Significance
In a colony of naked mole-rats, instead of a single breeding female (the queen) nursing alone, sexually immature members (subordinates) show positive alloparental pup care. Their response to pup vocalizations is not continuous, but rather is enhanced during the queen’s postpartum period through consumption of the pregnant queen’s feces. Furthermore, oral ingestion of the pregnant queen’s feces also induces an increase in the subordinates’ estradiol concentrations. Because subordinates’ responses to pup vocalizations were enhanced through the ingestion of nonpregnant queen’s feces amended with estradiol, we concluded that estradiol is the substance that enhances their responses to pup vocalizations in naked mole-rats. Moreover, these results suggest that naked mole-rats communicate the substance between the queen and subordinates through coprophagy.
Keywords: naked mole-rats, alloparental behavior, coprophagy, estradiol
Abstract
Naked mole-rats form eusocial colonies consisting of a single breeding female (the queen), several breeding males, and sexually immature adults (subordinates). Subordinates are cooperative and provide alloparental care by huddling and retrieving pups to the nest. However, the physiological mechanism(s) underlying alloparental behavior of nonbreeders remains undetermined. Here, we examined the response of subordinates to pup voice and the fecal estradiol concentrations of subordinates during the three reproductive periods of the queen, including gestation, postpartum, and nonlactating. Subordinate response to pup voice was observed only during the queen’s postpartum and was preceded by an incremental rise in subordinates’ fecal estradiol concentrations during the queen’s gestation period, which coincided with physiological changes in the queen. We hypothesized that the increased estradiol in the queen’s feces was disseminated to subordinates through coprophagy, which stimulated subordinates’ responses to pup vocalizations. To test this hypothesis, we fed subordinates either fecal pellets from pregnant queens or pellets from nonpregnant queens amended with estradiol for 9 days and examined their response to recorded pup voice. In both treatments, the subordinates exhibited a constant level of response to pup voice during the feeding period but became more responsive 4 days after the feeding period. Thus, we believe that we have identified a previously unknown system of communication in naked mole-rats, in which a hormone released by one individual controls the behavior of another individual and influences the level of responsiveness among subordinate adults to pup vocal signals, thereby contributing to the alloparental pup care by subordinates.
Naked mole-rats (Heterocephalus glaber) are eusocial mammals that exhibit reproductive hierarchy within their colonies. These rodents inhabit arid regions of East Africa and live in underground colonies that typically contain 60–80 individuals (1). Only one female (the queen) and one to three males are reproductively active, while the remaining colony members of both sexes (subordinates) do not participate in reproduction (2–5). In female subordinates, ovaries are small, thin, and flat structures, and feature mainly primordial and primary follicles (5). Lack of ovarian development is due to insufficient secretion of luteinizing hormone from the pituitary as a result of its insensitivity to gonadotropin-releasing hormone (4). Subordinates exhibit no mating behavior but provide coordinated support during foraging, colony defense, and care of the queen’s offspring (alloparenting). Alloparental pup care behavior displayed by subordinates includes licking, grooming, retrieving, and warming of pups (Fig. 1A) (1). In other species, such forms of caring behavior are more typically performed by birthing females in response to the drastic hormonal changes they experience during pregnancy, such as augmented release of progesterone and estrogen from gonads, which play key roles in the onset of these behaviors (6). Female subordinates of naked mole-rats, however, never experience delivery nor even gonadal maturation (1, 4, 5).
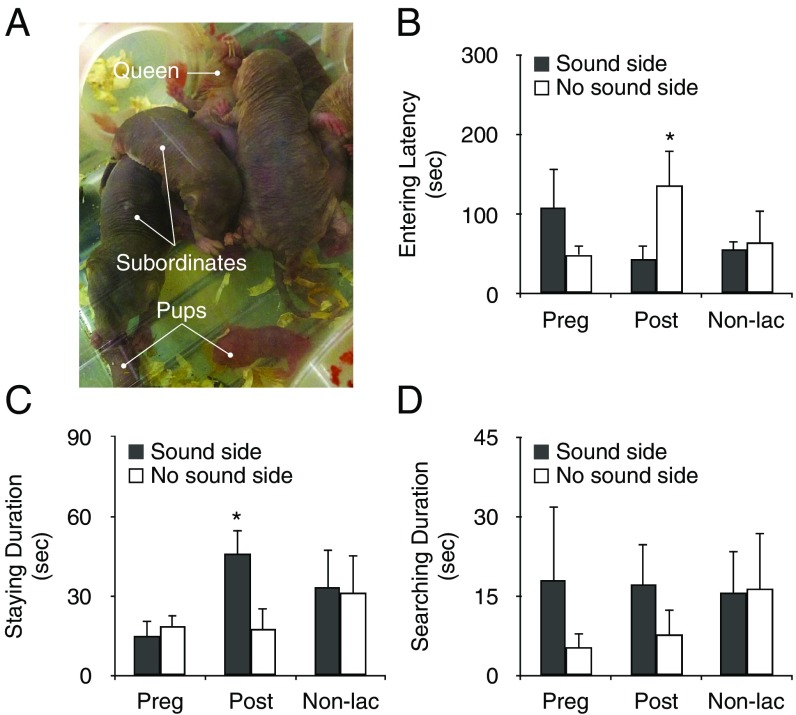
Fig. 1.
Subordinates enhanced responses to pup vocalizations during the postpartum period. (A) The subordinate naked mole-rats showed alloparental pup care, such as licking, grooming, and warming of the queen’s pups. (B–D) The approach response of subordinates to the side with a speaker (sound side, black) and the side without a speaker (no-sound side, white) during each reproduction period: gestation (n = 6), postpartum (n = 7), and nonlactation (n = 5). (B) Latency to enter a tube. (C) Time spent in a tube. (D) Time subordinates spent exploring the mesh. During the postpartum period, subordinates approached the sound side more frequently than the no-sound side of the tube. *P < 0.05. Data are represented as mean ± SEM.
One clue as to how subordinate mole-rats acquire alloparental pup care behavior is the enlargement of the nipples of subordinates after the queen gives birth (7), despite their having low levels of gonadal hormones preventing the development of mammary glands (8, 9). Here, we hypothesized that subordinates might acquire hormones, like progesterone and estrogen, from external sources, such as the ingestion of the queen’s feces. This is because naked mole-rats routinely perform coprophagy. As a means of maximizing limited resources, naked mole-rats often consume feces and induce other individuals, including the queen, to excrete feces (7, 10). Furthermore, gonadal steroids are chemically stable and are found in the feces of many mammals, including rodents (11). Therefore, in the present study, we tried to demonstrate that subordinates obtain gonadal hormones from the feces of the queen, which might, at least partially, account for their investment in alloparental pup care behavior.
First, to determine whether subordinates display alloparental activity at a constant rate, or if such activity is only enhanced after the queen has given birth, we compared responses to pup voice among the subordinates during the three periods of the queen’s reproductive cycle, consisting of the gestation, postpartum, and nonlactation periods. In rodents, pups vocalize when in distress, such as when separated from the nest, cold, or in response to unusual tactile stimulation (12, 13). The lactating female responds to pup voice and exhibits maternal behaviors, such as orienting, searching, and retrieving the pups (12, 14, 15). Therefore, we examined the responses of female subordinate naked mole-rats to reproduced pup voice by using a two-choice test during each phase of the queen’s reproduction. Next, we investigated whether ingestion of the feces excreted by the pregnant queen induces responses to pup voice in subordinate females by feeding pellets containing feces from either pregnant or nonpregnant queens to subordinates. We also evaluated estrogen and progesterone concentrations in the feces of subordinates during each phase of the queen’s reproductive cycle and when subordinates orally ingested estrogen and progesterone. Finally, we examined how subordinates responded to pup voice after being fed pellets containing feces from the nonpregnant queen amended with estradiol.
Results
Subordinates’ Response to Pup Vocalizations Increases During the Queen’s Postpartum Period.
When pup voice were reproduced during the postpartum period, subordinate latency to enter a tube was shorter for the tube with a speaker (sound side) than the tube without a speaker (no-sound side) [linear mixed models (LMM), Fvoice*period (2, 24.407) = 2.973, P = 0. 070; Bonferroni, P = 0.040] (Fig. 1B, SI Appendix, Fig. S1A, and Movie S1). The mean time spent in the tube during the postpartum period was longer on the sound side than on the no-sound side of the tube [LMM, Fvoice*period (2, 23.228) = 1.724, P = 0.200; Bonferroni, P = 0.032] (Fig. 1C). However, these reactions to reproduced pup voice were not observed during the gestation and nonlactation periods. As for mean time spent searching the mesh, there was no preference in regard to pup voice in any period [LMM, Fvoice*period (2, 22.052) = 0.353, P = 0.707] (Fig. 1D). Moreover, when other sounds were reproduced during the postpartum period, subordinates did not show the preference corresponding to the responses to pup voice (SI Appendix, Fig. S1 B–D). These results demonstrated that subordinate response to pup voice was enhanced only during the queen’s postpartum period. In addition, the results indicated the presence of an inducer of responses to pup voice that was dependent on the queen’s reproductive stage.
Responses to Pup Vocalizations in Subordinates Are Induced by Oral Ingestion of Pregnant Queen’s Feces.
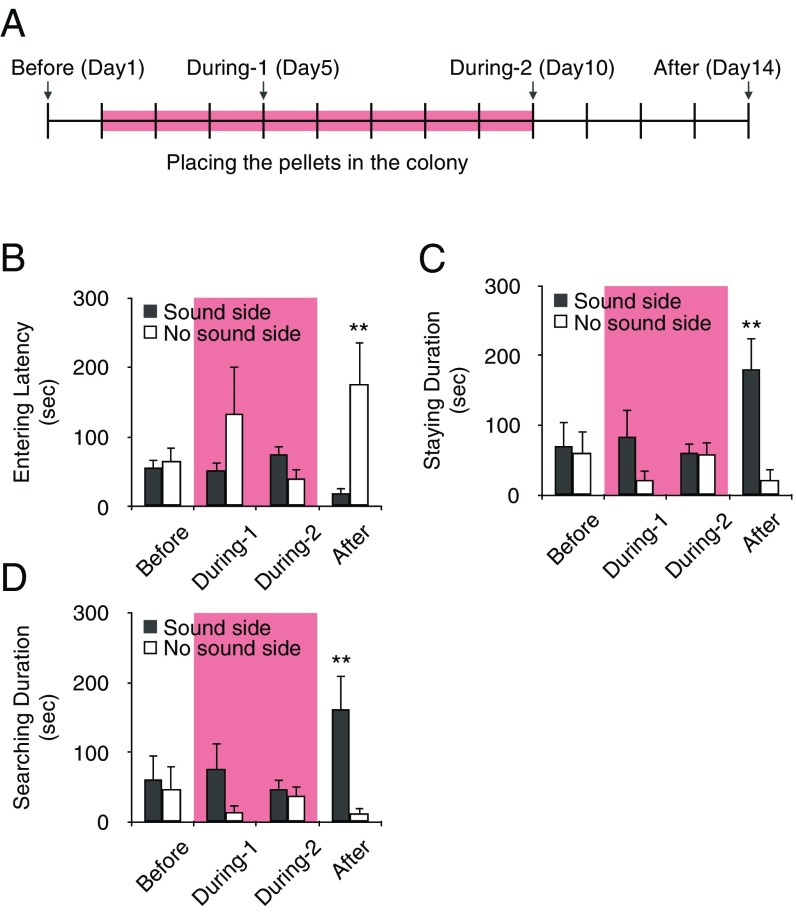
Naked mole-rats reingest partially digested resources through coprophagy—regular consumption of feces excreted by other individuals, including that from the queen (7, 10). Therefore, we hypothesized that the pregnant queen’s feces might contain the inducer of responses to pup vocalizations in subordinates during the queen’s postpartum period. To test this hypothesis, we investigated the response of subordinates to reproductions of pup voice by using a two-choice test before providing fecal pellets containing pregnant queen’s feces, during feeding on these fecal pellets, and after the removal of the pellets from the colony (Fig. 2A). Subordinate latency to enter a tube was shorter on the sound side than on the no-sound side of the tube after the pellets were removed, but not before or during their placement in the test box [LMM, Fvoice*stage (3, 28) = 2.805, P = 0.058; Bonferroni, P = 0.004] (Fig. 2B). Similarly, the mean time subordinates stayed in a tube or explored the mesh was longer on the sound side than on the no-sound side only after removal of the pellets [LMM, Fvoice*stage (3, 28) = 2.958, P = 0.049; Bonferroni, P = 0.002] [LMM, Fvoice*stage (3, 28) = 7.773, P = 0.096; Bonferroni, P = 0.001] (Fig. 2 C and D). These results indicated that the response to pup voice displayed by the subordinates was induced by oral ingestion of the pregnant queen’s feces.
Fig. 2.
Subordinates enhance responses to pup vocalizations by eating the pregnant queen’s feces. (A) Schematic diagram of food pellet placement and two-choice test. We conducted the test four times in 14 d and placed the pellets containing pregnant queen’s feces in the colony for 9 d (pink, from day 2 to day 10). (B–D) The approach response of subordinates to the sound (black) and no-sound (white) side of the tube at different time points: before (day 1, n = 5), during 1 (day 5, n = 5), during 2 (day 10, n = 5), and after (day 14, n = 5). (B) Latency to enter a tube. (C) Time spent inside a tube. (D) Time subordinates spent searching the mesh. After consuming the pellets, subordinates approached the side emitting the reproduced pup’s voice more frequently than the silent side of the tube. **P < 0.01. Data are represented as mean ± SEM.
Oral Ingestion of Estradiol by Subordinates Induces Its Increment in a Pattern Similar to That in the Queen Throughout Her Reproductive Stages.
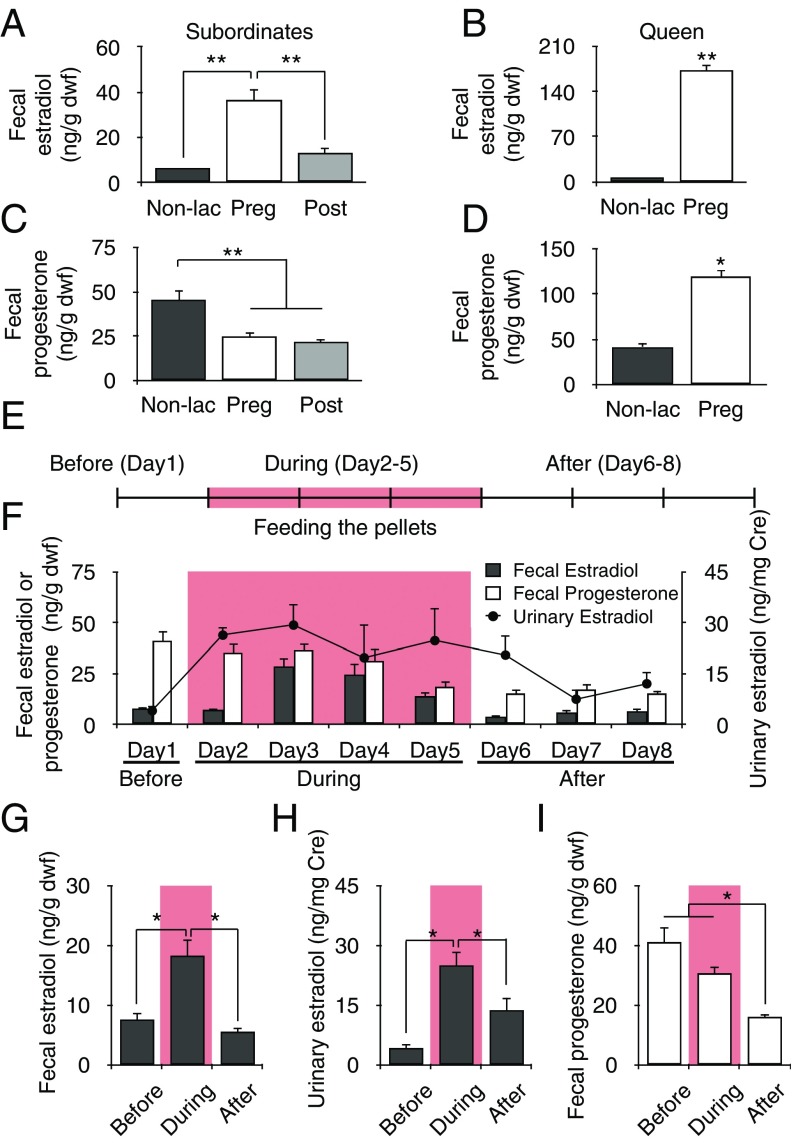
We measured fecal estradiol and progesterone and urinary estradiol concentrations of subordinates throughout the queen’s three reproductive periods. Both fecal and urinary estradiol concentrations were higher in subordinates during the queen’s gestation period than during the nonlactation period [LMM, Fperiod (2) = 33.335, P < 0.001; Bonferroni, P < 0.001, P < 0.001] (Fig. 3A and SI Appendix, Fig. S2), and queen fecal estradiol concentration was higher during the gestation period than the nonlactation period [paired t test, t(40.539) = 2.000, P = 0.001] (Fig. 3B). Although subordinate fecal progesterone concentrations were lower during the queen’s gestation period than the nonlactation period [LMM, Fperiod (2) = 10.889, P < 0.001; Bonferroni, P = 0.023, P = 0.004] (Fig. 3C), progesterone concentrations in the queen’s feces were higher during the gestation period than during the nonlactation period [paired t test, t(9.322) = 2.000, P = 0.011] (Fig. 3D). Taken together, estradiol concentrations in subordinates increased during the queen’s gestation period in accordance with the incremental rise in estradiol concentration in the queen’s feces. In contrast, fecal progesterone concentration in female subordinates moved in the opposite direction of that of the pregnant queen during pregnancy.
Fig. 3.
Subordinates can change their estradiol concentrations by oral ingestion. Fecal estradiol concentrations in subordinates (A) and the queen (B) during different reproductive periods. (A) Subordinates (n = 15) showed an increase in estradiol concentrations during the gestation period (white) compared with that in the nonlactation (black) and postpartum period (gray). (B) Estradiol concentration in the queen increased more during the gestation period (white, n = 3) than in the nonlactation period (black, n = 3). Fecal progesterone concentrations in subordinates (C) and the queen (D) during different reproductive periods. In subordinates (n = 15), fecal progesterone concentrations decreased during the gestation period (white) and postpartum period (gray) compared with that in the nonlactation period (black). (D) Progesterone concentration in the queen increased more during the gestation period (white, n = 3) than in the nonlactation period (black, n = 3). (E) Schematic diagram of the feeding of food pellets and sampling schedule. We sampled feces and urine from each subordinate for 8 d and fed the pellets containing estradiol and progesterone for 4 d (pink, from day 2 to day 5). (F) Fluctuations in fecal estradiol (black bar) and progesterone (white bar) and urinary estradiol (black circle) concentrations in subordinates; abbreviations follow the schematic diagram in E. Day 1, before (fecal estradiol, urinary estradiol: n = 5, 5); day 2, during (n = 5, 4); day 3, during (n = 5, 4); day 4, during (n = 5, 4); day 5, during (n = 5, 3); day 6, after (n = 5, 4); day 7, after (n = 5, 3); and day 8, after (n = 5, 4). (G) Fecal estradiol increased during feeding with pellets (n = 5, days 2–5) compared with before (n = 5, day 1) and after (n = 5, days 6–8) the feeding. (H) Urinary estradiol increased during feeding with pellets (n = 5) compared with that before (n = 5) and after (n = 5) feeding with pellets. (I) Fecal progesterone decreased after feeding with pellets (n = 5) compared with before (n = 5) and during (n = 5) feeding. *P < 0.05, **P < 0.01. Fecal estradiol concentrations [nanograms per gram of dry weight of feces (dwf)] or urinary estradiol concentrations [nanograms per milligram of creatinine (Cre)] are represented as mean ± SEM.
Although these results suggested estrogen to be the substance of interest in the feces excreted by the pregnant queen, it is unclear whether estradiol concentration in subordinates increased by coprophagy (i.e., oral ingestion of pregnant queen’s feces) or by some other means. To examine this, we fed female subordinates pellets containing estradiol and progesterone instead of the pregnant queen’s feces (Fig. 3E), and tracked the chronological changes in estradiol or progesterone concentrations in the feces and urine of the subordinates (Fig. 3F), finding that both fecal and urinary estradiol concentrations increased only when the pellets were fed to the subordinates [LMM, Fstage (2, 33) = 10.567, P < 0.001; Bonferroni, P = 0.049, P < 0.001] [LMM, Fstage (2, 25.543) = 7.905, P = 0.002; Bonferroni, P = 0.003, P = 0.048] (Fig. 3 G and H), whereas subordinate fecal progesterone concentrations decreased after feeding on the pellets [LMM, Fstage (2, 33) = 12.865, P < 0.001; Bonferroni, P = 0.001, P < 0.001] (Fig. 3I). These changes were similar to the increasing estradiol and decreasing progesterone concentrations observed in subordinates during the queen’s gestation period. Our results indicated that oral ingestion of gonadal hormones through coprophagy of the pregnant queen’s feces induces changes in estradiol levels in subordinates commensurate with the progress of the queen’s reproductive state.
Responses to Pup Vocalizations in Subordinates Are Induced by Oral Ingestion of Estradiol.
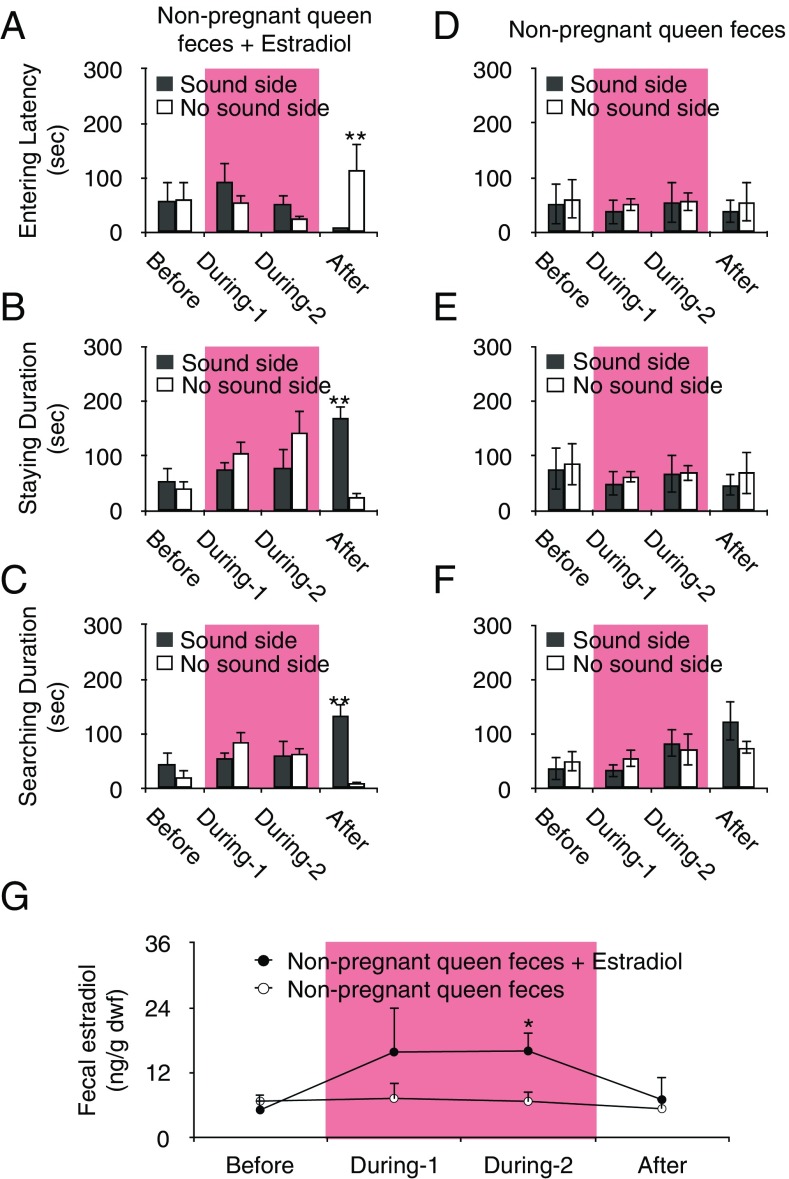
Finally, we examined subordinate response to reproduced pup voice after being fed pellets containing the nonpregnant queen’s feces either amended with estradiol or without the steroid. Female subordinates receiving pellets containing the nonpregnant queen’s feces amended with estradiol entered the tube on the sound side faster than on the no-sound side following removal of the pellets, but not before or as the pellets were provided [LMM, Fvoice*stage (3, 28) = 3.139, P = 0.041; Bonferroni, P = 0.008] (Fig. 4A). Similarly, mean time spent in the tube or searching the mesh was longer on the sound side than on the no-sound side only after the pellets were removed [LMM, Fvoice*stage (3, 28) = 6.392, P = 0.002; Bonferroni, P < 0.001] [LMM, Fvoice (1, 28) = 4.925, P = 0.035, Fvoice*stage (3, 28) = 6.861, P = 0.001; Bonferroni, P = 0.001] (Fig. 4 B and C). This behavioral response to pup voice was not observed when female subordinates were fed pellets containing nonpregnant queen’s feces without the steroid [LMM, Fvoice*stage (3, 28) = 0.048, P = 0.986] [LMM, Fvoice*stage (3, 28) = 0.945, P = 0.432] [LMM, Fvoice*stage (3, 28) = 0.031, P = 0.992] (Fig. 4 D–F). Moreover, fecal estradiol concentrations increased in female subordinates fed pellets of nonpregnant queen’s feces amended with estradiol during feeding compared with female subordinates fed pellets lacking the steroid [LMM, Ffecal_type*stage (1) = 2.078, P = 0.187; Bonferroni, P = 0.042] (Fig. 4G). These results demonstrated that estradiol is a molecule that is able to contribute alloparental behaviors in subordinates.
Fig. 4.
Subordinates’ responses to pup vocalizations is induced by estradiol from the pregnant queen. The approach response of subordinates fed pellets of nonpregnant queen’s feces amended (A–C) or not amended with estradiol (D–F) to the sound (black) and no-sound (white) side of the tube at each time point: before (day 1, n = 5), during 1 (day 5, n = 5), during 2 (day 10, n = 5), and after (day 14, n = 5). (A) Latency to enter a tube. (B) Time spent in a tube. (C) Time subordinates spent searching the mesh. (D) Latency to enter a tube. (E) Time spent in a tube. (F) Time subordinates spent searching the mesh. After consuming the pellets, subordinates approached the side emitting the reproduced pup’s voice more frequently than the silent side of the tube. (G) Fecal estradiol increased during feeding with pellets of nonpregnant queen’s feces amended with estradiol (n = 5, day 10) compared with that during feeding with pellets not amended with the steroid (n = 5, day 10). *P < 0.05, **P < 0.01. Data are represented as mean ± SEM.
Discussion
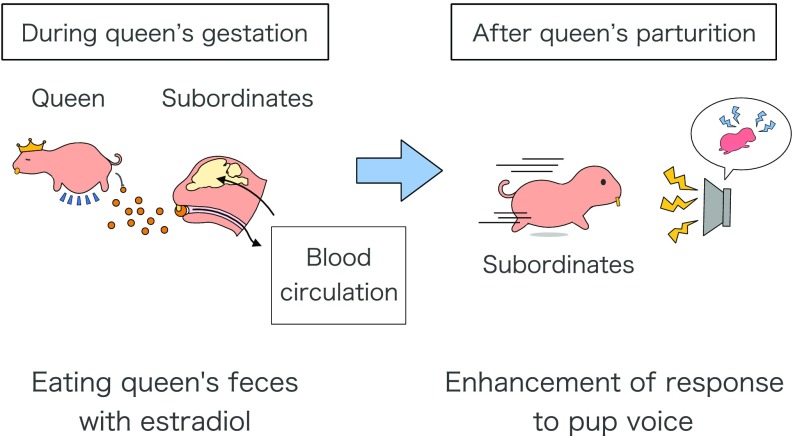
We demonstrated that the behavioral responses to pup voice by subordinates depended on the queen’s reproductive stage, and that such behaviors were enhanced after the birth of pups as the need for alloparental pup care increased. Moreover, pellets that were fed to the subordinates containing the feces of pregnant queens and pellets containing the feces of nonpregnant queens amended with estradiol induced similar behavioral responses in subordinates. Our results provide evidence of coprophagous-induced responses to pup voice that contribute to the alloparental pup care in naked mole-rats. In mice, it was suggested that estradiol in the urine disrupts the intrauterine implantation of fertilized ova in inseminated females (16); thus, a similar form of social communication exists using hormones. Based on the results of the present study, we hypothesize that the incremental rise in estradiol concentrations in subordinates by the oral ingestion of the pregnant queen’s feces induces alloparental pup care during the queen’s postpartum period (Fig. 5).
Fig. 5.
Schema of our hypothesis. Coprophagous-induced alloparental behavior in subordinate naked mole-rats. The blood estradiol concentrations in subordinates increase by oral ingestion of the pregnant queen’s feces. This hormonal change induces alloparental pup care behaviors in subordinates after parturition by the queen.
It is well known that in eusocial societies, dominant individuals induce cooperative behavior in subordinates through the release of pheromones (17–19). However, vomeronasal organs, which receive pheromones, are not well developed in naked mole-rats (20). In addition, naked mole-rat queens do not secrete pheromones that suppress gonadal development or reproduction in subordinates (21, 22), unlike other eusocial organisms and rodents, which use pheromones for sexual suppression (19, 23, 24). Instead of pheromonal communication, naked mole-rats appear to have developed a unique system of communication using hormones. This system might facilitate collaborative maternal care between the queen and subordinates, in which the queen conveys information about her reproductive stage using estrogen, with the hormone produced by the queen stimulating responses to pup vocalizations in subordinates.
According to the predominant theory, the priming of estrogen to the medial preoptic area (MPOA) is essential for the induction of maternal behavior in rodents, as the MPOA contains an abundance of estrogen receptors and lowers the threshold of maternal response activation via the administration of estrogen (25, 26). This provides additional support for our hypothesis. In contrast, it has been suggested that the maternal response in female rodents is maintained by stimuli from pups if estrogen levels fall, once they are maternally responsive (26). In the present study, estradiol concentrations in subordinates increased during the queen’s gestation period, and their response to pup voice was enhanced during the queen’s postpartum phase. Therefore, after being induced by estradiol from the queen’s feces during the queen’s gestation period, the maternal response in subordinates might be maintained during the queen’s postpartum period by pup stimulation. In addition, naked mole-rats are known to have several unique metabolic functions (27); in our study, subordinates exhibited characteristic progesterone metabolic activity. In contrast to estradiol, the fecal progesterone concentrations in subordinates decreased from the queen’s nonlactating period to her gestational period, even when fecal progesterone from the queen was increased. Similarly, fecal progesterone concentrations in subordinates decreased after consumption of pellets containing estradiol and progesterone. Because of this, we believe that subordinates are extremely efficient at converting progesterone into estrogen, a metabolic capability which might not only contribute to the production of estrogen to supplement that absorbed from ingestion of the queen’s feces, but also indirectly helps to induce the maternal response, given that high progesterone concentrations inhibit the onset of maternal behaviors primed by estrogen (28).
Previous studies examining endocrinological mechanisms of alloparental care among cooperative animals tended to focus on testosterone or glucocorticoids, neglecting potential roles of estrogen or progesterone (29). Research on social mole-rats and other cooperative species suggests that low testosterone is an important mechanism of subordinate alloparental behavior, especially as the unusually high testosterone in dominants appears to increase reproductive potential while reducing parental investment (30–33). A specific example from golden lion tamarins shows that subordinates have lower fecal testosterone and engaged in more alloparenting than dominants (34). However, another study of Damaraland mole-rats did not find a connection between alloparental care and experimental increases in testosterone (35). In meerkats, the aggression of dominant breeders toward subordinates is thought to increase stress hormones (glucocorticoids) in the latter. This physiological change may boost subordinate cooperation, as plasma cortisol concentrations were positively associated with pup food-provisioning rates in male meerkats (36). However, a recent long-term (19 y) pharmacological experiment found little support for the idea that dominant aggression is a manipulative tactic to raise subordinate cooperative behavior via effects on glucocorticoid levels (37). In this context, our findings offer a perspective on the endocrinological mechanism of alloparenting, suggesting that estrogen, rather than testosterone or glucocorticoids, may be a major regulating hormone.
Here, we identified a unique communication system in naked mole-rats, in which an endogenous hormone is transported to other individuals, where it regulates their behavior. Because vomeronasal organs in naked mole-rats are not well developed (20), it may be difficult for naked mole-rats to communicate with other individuals by means of nonvolatile pheromones. In addition, because the good ventilation is not always guaranteed in the naked mole-rats’ areas of inhabitance (such as underground tunnels), communication systems based on the use of volatile odorants may be unsuitable for organisms. Thus, naked mole-rats may have evolved a unique means of communication that does not rely on the use of volatile or nonvolatile odorants, but rather on a hormone. Using hormones instead of pheromones for communication may directly mediate the reproductive state and behavior of another individual. It is also interesting that this communication occurs through coprophagy, a habit that might have evolved as an adaptive strategy to maximize the use of resources under harsh semidesert conditions (38). It might be difficult for a single queen to distribute her feces to all members of the colony. However, some subordinates consistently remain in the nest, and the queen remains in the nest with increasing frequency during the gestation period (38). Thus, it is plausible that the nearby subordinates eat feces from the pregnant queen and become alloparents. Coprophagy near to the nest of the queen is enough to induce cooperation between a queen and subordinates for the growing pups. In conclusion, we think that this form of communication by naked mole-rats is highly suitable from an ecological perspective. In the future, we hope to clarify the neural basis of this unique form of social-behavioral signaling exhibited by naked mole-rats by investigating the functional neuronal network underlying this behavioral paradigm.
Materials and Methods
Animals.
Naked mole-rat individuals used in the experiments were bred at Keio University and Hokkaido University, and maintained in acrylic boxes (15 × 15 × 20 cm) with lids, which were connected by acrylic tubing (5 cm inner diameter, 16 cm long). The animals were fed apples, burdock, bananas, sweet potatoes, potatoes, carrots, and oatmeal ad libitum (water was not supplied), and housed at constant temperature (27–30 °C) under continuous light. Data were collected from female subordinates and queens that belonged to a colony consisting of more than 10 individuals that had lived in the colony for more than 6 mo (26.5 wk). Additional details about the individuals are provided in SI Appendix, Table S1. All procedures were approved by the Ethics Committees of Azabu University (approval no. 080214–5), Hokkaido University (approval no. 14–0065), and Keio University [approval no. 09212-(1)].
Experimental Design.
Experiment 1.
A two-choice test was used to examine the response of subordinates to pup voice (SI Appendix, Fig. S1A). Each subordinate was tested repeatedly during the three periods of the queen’s reproductive cycle, consisting of gestation, postpartum, and nonlactation. We defined “gestation” as the period until fetal movements in the queen’s abdomen were first observed (∼60–70 d after the start of the gestation period: that was about the last 10 d of pregnancy); “postpartum” was defined as the period from the 8th day to the 14th day after parturition; and “nonlactation” was defined as the period that began 80–90 d after parturition. For the two-choice test, we chose female subordinates (n = 7) among individuals which had not experienced the queen giving birth during their adulthood. The test consisted of two blocks, to which the subordinates were allowed to adapt for 15 min, following which they were exposed to a recording of pup voice for 5 min. During the 5 min of exposure vocalizations, we measured three behaviors: (i) latency to enter, defined as the time until the entire body of the subordinate was within a tube with a speaker (sound side) or a tube without a speaker (no-sound side); (ii) duration of the stay, the summation of time that the subordinate spent in the sound side or the no-sound side; and (iii) duration of the search, the summation of time the subordinate spent exploring the mesh on the sound side or the no-sound side. One obvious outlier during the pregnancy period and two obvious outliers during the nonlactation period were removed from the two-choice test. Fecal and urinary samples were collected immediately after natural defecation and urination, or by applying gentle pressure on the abdomen, during the three reproductive stages of the queen. Fecal samples were collected from all individuals included in the two-choice test and from eight other female subordinates that had not experienced the queen giving birth during their adulthood. Although we also attempted to collect urinary samples from the same 15 individuals used for fecal sampling, urinary samples were difficult to harvest; thus, only the urinary samples from 5 individuals that we were able to collect during both the queen’s nonlactation and gestation periods were included in the analysis. Additional details about these procedures are provided in SI Appendix, SI Materials and Methods.
Experiment 2.
The subordinates were fed pellets containing feces from the pregnant queen or pellets containing the feces of the nonpregnant queen ad libitum. The female subordinates (n = 5) that had previously experienced the queen giving birth were used in each experiment. The two-choice test was conducted four times: before the feeding commenced (1 d), twice during the feeding (5 d and 10 d), and after the termination of feeding (14 d). During the feeding, we visually confirmed that the female subordinates ingested the pellets. Additional details about pup voice preference test with feeding feces are provided in SI Appendix, SI Materials and Methods.
Experiment 3.
Five female subordinates were selected from a colony whose queen was in the nonlactation phase. We collected fecal and urinary samples from the subordinates for a total of 8 d, for four of which subordinates were fed pellets containing estradiol and progesterone. Subordinates consistently ingested ∼1.0 g of the pellets at each feeding. After feeding, we collected fecal and urinary samples for 3 d with a pipette immediately after each natural defecation and urination event or by applying gentle pressure to the abdomen.
Experiment 4.
The subordinates were fed pellets containing feces from the nonpregnant queen with or without estradiol ad libitum. The female subordinates (n = 5) that had previously experienced the queen giving birth were used in each experiment. The two-choice test was conducted four times: before the feeding commenced (1 d), twice during the feeding (5 d and 10 d), and after the termination of feeding (14 d). During the feeding, we visually confirmed that the female subordinates ingested the pellets. At each time, the fecal samples were collected immediately after each natural defecation or by applying gentle pressure on the abdomen.
Statistical Analysis.
All data were analyzed using SPSS Statistics 22 (IBM Corp.) software and presented as means ± SEM. Behavioral test data were analyzed using a LMM with Bonferroni as the multiple-comparison correction. Hormone concentrations were analyzed via paired t test or LMM with Bonferroni as the multiple-comparison correction. Obvious outliers were detected by Grubbs’ test; a single outlier was detected during the period of pregnancy and two outliers were detected during the nonlactation period in experiment 1. These outliers were removed from the analysis.
Supplementary Material
Acknowledgments
We thank Y. Seki, R. Tachibana, and K. Okanoya for their advice in setting up the acoustic systems and Y. Oiwa and Y. Fujimura for technical help and animal care. This work was supported by the Japan Society for the Promotion of Science, and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan Grant 26292167 (to K. Mogi); Grants 23248049, 25118007, and 15H02479 (to T.K.); and by a grant from the Funding Program for World-Leading Innovative R&D on Science and Technology program “Strategic Exploitation of Neuro-Genetics for Emergence of the Mind” from the Japanese Cabinet Office (to H.O.). H.O. is a founding scientist of SanBio Company, Ltd and K-Pharma, Inc.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720530115/-/DCSupplemental.
References
- 1.Jarvis JU. Eusociality in a mammal: Cooperative breeding in naked mole-rat colonies. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- 2.Faulkes CG, Abbott DH. The physiology of a reproductive dictatorship: Regulation of male and female reproduction by a single breeding female in colonies of naked mole-rats. In: Solomon NG, French JA, editors. Cooperative Breedings in Mammals. Cambridge Univ Press; Cambridge, UK: 1997. pp. 302–334. [Google Scholar]
- 3.Faulkes CG, Abbott DH, Jarvis JU. Social suppression of reproduction in male naked mole-rats, Heterocephalus glaber. J Reprod Fertil. 1991;91:593–604. doi: 10.1530/jrf.0.0910593. [DOI] [PubMed] [Google Scholar]
- 4.Faulkes CG, Abbott DH, Jarvis JU, Sherriff FE. LH responses of female naked mole-rats, Heterocephalus glaber, to single and multiple doses of exogenous GnRH. J Reprod Fertil. 1990;89:317–323. doi: 10.1530/jrf.0.0890317. [DOI] [PubMed] [Google Scholar]
- 5.Kayanja FIB, Jarvis JUM. Histological observations on the ovary, oviduct and uterus of the naked mole-rat. Z Saugetierkd. 1971;36:114–121. [Google Scholar]
- 6.Terkel J, Rosenblatt JS. Humoral factors underlying maternal behavior at parturition: Corss transfusion between freely moving rats. J Comp Physiol Psychol. 1972;80:365–371. doi: 10.1037/h0032965. [DOI] [PubMed] [Google Scholar]
- 7.Jarvis JUM, Sherman PW, Alexander RD. The Biology of the Naked Mole-Rat. Princeton Univ Press; Princeton: 1991. Reproduction of naked mole-rats; pp. 384–425. [Google Scholar]
- 8.Shyamala G, Ferenczy A. Mammary fat pad may be a potential site for initiation of estrogen action in normal mouse mammary glands. Endocrinology. 1984;115:1078–1081. doi: 10.1210/endo-115-3-1078. [DOI] [PubMed] [Google Scholar]
- 9.Tucker HA. Hormones, mammary growth, and lactation: A 41-year perspective. J Dairy Sci. 2000;83:874–884. doi: 10.3168/jds.S0022-0302(00)74951-4. [DOI] [PubMed] [Google Scholar]
- 10.Dyer BD. A hypothesis about the significance of symbionts as a source of protein in the evolution of eusociality in naked mole rats. Symbiosis. 1998;24:369–383. [Google Scholar]
- 11.Muir C, Spironello-Vella E, Pisani N, deCatanzaro D. Enzyme immunoassay of 17 beta-estradiol, estrone conjugates, and testosterone in urinary and fecal samples from male and female mice. Horm Metab Res. 2001;33:653–658. doi: 10.1055/s-2001-18692. [DOI] [PubMed] [Google Scholar]
- 12.Noirot E. Ultrasounds and maternal behavior in small rodents. Dev Psychobiol. 1972;5:371–387. doi: 10.1002/dev.420050410. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerberg B, Rosenthal AJ, Stark AC. Neonatal social isolation alters both maternal and pup behaviors in rats. Dev Psychobiol. 2003;42:52–63. doi: 10.1002/dev.10086. [DOI] [PubMed] [Google Scholar]
- 14.Smotherman WP, Bell RW, Starzec J, Elias J, Zachman TA. Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav Biol. 1974;12:55–66. doi: 10.1016/s0091-6773(74)91026-8. [DOI] [PubMed] [Google Scholar]
- 15.Ehret G. Categorical perception of mouse-pup ultrasounds in the temporal domain. Anim Behav. 1992;43:409–416. [Google Scholar]
- 16.deCatanzaro D, Beaton EA, Khan A, Vella E. Urinary oestradiol and testosterone levels from novel male mice approach values sufficient to disrupt early pregnancy in nearby inseminated females. Reproduction. 2006;132:309–317. doi: 10.1530/rep.1.00965. [DOI] [PubMed] [Google Scholar]
- 17.Billen J, Morgan ED. Pheromone communication in social insects: Sources and secretions. In: Meer RKV, Breed MD, Espelie KE, Winston ML, editors. Pheromone Communication in Social Insects Ants Wasps Bees, and Termites. Westview Press; Boulder, CO: 1998. pp. 3–33. [Google Scholar]
- 18.Holman L, Jørgensen CG, Nielsen J, d’Ettorre P. Identification of an ant queen pheromone regulating worker sterility. Proc Biol Sci. 2010;277:3793–3800. doi: 10.1098/rspb.2010.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuura K, et al. Identification of a pheromone regulating caste differentiation in termites. Proc Natl Acad Sci USA. 2010;107:12963–12968. doi: 10.1073/pnas.1004675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith TD, Bhatnagar KP, Dennis JC, Morrison EE, Park TJ. Growth-deficient vomeronasal organs in the naked mole-rat (Heterocephalus glaber) Brain Res. 2007;1132:78–83. doi: 10.1016/j.brainres.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Smith TE, Faulkes CG, Abbott DH. Combined olfactory contact with the parent colony and direct contact with nonbreeding animals does not maintain suppression of ovulation in female naked mole-rats (Heterocephalus glaber) Horm Behav. 1997;31:277–288. doi: 10.1006/hbeh.1997.1384. [DOI] [PubMed] [Google Scholar]
- 22.Faulkes CG, Abbott DH. Evidence that primer pheromones do not cause social suppression of reproduction in male and female naked mole-rats (Heterocephalus glaber) J Reprod Fertil. 1993;99:225–230. doi: 10.1530/jrf.0.0990225. [DOI] [PubMed] [Google Scholar]
- 23.Massey A, Vandenbergh JG. Puberty delay by a urinary cue from female house mice in feral populations. Science. 1980;209:821–822. doi: 10.1126/science.7190728. [DOI] [PubMed] [Google Scholar]
- 24.Kruczek M, Marchlewska-Koj A. Puberty delay of bank vole females in a high-density population. Biol Reprod. 1986;35:537–541. doi: 10.1095/biolreprod35.3.537. [DOI] [PubMed] [Google Scholar]
- 25.Champagne FA, Weaver ICG, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- 26.Numan M, Insel TR. The Neurobiology of Parental Behavior. 1st Ed Springer; New York: 2003. [Google Scholar]
- 27.Edrey YH, Park TJ, Kang H, Biney A, Buffenstein R. Endocrine function and neurobiology of the longest-living rodent, the naked mole-rat. Exp Gerontol. 2011;46:116–123. doi: 10.1016/j.exger.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Bridges RS, Russell DW. Steroidal interactions in the regulation of maternal behaviour in virgin female rats: Effects of testosterone, dihydrotestosterone, oestradiol, progesterone and the aromatase inhibitor, 1,4,6-androstatriene-3,17-dione. J Endocrinol. 1981;90:31–40. doi: 10.1677/joe.0.0900031. [DOI] [PubMed] [Google Scholar]
- 29.Schradin C, Vuarin P, Rimbach R. The neoteny-helper hypothesis: When to expect and when not to expect endocrine mechanisms to regulate allo-parental care? Physiol Behav. 2018;193:127–134. doi: 10.1016/j.physbeh.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Wingfiled JC, Hegner RE, Lewi DM. Circulating levels of luteinizing hormone and steroid hormones in relation to social status in the cooperatively breeding white-browed sparrow weaver, Plocepassev mahali. J Zool (Lond) 1991;225:43–58. [Google Scholar]
- 31.Pikus AE, Guindre-Parker S, Rubenstein DR. Testosterone, social status and parental care in a cooperatively breeding bird. Horm Behav. 2018;97:85–93. doi: 10.1016/j.yhbeh.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Clarke FM, Faulkes CG. Intracolony aggression in the eusocial naked mole-rat, Heterocephalus glaber. Anim Behav. 2001;61:311–324. [Google Scholar]
- 33.Clarke FM, Faulkes CG. Dominance and queen succession in captive colonies of the eusocial naked mole-rat, Heterocephalus glaber. Proc Biol Sci. 1997;264:993–1000. doi: 10.1098/rspb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bales KL, French JA, McWilliams J, Lake RA, Dietz JM. Effects of social status, age, and season on androgen and cortisol levels in wild male golden lion tamarins (Leontopithecus rosalia) Horm Behav. 2006;49:88–95. doi: 10.1016/j.yhbeh.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Zöttl M, et al. Allo-parental care in Damaraland mole-rats is female biased and age dependent, though independent of testosterone levels. Physiol Behav. 2018;193:149–153. doi: 10.1016/j.physbeh.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Carlson AA, et al. Cortisol levels are positively associated with pup-feeding rates in male meerkats. Proc Biol Sci. 2006;273:571–577. doi: 10.1098/rspb.2005.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dantzer B, et al. The influence of stress hormones and aggression on cooperative behaviour in subordinate meerkats. Proc Biol Sci. 2017;284:20171248. doi: 10.1098/rspb.2017.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarvis JU, Smotherman WP. Heterocephalus glaber. Am Soc Mammal. 2006;706:1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.