population is shown in Table 2. Exercise testing was performed in 80 of 157 (51%) patients and TTE in all 157 (100%) patients. The VF/SCD and sustained VT groups had significantly more impaired LVEF than NSVT group. Invasive coronary angiography was performed more often in the VT/SCD and sustained VT groups as compared to the NSVT group. Conversely, exercise stress testing was performed more often in the NSVT group than in the other groups. After adjudication of all non-CMR diagnostic information, a ‘no SHD’ diagnosis category was retained in 84 of 157 (54%) patients, an ‘ICM’ category in 39 of 157 (25%) patients, an ‘NICM’ category in 11 of 157 (7%) patients, an ‘ARVC’ category in 3 of 157 (2%) patients, an ‘HCM’ category in 2 of 157 (1%) patients, and a ‘other’ category in 18 of 157 (11%) patients. The ‘other’ category comprised (i) five surgically repaired congenital cardiomyopathy, (ii) eight suspected ARVC with non-definite diagnosis according to Task Force Criteria (‘borderline’ diagnosis in seven, ‘possible’ diagnosis in one), and (iii) five patients with mild ventricular dilatation and/or EF impairment and no regional wall motion abnormality on echocardiography, and without obstructive CAD on angiography, these findings being possibly arrhythmia-induced. Figure 1 illustrates the rate of diagnostic category assignments before CMR.
Table 2.
Non-CMR tests
| Total population (n = 157) | NSVT ( n = 52) | Sustained VT (n = 88) | VF/SCD (n = 17) | P-value | |
|---|---|---|---|---|---|
| Exercise testing performed | 80 (51%) | 39 (75%) | 37 (42%) | 4 (24%) | <0.001b,c,d |
| TTE performed | 157 (100%) | 52 (100%) | 88 (100%) | 17 (100%) | – |
| LV diameter (mm) | 53 ± 11 | 52 ± 8 | 54 ± 8 | 50 ± 12 | 0.20 |
| LV dilatation | 27 (17%) | 4 (8%) | 19 (22%) | 4 (24) | 0.03b |
| LVEF (%) | 54 ± 15 | 61 ± 14 | 51 ± 14 | 54 ± 17 | 0.003b |
| LVEF impairment | 44 (28%) | 6 (12%) | 31 (35%) | 7 (41%) | 0.006b,d |
| LV wall motion abnormality | 40 (25%) | 6 (12%) | 30 (34%) | 4 (24%) | 0.003b |
| Coronary angiography performed | 113 (72%) | 27 (52%) | 69 (79%) | 17 (100%) | 0.004b,d |
| Invasive angiography | 96 (61%) | 24 (46%) | 58 (66%) | 14 (82%) | 0.02b,d |
| Computed tomography | 17 (11%) | 3 (6%) | 11 (13%) | 3 (18%) | 0.13 |
| Normal coronary arteriesa | 47 (49%) | 17 (71%) | 21 (36%) | 9 (64%) | 0.007b,c |
| Non-obstructive CADa | 20 (21%) | 4 (17%) | 16 (28%) | 0 (0%) | 0.04b,c,d |
| Obstructive CADa | 29 (30%) | 3 (13%) | 21 (36%) | 5 (36%) | 0.09 |
CAD, coronary artery disease; CMR, cardiac magnetic resonance; EF, ejection fraction; LV, left ventricle; NSVT, non-sustained VT; TTE, trans-thoracic echocardiography; VF/SCD, ventricular fibrillation/sudden cardiac death; VT, ventricular tachycardia.
Refers to patients with coronary angiography.
Significant difference between NSVT and sustained VT groups.
Significant difference between sustained VT and VF/SCD groups.
Significant difference between NSVT and VF/SCD groups.
Figure 1.
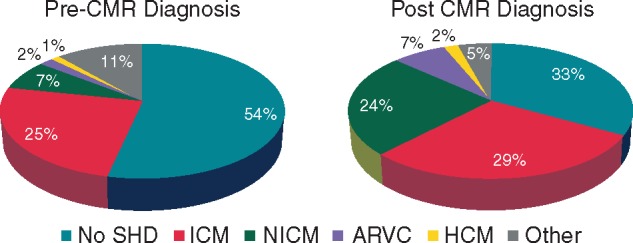
Diagnostic categories before and after CMR in 157 consecutive patients presenting with a first episode of ventricular arrhythmia (52 NSVT, 88 sustained VT, and 17 VF/SCD).
Cardiac magnetic resonance imaging
The mean delay between the first arrhythmic episode and the CMR study was 25 ± 17 days. Cardiac magnetic resonance findings according to the type of arrhythmia are listed in Table 3. Left ventricular ejection fraction was significantly lower and LVEDV significantly higher in the VF/SCD and sustained VT groups than in the NSVT group. Late gadolinium-enhanced imaging was abnormal in 101 of 157 (64%) patients, including 87 (55%) on the left ventricle and 20 (13%) on the right ventricle. Positive LGE was more frequent in sustained VT and VF/SCD than in NSVT patients. When positive, LGE were larger in those with sustained VT and VF/SCD than in those with NSVT. In the total population, the LGE patterns were subendocardial in 48 of 157 (31%) patients, subepicardial in 25 (16%) patients, midwall in 23 (15%) patients, and transmural in 29 (19%) patients. Cardiac magnetic resonance imaging identified a structural substrate for ventricular arrhythmia in 105 of 157 (67%) patients, including 20/52 (38%) of the patients with NSVT, 73/88 (83%) of those with sustained VT, and 12/17 (71%) of those with VF/SCD. Cardiac magnetic resonance was categorized as no SHD in 52 of 157 (33%) patients, ICM in 46 (29%), NICM in 38 (24%), ARVC in 11 (7%), HCM in 3 (2%), and other in 7 (4%) patients. The other category comprised five patients with surgically repaired congenital cardiomyopathy, and two with mild biventricular dilatation and/or EF impairment and negative LGE, these findings being possibly arrhythmia induced. Figure 1 illustrates the rate of assignment in each diagnostic category. Cardiac magnetic resonance findings in each diagnostic category are shown in Table 4.
Table 3.
CMR findings according to the type of arrhythmia
| NSVT (n = 52) | Sustained VT (n = 88) | VF/SCD (n = 17) | P-value | |
|---|---|---|---|---|
| Cine imaging | ||||
| LV EDV indexed (mL/m2) | 73 ± 22 | 87 ± 25 | 102 ± 42 | 0.04b,c,d |
| LV dilatation | 5 (10%) | 27 (31%) | 8 (47%) | 0.004b,d |
| LVEF (%) | 63 ± 10 | 52 ± 15 | 48 ± 21 | <0.001b,d |
| LVEF impairment | 6 (12%) | 37 (42%) | 8 (47%) | 0.001b,d |
| LV WMA | 6 (12%) | 43 (49%) | 7 (41%) | 0.006b,d |
| LV maximum thickness (mm) | 10.2 ± 0.8 | 10.1 ± 0.8 | 10.0 ± 0.5 | 0.82 |
| RV EDV indexed (mL/m2) | 73 ± 16 | 83 ± 30 | 79 ± 27 | 0.02b |
| RV dilatation | 3 (6%) | 17 (19%) | 2 (12%) | 0.03b |
| RVEF (%) | 55 ± 8 | 49 ± 11 | 53 ± 15 | 0.001b |
| RVEF impairment | 4 (8%) | 18 (20%) | 3 (18%) | 0.04b |
| RV WMA | 3 (6%) | 14 (16%) | 1 (6%) | 0.08 |
| LGE imaging | ||||
| LGE positive on LV | 16 (31%) | 61 (69%) | 10 (59%) | 0.04b,d |
| LGE extent on LV (N segments)b | 2.4 ± 2.1 | 4.0 ± 2.7 | 4.7 ± 2.9 | 0.04b,d |
| Subendocardial LGE | 7 (13%) | 34 (39%) | 7 (41%) | 0.007b,d |
| Non-ischaemic LGE | 9 (17%) | 28 (32%) | 4 (24%) | 0.06 |
| Midwall | 4 (8%) | 16 (18%) | 3 (18%) | 0.04b,d |
| Subepicardial | 5 (10%) | 18 (20%) | 2 (12%) | 0.04b |
| Striae-like | 5 (10%) | 23 (26%) | 3 (18%) | 0.02b |
| Focal or patchy | 3 (6%) | 4 (5%) | 2 (12%) | 0.09 |
| Transmural LGE | 3 (6%) | 23 (26%) | 3 (18%) | 0.01b,d |
| LGE positive on RV | 3 (6%) | 16 (18%) | 1 (6%) | 0.04b |
| LGE extent on RV (N segments)a | 1.3 ± 0.6 | 2.9 ± 2.0 | 2 | 0.20 |
| Final diagnosis after CMR | ||||
| No SHD | 32 (62%) | 15 (17%) | 5 (29%) | 0.02b,d |
| ICM | 7 (13%) | 33 (38%) | 6 (35%) | 0.04b,d |
| NICM | 8 (15%) | 26 (30%) | 4 (23%) | 0.06 |
| ARVC | 1 (2%) | 10 (11%) | 0 (0%) | 0.05 |
| HCM | 1 (2%) | 1 (1%) | 1 (6%) | 0.19 |
| Other | 3 (6%) | 3 (3%) | 1 (6%) | 0.51 |
| CMR diagnostic yield (% modified diagnoses) | 11 (21%) | 34 (39%) | 3 (18%) | 0.03b |
ARVC, arrhythmogenic right ventricular cardiomyopathy; CMR, cardiac magnetic resonance; EF, ejection fraction; HCM, hypertrophic cardiomyopathy; ICM, ischaemic cardiomyopathy; LGE, late gadolinium enhancement; LV, left ventricle; NICM, non-ischaemic cardiomyopathy; NSVT: non-sustained ventricular tachycardia; RV, right ventricle; SHD, structural heart disease; VF/SCD, ventricular fibrillation/sudden cardiac death; VT, ventricular tachycardia.
Refers to patients with positive LGE.
Significant difference between NSVT and sustained VT groups.
Significant difference between sustained VT and VF/SCD groups.
Significant difference between NSVT and VF/SCD groups.
Table 4.
CMR findings according to final diagnosis
| No SHD (n = 52) | ICM (n = 46) | NICM (n = 38) | ARVC (n = 11) | HCM (n = 3) | Other (n = 7) | |
|---|---|---|---|---|---|---|
| Cine imaging | ||||||
| LV EDV indexed (mL/m2) | 70 ± 15 | 93 ± 31 | 94 ± 31 | 83 ± 24 | 77 ± 11 | 80 ± 19 |
| LV dilatation | 0 (0%) | 22 (48%) | 14 (37%) | 0 (0%) | 0 (0%) | 4 (57%) |
| LVEF (%) | 65 ± 7 | 45 ± 15 | 53 ± 17 | 56 ± 10 | 51 ± 11 | 56 ± 19 |
| LVEF impairment | 0 (0%) | 32 (70%) | 17 (45%) | 0 (0%) | 1 (33%) | 1 (14%) |
| LV wall motion abnormality | 0 (0%) | 36 (78%) | 16 (42%) | 0 (0%) | 1 (33%) | 3 (43%) |
| LV maximum thickness (mm) | 10.4 ± 0.8 | 9.6 ± 1.0 | 10.0 ± 1.4 | 10.4 ± 0.5 | 15.7 ± 2.1 | 10.2 ± 0.8 |
| RV EDV indexed (mL/m2) | 75 ± 16 | 70 ± 18 | 80 ± 23 | 118 ± 35 | 63 ± 10 | 111 ± 53 |
| RV dilatation | 0 (0%) | 1 (3%) | 4 (11%) | 8 (73%) | 0 (0%) | 6 (86%) |
| RVEF (%) | 56 ± 9 | 53 ± 9 | 51 ± 10 | 32 ± 7 | 55 ± 9 | 45 ± 18 |
| RVEF impairment | 0 (0%) | 4 (9%) | 9 (24%) | 11 (100%) | 0 (0%) | 4 (57%) |
| RV wall motion abnormality | 0 (0%) | 1 (3%) | 1 (3%) | 11 (100%) | 0 (0%) | 5 (71%) |
| LGE imaginga | ||||||
| LGE positive on LV | 0 (0%) | 46 (100%) | 35 (92%) | 3 (27%) | 3 (100%) | 0 (0%) |
| LGE extent on LV (N segments)b | – | 4.7 ± 2.7 | 2.8 ± 2.5 | 2.0 ± 1.0 | 3.3 ± 1.5 | – |
| Anterior LV LGE | – | 17 (37%) | 6 (16%) | 0 (0%) | 3 (100%) | – |
| Septal LV LGE | – | 23 (50%) | 13 (34%) | 0 (0%) | 3 (100%) | – |
| Inferior LV LGE | – | 30 (65%) | 5 (13%) | 1 (9%) | 1 (33%) | – |
| Lateral LV LGE | – | 29 (63%) | 28 (74%) | 3 (27%) | 0 (0%) | – |
| Subendocardial LGE | – | 46 (100%) | 2 (5%) | 0 (0%) | 0 (0%) | – |
| Non-ischemic LGE | – | 0 (0%) | 35 (92%) | 3 (27%) | 3 (100%) | – |
| Midwall | – | – | 19 (50%) | 1 (9%) | 3 (100%) | – |
| Subepicardial | – | – | 23 (61%) | 2 (18%) | 0 (0%) | – |
| Striae-like | – | – | 29 (76%) | 2 (18%) | 0 (0%) | – |
| Focal or patchy | – | – | 6 (16%) | 0 (0%) | 3 (100%) | – |
| Transmural LGE | – | 26 (56%) | 2 (5%) | 0 (0%) | 0 (0%) | – |
| LGE positive on RV | 0 (0%) | 2 (4%) | 2 (5%) | 11 (100%) | 0 (0%) | 5 (71%) |
| LGE extent on RV (N segments)b | – | 1 ± 0 | 1 ± 0 | 3.7 ± 1.8 | – | 1.4 ± 0.5 |
| Anterior RV LGE | – | 0 (0%) | 1 (3%) | 6 (54%) | – | 5 (71%) |
| Lateral RV LGE | – | 0 (0%) | 1 (3%) | 11 (100%) | – | 0 (0%) |
| Inferior RV LGE | – | 2 (4%) | 0 (0%) | 7 (63%) | – | 0 (0%) |
| Septal RV LGE | 0 (0%) | 0 (0%) | 0 (0%) | – | 5 (71%) |
ARVC, arrhythmogenic right ventricular cardiomyopathy; CMR, cardiac magnetic resonance; EF, ejection fraction; HCM, hypertrophic cardiomyopathy; ICM, ischaemic cardiomyopathy; LGE, late gadolinium enhancement; LV, left ventricle; NICM, non-ischaemic cardiomyopathy; RV, right ventricle; SHD, structural heart disease.
CMR protocol inclusive of high-resolution LGE.
Refers to patients with positive LGE.
Diagnostic yield of pre-cardiac magnetic resonance and cardiac magnetic resonance studies
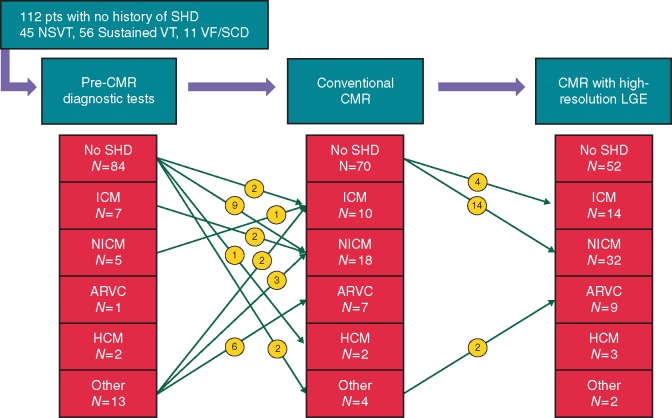
At the time of inclusion, a diagnosis of SHD was present in 45/157 (29%) of the patients and absent in 112 (71%) patients. None of the previously established diagnoses (0/45) were modified by the pre-CMR diagnostic workup, nor by CMR results. In the population with no known SHD at the time of inclusion (n = 112), the pre-CMR diagnostic management found SHD in 28 of 112 (25%) patients: ICM in 7 of 112 (6%), NICM in 5 of 112 (4%), ARVC in 1 of 112 (1%), HCM in 2 of 112 (2%), and other in 13 of 112 (12%) patients. The other category comprised eight suspected ARVC with non-definite diagnosis and five suspected arrhythmia-induced abnormalities. When including CMR results in the diagnosis workup, the rate of SHD significantly increased to 60 of 112 (54%) (P < 0.001). Of the 84 patients with no SHD as per non-CMR tests, CMR results revealed SHD in 32 of 84 (38%) patients. This included ICM in 6/84 (7%), NICM in 23/84 (27%), HCM in 1/84 (1%), and other in 2/84 (2%) (2 patients with suspected arrhythmia-induced cardiomyopathy). In addition CMR results modified the diagnosis of SHD retained after non-CMR tests in 16/112 patients (14% of the population with no known SHD at inclusion). This included (i) 8 of 112 (7%) patients with non-definite ARVC before CMR (categorized as other) and with definite ARVC diagnosis after CMR; (ii) 5 of 112 (4%) patients with suspected arrhythmia-induced abnormalities before CMR (categorized as other) in whom CMR showed LGE consistent with NICM in 3 and ICM in 2; (iii) 2 of 112 (2%) patients with markedly impaired LVEF, considered as ICM before CMR based on coronary angiography, in whom CMR showed LGE on midwall septum and no subendocardial scar, which was consistent with NICM; and (iv) 1 of 112 (1%) patient with dilated cardiomyopathy, considered as NICM before CMR based on negative angiography, in whom CMR showed large subendocardial scar in LAD territory, consistent with ICM. Thus, in the population with no known SHD at inclusion, CMR results modified the diagnostic category in 48 of 112 (43%) patients as compared to the pre-CMR diagnostic workup. When excluding patients with NSVT, CMR results modified the diagnostic category in 37 of 67 (55%) patients with no prior SHD history presenting with sustained VT or VF/SCD, as compared to pre-CMR workup. The diagnostic yield of non-CMR and CMR tests is illustrated in Figure 2. Examples of typical diagnostic changes are shown in Figure 3.
Figure 2.
Diagnostic yield of pre-CMR and CMR tests in the 112 patients with no prior history of structural heart disease.
Figure 3.
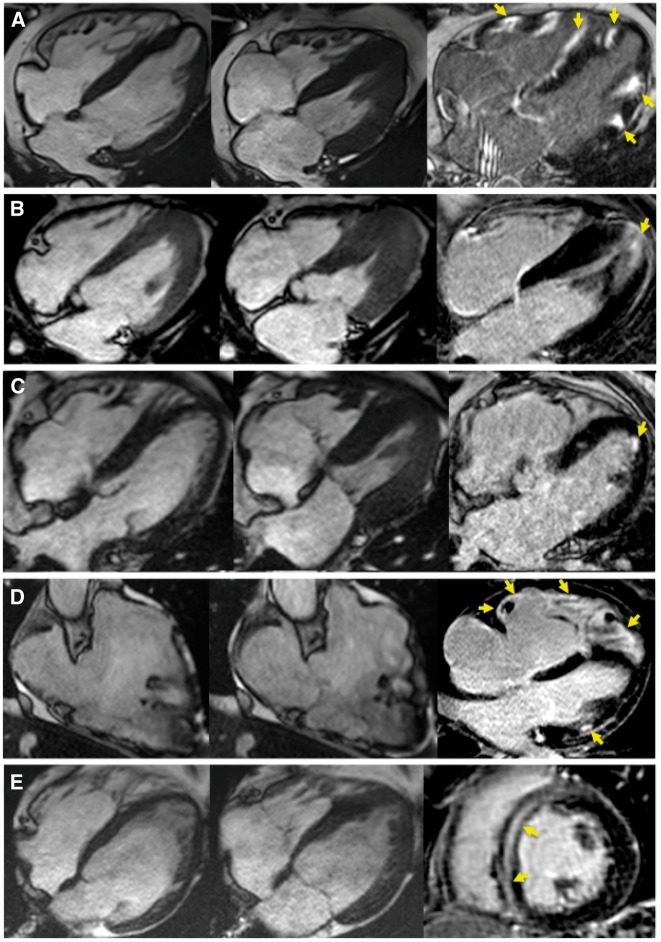
Examples of typical diagnostic changes introduced by CMR. Cine images at end diastole (left column), end systole (middle column), and LGE images (right column) are provided. (A) Image of a 44-year-old man with monomorphic sustained VT of RBBB morphology. TTE, coronary angiography, and cine MRI were negative. LGE showed multifocal substrate on LV and RV free walls and within the septum (arrows), categorized as NICM. The diagnosis of cardiac sarcoid was retained after transbronchial biopsy. (B) Image of a 34-year-old man with NSVT of unkown morphology, negative TTE, and non-specific T wave changes on ECG. Cine and LGE MR showed apical hypertrophy with midwall fibrosis (arrows), consistent with an apical form of HCM. (C) Image of a 58-year-old women with sustained VT of RBBB morphology. TTE, coronary angiography and cine MR were negative. LGE showed subendocardial scar on mid anterior LV, consistent with ICM. The patient had a history of severe asthma, and CMR results were instrumental in fulfilling the diagnostic criteria for eosinophilic granulomatosis with polyangiitis. (D) Image of a 41-year-old man with pre-CMR borderline ARVC diagnosis based on polymorphic sustained VT at exercise tesing, late potentials on SAECG, and inverted T waves in ECG leads V1–V2. Cine MR results fulfilled the criteria for definite ARVC by showing RV dilatation, EF impairment, and RVOT dyskinesia. LGE showed diffuse fibrosis on RV free wall, and focal midwall fibrosis on LV free wall (arrows). (E) Image of a 35-year-old man with frequent PVCs and NSVT on Holter. Coronary CT angiography showed normal arteries. TTE showed mild biventricular dilatation suggesting arrhythmia-induced cardiomyopathy. Cine and LGE MR showed mild biventricular dilatation and midwall fibrosis within the interventricular septum (arrows), categorized as NICM. CMR findings were instrumental in the decision to perform and obtain a positive genetic testing for laminopathy. SAECG, signal-averaged electrocardiogram; RVOT, right ventricular outflow tract; PVC: premature ventricular contraction.
Impact of high-resolution late gadolinium-enhanced imaging
In 20 cases (13% of the total population and 18% of the population with no SHD at inclusion), CMR-driven diagnostic changes were motivated by the finding of abnormal myocardial hyperenhancement consistent with scar tissue on high-resolution LGE imaging, while conventional LGE imaging was negative. The final diagnosis retained was ICM in 4 of 20 (20%), NICM in 14 of 20 (70%), and ARVC in 2 of 20 (10%) cases. In these ICM and NICM cases, scars were of limited size (1.2 ± 0.4 segments) and never transmural. In the two ARVC cases, high-resolution LGE was instrumental in making a decision on the presence of borderline wall motion abnormalities by showing clear co-localized fibrosis non-visible on conventional LGE images. An example of ARVC is shown in Figure 4. In the subgroup of patients categorized ‘no SHD’ after non-CMR diagnostic workup (n = 84), a relevant myocardial disease was found in 32 of 84 (38%) patients, and in 18 of these only the high-resolution LGE imaging was abnormal, conventional LGE and cine images being negative. Hence, the addition of high-resolution LGE to the conventional CMR protocol allowed a significant increase in the rate of SHD detected in this population with no apparent SHD as per pre-CMR tests (from 17% to 38%, P < 0.001). Examples of positive high-resolution LGE in otherwise negative patients are shown in Figure 5.
Figure 4.
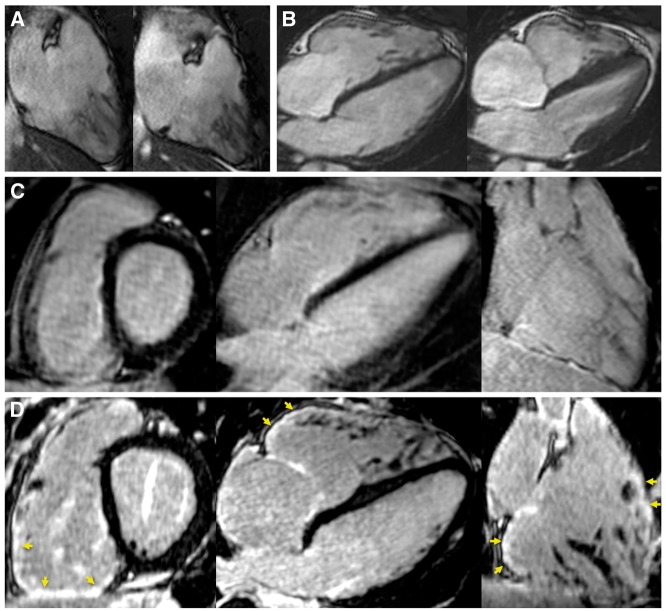
Example of ARVC diagnosis. A 20-year-old man with family history of premature sudden death in the brother. Pre-CMR workup retained a borderline ARVC diagnosis based on NSVT of RVOT morphology at exercise tesing, negative TTE, absence of repolarization or conduction abnormality on ECG, but positive late potentials on SAECG. Cine MR showed preserved RVEF, mild RV dilatation (103 mL/m2), and borderline wall motion abnormality on laterobasal and infero-basal RV (two-chamber view in A and four-chamber in B). Conventional LGE images were considered normal (C). Free-breathing LGE at higher spatial resolution showed focal fibrosis on infero-basal and laterobasal RV as well as on RVOT (arrows in D). The colocalization between fibrosis and the suspected wall motion abnormality was instrumental in retaining a minor Task Force Criterion for ARVC, fulfilling the criteria for definite ARVC diagnosis.
Figure 5.
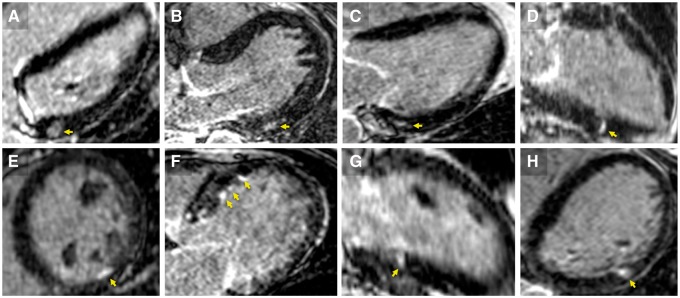
Examples of positive high-resolution LGE in otherwise negative patients. LGE of non-ischemic (arrows in A through D) and of ischaemic (in E through H) distribution are shown for eight patients. The arrythmia was NSVT in three (cases A, C, and E) and sustained VT in five (cases B, D, F, G, H). All of these patients had no prior history of SHD, negative TTE, cine MR and conventional LGE, and no obstructive CAD on coronary angiography. VT morphology was available in 5 cases (A, B, D, G, H) and matched substrate location in all five patients. Post-embolic microinfarction was suspected in (E) and (G) based on the documentation of AF episodes on 24 h Holter ECG. In the remaining six patients the aetiology remained uncertain.
Substrate location vs. ventricular tachycardia morphology
Of the 48 patients in whom CMR led to a diagnostic change, VT morphology could not be assessed in 16 of 48 (33%) patients: polymorphic VT in 8, VF/aborted sudden death with no documented monomorphic arrhythmia in 3, and no available documentation of VT in 5. VT morphology was available in the remaining 32 of 48 (70%) patients and matched substrate location in 28 of 32 (88%) patients. In the 30 patients with no SHD as per non-CMR diagnostic management, VT morphology was available in 21 of 30 (70%), and matched substrate location in 18 of 21 (86%) patients.
Characteristics of patients benefiting from cardiac magnetic resonance diagnosis
The characteristics of patients with and without a diagnosis modified at CMR are compared in Table 5. Among baseline characteristics, patients benefiting from CMR were more likely to have sustained VT and less likely to have a prior diagnosis of SHD. Among pre-CMR findings, patients benefiting from CMR showed less LVEF impairment, wall motion abnormalities and obstructive CAD. The diagnosis category retained before CMR was more likely ‘other’ or ‘no SHD’ and less likely ‘ICM’. Among the CMR findings patients benefiting from CMR diagnosis had less frequent and less large LGE, with the LGE pattern being more frequently non-ischemic, and less frequently ischaemic or transmural. The final diagnostic category was less frequently ‘no SHD’ and more frequently ‘NICM’ or ‘ARVC’. When comparing patients with a diagnosis modified based on conventional CMR to those with a diagnosis modified with the adjunction of high-resolution LGE, patients benefiting from high-resolution LGE presented more frequently with NSVT and less frequently with VT. The pre-CMR diagnosis was more often ‘No SHD’ and less often ‘other’. CMR cine imaging was more frequently normal, and LGE imaging showed smaller scars.
Table 5.
Characteristics of patients benefiting from CMR diagnosis
| CMR does not modify the diagnosis | CMR modifies the diagnosis | P-value | Diagnoses modified by conventional CMR | Diagnoses modified by high-resolution LGE | P-value | |
|---|---|---|---|---|---|---|
| Number of patients | 109 | 48 | 28 | 20 | ||
| Age (years) | 53 ± 18 | 55 ± 16 | 0.45 | 54 ± 17 | 57 ± 13 | 0.47 |
| Female gender | 29 (27%) | 11 (23%) | 0.63 | 6 (21%) | 5 (25%) | 0.78 |
| NSVT | 41 (38%) | 11 (23%) | 0.07 | 3 (11%) | 8 (40%) | 0.02 |
| Sustained VT | 54 (50%) | 34 (71%) | 0.01 | 24 (86%) | 10 (50%) | 0.007 |
| VF/SCD | 14 (13%) | 3 (6%) | 0.22 | 1 (4%) | 2 (10%) | 0.38 |
| Prior diagnosis of SHD | 45 (41%) | 0 (0%) | <0.001 | 0 (0%) | 0 (0%) | – |
| Pre CMR findings | ||||||
| LV dilatation on echo | 20 (18%) | 7 (15%) | 0.57 | 6 (21%) | 1 (5%) | 0.12 |
| LVEF impairment on echo | 37 (34%) | 7 (15%) | 0.01 | 6 (21%) | 1 (5%) | 0.12 |
| LV wall motion abnormality on echo | 36 (33%) | 4 (8%) | <0.001 | 4 (14%) | 0 (0%) | 0.08 |
| Obstructive CAD on angio | 26 (24%) | 3 (6%) | 0.008 | 3 (11%) | 0 (0%) | 0.14 |
| Diagnostic category after pre-CMR work-up | ||||||
| No SHD | 52 (48%) | 32 (67%) | 0.03 | 14 (50%) | 18 (90%) | 0.003 |
| ICM | 37 (34%) | 2 (4%) | <0.001 | 2 (7%) | 0 (0%) | 0.23 |
| NICM | 10 (9%) | 1 (2%) | 0.11 | 1 (4%) | 0 (0%) | 0.40 |
| HCM | 2 (2%) | 0 (0%) | 0.35 | 0 (0%) | 0 (0%) | – |
| ARVC | 3 (3%) | 0 (0%) | 0.25 | 0 (0%) | 0 (0%) | – |
| Other | 5 (5%) | 13 (27%) | <0.001 | 11 (39%) | 2 (10%) | 0.02 |
| CMR findingsa | ||||||
| LV dilatation | 28 (26%) | 12 (25%) | 0.93 | 11 (39%) | 1 (5%) | 0.006 |
| LVEF impairment | 40 (37%) | 11 (23%) | 0.09 | 10 (36%) | 1 (5%) | 0.01 |
| LV wall motion abnormality | 45 (41%) | 11 (23%) | 0.03 | 11 (39%) | 0 (0%) | <0.001 |
| RV dilatation | 9 (8%) | 13 (27%) | 0.002 | 10 (36%) | 3 (15%) | 0.12 |
| RVEF impairment | 16 (15%) | 9 (19%) | 0.52 | 9 (32%) | 0 (0%) | 0.004 |
| RV wall motion abnormality | 9 (8%) | 9 (19%) | 0.06 | 7 (25%) | 2 (10%) | 0.20 |
| LGE positive on LV | 50 (46%) | 37 (77%) | <0.001 | 19 (68%) | 18 (90%) | 0.07 |
| LGE extent on LV (N segments)b | 5.0 ± 2.7 | 2.2 ± 1.7 | <0.001 | 3.1 ± 2.0 | 1.2 ± 0.4 | <0.001 |
| LV LGE of ischaemic pattern | 39 (36%) | 9 (19%) | 0.03 | 5 (18%) | 4 (20%) | 0.95 |
| LV LGE of non-ischaemic pattern | 13 (12%) | 28 (58%) | <0.001 | 14 (50%) | 14 (70%) | 0.17 |
| LV LGE transmural | 28 (26%) | 1 (2%) | <0.001 | 1 (4%) | 0 (0%) | 0.40 |
| LV LGE positive on RV | 10 (9%) | 10 (21%) | 0.04 | 8 (29%) | 2 (10%) | 0.12 |
| LGE extent on RV (N segments)b | 2.5 ± 2.0 | 2.7 ± 1.8 | 0.82 | 3.0 ± 1.8 | 1.5 ± 0.7 | 0.31 |
| Diagnostic category after CMRa | ||||||
| No SHD | 52 (48%) | 0 (0%) | <0.001 | 0 (0%) | 0 (0%) | – |
| ICM | 37 (34%) | 9 (19%) | 0.05 | 5 (18%) | 4 (20%) | 0.86 |
| NICM | 10 (9%) | 28 (58%) | <0.001 | 14 (50%) | 14 (70%) | 0.17 |
| HCM | 2 (2%) | 1 (2%) | 0.92 | 1 (4%) | 0 (0%) | 0.40 |
| ARVC | 3 (3%) | 8 (17%) | 0.002 | 6 (21%) | 2 (10%) | 0.31 |
| Other | 5 (5%) | 2 (4%) | 0.91 | 2 (7%) | 0 (0%) | 0.23 |
ARVC, arrhythmogenic right ventricular cardiomyopathy; CMR, cardiac magnetic resonance; EF, ejection fraction; HCM, hypertrophic cardiomyopathy; ICM, ischaemic cardiomyopathy; LV, left ventricle; LGE, late gadolinium enhancement; NICM, non-ischaemic cardiomyopathy; NSVT, non-sustained ventricular tachycardia; RV, right ventricle; SHD, structural heart disease; VF/SCD, ventricular fibrillation/sudden cardiac death; VT, ventricular tachycardia.
CMR protocol inclusive of high-resolution LGE.
Refers to patients with positive LGE.
Impact of cardiac magnetic resonance results on patient management
The diagnostic information provided by CMR led to a modification of therapy in 19 patients, hence 12% of the total population, and 17% of patients with no known SHD at inclusion. This included the introduction of anti-arrhythmic medication in 11 patients, antiplatelet therapy in 9 patients, lipid-lowering agents in 9 patients, and angiotension-converting enzyme inhibitors in 2 patients. Implantable cardioverter defibrillator therapy was introduced in 97 of 157 (62%) patients in the total population. CMR was not instrumental in the decision to implant as it was in all cases indicated in the frame of secondary prevention. Catheter ablation was performed in 22 of 157 (18%) patients of the total population (6 patients with VF, 15 with sustained VT, and 1 with NSVT). Cardiac magnetic resonance data on cardiac anatomy and myocardial scar were integrated in three-dimensional mapping systems and used to provide guidance during the ablation procedure in 19 of these patients. LGE was positive in 18 of 19 patients and co-localized with abnormal findings at electroanatomical mapping in all cases. Patient outcome was analysed over a median follow-up period of 9 months (Q1–Q3: 7–24 months). To document arrhythmia recurrence, Holter ECG was performed during follow-up in 21 patients (most often motivated by clinical symptoms), and ICD reports were available for all implanted patients (n = 97). Ventricular arrhythmia recurrence was detected in 13 of 21 (62%) patients on follow-up Holter and 14 of 97 (14%) patients with ICDs received appropriate therapy during follow-up. In the total population, arrhythmia recurrence was either suspected from clinical symptoms or documented by ECG or ICD recordings in 31 of 157 (20%) patients. Arrhythmia recurrence did not differ between patients with or without a diagnostic change after CMR (P = 0.22). Four patients died during follow-up (one from non-cardiac cause, two from heart failure, and one from VT storm). All four had prior history of SHD and no diagnostic change after CMR.
Discussion
This study is to our knowledge the largest series evaluating the diagnostic yield of CMR in identifying the underlying cause of ventricular arrhythmias, and the first to introduce the use of free-breathing LGE imaging to detect arrhythmogenic substrates with higher spatial resolution. As compared to the first-line diagnostic strategy based on TTE and coronary angiography, the diagnostic yield of CMR is high, leading to a diagnostic change in over 40% of the patients with no prior history of SHD presenting with VT, VF, or SCD. Cardiac magnetic resonance is particularly useful in patients with negative diagnostic workup including TTE and coronary angiography, while it appears to have less diagnostic value in patients with a prior history of SHD. The addition of high-resolution LGE imaging to the CMR protocol significantly improves the performance of CMR in detecting smaller LV or RV substrates, particularly in patients with no SHD as per pre-CMR assessment. Results from CMR significantly impact patient management.
Diagnostic yield of cardiac magnetic resonance in ventricular arrhythmias
Most malignant VAs occur on structurally diseased hearts. According to the current guidelines, TTE and coronary angiography remain the first-line imaging methods for the diagnostic management of patients with VAs, while CMR should be considered when echocardiography does not provide accurate assessment of ventricular function and/or evaluation of structural changes.3 Prior studies have supported a broader use of CMR in the diagnostic management of cardiac diseases, firstly because cine MR can assess regional wall motion and ventricular volumes and EF with higher reproducibility as compared to TTE, and more importantly because LGE at CMR is the reference method for the detection of myocardial injury.15,17 This study shows that CMR has a high diagnostic yield in patients with no prior history of SHD experiencing a first episode of VT, VF, or SCD. The largest prior series of the kind is the one by White et al.4 who studied 82 patients with either SCD or sustained VT. These authors reported a 50% diagnostic yield of CMR as compared to the conventional diagnostic workup based on TTE and angiography, including 24% in patients with no SHD per non-CMR diagnostic tests. Our results are in line with this prior study, although the diagnostic yield of CMR is slightly lower (43%). This could be due to the inclusion of patients with NSVT in the present work, or to a more delayed use of CMR, potentially missing transient abnormalities at the acute stage. Nonetheless, our results also suggest that CMR should be more broadly employed after a first episode of NSVT, VT, or VF/SCD. The high rate of structural abnormalities in the studied population is also consistent with a recent study reporting abnormal LGE findings in over 70% of SCD survivors.18 This work also clarifies the population of patients particularly benefiting from CMR: while it has obviously less diagnostic value in patients with a prior diagnosis of SHD, CMR seems in contrast particularly critical in patients with negative TTE and coronary angiography. Interestingly, we found higher rates of structural abnormalities than expected in patients with NSVT. In this population, the high prevalence of positive LGE in otherwise normal patients supports a broader use of CMR, given the therapeutic implications. These subtle structural abnormalities might explain why some patients with NSVT and apparently normal hearts show adverse long-term outcome.19 However, we acknowledge that given the high and underestimated prevalence of asymptomatic NSVT episodes,20 our study was certainly subjected to a substantial selection bias. Further studies should focus on clarifying which patients with NSVT should undergo CMR.
Non-ischaemic cardiomyopathy diagnoses on cardiac magnetic resonance
Although ICM, ARVC and HCM diagnostic categories correspond to rather homogeneous disease entities, NICM encompasses a broad range of disease processes, and was the most prevalent diagnostic category introduced by CMR. A minority [14/38 (37%)] of NICM patients showed a dilated cardiomyopathy (DCM) pattern associating LV dilatation and LVEF impairment. In this population, LGE patterns consisted mostly of midwall striae within the septum or midwall patchy enhancements at RV insertion points, which is consistent with past CMR series in DCM,21 and prior histological reports.22 However, the prevalence of LGE [11/14 (79%)] was higher than previously reported21 and might reflect the arrhythmogenicity of focal scarring in DCM. Of note, although DCM can be diagnosed on TTE or cine MR, LGE might have an incremental diagnostic role, septal LGE being potentially suggestive of laminopathy23 or sarcoid,24 and LGE within the LV free wall potentially indicating a chronic inflammatory process.25 But besides this usual DCM pattern, most of NICM patients showed normal LV volume and EF and were diagnosed as NICM based on the finding of LGE of non-ischaemic distribution. These LGE patterns, rather involving the LV free wall on midwall or subepicardial layers, could be related to various mechanisms (acute viral myocarditis, chronic myocarditis, sarcoid or other systemic diseases, etc.). Unfortunately, endomyocardial biopsies could not be systematically performed to further document the aetiology. We acknowledge that in the absence of histopathological correlation, one could question the pathological nature of these imaging features. Indeed, little is known about the prevalence of such LGE findings in the general population. Nonetheless, the distribution of this substrate most often matched the VT morphology and co-localized with electrophysiological abnormalities at contact mapping in all cases in which such procedures were performed, strongly supporting its implication in the arrhythmia mechanism.
Use of high-resolution late gadolinium-enhanced imaging
The value of a free-breathing LGE method to image the arrhythmogenic substrate at higher spatial resolution is in our opinion the most novel finding of this work. Respiratory-navigated LGE methods were initially developed to image the left atrium.5 A prior report has shown their ability to image focal scar on the RV. Our results show that high-resolution LGE markedly improves the diagnostic yield of CMR in VAs, particularly in patients with NSVT, or in those with otherwise negative diagnostic tests. These populations are of great interest, because the finding of focal scar has immediate impact on therapy, potentially justifying by itself the introduction of antiarrhythmic drugs or the limitation of physical exercise.3 As expected, the patients benefiting the most from high-resolution LGE have smaller scars. Thus, future research in magnetic resonance methods should aim at further improving the spatial resolution of LGE images (e.g. by achieving infra-millimetric resolution), because this might translate into even higher rates of structural abnormalities detected in patients with VAs. Again, little is known about the prevalence of such LGE findings in the general population, and as little about their aetiology: post-inflammatory when non-ischaemic pattern? post-embolic, microvascular disease or vasospasm when ischaemic pattern? In this study, electrophysiological correlations (from ECG and contact mapping) clearly indicate that these scars were responsible for the documented arrhythmias. Given that most magnetic resonance imaging vendors now have a free-breathing LGE solution available, our study supports the systematic integration of the method in patients undergoing CMR to detect VA substrates, particularly when no prior SHD is known and when conventional LGE is negative.
Impact of cardiac magnetic resonance diagnosis on therapy
Abnormal CMR findings in patients with no known SHD and moderate or absent symptoms can have a direct impact on therapy.3 In our study, this comprised the introduction of antiplatelet therapy and lipid-lowering agents in patients with ICM, ACE inhibitors in patients with early stage DCM, or anti-arrhythmic medication in patients with NSVT. In contrast, the decision to implant ICDs is obviously not driven by CMR findings as it is indicated within the frame of secondary prevention.3 However, some of these patients may benefit from catheter ablation, and the ability to use high-resolution LGE data to guide the procedure justifies in our centre its systematic integration in the CMR protocol, even when the SHD is already known and conventional LGE positive.6,7 Indeed, a detailed three-dimensional description of scar architecture might become useful to assist catheter ablation in patients coming back with multiple shocks.
Study limitations
The first limitation of this work is the absence of systematic endomyocardial biopsies to obtain diagnostic confirmation, particularly in patients with NICM. Endomyocardial biopsy is not part of the routine diagnostic workup of NICM in our centre. In addition, the ability of the method to retrieve relevant diagnostic information in NICM with normal LV volume and EF might be limited because CMR indicates that these substrates are focal and mostly located on the LV free wall. A second limitation of this study is its study design inappropriate to evaluate the impact of CMR on patient outcome. This aspect was beyond the scope of this work. Further studies should be designed to document a potential impact of CMR on the outcome. Another limitation is the delay between arrhythmia episodes and CMR studies, possibly missing transient abnormalities at the acute stage. A shorter delay was not feasible for practical reasons. Regarding CMR methods, we acknowledge that the addition of T1 mapping and T2 imaging might have been useful to document diffuse substrate or acute oedema. The former was not available during the whole course of the study, and the latter was not part of the study protocol since CMR studies were most of the time not performed at the acute phase. Last, for practical reasons, the correlation between imaging and electroanatomical mapping studies was only available in a limited number of patients. This aspect was not the main scope of this study, and more detailed data on electrophysiological correlates of imaging in larger populations can be found elsewhere.6,8
Conclusions
In patients with no prior history of SHD presenting with VT, VF, or SCD, the diagnostic yield of CMR is high, leading to a diagnostic change in over 40% of the patients as compared to the first-line diagnostic strategy based on TTE and coronary angiography. CMR is particularly useful in patients with negative diagnostic workup including TTE and coronary angiography, while it appears to have less diagnostic value in patients with a prior history of SHD. The addition of high-resolution LGE imaging to the CMR protocol significantly improves the performance of CMR in detecting smaller LV or RV substrates, particularly in patients with no SHD as per pre-CMR assessment. Results from CMR significantly impact patient management.
Funding
This work was supported by l’Agence Nationale de la Recherche [Grant Agreements ANR-10-IAHU-04 and ANR-11-EQPX-0030] and by the European Research Council [Grant Agreement ERC n°715093].
Conflict of interest: none declared.
References
- 1. Zipes DP, Wellens HJ.. Sudden cardiac death. Circulation 1998;98:2334–51. [DOI] [PubMed] [Google Scholar]
- 2. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2006;8:746–837. [DOI] [PubMed] [Google Scholar]
- 3. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2015;17:1601–87. [DOI] [PubMed] [Google Scholar]
- 4. White JA, Fine NM, Gula L, Yee R, Skanes A, Klein G. et al. Utility of cardiovascular magnetic resonance in identifying substrate for malignant ventricular arrhythmias. Circ Cardiovasc Imaging 2012;5:12–20. [DOI] [PubMed] [Google Scholar]
- 5. Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN. et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119:1758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cochet H, Komatsu Y, Sacher F, Jadidi AS, Scherr D, Riffaud M. et al. Integration of merged delayed-enhanced magnetic resonance imaging and multidetector computed tomography for the guidance of ventricular tachycardia ablation: a pilot study. J Cardiovasc Electrophysiol 2013;24:419–26. [DOI] [PubMed] [Google Scholar]
- 7. Berte B, Denis A, Amraoui S, Yamashita S, Komatsu Y, Pillois X. et al. Characterization of the left-sided substrate in arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 2015;8:1403–12. [DOI] [PubMed] [Google Scholar]
- 8. Yamashita S, Sacher F, Mahida S, Berte B, Lim HS, Komatsu Y. et al. Image integration to guide catheter ablation in scar-related ventricular tachycardia. J Cardiovasc Electrophysiol 2016;27:699–708. [DOI] [PubMed] [Google Scholar]
- 9. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA. et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J 2010;31:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P. et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–79. [DOI] [PubMed] [Google Scholar]
- 11. Kou S, Caballero L, Dulgheru R, Voilliot D, De Sousa C, Kacharava G. et al. Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging 2014;15:680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gopinathannair R, Etheridge SP, Marchlinski FE, Spinale FG, Lakkireddy D, Olshansky B.. Arrhythmia-induced cardiomyopathies: mechanisms, recognition, and management. J Am Coll Cardiol 2015;66:1714–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson 2013;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R. et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 2015;17:29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ.. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 2005;26:1461–74. [DOI] [PubMed] [Google Scholar]
- 16. Miller JM, Jain R, Dandamudi G, Kambur TR.. Electrocardiographic localization of ventricular tachycardia in patients with structural heart disease. Card Electrophysiol Clin 2017;9:1–10. [DOI] [PubMed] [Google Scholar]
- 17. Rehwald WG, Fieno DS, Chen EL, Kim RJ, Judd RM.. Myocardial magnetic resonance imaging contrast agent concentrations after reversible and irreversible ischemic injury. Circulation 2002;105:224–9. [DOI] [PubMed] [Google Scholar]
- 18. Neilan TG, Farhad H, Mayrhofer T, Shah RV, Dodson JA, Abbasi SA. et al. Late gadolinium enhancement among survivors of sudden cardiac arrest. JACC Cardiovasc Imaging 2015;8:414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin CY, Chang SL, Chung FP, Chen YY, Lin YJ, Lo LW. et al. Long-term outcome of non-sustained ventricular tachycardia in structurally normal hearts. PLoS One 2016;11:e0160181.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Solomon MD, Yang J, Sung SH, Livingston ML, Sarlas G, Lenane JC. et al. Incidence and timing of potentially high-risk arrhythmias detected through long term continuous ambulatory electrocardiographic monitoring. BMC Cardiovasc Disord 2016;16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCrohon JA, Moon JC, Prasad SK.. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation 2003;108:54–9. [DOI] [PubMed] [Google Scholar]
- 22. Roberts WC, Siegel RJ, McManus BM.. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am J Cardiol 1987;60:1340–55. [DOI] [PubMed] [Google Scholar]
- 23. Holmström M, Kivistö S, Heliö T, Jurkko R, Kaartinen M, Antila M. et al. Late gadolinium enhanced cardiovascular magnetic resonance of lamin A/C gene mutation related dilated cardiomyopathy. J Cardiovasc Magn Reson 2011;13:30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA. et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009;120:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Cobelli F, Pieroni M, Esposito A, Chimenti C, Belloni E, Mellone R. et al. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol 2006;47:1649–54. [DOI] [PubMed] [Google Scholar]