Significance
During search, information about the similarity of stimuli to the searched-for target is thought to be encoded in spatial priority maps, which signal the behavioral relevance of stimuli across the visual field. The frontal eye field (FEF) and the lateral intraparietal area (LIP) have both been reported to hold priority maps, but it is unknown whether the two areas have identical or distinct roles in encoding attentional priority. Here, we show that whereas LIP responses reflect the similarity of stimuli to the target, FEF responses integrate perceptual relevance with oculomotor decisions. Moreover, although feature-based attention effects are stronger within FEF, they emerge at similar latencies in subpopulations of the two areas, implying parallel processing of priority information.
Keywords: visual search, frontal eye field, lateral intraparietal area, feature-based attention, saccades
Abstract
When searching for an object in a crowded scene, information about the similarity of stimuli to the target object is thought to be encoded in spatial priority maps, which are subsequently used to guide shifts of attention and gaze to likely targets. Two key cortical areas that have been described as holding priority maps are the frontal eye field (FEF) and the lateral intraparietal area (LIP). However, little is known about their distinct contributions in priority encoding. Here, we compared neuronal responses in FEF and LIP during free-viewing visual search. Although saccade selection signals emerged earlier in FEF, information about the target emerged at similar latencies in distinct populations within the two areas. Notably, however, effects in FEF were more pronounced. Moreover, LIP neurons encoded the similarity of stimuli to the target independent of saccade selection, whereas in FEF, encoding of target similarity was strongly modulated by saccade selection. Taken together, our findings suggest hierarchical processing of saccade selection signals and parallel processing of feature-based attention signals within the parietofrontal network with FEF having a more prominent role in priority encoding. Furthermore, they suggest discrete roles of FEF and LIP in the construction of priority maps.
Selective attention allows us to focus on a subset of sensory inputs to limit visual processing to objects or locations that are most relevant to behavior. Converging evidence from brain imaging, recording, and lesion studies has established that prefrontal (PFC) and parietal cortical areas are critical components of this selection process (1–3). Two areas in particular within the parietofrontal network, the lateral intraparietal area (LIP) and the frontal eye field (FEF), have been implicated in the control of spatial orienting to locations of interest via saccades and via covert deployment of attention. It is widely held that neurons in these two areas integrate information about the physical properties of stimuli and current behavioral goals to construct a priority map in which stimuli or spatial locations are represented by a level of activity that reflects their attentional priority (3–5).
Attention can be allocated to spatial and nonspatial visual features (spatial and feature-based attention, respectively). Recent studies have suggested hierarchical processing of spatial attention signals in the parietofrontal network, with FEF (and lateral PFC) being the origin of such signals in endogenous attention and LIP in exogenous attentional processes (6, 7) (but see ref. 8). Few studies, however, have dissociated feature from spatial attention signals in visual search tasks. These have shown that neuronal responses in FEF and LIP are modulated by attention to objects and features (9–11). However, a direct comparison of how feature-based attention signals are processed within the FEF–LIP circuit is missing and the specific contributions of FEF and LIP in the construction of priority maps remain elusive.
By some accounts, priority-related information is directed from LIP to FEF, which in turn generates attentional and gaze shift signals (12, 13). Connectional and functional data support a role of LIP in encoding visual features, particularly when they are task relevant, by integrating feature information from upstream visual areas (14–20). Thus, LIP neurons are well suited to compute the relative priority of stimuli based on their features and could therefore act as a source of feature attention signals to FEF and possibly extrastriate areas. Conversely, it is also possible that FEF neurons receive feature attention signals from other PFC regions (9) and send this information to LIP. Alternatively, feature attention effects could emerge at the same time in the two areas, suggesting parallel rather than serial encoding of feature attention within the parietofrontal network.
To test whether spatial and feature-based attention signals are processed in a serial or parallel manner within the FEF–LIP circuit, we recorded neuronal responses in the two areas simultaneously in a visual search task with unconstrained eye movements. Our results show earlier and more pronounced spatial selection effects in FEF, consistent with hierarchical processing of saccade selection signals. Feature attention effects were more robustly encoded in FEF; however, they emerged simultaneously in subpopulations of FEF and LIP neurons. Thus, our results do not support serial processing of priority signals from LIP to FEF. Furthermore, we reveal differences in priority encoding in the two areas. In LIP, the similarity of stimuli to the target is encoded independently of saccade selection, whereas in FEF target similarity encoding is strongly influenced by spatial selection.
Results
We trained two monkeys in a free-viewing visual search task (Fig. 1A). Briefly, the animals were required to fixate a central spot, which was subsequently replaced by a cue corresponding to the search target. Following a variable delay period, an array of eight stimuli appeared on the screen. One of the stimuli was the target and the remaining were distractors, two of which shared the target’s color and one the target’s shape. The remaining distractors did not share features with the target. Monkeys were allowed to freely scan the array to locate the target and were rewarded after fixating it for 700 ms. The behavioral search pattern indicated that both animals used the target’s features to guide the search (SI Appendix).
Fig. 1.
Schematic representation of the behavioral task. (A) Sequence of events in the free-viewing visual search task. Monkeys were required to fixate the central fixation spot and subsequently the cue that indicated the target stimulus. Following a delay period, monkeys were allowed to scan the array to locate and fixate the target. The solid lines indicate a hypothetical eye position trace (saccades to different stimuli in the array). (B) Conditions used to study feature attention. The oval indicates a hypothetical neuronal RF during the initial fixation. Note that stimuli in the RF are the same in all displays. However, depending on the identity of the target (shown below each display), the task relevance of the RF stimuli is different in the different trials.
Feature Attention Modulates Neuronal Responses in FEF and LIP.
We recorded neuronal activity from 590 visually responsive units in FEF (330 from monkey PT and 260 from monkey FN) and 542 units in LIP (297 from monkey PT and 245 from monkey FN). Responses were qualitatively similar in the two monkeys; thus, they were combined. We first asked whether feature-based attention modulated responses in the two areas. To this end, we considered four conditions depending on the similarity of the response field (RF) stimulus to the target (Fig. 1B). These comprised trials in which the RF stimulus during the initial fixation was (i) the target (“target”), (ii) a distractor that shared the target’s color (“share color”), (iii) a distractor that shared the target’s shape (“share shape”), and (iv) distractor(s) that did not share features with the target (“no share”). To isolate the effects of feature attention from those of saccade execution, we considered only trials in which the saccade was made away from the RF.
Neuronal responses were modulated by feature attention in both FEF and LIP. We found that 211 units in FEF and 149 units in LIP (FEF, 54%; LIP, 46% of units with enough trials to be included in the analysis) showed a significant effect of target similarity in their responses (one-way ANOVA, P < 0.05; 50-ms sliding windows moved at 25-ms step, 25–200 ms following array onset). At the population level, average neuronal responses to the target were significantly enhanced compared with those to irrelevant distractors by 15.4% in FEF and by 4.3% in LIP (average response in a window 150–200 ms following array onset; FEF, P < 0.001; LIP, P < 0.01, paired t test; Fig. 2 A and B). Similar results were obtained using earlier time windows (e.g., 130–200 ms following array onset). Responses to distractors that shared the target’s color or shape were intermediate between those to the target and irrelevant distractors. At the level of individual units, we quantified the difference between responses in the target and no share conditions by calculating a feature attention index. Attention indices were significantly different from zero in both FEF and LIP (Fig. 2E; P < 0.001 in both cases, Wilcoxon signed-rank test). To determine whether individual units showed a significant effect of target similarity, we pooled responses in the share color and share shape conditions and calculated a feature attention index between target and share as well as share and no share conditions. The differences between share and no share conditions were significantly different from zero across units in both areas (FEF, median, 0.039; P < 0.001; LIP, median, 0.014; P < 0.05, Wilcoxon signed-rank test). Similarly, differences between the target and share conditions were significantly different from zero in FEF; however, they did not reach significance in LIP (FEF, median, 0.031; P < 0.001; LIP, median, 0.012; P = 0.055, Wilcoxon signed-rank test). These results suggest that responses in both areas are enhanced for targets relative to irrelevant distractors and that they are intermediate for stimuli that share features with the target. Thus, they provide support to the notion that both FEF and LIP hold a map of attentional priority. However, a comparison of the effect magnitude in the two areas revealed that the target-related effect was significantly larger in FEF compared with LIP (Fig. 2F; median, FEF, 0.07; median, LIP, 0.02; P < 0.001, Wilcoxon rank-sum test). When we matched the RF stimuli across the four conditions, results were qualitatively and statistically equivalent to those obtained above (SI Appendix, Fig. S2).
Fig. 2.
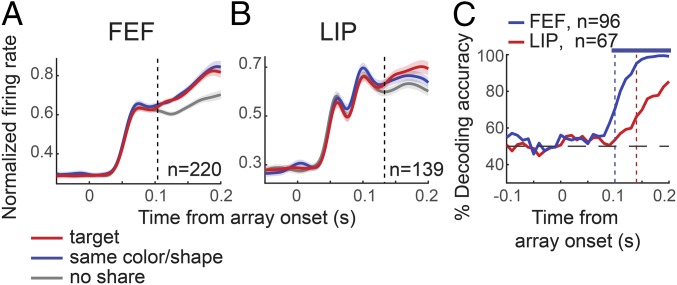
Feature-based attention effects during the first fixation when the first saccade was made away from the RF. (A and B) Normalized population average firing rates aligned on array onset in target (red), share color (green), share shape (blue), and no share trials (gray), in FEF (A) and LIP (B). The dotted vertical lines indicate latency of feature attention effects at the population level. Error bars (shaded area around each line) represent ±SEM. (C and D) Responses aligned on saccade initiation in FEF (C) and LIP (D). (E) Distribution of feature attention indices in LIP (Top) and FEF (Bottom). The colored bars correspond to units with significant modulation of activity between the target and no share conditions. The arrows indicate the median of the distribution. (F) Cumulative distribution of feature attention indices in FEF (blue) and LIP (red). (G) Cumulative distribution of feature attention latencies in FEF (blue) and LIP (red) computed from individual units. (H) Time-resolved decoding accuracy for feature attention in FEF (blue) and LIP (red). Classification between target, share color/shape, and no share was based on 20 trials from each condition (chance is at 33.3%). The dotted lines indicate the time at which decoding performance in each population exceeded chance. The blue horizontal line at the top of the graph indicates periods that classification accuracy was significantly higher for FEF compared with LIP (P < 0.001, permutation test).
Next, we examined the relative onset of feature attention effects in the two areas. We reasoned that if LIP activity signals the attentional priority of stimuli, which is subsequently sent to FEF to guide saccades, feature attention effects should emerge earlier in LIP. At the population level, feature attention latencies emerged significantly earlier in FEF. Specifically, significant differences in activity between the target and no share conditions were found as early as 125 and 167 ms following array onset, in FEF and LIP, respectively (P < 0.01, permutation test; Fig. 2 A and B). These feature attention effects occurred before the onset of the first saccade in both FEF and LIP, with effects reaching statistical significance at 45 and 25 ms, respectively, before the initiation of the first saccade (Fig. 2 C and D).
To test whether this earlier onset of effects was due to the larger feature attention effects in FEF, we estimated latencies for a subpopulation of FEF and LIP units with target-related enhancements of at least 10% (window 100–200 ms following array onset). We identified 179 such units in FEF and 107 units in LIP. When we averaged responses across these units and calculated the latency of the feature effect, we found that attentional latencies were not significantly different in the two areas (FEF, 90 ms; LIP, 98 ms; P = 0.28, permutation test; Fig. 3 A and B). Similar results were obtained when we limited analysis to units in the two areas with similar effect size (10–30% enhancement in target condition; P = 0.8, permutation test). Consistent with this finding, feature attention latencies computed for individual units either from the whole population or from the subpopulations with target-related enhancements greater than 10% were not different in the two areas (Figs. 2G and 3C). More specifically, we were able to reliably estimate a feature attention latency in 153 neurons in FEF and 60 neurons in LIP, which met the criteria for the determination of feature attention latency (significant difference in activity between target and no share conditions for a minimum of 30 consecutive 1-ms bins). The distributions of latencies in the two areas were not significantly different (FEF, median, 162 ms; LIP, median, 161 ms; P = 0.7, permutation test; Fig. 2G). However, the proportion of units in which a feature attention latency could be estimated was significantly higher in FEF than that in LIP (FEF, 38.7%; LIP, 18.5%; P < 0.001, χ2 test). Similar results were obtained when we considered feature attention effects during subsequent fixations (SI Appendix, Figs. S3 and S4).
Fig. 3.
Feature-based attention effects on FEF and LIP neurons with target-related enhancements. Normalized population average firing rates in (A) FEF and (B) LIP aligned on array onset when the first saccade was directed away from the RF. Only units with target-related enhancements of 10% or above are included. Responses to share color and share shape distractors were similar; thus, they were combined (blue). (C) Cumulative distribution of feature attention latencies in FEF (blue) and LIP (red) computed from individual units of this subpopulation. Other conventions are as in Fig. 2.
Overall, our results indicate that feature-based attention modulates responses in both areas, but effects are more pronounced and more widespread within the FEF population, and as a result, they can be identified earlier in FEF at the population level. However, a population of LIP units with large enough feature selection effects discriminates the target from irrelevant distractors as early as FEF. We found no evidence that these LIP units were spatially clustered within LIP. Neither the depth at which they were encountered nor their position on the anterolateral-to-posteriomedial axis differed from that of nonselective units.
Given that both FEF and LIP modulate their responses according to the similarity of the RF stimulus to the target, we sought to determine whether information about target similarity can be reliably decoded from neuronal responses in the two areas on a trial-by-trial basis. To this end, we used a linear support vector machine (SVM) algorithm that classifies responses based on the pattern of activity on individual trials across the whole population. We asked whether we can predict if (i) the target, (ii) a distractor that shared the target’s color or shape, or (iii) irrelevant distractor(s) were inside the RF of the recorded neurons. As in the univariate analysis above, we isolated feature attention from saccade goal selection by considering trials in which the first saccade was made away from the RF. Neuronal responses were classified into three classes with equal prior probabilities; thus, chance performance was 33.3%. The similarity of the stimulus in the RF to the target could be decoded robustly from both areas with accuracies significantly above chance (Fig. 2H). In agreement with the results presented above, decoding performance was higher (101–118 and 131–200 ms; P < 0.001, permutation test) and reached significance earlier for the FEF population (FEF, 110 ms; LIP, 120 ms; P < 0.01, permutation test). Decoding accuracies approached 100% in the FEF when classification was limited to two categories (target in RF, irrelevant distractor in RF; SI Appendix, Fig. S5). These results provide additional evidence that feature attention-related information is encoded earlier and more robustly by the FEF population compared with LIP.
Feature Attention Effects with the Saccade Directed to the RF.
To dissociate spatial from feature-based attention effects, feature attention modulations are typically measured with attention directed away from the RF (9–11, 21, 22). In free-gaze visual search tasks, target or feature-related enhancements, such as the ones reported here, suggest that a stimulus-related response enhancement at the level of individual units does not always lead to a saccade toward that stimulus. It is conceivable that a stronger or earlier feature attention effect might lead to selection of the RF stimulus as the goal of the next saccade. Moreover, if information about the attended feature from LIP is sent to FEF to guide saccades to the appropriate location, one could expect that activity in FEF might not dissociate between target and distractors when saccades are directed to the RF stimulus.
To test these ideas, we selected initial fixations that preceded saccades to the RF and compared responses across three conditions depending on whether the RF stimulus was (i) the target, (ii) a share stimulus, and (iii) a no share distractor. At the population level, activity in both areas showed a significant effect of target similarity (FEF, P < 0.001; LIP, P < 0.05, one-way ANOVA; window, 150–200 ms following array onset; Fig. 4 A and B). Post hoc tests revealed that responses to the target were significantly enhanced relative to those to irrelevant distractors in both areas (average enhancement: FEF, 17.3%; P < 0.001; LIP, 11.5%; P < 0.05, Tukey–Kramer post hoc test). Pairwise comparisons between responses in the target and share conditions and between the share and no share conditions revealed that at the population level, only the difference between FEF responses in the share and no share conditions reached significance (P < 0.001, Tukey–Kramer post hoc test). However, quantification of the effects at the level of individual units offered additional insight. Specifically, we confirmed that the enhancement in the target condition compared with the no share condition was significant across units (median index: FEF, 0.075; LIP, 0.04; P < 0.001 in both areas, Wilcoxon signed-rank test). Attention indices were significantly higher in FEF (P < 0.05, Wilcoxon rank-sum test). Similar indices were calculated to quantify the size of the effect between target and share as well as between share and no share conditions. In LIP, the distribution of both indices was shifted toward positive values and the medians were significantly different from zero (median index, share vs. no share, 0.027; target vs. share, 0.026; P < 0.05 in both cases, Wilcoxon signed-rank test). In FEF, the effect between share and no share conditions was significant (median index, 0.072; P < 0.001, Wilcoxon signed-rank test), whereas that between target and share conditions was not (median index, 0.001; P = 0.7, Wilcoxon signed-rank test). These results suggest that the behavioral relevance of the stimulus modulates responses in both areas even when the saccade is directed to the RF stimulus, with the target inducing an enhancement of neuronal responses on average. Thus, neither FEF nor LIP carries exclusively information about the spatial goal of the saccade. Interestingly, however, whereas in LIP, stimuli appear to be represented by a level of activity that reflects their similarity to the target, consistent with the idea of a priority map, in FEF, stimuli that are likely targets (target and share color/shape) are represented by the same level of activity when the saccade is directed to them. The distribution of effects between target and share color/shape distractor in RF conditions is shown in SI Appendix, Fig. S6. Similar results were obtained for late search fixations that preceded saccades to the RF (SI Appendix, Fig. S7). We also examined the latency of target-related enhancements relative to irrelevant (no share) distractors. Similar to our results in saccade away trials, the target-related enhancement at the population level emerged significantly earlier in the FEF compared with LIP (FEF, 104 ms; LIP, 133 ms; P < 0.01, permutation test; Fig. 4 A and B). However, latencies calculated from individual units were not significantly different in the two areas (median latency, FEF, 135 ms; LIP, 150 ms; P = 0.6, permutation test), although a significantly larger proportion of FEF units gave reliable latency estimates (FEF, 27.9%; LIP, 16%; P < 0.001, χ2 test).
Fig. 4.
Feature-based attention effects during the first fixation when the subsequent saccade was directed to stimuli in the RF. Normalized population average firing rates in (A) FEF and (B) LIP. (C) Decoding accuracy for target vs. no share conditions across the FEF and LIP populations shown in a time-resolved manner. Classification is based on 20 trials from each condition. Chance is at 50%. The blue horizontal line at the top of the graph indicates periods that classification accuracy was significantly higher for FEF compared with LIP (P < 0.001, permutation test). Other conventions are as in Figs. 2 and 3.
Decoding the behavioral relevance of the stimulus in the RF confirmed that the presence of the target over an irrelevant distractor can be reliably predicted from population activity in both FEF and LIP when the saccade is directed to the RF (Fig. 4C). Decoding performance was above chance (50%) in both areas; however, it was significantly higher (92–200 ms; P < 0.001, permutation test) and exceeded chance levels earlier for the FEF population (FEF, 100 ms; LIP, 140 ms; P < 0.001, permutation test).
We sought to determine whether the latency or the magnitude of feature attention effects are influenced by the direction of the impending saccade. To this end, we first asked whether neuronal responses can distinguish the target earlier when the RF stimulus is selected as the saccade goal and compared feature attention latencies with the saccade directed to and away from the RF. When the saccade was directed to the RF, feature attention effects emerged 21 ms earlier in the FEF and 34 ms earlier in LIP (P < 0.05 in both cases, permutation test). The magnitude of the effect, however, did not differ significantly between the two conditions (P > 0.1 for both FEF and LIP, Wilcoxon rank-sum test; window 150–200 ms following array onset). By contrast, we found that in FEF, the relative difference in responses to the target and share distractors depended on whether the stimulus that shared features with the target would be fixated or not. Specifically, an index that quantified the difference between the two conditions (target in RF vs. share distractor in RF) was computed for each unit for saccades to and away from the RF. In LIP, the distributions of these indices were not significantly different for saccades to and away from the RF (median index, saccade to RF, 0.025; saccade away from RF, 0.011; P = 0.51, Wilcoxon rank-sum test). In FEF, however, the distributions of indices for saccades to and away from the RF were significantly different (median index, saccade to RF, 0.002; saccade away from RF, 0.037; P < 0.001, Wilcoxon rank-sum test). Thus, in FEF, the relative difference in responses to the target and to a stimulus that shares features with the target was modulated by the decision to fixate the latter.
Overall, our results suggest that the target modulates FEF and LIP responses independent of saccade goal location. This supports the idea that parallel mechanisms that signal the behavioral relevance of stimuli before these are overtly attended, play a prominent role in the guidance of visual search. However, when stimuli in the RF are selected to be fixated, target-related enhancements occur earlier, indicating that the relative onset of feature-based attention modulations in FEF and LIP influences behavioral outcome. This will be further explored in the following section.
Activity in FEF and LIP Reflects Behavioral Performance.
When we scan a visual scene, saccades are performed extremely fast. It is therefore likely that the first saccade goal is determined before feature attention effects can influence the decision. Accordingly, an enhanced response to the target during the initial fixation could have guided target selection during the next fixation. In fact, previous studies have confirmed such a relationship between response enhancements in FEF activity and future saccades (11, 23). We therefore asked whether responses in FEF and LIP are enhanced when the target is identified with fewer saccades. Fig. 5 shows population average responses to the target in the RF when the first saccade was made away from the RF and the second saccade was directed either toward the target in the RF or away from the RF. In both FEF and LIP, responses were higher when the target was identified with two saccades than when it was identified with more than two saccades (Fig. 5 A and B; P < 0.001 in both areas, paired t test; window 150–200 ms following array onset). Indices that quantified the difference in responses between the two conditions were significantly different from zero in both areas (P < 0.001, Wilcoxon signed-rank test); however, the effect size was not significantly different between the two areas (median, FEF, 0.06; LIP, 0.047; P = 0.16, Wilcoxon rank-sum test). Although the effect at the population level emerged earlier in FEF, the difference in latencies between the two areas was not significant (FEF, 135 ms; LIP, 158 ms following array onset; P = 0.78, permutation test). We also confirmed that this enhanced response was not due to multiple saccade planning (SI Appendix, Fig. S8).
Fig. 5.
Relationship of feature-based attention response modulation to future saccades. (A and B) Normalized population average firing rates in FEF (A) and LIP (B), during the first fixation with the target in the RF and the first saccade executed away from the RF. Responses when the second saccade was directed to the target in the RF (red) are compared with those when the second saccade was directed away from the RF (gray). (C) Time-resolved decoding of feature-based attention information (target vs. no share trials) from FEF (blue) and LIP (red) responses using trials in which the target was identified with up to two saccades (solid lines) or with more than two saccades (dashed lines). Classification is based on 20 trials from each condition. Chance is at 50%. The dotted vertical lines indicate the times at which decoding performance exceeded chance in trials that the target was found with a maximum of two saccades. When the target was identified after more than two saccades, accuracy did not exceed chance. The horizontal lines at the top of the graph indicate periods during which classification accuracy was significantly higher when using trials in which the target was identified with up to two saccades compared with using trials in which the target was identified with more than two saccades in FEF (blue) and LIP (red) (P < 0.001, permutation test). Other conventions are as in Fig. 2.
Another way to explore the relationship between feature attention-induced modulations and behavior, is by estimating in neuronal responses the amount of information about the task relevance of the stimulus relative to the number of saccades needed to fixate the target. We decoded whether the target or an irrelevant distractor was in the RF from population activity in the two areas, and compared classification accuracy using trials in which the target was found with up to two saccades and trials in which the target was found with more than two saccades (Fig. 5C). We included only trials in which the first saccade was made away from the RF. When the animals located the target with a maximum of two saccades, the behavioral relevance of the stimulus (target or distractor) could be reliably decoded from FEF and LIP activity. In agreement with the more prominent effects observed in FEF during feature-based attention, decoding performance was higher and reached significance earlier in FEF compared with LIP (latency: FEF, 110 ms; LIP, 140 ms; P < 0.001, permutation test). By contrast, when the target was found with more than two saccades, classification accuracy did not reach significance in either area. Overall, these results provide strong evidence that information about the target carried by neuronal populations in FEF and LIP predicts and likely guides behavior during search.
Saccade Goal Selection.
Besides representing the behavioral priority of stimuli, FEF and LIP have been implicated in saccade goal selection (11, 24, 25). Both areas have direct projections to premotor oculomotor structures (26, 27). However, to our knowledge, no study has directly compared saccade goal selection latencies in the two areas. We contrasted responses before a saccade to the RF to those before a saccade away from the RF, regardless of the behavioral relevance of the RF stimuli (Fig. 6). We found that saccade goal selection modulated responses in both FEF and LIP (mean enhancement when the saccade was executed to the RF, 24.1% in FEF and 11.2% in LIP; P < 0.001 in both areas, paired t test; window 150–200 ms following array onset; Fig. 6 A and B). However, the magnitude of the effect was significantly larger in FEF compared with LIP across units (median modulation index: FEF, 0.86; LIP, 0.47; P < 0.001, Wilcoxon rank-sum test). Saccade goal selection times at the population level were shifted significantly earlier in FEF compared with LIP (FEF, 90 ms; LIP, 120 ms; P < 0.001, permutation test) and emerged long before the initiation of the first saccade (Fig. 6 C and D; FEF, 142 ms; LIP, 83 ms before saccade onset). The cumulative distribution of saccade goal selection latencies was also shifted toward earlier times for FEF units (Fig. 6E; median, FEF, 135 ms; LIP, 151 ms following array onset; P < 0.001, permutation test). To test whether units with large effects in the two areas select the saccade goal at similar latencies, we recalculated latencies for a subpopulation of FEF and LIP units with response enhancement greater than 10% during saccades to the RF (247 FEF units and 131 LIP units; window 150–200 ms following array onset). Latencies were shifted significantly earlier for the FEF in this subset of sites too (median latency: FEF, 132 ms; LIP, 138 ms; P < 0.05, permutation test), and this result also held for equal population sizes, confirming that the FEF encodes the goal of the upcoming saccade before LIP.
Fig. 6.
Saccade goal selection effects around the first saccade. (A and C) Normalized population average firing rates in FEF aligned on array onset (A) and on first saccade onset (C). (B and D) Normalized population average firing rates in LIP aligned on array onset (B) and on first saccade onset (D). Responses when the saccade was directed to the RF (red lines) are compared with those when the saccade was directed away from the RF (gray lines). (E) Cumulative distribution of saccade goal selection latencies in FEF and LIP computed from individual units. (F) Time-resolved decoding accuracy of saccade direction (to vs. away from RF). Classification is based on 50 trials from each condition. Chance is at 50%. Dotted lines indicate the times at which decoding performance exceeded chance. Other conventions are as in Fig. 2.
The more prominent and earlier involvement of FEF in spatial selection was also confirmed by a decoding analysis using an SVM classifier and trials in which the first saccade was directed toward or away from the RF (50% chance accuracy). Decoding performance was significantly higher in FEF compared with LIP and exceeded chance levels earlier for the FEF population (FEF, 70 ms; LIP, 90 ms following array onset; P < 0.001, permutation test; Fig. 6F). Overall, our results confirm a more prominent role of FEF in saccade selection compared with LIP and support hierarchical processing of saccade goal selection signals within the FEF–LIP circuit.
Relative Latency of Feature Attention and Saccade Goal Selection Effects.
Having demonstrated that activity in FEF and LIP is modulated by feature-based attention and saccade goal selection, we next compared the latencies of feature attention and saccade direction effects during the initial fixation. To this end, we estimated the amount of information about these processes carried by neuronal responses in the two areas, using the percentage of explained variance metric (ωPEV). By means of a three-way ANOVA, we quantified how much of the variance in each unit’s firing rate can be explained by each of the following three factors: (i) the visual content of the display (irrespective of behavioral relevance of stimuli), (ii) the behavioral relevance of stimuli in the RF (feature attention), and (iii) saccade goal selection (overt spatial attention), that is, whether the saccade was made toward or away from the RF.
The visual features of the stimuli in the array, irrespective of their behavioral relevance, exerted the earliest influence on neuronal activity as early as 95 ms following array onset in both areas (Fig. 7). The influence of spatial selection on neuronal activity was the next that reached significance. In agreement with the results presented in the previous sections, information about the saccade goal location appeared significantly earlier in FEF (FEF, 115 ms; LIP, 135 ms; P < 0.01, permutation test). Finally, the behavioral relevance of stimuli in the RF (similarity to target) was the last to influence neuronal responses in the two areas and appeared significantly earlier in FEF (FEF, 145 ms; LIP, 175 ms; P < 0.001, permutation test). The difference in latencies among all three factors was significant within each area (P < 0.001 for all comparisons, permutation test with Bonferroni–Holm correction for multiple comparisons). Interestingly, saccade goal location had a significantly earlier influence on neuronal activity compared with feature attention. This finding indicates that, on average, the saccade goal is determined before the encoding of stimulus relevance, at least during the initial fixation, in agreement with previous studies (10, 11). However, stimulus relevance is encoded before the onset of the first saccade, and this information is available to guide subsequent saccades as suggested by results shown in previous sections. In line with this, we found that, in FEF, feature attention is encoded before the goal of the second saccade during the initial fixation, while in LIP, feature attention and the second saccade goal are encoded at similar times (SI Appendix, Fig. S9).
Fig. 7.
Neural information about different task variables. Information is measured as the percentage in spiking variance explained by each variable independently (ωPEV). Average z-scored ωPEV values in (A) FEF and (B) LIP are shown for units with significant PEV values for display (gray), saccade goal location (red), and feature-based attention (blue). The dashed horizontal line indicates the 95% confidence limit (z-score PEV, 1.645). Error bars represent ±SEM.
Discussion
In the present study, we examined how spatial selection and feature-based priority signals are processed within FEF and LIP, two areas that have been implicated in the construction of priority maps. By some accounts, LIP responses encode the relative priority of stimuli or locations in the visual field, and downstream areas use this information depending on the behavioral context (10, 12, 13). Accordingly, these signals are fed to oculomotor structures to select the goal of saccadic eye movements and to visual areas to selectively enhance the neuronal representation of attended stimuli for detailed visual processing. By recording simultaneously from the FEF, an oculomotor-related area, and LIP, during a free-viewing visual search task, we provide evidence that does not support serial processing of priority related signals from LIP to FEF. Moreover, our results reveal differences in priority encoding in the two areas.
The concept of a priority map posits that bottom-up information about the features of the visual stimulus from early visual cortices (feature maps), and information about prior knowledge and current behavioral goals from higher-order areas, is integrated into a map of the visual field where objects, features, or locations are represented by a level of activity that reflects their relative priority (4, 5, 28). This idea can be tested in visual search paradigms in which spatial attention can be dissociated from feature-based attention, and attention effects on neuronal responses can be directly linked to behavior. For feature-based attention, we confirmed that, in both FEF and LIP, target stimuli were associated with higher responses compared with irrelevant distractors, whereas distractors that shared features with the target (either color or shape) induced intermediate responses. These results are consistent with earlier studies, which have reported object (9–11) and feature attention effects (11, 19, 29) in FEF and LIP. By comparing the magnitude and latency of attention effects in the two areas, we found that object and feature attention effects were more pronounced and emerged earlier in the FEF population compared with LIP. However, a substantial proportion of LIP units that showed similar magnitude of effects as those in an FEF population encoded the target at a similar latency with the FEF. Thus, our results demonstrate that LIP comprises heterogeneous neuronal populations and that a subset of neurons within LIP encodes target similarity as early as the FEF. A diversity of responses in LIP has also been reported in previous studies, which have shown that distinct LIP populations can independently encode or multiplex sensory and cognitive signals (30–33). In our study, LIP sites that encoded feature-based attention were not anatomically clustered but were intermingled with sites that showed no effect of feature-based attention. Such spatial intermingling of LIP neurons with different properties has been reported previously (30, 31). Future studies should directly test whether this diverse pattern of responses can be mapped onto distinct cell types or networks with identifiable functional and/or connectional signatures.
What might be the source of the feature-based attention effects observed in FEF and LIP? Previous studies have reported earlier feature and object attention effects in FEF compared with V4 (11) or IT (9). These results together with ours suggest that feature-based attention signals emerge outside the visual cortices. Indeed, a recent study showed that inactivation of a prefrontal area, the ventral prearcuate (VPA) region, impairs the ability of monkeys to locate search targets and eliminates the target-related enhancement in FEF (9). Thus, VPA could be a source of object or feature-based attention signals to FEF. Consistent with this idea, VPA neurons carry an attentional template of the searched-for object during the delay and search periods (9), in line with the suggested close link between working memory and priority maps (12, 34). Whether VPA is also a source of direct signals to LIP should be tested in future studies.
Our finding that a subpopulation of LIP neurons encodes the target at similar latencies with the FEF suggests that object- or feature-based attention effects are the result of concurrent processing of feature attention signals in distinct populations within the two areas. Concurrent activation of prefrontal and parietal cortices has been reported before (8) and inactivation studies have revealed an interdependence of signals in the two areas (35, 36), possibly mediated through the abundant reciprocal connections between FEF and LIP (37–41). It is thus possible that, rather than being a sign of redundancy, parallel processing of feature-based attention signals in FEF and LIP underlies distributed processing of priority signals across the two regions (42). Distributed processing of attentional signals could also explain the mild effects observed with lesions limited to a single area. Indeed, prefrontal lesions result in attentional deficits but do not abolish attentional function and reduce but do not eliminate attentional modulation of neuronal responses and gamma synchrony in area V4 (43, 44).
Although our results clearly refute the hypothesis that priority signals are sent from LIP to FEF, they cannot conclusively rule out the possibility that information about attentional priority based on target similarity is sent from FEF to LIP. Feature-based attention effects were more widespread in FEF and emerged earlier within the FEF population compared with LIP. This was consistently observed in all different analyses when the entire populations were considered. Although latencies measured for units with substantial target-related modulation did not differ between the two areas, one could argue that due to the abundant monosynaptic connections between the two areas, the difference in latencies was too small to be detected by our statistical methods. In fact, Bichot et al. (9) also failed to find a statistically significant difference in object-based attention latencies between VPA and FEF. Still, effects in VPA were more widespread and inactivation of VPA abolished object-based attention effects in FEF, pointing to VPA as a source of feature attention signals to FEF. Thus, the current data may be insufficient to conclusively rule out a role of FEF in driving feature-based attention effects in a subpopulation of LIP neurons.
While our finding of enhanced responses to targets relative to irrelevant distractors was consistent for saccades directed to the RF and away from it, we found a striking difference in target similarity encoding in the two areas when saccades were directed to the RF. In LIP, neurons encoded the similarity of RF stimuli to the target even when these were selected as saccade goals. This result is consistent with the fact that a fraction of LIP neurons signals the “match” status of stimuli in a match-to-sample task (31). By contrast, although responses of FEF neurons dissociated targets from irrelevant distractors, responses were identical for targets and stimuli that shared features with the target when the saccade was directed to them. The difference in activity between targets and irrelevant distractors argues against an exclusive motor role of FEF neurons during visual search. Why then were responses to the target and to stimuli that shared color or shape with the target indiscriminable when the saccade was directed to the RF? One possibility is that distractors, which shared features with the target, were selected as the goal of the next saccade when they were considered potential targets and required further processing to be compared with the attentional template. In that case, the relative priority of targets and similar-to-target stimuli would be equal. In line with this interpretation, we found that the median fixation duration for share relative to no share distractors was significantly enhanced (SI Appendix, Behavioral Data), suggesting that animals spent more time on these stimuli. This additional fixation time could facilitate comparison with the memorized target. This finding is in agreement with a previous study, which has also shown that, in a conjunction task, stimuli that share features with the target tend to be fixated for longer time compared with irrelevant distractors (21). Conceivably, distractors that did not share any features with the target could be fixated during search while sampling the array even though their relative priority was low as reflected in the neuronal responses.
Thus, our finding that FEF activity cannot dissociate between likely targets when these are selected as saccade goals indicates that FEF responses are closer to the output of the visuomotor transformation that selects locations of interest based on the behavioral relevance of stimuli. We propose that a likely difference between the priority maps in LIP and FEF is that, in LIP, priority is defined based on the physical properties of stimuli and their similarity to the target whereas, in FEF, priority is modulated by the decision to fixate a likely target.
We also examined how saccade selection affects neuronal responses in the two areas during search. Both FEF and LIP modulated their responses with the direction of the executed saccade, but spatial selection signals were more pronounced and arose earlier in FEF. The response modulation we observed is consistent with a role of both areas in spatial attention and saccade execution (7, 24, 33, 45–52). FEF and LIP are known to project to the superior colliculus (SC), conveying visual as well as saccadic signals (53, 54). Here, we show that FEF neurons encode the goal of the next saccade before LIP neurons suggesting serial processing of saccade selection signals. Moreover, response modulation was stronger in FEF compared with LIP. These results are consistent with inactivation and lesion data that have suggested a more prominent role of FEF in the control and guidance of saccadic eye movements. Compared with FEF inactivation, lesions in LIP produce milder or no deficits in visually and memory guided saccades to single targets (13, 55–59).
A comparison of the relative onset of the different signals carried by the FEF and LIP neuronal populations revealed a temporal succession of visual, motor, and cognitive signals in both areas. Information about the visual features of stimuli was encoded first, but at latencies close to 100 ms following array onset. Considering the early visual response latencies in both areas (60, 61), this relatively late stimulus selectivity is rather surprising. Although we cannot rule out the possibility that such a late stimulus selectivity arises due to the large number of stimuli in the array, we favor the view that the observed effect is not a purely feature selective one but rather one that emerges due to the feature demands of the particular task. In support of this, we found that less than 10% of units in either area showed feature selectivity in windows up to 150 ms when the saccade was directed away from the RF and the stimulus in the RF was an irrelevant distractor. The saccade direction signal was the next to affect neuronal responses in both areas and emerged before the feature-based attention signal. This finding is consistent with previous studies (10, 11) and suggests a strong influence of serial scanning strategies in the free-viewing search task. Animals took advantage of the fact that saccades were not penalized and moved their eyes to scan the array before information about the behavioral relevance of stimuli was processed. This has also been pointed out before (10). Two findings in our study can explain how a relatively late feature attention signal could be used to guide the search. First, feature attention signals were found to influence subsequent saccades and determined how fast the target would be selected. In line with this, we found that, at least in the FEF, feature attention signals emerged before signals related to the goal of the second saccade. Second, when the saccade was directed to the RF stimulus, information about stimulus relevance emerged at significantly earlier times. Thus, both the relative onset of feature-based attention effects and their magnitude determines how fast the target will be found. Our study shows that both FEF and LIP contribute to this process.
In conclusion, our data point to parallel processing of feature-based attention signals in FEF and LIP and highlight differences in the way priority signals are encoded in the two areas. First, priority information based on feature similarity with the target is encoded more robustly within the FEF compared with LIP. Second, whereas activity in LIP reflects the similarity of stimuli to the target, FEF activity integrates perceptual relevance with oculomotor decisions and more closely reflects behavioral decisions that are implemented through saccades.
Methods
Subjects and Surgical Procedures.
Two female rhesus monkeys (Macaca mulatta) weighing 4–6 kg were used in the study. Both animals were purposely bred by authorized suppliers within the European Union (Deutsches Primatenzentum and Cyno Consulting). Experiments were carried out at facilities approved by the Veterinary Authorities of the Region of Crete (Medical School, University of Crete, EL91-BIOexp-06) and complied with the European (Directive 2010/63/EU and its amendments) and national (Presidential Decree 56/2013) laws on the protection of animals used for scientific purposes. Experimental protocols were approved by the Institutional Experimental Protocol Evaluation Committee (Approval 6170/7-5-2014).
The monkeys were implanted with a titanium post to fix the head. Following training, two titanium recording chambers were implanted, one over the FEF and one over LIP [stereotaxic coordinates: monkey PT: FEF, anteroposterior (AP) 27, mediolateral (ML) 17.5; LIP, AP −6, ML 12; monkey FN: FEF, AP 26, ML 18; LIP, AP −3, ML 12, based on MRI scans obtained before surgery]. Surgical procedures were performed under general anesthesia and aseptic conditions.
Behavioral Tasks.
Monkeys were seated in front of a CRT monitor (resolution, 800 × 600; refresh rate, 100 Hz) at a distance of 36 cm, inside a dark isolation box. Stimulus presentation and monitoring of behavioral parameters were controlled by the MonkeyLogic software package (62). Eye position was monitored by an infrared-based tracking system (ETL-200; ISCAN) and was sampled at 120 Hz.
In the free-gaze visual search task, stimuli in each session were generated pseudorandomly from a pool of eight shapes and eight colors. Stimuli were 1.6 × 1.6° in size and were matched for the number of pixels. Colors were matched for luminance (∼7 cd/m2) and were red, green, blue, yellow, orange, purple, magenta, and cyan. Stimuli were presented on a dark background (0.11 cd/m2). To initiate the trial, monkeys had to fixate a square spot (0.75° × 0.75°) at the center of the screen for 300–500 ms within a 2.5° diameter window. Subsequently, the fixation spot was replaced by the cue, which indicated the target that the animals had to locate during the search. After 800 ms, the cue was replaced by the fixation spot and the monkeys were required to maintain central fixation for 500–1,000 ms. Following the delay period, a search array of eight stimuli appeared. One of the stimuli was the target, and the remaining were distractors. Two distractors shared the target’s color, one shared the target’s shape, and the remaining four distractors did not have common features with the target. Animals were allowed to freely move their eyes to scan the array to locate the target within 3.5 s. When they identified the target, they had to fixate it for 700 ms to receive water or juice reward. If the monkeys broke fixation during the initial fixation, cue, and delay periods or failed to fixate the target within 3.5 s, the trial was aborted. Search array stimuli were presented in a rhombus arrangement at an eccentricity of 6–11° depending on the RF eccentricity of the recorded neurons. This particular arrangement allowed us to analyze responses beyond the first saccade as distances between each stimulus and all of the other stimuli in the array are fixed and can be easily reproduced in a center-out memory guided saccade task that was used for RF mapping.
To map RFs of the recorded neurons and further characterize neuronal properties, we used a fixation task and a memory-guided saccade task. In the fixation task, monkeys had to keep their gaze on a central spot (0.75° × 0.75°) within a 2.5° diameter window while circular stimuli (3° in diameter) were flashed for 100 ms at different locations throughout the visual field. The stimuli appeared at 6, 8, and 12° eccentricity and in eight directions, namely, 0 (horizontal right), 45, 90 (vertical up), 135, 180, 225, 270, and 315°. Once the eccentricity eliciting the max response was determined, a memory-guided saccade task was used to identify units with visual and/or motor responses and further map the extent of their RFs. In the memory-guided saccade task, monkeys had to fixate a 0.75° × 0.75° central spot within a 2.5° diameter window. A target stimulus flashed for 150 ms in one of 24 possible positions. After a variable delay of 500–1,000 ms and while animals were still fixating centrally, the fixation spot was extinguished (go cue) and monkeys were rewarded for making a saccade to the memorized position of the peripheral target within a 4° diameter window centered on the position of the previously presented target. The 24 possible positions were arranged in an inner (8 positions) and an outer (16 positions) rhombus. The inner rhombus comprised the eight search array locations and included the preferred position for most recorded units. In the outer rhombus, stimuli were presented at eccentricities that were twice those of the inner rhombus. Using this arrangement, the stimuli distances from the central fixation spot in the memory-guided saccade task corresponded to all possible distances between stimuli locations in the search task. This allowed us to estimate the translated RF during each fixation in the search task.
Recordings.
Neural activity was simultaneously recorded from FEF and LIP using the Omniplex system (Plexon). On a given day, up to four glass-coated tungsten microelectrodes (impedance, 1–1.5 MΩ; Alpha-Omega Engineering) were positioned through a grid system over each area and were advanced through the dura by a four-channel microdrive system (NAN Instruments). Signals were filtered between 300 Hz−8 kHz, amplified, and digitized at 40 kHz to obtain spike data. Spike waveforms were sorted off-line to isolate waveforms from single neurons using template matching and principal component analysis. If there was no clear isolation based on waveform projections on the principal component space or it was not possible to keep isolation on a single neuron throughout the entire session, multiunit activity was also accepted. In each session, electrodes were advanced through the dura until we recorded visual responses to flashing stimuli at 6°–12° eccentricities, in the fixation task. Subsequently, responses were recorded in the memory-guided saccade task and in the visual search task. The location of recordings in both FEF and LIP was estimated at the end of the experiments from MRI scans, the chambers’ angles relative to the coronal and sagittal planes, and the depth of each recording site. FEF recordings were located in the anterior bank of the arcuate sulcus, and LIP recordings in the lateral bank of the intraparietal sulcus. To ensure that parietal recordings did not include sites in 7a at the lip of the sulcus, LIP recordings were obtained at depths between 3 and 7 mm from the first activity encountered in each electrode, within the lateral bank of the intraparietal sulcus. To verify that our frontal recordings were in the FEF, we also electrically stimulated FEF sites at the end of the experiments in both monkeys and elicited eye movements with currents lower than 50 μA using 70-ms trains of biphasic pulses (duration, 500 µs) at 350 Hz (63).
Data Analysis.
Spike density functions were generated by convolving spikes with a Gaussian filter (sigma, 10 ms). We determined the RF of each unit (visual and/or movement field) by examining neuronal responses in the memory-guided saccade task. To assess visual activity in the memory-guided saccade task, we compared responses 50–150 ms following target onset to baseline activity −150–0 ms before target onset (paired t test, P < 0.01). To determine saccade-related activity, responses −100–20 ms around the onset of the saccade were compared with activity −150–0 ms before the go cue (paired t test, P < 0.01). Using these criteria, we found that 77% of LIP and 73% of FEF units showed significant enhancements in activity following the onset of a visual stimulus in at least one location while 42% of LIP and 66% of FEF units showed a significant saccade-related enhancement for at least one saccade vector during the memory-guided saccade task. Locations of stimuli that elicited significant visual/motor responses were defined to be in the visual/motor RF, respectively. Only units with significant visual responses both in the memory-guided saccade and the search task (50–150 ms following the array onset relative to −150–0 ms before array onset) were included in the feature attention analyses (Figs. 2–5), whereas units with visual and/or motor-related activity were included in the analysis of saccade goal selection (Fig. 6). Units with less than five trials in either of the conditions under study were excluded from the analysis.
Neuronal responses were aligned either on array onset (or stimulus onset in the memory-guided saccade task) or on saccade onset. To determine saccade onset, we considered the horizontal (x) and vertical (y) eye position signals during a period ranging from the initial fixation to the offset of the array. We calculated the instantaneous vx and vy velocities and used a velocity threshold of 50°/s combined with an amplitude threshold of 0.4° for the detection of saccades.
To study feature attention, we considered four conditions: (i) the target was in the RF (target), (ii) a distractor that shared the target’s color was in the RF (share color), (iii) a distractor that shared the target’s shape was in the RF (share shape), and (iv) the RF included distractor(s) that did not share features with the target (no share) (Fig. 1B). Responses to share color and share shape conditions were similar; thus, for certain analyses, they were combined. If the RF included more than one stimuli, in the first three conditions, we considered fixations during which only one relevant stimulus was inside the RF (either the target, or a share color/shape distractor) and all other stimuli were irrelevant distractors. To compare responses among conditions, we matched fixations so that the same stimuli were in the RF across the different conditions, and only their behavioral relevance changed in each condition. This procedure resulted into fewer trials being included in the analysis; however, results were quantitatively similar to those obtained without restricting analysis to stimuli-matched fixations. Thus, to increase statistical power, the results presented throughout the manuscript were obtained using all available trials in each condition, unless stated otherwise in the text. To calculate population average responses in Figs. 2–4, firing rates of individual units were normalized by dividing by the maximum response in the target condition 50–150 ms following array onset or −150–0 ms relative to saccade onset. When two conditions were compared at the population level (Figs. 5 and 6), data were normalized by dividing by the maximum response in either condition.
To obtain quantitative measurements of the size of the effect, contrast ratio indices between two conditions were calculated for each unit as follows: (FRcnd1 − FRcnd2)/(FRcnd1 + FRcnd2), where FR is the average firing rate in the interval 150–200 ms following array onset.
The latency of feature attention effects at the level of individual sites was determined to be the first of 30 consecutive 1-ms bins with significantly different responses across trials between the target and no share conditions (unpaired t test, P < 0.05). Similarly, latencies of saccade goal selection effects were calculated by comparing responses in the saccade to vs. away from the RF conditions (unpaired t test, P < 0.01). At the population level, latencies were estimated in the same manner by comparing responses between the two conditions across units, using a paired t test. To test whether the observed latency difference between FEF and LIP at the population level was significant, we employed a two-sided permutation test and 1,000 iterations. The distributions of latencies across individual units were also compared using a two-sided permutation test and 1,000 iterations. We confirmed that the Gaussian kernel used for smoothing did not affect our conclusions on the relative latency differences and the statistical significance of latency differences between the two areas. To this end, all relevant analyses and latency estimates were repeated using unsmoothed spike trains averaging activity within 10-ms nonoverlapping bins. We subsequently estimated the latency of the effects by asking for significant differences in at least three consecutive 10-ms bins (using a Wilcoxon signed-rank test for latencies at the population level and a Wilcoxon rank-sum test for latencies computed for individual units). All conclusions were the same.
A linear SVM classifier (64) was used to decode feature attention- and saccade goal selection-related information from neuronal responses. Estimates of decoding performance were based on artificial neuronal populations by combining units recorded over different sessions. Classification accuracy was estimated using a 5-fold cross-validation procedure (65). Before classification, data were scaled between 0 and 1 to ensure that classification was not influenced by the absolute magnitude of the responses. The same scaling was applied to the train and test sets. To compute the time course of decoding accuracy, we calculated spike counts within 100-ms windows advanced in 10-ms steps. The input to the classifier in each time window was an N × T matrix, where N is the number of units and T is the number of trials. Typically, the number of units, N, was higher than the number of trials, T. Due to the dynamic nature of the task, the number of trials included in the analysis was small relative to the number of trials completed in each session. Specifically, the number of trials included in the analysis depended on (i) the behavioral relevance of the stimuli in the RF and (ii) whether the saccade was made toward or away from the RF. The fact that N is greater than T can be detrimental for many classifiers due to the “curse of dimensionality” (65, 66). However, kernel methods such as SVM are more resilient to this problem as is evident from the high decoding accuracies obtained in this study. A resampling procedure was used to select an equal number of trials from each condition (n = 50) so that prior probabilities were equal for each class. To calculate the latency at which classification accuracy started to deviate from chance, we computed for each time window the null accuracy distribution by shuffling the indices of trials assigned to the different classes. For each time window, this was repeated 500 times. The time at which decoding accuracy deviated from chance was determined as the first of five consecutive windows in which accuracy was significantly above chance (P < 0.05). Significant differences between latencies in the two areas were assessed using a two-sided permutation test and 1,000 iterations. To ensure that differences in the decoding performance between the two areas were not due to differences in the size of the two populations, we repeated the decoding procedure by matching the number of units in the two areas using a resampling procedure (repeated 50 times) and obtained qualitatively and statistically similar results. Note that the number of units included in each decoding analysis differs from that used for the population average firing rate graphs (see, for example, Figs. 2 and 4–6). This is due to the fact that, in the decoding analyses, classification requires an equal number of trials from each condition (20 trials for the analyses in Figs. 2, 4, and 5 and 50 trials for the analysis in Fig. 6). Thus, only units with the required number of trials for all conditions contributed to the population used for each decoding analysis.
To quantify the information carried by individual units’ responses about task parameters, we used the percentage of explained variance (PEV) statistic (67). PEV reflects the amount of variance in the unit’s firing rate that can be explained by the different task variables. In accordance with previous studies (68, 69), we used the ω2 statistic that is an unbiased measure of explained variance defined as follows: ω2 = (SSbetween groups − df × MSE)/(SStotal + MSE), where df is degrees of freedom, SSbetween groups is the sum of squares between groups (levels), SStotal is the total sum of squares, and MSE is the mean square error. These quantities were calculated for each unit by performing a three-way ANOVA across trials. The first factor of the ANOVA was the search display (up to 18 levels) that quantified information about the identity of stimuli irrespective of their behavioral relevance. The second factor was the direction of the first saccade, that is, whether it was made toward or away from the RF (two levels). The third factor was the behavioral relevance of the RF stimuli, that is, whether the trial was a target, share color/shape, or no share trial (three levels). Information about each factor was estimated independently.
To quantify neural information in a time-resolved manner, spike counts were calculated within 50-ms sliding windows, advanced in 10-ms steps, and ω2 was computed in each window. For each unit, a null distribution of ω2 values was estimated by shuffling the trials corresponding to the different levels of each factor and repeating this procedure for 500 times. Subsequently, PEV values were z-scored by subtracting the mean and dividing by the SD of the null distribution and were then averaged across the population of selective units. Only units with PEV values greater than the 95th percentile of the null distribution in at least three consecutive windows were included in the population average for each factor. We determined the latency of the influence of each factor on neuronal activity as the time that z-score PEV values exceeded 1.645.
The data described in this paper are available upon reasonable request.
Supplementary Material
Acknowledgments
This work was supported by the actions “Supporting Postdoctoral Researchers,” Grant LS5_1325 (to P.S.), and ARISTEIA II, Grant 2988 (to G.G.G.) of the Operational Program “Education and Lifelong Learning,” cofinanced by the European Social Fund and the Greek State; by a FONDATION SANTE Grant (to G.G.G.); and by the project “Advanced Research Activities in Biomedical and Agroalimentary Technologies” (MIS 5002469), which is implemented under the “Action for the Strategic Development on the Research and Technological Sector,” funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (National Strategic Reference Framework 2014–2020) and cofinanced by Greece and the European Union (European Regional Development Fund). P.S. was partially supported by a Stavros Niarchos Foundation–Foundation for Research and Technology Hellas Fellowship (Project ARCHERS). S.P. was partially supported by a PhD fellowship through the action “Research Programs for Excellence State Scholarships Foundation-SIEMENS.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1804643115/-/DCSupplemental.
References
- 1.Paneri S, Gregoriou GG. Top-down control of visual attention by the prefrontal cortex. Functional specialization and long-range interactions. Front Neurosci. 2017;11:545. doi: 10.3389/fnins.2017.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire RF, Noudoost B, Schafer RJ, Moore T. Prefrontal contributions to visual selective attention. Annu Rev Neurosci. 2013;36:451–466. doi: 10.1146/annurev-neuro-062111-150439. [DOI] [PubMed] [Google Scholar]
- 3.Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb J. From thought to action: The parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53:9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- 6.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 7.Ibos G, Duhamel JR, Ben Hamed S. A functional hierarchy within the parietofrontal network in stimulus selection and attention control. J Neurosci. 2013;33:8359–8369. doi: 10.1523/JNEUROSCI.4058-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsuki F, Constantinidis C. Early involvement of prefrontal cortex in visual bottom-up attention. Nat Neurosci. 2012;15:1160–1166. doi: 10.1038/nn.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bichot NP, Heard MT, DeGennaro EM, Desimone R. A source for feature-based attention in the prefrontal cortex. Neuron. 2015;88:832–844. doi: 10.1016/j.neuron.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ipata AE, Gee AL, Bisley JW, Goldberg ME. Neurons in the lateral intraparietal area create a priority map by the combination of disparate signals. Exp Brain Res. 2009;192:479–488. doi: 10.1007/s00221-008-1557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H, Desimone R. Feature-based attention in the frontal eye field and area V4 during visual search. Neuron. 2011;70:1205–1217. doi: 10.1016/j.neuron.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zelinsky GJ, Bisley JW. The what, where, and why of priority maps and their interactions with visual working memory. Ann N Y Acad Sci. 2015;1339:154–164. doi: 10.1111/nyas.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baizer JS, Ungerleider LG, Desimone R. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J Neurosci. 1991;11:168–190. doi: 10.1523/JNEUROSCI.11-01-00168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- 16.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 17.Ungerleider LG, Galkin TW, Desimone R, Gattass R. Cortical connections of area V4 in the macaque. Cereb Cortex. 2008;18:477–499. doi: 10.1093/cercor/bhm061. [DOI] [PubMed] [Google Scholar]
- 18.Sereno AB, Maunsell JH. Shape selectivity in primate lateral intraparietal cortex. Nature. 1998;395:500–503. doi: 10.1038/26752. [DOI] [PubMed] [Google Scholar]
- 19.Ibos G, Freedman DJ. Interaction between spatial and feature attention in posterior parietal cortex. Neuron. 2016;91:931–943. doi: 10.1016/j.neuron.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toth LJ, Assad JA. Dynamic coding of behaviourally relevant stimuli in parietal cortex. Nature. 2002;415:165–168. doi: 10.1038/415165a. [DOI] [PubMed] [Google Scholar]
- 21.Bichot NP, Rossi AF, Desimone R. Parallel and serial neural mechanisms for visual search in macaque area V4. Science. 2005;308:529–534. doi: 10.1126/science.1109676. [DOI] [PubMed] [Google Scholar]
- 22.Treue S, Martínez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- 23.Phillips AN, Segraves MA. Predictive activity in macaque frontal eye field neurons during natural scene searching. J Neurophysiol. 2010;103:1238–1252. doi: 10.1152/jn.00776.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. J Neurosci. 2006;26:3656–3661. doi: 10.1523/JNEUROSCI.5074-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson KG, Bichot NP, Schall JD. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J Neurophysiol. 1997;77:1046–1050. doi: 10.1152/jn.1997.77.2.1046. [DOI] [PubMed] [Google Scholar]
- 26.Lynch JC, Graybiel AM, Lobeck LJ. The differential projection of two cytoarchitectonic subregions of the inferior parietal lobule of macaque upon the deep layers of the superior colliculus. J Comp Neurol. 1985;235:241–254. doi: 10.1002/cne.902350207. [DOI] [PubMed] [Google Scholar]
- 27.Fries W. Cortical projections to the superior colliculus in the macaque monkey: A retrograde study using horseradish peroxidase. J Comp Neurol. 1984;230:55–76. doi: 10.1002/cne.902300106. [DOI] [PubMed] [Google Scholar]
- 28.Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci. 2001;2:194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- 29.Bichot NP, Schall JD. Effects of similarity and history on neural mechanisms of visual selection. Nat Neurosci. 1999;2:549–554. doi: 10.1038/9205. [DOI] [PubMed] [Google Scholar]
- 30.Premereur E, Vanduffel W, Janssen P. Functional heterogeneity of macaque lateral intraparietal neurons. J Neurosci. 2011;31:12307–12317. doi: 10.1523/JNEUROSCI.2241-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibos G, Freedman DJ. Sequential sensory and decision processing in posterior parietal cortex. eLife. 2017;6:e23743. doi: 10.7554/eLife.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meister ML, Hennig JA, Huk AC. Signal multiplexing and single-neuron computations in lateral intraparietal area during decision-making. J Neurosci. 2013;33:2254–2267. doi: 10.1523/JNEUROSCI.2984-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- 34.Yu Q, Shim WM. Occipital, parietal, and frontal cortices selectively maintain task-relevant features of multi-feature objects in visual working memory. Neuroimage. 2017;157:97–107. doi: 10.1016/j.neuroimage.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 35.Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J Neurophysiol. 2000;83:1550–1566. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- 36.Quintana J, Fuster JM, Yajeya J. Effects of cooling parietal cortex on prefrontal units in delay tasks. Brain Res. 1989;503:100–110. doi: 10.1016/0006-8993(89)91709-5. [DOI] [PubMed] [Google Scholar]
- 37.Barbas H, Mesulam MM. Organization of afferent input to subdivisions of area 8 in the rhesus monkey. J Comp Neurol. 1981;200:407–431. doi: 10.1002/cne.902000309. [DOI] [PubMed] [Google Scholar]
- 38.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- 39.Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: Convergence and segregation of processing streams. J Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to posterior cortical areas from the macaque frontal eye fields. J Comp Neurol. 1995;353:291–305. doi: 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- 41.Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- 42.Katsuki F, Constantinidis C. Unique and shared roles of the posterior parietal and dorsolateral prefrontal cortex in cognitive functions. Front Integr Nuerosci. 2012;6:17. doi: 10.3389/fnint.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregoriou GG, Rossi AF, Ungerleider LG, Desimone R. Lesions of prefrontal cortex reduce attentional modulation of neuronal responses and synchrony in V4. Nat Neurosci. 2014;17:1003–1011. doi: 10.1038/nn.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi AF, Bichot NP, Desimone R, Ungerleider LG. Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. J Neurosci. 2007;27:11306–11314. doi: 10.1523/JNEUROSCI.2939-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirpour K, Ong WS, Bisley JW. Microstimulation of posterior parietal cortex biases the selection of eye movement goals during search. J Neurophysiol. 2010;104:3021–3028. doi: 10.1152/jn.00397.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cutrell EB, Marrocco RT. Electrical microstimulation of primate posterior parietal cortex initiates orienting and alerting components of covert attention. Exp Brain Res. 2002;144:103–113. doi: 10.1007/s00221-002-1032-x. [DOI] [PubMed] [Google Scholar]
- 47.Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci USA. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature. 2011;474:372–375. doi: 10.1038/nature09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- 50.Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- 51.Gregoriou GG, Gotts SJ, Desimone R. Cell-type-specific synchronization of neural activity in FEF with V4 during attention. Neuron. 2012;73:581–594. doi: 10.1016/j.neuron.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferraina S, Paré M, Wurtz RH. Comparison of cortico-cortical and cortico-collicular signals for the generation of saccadic eye movements. J Neurophysiol. 2002;87:845–858. doi: 10.1152/jn.00317.2001. [DOI] [PubMed] [Google Scholar]
- 54.Wurtz RH, Sommer MA, Paré M, Ferraina S. Signal transformations from cerebral cortex to superior colliculus for the generation of saccades. Vision Res. 2001;41:3399–3412. doi: 10.1016/s0042-6989(01)00066-9. [DOI] [PubMed] [Google Scholar]
- 55.Wardak C, Olivier E, Duhamel JR. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J Neurosci. 2002;22:9877–9884. doi: 10.1523/JNEUROSCI.22-22-09877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dias EC, Segraves MA. Muscimol-induced inactivation of monkey frontal eye field: Effects on visually and memory-guided saccades. J Neurophysiol. 1999;81:2191–2214. doi: 10.1152/jn.1999.81.5.2191. [DOI] [PubMed] [Google Scholar]
- 57.Li CS, Mazzoni P, Andersen RA. Effect of reversible inactivation of macaque lateral intraparietal area on visual and memory saccades. J Neurophysiol. 1999;81:1827–1838. doi: 10.1152/jn.1999.81.4.1827. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Yttri EA, Snyder LH. Intention and attention: Different functional roles for LIPd and LIPv. Nat Neurosci. 2010;13:495–500. doi: 10.1038/nn.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sommer MA, Tehovnik EJ. Reversible inactivation of macaque frontal eye field. Exp Brain Res. 1997;116:229–249. doi: 10.1007/pl00005752. [DOI] [PubMed] [Google Scholar]
- 60.Bisley JW, Krishna BS, Goldberg ME. A rapid and precise on-response in posterior parietal cortex. J Neurosci. 2004;24:1833–1838. doi: 10.1523/JNEUROSCI.5007-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pouget P, Emeric EE, Stuphorn V, Reis K, Schall JD. Chronometry of visual responses in frontal eye field, supplementary eye field, and anterior cingulate cortex. J Neurophysiol. 2005;94:2086–2092. doi: 10.1152/jn.01097.2004. [DOI] [PubMed] [Google Scholar]
- 62.Asaad WF, Santhanam N, McClellan S, Freedman DJ. High-performance execution of psychophysical tasks with complex visual stimuli in MATLAB. J Neurophysiol. 2013;109:249–260. doi: 10.1152/jn.00527.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- 64.Chang C-C, Lin C-J. LIBSVM: A library for support vector machines. ACM Trans Intell Syst Technol. 2011;2:27. [Google Scholar]
- 65.Duda RO, Hart PE, Stork DG. Pattern Classification. 2nd Ed Wiley; New York: 2001. [Google Scholar]
- 66.Sapountzis P, Gregoriou GG. Neural signatures of attention: Insights from decoding population activity patterns. Front Biosci. 2018;23:221–246. doi: 10.2741/4588. [DOI] [PubMed] [Google Scholar]
- 67.Olejnik S, Algina J. Generalized eta and omega squared statistics: Measures of effect size for some common research designs. Psychol Methods. 2003;8:434–447. doi: 10.1037/1082-989X.8.4.434. [DOI] [PubMed] [Google Scholar]
- 68.Buschman TJ, Siegel M, Roy JE, Miller EK. Neural substrates of cognitive capacity limitations. Proc Natl Acad Sci USA. 2011;108:11252–11255. doi: 10.1073/pnas.1104666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siegel M, Buschman TJ, Miller EK. Cortical information flow during flexible sensorimotor decisions. Science. 2015;348:1352–1355. doi: 10.1126/science.aab0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.