Significance
Seroprevalence studies are important for determining risk factors for infection and for assessing the susceptible fraction of a population after an epidemic has swept through a population. Here, Zika virus (ZIKV) seroprevalence was estimated among participants of a pediatric cohort, a separate household cohort, and an adult-only subset from the household cohort, in Managua, Nicaragua, after the 2016 Zika epidemic, yielding 36, 46, and 56% seropositivity, respectively. To our knowledge, this is the only ZIKV seroprevalence study reported in Central America and the largest in the Americas and in a pediatric population to date. The explosive 2016 Zika epidemic resulted in a high level of seropositivity to ZIKV, making a second large Zika epidemic in Managua in the near future unlikely.
Keywords: Zika virus, seroprevalence, cohort, risk factor, spatial analysis
Abstract
In 2015, a Zika epidemic in Brazil began spreading throughout the Americas. Zika virus (ZIKV) entered Managua, Nicaragua, in January 2016 and caused an epidemic that peaked in July–September 2016. ZIKV seropositivity was estimated among participants of pediatric (n = 3,740) and household (n = 2,147) cohort studies, including an adult-only subset from the household cohort (n = 1,074), in Managua. Seropositivity was based on a highly sensitive and specific assay, the Zika NS1 blockade-of-binding ELISA, which can be used in dengue-endemic populations. Overall seropositivity for the pediatric (ages 2–14), household (ages 2–80), and adult (ages 15–80) cohorts was 36, 46, and 56%, respectively. Trend, risk factor, and contour mapping analyses demonstrated that ZIKV seroprevalence increased nonlinearly with age and that body surface area was statistically associated with increasing seroprevalence in children. ZIKV seropositivity was higher in females than in males across almost all ages, with adjusted prevalence ratios in children and adults of 1.11 (95% CI: 1.02–1.21) and 1.14 (95% CI: 1.01–1.28), respectively. No household-level risk factors were statistically significant in multivariate analyses. A spatial analysis revealed a 10–15% difference in the risk of ZIKV infections across our 3-km-wide study site, suggesting that ZIKV infection risk varies at small spatial scales. To our knowledge, this is the largest ZIKV seroprevalence study reported in the Americas, and the only one in Central America and in children to date. It reveals a high level of immunity against ZIKV in Managua as a result of the 2016 epidemic, making a second large Zika epidemic unlikely in the near future.
In February of 2016, the World Health Organization (WHO) announced a Public Health Emergency of International Concern (1) after the explosive transmission of Zika virus (ZIKV) due to concern that ZIKV infection was linked to congenital and developmental syndromes in the Americas and the Pacific Islands (2–7). A few months later, ZIKV infection was declared a cause of microcephaly and other serious congenital disorders (8–10). ZIKV, first isolated in 1947 from a sentinel rhesus monkey in the Ziika forest, Uganda, is a re-emerging virus of the Flavivirus genus within the Flaviviridae family (11). The virus is primarily transmitted by infected Aedes aegypti mosquitoes, although sexual and perinatal/vertical transmission also occurs (12, 13). Symptomatic ZIKV infection is characterized by mild disease such as low-grade fever, rash, and/or conjunctivitis. Maternal ZIKV infection during pregnancy can lead to congenital Zika syndrome in fetuses and infants, which is characterized by severe microcephaly, a decrease in brain tissue, congenital contractures, and ocular malformations (8–10, 14, 15). ZIKV is related to other flaviviruses of public health concern, including yellow fever and West Nile viruses, and is most closely related to dengue virus (DENV). The antibody response to flavivirus infections is highly cross-reactive, representing a significant challenge for unambiguous detection of prior infection by serological assays and for determining the virus-specific seroprevalence in regions where flaviviruses cocirculate (16).
Until recently, Zika was considered a neglected tropical disease ecologically constrained to Africa and Southeast Asia. However, following several Zika epidemics in the Pacific Islands, a cluster of acute exanthematic cases of unknown etiology was reported in Brazil in March of 2015 (17). Thereafter, Brazil experienced a large Zika epidemic, notable in the Northeast Region for the size of the epidemic and its association with microcephaly and other congenital birth defects (7). ZIKV quickly spread to other Latin American and Caribbean countries, resulting in ∼800,000 suspected and confirmed autochthonous cases as of early January 2018 (18); however, these official Zika case numbers probably underestimate the true number of Zika cases, as Zika disease is relatively mild in the general population and therefore likely to be underreported. In Nicaragua as well as its capital, Managua, the first autochthonous Zika case was reported in January 2016. In Managua, several months of isolated cases were followed by an explosive epidemic peaking between July and September of the same year (19, 20).
Seroprevalence studies are important to document the occurrence of epidemics and to assess the fraction of a population that remains susceptible to future infection. Furthermore, seroprevalence studies allow for the determination of risk factors for infection in the sampled population (21). To date, no studies have documented the impact of the Zika epidemic in Central America, although high seropositivity rates have been reported in Brazil and tropical areas in Bolivia (22, 23).
In this study, we performed cross-sectional seroprevalence studies of anti-ZIKV antibodies in two cohorts operating within the catchment area of the Health Center Sócrates Flores Vivas (HCSFV), in Managua, Nicaragua. Our study used a new competition ELISA to detect total anti-ZIKV NS1 antibodies. The assay distinguishes between ZIKV and DENV infections with high sensitivity and specificity for up to several years postinfection and has been validated in six countries (including Nicaragua) (24, 25). In particular, the assay has a 95% sensitivity and a specificity against DENV of 89% in patient samples >20 d after the onset of symptoms compared with RT-PCR results. In late convalescent samples (e.g., >7 mo post-illness as in the samples used in the present study), the assay’s sensitivity and specificity rose to 96 and 92%, respectively, in a validation study performed in Nicaragua (24, 25). This study aimed to (i) determine the seroprevalence of anti-ZIKV antibodies after the first Zika epidemic in Managua, (ii) describe the age patterns of ZIKV infection, (iii) identify potential risk factors that correlate with ZIKV infection, and (iv) characterize the neighborhood-specific spatial distribution of ZIKV seroprevalence within our study populations.
Materials and Methods
Ethics Statement.
The Pediatric Dengue Cohort Study (PDCS) was reviewed and approved by the Institutional Review Boards (IRBs) of the University of California, Berkeley; the University of Michigan; and the Nicaraguan Ministry of Health. The Household Influenza Cohort Study (HICS) was reviewed and approved by University of Michigan and the Nicaraguan Ministry of Health IRBs. Parents or legal guardians of all pediatric subjects provided written informed consent. Participants aged 6–14 y provided oral assent, and participants aged 15–17 provided written assent. Participants 18 y of age and older provided written informed consent.
Study Population.
Study participants from both the PDCS (n = ∼3,700, 2- to 14-y-olds in 2,127 households) and HICS (n = ∼2,000, 2- to 80-y-olds in 433 households) cohorts reside in the catchment area of the HCSFV in District II of Managua, a low-to-middle income socioeconomic status (SES) area with an illiteracy rate of 7% and where >90% of the homes have running water and a sewage system (26). The HCSFV serves ∼62,000 residents of 18 neighborhoods. In 2015, the populations of District II aged 2–14 and ≥15 were estimated at 14,240 and 45,312 persons, respectively. The PDCS is an ongoing study of DENV, ZIKV, and chikungunya virus (CHIKV) infection (27, 28), currently in its 15th continuous year. The HICS is an ongoing family cohort study of influenza (28). Both PDCS and HICS participants receive their primary health care at the HCSFV, and a routine blood sample is collected annually from healthy participants. Study participants were restricted to those with blood samples collected during the annual sampling performed in February–May 2017 or at enrollment into the HICS in May–July 2017 (SI Appendix, Fig. S1).
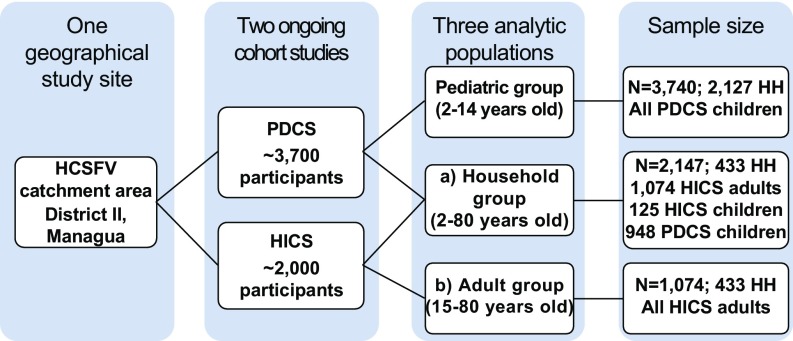
PDCS participants aged 2–14 y (median age: 7) are referred to collectively as the pediatric group (n = 3,740) (Fig. 1). HICS participants were split into (i) an adult-only subset aged 15–80 y (median age: 32), referred to as the adult group (n = 1,074), to complement analyses of the pediatric group and (ii) the full HICS cohort (2–80 y old, median age: 15), referred to as the household group to conduct analyses across the full spectrum of age within a population (n = 2,147) defined by membership in 433 HICS households. Of the latter, 125 children are enrolled only in the HICS cohort, while 948 children are in the PDCS. The PDCS and HICS studies coordinated efforts such that only one blood sample was collected from each dually enrolled child. Age 15 was chosen as the boundary point between pediatric and adult participants because PDCS participants age out of the pediatric cohort on their 15th birthday.
Fig. 1.
Flowchart of study design and sample size of the participating pediatric, household, and adult groups in Managua, Nicaragua, 2017. The PDCS and the HICS participants reside in the catchment area of the HCSFV in District II of Managua, Nicaragua. For both cohort studies, a routine blood sample was collected from healthy participants between February and July of 2017. The pediatric group (2–14 y old) consisted of the PDCS annual samples (n = 3,740). HICS participants were split into (a) the full HICS cohort (2–80 y old), referred to as the household group, to conduct analyses across the full spectrum of age within a single population (n = 2,147) defined by membership in 433 households and (b) an adult-only subset consisting of HICS participants aged 15–80, referred to as the adult group (n = 1,074), to complement analyses of the pediatric group. Of the latter, 125 children are enrolled only in the HICS cohort, while 948 children are in the PDCS. HH, households.
Survey Data.
During the annual sampling, surveys were administered to collect data on participants’ demographics as well as potential household and personal risk factors for ZIKV infection (SI Appendix, SI Materials and Methods).
Laboratory Methods.
All laboratory samples were processed at the Laboratorio Nacional de Virología of the Centro Nacional de Diagnóstico y Referencia in Managua. Anti-ZIKV antibodies were measured by the Zika NS1 blockade-of-binding (BOB) ELISA, a competitive ELISA detecting total anti-ZIKV NS1 antibodies via competition with a highly specific human anti-ZIKV NS1 monoclonal antibody (16, 24, 25) (SI Appendix, SI Materials and Methods).
Statistical Analysis.
All data were analyzed using R version 3.4.2 (29).
ZIKV seroprevalence.
Intercept-only logistic regression models were used within a generalized estimating equations (GEE) framework to estimate the log-odds of ZIKV seropositivity in the three analytic groups, averaging across households. GEE models used the household ID variable as the clustering unit, assumed an exchangeable correlation structure, and estimated confidence intervals (CI) using robust variance estimators (30). Estimated log-odds were then transformed to the probability scale for the point and interval estimates of ZIKV seropositivity.
Age trend and breakpoint analysis.
Generalized additive models (GAMs) are quadratically penalized generalized linear models (GLMs) that use smooth functions of the dependent variables to fit the data with less error than traditional GLMs (31). GAMs with thin plate regression splines were used to model and graph the functional association of age and ZIKV seroprevalence in the three groups (32). Smoothing parameters were estimated using generalized cross-validation, and basis dimensions were tested to ensure appropriate smoothing (33). A quantile-based, 95% confidence band was estimated by nonparametrically cluster bootstrapping the data 10,000 times at the household level to account for clustering. Age trends are presented for all three groups and stratified by sex. Breakpoint analysis summarizes data with a series of linear regressions joined together at breakpoints (34). We used the segmented R package to estimate the major breakpoints in the age trend curves as well as the average value of linear slopes between the breakpoints.
Risk factor analysis.
Modified Poisson regression was used to estimate crude and multivariate-adjusted prevalence ratios (PRs) in the pediatric and adult groups (35). GEE models were parameterized as previously indicated. A literature search guided the decisions of which household- and individual-level variables to include in the models. Considering that mosquitoes prefer to feed on larger targets, other conditions remaining the same (36–38), we compared separate GEE Poisson models for ZIKV seroprevalence with height, body mass index (BMI), BMI percentiles, and estimated body surface area (BSA) with the QICu statistic (39). Pediatric BMI accounted for age and sex and was compared with WHO reference populations (40, 41), and BSA was estimated using Mosteller’s formula (42). The variable that induced the smallest QICu value (BSA) was included in the risk factor analysis. Crude and adjusted PRs were estimated for age, BSA, an SES proxy, daily hours without water, presence of a water faucet inside the home, school attendance period (pediatric cohort only), water storage (adult cohort only), and knowledge about ZIKV (adult cohort only). Potential factors associated with ZIKV seropositivity were explored separately in the pediatric and adult groups, as children and adults may have different risk factors for infection (26). The potential for age and BSA to interact and synergistically contribute to ZIKV seroprevalence was explored using a 3D risk surface produced from a GAM model with a 2D smoothing spline on age and BSA. GAM models were conditioned on SES, presence of an interior faucet, and hours without running water. Three-dimensional contour maps were produced for the pediatric cohort (where the sample size was largest) and the household group (to span the entire age range in our study).
Mapping seroprevalence.
To examine spatial trends in our study site, neighborhood-specific, intercept-only GEE models were fit to estimate neighborhood-specific seroprevalence levels, as in the overall analysis.
Results
Study Participants.
ZIKV seroprevalence was estimated for the PDCS and HICS cohorts (SI Appendix, Table S1). The male-to-female ratio was ∼1:1 in the pediatric cohort (PDCS), 1:2.5 in the adult group (HICS), and 1:1.6 in the household group (HICS). The HICS population had more females than males because there were more adult women living in the households than adult men, more women than men were home during recruitment, and adult men were more likely to decline to participate. The mean number of participants per household was 1.76 (SD 1.13) in the pediatric group, 2.48 (SD 1.49) in the adult group, and 4.96 (SD 2.49) in the household group. Based on WHO guidelines (40, 41), approximately one-third (32.3%) of the children in the pediatric group were overweight or obese (BMI score ≥ 85 percentile), whereas almost three-quarters (71.7%) of the adult participants were overweight or obese (BMI score > 25). Less than 4% of either cohort’s participants were underweight (SI Appendix, Tables S2 and S3).
ZIKV Seroprevalence.
The pediatric group had an overall ZIKV seroprevalence of 36.1% (95% CI: 34.5, 37.8) across households. The household and adult-only groups had a seroprevalence of 45.8% (95% CI: 43.4, 48.3) and 56.4% (95% CI: 53.1, 59.6), respectively.
Age Trend Analysis.
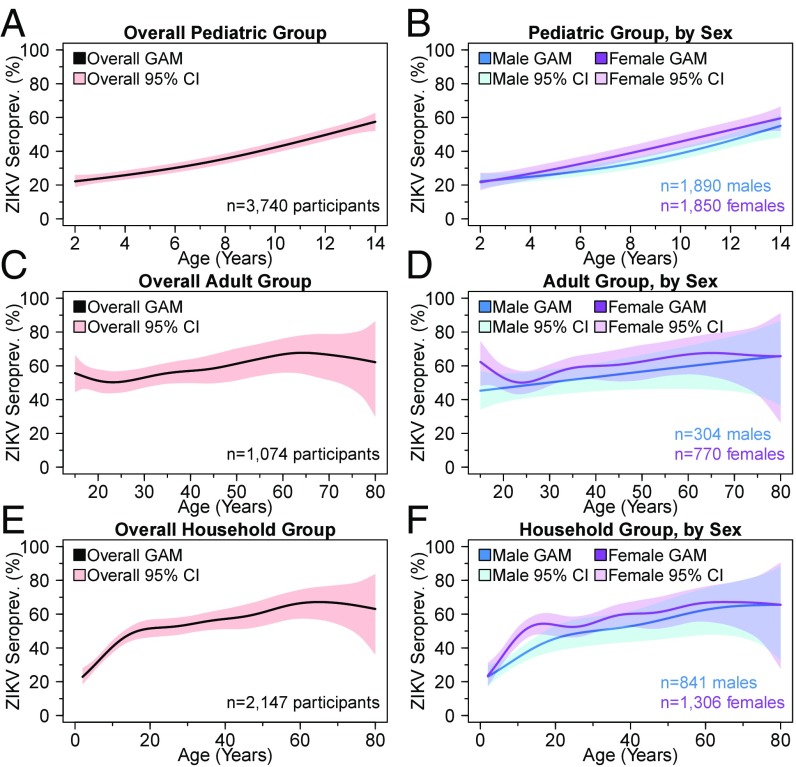
Across all three groups, overall ZIKV seroprevalence had nonlinear associations with age (Fig. 2). In the pediatric cohort, we observed a sharp rise in ZIKV seroprevalence from ∼22% at age 2 to 58% at age 14, overall and for both sexes (Fig. 2A). Breakpoint analysis indicated that the overall slope after age 6.4 is ∼2.2 times that of the slope before age 6.4. ZIKV seroprevalence was higher for girls than for boys for children past age 3 (Fig. 2B). In the adult cohort, a cubic relationship was observed for the overall ZIKV seroprevalence, with a local maximum of 56% at age 15, followed by a decrease coinciding with early reproductive age (global minima: 50% seroprevalence at age 23) and then a slow rise in seroprevalence during adulthood (Fig. 2C). Overall seroprevalence peaked at 68% at age 64 and plateaued thereafter. However, the male seroprevalence trend line displayed different behavior than the overall trend line (Fig. 2D). Whereas the male trend line increased linearly across age, the female trend line displayed a U-shaped relationship between the ages of 15 and 34 with a nadir of 50% estimated seroprevalence at age 24.
Fig. 2.
Overall and sex-specific ZIKV seroprevalence trend lines by age. ZIKV seroprevalence was graphed based on GAM trend curves, with 95% confidence bands estimated from cluster bootstrapping 10,000 at the household level for the pediatric (A and B), adult (C and D), and household (E and F) groups. Overall (pink) as well as sex-specific (blue and purple) trend lines are presented.
To resolve differences in seroprevalence trends between the pediatric and adult groups, as well as to increase the precision of seroprevalence estimates during early adulthood, we also analyzed seroprevalence age trends within the entire household group (Fig. 2 E and F). Overall, a sharp rise in seroprevalence was observed during childhood, as in the pediatric group. Thereafter, the slope of seroprevalence with respect to age gradually increased from 50% at age 17 to its peak of 67% at age 65 (Fig. 2E). The smooth behavior of the household group’s seroprevalence curve around age 15 indicates that our results are robust to the age boundaries of the PDCS and HICS groups. In the sex-specific analysis, females were observed to have a higher estimated seroprevalence than males across most of the 2- to 80-y age range, with a dip from ages 17–24 (Fig. 2F).
Risk Factor Analysis.
Estimated BSA was shown to be an overall stronger predictor of ZIKV infection status than other body-size variables (SI Appendix, Table S4), including obesity status, which was itself significantly associated with ZIKV seroprevalence in preliminary univariate analyses. Therefore, BSA was the only body size variable included in the risk factor analysis. In the univariate analyses for the pediatric group, age, female sex, BSA, and school attendance period were significantly associated with ZIKV seroprevalence. In the univariate analysis, age showed a strong, linear association with increasing ZIKV seroprevalence. In a multivariable model without BSA, age was statistically significant; however, age lost its significance after adjusting for BSA. Only female sex and BSA were significantly associated with ZIKV seroprevalence in the full multivariate analysis (SI Appendix, Table S5). Averaging across households, female children had a significantly higher seroprevalence than male children with prevalence ratios of 1.11 (95% CI: 1.02, 1.21) after adjusting for age, BSA, an SES proxy, and household water availability variables. Seroprevalence significantly increased from an adjusted prevalence ratio of 0.73 (95% CI: 0.55, 0.97) in the lowest BSA quintile to an adjusted prevalence ratio of 1.28 (95% CI: 1.07, 1.52) in the highest quintile. Participants with the lowest SES level had a marginally significant positive association with ZIKV seroprevalence.
In both the univariate and multivariate analyses of the adult group, increasing age was associated with increasing ZIKV seroprevalence (SI Appendix, Table S6). Averaging across households, the ZIKV seroprevalence for adults aged 60–74 was 1.24 (95% CI: 1.03, 1.49) times that of the 30- to 44-y-old adults, adjusting for BSA, an SES proxy, and household water availability variables. After adjustment, the ZIKV seroprevalence in adult females was 1.14 (95% CI: 1.01, 1.28) times that of adult males. Although ZIKV seroprevalence generally increased with BSA in both the univariate and multivariate analyses of adult participants, its association was nonsignificant and weaker than in the pediatric participants. As before, the SES proxy variable and household water availability variables were not statistically significant.
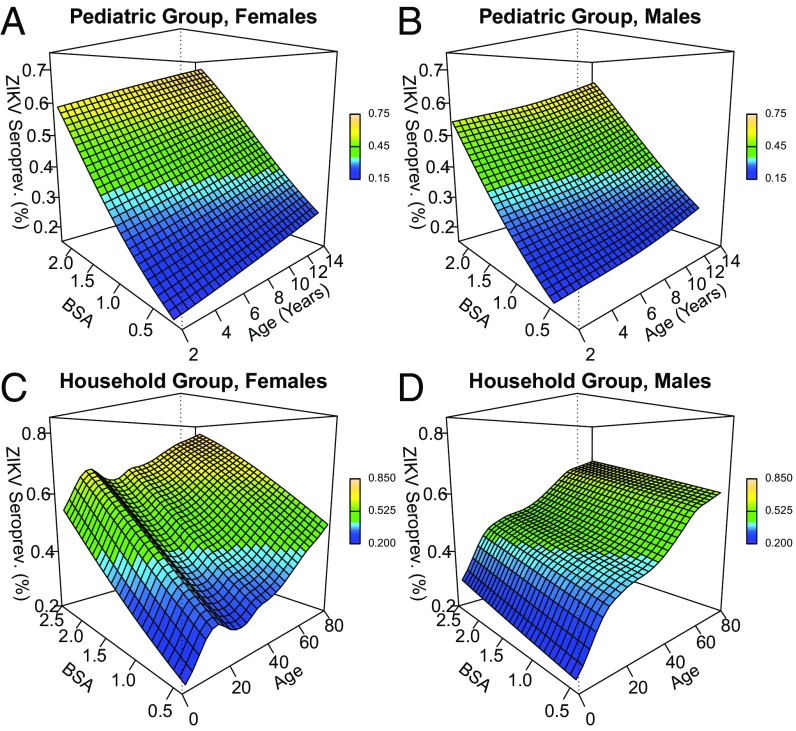
To explore the potential for synergistic, statistical interaction between age and BSA on ZIKV seroprevalence without arbitrarily categorizing either continuous variable, GAM models that used 2D smoothing splines on age and BSA were used to graph this 3D association (Fig. 3). The association of age and ZIKV seroprevalence from the trend analysis held across all levels of BSA in the pediatric group (Fig. 3 A and B). The steeper slope of the BSA–seroprevalence association, relative to the age–seroprevalence association, implies, as the risk factor analysis did, that BSA had a stronger association with ZIKV seroprevalence than age. Conditional on age, the association of BSA with ZIKV seroprevalence was nearly linear. Combinations of age and BSA that synergistically increased (or decreased) the conditional probability of ZIKV infection would appear as quadratic peaks (or troughs) on the 3D risk surface. No such quadratic contours were apparent in the risk surfaces of the pediatric group, implying a lack of statistical interaction between age and BSA. Hence, age and BSA contributed linearly to ZIKV seroprevalence and did not display statistical interaction in their association with ZIKV seroprevalence in our pediatric participants. In the household group, ZIKV seroprevalence increased linearly with BSA for both males and females, conditional on age (Fig. 3 C and D). However, conditional on age, the slope of the BSA–ZIKV seroprevalence association was between 1.5 and 1.9 times greater among females than males, suggesting that BSA had a stronger association with ZIKV seroprevalence in females than in males across all ages after accounting for an SES proxy and household water availability variables. Conditional on BSA, the age trends identified in the trend analysis likewise held for males and females in the household group, where age and BSA appeared to be nonlinearly associated with ZIKV seroprevalence, without statistical interaction.
Fig. 3.
Three-dimensional sex-specific contour maps (a.k.a. risk surfaces) for ZIKV seroprevalence as a function of estimated BSA and age. The underlying GAM was adjusted for an SES proxy, hours without water, and location of the water faucet. Combinations of age and BSA that synergistically increased or decreased the conditional probability of ZIKV infection would appear as quadratic peaks or troughs on the 3D risk surface, respectively. No such quadratic peaks were observed in any population. The contour maps for females and males in the pediatric group were nearly planar (A and B). In the household group, ZIKV seroprevalence increased linearly with BSA for both females and males, conditional on age (C and D).
Spatial Distribution.
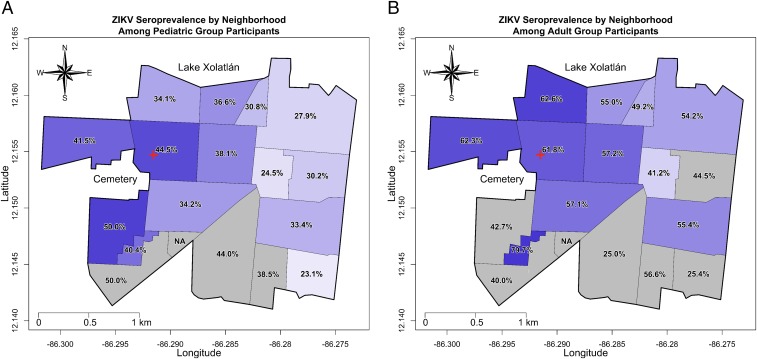
The neighborhood-specific ZIKV seroprevalence was estimated and mapped for the pediatric and adult groups (Fig. 4). The seroprevalence in a given neighborhood was ∼20% higher for the adult group compared with the pediatric group. The overall seroprevalence estimates indicated a similar 20% difference between the adult and pediatric groups, suggesting that this overall difference between children and adults did not vary by neighborhood. A west-to-east seroprevalence pattern was detected in both cohorts, with the western neighborhoods near the cemetery having a seroprevalence ∼10–15% higher than the eastern neighborhoods. That such a difference was observed over a study area that is 3 km across its widest point suggests that spatial variation in ZIKV seroprevalence may exist at small geographical scales. Due to the low number of study participants from the southern neighborhoods, it was impossible to ascertain whether a north-to-south seroprevalence gradient exists in our study site.
Fig. 4.
Map of neighborhood-specific ZIKV seroprevalence estimates for the pediatric and adult groups. The ZIKV seroprevalence estimates for each neighborhood were mapped for the pediatric group (A) and the adult group (B). The shade of each neighborhood corresponds to its ZIKV seroprevalence (darker shading corresponding to higher seroprevalence). Neighborhoods shaded in gray represent those in which the point estimate may be statistically inconsistent as a result of a low sample size. The red cross signifies the location of the HCSFV.
Discussion
To our knowledge, to date this is the largest ZIKV seroprevalence study in the Americas and the only one reported in Central America and in a sizable pediatric population from the recent Zika epidemic. We document a high number of ZIKV infections that occurred during the Zika epidemic that swept through Managua, Nicaragua, peaking during July–September of 2016, after the virus was first identified in the country and its capital in January of 2016. ZIKV seropositivity was estimated among participants of a pediatric cohort (n = 3,740; 36%) and a full household-based cohort (n = 2,147; 46%). Seropositivity was also analyzed in an adult-only subset of the household cohort (n = 1,074; 56%). Importantly, seropositivity was based on a highly sensitive and specific assay, the Zika NS1 BOB ELISA, which can be used in a dengue-endemic population (24, 25). The high seropositivity estimates across our study populations suggest that ZIKV transmission throughout Managua’s ZIKV-naive population caused a widespread and explosive epidemic over a 3-mo period.
High seropositivity rates after de novo introduction of ZIKV into a population have been previously observed. Aubry et al. (43) found seroprevalence to be 49% in the general population and 66% in children after the 2014–2015 Zika epidemic in French Polynesia. In the Americas, the post-2015–2016 epidemic ZIKV seroprevalence was found to be 63% in Salvador, Brazil (23), and 39 and 21.5% in Beni and Santa Cruz de la Sierra, respectively, two tropical regions of Bolivia (22). However, in contrast to our findings in both the PDCS and HICS cohort populations, none of these studies found a significant association between ZIKV seroprevalence and sex, either overall or in subgroups of their participants. Prior studies (22, 23, 43) (n = 814; n = 196; n = 633, respectively) may not have been sufficiently powered or sampled in such a way as to detect the modest, but consistently higher, seroprevalence that we observed among females compared with males. However, the only South American ZIKV seroprevalence study (23) that provided complete, sex-stratified data observed a higher, but nonsignificant, seroprevalence among females (64 vs. 61%, n = 633). Different societal norms between the sexes, such as clothing norms, may also contribute to sex-specific infection patterns, which could explain the differences between studies regarding the association of ZIKV seroprevalence and sex.
A recently published seroprevalence study from Brazil (23) observed a mostly flat relationship with age—as would be expected of a de novo pathogen introduction into a fully susceptible population. However, Netto et al. (23) preferentially sampled adult participants. We show a sharp rise with age in our pediatric group and a flatter, but slowly increasing, seroprevalence across adulthood. Interestingly, similar increases in seroprevalence with age were observed in seroprevalence studies of anti-CHIKV antibodies after the 2014–2015 chikungunya epidemic in Managua’s then-naive population (26, 44, 45). CHIKV is an alphavirus, which should preclude the possibility of anti-CHIKV antibodies cross-reacting with anti-DENV antibodies to produce the age trends similar to the ones that we observed; this similarity implies that our age trends are not due to nonspecific antibody measurement by the Zika NS1 BOB ELISA.
We found that BSA was statistically associated with increasing seroprevalence in children and that, conditional on age, ZIKV seroprevalence increased linearly with increasing BSA. The documented preference of mosquitoes to feed on larger targets (36–38), the trend analysis, and the extended risk factor analysis all suggest that the large and continual increases in BSA during childhood and adolescence are primarily responsible for the sharp rise in ZIKV seroprevalence during that period. Aedes mosquitoes, the only known vectors for arboviruses in Nicaragua (46), are attracted to carbon dioxide and other components associated with human respiration and perspiration, which aid female Aedes mosquitoes in locating a blood meal (36, 47). Larger and heavier persons expel more of these attractive components, explaining, together with exposed skin surface area, the general trend for older individuals to have a higher seroprevalence than younger individuals. In the univariate analysis, attending school in the afternoon was significantly associated with higher seroprevalence levels, but not in the multivariate analysis. In our population, older children tend to go to school in the afternoon, and since older age in children implies greater BSA, this could account for the lack of significant association when BSA is included in the model. However, we could not explain why female ZIKV seropositivity was higher than male seropositivity across almost all ages, nor why female seropositivity notably rose and then dipped during early reproductive age, in contrast to the linear seroprevalence trend observed in males. We did not find any graphical evidence of statistical interaction between BSA and age, even in children. This suggests that our age variable, after accounting for BSA, may be a serving as a proxy for other factors. Potential factors underlying the age–seroprevalence patterns that we observed include increased mobility in adolescence and adulthood that could lead to more frequent encounters with mosquito populations outside the home, as well as public health messaging regarding the potentially adverse consequences of ZIKV infection during pregnancy.
At least one study has documented a statistically significant association between low SES and both ZIKV infection and disease (23). Two ecological studies have shown statistically significant associations between Zika-associated incident microcephaly measures and both poor living conditions and low per capita GDP (48, 49). Increasing levels of poverty were nonsignificantly associated with increasing ZIKV seropositivity in our study. Although it is possible that SES is related to ZIKV disease manifestation and not ZIKV infection, our two cohort studies were based in overall low-to-middle–SES neighborhoods, which likely explains why our SES proxy variable was nonsignificant.
Our spatial analysis found up to a 15% difference in the risk of ZIKV infection across a 3-km-wide study area. The thousands of homes in our study site, combined with accurate assessments of ZIKV infection outcomes, provide for a high resolution of spatial infection patterns at the neighborhood level. Our analysis shows a clear west–east seroprevalence gradient in our study site, suggesting that the general cemetery in the western region of our site may be an environmental source that amplifies local acquisition of ZIKV infection. Cemeteries have been well established as ecosystems for mosquito development according to a recent review of 30 papers (50). Vegetation and freshly cut flowers provide energy in the form of sugars for adult mosquitoes; visitors, cemetery caretakers, and stray animals provide an ample supply of blood meals for female mosquitoes; and flower vases and other water containers provide ideal sites for larval and pupal development. Previous ZIKV spatial analyses have mostly been based on case data or less sensitive serological assays (23, 51–53), making direct comparisons between the results of our spatial analysis and the published literature difficult. A follow-up study is examining the spatial distribution of ZIKV infections and disease cases at finer spatial scales than was possible here.
Our study was strengthened by the large sample sizes across two cohort studies, participants ranging from 2 to 80 y of age, sensitive and specific measurements of anti-ZIKV antibodies using the Zika NS1 BOB ELISA, and robust analysis of age trends and risk factors. Nonetheless, our study had several limitations. First, our results may not be representative of the ZIKV infection experience across all of Managua as a result of the skew in gender-specific participation rates in the HICS cohort. Second, we did not collect information regarding average length of time spent at home and knowledge of the effects of ZIKV infection during pregnancy, which might explain some of the age trends that we estimated. Third, southern neighborhoods in our sample were under-represented in our study, which precluded us from estimating seroprevalence with precision in those neighborhoods.
In summary, this study determined ZIKV seroprevalence and risk factors in both children and adult populations using a laboratory method with minimal cross-reactivity with DENV. We report household-averaged ZIKV seroprevalences of 36% and 56% in children and adults, respectively. BSA was a strong predictor of ZIKV seroprevalence, especially for children, and should be considered for inclusion in future arboviral seroprevalence studies. Altogether, this study indicates that a high level of protective immunity against ZIKV has developed in Managua’s population as a result of the large 2016 epidemic, making a large Zika epidemic in Managua unlikely in the near future.
Supplementary Material
Acknowledgments
We thank Davide Corti at Humabs Biomed SA, subsidiary of Vir Biotechnology, Inc., for his generous donation of biotinylated ZKA35 monoclonal antibody. We thank the outstanding study team of the Nicaraguan Pediatric Dengue Cohort Study and the Household Influenza Cohort Study at the Centro de Salud Sócrates Flores Vivas (Nicaraguan Ministry of Health), the Laboratorio Nacional de Virología at the Centro Nacional de Diagnóstico y Referencia (Nicaraguan Ministry of Health), and the Sustainable Sciences Institute, as well as the study participants and their families. We also thank the Nicaraguan Ministry of Health for providing surveillance data for the Zika cases in Managua that were tested and confirmed by rRT-PCR. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health via Grants P01AI106695 (to E.H.), R01AI099631 (to A.B.), and R01AI121721 (to A.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1804672115/-/DCSupplemental.
References
- 1.WHO 2016 WHO statement on the first meeting of the International Health Regulations (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations: World Health Organization. Available at www.who.int/en/news-room/detail/01-02-2016-who-statement-on-the-first-meeting-of-the-international-health-regulations-%282005%29-%28ihr-2005%29-emergency-committee-on-zika-virus-and-observed-increase-in-neurological-disorders-and-neonatal-malformations. Accessed January 3, 2018.
- 2.Schuler-Faccini L, et al. Brazilian Medical Genetics Society–Zika Embryopathy Task Force Possible association between Zika virus infection and microcephaly: Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:59–62. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- 3.Cauchemez S, et al. Association between Zika virus and microcephaly in French Polynesia, 2013-15: A retrospective study. Lancet. 2016;387:2125–2132. doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleber de Oliveira W, et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy: Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:242–247. doi: 10.15585/mmwr.mm6509e2. [DOI] [PubMed] [Google Scholar]
- 5.Brasil P, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mlakar J, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 7.Heukelbach J, Alencar CH, Kelvin AA, de Oliveira WK, Pamplona de Góes Cavalcanti L. Zika virus outbreak in Brazil. J Infect Dev Ctries. 2016;10:116–120. doi: 10.3855/jidc.8217. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects: Reviewing the evidence for causality. N Engl J Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 9.Krauer F, et al. WHO Zika Causality Working Group Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barré syndrome: Systematic review. PLoS Med. 2017;14:e1002203. doi: 10.1371/journal.pmed.1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medina MT, Medina-Montoya M. New spectrum of the neurologic consequences of Zika. J Neurol Sci. 2017;383:214–215. doi: 10.1016/j.jns.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 11.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 12.Foy BD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17:880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabata T, et al. Zika virus replicates in proliferating cells in explants from first-trimester human placentas, potential sites for dissemination of infection. J Infect Dis. 2017;217:1202–1213. doi: 10.1093/infdis/jix552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rozé B, et al. Guillain-Barré Syndrome Zika Working Group of Martinique Guillain-Barré syndrome associated with Zika virus infection in Martinique in 2016: A prospective study. Clin Infect Dis. 2017;65:1462–1468. doi: 10.1093/cid/cix588. [DOI] [PubMed] [Google Scholar]
- 15.Costello A, et al. Defining the syndrome associated with congenital Zika virus infection. Bull World Health Organ. 2016;94:406–406A. doi: 10.2471/BLT.16.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stettler K, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 17.ProMED-PORT 2015 Doença desconhecida - Brasil (02) (BA), surto? [Unknown disease - Brazil (02) (BA), outbreak?] Available at www.promedmail.org/direct.php?id=20150325.3253769. Accessed June 7, 2018. Portuguese.
- 18.Pan American Health Organization 2018 PAHO Zika Cumulative Cases - 4 January 2018. Available at https://www.paho.org/hq/index.php?option=com_content&view=article&id=12390&Itemid=42090%EF%83%A1=en. Accessed February 27, 2018.
- 19.Nicaragua Ministry of Health 2016 Epidemiological Bulletin No. 52. Available at www.minsa.gob.ni/index.php/repository/Descargas-MINSA/Direcci%C3%B3n-General-Vigilancia-de-la-Salud-P%C3%BAblica/Boletines/Boletines-2017/Bolet%C3%ADn-Epidemiol%C3%B3gico-Semana-No.-52/. Accessed December 20, 2017.
- 20.Pan American Health Organization/World Health Organization 2017 Zika: Epidemiological Report: Nicaragua. Available at https://www.paho.org/hq/dmdocuments/2017/2017-phe-zika-situation-report-nic.pdf. Accessed February 27, 2018.
- 21.WHO 2017 Standardized Protocol: Cross-sectional seroprevalence study of Zika virus infection in the general population. Available at www.who.int/reproductivehealth/zika/ZIKV-cross-sectional-protocol-v1.9.pdf?ua=1. Accessed January 4, 2018.
- 22.Saba Villarroel PM, et al. Zika virus epidemiology in Bolivia: A seroprevalence study in volunteer blood donors. PLoS Negl Trop Dis. 2018;12:e0006239. doi: 10.1371/journal.pntd.0006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netto EM, et al. High Zika virus seroprevalence in Salvador, Northeastern Brazil limits the potential for further outbreaks. MBio. 2017;8:e01390-17. doi: 10.1128/mBio.01390-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balmaseda A, et al. Antibody-based assay discriminates Zika virus infection from other flaviviruses. Proc Natl Acad Sci USA. 2017;114:8384–8389. doi: 10.1073/pnas.1704984114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balmaseda A, et al. Comparison of four serological methods and two reverse transcription-PCR assays for diagnosis and surveillance of Zika virus infection. J Clin Microbiol. 2018;56:e01785-17. doi: 10.1128/JCM.01785-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuan G, et al. Seroprevalence of anti-chikungunya virus antibodies in children and adults in Managua, Nicaragua, after the first chikungunya epidemic, 2014-2015. PLoS Negl Trop Dis. 2016;10:e0004773. doi: 10.1371/journal.pntd.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balmaseda A, et al. Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J Infect Dis. 2010;201:5–14. doi: 10.1086/648592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon A, et al. The Nicaraguan pediatric influenza cohort study: Design, methods, use of technology, and compliance. BMC Infect Dis. 2015;15:504. doi: 10.1186/s12879-015-1256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Core Team 2017 R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna), Version 3.4.2. Available at https://www.R-project.org/. Accessed January 3, 2018.
- 30.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 31.Hastie T, Tibshirani R. Generalized additive models. Stat Sci. 1986;1:297–310. doi: 10.1177/096228029500400302. [DOI] [PubMed] [Google Scholar]
- 32.Wood SN. Thin plate regression splines. J R Stat Soc Series B Stat Methodol. 2018;65:95–114. [Google Scholar]
- 33.Wood SN. Generalized Additive Models: An Introduction with R. 2nd Ed Chapman and Hall/CRC Press; Boca Raton, FL: 2017. [Google Scholar]
- 34.Vito Michele Rosario M, Scienze Economiche Ae S, Muggeo V. Segmented: An R package to fit regression models with broken-line relationships. R News. 2018;8:20–25. [Google Scholar]
- 35.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22:661–670. doi: 10.1177/0962280211427759. [DOI] [PubMed] [Google Scholar]
- 36.Liebman KA, et al. Determinants of heterogeneous blood feeding patterns by Aedes aegypti in Iquitos, Peru. PLoS Negl Trop Dis. 2014;8:e2702. doi: 10.1371/journal.pntd.0002702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrington LC, et al. Heterogeneous feeding patterns of the dengue vector, Aedes aegypti, on individual human hosts in rural Thailand. PLoS Negl Trop Dis. 2014;8:e3048. doi: 10.1371/journal.pntd.0003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryan GRP, Boreham PFL, Joan H. The relationship of host size to feeding by mosquito of the Anopheles gambiae Giles complex (Diptera: Culicidae) Bull Entomol Res. 1980;70:133–144. [Google Scholar]
- 39.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 40.de Onis M, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Onis M, Garza C, Onyango AW, Rolland-Cachera MF. le Comité de nutrition de la Société française de pédiatrie [WHO growth standards for infants and young children] Arch Pediatr. 2009;16:47–53. French. doi: 10.1016/j.arcped.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 43.Aubry M, et al. Zika virus seroprevalence, French Polynesia, 2014-2015. Emerg Infect Dis. 2017;23:669–672. doi: 10.3201/eid2304.161549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dirección General de Vigilancia para la Salud, Ministerio del Poder Ciudadano para la Salud de Nicaragua, Managua, Nicaragua Ministerio del Poder Ciudadano para la Salud de Nicaragua [Chikungunya seroprevalence and clinical case rate in Nicaragua, 2014-2015] Rev Panam Salud Publica. 2017;41:e59. Spanish. doi: 10.26633/RPSP.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balmaseda A, et al. Clinical attack rate of chikungunya in a cohort of Nicaraguan children. Am J Trop Med Hyg. 2016;94:397–399. doi: 10.4269/ajtmh.15-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arosteguí J, et al. Beyond efficacy in water containers: Temephos and household entomological indices in six studies between 2005 and 2013 in Managua, Nicaragua. BMC Public Health. 2017;17:434. doi: 10.1186/s12889-017-4296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Logan JG, Birkett MA. Semiochemicals for biting fly control: Their identification and exploitation. Pest Manag Sci. 2007;63:647–657. doi: 10.1002/ps.1408. [DOI] [PubMed] [Google Scholar]
- 48.Souza WV, et al. Microcephaly epidemic related to the Zika virus and living conditions in Recife, Northeast Brazil. BMC Public Health. 2018;18:130. doi: 10.1186/s12889-018-5039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali S, et al. Environmental and social change drive the explosive emergence of Zika virus in the Americas. PLoS Negl Trop Dis. 2017;11:e0005135. doi: 10.1371/journal.pntd.0005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vezzani D. Review: Artificial container-breeding mosquitoes and cemeteries: A perfect match. Trop Med Int Health. 2007;12:299–313. doi: 10.1111/j.1365-3156.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Morales AJ, et al. Spatial distribution of Zika virus infection in Northeastern Colombia. Infez Med. 2017;25:241–246. [PubMed] [Google Scholar]
- 52.Rodriguez-Morales AJ, et al. Mapping Zika virus infection using geographical information systems in Tolima, Colombia, 2015-2016. F1000 Res. 2016;5:568. doi: 10.12688/f1000research.8436.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haque U, Ball JD, Zhang W, Khan MMH, Treviño C JA. Clinical and spatial features of Zika virus in Mexico. Acta Trop. 2016;162:5–10. doi: 10.1016/j.actatropica.2016.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.