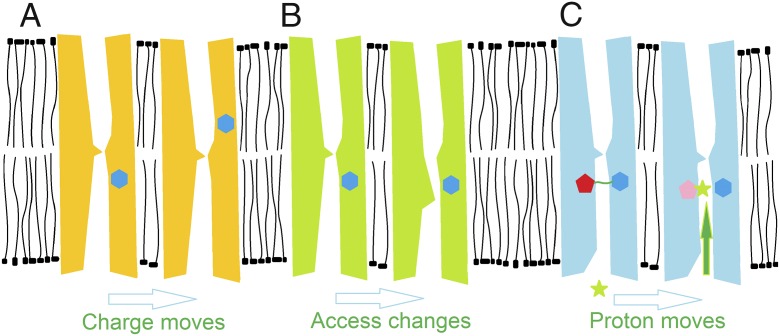
The voltage-gated proton channel (HV1) is a unique molecule that resides at the interface between ion channels and bioenergetic molecules that use proton gradients to store or transduce energy. HV1 plays key roles in the health and disease of diverse tissues and species (1). Important information regarding the physical components of ion-channel gating (opening and closing, which in turn activate or terminate flow of ionic current through the pore) can be obtained by measuring the size, kinetics, and voltage dependence of gating currents. Fig. 1 illustrates different mechanisms by which gating currents might be generated. In their landmark voltage-clamp studies of sodium and potassium channels in squid axons in the mid-20th century, Hodgkin and Huxley (2) deduced that (i) ionic current flowed through discreet sites (channels) in the membrane; (ii) these permeation pathways were gated, opening and closing in response to changes in membrane potential; and (iii) this process most likely involved the movement of charged groups across part or all of the transmembrane electric field. They recognized that the movement of charges within the electric field should generate a small capacitive current, but it was smaller than they could detect. The predicted gating currents were measured in sodium channels two decades later (3–6). Sodium-channel gating currents were ∼300 times smaller than the ionic current and were mostly over in 1 ms (3). Several properties of proton channels frustrated attempts to measure their gating currents until recently. Carmona et al. (7) report gating currents in HV1 from the sea squirt Ciona intestinalis. A contemporaneous study by De La Rosa and Ramsey (8) reports gating currents in human HV1. The clever experimental approaches devised by these groups to overcome the intransigence of HV1 and their conclusions are discussed below. Both studies advance our understanding of the gating of this unique channel. The most surprising result of the Carmona et al. study was that a Cole–Moore effect was observed in both ionic and gating currents from monomeric constructs.
Fig. 1.
Three physical representations of how gating currents might arise. The cartoons depict channels in a membrane viewed from the side. In each pair, the resting state is on the Left, and an activated state leading to current flow is on the Right. (A) Positively charged groups (blue hexagons) within the protein physically move outward. If most of the membrane potential drops across a short central “hydrophobic gasket,” then the charges need only move from the internal to the external aqueous vestibule to effectively transfer the charge across the entire membrane electric field. (B) Charges within the protein need not themselves move at all if the protein and its electric field are rearranged, for example, if the accessibility of charged groups switches between internal and external. (C) A proton (green star) might move from the bulk internal solution through a crevice where it could protonate an acidic group (red pentagon). If the crevice is narrow, the proton would cross part of the membrane electric field to reach the protonation site. A contribution of proton movement to gating currents has been proposed for K+ channels (32) as well as for HV1 (12, 18).
Strategies for Measuring Gating Currents from HV1
Measurement of gating currents in HV1 did not occur until a decade after the gene was identified (9, 10). Gating currents are measured in other voltage-gated ion channels by first eliminating ionic currents either by blocking the current or by eliminating the permeant ion. Gating currents reflect the repositioning of a small number of charges within the protein each time the channel opens or closes, and thus would be dwarfed by the much larger ionic currents that flow at a high rate as long as the channel is open. No effective inhibitors of HV1 act by simple pore occlusion (block). The most potent inhibitor, Zn2+, profoundly alters the voltage dependence and kinetics of gating (11), the very subjects about which gating currents are meant to enlighten us. It is impossible to eliminate the permeant ion, H+. Reducing its concentration strongly shifts the voltage at which channel gating occurs (12), and extremely high pH exerts deleterious effects on channel and membrane integrity (personal observation). Both groups (7, 8) eliminated most outward ionic current by introducing an Asn→Arg mutation into the S4 transmembrane helix (N264R or N214R). Because inward ionic currents are still observable, this mutation permits indirect determination of the voltage dependence of gating via ionic “tail currents” seen after repolarization. This information is vitally important, because most mutations to HV1 alter the gating kinetics and/or voltage dependence of its ionic currents (13), and gating currents are meaningful only if we know the details of gating in the mutant studied.
A second problem in detecting gating currents in HV1 is that these channels open very slowly in most species, especially mammals (14). Not only are gating currents intrinsically small, but for HV1 these currents are distributed over a timescale of seconds. For example, human eosinophils have the highest level of HV1 expression in any native cell of 100–200 pA/pF (15, 16). A whole-cell proton conductance of 1.5 nS at pHi 6.5, given a single-channel conductance at pHi 6.5 of 38 fS (17), reflects 40,000 channels per eosinophil. If each channel dimer moves six electronic charges (e0) across the membrane during opening (18), 3.8 × 10−14 C will move, and distributed over 1 s, the gating current will average just 38 fA, far too small to detect reliably. Carmona et al. solved this problem by studying HV1 as a monomer, rather than the dimer as occurs in most species (19–21). Monomeric constructs open substantially faster than dimers (19, 21, 22), and consequently gating currents could be detected in the monomer but not in the dimer. De La Rosa and Ramsey (8) used dimeric channels but introduced a second mutation (W207A) that accelerates channel opening by 100-fold (23). One property of HV1 facilitates gating current measurements, namely the small unitary conductance of ionic currents, two to three orders of magnitude smaller than most voltage-gated ion channels (17), which reduces the amplitude of ionic currents relative to gating currents.
What Are the Features of HV1 Gating Currents?
Gating currents should exhibit kinetics appropriate to those of current turn-on. They should saturate with large depolarizations (5). The currents reported by Carmona et al. meet these criteria. Equality of the integrals of ON and OFF gating currents measured during depolarization and repolarization is a strong criterion that shows the gating currents are genuine, because the same charges that rearrange to open the channel should return to their resting position upon repolarization (4). Because the mutation introduced eliminated outward but not inward ionic currents, comparing ON and OFF gating charge was not feasible. Carmona et al. did manage to detect OFF gating currents using short pulses, but these unexpectedly were only 1.4% of the magnitude of the ON currents (7). Almost all of the OFF gating current was trapped, most likely due to slowly reversible occlusion of the pore by the side chain of Arg that replaced Asn. The full gating charge was eventually recovered after prolonged hyperpolarization (7). Changes in pH shifted the voltage dependence of both ionic and gating currents (8). Replacing the first of three Arg in the S4 helix with Ala reduced the measured gating currents (8), as expected (24). Finally, the gating charge was decreased in the monomer compared with the dimer (8), although by much less than the 50% reported in studies in which gating charge was estimated by the limiting slope of the gH–V relationship (24, 25). Taken together, the evidence supports that genuine gating currents for HV1 have been demonstrated. This represents a major achievement that advances the field.
Unfortunately, the difficulty in determining the number of channels due to their minuscule unitary conductance (17) precludes accurate estimates of the quantity of gating charge transferred by each channel during opening. Another limitation of both studies results from the Asn→Arg mutation used to eliminate outward current. Replacing a neutral amino acid with a charged one inevitably alters the electric field within the protein, and thus the information gained applies strictly to the mutant channel. Heisenberg’s uncertainty principle aside, most global functions and properties of the mutant channels appear normal, and the new class of information obtained is extremely welcome.
Cole–Moore Effect
Hodgkin and Huxley (2) described the sigmoid onset of sodium and potassium currents upon depolarization using a mathematical model that can be envisioned to mean that channel opening occurs only after multiple identical subunits undergo a conformational change. Potassium channel opening was described by n4, where the parameter n increased from 0 to 1 exponentially with time. Decades later, the potassium channel was determined to be a homotetramer (26). Cole and Moore (27) showed that the delay in the onset of potassium current increased with increasingly negative prepulses, describable by an n25 model. No one imagines there are 25 subunits, but a general conclusion from the “Cole–Moore effect” is that each channel must undergo multiple voltage-dependent transitions between closed states before it opens (28). HV1 display distinctly sigmoidal activation kinetics in most species. The only quantitative model of voltage- and pH-dependent gating of HV1 postulated two protomers (12). Furthermore, HV1 exists as a dimer with cooperative gating, suggesting that both monomers must undergo a conformational change before either conducts (29, 30). In fact, the sigmoidal onset of current fits n2 kinetics rather nicely (29). Finally, when HV1 is forced into a monomeric lifestyle by truncating the C terminus, the current turns on faster and exponentially (22, 29). The interpretation has been that the sigmoidal turn-on of proton current reflects cooperative gating of the dimer. It is thus surprising that Carmona et al. (7) find that both ionic and gating currents of their monomeric construct exhibited a Cole–Moore effect. This result indicates that multiple closed states are traversed within each monomer and raises doubts whether the sigmoid turn-on of H+ current is due exclusively to cooperative gating of the dimer. Recently, a snail HV1 was identified that appears to exist as a dimer yet activates exponentially (31). The gating of HV1 is evidently more complicated than we imagined.
Acknowledgments
This research was supported by NIH Grants GM121462 and GM126902.
Footnotes
The author declares no conflict of interest.
See companion article on page 9240.
References
- 1.DeCoursey TE. Voltage-gated proton channels: Molecular biology, physiology, and pathophysiology of the HV family. Physiol Rev. 2013;93:599–652. doi: 10.1152/physrev.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong CM, Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973;242:459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong CM, Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974;63:533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keynes RD, Rojas E. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J Physiol. 1974;239:393–434. doi: 10.1113/jphysiol.1974.sp010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider MF, Chandler WK. Voltage dependent charge movement of skeletal muscle: A possible step in excitation-contraction coupling. Nature. 1973;242:244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- 7.Carmona EM, et al. Gating charge displacement in a monomeric voltage-gated proton (HV1) channel. Proc Natl Acad Sci USA. 2018;115:9240–9245. doi: 10.1073/pnas.1809705115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De La Rosa V, Ramsey IS. Gating currents in Hv1 proton channels. Biophys J. 2018;114:2844–2854. doi: 10.1016/j.bpj.2018.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 10.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherny VV, DeCoursey TE. pH-dependent inhibition of voltage-gated H+ currents in rat alveolar epithelial cells by Zn2+ and other divalent cations. J Gen Physiol. 1999;114:819–838. doi: 10.1085/jgp.114.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherny VV, Markin VS, DeCoursey TE. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J Gen Physiol. 1995;105:861–896. doi: 10.1085/jgp.105.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeCoursey TE, Morgan D, Musset B, Cherny VV. Insights into the structure and function of HV1 from a meta-analysis of mutation studies. J Gen Physiol. 2016;148:97–118. doi: 10.1085/jgp.201611619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 15.Gordienko DV, et al. Voltage-activated proton current in eosinophils from human blood. J Physiol. 1996;496:299–316. doi: 10.1113/jphysiol.1996.sp021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrenzel J, Lew DP, Krause KH. Proton currents in human eosinophils. Am J Physiol. 1996;271:C1861–C1871. doi: 10.1152/ajpcell.1996.271.6.C1861. [DOI] [PubMed] [Google Scholar]
- 17.Cherny VV, Murphy R, Sokolov V, Levis RA, DeCoursey TE. Properties of single voltage-gated proton channels in human eosinophils estimated by noise analysis and by direct measurement. J Gen Physiol. 2003;121:615–628. doi: 10.1085/jgp.200308813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeCoursey TE. Voltage and pH sensing by the voltage-gated proton channel, HV1. J R Soc Interface. 2018;15:20180108. doi: 10.1098/rsif.2018.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch HP, et al. Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci USA. 2008;105:9111–9116. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SY, Letts JA, Mackinnon R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc Natl Acad Sci USA. 2008;105:7692–7695. doi: 10.1073/pnas.0803277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tombola F, Ulbrich MH, Isacoff EY. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58:546–556. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musset B, et al. Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. J Physiol. 2010;588:1435–1449. doi: 10.1113/jphysiol.2010.188318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherny VV, et al. Tryptophan 207 is crucial to the unique properties of the human voltage-gated proton channel, hHV1. J Gen Physiol. 2015;146:343–356. doi: 10.1085/jgp.201511456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez C, Rebolledo S, Perez ME, Larsson HP. Molecular mechanism of voltage sensing in voltage-gated proton channels. J Gen Physiol. 2013;141:275–285. doi: 10.1085/jgp.201210857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujiwara Y, et al. The cytoplasmic coiled-coil mediates cooperative gating temperature sensitivity in the voltage-gated H+ channel Hv1. Nat Commun. 2012;3:816. doi: 10.1038/ncomms1823. [DOI] [PubMed] [Google Scholar]
- 26.MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- 27.Cole KS, Moore JW. Potassium ion current in the squid giant axon: Dynamic characteristic. Biophys J. 1960;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zagotta WN, Hoshi T, Aldrich RW. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J Gen Physiol. 1994;103:321–362. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez C, Koch HP, Drum BM, Larsson HP. Strong cooperativity between subunits in voltage-gated proton channels. Nat Struct Mol Biol. 2010;17:51–56. doi: 10.1038/nsmb.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tombola F, Ulbrich MH, Kohout SC, Isacoff EY. The opening of the two pores of the Hv1 voltage-gated proton channel is tuned by cooperativity. Nat Struct Mol Biol. 2010;17:44–50. doi: 10.1038/nsmb.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas S, et al. Exotic properties of a voltage-gated proton channel from the snail Helisoma trivolvis. J Gen Physiol. 2018;150:835–850. doi: 10.1085/jgp.201711967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kariev AM, Green ME. Voltage gated ion channel function: Gating, conduction, and the role of water and protons. Int J Mol Sci. 2012;13:1680–1709. doi: 10.3390/ijms13021680. [DOI] [PMC free article] [PubMed] [Google Scholar]