Significance
Islands are hotspots of alien species invasions, and their distinct biodiversity is particularly vulnerable to invading species. While isolation has shaped natural colonization of islands for millions of years, globalization in trade and transport has led to a breakdown of biogeographical barriers and subsequent colonization of islands by alien species. Using a large dataset of 257 subtropical and tropical islands, we show that alien richness increases with increasing isolation of islands. This pattern is consistent for plants, ants, mammals, and reptiles, and it cannot simply be explained by island economics and trade alone. Geographical isolation does not protect islands from alien species, and island species richness may reach a new dynamic equilibrium at some point, likely at the expense of many endemic species.
Keywords: island biogeography, alien species, isolation, island invasibility, naturalization
Abstract
One of the best-known general patterns in island biogeography is the species–isolation relationship (SIR), a decrease in the number of native species with increasing island isolation that is linked to lower rates of natural dispersal and colonization on remote oceanic islands. However, during recent centuries, the anthropogenic introduction of alien species has increasingly gained importance and altered the composition and richness of island species pools. We analyzed a large dataset for alien and native plants, ants, reptiles, mammals, and birds on 257 (sub) tropical islands, and showed that, except for birds, the number of naturalized alien species increases with isolation for all taxa, a pattern that is opposite to the negative SIR of native species. We argue that the reversal of the SIR for alien species is driven by an increase in island invasibility due to reduced diversity and increased ecological naiveté of native biota on the more remote islands.
Islands harbor a disproportionately high number of evolutionarily unique, geographically restricted species, and thus contribute significantly to global biodiversity (1). Native species richness on islands, which arose through colonization events and evolution over geological time scales, follows positive species–area relationships and negative species–isolation relationships (SIRs), as predicted by the theory of island biogeography (2–5). While the negative SIR for native species is a well-documented pattern in ecology (2, 6, 7), it is less clear whether or how the number of alien species on islands is related to isolation.
On the one hand, globalization in trade and transport has considerably reduced the effective isolation of islands worldwide and has led to a breakdown of biogeographical barriers (8). While natural dispersal to remote islands is extremely rare and has had a strong influence on island native species richness and composition, human-aided transport increases the frequency of introduction events by orders of magnitude; as a result, SIR patterns may decrease or even vanish (2, 9). Alternately, economic theory predicts that insularity (characterized by smallness and remoteness) has a strong effect on the socioeconomic structure of an island (10). Small markets, dependence on sea and air transport, and exclusion from major transport routes, together with higher costs generally, mean that fewer commodities are transported to more remote islands (10). Hence, fewer intentional and accidental alien introductions (i.e., lower propagule and colonization pressures), and thus lower colonization rates, might be expected for more remote islands (11). Still, another line of reasoning suggests that invasibility should be highest on the most remote islands because their impoverished and evolutionarily naive biota provide greater ecological opportunities for introduced species to establish (12–14). Further, alien species establishment might lead to the extinction of native species through enhanced competition or predation, thereby increasing the establishment odds for additional aliens. These hypotheses would predict alien species richness on islands to be positively correlated, negatively correlated, or uncorrelated with isolation, depending on the balance between colonization pressure and establishment probabilities. Empirical studies have so far provided ambiguous results, with no correlations [for plants (15, 16) and birds (16)] or positive correlations [for birds (17), plants (18), and ants (19)] between alien species richness and island isolation. Since these studies vary in methods, predictor variables, and spatial and taxonomic extent, we are so far unable to draw general conclusions regarding the SIR for alien versus native species.
Here, we compiled the most comprehensive datasets of naturalized alien (i.e., those species established outside their native range and forming self-sustainable populations [sensu Blackburn et al. (20)]) and native species numbers currently available for vascular plants, ants, reptiles, mammals, and birds on subtropical and tropical islands (between 30°N and 30°S latitudes) (Fig. 1, number of islands: vascular plants = 108, ants = 89, mammals = 125, reptiles = 75, and birds = 87; SI Appendix, Table S9) and assessed the SIR. We restricted the data to subtropical and tropical islands to reduce the confounding effects of differences in climatic conditions between islands. Further, remoteness of islands exhibits a strong latitudinal gradient as the spherical shape of Earth and the distribution of continental land masses result in far fewer remote islands at higher latitudes, at least in the Northern Hemisphere. Nevertheless, in our analysis, we account for the effects of various important factors such as island size, climatic and topographic heterogeneity, and human impact [e.g., per capita gross domestic product (GDP)] by using them as additional predictor variables in generalized linear mixed effects models (GLMMs). Since comprehensive data on introduction effort do not exist at such a macroecological scale for most of these taxonomic groups (with the exception of birds, as stated below) and robust data on imported products (i.e., a proxy for propagule and colonization pressure) for all of the analyzed islands are lacking, we analyzed the correlation of commodity import and geographical isolation based on a subset of islands, using World Bank trade data (21) (additional analysis is provided in SI Appendix, Tables S7 and S8).
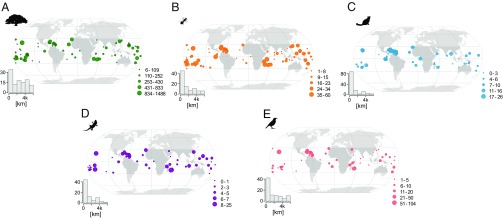
Fig. 1.
Geographical distribution of tropical and subtropical islands used in the study for vascular plants (A), ants (B), mammals (C), reptiles (D), and birds (E). Symbol size scales with the number of naturalized alien species. The histograms show the frequencies of island distance to the mainland for the four taxonomic groups. The number of islands included in the analysis differs among the taxonomic groups (vascular plants = 108, ants = 89, mammals = 125, reptiles = 75, and birds = 87). Dark gray colors indicate the area between the latitudes 30°S and 30°N of the equator. Pictograms courtesy of PhyloPic (www.phylopic.org); (A) Tracy A. Heath, (C and D) Steven Traver, and (E) Ferran Sayol.
Results and Discussion
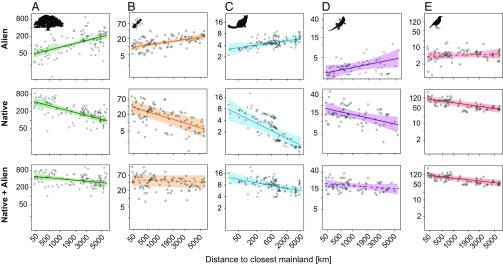
Across all five taxonomic groups, we found that island isolation had contrasting effects on native and naturalized alien species richness. While native species richness decreased with isolation, consistent with island-biogeography theory (2, 3, 22), alien species richness increased with isolation for all taxonomic groups except birds, where alien numbers and isolation were uncorrelated (Figs. 2 and 3 and SI Appendix, Tables S1A and S6). Consequently, for all taxonomic groups, SIRs were markedly weaker when assessed with combined native and alien richness (Figs. 2 and 3 and SI Appendix, Table S1).
Fig. 2.
Alien and native species richness on islands dependent on island isolation for vascular plants (A), ants (B), mammals (C), reptiles (D), and birds (E). Shown are partial residual plots of the species richness–isolation relationships for naturalized alien (Top), native (Middle), and total (Bottom) species richness (log–log space). GLMMs with a Poisson-distributed response were applied to additionally account for island size, heterogeneity (elevational range), climate (temperature and precipitation), and human impact (per capita GDP). Each column represents one taxonomic group. Shading around the regression line indicates its 95% confidence interval. Dashed lines indicate insignificant results. Pictograms courtesy of PhyloPic (www.phylopic.org); (A) Tracy A. Heath, (C and D) Steven Traver, and (E) Ferran Sayol.
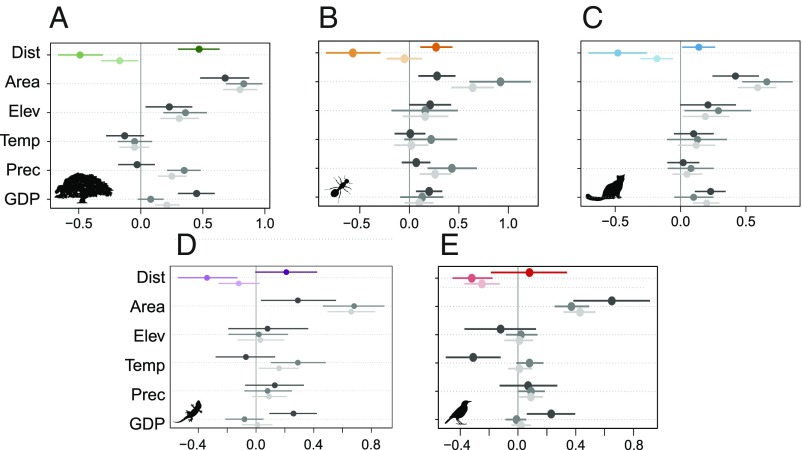
Fig. 3.
Regression coefficients and 95% confidence limits for the standardized predictor variables in the GLMMs for vascular plants (A), ants (B), mammals (C), reptiles (D), and birds (E). Dark colors represent the estimates for naturalized alien species, medium colors represent the estimates for native species, and light colors represent the estimates for all species. Area, island area; Dist, distance to the closest mainland; Elev, elevational range; GDP, per capita GDP (the full model output is provided in SI Appendix, Table S1A); Prec, annual precipitation sum; Temp, mean annual temperature. Pictograms courtesy of PhyloPic (www.phylopic.org); (A) Tracy A. Heath, (C and D) Steven Traver, and (E) Ferran Sayol.
Effects of the other predictor variables on species richness were as expected: The numbers of both native and naturalized alien species increased with island area (Fig. 3 and SI Appendix, Table S1). Socioeconomic development (measured as per capita GDP) did not affect native species richness, but it had a significant positive effect on alien species richness of all taxonomic groups (Fig. 3 and SI Appendix, Table S1A). For plants and mammals, the effect of per capita GDP was still significant when considering alien and native richness together. Due to the focus on (sub) tropical islands, climate effects were minor; only the richness of native reptile species and alien bird species decreased with mean annual temperature. Native bird, ant, and vascular plant species richness increased with annual precipitation (Fig. 3 and SI Appendix, Table S1A). Moreover, species richness of alien and native vascular plants and mammals was positively related to topographic heterogeneity (Fig. 3 and SI Appendix, Table S1A). The robustness of our results was confirmed by a sensitivity analysis that removed potential biases introduced by differences in geographical coverage, sampling intensity, and data quality (SI Appendix, Table S2). As it has been suggested that some large islands may act as source regions for other islands, we also used an alternative isolation metric that considered seven larger islands of the Malay Archipelago (Sumatra, Kalimantan, Java, Mindanao, Luzon, Sulawesi, and New Guinea; SI Appendix, Fig. S3) as potential source regions in addition to the mainland. In this case, the isolation effect decreased slightly and became marginally significant only for reptile species (P = 0.078; SI Appendix, Table S1B).
One possible mechanism underpinning the positive SIRs for alien species richness is a systematic decrease in the resistance of resident biota to the colonization by new species with increasing geographical isolation. This hypothesis was first proposed by Elton (12) and later explicated, for example, by Simberloff (13) and Denslow (14). Arguments in favor of this mechanism emphasize that different resource use of native and alien species is crucial for successful establishment of the latter (23), and that this divergence likely increases with geographical (and hence evolutionary) isolation. Moreover, particular functional groups, especially large predators and herbivores (24) but also pathogens and parasites (25), are generally rarer or absent from remote islands. This leads to reduced enemy-escape responses [e.g., island tameness in lizards (26)]. As a consequence, introduced predators might have easier access to resident prey, and introduced species might experience less predation, herbivory, and pathogen pressure [“enemy release” hypothesis (27)]. In addition, alien species introduce traits that native island biotas have not been exposed to previously [e.g., certain allelopathic secondary chemical compounds (28)] and to which they are naive [“novel weapons” hypothesis (29)], a phenomenon that may increase with isolation as native species become more evolutionarily distinct (26). Furthermore, as isolated islands usually have a reduced phylogenetic diversity (30), the species there might have experienced less competition, and therefore be competitively inferior to alien species from regions with a high phylogenetic diversity [“evolutionary imbalance” hypothesis (31)]. Taken together, these mechanisms may well drive a strong positive correlation between geographical isolation and successful establishment of new arrivals, and hence drive the positive alien species-isolation patterns we found. The absence of the positive SIR in alien birds indicates that native bird faunas on remote islands might not be as depauperate and naive as for other taxonomic groups. Moreover, a more generalist behavior in passerine birds on isolated islands (32, 33) potentially leads to higher occupation of the available niche space and, consequently, reduced opportunity for newly introduced birds to establish.
However, variation in propagule and colonization pressure might also affect the establishment probabilities of alien species (11). In a study on birds, Blackburn et al. (17) argued that remote islands generally lack native species useful for farming, hunting, or aesthetic purposes, which might have led to a greater number of intentional releases of alien birds (i.e., higher colonization pressure), driving a positive alien SIR. In our analysis, however, we could not confirm this hypothesis for birds on (sub) tropical islands, as there was no relationship between colonization pressure (measured as the number of all bird species introduced to an island) and distance to the mainland (SI Appendix, Table S1C). Our contrasting finding might result from the following: (i) improved accessibility to data and information on islands over the last decade, (ii) a different study region, and (iii) different sample size and predictor variables. For groups other than birds, the effect of colonization pressure on alien richness remains difficult to test, since reliable data on introduction events do not exist. However, we consider it unlikely that increasing propagule pressure on more remote islands is the dominant driver of the positive correlation between alien richness and isolation for several reasons. First, by definition, alien species have to be introduced to an island by human agency (20). Therefore, statistics on imported commodities are useful indicators for the number of intentional as well as unintentional introductions. An analysis of the World Bank global trade data shows that imports decline with increasing island isolation (10, 32) (additional analysis is provided in SI Appendix, Table S7). Thus, import volumes indicate that colonization pressure does not increase with geographical isolation. Second, since colonization pressure is positively correlated with GDP (34), our analyses also partly correct for varying propagule and colonization pressure by including GDP in the regression models. Third, the positive correlation between alien richness and isolation was consistent across four taxonomic groups, including one where species are not commercially used, and thus are rarely introduced on purpose.
In conclusion, we show that naturalized alien species have markedly changed fundamental biogeographical patterns of species richness on islands around the world. The breakdown of biogeographical dispersal barriers, due to human activities, has weakened the classical SIRs. While this pattern has previously been shown for Anolis lizards in the Caribbean (35), we show here that it holds across the tropics and subtropics for four of five taxonomic groups. Globalization in trade and transport will increasingly decouple geographical distance from isolation. As a consequence, immigration rates will increase even on remote islands, which will become packed with species, as the theory of island biogeography predicts for equivalently sized but less isolated islands (2).
SIRs for alien species have not just vanished; they have become inverted compared with the SIRs for native species, and there is a clear congruency of low native diversity and disproportionately high naturalized alien numbers on remote islands. Since trade data and analysis of introduction effort provide no convincing evidence of increasing colonization pressure, we argue that the reversed alien SIR is rather driven by a systematic increase in invasibility among more isolated (sub) tropical islands.
Methods
Global Island Distribution.
The dataset comprises a total of 257 (sub) tropical islands and island groups [i.e., archipelagos (hereafter also referred to as islands)] of oceanic and continental origin. We focus on (sub) tropical islands between 30°N and 30°S latitudes to minimize confounding effects of interisland climatic differences. We used archipelago data, where available, to increase consistency across datasets. Analyses including all islands without such an archipelago grouping yielded similar results. Following Santos et al. (36), archipelagos should generally exhibit similar characteristics relevant for species–area relationships as their constituent islands, justifying their use in biogeographical and macroecological studies.
Datasets.
The number and identities of the islands differed among taxonomic groups (SI Appendix, Table S9), including 108 islands for vascular plants, 89 islands for ants, 125 islands for mammals, 75 islands for reptiles, and 87 islands for birds. Species lists of native and naturalized alien species (i.e., species established outside their native range and forming self-sustainable populations [sensu Blackburn et al. (20)]) were compiled from various sources (SI Appendix, Table S6). For birds, we additionally extracted numbers of all birds introduced on an island from the Global Avian Invasion Atlas (GAVIA) database (37), as a measure for introduction effort. Large data compilations may be affected by biases in data quality and completeness [i.e., varying sampling strategies, differences in taxonomic concepts (38, 39)]. To address these issues, we compiled complete species lists, where available, based on recent database projects that ensure taxonomic standardization [e.g., using The Plant List for vascular plants (40)] and supplemented the dataset with species richness data from different sources where no full species lists could be compiled. In the case of conflicting data, we used the most up-to-date and detailed sources.
Potential effects of variation in data reliability were tested using a sensitivity analysis (discussed below). Each island was assigned to a geographical region following the Biodiversity Information Standards (historically known as the Taxonomic Databases Working Group; TDWG) classification (41) (SI Appendix, Table S4). For all islands, we compiled eight predictor variables that represented socioeconomic (human population density and per capita GDP), climatic (mean annual temperature and annual precipitation sum), and geographical (island area, elevational range, and distance to the mainland) variables. Distance to the mainland was calculated as the shortest geodesic distance to a continent, excluding Antarctica. As it has been suggested that several large islands (e.g., New Guinea) may have acted as species sources for other islands, we calculated an alternative distance metric that includes the seven large islands of the Malay Archipelago as potential source regions (SI Appendix, Fig. S3). The geographical distance is just one metric, and ocean currents, winds, and the richness of source regions also influence immigration rates for native species (22). However, these additional variables are arguably less relevant for aliens as they are introduced through human transport. Therefore, we decided to use the shortest geographical distance to the mainland as our measure of isolation. Island area and elevational ranges were calculated for each island and island group. In the case of island groups, the cumulative terrestrial surface area of all islands was used. Island area ranged from 5.11 to 110,730 km2, with a median size of 280 km2. Data on current climate for each region were derived from WorldClim 2.0 (42). Data on human population density were derived from the History Database of the Global Environment (HYDE) database (43), and per capita GDP was derived from a study by Gennaioli et al. (44), the World Bank (21), and the United Nations (45) (SI Appendix, Table S3). The Pearson correlation coefficients between all predictor variables were below 0.7, except for ants and reptiles, where elevation and area had a Pearson correlation coefficient of 0.7 (SI Appendix, Table S10). We therefore reran the analyses excluding elevation, resulting in little change in the results. For alien reptiles, the relationship with distance to the mainland became just marginally significant (P = 0.053).
Statistical Analysis.
We analyzed the dependence of alien and native species richness (species numbers) on distance to the mainland, island area, elevational range, mean annual temperature, annual precipitation sum, per capita GDP, and human population density as predictor variables by means of GLMMs with a Poisson-distributed response (species richness) and the canonical log link function. Human population density, a frequently used surrogate of human impact (e.g., refs. 15, 16), was never significant, and was thus excluded from the analyses. A random effect intercept term with the TDWG level 4 region as a grouping factor acknowledged political/socioeconomic groupings among regions, and a random effect intercept term for island geological setting [i.e., oceanic islands vs. islands situated on continental shelves (46)] accounted for possible differences in colonization due to historic connections with continents (22). We additionally ran the models including oceanic islands only. The results did not change for native species of all taxonomic groups. For alien reptiles (P = 0.071) and mammals (P = 0.628), the relationship with distance to the nearest mainland became nonsignificant, but a positive trend remained. This most likely resulted from a truncation of the isolation gradient by excluding continental islands, as well as from a reduction of the sample size. Finally, an additional observation-level random effect term accounted for overdispersion (47). To improve symmetry and linearity, and to stabilize variances, numerical predictors were subjected to appropriate transformations (natural log) for island area, elevational range, and distance to the mainland (square root for precipitation sum and per capita GDP), and finally standardized (scaled to a mean of 0 and SD of 1). The magnitude of regression coefficients therefore represented the relative effect size. We fitted individual models for alien, native, and total (alien plus native) species numbers for every taxonomic group. Additionally, we fitted models for all introduced birds as a response. Model residuals were assessed for spatial autocorrelation by using spline (cross-) correlograms, and no spatial autocorrelation was found (SI Appendix, Figs. S1 and S2).
All statistical analyses were performed using R (version 3.3.1). For GLMM analyses, we used the function glmer from the package lme4 for fitting (48) and the function effect from the package effects for partial effect plots. For spline correlograms, we used the function spline.correlog from the package ncf (49).
Sensitivity Analysis.
To test the robustness of the assessed relationships between alien species richness and island isolation, we performed a sensitivity analysis. The aim of this analysis was to exclude systematic biases in the data that might stem from heterogeneous sampling intensity or overrepresentation of selected geographical regions, as well as from variable data quality depending on data sources. Therefore, we first systematically excluded islands of a given geographical region (based on TDWG level 2 classifications) from the datasets. Then, the number of excluded islands was resampled from the remaining islands to ensure constant sample sizes. Subsequently, we fitted the same GLMMs as were used for the main analysis to the resampled datasets. This procedure was repeated 500 times, and 95% confidence intervals were calculated for the regression coefficients (SI Appendix, Table S2). Similarly, we assessed source reliability by assigning all sources hierarchically to seven categories: (i) peer-reviewed publications, (ii) scientific monographs and books, (iii) reports of renowned and established organizations (e.g., the Convention on Biological Diversity, International Union for Conservation of Nature), (iv) reports from gray literature, (v) renowned webpage repositories (e.g., Caribherp, Charles Darwin Foundation), (vi) other webpages, and (vii) personal communications. We then excluded less reliable data sources (i.e., categories vi and vii), resampled from the remaining islands, and recalculated the models.
The exclusion of islands and references revealed no qualitative difference in the SIR (i.e., the positive trend of the relationship remained; SI Appendix, Table S2). However, for alien mammals and alien reptiles, the regression coefficient for isolation dropped more strongly when excluding islands of some selected regions (e.g., mammals: western Indian Ocean and Australian islands; reptiles: north-central and northwestern Pacific islands). However, the positive trend of the relationship remained. For the reptile data, which were more sensitive to the exclusion of certain islands compared with other groups, the relation to distance was least significant for the whole dataset (P = 0.049; SI Appendix, Table S1) in the first place and had the lowest sample size of all groups. Systematics and taxonomy of reptiles changed radically in the last decades (50), and it is possible that these changes might not have been fully acknowledged by all data sources used in this study, making the species numbers less robust. Moreover, the global reptile distribution seems to be more erratic than in other groups, even for native species. For instance, Hawaii has no native reptiles, but in similar remote islands, such as Samoa or the Cook Islands, native reptiles are present. However, even the exclusion of all Caribbean islands (56 islands for mammals and 30 islands for reptiles) did show a strengthening, rather than a weakening, of the positive SIR for alien species. We did not run a sensitivity analysis for birds, since the relationship of alien species richness with isolation was nonsignificant.
Data Availability.
All data analyzed during the current study are included in this published article and SI Appendix, Table S4 or in the sources where the available datasets are are provided (SI Appendix, Tables S3 and S5).
Supplementary Material
Acknowledgments
We are indebted to the many colleagues and collaborators who helped us in compiling the native and alien species data. This study was supported by Grant I2086-B16 from the Austrian Science Foundation FWF (to D.M., B.L., F.E., S.D., and T.M.), Grant KL 1866/9-1 (to M.v.K. and W.D.) and Grant SE 1891/2-1 (to H.S.) from the Deutsche Forschungsgemeinschaft, and Grant FZT 118 (to the German Centre for Integrative Biodiversity Research) supporting M.W. P.P. and J.P. were supported by the Czech Science Foundation (Project 14-36079G, Centre of Excellence for Plant Diversity Analysis and Synthesis; PLADIAS), the Czech Academy of Sciences (Long-Term Research Development Project RVO 67985939), and the Praemium Academiae Award from The Czech Academy of Sciences (to P.P.). C.C. was supported by a postdoctoral grant from Fundo Europeu de Desenvolvimento Regional (FEDER) funds through the Operational Competitiveness Factors Programme “COMPETE” and by National Funds through the Foundation for Science and Technology (FCT) within the framework of project PTDC/AAG-GLO/0463/2014-POCI-01-0145-FEDER-016583. E.P.E. was supported by subsidy funding to the Okinawa Institute of Science and Technology and a Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (17K15180). F.H. was supported by the Young Investigator Award of the Faculty of Life Sciences, University of Vienna, Austria. Pictograms were derived from the PhyloPic website (www.phylopic.org). No changes were applied to the pictures.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1804179115/-/DCSupplemental.
References
- 1.Kier G, et al. A global assessment of endemism and species richness across island and mainland regions. Proc Natl Acad Sci USA. 2009;106:9322–9327. doi: 10.1073/pnas.0810306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacArthur R, Wilson EO. The Theory of Island Biogeography. Princeton Univ Press; Princeton: 1967. [Google Scholar]
- 3.Whittaker RJ, Triantis KA, Ladle RJ. A general dynamic theory of oceanic island biogeography. J Biogeogr. 2008;35:977–994. [Google Scholar]
- 4.Borregaard MK, et al. Oceanic island biogeography through the lens of the general dynamic model: Assessment and prospect. Biol Rev Camb Philos Soc. 2017;92:830–853. doi: 10.1111/brv.12256. [DOI] [PubMed] [Google Scholar]
- 5.Weigelt P, Steinbauer MJ, Cabral JS, Kreft H. Late Quaternary climate change shapes island biodiversity. Nature. 2016;532:99–102. doi: 10.1038/nature17443. [DOI] [PubMed] [Google Scholar]
- 6.Weigelt P, Jetz W, Kreft H. Bioclimatic and physical characterization of the world’s islands. Proc Natl Acad Sci USA. 2013;110:15307–15312. doi: 10.1073/pnas.1306309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whittaker RJ, Fernández-Palacios JM. Island Biogeography: Ecology, Evolution, and Conservation. Oxford Univ Press; London: 2007. [Google Scholar]
- 8.Capinha C, et al. Diversity, biogeography and the global flows of alien amphibians and reptiles. Divers Distrib. 2017;23:1313–1322. [Google Scholar]
- 9.Pysek P, et al. Disentangling the role of environmental and human pressures on biological invasions across Europe. Proc Natl Acad Sci USA. 2010;107:12157–12162. doi: 10.1073/pnas.1002314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deidda M. Insularity and economic development: A survey. Int Rev Econ. 2015;63:107–128. [Google Scholar]
- 11.Lockwood JL, Cassey P, Blackburn TM. The more you introduce the more you get: The role of colonization pressure and propagule pressure in invasion ecology. Divers Distrib. 2009;15:904–910. [Google Scholar]
- 12.Elton CS. The Ecology of Invasions by Animals and Plants. Univ of Chicago Press; Chicago: 1958. [Google Scholar]
- 13.Simberloff D. Why do introduced species appear to devastate islands more than mainland areas? Pac Sci. 1995;49:87–97. [Google Scholar]
- 14.Denslow JS. Weeds in paradise: Thoughts on the invasibility of tropical islands. Ann Mo Bot Gard. 2003;90:119–127. [Google Scholar]
- 15.Traveset A, Kueffer C, Daehler CC. Global and regional nested patterns of non-native invasive floras on tropical islands. J Biogeogr. 2014;41:823–832. [Google Scholar]
- 16.Blackburn TM, Delean S, Pyšek P, Cassey P, Field R. On the island biogeography of aliens: A global analysis of the richness of plant and bird species on oceanic islands. Glob Ecol Biogeogr. 2016;25:859–868. [Google Scholar]
- 17.Blackburn TM, Cassey P, Lockwood JL. The island biogeography of exotic bird species. Glob Ecol Biogeogr. 2008;17:246–251. [Google Scholar]
- 18.Denslow JS, Space JC, Thomas PA. Invasive exotic plants in the tropical Pacific islands: Patterns of diversity. Biotropica. 2009;41:162–170. [Google Scholar]
- 19.Roura-Pascual N, Sanders NJ, Hui C. The distribution and diversity of insular ants: Do exotic species play by different rules? Glob Ecol Biogeogr. 2016;25:642–654. [Google Scholar]
- 20.Blackburn TM, et al. A proposed unified framework for biological invasions. Trends Ecol Evol. 2011;26:333–339. doi: 10.1016/j.tree.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 21. World Bank (2015) Dataset: GDP per capita (current US$). World Bank Natl accounts data OECD Natl Accounts data files. Available at https://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed April 8, 2016.
- 22.Weigelt P, Kreft H. Quantifying island isolation–Insights from global patterns of insular plant species richness. Ecography. 2013;36:417–429. [Google Scholar]
- 23.Drenovsky RE, et al. A functional trait perspective on plant invasion. Ann Bot. 2012;110:141–153. doi: 10.1093/aob/mcs100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkinson IAE. Introduced Animals and Extinctions. Conservation for the Twenty-First Century. Oxford Univ Press; New York: 1989. [Google Scholar]
- 25.Apanius V, Yorinks N, Bermingham E, Ricklefs RE. Island and taxon effects in parasitism and resistance of Lesser Antillean birds. Ecology. 2000;81:1959–1969. [Google Scholar]
- 26.Cooper WE, Jr, Pyron RA, Garland T., Jr Island tameness: Living on islands reduces flight initiation distance. Proc Biol Sci. 2014;281:20133019. doi: 10.1098/rspb.2013.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol. 2002;17:164–170. [Google Scholar]
- 28.Cappuccino N, Arnason JT. Novel chemistry of invasive exotic plants. Biol Lett. 2006;2:189–193. doi: 10.1098/rsbl.2005.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callaway RM, Ridenour WM. Novel weapons: Invasive success and the evolution of increased competitive ability. Front Ecol Environ. 2004;2:436–443. [Google Scholar]
- 30.Weigelt P, et al. Global patterns and drivers of phylogenetic structure in island floras. Sci Rep. 2015;5:12213. doi: 10.1038/srep12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fridley JD, Sax DF. The imbalance of nature: Revisiting a Darwinian framework for invasion biology. Glob Ecol Biogeogr. 2014;23:1157–1166. [Google Scholar]
- 32.Valido A, Dupont YL, Olesen JM. Bird-flower interactions in the Macaronesian islands. J Biogeogr. 2004;31:1945–1953. [Google Scholar]
- 33.Diamond JM. Ecological consequences of island colonization by southwest Pacific birds, I. Types of niche shifts. Proc Natl Acad Sci USA. 1970;67:529–536. doi: 10.1073/pnas.67.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dyer EE, et al. The global distribution and drivers of alien bird species richness. PLoS Biol. 2017;15:e2000942. doi: 10.1371/journal.pbio.2000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helmus MR, Mahler DL, Losos JB. Island biogeography of the Anthropocene. Nature. 2014;513:543–546. doi: 10.1038/nature13739. [DOI] [PubMed] [Google Scholar]
- 36.Santos AMC, et al. Are species-area relationships from entire archipelagos congruent with those of their constituent islands? Glob Ecol Biogeogr. 2010;19:527–540. [Google Scholar]
- 37.Dyer EE, Redding DW, Blackburn TM. The global avian invasions atlas, a database of alien bird distributions worldwide. Sci Data. 2017;4:170041. doi: 10.1038/sdata.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hortal J, Lobo JM, Jiménez-Valverde A. Limitations of biodiversity databases: Case study on seed-plant diversity in Tenerife, Canary Islands. Conserv Biol. 2007;21:853–863. doi: 10.1111/j.1523-1739.2007.00686.x. [DOI] [PubMed] [Google Scholar]
- 39.Jones OR, Purvis A, Quicke DLJ. Latitudinal gradients in taxonomic overdescription rate affect macroecological inferences using species list data. Ecography. 2012;35:333–340. [Google Scholar]
- 40.Kalwij JM. Review of ‘The Plant List, a working list of all plant species.’. J Veg Sci. 2012;23:998–1002. [Google Scholar]
- 41.Brummitt RK. World Geographical Scheme for Recording Plant Distributions. 2nd Ed Carnegie Mellon University; Pittsburgh: 2001. [Google Scholar]
- 42.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high-resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 43.Klein Goldewijk K, Beusen A, Van Drecht G, De Vos M. The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Glob Ecol Biogeogr. 2011;20:73–86. [Google Scholar]
- 44.Gennaioli N, La Porta R, Lopez De Silanes F, Shleifer A. Growth in regions. J Econ Growth. 2014;19:259–309. [Google Scholar]
- 45.United Nations Statistics Division 2015 Dataset: Per capita GDP at current prices–US dollars. Natl Accounts Estim Main Aggregates. Available at data.un.org/Data.aspx?q=gdp+per+capita&d=SNAAMA&f=grID%3a101%3bcurrID%3aUSD%3bpcFlag%3a1. Accessed April 8, 2016.
- 46.Cody ML. Plants on Islands–Diversity and Dynamics on a Continental Archipelago. Univ of California Press; Oakland, CA: 2006. [Google Scholar]
- 47.Warton DI, Hui FKC. The arcsine is asinine: The analysis of proportions in ecology. Ecology. 2011;92:3–10. doi: 10.1890/10-0340.1. [DOI] [PubMed] [Google Scholar]
- 48.Bates D, Maechler M, Bolker BM, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 49.Bjornstad ON. 2016 ncf: Spatial Nonparametric Covariance Functions. R Package, Version 1.1-7. Available at https://cran.r-project.org/web/packages/ncf/index.html. Accessed July 28, 2016.
- 50.Meiri S, Chapple DG. Biases in the current knowledge of threat status in lizards, and bridging the ‘assessment gap.’. Biol Conserv. 2016;204:6–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed during the current study are included in this published article and SI Appendix, Table S4 or in the sources where the available datasets are are provided (SI Appendix, Tables S3 and S5).