Significance
Many occlusive vascular diseases in humans are largely dependent upon vascular smooth muscle cell (VSMC) phenotypic switching from a contractile to a proliferative phenotype, contributing to the formation of intimal lesions that eventually block the blood flow. Previous studies showed that the long noncoding RNA (lncRNA) NEAT1 is critical for tumorigenesis. In this report, we showed that NEAT1 expression was not only induced in VSMCs during phenotypic switching but functionally was critical for the smooth muscle phenotypic change. Our study demonstrates an unexpected role of the lncRNA NEAT1 in VSMCs and suggests that NEAT1 is a novel therapeutic target for treating occlusive vascular diseases in humans.
Keywords: long noncoding RNA, smooth muscle cells, phenotypic switching, gene expression, epigenetic regulation
Abstract
In response to vascular injury, vascular smooth muscle cells (VSMCs) may switch from a contractile to a proliferative phenotype thereby contributing to neointima formation. Previous studies showed that the long noncoding RNA (lncRNA) NEAT1 is critical for paraspeckle formation and tumorigenesis by promoting cell proliferation and migration. However, the role of NEAT1 in VSMC phenotypic modulation is unknown. Herein we showed that NEAT1 expression was induced in VSMCs during phenotypic switching in vivo and in vitro. Silencing NEAT1 in VSMCs resulted in enhanced expression of SM-specific genes while attenuating VSMC proliferation and migration. Conversely, overexpression of NEAT1 in VSMCs had opposite effects. These in vitro findings were further supported by in vivo studies in which NEAT1 knockout mice exhibited significantly decreased neointima formation following vascular injury, due to attenuated VSMC proliferation. Mechanistic studies demonstrated that NEAT1 sequesters the key chromatin modifier WDR5 (WD Repeat Domain 5) from SM-specific gene loci, thereby initiating an epigenetic “off” state, resulting in down-regulation of SM-specific gene expression. Taken together, we demonstrated an unexpected role of the lncRNA NEAT1 in regulating phenotypic switching by repressing SM-contractile gene expression through an epigenetic regulatory mechanism. Our data suggest that NEAT1 is a therapeutic target for treating occlusive vascular diseases.
Vascular smooth muscle cells (VSMCs) are a major component of the vascular wall. In response to vascular injury, VSMCs may switch from a contractile to a “synthetic” phenotype that is characterized by increased motility and proliferation as well as reduced expression of contractile proteins such as calponin (CNN1), SM22α (transgelin, TAGLN), SM MHC (myosin heavy chain, MYH11), SM α-actin (ACTA2), and Hic-5 (TGFB1I1) (1). Many occlusive vascular diseases in humans, including intimal hyperplasia after angioplasty, atherosclerosis, and restenosis following vascular interventions, are largely dependent upon VSMC phenotype modulation, contributing to progression of intimal lesions that compromise vessel patency (1, 2). Although many studies have shown that, following arterial injury and in atherosclerosis neointima, VSMCs mostly originate from the local vessel wall (3–10), there remains a lack of knowledge of the factors that can control the switch of SM phenotype in vascular diseases.
Previous studies have shown that serum response factor (SRF) is a critical transcription factor for regulating expression of many SM-specific contractile genes (11). SRF is a multifunctional protein that not only binds a highly conserved cis-regulatory element CC(A/T)6GG, termed a CArG box that can be found in most SM-specific gene loci, but also interacts with a wide variety of accessory cofactors (11). WDR5 (WD Repeat Domain 5), a long noncoding RNA (lncRNA) binding protein (12) and a critical adaptor protein in histone 3 lysine 4 (H3K4) methyltransferase complexes (13), has been shown to play a crucial role in SM differentiation by promoting active chromatin, thereby allowing SRF to bind to CArG boxes of SM-specific genes (14). However, how the function of WDR5 is regulated to control SM-contractile protein gene expression is largely unknown.
In sharp contrast to the <3% of human genomic DNA that is transcribed and ultimately translated into protein, >80% of genomic DNA is capable of being transcribed but does not encode for protein (15, 16). Depending upon the length of the transcript, noncoding RNA can be divided into small (<200 nt) and long noncoding RNA (lncRNA, >200 nt) (17). Originally, the large number of noncoding transcripts were regarded as “junk” products, but emerging evidence suggests that a significant portion of lncRNAs are critical for control of gene expression during development and under pathological conditions (17). Despite some recent progress (18–21), most of the lncRNAs that are functionally important in regulating VSMC phenotypic switching remain to be identified.
Paraspeckles are nuclear bodies that are typically detected as foci in close proximity to nuclear speckles and are enriched with RNA-binding proteins such as splicing factor proline/glutamine-rich (SFPQ), paraspeckle component 1 (PSPC1), and non-POU domain containing octamer binding (NONO) that binds to the lncRNA NEAT1 (nuclear paraspeckle assembly transcript 1) (22). In humans, NEAT1 has two splice variants, NEAT1_1 (V1) (∼3,700 nt) and NEAT1_2 (V2) (∼23K nt) (22, 23). Although the homology of NEAT1 exons among mammals is relatively low, the function of NEAT1 in paraspeckle formation is conserved from mouse to human (22, 23). Both of the NEAT1 variants can be found in paraspeckles, where they bind resident proteins to maintain architectural integrity of the paraspeckle (22, 24), although a recent study showed that NEAT1 V1 can be also found in microspeckles (25). Previous studies have demonstrated that paraspeckles are not only subnuclear structures but also regulate the expression of a number of genes via the sequestration of specific proteins and RNAs (26–28). In particular, the paraspeckle lncRNA NEAT1 is critical for IL-8 production in response to viral infection (28) and for promoting tumorigenesis in breast and prostate cancers (29, 30). However, little is known about the functional role of NEAT1 in VSMCs during VSMC phenotypic modulation.
In this study, we discovered that the lncRNA NEAT1 is induced in response to arterial injury and is a previously unrecognized coordinator of VSMC phenotype, which can down-regulate contractile gene expression and promote VSMC proliferation and migration through regulating the lncRNA binding protein WDR5.
Methods
Rat balloon angioplasty was carried out as previously described (31, 32). RNA-FISH (fluorescence in situ hybridization) was performed following the protocol as previously described (33). NEAT1 KO (knockout) mice were generated and maintained in C57BL/6 strain background, and left carotid artery ligation injury was carried out as previously described (32, 34). The use of experimental rats and mice for arterial injury procedures, including BSL-2 viral work, was approved by the Institutional Animal Care and Use Committee and Biosafety committees at Augusta University. Full materials and methods are detailed in SI Appendix.
Results
NEAT1 Expression Is Increased in VSMCs Following Arterial Injury.
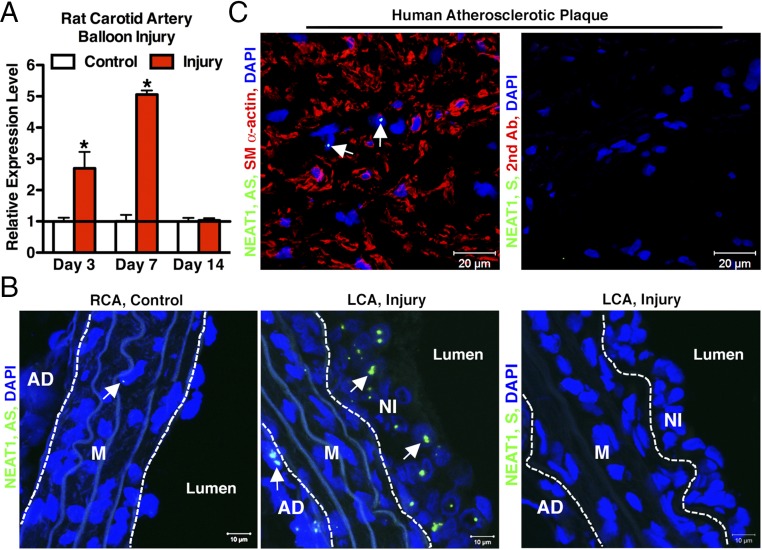
A mounting body of evidence suggests the lncRNA NEAT1 plays a critical role in tumorigenesis by promoting cell proliferation and migration, but little is known about whether NEAT1 has any function in VSMCs. As an initial step to examine the function of NEAT1 in VSMCs, we performed qRT-PCR assay to assess NEAT1 expression by using the RNA samples harvested from control or carotid arteries injured by a balloon denudation, a model resembling human angioplasty procedures in which VSMCs convert from a contractile to synthetic phenotype and contribute to neointima formation (35). The qRT-PCR results revealed that NEAT1 was significantly elevated twofold and fivefold 3 and 7 d postinjury, respectively, during the onset of maximal VSMC proliferation and migration (35, 36) (Fig. 1A). RNA-FISH assays further revealed that NEAT1 was rarely detectable in the medial VSMCs of control intact arteries (Fig. 1B, Left and SI Appendix, Fig. S1), but were readily detectable in the nuclei of neointimal cells (Fig. 1B, Middle, arrows), which are mostly dedifferentiated VSMCs (35). Similarly, we also found that NEAT1 is readily detectable in human advanced carotid atherosclerotic plaque (Fig. 1C, Left), suggesting a conservation of the potential NEAT1 function between rodent and humans. Together, these data demonstrate that NEAT1 expression is induced in neointimal VSMCs in response to arterial injury.
Fig. 1.
NEAT1 is induced in balloon-injured rat carotid arteries. (A) The qRT-PCR was performed to assess NEAT1 expression in rat carotid artery post-balloon injury at the time as indicated; n = 5 rats for each time point. *P < 0.05. Error bars indicate standard deviation. (B) Seven days after rat carotid artery balloon injury, control right common carotid artery (RCA) (Left) or injured left common carotid (LCA) (Middle) were sectioned for RNA-FISH to visualize NEAT1 (green), as indicated by arrows. Cell nuclei were stained with DAPI (blue). The section hybridized with NEAT1 sense (S) probe was used as a negative control (Right). Dashed lines denote external or internal elastic lamina. AD, adventitia layer; M, media layer; NI, neointima layer. The representative images shown are chosen from three rats. (C) RNA-FISH/IF were performed to stain NEAT1 (green, arrows) and SM α-actin (red) in human carotid endarterectomy specimen (Left). Sections with NEAT1 S probe and second antibody (Right) served as negative control. The representative images shown are selected from three human specimens.
NEAT1 Expression Is Induced During SM Phenotypic Modulation in Vitro.
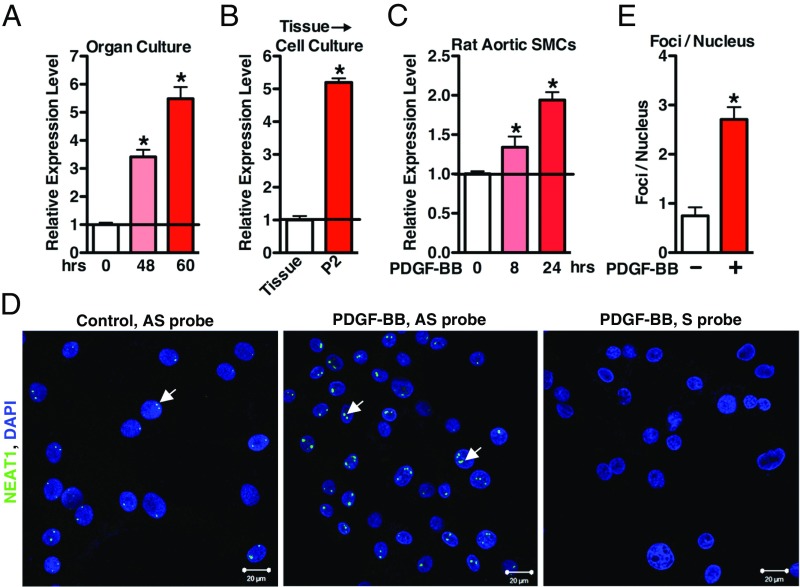
To extend the in vivo findings (Fig. 1) to in vitro, we next sought to determine the expression level of NEAT1 during SM phenotypic switching in in vitro settings. In culture, rat aortic tissue undergoes a dramatic switch toward a synthetic proliferative phenotype (37, 38) that is associated with significantly increased expression of NEAT1 in a time-dependent manner (Fig. 2A). During dedifferentiation of VSMCs induced by primary culture (37), NEAT1 expression was significantly induced (Fig. 2B). In response to PDGF-BB, a growth factor known to further inhibit SM contractile protein expression while promoting VSMC proliferation and migration (39), NEAT1 expression was significantly up-regulated in a time-dependent manner (Fig. 2C). RNA-FISH assays further revealed the induced expression of NEAT1 in PDGF-BB−treated rat aortic primary VSMCs (Fig. 2 D and E). Taken together, these data demonstrate that NEAT1 expression is induced in phenotypically modulated synthetic VSMCs in vitro.
Fig. 2.
NEAT1 is induced in response to SM phenotypic switching in vitro. (A) The qRT-PCR was performed to evaluate NEAT1 expression using the aortic tissues either from the freshly isolated rat thoracic aorta (0 h) or rat thoracic aorta cultured in 20% FBS for the times as indicated; n = 4. *P < 0.05. (B) The qRT-PCR was performed to assess NEAT1 expression in fresh rat aortic tissue or second passage enzymatically dispersed rat aortic VSMCs; n = 4. *P < 0.05. (C) Rat primary aortic VSMCs were treated with PDGF-BB (25 ng/mL) for 8 h or 24 h as indicated, and NEAT1 expression was measured by qRT-PCR; n = 3. *P < 0.05. (D) Rat aortic SMCs were treated without (Left) or with (Middle) PDGF-BB (25 ng/mL) for 24 h, and then RNA-FISH assays were performed to visualize NEAT1 expression. Arrows indicate the representative NEAT1 signal. Cells treated with PDGF-BB and hybridized with NEAT1 sense (S) probe served as the negative control (Right). (E) The numbers of RNA-FISH NEAT1 foci per cell with or without PDGF-BB treatment in HCASMCs (in D) were manually counted and plotted; n = 3. *P < 0.05.
NEAT1 Suppresses SM-Specific Gene Expression.
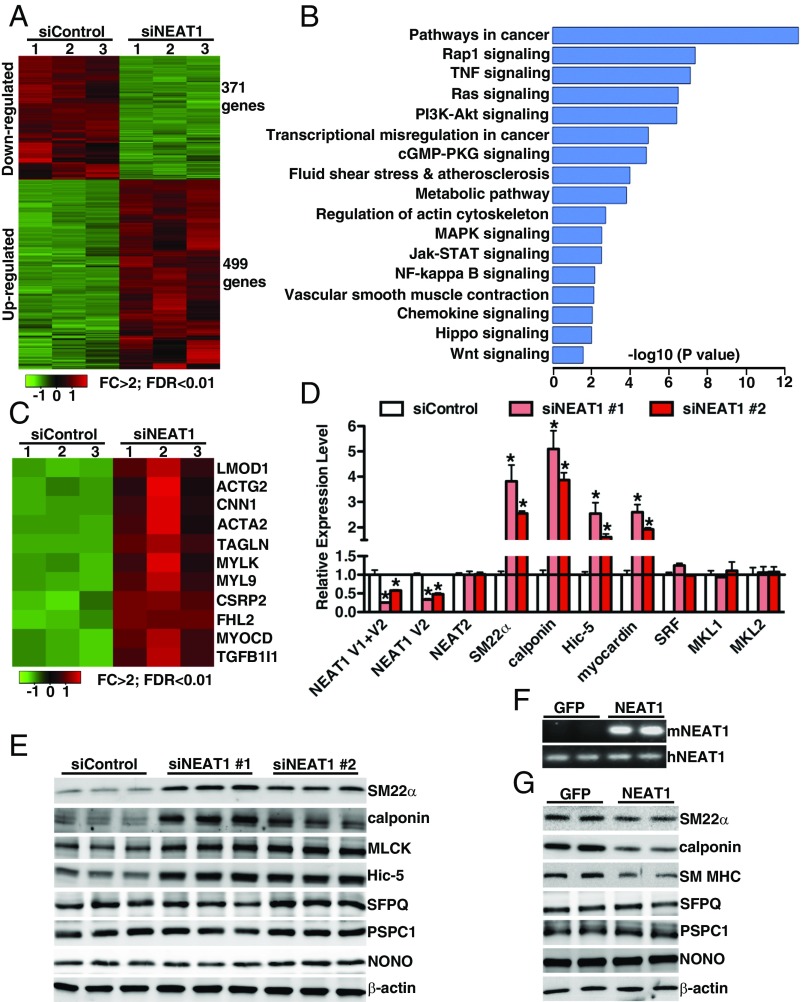
Since NEAT1 expression was elevated in tandem with the reduction of SM-specific markers during SM phenotypic switching in vivo and in vitro, we next sought to investigate the function of NEAT1 in SM-specific gene expression by loss- or gain-of-function assays. First, we examined the effects of NEAT1 depletion on VSMC transcriptome by performing RNA-sequencing (RNA-seq) analysis. RNA-seq data revealed that silencing NEAT1 results in significant down-regulation of both V1 and V2 isoforms but not its adjacent lncRNA NEAT2 (also known as MALAT1; SI Appendix, Fig. S2). Using a twofold cutoff and false discovery rate (FDR) of <0.01 threshold for inclusion, RNA-seq data further revealed that silencing NEAT1 in HCASMCs (human coronary artery smooth muscle cells) significantly down-regulated 371 genes while up-regulating 499 genes (Fig. 3A and Dataset S1). Gene ontology (GO) enrichment analysis revealed that these genes are associated with SM contraction in addition to many signaling pathways related to tumorigenesis such as Hippo signaling (32). Interestingly, among those genes up-regulated after silencing NEAT1, there are a large set of SRF-dependent genes encoding SM contractile proteins such as calponin (CNN1), SM22α (TAGLN), SM MHC (MYH11), SM α-actin (ACTA2), Hic-5 (TGFB1I1), and myocardin (MYOCD), a potent SRF cofactor critical for promoting VSMC myogenesis (40) (Fig. 3C). To further validate the findings, depletion of NEAT1 using an additional siRNA duplex also significantly increased expression of SM22α, calponin, Hic-5, and myocardin at both mRNA and protein levels in HCASMCs, without affecting the expression of paraspeckle NEAT1-binding proteins such as SFPQ, PSPC1, and NONO (Fig. 3 D and E). Moreover, silencing NEAT1 did not significantly alter other key factors critical for regulation of SM-specific gene expression, including SRF, MKL1, and MKL2 (Fig. 3D). The induction of endogenous SM-specific gene expression resulting from silencing NEAT1 in VSMCs is unlikely an off-target effect, since two different NEAT1 silencing duplexes have similar results. Furthermore, overexpression of mouse NEAT1 V1 was sufficient to reduce SM-specific gene expression in HCASMCs while having no effect on paraspeckle protein expression (Fig. 3 F and G). As a recent study showed that the paraspeckle protein SFPQ mediates NEAT1 function to regulate IL-8 expression in response to viral infection (28), we next sought to determine whether SFPQ plays a role in regulating SM-specific gene expression by transfection of silencing duplexes against SFPQ into HCASMCs. Data from this experiment revealed that, in contrast to silencing NEAT1, depletion of SFPQ did not have any effects on the endogenous SM contractile gene expression (SI Appendix, Fig. S3). Taken together, these data demonstrate that NEAT1 plays a critical role in repressing SM-specific gene expression independent of its paraspeckle binding protein SFPQ.
Fig. 3.
NEAT1 attenuates SM-specific gene expression. (A) Heat map of mRNAs differentially expressed between control and NEAT1 knockdown HCASMCs, as revealed by RNA-seq with cutoff of FDR < 0.01 and FC (fold change) >2; n = 3 each group. (B) GO analysis of the differentially expressed genes between silencing control and silencing NEAT1 in HCAMSCs. (C) Heat map of significantly up-regulated SRF-dependent contractile genes in silencing NEAT1 compared with silencing control in HCASMCs. HCASMCs were transduced with scrambled control siRNA or two different NEAT1 siRNA duplexes and harvested for (D) qRT-PCR or (E) Western blotting analysis as indicated. *P < 0.05. (F) RT-PCR was performed to validate the exogenous mouse NEAT1 expression using the mouse or human NEAT1 specific primers after control GFP or mouse NEAT1 V1 adenovirus was transduced into HCASMCs for 48 h. (G) Western blot was performed to examine protein expression in GFP or NEAT1 overexpressing HCASMCs.
NEAT1 Promotes VSMC Proliferation and Migration.
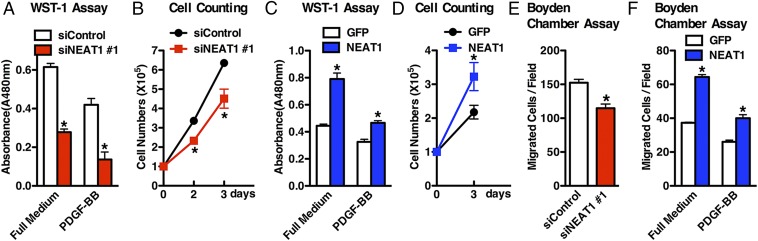
KO of NEAT1 in female mice impairs alveolar cell proliferation, resulting in the defects of mammary gland development (41). Recent studies also demonstrated that NEAT1 promotes prostate and breast cancer cell proliferation and survival (29, 30). Consistent with this, NEAT1 is induced in arterial injury (Fig. 1) where neointimal VSMCs are known to be proliferative. We therefore directly tested whether NEAT1 is able to affect VSMC proliferation by silencing endogenous NEAT1 or overexpression of NEAT1 in HCASMCs. Data from these experiments revealed that the rates of HCASMC proliferation were inhibited by silencing endogenous NEAT1 expression and increased by NEAT1 overexpression, supporting a positive role for the NEAT1 in regulating VSMC proliferation (Fig. 4 A–D). Similarly, by using Boyden chamber assay, we found that silencing endogenous NEAT1 in HCASMCs significantly impaired VSMC migration (Fig. 4E), while overexpression of NEAT1 significantly promoted VSMC migration in both full medium and PDGF-BB−treated conditions (Fig. 4F). Taken together, these data demonstrate that NEAT1 plays an integral role in SM phenotypic modulation by suppressing SM contractile gene expression while promoting VSMC proliferation and migration.
Fig. 4.
NEAT1 is required and sufficient to promote VSMC proliferation and migration. (A) Control or NEAT1 silencing duplex #1 was transfected into HCASMCs in full medium or PDGF-BB−containing medium (25 ng/mL), and cell proliferation was measured using the cell proliferation WST-1 kit (Roche); n = 3. *P < 0.05. (B) HCASMCs after transfection with control or NEAT1 #1 silencing duplex were counted at each time point as indicated; n = 3. *P < 0.05. Adenovirus encoding NEAT1 or control GFP were transduced into HCASMCs, and then the proliferation was measured as described in A by (C) WST-1 assay or (D) cell counting; n = 3. *P < 0.05. (E) Quantification of Boyden chamber assay to assess cell migration after 8-h plating of silencing duplex transfected or (F) viral transduced HCASMCs; n = 3. *P < 0.05.
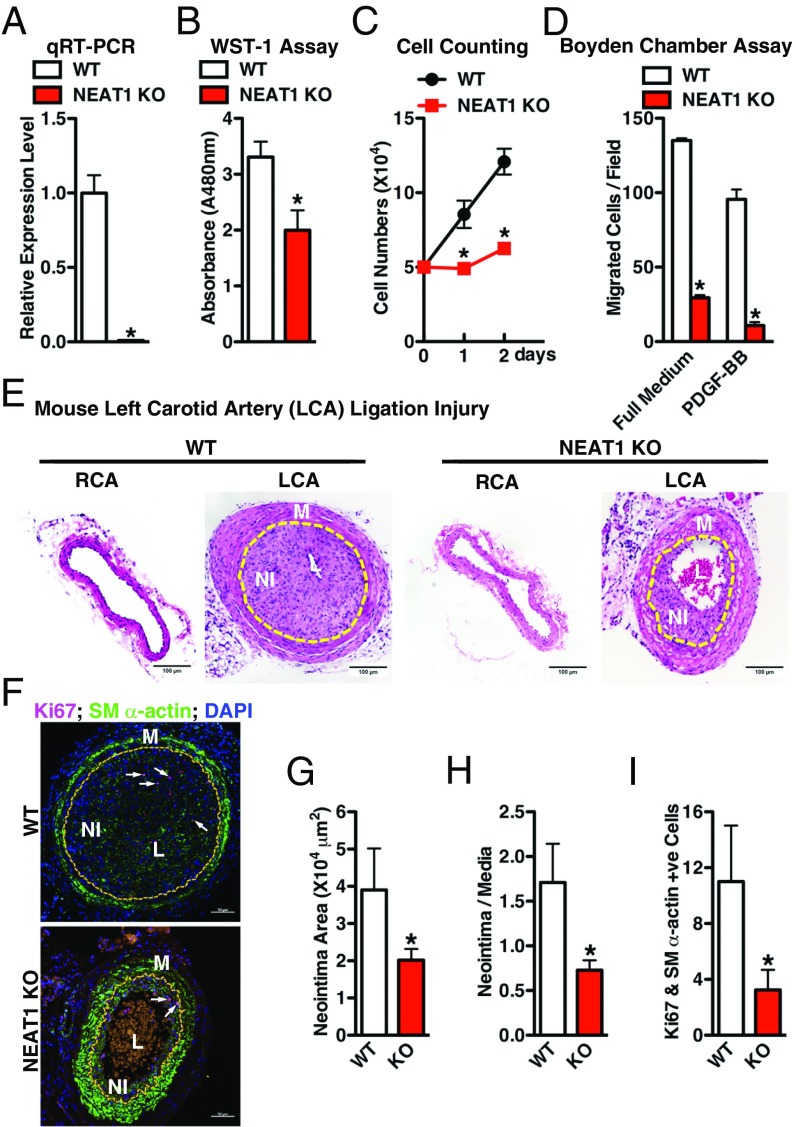
KO of NEAT1 Significantly Impairs VSMC Proliferation and Migration and Attenuates Neointima Formation After Carotid Artery Ligation Injury in Mice.
In vitro gain- and loss-of-function assays suggest a unique role of the paraspeckle lncRNA NEAT1 in suppressing SM-specific gene expression while promoting VSMC migration and proliferation (Figs. 3 and 4). Therefore, we next examined the functional role of NEAT1 in neointima formation in vivo under pathological conditions using NEAT1 KO mice (34). NEAT1 KO mice do not exhibit apparent gross abnormalities at baseline except for dysfunction of the corpus luteum (42) and in mammary gland development in the female KO mice (41). We confirmed that NEAT1 expression is completely depleted in the KO aorta (Fig. 5A). We further demonstrated that the VSMCs cultured from NEAT1 KO mice show decreased cell proliferation capability (Fig. 5 B and C) and migration (Fig. 5D) compared with WT VSMCs. Moreover, the neointima formed in response to carotid artery ligation for 28 d was significantly reduced in KO mice compared with the WT counterparts (Fig. 5 E–H). Immunofluorescence (IF) staining of proliferation marker Ki67 and SM-contractile gene SM α-actin further revealed that the NEAT1 deficiency attenuated VSMC proliferation after ligation injury (Fig. 5I). Taken together, these data demonstrate that the lncRNA NEAT1 plays a critical role for SM phenotypic switching in vitro and in vivo.
Fig. 5.
KO of NEAT1 impairs VSMC proliferation and migration and attenuates neointima formation induced by ligation injury in mice. (A) The qRT-PCR was performed to measure NEAT1 expression in thoracic aorta from WT or NEAT1 KO mice; n = 4. *P < 0.05. Primary aortic VSMCs prepared from WT or NEAT1 KO mice were subjected to (B) WST-1 assay and (C) cell number counting to measure SMC proliferation or (D) Boyden chamber transwell assay to measure SMC migration, as indicated; n = 3. *P < 0.05. (E) Adult WT or NEAT1 KO mice were subjected to carotid artery ligation injury (LCA) for 28 d. Representative hematoxylin- and eosin-stained sections show less neointima formation in the KO mice compared with WT. Neointima lesions are outlined with yellow lines. L, lumen; M, media layer; NI, neointima layer. (F) Costaining of proliferative marker Ki67 (red) and SM-specific marker SM α-actin (green) was performed in the ligation-injured LCA (28 d postinjury) from WT or NEAT1 KO mouse. Nuclei were counterstained with DAPI (blue). Arrows point to the representative Ki67 positive cells in neointima. Neointima lesions were outlined with yellow lines. Statistical analysis of (G) neointima area and (H) neointima/media layer ratio of sections shown in F using ImageJ software; n = 7. *P < 0.05. (I) Quantification of the Ki67 positive SMCs within the neointima area of the WT or NEAT1 KO LCA as shown in F; n = 7. *P < 0.05.
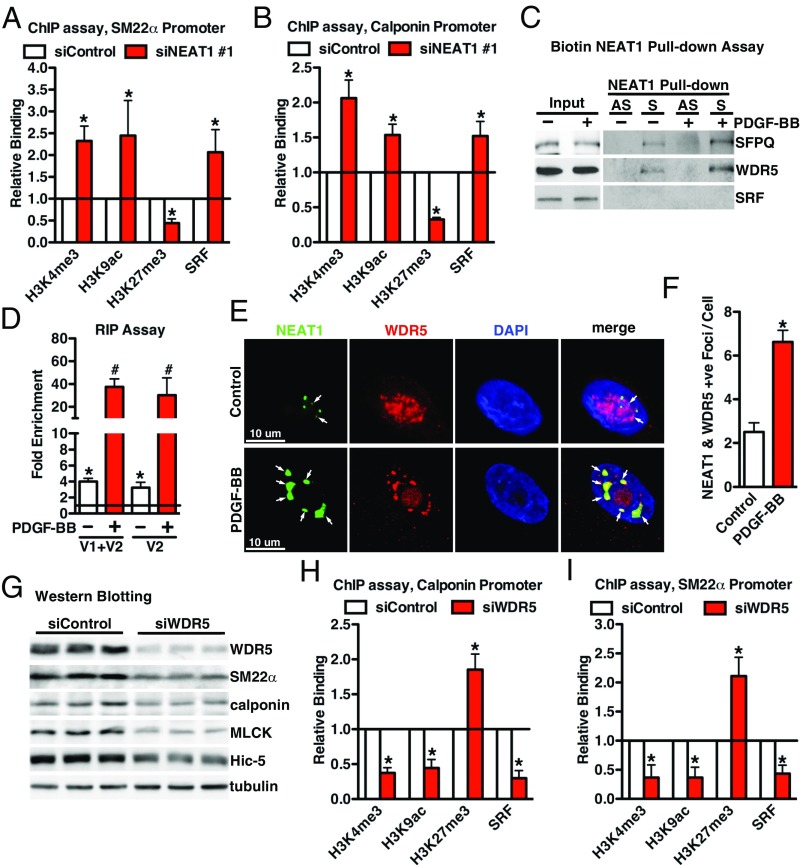
NEAT1 Induces an Inactive Chromatin State Within SM-Specific Gene Promoters by Binding to the Epigenetic Activator WDR5.
Data described above demonstrated a function of NEAT1 in SM-contractile gene expression in VSMCs; therefore, we next sought to determine the mechanism by which NEAT1 represses SM-specific gene expression. We first knocked down NEAT1 in HCASMCs, and ChIP assays were performed to examine the chromatin status by using a variety of antibodies against modified histones, including transcriptionally active marks H3K4me3, H3K9ac and the repressive mark H3K27me3. Since SRF binding to the CArG boxes within SM-specific gene promoters is the prerequisite to induce expression of these genes, we also evaluated SRF binding to CArG box regions within SM-specific gene promoters by using anti-SRF antibody. Data from these ChIP assays revealed that depletion of NEAT1 significantly promoted the enrichment of active histone modifications (H3K4me3 and H3K9ac) while decreasing the enrichment of inactive modification (H3K4me3) within CArG regions of SM-specific genes, calponin, and SM22α, resulting in increased binding of SRF to the CArG box regions of these genes (Fig. 6 A and B). To further dissect the mechanism by which NEAT1 could affect these histone modifications, we next sought to determine which factors bind to NEAT1 in VSMCs. Biotin RNA pull-down assays using nuclear protein extracted from PDGF-BB−treated or nontreated HCASMCs revealed that NEAT1 lncRNA bound WDR5 (12, 13, 43), a critical adaptor protein within MLL (Mixed Lineage Leukemia) H3K4 methylase complexes that previously has been shown to activate SM-specific gene expression (14, 44), and that this binding increased in PDGF-BB−treated cells (Fig. 6C). In contrast, SRF was absent from the NEAT1 RNA/protein complex (Fig. 6C) and was not colocalized with NEAT1, as shown by RNA-FISH/IF assay (SI Appendix, Fig. S4). Data from RNA immunoprecipitation (RIP) assays further demonstrated that NEAT1 was associated with WDR5 at basal level, but also the binding was significantly enhanced by treatment with PDGF-BB (Fig. 6D). Consistently, RNA-FISH/IF revealed that PDGF-BB treatment not only induced NEAT1 expression but promoted colocalization of NEAT1 and WDR5 (Fig. 6 E and F). Since PDGF-BB can promote NEAT1 binding with WDR5, we next directly assessed WDR5 function in VSMCs by knocking down endogenous WDR5 in HCASMCs. Protein lysate was harvested for Western blot 48 h after transfection of silencing RNA duplex against WDR5. Data from these experiments demonstrated that knockdown of WDR5 significantly decreased endogenous SM-specific protein expression (Fig. 6G). This effect is most likely due to the condensed chromatin configuration induced by depletion of WDR5 as shown by the decreased enrichment of active histone modifications (H3K4me3 and H3K9ac) and SRF binding along with enhanced inactive modification (H3K4me3) within CArG regions of SM-specific genes, calponin, and SM22α (Fig. 6 H and I). Taken together, these data suggest that NEAT1 functions through binding with WDR5 to repress SM-specific gene expression in VSMCs.
Fig. 6.
NEAT1 functions through binding to WDR5 in VSMCs. Control or NEAT1 silencing duplex #1 was transfected into HCASMCs, and chromatin was harvested for ChIP using the antibodies as indicated to determine the histone modifications or SRF binding to the CArG regions within (A) SM22α or (B) calponin gene regulatory regions; n = 3. *P < 0.05. (C) Biotin labeled sense (S) or antisense (AS) human NEAT1 was incubated with the nuclear protein from HCASMCs treated with or without PDGF-BB (25 ng/mL, 24 h), and Western blotting was performed to examine the proteins binding to NEAT1 by the indicated antibodies. (D) RIP assays were performed to determine the association between NEAT1 and WDR5 with or without PDGF-BB treatment (25 ng/mL, 24 h). Primers designed for NEAT1 V1 or V2 were utilized to measure the relative NEAT1 binding to WDR5. Samples immunoprecipitated with IgG served as control (set to 1). (E) RNA-FISH/IF was performed to determine the colocalization of NEAT1 (green) and WDR5 (red) with or without PDGF-BB treatment (25 ng/mL, 24 h). Arrows point to the colocalized NEAT1 and WDR5. (F) Quantification of the colocalized NEAT1 and WDR5 foci each cell as shown in D; n = 40 cells in each group. *P < 0.05. (G) Western blot was performed to assess SM-specific gene expression after knockdown of WDR5 in HCASMCs for 48 h. (H) ChIP assays were performed as described in A and B, except HCASMCs were transfected with silencing duplexes against WDR5 to determine epigenetic marks or SRF binding within calponin or SM22α gene (I); n = 3. *P < 0.05.
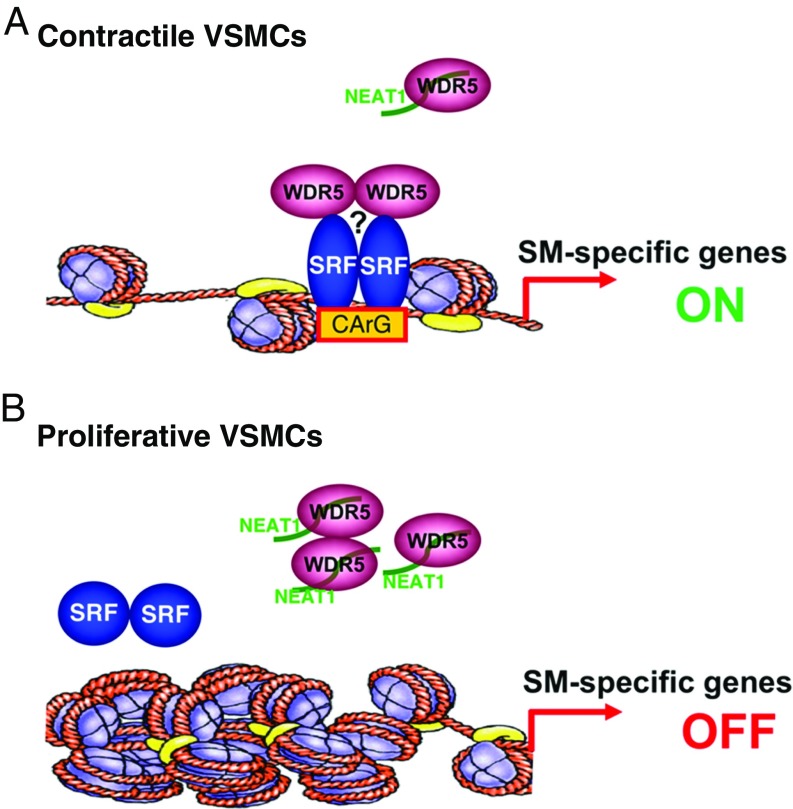
In summary, our data suggest that, in contractile VSMCs, NEAT1 is expressed at a low level. Under these conditions, the WDR5 component of the MLL histone methytransferase complex is free to facilitate an “open” configuration chromatin at the promoters of SM-specific genes, thereby promoting SRF binding and transcriptional activation (Fig. 7A). In contrast, in response to the stimuli that promote VSMC dedifferentiation and proliferation such as PDGF-BB, induced expression of lncRNA NEAT1 sequestrates WDR5 from SM-specific gene loci, thereby initiating an epigenetic “off” state and consequently impairing SRF accessibility to the CArG boxes, resulting in down-regulation of SM-specific gene expression (Fig. 7B).
Fig. 7.
Schematic summary of this study. (A) In contractile VSMCs, NEAT1 is expressed at a low level, freeing the histone modifier WDR5 to trimethylate histone H3K4 at the promoters of genes encoding SM contractile proteins, thereby opening the chromatin at these loci, facilitating SRF binding and transcriptional activation. WDR5 may possibly function through other cofactors to modulate SM contractile gene expression, as indicated by a question mark. (B) In contrast, in response to vascular injury or mitogens such as PDGF-BB, the increased expression of lncRNA NEAT1 sequestrates WDR5 from MLL complexes at the promoters of genes encoding SM-specific contractile proteins, initiating an epigenetic off state and consequently impairing SRF accessibility to the CArG boxes, resulting in down-regulation of SM-specific gene expression.
Discussion
This study provides evidence demonstrating a critical role for the paraspeckle-specific lncRNA NEAT1 in promoting VSMC proliferation, migration, and dedifferentiation during phenotypic switching. Paraspeckles are mammal-specific nuclear bodies that can be found in most cells cultured in vitro (45). NEAT1 is essential for the architectural integrity of paraspeckle by binding to paraspeckle proteins such as SFPQ (22, 24). Our current study revealed an unexpected role of NEAT1 in SM phenotypic switching independent of its paraspeckle binding protein SFPQ (SI Appendix, Fig. S3). We found that expression of NEAT1 is induced during phenotypic modulation in VSMCs in vitro and in vivo (Figs. 1 and 2). Loss- and gain-of-function NEAT1 assays revealed that NEAT1 not only promotes proliferation and migration of VSMCs but also decreases the expression of SM-specific contractile proteins (Figs. 3–5). In contrast to the recent study which showed that the paraspeckle protein SFPQ mediates NEAT1 ability to regulate IL-8 expression in response to viral infection (28), our data show that deletion of NEAT1 binding protein SFPQ has no effects on SM-contractile protein gene expression (SI Appendix, Fig. S3). It is unclear whether the effects of NEAT1 on SM-specific gene expression are dependent upon its function for the paraspeckle formation. Interestingly, a previous study showed that NEAT2, a neighboring lncRNA to NEAT1, is highly expressed in endothelial cells and is critical for angiogenesis (46). Furthermore, knockdown of NEAT2 in VSMCs has been shown to attenuate VSMC proliferation and migration (47), similar to our findings for NEAT1. Together with the recent report demonstrating that knockdown of NEAT1 in endothelial cells impaired the integrity and increased the permeability of the blood−tumor barrier (48), it appears that lncRNA expression from the clustered NEAT1 and NEAT2 loci is important for both endothelial cells and VSMC phenotype.
Our mechanistic studies demonstrated that, in response to PDGF-BB, a potent mitogen promoting SM phenotypic modulation (39), NEAT1 bound the key chromatin modifier WDR5 and led to decreased histone H3K4me3 and H3K9ac at the promoters of SM-specific genes, thereby initiating an epigenetic off state and resulting in down-regulation of SM-specific gene expression (Figs. 6 and 7). Our data also suggested that silencing of NEAT1-mediated SM gene expression occurs through promoting SRF binding to CArG boxes, not through its expression level (Figs. 3D and 6 A and B). A recent study showed that the SWI/SNF chromatin-remodeling complexes were identified as paraspeckle components that interact with NEAT1 (49). Our previous studies demonstrated that Brahma (Brm) or Brahma-like gene 1 (Brg1), the ATPase subunits of the SWI/SNF ATP-dependent chromatin remodeling complex, is required for the expression of SM-specific genes by promoting SRF/myocardin complex binding to the promoters of these genes through facilitating an open chromatin state within these gene loci (50). Interestingly, Capture Hybridization Analysis of RNA Targets - Mass Spectrometry data from the previous report suggested an association between NEAT1, BRG1, and WDR5 in prostate cancer cells (29). Therefore, it is possible that sequestration of Brg1 by NEAT1 in paraspeckles is an alternative mechanism accounting for the regulation of SM-specific gene mediated by NEAT1 in VSMCs. Furthermore, our RNA-seq data demonstrated that silencing NEAT1 in HCASMCs significantly augmented expression of myocardin mRNA (Fig. 3 C and D). Myocardin is a powerful SRF cofactor that is sufficient to promote SM-specific gene expression in multiple cells (40, 51). Interestingly, overexpression of myocardin facilitated an open status of chromatin in SM-specific gene loci to allow SRF binding and an active transcription of these genes (40), similar to the results after silencing NEAT1 in VSMCs (Fig. 6 A and B). Although we did not have the direct evidence to show that myocardin bind to NEAT1 in vitro, it is possible that NEAT1 can recruit myocardin through other bridging factors in vivo, thereby displacing myocardin from the SM gene. Therefore, it is likely that the augmentation of SM-specific gene expression induced by silencing NEAT1 results from a combination of myocardin induction and/or liberation of WDR5, Brg1, and myocardin from NEAT1 complexes in VSMCs.
It has been documented that, in many solid tumors, including those found in prostate cancer, lung cancer, esophageal cancer, colorectal cancer, and hepatocellular carcinoma, the paraspeckle lncRNA NEAT1 is overexpressed and NEAT1 promotes tumor cell proliferation (52). In the current study, we found that NEAT1 was also induced following vascular injury. Consistent with the function of NEAT1 in promoting cancer cell proliferation, we found overexpression of NEAT1 enhanced VSMC proliferation, while knockdown or KO of NEAT1 attenuated VSMC proliferation and migration, thereby diminishing neointima formation after vascular injury (Figs. 4 and 5). GO analysis of mRNAs whose expressions were altered following knockdown of NEAT1 revealed that NEAT1 affects expression of many genes related to cell cycle and the Hippo-YAP pathway in VSMCs (Fig. 3B) (32), suggesting that NEAT1-mediated regulation of VSMC proliferation likely occurs through multiple mechanisms, including modulating of Hippo pathway activity and expression of cell cycle genes. Interestingly, we found that NEAT1 expression was induced in adventitial cells after vascular injury (Fig. 1B and SI Appendix, Fig. S1). Since there is emerging evidence suggesting adventitial cells may be attributed to neointima formation in addition to media layer VSMCs (53), future studies are needed to investigate the potential role of NEAT1 in adventitial cells.
Activation of hypoxia-inducible factor (HIF) is common in many types of solid tumors. A recent study demonstrated that NEAT1 was a direct transcriptional target of HIF in many breast cancer cell lines and in solid tumors, thereby conferring HIF-dependent gene regulation and eventually leading to accelerated cellular proliferation (30). In addition, NEAT1 was identified as a direct transcriptional target of ERα (estrogen receptor alpha) and as an important mediator for maintenance of prostate cancer progression (29). Furthermore, the transcription factor C/EBPβ was critical for NEAT1 expression during acute promyelocytic leukemia cell differentiation induced by all-trans retinoic acid (54). In this study, we found that NEAT1 was significantly induced during SM phenotypic modulation in vivo and in vitro (Figs. 1 and 2). It will be important to investigate the underlying mechanisms by which NEAT1 is induced in response to vascular injury and in response to mitogens such as PDGF-BB.
In summary, this study has revealed an unexpected role of NEAT1 in promoting VSMC phenotypic modulation that is mediated, at least in part, through NEAT1’s regulation of MLL histone methyltransferase complexes. These findings suggest that NEAT1 is a potential therapeutic target for treating occlusive vascular diseases.
Supplementary Material
Acknowledgments
We thank Dr. Paul Herring for a critical reading of the manuscript. We also thank Dr. David L. Spector (Cold Spring Harbor Laboratory) for providing the pGEMTE vector containing full-length human NEAT1 V1 sequence. We thank the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with genomic analysis. The Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine is partially supported by National Cancer Institute Cancer Center Support Grant P30 CA91842 to the Siteman Cancer Center and by The Washington University Institute of Clinical and Translational Sciences (ICTS)/Clinical and Translational Science Award (CTSA) Grant UL1TR000448 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The work at the J.Z. laboratory is supported by grants from the National Heart, Lung, and Blood Institute, NIH. J.Z. is an Established Investigator of the American Heart Association. This publication is solely the responsibility of the authors and does not necessarily represent the official view of NCRR or NIH.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA-seq data generated in this study have been deposited in the Sequences Read Archive at the NCBI (accession no. SRP154294).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803725115/-/DCSupplemental.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Owens GK. Molecular control of vascular smooth muscle cell differentiation. Acta Physiol Scand. 1998;164:623–635. doi: 10.1111/j.1365-201x.1998.tb10706.x. [DOI] [PubMed] [Google Scholar]
- 3.Bentzon JF, et al. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2696–2702. doi: 10.1161/01.ATV.0000247243.48542.9d. [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, et al. Smooth muscle cells in transplant atherosclerotic lesions are originated from recipients, but not bone marrow progenitor cells. Circulation. 2002;106:1834–1839. doi: 10.1161/01.cir.0000031333.86845.dd. [DOI] [PubMed] [Google Scholar]
- 5.Nemenoff RA, et al. SDF-1α induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2011;31:1300–1308. doi: 10.1161/ATVBAHA.111.223701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herring BP, Hoggatt AM, Burlak C, Offermanns S. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc Cell. 2014;6:21. doi: 10.1186/2045-824X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang M, Liang A, Wang Y, Jiang J, Cheng J. Smooth muscle cells from the anastomosed artery are the major precursors for neointima formation in both artery and vein grafts. Basic Res Cardiol. 2014;109:431. doi: 10.1007/s00395-014-0431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwata H, et al. Bone marrow-derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages. Circulation. 2010;122:2048–2057. doi: 10.1161/CIRCULATIONAHA.110.965202. [DOI] [PubMed] [Google Scholar]
- 9.Daniel JM, et al. Time-course analysis on the differentiation of bone marrow-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol. 2010;30:1890–1896. doi: 10.1161/ATVBAHA.110.209692. [DOI] [PubMed] [Google Scholar]
- 10.Chappell J, et al. Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contributes to neointimal formation in mouse injury and atherosclerosis models. Circ Res. 2016;119:1313–1323. doi: 10.1161/CIRCRESAHA.116.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miano JM. Serum response factor: Toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 12.Wang KC, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang YW, et al. Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. eLife. 2014;3:e02046. doi: 10.7554/eLife.02046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gan Q, et al. WD repeat-containing protein 5, a ubiquitously expressed histone methyltransferase adaptor protein, regulates smooth muscle cell-selective gene activation through interaction with pituitary homeobox 2. J Biol Chem. 2011;286:21853–21864. doi: 10.1074/jbc.M111.233098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djebali S, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulitsky I, Bartel DP. lincRNAs: Genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fatica A, Bozzoni I. Long non-coding RNAs: New players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 18.Bell RD, et al. Identification and initial functional characterization of a human vascular cell-enriched long noncoding RNA. Arterioscler Thromb Vasc Biol. 2014;34:1249–1259. doi: 10.1161/ATVBAHA.114.303240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu G, et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130:1452–1465. doi: 10.1161/CIRCULATIONAHA.114.011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung A, et al. Novel long noncoding RNAs are regulated by angiotensin II in vascular smooth muscle cells. Circ Res. 2013;113:266–278. doi: 10.1161/CIRCRESAHA.112.300849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J, et al. MYOSLID is a novel serum response factor-dependent long noncoding RNA that amplifies the vascular smooth muscle differentiation program. Arterioscler Thromb Vasc Biol. 2016;36:2088–2099. doi: 10.1161/ATVBAHA.116.307879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naganuma T, Hirose T. Paraspeckle formation during the biogenesis of long non-coding RNAs. RNA Biol. 2013;10:456–461. doi: 10.4161/rna.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bond CS, Fox AH. Paraspeckles: Nuclear bodies built on long noncoding RNA. J Cell Biol. 2009;186:637–644. doi: 10.1083/jcb.200906113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemson CM, et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009;33:717–726. doi: 10.1016/j.molcel.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R, Harvey AR, Hodgetts SI, Fox AH. Functional dissection of NEAT1 using genome editing reveals substantial localization of the NEAT1_1 isoform outside paraspeckles. RNA. 2017;23:872–881. doi: 10.1261/rna.059477.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasanth KV, et al. Regulating gene expression through RNA nuclear retention. Cell. 2005;123:249–263. doi: 10.1016/j.cell.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 27.Hirose T, et al. NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol Biol Cell. 2014;25:169–183. doi: 10.1091/mbc.E13-09-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamura K, et al. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol Cell. 2014;53:393–406. doi: 10.1016/j.molcel.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Chakravarty D, et al. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun. 2014;5:5383. doi: 10.1038/ncomms6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choudhry H, et al. Tumor hypoxia induces nuclear paraspeckle formation through HIF-2α dependent transcriptional activation of NEAT1 leading to cancer cell survival. Oncogene. 2015;34:4482–4490. doi: 10.1038/onc.2014.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu F, et al. MicroRNA-15b/16 attenuates vascular neointima formation by promoting the contractile phenotype of vascular smooth muscle through targeting YAP. Arterioscler Thromb Vasc Biol. 2015;35:2145–2152. doi: 10.1161/ATVBAHA.115.305748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, et al. The induction of yes-associated protein expression after arterial injury is crucial for smooth muscle phenotypic modulation and neointima formation. Arterioscler Thromb Vasc Biol. 2012;32:2662–2669. doi: 10.1161/ATVBAHA.112.254730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunting KM, Nalloor RI, Vazdarjanova A. Influence of isoflurane on immediate-early gene expression. Front Behav Neurosci. 2016;9:363. doi: 10.3389/fnbeh.2015.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagawa S, Naganuma T, Shioi G, Hirose T. Paraspeckles are subpopulation-specific nuclear bodies that are not essential in mice. J Cell Biol. 2011;193:31–39. doi: 10.1083/jcb.201011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 36.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983;49:208–215. [PubMed] [Google Scholar]
- 37.Wang X, et al. Transforming growth factor-β1-induced transcript 1 protein, a novel marker for smooth muscle contractile phenotype, is regulated by serum response factor/myocardin protein. J Biol Chem. 2011;286:41589–41599. doi: 10.1074/jbc.M111.250878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng JP, Ju D, Shen J, Yang M, Li L. Disruption of actin cytoskeleton mediates loss of tensile stress induced early phenotypic modulation of vascular smooth muscle cells in organ culture. Exp Mol Pathol. 2010;88:52–57. doi: 10.1016/j.yexmp.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res. 1992;71:1525–1532. doi: 10.1161/01.res.71.6.1525. [DOI] [PubMed] [Google Scholar]
- 40.Miano JM. Myocardin in biology and disease. J Biomed Res. 2015;29:3–19. doi: 10.7555/JBR.29.20140151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Standaert L, et al. The long noncoding RNA Neat1 is required for mammary gland development and lactation. RNA. 2014;20:1844–1849. doi: 10.1261/rna.047332.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagawa S, et al. The lncRNA Neat1 is required for corpus luteum formation and the establishment of pregnancy in a subpopulation of mice. Development. 2014;141:4618–4627. doi: 10.1242/dev.110544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez JA, et al. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell. 2013;152:743–754. doi: 10.1016/j.cell.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wysocka J, et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121:859–872. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 45.Fox AH, et al. Paraspeckles: A novel nuclear domain. Curr Biol. 2002;12:13–25. doi: 10.1016/s0960-9822(01)00632-7. [DOI] [PubMed] [Google Scholar]
- 46.Michalik KM, et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014;114:1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 47.Yu CK, Xu T, Assoian RK, Rader DJ. Mining the stiffness-sensitive transcriptome in human vascular smooth muscle cells identifies long noncoding RNA stiffness regulators. Arterioscler Thromb Vasc Biol. 2018;38:164–173. doi: 10.1161/ATVBAHA.117.310237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo J, et al. Long non-coding RNA NEAT1 regulates permeability of the blood-tumor barrier via miR-181d-5p-mediated expression changes in ZO-1, occludin, and claudin-5. Biochim Biophys Acta. 2017;1863:2240–2254. doi: 10.1016/j.bbadis.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Kawaguchi T, et al. SWI/SNF chromatin-remodeling complexes function in noncoding RNA-dependent assembly of nuclear bodies. Proc Natl Acad Sci USA. 2015;112:4304–4309. doi: 10.1073/pnas.1423819112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou J, et al. The SWI/SNF chromatin remodeling complex regulates myocardin-induced smooth muscle-specific gene expression. Arterioscler Thromb Vasc Biol. 2009;29:921–928. doi: 10.1161/ATVBAHA.109.187229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci USA. 2003;100:7129–7134. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu X, Li Z, Zheng H, Chan MT, Wu WK. NEAT1: A novel cancer-related long non-coding RNA. Cell Prolif. 2017;50:e12329. doi: 10.1111/cpr.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kramann R, et al. Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell. 2016;19:628–642. doi: 10.1016/j.stem.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, et al. C/EBPbeta contributes to transcriptional activation of long non-coding RNA NEAT1 during APL cell differentiation. Biochem Biophys Res Commun. 2018;499:99–104. doi: 10.1016/j.bbrc.2017.10.137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.