Abstract
The numerous post-transcriptional modifications of tRNA play a crucial role in tRNA function. While most modifications are introduced to tRNA independently, several sets of modifications are found to be interconnected such that the presence of one set of modifications drives the formation of another modification. The vast majority of these modification circuits are found in the anticodon loop (ACL) region where the largest variety and highest density of modifications occur compared to the other parts of the tRNA and where there is relatively limited sequence and structural information. We speculate here that the modification circuits in the ACL region arise to enhance enzyme modification specificity by direct or indirect use of the first modification in the circuit as an additional recognition element for the second modification. We also describe the five well-studied modification circuits in the ACL, and outline possible mechanisms by which they may act. The prevalence of these modification circuits in the ACL and the phylogenetic conservation of some of them suggest that a number of other modification circuits will be found in this region in different organisms.
Keywords: 3-methylcytidine, wybutosine, 5-methylcytidine, 2′-O-methylguanosine
tRNAs are heavily post-transcriptionally modified, and are modified at different stages of tRNA biogenesis (Nishikura and De Robertis 1981; Jiang et al. 1997; Phizicky and Hopper 2010; Ohira and Suzuki 2011), and these modifications are important for tRNA function in translation. While most modifications are introduced to tRNA independently, several modification circuits have been identified in which one or more modifications stimulates formation of a subsequent modification. All of the well-studied examples of this ordered modification occur in the anticodon loop (ACL) region of the tRNA, but it is not known why ordered modification occurs, or why it is seemingly more prevalent in the ACL region. Here we propose that this propensity for ordered modification evolved in the ACL region because of the requirement for specificity of these modifications, combined with the relative lack of distinctive information in the ACL region.
Of the numerous tRNA modifications found in different organisms, the largest variety of modifications and the highest modification density occurs in the ACL region. For example, of the 25 chemically distinct modifications found in cytoplasmic tRNAs in the yeast Saccharomyces cerevisiae, 15 are found in the 9 nucleotides (nt) of the ACL region, comprising loop residues N32–N38 and the closing base pair N31–N39, while 16 are found in the remaining 67 or more nucleotides in the main body of the tRNA, comprising the acceptor stem, D-stem–loop, the bulk of the anticodon stem (from pairs N27–N43 to N30–N40), the T-stem–loop, and the variable arm (Fig. 1A,B). Six of these modifications are found in both regions. A similar biased distribution of tRNA modifications is widely found in other organisms: Of the 28 distinct tRNA modifications in Escherichia coli, 21 are found in the ACL region, while eight are found in the main body; and of the 28 distinct modifications in cytoplasmic tRNAs in humans, 17 are in the ACL region and 17 are in the main body (Machnicka et al. 2014). Overall modification density is also heavily biased toward the ACL region in all organisms. Thus, among sequenced eukaryotic cytoplasmic tRNAs, on average 30% of the ACL region residues are modified, while only 14.8% of the residues in the main body are modified (29.8% and 14.7% in yeast), and these percentages are similarly skewed in bacteria (15.0% in the ACL region and 5.7% in the main body) and in archaea (12.8% and 9.6%, respectively) (Machnicka et al. 2014).
FIGURE 1.
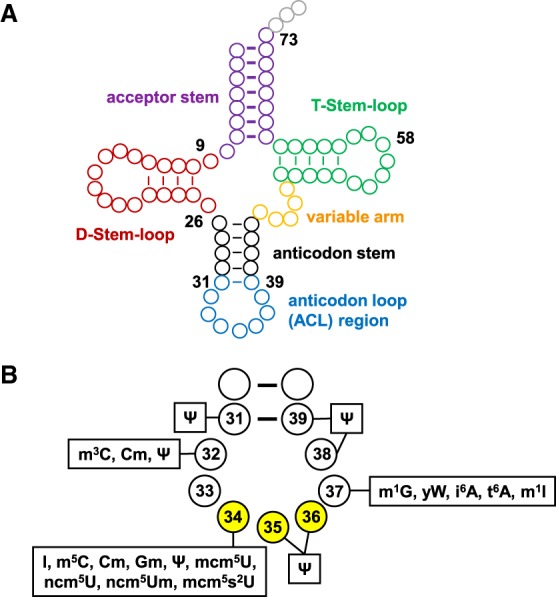
Schematic of tRNA and the biochemically distinct modifications found in the S. cerevisiae ACL region. (A) The secondary structure of tRNA. Each circle represents a residue and is color-coded based on subdomains as indicated. (B) Schematic of modifications found in each residue of the S. cerevisiae ACL region.
The enrichment of the variety and density of modifications in the ACL region is consistent with their important and varied roles during translation (Phizicky and Hopper 2010; Gu et al. 2014; Grosjean and Westhof 2016). A number of ACL region modifications affect mRNA decoding or reading frame maintenance, by modulating codon:anticodon interactions and fine-tuning local structure during translation (Björk et al. 1989, 2007; Urbonavicius et al. 2001; Lecointe et al. 2002; Murphy and Ramakrishnan 2004; Waas et al. 2007; Weixlbaumer et al. 2007; Johansson et al. 2008; El Yacoubi et al. 2011; Maehigashi et al. 2014; Lorenz et al. 2017). Specifically, to ensure the efficiency and accuracy of translation, all tRNAs adopt a canonical U-turn structure in the ACL to promote a stable codon–anticodon interaction in the ribosome A-site (Auffinger and Westhof 2001), and many ACL region modifications reinforce formation of this conserved loop structure by preventing base-pairing between nucleotides in the ACL, and by improving stacking interactions with neighboring residues (Murphy et al. 2004; Agris 2008). In addition, some ACL region modifications have a crucial role in ensuring charging fidelity by serving as charging determinants or anti-determinants (Muramatsu et al. 1988; Pütz et al. 1994). The importance of ACL modifications relative to body modifications is underscored by their preferential phylogenetic retention. For example, in the human unicellular endosymbiont Candidatus Riesia pediculicola, which has a streamlined genome and likely a minimal tRNA modification set, the ACL modifications have been retained, whereas the body modifications have been lost (de Crécy-Lagard et al. 2012).
Many modification enzymes target a single base at specific positions
Most tRNA modification enzymes that modify body residues in the tRNA target a specific base, albeit by different mechanisms. For example, the yeast tRNA pseudouridine (Ψ) synthase Pus4 modifies U55 by recognizing the identity of all the universally conserved nucleotides in the T-loop and its proximal stem as well as the structure of a portion of the T-arm (Becker et al. 1997), and consistently modifies mRNAs with a similar stem–loop motif (Lovejoy et al. 2014). Similarly, the yeast tRNA 5-methyluridine (m5U) methyltransferase modifies U54 by recognizing several conserved nucleotides in the T stem–loop as well as the stacked T-stem and acceptor stem (Becker et al. 1997); the yeast 7-methylguanosine (m7G) tRNA methyltransferase Trm8/Trm82 recognizes the local structure around the variable loop and especially the D arm and T arm to modify residue G46 in the third residue of variable loops of 5 nt (Leulliot et al. 2008), similar to the Aquifex aeolicus m7G MTase (Okamoto et al. 2004); and the E. coli U20 dihydrouridine synthase DusC interacts with the D and T stem–loops to orient tRNA and U20 near its catalytic site (Byrne et al. 2015). However, for some body modifications, the specificity of the enzymes is less clear. For example, the yeast 1-methylguanosine (m1G) methyltransferase Trm10 modifies 13 of 19 tRNA species that have a G9 residue, with no obvious common sequence element, although Trm10 substrate recognition seems to depend on an as yet undefined structural conformation, since tRNAs with an extended variable loop are consistently unmodified (Swinehart et al. 2013). Similar arguments may explain the specificity of Trm3 for 2′-O-methylation of G18 on a subset of yeast tRNAs (Cavaillé et al. 1999), and of archaeosine tRNA-guanine transglycosylase, which modifies G15 on a subset of tRNAs through an alternative λ-form tRNA conformation (Ishitani et al. 2003).
In the ACL region, some modification enzymes also only require the presence of the correct nucleotide at the residue to be modified. One such example is yeast Tad2/3, which in the context of tRNA catalyzes inosine (I) formation in all eight sequenced tRNA with A34 (Gerber and Keller 1999). Similarly, E. coli TruA recognizes and pseudouridylates U38, U39, and U40 of the anticodon stem–loop, using the elbow and the D-stem of the tRNA to establish the orientation of tRNA and position the modification sites near the catalytic center (Hur and Stroud 2007), and its family member yeast Pus3 appears to modify all tRNAs with U38 or U39 (Machnicka et al. 2014). Slightly different recognition mechanisms involving relatively simple rules are used by some other ACL region enzymes. For example, enzymes responsible for t6A37 (N6-threonylcarbamoyl adenosine) specifically recognize tRNAs with U36 residues in all domains of life (Deutsch et al. 2012; Miyauchi et al. 2013). Another possible example in this category is the tRNA i6A37 (N6-isopentenyladenosine) synthase, which requires A36–A37–A38 and likely some other secondary elements (Persson et al. 1994; Motorin et al. 1997).
Consistent with their lack of discrimination among different tRNA species, many modifications are usually made independently of other modifications, since deletion of one modifying enzyme often does not alter levels of other modifications in the cell (Huang et al. 2005; Wilkinson et al. 2007; Kotelawala et al. 2008; Guy et al. 2012; Han et al. 2015).
Some modification enzymes require prior modifications at other residues for efficient modification
In contrast to tRNA body residues, some residues in the ACL differ greatly in their modifications in different tRNAs. Thus, U34 has five different fates in different yeast tRNAs: It can be unmodified, or it is modified to one of four different derivatives (Fig. 1B). Similarly, C32 is unmodified or modified to either of two different derivatives in different tRNAs; A37 is unmodified or modified to three different derivatives; and G37 is modified to either of two derivatives.
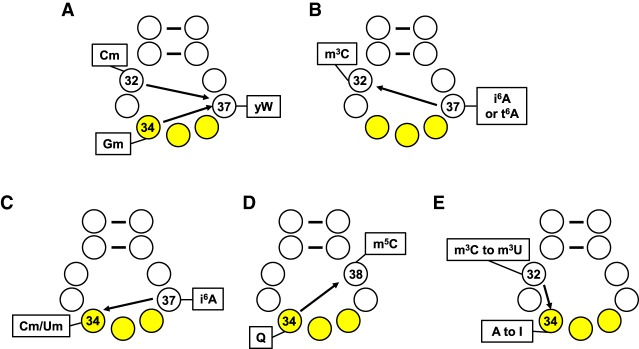
This variability of modification of different ACL residues in different tRNAs requires unique mechanisms of recognition. As we document further below, a subset of these mechanisms involves prior modification of other residues (Fig. 2). Recent examples of such modification circuits include wybutosine (yW) formation at m1G37 of tRNAPhe in Schizosaccharomyces pombe, S. cerevisiae and humans, which is greatly stimulated by 2′-O-methylated C32 (Cm) and G34 (Gm) (Guy and Phizicky 2015; Guy et al. 2012, 2015); 3-methycytidine (m3C) at C32, which is greatly stimulated by prior i6A37 formation in S. pombe, S. cerevisiae, and likely mouse (Arimbasseri et al. 2016; Han et al. 2017), or by prior t6A37 (Han et al. 2017); Cm34 or Um34 modification of E.coli tRNALeu(CAA) and tRNALeu(UAA), which is stimulated by i6A37; 5-methycytidine (m5C) at C38 in S. pombe and Dictyostelium discoideum, which is stimulated by prior queuosine (Q) formation at residue 34 (Muller et al. 2015); and A to I editing at N34 of Trypanosoma brucei tRNAThr(AGU), which is stimulated by prior m3C32 formation and then deamination to form m3U (Rubio et al. 2006, 2017). Note that some other complex modifications require a multistep reaction, and the dependence of a latter step reaction on the prior modification intermediates at the same residue (Grosjean et al. 1995; Morl et al. 1995) is not considered as a modification circuit by this review, and will not be further discussed.
FIGURE 2.
Schematic of the five anticodon loop circuits discussed in this review. (A) Cm32 and Gm34 drive yW37 formation in tRNAPhe of S. pombe, S. cerevisiae, and humans. (B) i6A37 drives m3C32 formation in tRNASer of S. pombe and S. cerevisiae, and t6A37 drives m3C32 formation in tRNAThr of S. cerevisiae. (C) i6A37 drives Cm34 and Um34 formation in tRNALeu(CAA) and tRNALeu(UAA) of E. coli. (D) Q34 drives m5C38 formation in tRNAAsp of S. pombe and D. discoideum. (E) C32 to m3C32 to m3U32 drives I34 formation in tRNAThr(AGU) of T. brucei.
Although a few modification circuits are reported to occur in the tRNA main body, all of them are found in Thermus thermophilus, an extreme thermophilic eubacteria of which tRNAs are adapted to high growth temperatures (Yokoyama et al. 1987). Examples include formation of 2-thioribothymidine (s2T) from ribothymidine (rT) at U54, which is stimulated by 1-methyladenosine (m1A) at A58 (Shigi et al. 2006); Gm18 and m1G37, which are stimulated by m7G46 at higher culture temperatures (Tomikawa et al. 2010); and 5-methyl-2-thiouridine (m5s2U) at U54 and m1A58, which are negatively regulated by Ψ55 at lower culture temperatures (Ishida et al. 2011). The latter two circuits are thought to be part of a network that maintains the proper balance of tRNA modifications and responds to temperature changes (Ishida et al. 2011).
A hypothesis to explain the prevalence of ACL region modification circuits
It is intriguing to consider why these modification circuits tend to occur in the ACL region, but not in other parts of a tRNA. One possible explanation is that ACL region modifications introduced first may act as additional recognition elements for other modifications in response to the requirement for modifications with great variation and high density in this region, combined with the lack of variability in the local sequence and structure. Indeed, the ACL sequence outside the anticodon itself has limited variation: N33 is almost always a uridine in elongator tRNAs; N37 is almost always a purine; and the vast majority of N32–N38 combinations are C-A, U-A, or U-U (Auffinger and Westhof 1999; Marck and Grosjean 2002). Of the 2726 tDNA gene sequences in the tRNA database, 48% of N32–N38 pairs are C-A, 18% are U-A, and 11% are U-U (Auffinger and Westhof 1999; Marck and Grosjean 2002); while in yeast 52% of N32–N38 pairs are C-A, 19% are U-A, and 19% are U-C (Fig. 3; Jühling et al. 2009). Thus, the majority of sequence variation in the ACL region comes from the anticodon sequence, which is a unique signature for each tRNA species and is often important for tRNA synthetase recognition (Kisselev 1985; Giegé et al. 1998). Moreover, as addressed above, the universal conservation of anticodon stem–loop structure is selected by the translation machinery, so structural information in this region is unlikely to be useful for individual enzyme specificity. The conserved sequence and structural similarities in the ACL region provide little room for substrate recognition of ACL modifying enzymes, if their specificities solely come from sequence elements around the modification site. Therefore, it is reasonable to speculate that evolution may have selected for modification circuits in which modifications introduced first positively or negatively regulate formation of other nearby modifications, adding another layer of complexity in the ACL region to enhance enzyme modification specificity.
FIGURE 3.
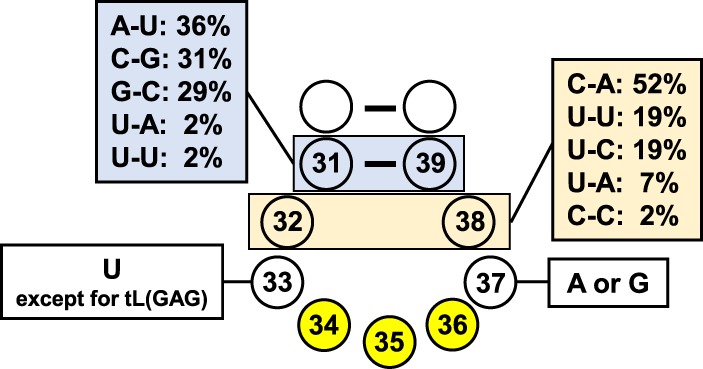
Schematic of the S. cerevisiae ACL landscape.
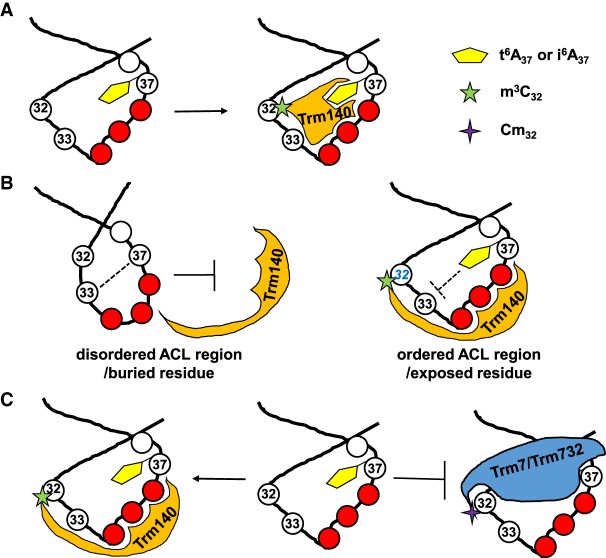
Three mechanisms might explain the observed ordered modification circuits in ACLs (Fig. 4). First, the initial modification might directly act as a recognition element for the subsequent modification enzyme, much like bromodomains found in chromatin-associated proteins and nuclear acetyltransferases, which bind acetyl-lysine (Zeng and Zhou 2002). A classical example of this mechanism of ordered modification is queuosine formation at residue 34 in marsupial mitochondrial tRNAAsp. This modification requires prior deamination of C35 of the encoded GCC anticodon to form a GUC anticodon, generating the U33G34U35 recognition sequence for tRNA guanine transglycosylase to catalyze queuosine formation (Morl et al. 1995; Börner et al. 1996; Xie et al. 2003). Second, the initial modification might alter the structure of the ACL to present a structure that is itself recognized or properly exposes the target residue for the subsequent modification enzyme. Third, an initial modification might prevent a particular subsequent modification from occurring, allowing a different modification enzyme to act in its stead. Although the precise mechanism by which any of the recently established ordered modification circuits is not yet known, we describe below what is known in each case.
FIGURE 4.
Three possible mechanisms to explain the ordered modification circuits in the ACL region, using the i6A37/t6A37-m3C32 circuit as an example. (A) The initial modification (i6A37 or t6A37) directly acts as a recognition element for the subsequent modification enzyme (Trm140 for m3C32 modification). (B) The initial modification (i6A37 or t6A37) alters the structure of the ACL to present a structure that is itself recognized or properly exposes the target residue (C32) for the subsequent modification enzyme (Trm140). (C) The initial modification (i6A37 or t6A37) prevents a subsequent modification from occurring (Cm32 by Trm7/732), allowing a different modification enzyme (Trm140) to act instead.
The yW37 modification, or derivatives of it, is almost universally found on tRNAPhe in eukaryotes, and not on any other tRNA (Machnicka et al. 2013), and it is known that the GAA anticodon sequence is necessary for yW formation in oocytes (Droogmans and Grosjean 1987). yW formation is the last step of tRNAPhe maturation, and the critical 2′-O-methylation of C32 and N34 required for efficient yW formation (Guy et al. 2012) is part of an intricate tRNAPhe maturation pathway in S. cerevisiae (Ohira and Suzuki 2011). These steps include initial export of unspliced pre-tRNA from the nucleus to the cytoplasm (Sarkar and Hopper 1998), tRNA splicing on the mitochondrial surface (Yoshihisa et al. 2007), 2′-O-methylation of C32 and N34 by Trm7/Trm732 and Trm7/Trm734 (Guy et al. 2012), retrograde transport of the tRNA back to the nucleus (Takano et al. 2005; Murthi et al. 2010), formation of m1G37, reexport of the tRNAPhe to the cytoplasm, and then further modification of m1G37 by Tyw1, Tyw2, Tyw3, and Tyw4 to form yW (Noma et al. 2006; Ohira and Suzuki 2011). There is an apo structure of Tyw1, which catalyzes the first step of yW from m1G37, from Methanococcus jannaschii and Pyrococcus horikoshii (Goto-Ito et al. 2007; Suzuki et al. 2007); however, little insight is provided for the specificity of the enzyme.
The dependence of yW37 formation on prior Cm32 and Gm34 modification in S. cerevisiae, S. pombe, and humans (Guy and Phizicky 2015; Guy et al. 2012, 2015) could be explained by one of the first two mechanisms mentioned above: direct Tyw1 recognition of Cm32 and Gm34; and Tyw1 recognition of the prestructured ACL region possibly including increased Tyw1 access to m1G37. Both models would be consistent with the partial modification of yW observed in trm734Δ mutants, which have the Cm32 modification but not Gm34 and trm732Δ mutants, which have Gm34, but not Cm32 (Guy and Phizicky 2015; Guy et al. 2012, 2015).
The m3C32 modification is found in almost all sequenced tRNASer and tRNAThr species with C32, as well as in mammalian tRNAArg(CCU) and tRNAArg(UCU), but not in other tRNA species. In S. cerevisiae, a single Trm140 homolog catalyzes m3C32 formation in all six of its tRNAThr and tRNASer substrates (D'Silva et al. 2011; Noma et al. 2011), whereas in S. pombe there are two paralogs, one responsible for tRNASer modification and the other for tRNAThr modification (Arimbasseri et al. 2016). S. cerevisiae Trm140 has two recognition modes for its substrates, and each involves t6A and/or i6A: Trm140 recognizes the sequence element G35–U36–t6A37 of tRNAThr substrates, and this sequence element is necessary and sufficient for m3C modification of another tRNA species (Han et al. 2017). In contrast, Trm140 recognizes tRNASer species through interaction with seryl-tRNA synthetase and the distinctive tRNASer variable loop recognized by SerRS (Himeno et al. 1997), as well as by either t6A37 or i6A37 (Han et al. 2017). In both sets of substrates, A37 modifications (either i6A or t6A) are stimulatory, but not absolutely necessary for m3C32 formation, and available data suggests that each element of tRNAThr and tRNASer recognition contributes independently to Trm140 recognition, including t6A and i6A (Han et al. 2017). A similar result was previously observed in S. pombe in which i6A37 stimulates m3C32 formation for all three tRNASer species with the modification (Arimbasseri et al. 2016).
The role of t6A37 or i6A37 in stimulating m3C32 modification by Trm140 family members is unclear, but it seems unlikely that S. cerevisiae Trm140 directly recognizes both modifications, since they are chemically very distinct: The isopentenyl group of i6A is much more hydrophobic than the acidic and polar threonyl group found in t6A. A more plausible explanation is that both i6A and t6A facilitate formation of the proper structure of the tRNA ACL, allowing for Trm140 recognition and m3C32 modification. Indeed, t6A37 has been shown to have a role in preordering the ACL by preventing base-pairing between U33 and A37, and by enhancing stacking interactions between A37 and A38 (Murphy et al. 2004). It is also possible that the bulky t6A and i6A modifications act as a negative recognition element for Trm7/Trm732, to prevent Cm32 modification in S. cerevisiae (Guy et al. 2012), although its recognition elements are not yet known.
Similarly, any of the three mechanisms might also be used to explain the dependence of Cm34 and Um34 on prior i6A37 modification in E.coli. The E. coli N34 2′-O-methytransferase TrmL catalyzes this methyl transfer reaction on its two substrates, tRNALeu(CAA) and tRNALeu(UAA) (Liu et al. 2013). The presence of i6A37, which is catalyzed by MiaA (Soderberg and Poulter 2001), strongly stimulates formation of Cm34 and Um34, since in vitro transcribed tRNALeu(CAA) and tRNALeu(UAA) without modifications are not substrates of TrmL, while the same tRNA transcripts are efficient TrmL substrates if they are premodified by recombinant MiaA (Zhou et al. 2015). In the same study, the sequence A36–A37–A38 was shown to be important for Cm34 and Um34 formation; however, whether this sequence element is necessary for recognition by TrmL itself or for the i6A37 modification is unclear, since the A36–A37–A38 motif is the known determinant for MiaA (Soderberg and Poulter 2001).
For m5C38 modification of tRNAAsp in S. pombe, the stimulatory role of Q34 has been demonstrated both in vivo and in vitro with purified Pmt1 methyltransferase (Muller et al. 2015). This experiment suggests either that Pmt1 directly interacts with Q34, or that Q34 appropriately affects the ACL structure of tRNAAsp. The crystal structures of several Dnmt2 homologs have been solved, but unfortunately without the tRNA substrate (Dong et al. 2001; Schulz et al. 2012; Li et al. 2013). Based on a modeled tRNAAsp–Dnmt2 structure, it is also possible that Q34 could alter the geometry of the ACL, allowing for better interactions between Dnmt2 and tRNA. Nonetheless, Q34 cannot be the sole determinant, since the tRNA guanine transglycosylase (TGT) that exchanges G with Q in tRNAs acts on all tRNAs with a GUN anticodon (Katze et al. 1982), whereas m5C38 is specific for tRNAAsp.
Deamination of A34 to inosine in tRNAThr(AGU) in T. brucei is stimulated by C32 deamination to uridine, based on the observation that in vitro transcribed tRNAThr(AGU) with U32 is edited to I34 with higher efficiency and initial rate than transcripts with C32 (Rubio et al. 2006). Remarkably, recent results show that the initial C to U editing step is preceded by m3C modification by a complex of the m3C methyltransferase Trm140 and the deaminase ADAT2/3, which catalyzes both reactions (Rubio et al. 2017; McKenney et al. 2018). While formation of m3C32 followed by formation of m3U32 occurs in the nucleus prior to 5′ leader removal and export of tRNA into cytoplasm, A to I editing at the wobble residue occurs in the cytoplasm (Gaston et al. 2007). As in the other cases, it is unclear how m3U32 stimulates the subsequent A34 deamination.
In summary, we have documented a large number of modifications in the ACL region that depend on prior modifications in the tRNA, and have proposed that the modification circuits may have evolved so that the second modification in the circuit can use additional recognition sites directly or indirectly from the first modification to achieve specificity. This helps resolve the dilemma in the ACL region of the need for different modifications at the same residue, combined with the lack of sufficient sequence variation or structural information to obtain the desired specificity. In principle, the anticodon sequence or set of anticodon sequences can provide some of the required specificity, but not always, such as in the case of m3C modification, which acts on tRNAs with very different anticodons. In all of these documented cases, lack of the first modifications in nonsubstrate tRNAs would also prevent the second modifications in these tRNAs, thereby improving overall specificity of modifications in the entire tRNA population. This set of five modification circuits within the ACL, and the phylogenetic conservation of two of them, suggest the existence of other modification circuits in the ACLs of different tRNA species or organisms, driven in part by the need for specific substrate recognition.
ACKNOWLEDGMENTS
We thank Elizabeth Grayhack for valuable comments on the manuscript. This work was supported by National Institute of General Medical Sciences, National Institutes of Health grant GM052347 to E.M.P.
Footnotes
Article is online at http://www.rnajournal.org/cgi/doi/10.1261/rna.067736.118.
REFERENCES
- Agris PF. 2008. Bringing order to translation: the contributions of transfer RNA anticodon-domain modifications. EMBO Rep 9: 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimbasseri AG, Iben J, Wei FY, Rijal K, Tomizawa K, Hafner M, Maraia RJ. 2016. Evolving specificity of tRNA 3-methyl-cytidine-32 (m3C32) modification: a subset of tRNAsSer requires N6-isopentenylation of A37. RNA 22: 1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffinger P, Westhof E. 1999. Singly and bifurcated hydrogen-bonded base-pairs in tRNA anticodon hairpins and ribozymes. J Mol Biol 292: 467–483. [DOI] [PubMed] [Google Scholar]
- Auffinger P, Westhof E. 2001. An extended structural signature for the tRNA anticodon loop. RNA 7: 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HF, Motorin Y, Sissler M, Florentz C, Grosjean H. 1997. Major identity determinants for enzymatic formation of ribothymidine and pseudouridine in the TΨ-loop of yeast tRNAs. J Mol Biol 274: 505–518. [DOI] [PubMed] [Google Scholar]
- Björk GR, Wikström PM, Byström AS. 1989. Prevention of translational frameshifting by the modified nucleoside 1-methylguanosine. Science 244: 986–989. [DOI] [PubMed] [Google Scholar]
- Björk GR, Huang B, Persson OP, Byström AS. 2007. A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA 13: 1245–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börner GV, Mörl M, Janke A, Pääbo S. 1996. RNA editing changes the identity of a mitochondrial tRNA in marsupials. EMBO J 15: 5949–5957. [PMC free article] [PubMed] [Google Scholar]
- Byrne RT, Jenkins HT, Peters DT, Whelan F, Stowell J, Aziz N, Kasatsky P, Rodnina MV, Koonin EV, Konevega AL, et al. 2015. Major reorientation of tRNA substrates defines specificity of dihydrouridine synthases. Proc Natl Acad Sci 112: 6033–6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillé J, Chetouani F, Bachellerie JP. 1999. The yeast Saccharomyces cerevisiae YDL112w ORF encodes the putative 2′-O-ribose methyltransferase catalyzing the formation of Gm18 in tRNAs. RNA 5: 66–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crécy-Lagard V, Marck C, Grosjean H. 2012. Decoding in Candidatus Riesia pediculicola, close to a minimal tRNA modification set? Trends Cell Mol Biol 7: 11–34. [PMC free article] [PubMed] [Google Scholar]
- Deutsch C, El Yacoubi B, de Crécy-Lagard V, Iwata-Reuyl D. 2012. Biosynthesis of threonylcarbamoyl adenosine (t6A), a universal tRNA nucleoside. J Biol Chem 287: 13666–13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong A, Yoder JA, Zhang X, Zhou L, Bestor TH, Cheng X. 2001. Structure of human DNMT2, an enigmatic DNA methyltransferase homolog that displays denaturant-resistant binding to DNA. Nucleic Acids Res 29: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans L, Grosjean H. 1987. Enzymatic conversion of guanosine 3′ adjacent to the anticodon of yeast tRNAPhe to N1-methylguanosine and the wye nucleoside: dependence on the anticodon sequence. EMBO J 6: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Silva S, Haider SJ, Phizicky EM. 2011. A domain of the actin binding protein Abp140 is the yeast methyltransferase responsible for 3-methylcytidine modification in the tRNA anti-codon loop. RNA 17: 1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi B, Hatin I, Deutsch C, Kahveci T, Rousset JP, Iwata-Reuyl D, Murzin AG, de Crécy-Lagard V. 2011. A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J 30: 882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston KW, Rubio MA, Spears JL, Pastar I, Papavasiliou FN, Alfonzo JD. 2007. C to U editing at position 32 of the anticodon loop precedes tRNA 5′ leader removal in trypanosomatids. Nucleic Acids Res 35: 6740–6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber AP, Keller W. 1999. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science 286: 1146–1149. [DOI] [PubMed] [Google Scholar]
- Giegé R, Sissler M, Florentz C. 1998. Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res 26: 5017–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto-Ito S, Ishii R, Ito T, Shibata R, Fusatomi E, Sekine SI, Bessho Y, Yokoyama S. 2007. Structure of an archaeal TYW1, the enzyme catalyzing the second step of wye-base biosynthesis. Acta Crystallogr D Biol Crystallogr 63: 1059–1068. [DOI] [PubMed] [Google Scholar]
- Grosjean H, Westhof E. 2016. An integrated, structure- and energy-based view of the genetic code. Nucleic Acids Res 44: 8020–8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H, Constantinesco F, Foiret D, Benachenhou N. 1995. A novel enzymatic pathway leading to 1-methylinosine modification in Haloferax volcanii tRNA. Nucleic Acids Res 23: 4312–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Begley TJ, Dedon PC. 2014. tRNA modifications regulate translation during cellular stress. FEBS Lett 588: 4287–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy MP, Phizicky EM. 2015. Conservation of an intricate circuit for crucial modifications of the tRNAPhe anticodon loop in eukaryotes. RNA 21: 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy MP, Podyma BM, Preston MA, Shaheen HH, Krivos KL, Limbach PA, Hopper AK, Phizicky EM. 2012. Yeast Trm7 interacts with distinct proteins for critical modifications of the tRNAPhe anticodon loop. RNA 18: 1921–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy MP, Shaw M, Weiner CL, Hobson L, Stark Z, Rose K, Kalscheuer VM, Gecz J, Phizicky EM. 2015. Defects in tRNA anticodon loop 2′-O-methylation are implicated in nonsyndromic X-linked intellectual disability due to mutations in FTSJ1. Hum Mutat 36: 1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Kon Y, Phizicky EM. 2015. Functional importance of Ψ38 and Ψ39 in distinct tRNAs, amplified for tRNAGln(UUG) by unexpected temperature sensitivity of the s2U modification in yeast. RNA 21: 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Marcus E, D'Silva S, Phizicky EM. 2017. S. cerevisiae Trm140 has two recognition modes for 3-methylcytidine modification of the anticodon loop of tRNA substrates. RNA 23: 406–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeno H, Yoshida S, Soma A, Nishikawa K. 1997. Only one nucleotide insertion to the long variable arm confers an efficient serine acceptor activity upon Saccharomyces cerevisiae tRNALeu in vitro. J Mol Biol 268: 704–711. [DOI] [PubMed] [Google Scholar]
- Huang B, Johansson MJ, Byström AS. 2005. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA 11: 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur S, Stroud RM. 2007. How U38, 39, and 40 of many tRNAs become the targets for pseudouridylation by TruA. Mol Cell 26: 189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K, Kunibayashi T, Tomikawa C, Ochi A, Kanai T, Hirata A, Iwashita C, Hori H. 2011. Pseudouridine at position 55 in tRNA controls the contents of other modified nucleotides for low-temperature adaptation in the extreme-thermophilic eubacterium Thermus thermophilus. Nucleic Acids Res 39: 2304–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani R, Nureki O, Nameki N, Okada N, Nishimura S, Yokoyama S. 2003. Alternative tertiary structure of tRNA for recognition by a posttranscriptional modification enzyme. Cell 113: 383–394. [DOI] [PubMed] [Google Scholar]
- Jiang HQ, Motorin Y, Jin YX, Grosjean H. 1997. Pleiotropic effects of intron removal on base modification pattern of yeast tRNAPhe: an in vitro study. Nucleic Acids Res 25: 2694–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MJ, Esberg A, Huang B, Björk GR, Byström AS. 2008. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol Cell Biol 28: 3301–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jühling F, Mörl M, Hartmann RK, Sprinzl M, Stadler PF, Pütz J. 2009. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res 37: D159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze JR, Basile B, McCloskey JA. 1982. Queuine, a modified base incorporated posttranscriptionally into eukaryotic transfer RNA: wide distribution in nature. Science 216: 55–56. [DOI] [PubMed] [Google Scholar]
- Kisselev LL. 1985. The role of the anticodon in recognition of tRNA by aminoacyl-tRNA synthetases. Prog Nucleic Acid Res Mol Biol 32: 237–266. [DOI] [PubMed] [Google Scholar]
- Kotelawala L, Grayhack EJ, Phizicky EM. 2008. Identification of yeast tRNA Um44 2′-O-methyltransferase (Trm44) and demonstration of a Trm44 role in sustaining levels of specific tRNASer species. RNA 14: 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecointe F, Namy O, Hatin I, Simos G, Rousset JP, Grosjean H. 2002. Lack of pseudouridine 38/39 in the anticodon arm of yeast cytoplasmic tRNA decreases in vivo recoding efficiency. J Biol Chem 277: 30445–30453. [DOI] [PubMed] [Google Scholar]
- Leulliot N, Chaillet M, Durand D, Ulryck N, Blondeau K, van Tilbeurgh H. 2008. Structure of the yeast tRNA m7G methylation complex. Structure 16: 52–61. [DOI] [PubMed] [Google Scholar]
- Li S, Du J, Yang H, Yin J, Ding J, Zhong J. 2013. Functional and structural characterization of DNMT2 from Spodoptera frugiperda. J Mol Cell Biol 5: 64–66. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Zhou M, Fang ZP, Wang M, Zhou XL, Wang ED. 2013. The tRNA recognition mechanism of the minimalist SPOUT methyltransferase, TrmL. Nucleic Acids Res 41: 7828–7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz C, Lünse CE, Mörl M. 2017. tRNA modifications: impact on structure and thermal adaptation. Biomolecules 7: E35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy AF, Riordan DP, Brown PO. 2014. Transcriptome-wide mapping of pseudouridines: pseudouridine synthases modify specific mRNAs in S. cerevisiae. PLoS One 9: e110799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, et al. 2013. MODOMICS: a database of RNA modification pathways—2013 update. Nucleic Acids Res 41: D262–D267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnicka MA, Olchowik A, Grosjean H, Bujnicki JM. 2014. Distribution and frequencies of post-transcriptional modifications in tRNAs. RNA Biol 11: 1619–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehigashi T, Dunkle JA, Miles SJ, Dunham CM. 2014. Structural insights into +1 frameshifting promoted by expanded or modification-deficient anticodon stem loops. Proc Natl Acad Sci 111: 12740–12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck C, Grosjean H. 2002. tRNomics: analysis of tRNA genes from 50 genomes of Eukarya, Archaea, and Bacteria reveals anticodon-sparing strategies and domain-specific features. RNA 8: 1189–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney KM, Rubio MAT, Alfonzo JD. 2018. Binding synergy as an essential step for tRNA editing and modification enzyme codependence in Trypanosoma brucei. RNA 24: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi K, Kimura S, Suzuki T. 2013. A cyclic form of N6-threonylcarbamoyladenosine as a widely distributed tRNA hypermodification. Nat Chem Biol 9: 105–111. [DOI] [PubMed] [Google Scholar]
- Morl M, Dorner M, Paabo S. 1995. C to U editing and modifications during the maturation of the mitochondrial tRNAAsp in marsupials. Nucleic Acids Res 23: 3380–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motorin Y, Bec G, Tewari R, Grosjean H. 1997. Transfer RNA recognition by the Escherichia coli isopentenyl-pyrophosphate:tRNA-isopentenyl transferase: dependence on the anticodon arm structure. RNA 3: 721–733. [PMC free article] [PubMed] [Google Scholar]
- Muller M, Hartmann M, Schuster I, Bender S, Thuring KL, Helm M, Katze JR, Nellen W, Lyko F, Ehrenhofer-Murray AE. 2015. Dynamic modulation of Dnmt2-dependent tRNA methylation by the micronutrient queuine. Nucleic Acids Res 43: 10952–10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T, Nishikawa K, Nemoto F, Kuchino Y, Nishimura S, Miyazawa T, Yokoyama S. 1988. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature 336: 179–181. [DOI] [PubMed] [Google Scholar]
- Murphy FV IV, Ramakrishnan V. 2004. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat Struct Mol Biol 11: 1251–1252. [DOI] [PubMed] [Google Scholar]
- Murphy FV IV, Ramakrishnan V, Malkiewicz A, Agris PF. 2004. The role of modifications in codon discrimination by tRNALysUUU. Nat Struct Mol Biol 11: 1186–1191. [DOI] [PubMed] [Google Scholar]
- Murthi A, Shaheen HH, Huang HY, Preston MA, Lai TP, Phizicky EM, Hopper AK. 2010. Regulation of tRNA bidirectional nuclear-cytoplasmic trafficking in Saccharomyces cerevisiae. Mol Biol Cell 21: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K, De Robertis EM. 1981. RNA processing in microinjected Xenopus oocytes. Sequential addition of base modifications in the spliced transfer RNA. J Mol Biol 145: 405–420. [DOI] [PubMed] [Google Scholar]
- Noma A, Kirino Y, Ikeuchi Y, Suzuki T. 2006. Biosynthesis of wybutosine, a hyper-modified nucleoside in eukaryotic phenylalanine tRNA. EMBO J 25: 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A, Yi S, Katoh T, Takai Y, Suzuki T, Suzuki T. 2011. Actin-binding protein ABP140 is a methyltransferase for 3-methylcytidine at position 32 of tRNAs in Saccharomyces cerevisiae. RNA 17: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira T, Suzuki T. 2011. Retrograde nuclear import of tRNA precursors is required for modified base biogenesis in yeast. Proc Natl Acad Sci 108: 10502–10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Watanabe K, Ikeuchi Y, Suzuki T, Endo Y, Hori H. 2004. Substrate tRNA recognition mechanism of tRNA (m7G46) methyltransferase from Aquifex aeolicus. J Biol Chem 279: 49151–49159. [DOI] [PubMed] [Google Scholar]
- Persson BC, Esberg B, Olafsson O, Björk GR. 1994. Synthesis and function of isopentenyl adenosine derivatives in tRNA. Biochimie 76: 1152–1160. [DOI] [PubMed] [Google Scholar]
- Phizicky EM, Hopper AK. 2010. tRNA biology charges to the front. Genes Dev 24: 1832–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pütz J, Florentz C, Benseler F, Giegé R. 1994. A single methyl group prevents the mischarging of a tRNA. Nat Struct Biol 1: 580–582. [DOI] [PubMed] [Google Scholar]
- Rubio MA, Ragone FL, Gaston KW, Ibba M, Alfonzo JD. 2006. C to U editing stimulates A to I editing in the anticodon loop of a cytoplasmic threonyl tRNA in Trypanosoma brucei. J Biol Chem 281: 115–120. [DOI] [PubMed] [Google Scholar]
- Rubio MA, Gaston KW, McKenney KM, Fleming IM, Paris Z, Limbach PA, Alfonzo JD. 2017. Editing and methylation at a single site by functionally interdependent activities. Nature 542: 494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Hopper AK. 1998. tRNA nuclear export in Saccharomyces cerevisiae: in situ hybridization analysis. Mol Biol Cell 9: 3041–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz EC, Roth HM, Ankri S, Ficner R. 2012. Structure analysis of Entamoeba histolytica DNMT2 (EhMeth). PLoS One 7: e38728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigi N, Suzuki T, Terada T, Shirouzu M, Yokoyama S, Watanabe K. 2006. Temperature-dependent biosynthesis of 2-thioribothymidine of Thermus thermophilus tRNA. J Biol Chem 281: 2104–2113. [DOI] [PubMed] [Google Scholar]
- Soderberg T, Poulter CD. 2001. Escherichia coli dimethylallyl diphosphate:tRNA dimethylallyltransferase: site-directed mutagenesis of highly conserved residues. Biochemistry 40: 1734–1740. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Noma A, Suzuki T, Senda M, Senda T, Ishitani R, Nureki O. 2007. Crystal structure of the radical SAM enzyme catalyzing tricyclic modified base formation in tRNA. J Mol Biol 372: 1204–1214. [DOI] [PubMed] [Google Scholar]
- Swinehart WE, Henderson JC, Jackman JE. 2013. Unexpected expansion of tRNA substrate recognition by the yeast m1G9 methyltransferase Trm10. RNA 19: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A, Endo T, Yoshihisa T. 2005. tRNA actively shuttles between the nucleus and cytosol in yeast. Science 309: 140–142. [DOI] [PubMed] [Google Scholar]
- Tomikawa C, Yokogawa T, Kanai T, Hori H. 2010. N7-methylguanine at position 46 (m7G46) in tRNA from Thermus thermophilus is required for cell viability at high temperatures through a tRNA modification network. Nucleic Acids Res 38: 942–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbonavicius J, Qian Q, Durand JM, Hagervall TG, Björk GR. 2001. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J 20: 4863–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waas WF, Druzina Z, Hanan M, Schimmel P. 2007. Role of a tRNA base modification and its precursors in frameshifting in eukaryotes. J Biol Chem 282: 26026–26034. [DOI] [PubMed] [Google Scholar]
- Weixlbaumer A, Murphy FV IV, Dziergowska A, Malkiewicz A, Vendeix FA, Agris PF, Ramakrishnan V. 2007. Mechanism for expanding the decoding capacity of transfer RNAs by modification of uridines. Nat Struct Mol Biol 14: 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ML, Crary SM, Jackman JE, Grayhack EJ, Phizicky EM. 2007. The 2′-O-methyltransferase responsible for modification of yeast tRNA at position 4. RNA 13: 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Liu X, Huang RH. 2003. Chemical trapping and crystal structure of a catalytic tRNA guanine transglycosylase covalent intermediate. Nat Struct Biol 10: 781–788. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Watanabe K, Miyazawa T. 1987. Dynamic structures and functions of transfer ribonucleic acids from extreme thermophiles. Adv Biophys 23: 115–147. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T, Ohshima C, Yunoki-Esaki K, Endo T. 2007. Cytoplasmic splicing of tRNA in Saccharomyces cerevisiae. Genes Cells 12: 285–297. [DOI] [PubMed] [Google Scholar]
- Zeng L, Zhou MM. 2002. Bromodomain: an acetyl-lysine binding domain. FEBS Lett 513: 124–128. [DOI] [PubMed] [Google Scholar]
- Zhou M, Long T, Fang ZP, Zhou XL, Liu RJ, Wang ED. 2015. Identification of determinants for tRNA substrate recognition by Escherichia coli C/U34 2′-O-methyltransferase. RNA Biol 12: 900–911. [DOI] [PMC free article] [PubMed] [Google Scholar]