Significance
The multiproxy approach represents a novel methodology and a unique opportunity to obtain a more detailed view of ancient resource use. Our multiproxy study, carried out on gomphotheres from Chile, widens potential occupied habitats to closed-canopy forests. This habitat variability supports the hypothesis that the diet of gomphotheres appears to be more constrained by resource availability than by the potential dietary range. We strongly recommend the use of a multiproxy approach, where morphology analyses are complemented by other sources of information. This approach prevents misleading conclusions about the origin of the proxy’s signal from arising, such as a leaf-browsing diet inferred from the dental calculus and microwear not necessarily being indicative of humidity.
Keywords: stable isotopes, dental calculus, dental microwear
Abstract
Proboscideans are so-called ecosystem engineers and are considered key players in hypotheses about Late Pleistocene megafaunal extinctions. However, knowledge about the autoecology and chronology of the proboscideans in South America is still open to debate and raises controversial views. Here, we used a range of multiproxy approaches and new radiocarbon datings to study the autoecology of Chilean gomphotheres, the only group of proboscideans to reach South America during the Great American Biotic Interchange (∼3.1 to 2.7 million years before present). As part of this study, we analyzed stable isotopes, dental microwear, and dental calculus microfossils on gomphothere molars from 30 Late Pleistocene sites (31° to 42°S). These proxies provided different scales of temporal resolution, which were then combined to assess the dietary and habitat patterns of these proboscideans. The multiproxy study suggests that most foraging took place in relatively closed environments. In Central Chile, there is a positive correlation between lower δ13C values and an increasing consumption of arboreal/scrub elements. Analyses of dental microwear and calculus microfossils have verified these leaf-browsing feeding habits. From a comparative perspective, the dietary pattern of South American gomphotheres appears to be constrained more by resource availability than by the potential dietary range of the individual taxa. This multiproxy study is aimed at increasing knowledge of the life history of gomphotheres and thus follows an issue considered one of the greatest challenges for paleontology in South America, recently pointed out by the need to thoroughly understand the role of ecological engineers before making predictions about the consequences of ecosystem defaunation.
Based on the dental morphology evolution of herbivorous mammals, different dietary categories have been recognized (i.e., browsers, grazers, and mixed feeders), which have allowed the interpretation of dietary patterns of mammalian lineages in evolutionary terms (1). However, dietary patterns can change significantly over ontogenetic and ecological timescales (2), and therefore there might be a decoupling between dental morphology and dietary preferences. This decoupling has been observed with the increasing application of other independent, nonmorphological proxies (3), which have evidenced “unexpected” dietary records either in extinct or modern taxa.
This demonstrated that variability in dietary patterns may correspond to some type of dietary plasticity, inherent to the taxon under study (e.g., the presence of putative cellulose-digesting microbes) (4), which could be more determining than dental morphology. A drastic environmental change may also trigger a shift in the resource and habitat use for a specific taxon, which might remain undetected if only dental morphology is investigated.
To overcome these current limitations, multiple sources of evidence can provide a wider perspective to infer the dietary preferences of a specific taxon and the environment where it lived. The application of a multiproxy investigation to a single taxon and to a single anatomical element provided solid data on resource and habitat use, as well as on the lifestyle of extinct fauna, leading to the formulation of previously unasked questions.
We performed a multiproxy study approach to better characterize the diet of Notiomastodon platensis from Chile (probably the only species of gomphothere that inhabited Chile), since they are known to have developed a flexible lifestyle, which lay behind their successful spread into South America (5). We focused on Central Chilean territory (31° to 42°S) (Fig. 1) due to two factors. First, from a paleoenvironmental point of view, this area represents one of the most thoroughly researched areas of South America (SI Appendix, Fig. S1). This, in turn, provides us with a greater and more precise number of proxies derived from different paleontological disciplines to deal with. Second, the exceptional preservation of the paleontological material found in this area guarantees the successful integration of different proxies. We have analyzed three proxies to study gomphotheres from different Chilean paleontological sites spanning an age between ∼30,000 and 12,000 calibrated years before present (cal y B.P.). The proxies considered in this study are: (i) stable isotope analysis (SIA), (ii) dental microwear analysis (DMA), and (iii) analysis of microfossils from dental calculus (AMDC) (Dataset S1). The advantage of this multiproxy approach over others lies in that it allows the interpretation of dietary patterns at different times in the individual’s life history. Moreover, the fact that the studied gomphotheres have been found at different time periods (SI Appendix, Fig. S2) enables us to evaluate environmental and climatic shifts that may have happened in Chile between ∼30,000 and 12,000 cal y B.P.
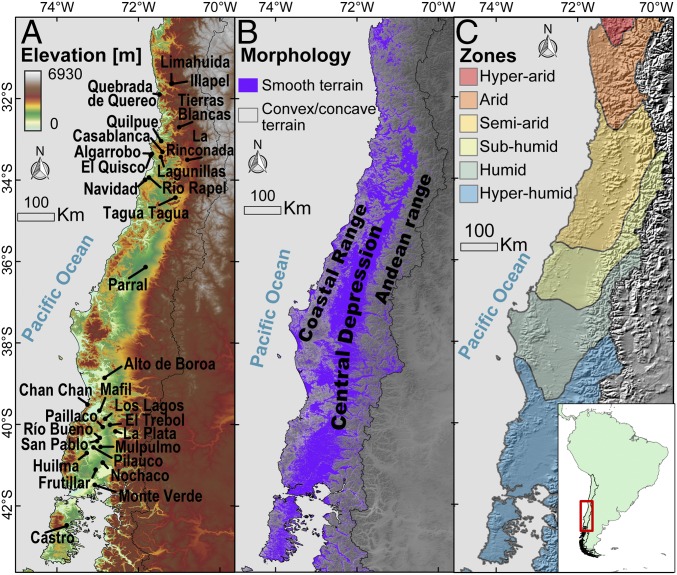
Fig. 1.
Geographical setting of the fossil record of the gomphotheres analyzed in this study. (A) Fossil sites that provided the Notiomastodon molars for this study. (B) Location of the main geomorphic units of the South-Central Chile continental margin. The smooth areas were the most likely habitats for Notiomastodon. We highlighted those areas in purple by classifying the land-surface topography of the Chilean territory lower than 2,000 meters above sea level. To this end, we defined two land-surface classes according to their cross-sectional curvature (52) (SI Appendix). (C) Modern latitudinal gradient of aridity/humidity regions for the study area, from Casanova et al. (52).
Gomphotheres are one of the best-studied Pleistocene megafaunal groups in South America and have been classified as browsers based on their dental morphology (6). Although their bunodont dental pattern suggests they consumed a soft diet, it has been suggested that gomphothere molars contain anatomical elements that could have enabled them to incorporate herbs into their diets (7). Additionally, differences in the anatomical structure of the wear facets may suggest strong contrasts in the chewing phases of different gomphothere species of the same genus (8). Previous studies have indicated a large dietary niche of gomphotheres in South America, ranging from a grassland to mosaic habitats (9). This led researchers to describe them as generalists and opportunists in terms of their dietary behavior (10). Lucas et al. (11), for instance, suggested that Stegomastodon might have shown one of the most complex morphological adaptations for grazing, and a recent study on mandibular elements further verified that the bunodont proboscideans in South America possessed morphological features consistent with a grazing diet (12). Comparatively, modern elephants have a grazing dental morphology (13). However, they show a mixed-feeder behavior with a tendency toward browsing (14). Recently, differences in the gastrointestinal tract and enamel ridges have explained the higher proportion of herbs in the diet of Elephas maximus than in that of Loxodonta africana (15). These differences are one of the causes behind the seasonal dietary shift observed in African savannah elephants, which show a preference for herbs during the wet season but rely on browsing during the dry season (16).
Following these lines of evidence, observed in extinct and extant proboscideans, South American gomphotheres constitute an excellent case study to evaluate the range of phenotypical plasticity within the group. If they were generalists and opportunists, their dietary composition should reflect local environmental conditions rather than an optimal adaptive diet (17). Furthermore, because proboscideans are described as ecological engineers (18), they constitute a key species to be taken into account when undertaking paleoenvironmental evaluations. This is due to the nature of megaherbivores that reach populations with high biomass (19). The presence or absence of this taxon in a given territory could give rise to major alterations in the structure and function of the ecosystem (19). Additionally, as obligate drinkers, the dental enamel of gomphotheres is potentially a good indicator of local meteoric water δ18O values (δ18Omw) (20), which can be linked to hydrological conditions within a particular climate regime.
The main aim of our study is to determine the autoecology of gomphotheres from Central Chile, assessing whether there is a consensus in the interpretation of diet and habitat characterized by each of the analyzed proxies. In this way, our proposal would enable us to evaluate whether the information provided by our proxies corresponds to (i) a dietary pattern extended over a long period of time; (ii) the presence of microhabitats; and/or (iii) if there was any evidence of an environmental change across the studied time span.
Results
Stable Isotope Analyses.
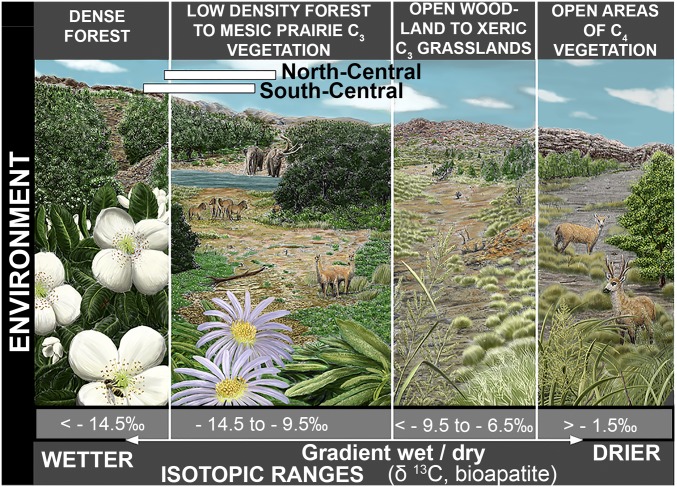
These results point to the overall existence of drier and warmer conditions in the North-Central (NC) region (SI Appendix, Tables S1–S3), with a mosaic-type vegetation cover (Fig. 2) and a more humid and cold (SI Appendix, Tables S1–S3) environment in the South-Central (SC) region with the presence of a higher proportion of arboreal elements (Fig. 2) (see Dataset S1 for more details).
Fig. 2.
Isotopic range of δ13C (‰, Vienna Pee Dee Belemnite) values in bioapatite from our study area, corresponding to different vegetation types: (i) dense forest (e.g., Valdivian forest), Eucryphia cardifolia; (ii) low-density forest to mesic prairie C3 vegetation (e.g., northwestern Chilean Patagonia) with Nothofagus dombeyi (arboreal) and Aster vahlii and Adenocaulon chilense (grasses); (iii) open woodland to xeric C3 grasslands (e.g., Patagonian steppe) with Stipa speciosa and Poa lanuginosa; and (iv) open vegetation areas composed of C4 (e.g., Corrientes, Argentina) with Panicum grumosum (grasses). The arrows indicate a gradient of humidity/aridity. Values between −6.5 and −1.5‰ correspond to C3-C4 open areas. The white bars represent the isotopic range shown by North-Central and South-Central Chilean gomphotheres. Illustration is by Eloy Manzanero.
Dental Microwear Analysis.
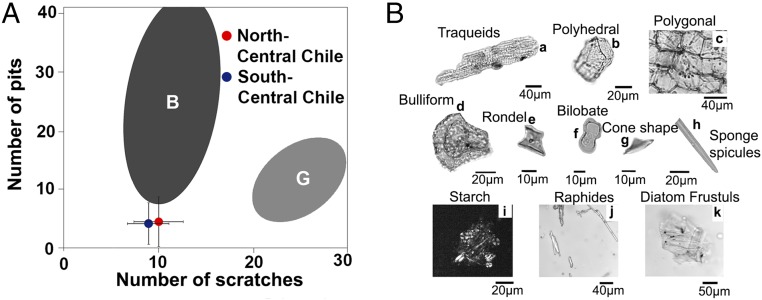
SI Appendix, Fig. S3 shows photomicrographs of N. platensis tooth enamel, which generally displays high percentages of coarse and hypercoarse scratches and low numbers of pits and fine scratches (SI Appendix, Tables S4 and S5). Compared with known values of the average number of pits versus the average number of scratches per taxon for extant ungulates (21), it is observed that our results do not fall in the 95% confidence ellipse of the extant leaf browsers (Fig. 3A) (see SI Appendix for more details).
Fig. 3.
(A) Bivariate plot of the average numbers of pits and scratches in Notiomastodon samples from Chile. Bars correspond to SD (±1 SD). Gray areas correspond to the Gaussian confidence ellipses (P = 0.95) on the centroid for extant leaf browsers (B) and grazers (G) from Solounias and Semprebon (21). (B) Recovered plant microfossils from the dental calculus of gomphotheres. Arboreal morphotypes: a–c. Poaceae morphotype: d–f. Cyperaceae morphotype: g. Other microfossils: h–k.
Analysis of Microfossils from Dental Calculus.
Most of the sample (88%) shows a predominance of tree and shrub elements (SI Appendix, Table S6). Morphotypes such as polygonal, polyhedral, spherical, or vascular tissue (Fig. 3B) support a diet based on trees and shrubs (SI Appendix, Fig. S4) (see SI Appendix for more details).
Discussion
The results of the multiproxy approach, alongside the wide dietary range previously inferred for gomphotheres (N. platensis and Cuvieronius hyodon) from South America (22–24), allow us to support the hypothesis that the diet of this proboscidean appears to be more greatly influenced by resource availability than by the potential dietary range of the taxon (SI Appendix, Fig. S5A). However, in gomphotheres with more positive values from the NC region, near the upper threshold of the −9.5‰ value, nonisotopic proxies still showed a leaf-browsing diet. From this logic, future multiproxy studies of other regions in South America—as in the Pampean Region, which supported an extraordinary fauna of large mammals during the Final Pleistocene (25)—could show evidence similar to that registered in the NC region of Chile. This demonstrates the high potential of combining different types of dietary proxies on vertebrate taxa to attain a deeper perspective of ancient resource use.
North-Central Chile.
Although isotopic values showed a high degree of variability, some trends can, nonetheless, be observed. δ13Cbio values tend to be lower, whereas δ18O values are intermediate compared with those from other South American areas. δ15N values show different trends in the NC and SC regions, with the former depicting higher values (closer to those shown by the Pampean taxa) and the latter depicting lower values (closer to those recorded by southern Patagonian taxa) (SI Appendix, Fig. S5B). A first interpretation of this landscape suggests the predominance of a woodland-mesic environment C3 grassland. However, the DMA and AMDC point to a significant presence of trees as shown by most samples (SI Appendix, Table S6).
Although the general pattern of climate and environmental changes tended toward aridization, the Pleistocene–Early Holocene transition was neither continuous nor gradual but rather complex and with reversals and unique/particular in different regions (26). This is reflected by the environmental variability evidenced in the studied specimens from the NC region.
The sample from the Quereo site (31°S) is one of the most informative (12,980 to 12,700 cal y B.P.; 2 σ) (Dataset S1). Although this sample is consistent with a woodland-mesic C3 grassland environment, its δ13Cbio value is near the upper-threshold −9.5‰ value, which would indicate a more open and drier environment. Its collagen isotopic values point to an arid environment (Dataset S1). The estimated mean annual temperature is close to that obtained from sea-surface temperature estimations for the Late Pleistocene (27), whereas δ18Omw and mean annual precipitation values were similar to current values (arid environment; Fig. 1). Pollen analyses allowed Villagrán and Varela (28) to carry out a paleoenvironmental reconstruction of the Quereo area. This study detected more humid conditions than at present until 11,200 cal y B.P. The DMA and AMDC showed a leaf-browser diet for the Quereo specimen, which may initially indicate some degree of humidity. However, the multiproxy study indicated that the Quereo individual may have lived in a habitat with climatic and environmental conditions similar to those observed today in the area (more arid than during the Last Glacial Termination), with a predominance of a woodland and shrub stratum adapted to semiarid conditions. Following this logic, similar reasoning could be applied to the gomphothere sample from the Illapel site (31°S), as it showed similar isotopic values (δ13Cbio). In addition, both presented 100% of a tree phytolith morphotype.
The stable isotope values from Casablanca (33°S) (Pleistocene/Holocene) pointed to a xeric and warm environment (Dataset S1). Moreover, the AMDC output showed a dominant consumption of herbs. This individual indicates that it is possible to find a grazing diet among the megafauna toward the end of the Pleistocene due to the increase in grassland. However, the low number of scratches and the presence of hypercoarse scratches suggest a browsing behavior during the last days or weeks before death. The latter interpretation agrees well with other gomphotheres in the same area: The individual from the Lagunillas site (33°S) indicated mixed-feeding (trees and herbs) (from AMDC) and browsing behavior (from DMA). Gomphotheres from the Rapel and Tierras Blancas sites (33°S) (Pleistocene/Holocene) fed in closed woodland according to their δ13Cbio signal and lived under warm conditions according to mean annual temperature values (Dataset S1). The gomphotheres from the Rapel, Algarrobo, and Navidad sites display a leaf-browser diet (from DMA and AMDC). Probably, under warmer and drier environmental conditions, as is the case today along the coast of the NC region, the strong oceanic influence gave rise to a permanently high level of humidity, leading to patches of deciduous and evergreen forests in the NC region; remnants of rainforest dated to the Late Pleistocene have been found along the coast (29). Moreover, the hilly orography from coastal areas to the Andes Range between 31° and 33°S (Fig. 1) are today dominated by a Mediterranean xeric-oceanic bioclimate, exhibiting from desert scrub all the way to sclerophyllous forest. This orographic feature may have allowed swamp forests and coastal wetlands to persist into the Early Holocene. Therefore, despite the significant increase in grassland, at least up to 8,700 cal y B.P. (26), orographic and oceanographic features enabled woodland-type microhabitats (such as interglacial refugia) to persist.
The samples from the Tagua Tagua site (34°S) and the Parral site (36°S) showed an environmental and dietary pattern in agreement with the persistence of a forested landscape until ∼13,800 cal y B.P. (SI Appendix, Fig. S1). Additionally, the multiproxy analyses of the selected specimens point to a leaf-browser diet. Moreover, most of the samples presented a mean annual precipitation >1,000 mm/y. One very informative sample from the Tagua Tagua site may be reflecting what can be considered the closest approximation to the environment of the Last Glacial Termination: sample SGO.PV.47k (13,810 to 14,520 cal y B.P.; 2 σ). Sample SGO.PV.256 (13,420 to 13,720 cal y B.P.; 2 σ), probably coming from the Tagua Tagua site (to be confirmed), also exhibits the same environmental signal as the SGO.PV.47k sample: Both are linked to a woodland-mesic C3 grassland environment, while also coinciding with a leaf-browser diet. In addition, in both samples, the mean annual temperature is similar to the sea-surface temperature of the Last Glacial Termination (SI Appendix, Fig. S1). However, as observed in all of the samples from the NC region, their relatively high δ15N values could reflect some degree of aridity. Therefore, this multiproxy study helps us to more precisely infer the habitat of N. platensis as being a humid and wooded environment. Despite this observed pattern, there are some samples from the Tagua Tagua site that showed some degree of variability. For example, the SGO.PV.47c1 sample pointed to dry areas with open C3-C4 vegetation. Estimated mean annual temperature and mean annual precipitation (Dataset S1) values support dry conditions. As deduced from the AMDC, during the final stage of its life, this particular individual seems to have lived in a wetland environment comprising trees, shrubs, and herbs. This sample also showed the highest number of fine scratches from the microwear database (SI Appendix, Table S4), although the presence of coarse and hypercoarse scratches could indicate a mixed-feeding diet, which is in agreement with a roughly even percentage of tree, shrub, and herb elements (SI Appendix, Table S6). Just like some of the NC gomphotheres, which consumed the Cyperacea taxon, the SGO.PV.47c1 sample did not show evidence that wetlands may have given rise to low δ13C values (30). Therefore, this gomphothere shows variability in its diet throughout its life, obtaining food items from open areas to wetlands. This individual is likely a reflection of the Pleistocene–Holocene transition.
South-Central Chile.
Notiomastodon samples from the SC region showed intermediate δ18O, low δ13Cbio-coll, and low δ15N values, in agreement with a temperate environment dominated by C3 plants and with a significant woodland cover (31). When the collagen δ13C diet-to-tissue trophic discrimination of ∼5‰ (32) is applied, the estimated mean vegetation δ13C value retrieved from SC gomphotheres was −27.7 ± 0.7‰. This mean δ13C value roughly coincides with the threshold value that delimits forest and woodland conditions (∼−27.5‰) (33), and it is placed close to the high-end member of the δ13C range obtained for modern forest and shrub vegetation analyzed in the same area (−40.2 to −27.9‰) (34). When the collagen δ15N diet-to-tissue trophic discrimination of ∼3‰ (32) is applied, the estimated mean vegetation δ15N value of SC gomphotheres was 1.0 ± 2.1‰. This value agrees well with the modern vegetation δ15N values of shrub and forest areas, which range between −9.9 and 3.5‰ (34). This mean vegetation δ15N value calculated from SC gomphotheres corresponds to either plants that do not fix nitrogen, nitrogen-fixing plants, or plants that grow in association with mycorrhizae in areas where precipitation is greater than 1,000 mm/y (35). However, the DMA and AMDC of SC individuals indicate that they preferably consumed non–nitrogen-fixing plants such as shrubs and trees. This seems consistent with the observed low δ13C values depicted by the SC gomphotheres and, in turn, with the presence of a closed canopy in the north Patagonian rainforest (SI Appendix, Fig. S1).
When calculating the mean annual precipitation, a wide variability is gleaned from the SC gomphotheres (Dataset S1), probably due to the chronological differences among the selected samples and sites. The mean annual temperature of the dated samples (Dataset S1) fits well with those recorded from marine proxies (SI Appendix, Fig. S1) except for the mean annual temperature (23 °C) estimated from the Pilauco MHMOPI/628 sample (13,240 to 15,640 cal y B.P.; 2 σ), which is unusually high. This sample raises special interest, as it comes from a site characterized by an increased abundance of nonarboreal elements (24). In addition (and contrary to what is observed in the general SC trend), δ15N values are high in the Pilauco samples (24). However, the multiproxy analysis carried out on the MHMOPI/628 specimen points to a leaf-browser diet, and its δ13C value indicates a forested environment.
In the SC region, during the Last Glacial Maximum, the climate was cold and humid, characterized by the increase in cold-resistant hygrophilous herbs and Moorland Magellanic communities (SI Appendix, Fig. S1). However, intercalated climate was colder and drier, characterized by the increase in Graminae-Compositae. Therefore, we should expect the gomphotheres to have had grazer behavior. Conversely, short-time episodes characterized by a warmer climate have also been detected. This increase in temperature triggered the appearance of forested areas. The multiproxy study of the samples from the Nochaco and Mulpulmo sites showed a δ13C signal for wooded areas for that time period. The opposite of what would be expected of a leaf-browsing diet appears to have taken place. Take, for example, the gomphothere from the El Trébol site (UAChPVTR1) (16 °C): Although it originates from a pre-Last Glacial Maximum interstadial period, when forests with conifers would have had a wider and continuous distribution than at present, populating the lowlands of the Central Depression (36), it shows mixed-feeding data from AMDC. The same is true for the gomphothere from the Choroico site (mixed-feeding data from AMDC); however, the δ13C values showed a closed-canopy environment from the Last Glacial Termination. On the one hand, perhaps, as has been observed in some modern elephants, gomphotheres did not consume food in proportion to its local abundance (e.g., due to variations in vegetation palatability throughout the year) (37). Alternatively, the microhabitats characterized by a mosaic habitat could have been common within a forested regional context of vegetation, which had already been established from ∼16,800 cal y B.P.
Comparison Between North-Central and South-Central Chile.
Significant differences emerge when comparing NC and SC gomphothere isotopic values. δ13C values indicated a more wooded-to-forested environment in the SC region, although in both areas the δ13C values revealed mostly a browsing dietary behavior. Estimated modern equivalent dietary (δ13Cdiet,meq) values and mean annual precipitation, along with δ15N values, point to a strong latitudinal gradient between the NC (lower precipitation) and the SC (higher precipitation) areas. Today, the climatic system in place could explain the differences noted in the δ15N values (38). However, for the Late Pleistocene, the climate was homogeneous in both areas (SI Appendix, Fig. S1). Unfortunately, the NC region lacks radiocarbon datings, which hinders a more robust interpretation. The complexity of the problem increases when we take into account that within a context of high humidity there are high δ15N values. Thus, in terms of the collagen δ15N diet-to-tissue trophic discrimination, the values of the gomphotheres from the Pilauco site (δ15N = 4 ± 0.9‰) (34) do not overlap with those of modern vegetation (δ15N = −1.9 ± 3.2‰) (34). The reason behind the difference between the gomphotheres from the Pilauco site and the other sites from the SC region (38° to 42°S) (δ15N = 0.6 ± 1.8‰; this study) should be sought for other factors such as, for example, by fires, grazing intensity, and coprophagy or the fertilization of the vegetation located in regular migratory routes (31), among others.
Although in the NC region there are samples that reflect both cold and warm conditions, overall NC and SC gomphothere δ18Omw and mean annual temperature values agree well with interglacial environments. This includes the modern interglacial situation where annual mean δ18Omw values for some nearby meteorological stations are in line with those obtained by gomphotheres. By taking into account the average pattern shown by our multiproxy approach on NC and SC gomphotheres, it can be inferred that atmospheric circulation patterns, δ18Omw values, and temperatures—at least during the Last Glacial Termination—were similar to those today.
Overall, the interpretation of the AMDC and DMA results is similar in both areas: A diet dominated by the consumption of forested vegetation is compatible with the trend toward the decrease in δ13C values. The presence of herbs in smaller percentages agrees with the isotopic range detected in the majority of the samples. The high proportion of samples with hypercoarse scratches suggests gomphotheres fed on tree bark, but it is also plausible that these scratches, when coupled with the presence of puncture pits, were caused by phytoliths present in hard fruit and seed coats. However, neither NC nor SC gomphotheres record puncture pits.
To date, there is an increasing number of studies evidencing a eurybiomic and generalist pattern in proboscidean behavior (39). In this context, as suggested for mastodons and mammoths from North America (31), a change in vegetation may have not been a determining and fatal factor in the extinction of South American gomphotheres. However, there are several limiting factors for biotic expansion. One of these factors may be the increasing need for larger species to obtain food of sufficient quality (40). Thus, because it has been found that nitrogen limitation is a key factor that influences the fitness of mammalian herbivores, a central question arises: What adverse effects on the biology of the gomphotheres might have been generated by a shift toward a more grazing behavior (low protein consumption and increase of abrasive feed)? Studies of African savannah elephants show that they feed on grass during the wet season but rely on browsing during the dry season (14). This dietary preference is related to a wider home range in the wet season than in the dry season (41). Codron et al. (42) suggest that switching between C3 browsing and C4 grazing over extended timescales helps modern elephants survive through environmental change, and could even allow for the recovery of overused resources to take place. Focusing on this logic, because this multiproxy study extends the dietary range (i.e., closed-canopy) of gomphotheres in South America, probably a set of adaptive features in gomphotheres must have acted synergistically to have been able to consume the different types of vegetation, for example from the consumption of C4 leaves with thick cell walls (43) in the Brazilian Intertropical Region (6° to 15°S) (22) to the consumption of woody vegetation with more plant secondary metabolites (44) in northwestern Chilean Patagonia (38° to 42°S) (24). The large record from Central Chile showing a leaf-browsing diet could suggest the presence of physiological adaptations in gomphotheres, which today are being considered key in the nutritional evolution of modern elephants: Because arboreal vegetation has more nutrients than herbs but more secondary metabolites with nocuous effects on nitrogen digestion, elephants have evolved tannin-binding proteins as a way of dealing with the negative effects of tannins; this would therefore increase the amount of available crude protein, which can greatly affect the carrying capacity (44).
Finally, because the registry of a generalist dietary pattern in the proboscideans continues to be a tendency in studies that use taxon-free proxies, two questions of ecological-evolutionary nature should guide future research: (i) What kinds of factors were decisive for a single taxon of proboscideans to have inhabited South America, and (ii) why was the flexible lifestyle of gomphotheres not enough to avoid their extinction?
Conclusions
This research involved a multiproxy analysis of gomphothere molars found in Chilean paleontological sites located between 31° and 42°S. These proxies provide different scales of temporal resolution, which were combined to assess dietary and habitat patterns. There is agreement between the range of the dietary resources registered during the first years of formation of the bioapatite in dental enamel and the diet registered during the final week or months of the gomphothere’s life: a dietary pattern dominated by the consumption of trees and shrubs with lower percentages of herbs in almost all of the samples.
The estimated δ13Cdiet,meq values and mean annual precipitation, along with δ15N values, point to a strong latitudinal gradient. However, some samples with high δ15N values might be explained by nonclimatic causes, since other proxies showed a high degree of humidity. Overall, NC and SC gomphothere δ18Omw and mean annual temperature values agree well with interglacial environmental conditions.
Habitat differences in South America support the hypothesis that the diet of gomphotheres appears to be more constrained by resource availability than by the potential dietary range of the taxa. Our multiproxy study has shown that, while the δ13C values are indicative of more open and xeric areas, the consumption of trees and shrubs in some episodes of the gomphotheres’ lives cannot be ruled out. We have observed that the opposite is true as well: Some extent of herb consumption may still have been possible when mean δ13C values point to closed-canopy and humid areas.
Finally, the information provided by this multiproxy study positions Central Chile as one of the “hotspots” of South America where further investigation should be encouraged to obtain a more in-depth knowledge of glacial and interglacial refugia, resource use, and the potential habitat shrinkage that finally led to the megafaunal extinction recorded at the end of the last Ice Age.
Materials and Methods
Materials.
A multiproxy approach involving stable isotope analysis, dental microwear analysis, and analysis of microfossils from dental calculus was carried out on 79 teeth of the gomphothere N. platensis. The three proxies were analyzed on a total of 15 teeth; two proxies were analyzed on 29 teeth; and one proxy was analyzed on 35 teeth (Dataset S1). A new set of radiocarbon dates is presented in this study (SI Appendix, Table S7). The samples selected for this study come from 30 sites located at latitudes between 31° and 36°S and between 38° and 42°S (SI Appendix, Fig. S1).
Methods.
SIA.
Samples for oxygen and carbon stable isotope analyses on bioapatite carbonate were treated following the analytical procedures described in Tornero et al. (45). Collagen extraction followed original protocols already listed by Longin (46) and later modified by Bocherens et al. (47).
DMA.
We followed Asevedo et al. (48) and sampled unaltered regions of the enamel on the occlusal surface. Second upper and lower molars were preferentially selected. The microwear features were examined using the protocol developed by Solounias and Semprebon (21).
AMDC.
The extraction of microfossils from the calculus samples was carried out using the chemical processing method described by Wesolowski et al. (49). To calculate the concentration of microfossils, we used Maher’s method (50) as modified by Wesolowski et al. (51).
For more details on the methodology used in this study, see SI Appendix.
Supplementary Material
Acknowledgments
Our manuscript benefited from the professional editing of Dr. Pía Spry-Marqués. We are grateful for the funding provided from Fondecyt Grant 1150738 (to M.P.). C.T. acknowledges the Beatriu de Pinós postdoctoral fellowship (BP-MSCA Cofound Code 2016-00346 from the AGAUR, Goverment of Catalonia, Spain). We are also grateful for the funding provided by CGL 2016-80000-B (MINECO) and 2014-SGR-901 and 2017-SGR-836 (AGAUR).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1804642115/-/DCSupplemental.
References
- 1.Lister A-M. Behavioural leads in evolution: Evidence from the fossil record. Biol J Linn Soc Lond. 2014;112:315–331. [Google Scholar]
- 2.Davis M, Pineda Munoz S. The temporal scale of diet and dietary proxies. Ecol Evol. 2016;6:1883–1897. doi: 10.1002/ece3.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews P, Hixson S. Taxon-free methods of palaeoecology. Ann Zool Fennici. 2014;51:269–284. [Google Scholar]
- 4.Zhu L, Wu Q, Dai J, Zhang S, Wei F. Evidence of cellulose metabolism by the giant panda gut microbiome. Proc Natl Acad Sci USA. 2011;108:17714–17719. doi: 10.1073/pnas.1017956108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mothe D, et al. Sixty years after ‘The mastodonts of Brazil’: The state of the art of South American proboscideans (Proboscidea, Gomphotheriidae) Quat Int. 2017;443:52–64. [Google Scholar]
- 6.Fox D-L, Fisher D-C. Dietary reconstruction of Miocene Gomphotherium (Mammalia, Proboscidea) from the Great Plains region, USA, based on the carbon isotope composition of tusk and molar enamel. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;206:311–335. [Google Scholar]
- 7.Rivals F, Mol D, Lacombat F, Lister A-M, Semprebon G-M. Resource partitioning and niche separation between mammoths (Mammuthus rumanus and Mammuthus meridionalis) and gomphotheres (Anancus arvernensis) in the Early Pleistocene of Europe. Quat Int. 2015;379:164–170. [Google Scholar]
- 8.Calandra I, Göhlich U-B, Merceron G. How could sympatric megaherbivores coexist? Example of niche partitioning within a proboscidean community from the Miocene of Europe. Naturwissenschaften. 2008;95:831–838. doi: 10.1007/s00114-008-0391-y. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez B, Prado J-L, Alberdi M-T. Feeding ecology, dispersal, and extinction of South American Pleistocene gomphotheres (Gomphotheriidae, Proboscidea) Paleobiology. 2004;30:146–161. [Google Scholar]
- 10.Pérez-Crespo V-A, Prado J-L, Alberdi M-T, Arroyo-Cabrales J, Johnson E. Diet and habitat for six American Pleistocene proboscidean species using carbon and oxygen stable isotopes. Ameghiniana. 2016;71:39–51. [Google Scholar]
- 11.Lucas S-G, Aguilar R-H, Spielmann J-A. Stegomastodon (Mammalia, Proboscidea) from the Pliocene of Jalisco, Mexico and the species-level taxonomy of Stegomastodon. New Mexico Museum of Natural History and Science Bulletin. 2011;53:517–553. [Google Scholar]
- 12.Borges-Silva L, Mothé D, Avilla L-S. A morfometria mandibular e sua evolução no habito pastador dos gonfoteridos trilofodontes brevirostrinos (Proboscidea: Gomphotheriidae) das Americas. In: Riff D, et al., editors. XXIV Congresso Brasileiro de Paleontologia, Boletim de Resumos. Paleontologia em destaque, Ediçao especial; Crato, Brazil: 2015. pp. 180–181. [Google Scholar]
- 13.Maglio V-J. Origin and evolution of the Elephantidae. Proc Am Philos Soc. 1973;63:1–149. [Google Scholar]
- 14.Cerling T-E, Harris J-M, Leakey M-G. Browsing and grazing in elephants: The isotope record of modern and fossil proboscideans. Oecologia. 1999;120:364–374. doi: 10.1007/s004420050869. [DOI] [PubMed] [Google Scholar]
- 15.Clauss M, et al. Observations on the length of the intestinal tract of African Loxodonta africana (Blumenbach 1797) and Asian elephants Elephas maximus (Linné 1735) Eur J Wildl Res. 2007;53:68–72. [Google Scholar]
- 16.Cerling T-E, Wittemyer G, Ehleringer J-R, Remien C-H, Douglas-Hamilton I. History of animals using isotope records (HAIR): A 6-year dietary history of one family of African elephants. Proc Natl Acad Sci USA. 2009;106:8093–8100. doi: 10.1073/pnas.0902192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Wang Y, Janis C-M, Goodall R-H, Purnell M-A. An examination of feeding ecology in Pleistocene proboscideans from southern China (Sinomastodon, Stegodon, Elephas), by means of dental microwear texture analysis. Quat Int. 2016;445:60–70. [Google Scholar]
- 18.Barnosky A-D, et al. Variable impact of Late-Quaternary megafaunal extinction in causing ecological state shifts in North and South America. Proc Natl Acad Sci USA. 2016;113:856–861. doi: 10.1073/pnas.1505295112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malhi Y, et al. Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc Natl Acad Sci USA. 2016;113:838–846. doi: 10.1073/pnas.1502540113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovács J, et al. Pliocene and Early Pleistocene paleoenvironmental conditions in the Pannonian Basin (Hungary, Slovakia): Stable isotope analyses of fossil proboscidean and perissodactyl teeth. Palaeogeogr Palaeoclimatol Palaeoecol. 2015;440:455–466. [Google Scholar]
- 21.Solounias N, Semprebon G. Advances in the reconstruction of ungulate ecomorphology with application to early fossil equids. Am Mus Novit. 2002;(3366):1–49. [Google Scholar]
- 22.Dantas M, et al. Isotopic paleoecology of the Pleistocene megamammals from the Brazilian Intertropical Region: Feeding ecology (δ13C), niche breadth and overlap. Quat Sci Rev. 2017;170:152–163. [Google Scholar]
- 23.Domingo L, Prado J-L, Alberdi M-T. The effect of paleoecology and paleobiogeography on stable isotopes of Quaternary mammals from South America. Quat Sci Rev. 2012;55:103–113. [Google Scholar]
- 24.González-Guarda E, et al. Late Pleistocene ecological, environmental and climatic reconstruction based on megafauna stable isotopes from northwestern Chilean Patagonia. Quat Sci Rev. 2017;170:188–202. [Google Scholar]
- 25.Prado J-L, Alberdi M-T. Quaternary mammalian faunas of the Pampean Region. Quat Int. 2010;212:176–186. [Google Scholar]
- 26.Maldonado A, et al. Early Holocene climate change and human occupation along the semiarid coast of north-central Chile. J Quat Sci. 2010;25:985–988. [Google Scholar]
- 27.Kaiser J, Schefuß E, Lamy F, Mohtadi M, Hebbeln D. Glacial to Holocene changes in sea surface temperature and coastal vegetation in north central Chile: High versus low latitude forcing. Quat Sci Rev. 2008;27:2064–2075. [Google Scholar]
- 28.Villagrán C, Varela J. Palynological evidence for increased aridity on the central Chilean coast during the Holocene. Quat Res. 1990;34:198–207. [Google Scholar]
- 29.Valero-Garcés B-L, et al. Palaeohydrology of Laguna de Tagua Tagua (34°30′ S) and moisture fluctuations in central Chile for the last 46,000 yr. J Quat Sci. 2005;20:625–641. [Google Scholar]
- 30.Kohn M-J. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc Natl Acad Sci USA. 2010;107:19691–19695. doi: 10.1073/pnas.1004933107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metcalfe J-Z, Longstaffe F-J, Hodgins G. Proboscideans and paleoenvironments of the Pleistocene Great Lakes: Landscape, vegetation, and stable isotopes. Quat Sci Rev. 2013;76:102–113. [Google Scholar]
- 32.Koch P-L. Isotopic study of the biology of modern and fossil vertebrates. In: Michener R, Lajtha K, editors. Stable Isotopes in Ecology and Environmental Science. 2nd Ed. Blackwell; Boston: 2007. pp. 99–154. [Google Scholar]
- 33.Drucker D, Bocherens H, Bridault A, Billiou D. Carbon and nitrogen isotopic composition of red deer (Cervus elaphus) collagen as a tool for tracking palaeoenvironmental change during the Late-Glacial and Early Holocene in the northern Jura (France) Palaeogeogr Palaeoclimatol Palaeoecol. 2003;195:375–388. [Google Scholar]
- 34.Petermann-Pichincura A, González-Guarda E. Dónde vivían y qué comían?: Un estudio multiproxy del hábitat y la dieta de los proboscideos en Chile. In: Rubilar-Rogers D, Otero R, editors. Primera Reunión de Paleontología de Vertebrados de Chile. Libro de Resúmenes. MNHN, Santiago; Chile: 2017. p. 49. [Google Scholar]
- 35.Fox-Dobbs K, Leonard J-A, Koch P-L. Pleistocene megafauna from eastern Beringia: Paleoecological and paleoenvironmental interpretations of stable carbon and nitrogen isotope and radiocarbon records. Palaeogeogr Palaeoclimatol Palaeoecol. 2008;261:30–46. [Google Scholar]
- 36.Villagrán C, Hinojosa L-F, Llorente-Bousquets J, Morrone J-J. Esquema biogeográfico de Chile. In: Llorente Bousquets J, Morrone J-J, editors. Regionalización Biogeográfica en Iberoamérica y Tópicos Afines. Primeras Jornadas Biogeográficas de la Red Iberoamericana de Biogeografía y Entomología Sistemática. Las Prensas de Ciencias. UNAM; Mexico City: 2005. pp. 551–557. [Google Scholar]
- 37.Codron J, et al. Elephant (Loxodonta africana) diets in Kruger National Park, South Africa: Spatial and landscape differences. J Mammal. 2006;87:27–34. [Google Scholar]
- 38.Amundson R, et al. Global patterns of the isotopic composition of soil and plant nitrogen. Global Biogeochem Cycles. 2003;17:1031. [Google Scholar]
- 39.Haiduc B-S, Răţoi B-G, Semprebon G-M. Dietary reconstruction of Plio-Pleistocene proboscideans from the Carpathian Basin of Romania using enamel microwear. Quat Int. 2018;467:222–229. [Google Scholar]
- 40.Shrader A-M, Bell C, Bertolli L, Ward D. Forest or the trees: At what scale do elephants make foraging decisions? Acta Oecol. 2012;42:3–10. [Google Scholar]
- 41.Thomas B, Holland J-D, Minot E-O. Elephant (Loxodonta africana) home ranges in Sabi Sand Reserve and Kruger National Park: A five-year satellite tracking study. PLoS One. 2008;3:e3902. doi: 10.1371/journal.pone.0003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Codron J, et al. Stable isotope series from elephant ivory reveal lifetime histories of a true dietary generalist. Proc Biol Sci. 2012;279:2433–2441. doi: 10.1098/rspb.2011.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clauss M, Kaiser T, Hummel J. The morphophysiological adaptations of browsing and grazing mammals. In: Gordon IJ, Prins HHT, editors. The Ecology of Browsing and Grazing. Springer; Berlin: 2008. pp. 47–88. [Google Scholar]
- 44.Schmitt M-H, Ward D, Shrader A-M. Incorporating secondary metabolites, tannin-binding proteins, and diet breadth into carrying-capacity models for African elephants. Ecol Modell. 2016;332:8–18. [Google Scholar]
- 45.Tornero C, et al. Seasonality and season of birth in Early Eneolithic sheep from Cheia (Romania): Methodological advances and implications for animal economy. J Archaeol Sci. 2013;40:4039–4055. [Google Scholar]
- 46.Longin R. New method of collagen extraction for radiocarbon dating. Nature. 1971;230:241–242. doi: 10.1038/230241a0. [DOI] [PubMed] [Google Scholar]
- 47.Bocherens H, et al. Biogéochimie isotopique (13C, 15N, 18O) et paléoécologie des ours pléistocènes de la grotte d’Aldène. Bull Mus Anthropol Prehist Monaco. 1991;34:29–49. [Google Scholar]
- 48.Asevedo L, Winck G-R, Mothé D, Avilla L-S. Ancient diet of the Pleistocene gomphothere Notiomastodon platensis (Mammalia, Proboscidea, Gomphotheriidae) from lowland mid-latitudes of South America: Stereomicrowear and tooth calculus analyses combined. Quat Int. 2012;255:42–52. [Google Scholar]
- 49.Wesolowski V, de Souza SMFM, Reinhard KJ, Ceccantinni G. Grânulos de amido e tólitos em cálculos dentários humanos: Contribuiçaõ ao estudo do modo de vida e subsistência de grupos sambaquianos do litoral sul do Brasil. Rev Museu Arqueol Etnol. 2007;17:191–210. [Google Scholar]
- 50.Maher L-J. Statistics for microfossil concentration measurements employing samples spiked with marker grains. Rev Palaeobot Palynol. 1981;32:153–191. [Google Scholar]
- 51.Wesolowski V, de Souza S-M-F-M, Reinhard K-J, Ceccantini G. Evaluating microfossil content of dental calculus from Brazilian sambaquis. J Archaeol Sci. 2010;37:1326–1338. [Google Scholar]
- 52.Casanova M, Salazar O, Seguel O, Luzio W. The Soils of Chile. Springer Netherlands; Dordrecht: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.