ABSTRACT
Immunotherapies have shown promising results in certain cancer patients. For hepatocellular carcinoma (HCC), the multiplicity of an immunotolerant microenvironment within both the tumor, and the liver per se may limit the efficacy of cancer immunotherapies. Since radiation induces immunogenic cell death and inflammatory reactions within the tumor microenvironment, we hypothesized that a combination therapy of radiation and lasting local immunostimulating agents, achieved by intratumoral injection of an adenoviral vector encoding interleukin 12, may reverse the immunotolerant microenvironment within a well-established orthotopic HCC toward a state favorable for inducing antitumor immunities. Our data showed that radiation and IL-12 combination therapy (RT/IL-12) led to dramatic tumor regression in animals bearing large subcutaneous or orthotopic HCC, induced systemic effect against distant tumor, and significantly prolonged survival. Radiation monotherapy induced tumor regression at early times but afterwards most tumors regained exponential growth, while IL-12 monotherapy only delayed tumor growth. Mechanistic studies revealed that RT/IL-12 increased expression of MHC class II and co-stimulatory molecules CD40 and CD86 on tumor-infiltrating dendritic cells, suggesting an improvement of their antigen presentation activity. RT/IL-12 also significantly reduced accumulation of tumor-infiltrating myeloid-derived suppressor cells (MDSCs) and impaired their suppressive functions by reducing production of reactive oxygen species. Accordingly, tumor-infiltrating CD8+ T cells and NK cells were significantly activated toward the antitumor phenotype, as revealed by increased expression of CD107a and TNF-α. Together, our data showed that RT/IL-12 treatment could reset the intratumoral immunotolerant state and stimulate activation of antitumor cellular immunity that is capable of eliminating large established HCC tumors.
Keywords: Orthotopic HCC, tumor microenvironment, MDSC, combination therapy, immunomodulation, radiotherapy, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) is often diagnosed at advanced stages1 and is one of the most difficult cancers to treat in advanced disease. Surgical resection and liver transplantation are considered curative therapies, but only a small subset of patients are candidates for surgery.2 HCC is an immunogenic liver lesion as evidenced by the presence of many neoantigens and intratumoral accumulation of effector T cells.3 However, the most HCC cancer patients do not respond to immunotherapies, likely due to the multiplicity of immunosuppressive factors, which are present within the large well-established tumors and in the liver, where robust immune responses are typically suppressed.4 It is well established that tumors employ several immunosuppressive mechanisms to suppress immune responses. For example, increased accumulation of immature dendritic cells (DCs) in tumors may result in presentation of tumor antigens without co-stimulation to naïve T cells and leads to antigen-specific anergy instead of immunity.5 Furthermore, tumor-associated myeloid-derived suppressor cells (MDSCs) not only support tumor growth and metastasis but also possess powerful immunosuppressive activities.6,7 Reducing the number of MDSCs in tumor-bearing mice markedly improved the antitumor responses.8 In addition to tumor-associated immunosuppression, the liver per se is considered as an immunotolerant organ that can promote immunological tolerance to foreign antigens (Ag) and elicit Ag-induced apoptosis of activated CD8 T cells.9 Therefore, the efficacy of an immunotherapeutic agent is likely to be reduced on encountering these immunosuppressive processes within HCC microenvironment.
Radiation is standard treatment for many cancers. It has been traditionally used to locally eradicate tumor cells and alter tumor or tumor stroma architecture via DNA double-strand breaks or induction of apoptosis. In addition to its widely established tumoricidal effect, increasing evidence demonstrates that radiation can initiate an immune stimulus to induce antitumor responses.10 Combination of radiation and various immunotherapies have been under investigation in the clinic.11 Among immune intervention therapies, interleukin-12 (IL-12) is considered as the most potent cytokine in triggering antitumor immune responses.12,13 IL-12 is critical in the activation of innate immunity, including antigen-presenting activity of DCs, and subsequent activation of T-helper 1 cell (Th1) immunity, and in promoting the killing function of cytotoxic T lymphocytes and natural killer cells.14 The improved therapeutic efficacy of RT in combination with IL-12 (RT/IL-12) has been demonstrated in several preclinical tumor models, including HCC.15–18 However, these early studies were predominantly conducted in models with relatively small subcutaneous tumors. Whether RT/IL-12 has therapeutic benefit against more clinically relevant HCC tumor models, that is, large established tumors growing in the liver environment, remains to be explored.
In this study, we were able to modulate immunosuppressive cells within the liver tumor microenvironment to recover antitumor immunity. Specifically, we investigated the therapeutic effect of RT and IL-12 combination therapy on large orthotopically transplanted HCC tumors. Remarkably, our results show that RT/IL-12 therapy led to significant tumor regression in animals, which was caused by increasing the maturation and activation status of DCs, reducing accumulation and suppressive functions of tumor-infiltrating MDSCs, as well as increasing activation and accumulation of tumor-infiltrating CD8+ T cells and the cytotoxic activities of both CD8+ T cells and natural killer (NK) cells. Our study offers exciting insights into the rational design of combinatorial therapy, and demonstrates that radiation and IL-12 together presents a powerful potential alternative therapy against advanced HCC.
Results
Combination of radiation and local IL-12 confers synergistic antitumor activity
The therapeutic efficacies of cancer immunotherapies in preclinical studies are often limited to small tumors that lack the immunosuppressive microenvironment present in the large well-established tumors.19 To investigate whether the combination of radiation and IL-12 can induce synergistic antitumor effects against large tumors, BALB/c mice were injected subcutaneously (s.c.) with BNL-P2 HCC cells and the tumors were allowed to establish until ≥ 10 mm in diameter (volumes between 150 and 200 mm3) for 14 days and then treated with the following regimens: (1) a single dose of radiation (10 Gy), (2) a single dose of adenoviral vector encoding a single-chain murine IL-12 (Ad/IL-12; 1 × 108 p.f.u) by intratumoral injection, (3) a combination of both radiation and Ad/IL-12 (RT/IL-12) or (4) untreated. A titered dose of Ad/IL-12 was used in this study to decrease vector spillover from the injected tumors to other organs to avoid potential IL-12-associated systemic toxicity.20 As shown in Figure 1(a), single therapy of radiation or IL-12 significantly suppressed tumor growth, the mean tumor volume on day 35 being 375 ± 47 mm3 (p < 0.001) in the radiation group and 415 ± 78 mm3 in the IL-12 group (p < 0.001), compared with 1,598 ± 151 mm3 in the untreated group. Radiation therapy induced significant tumor regression in most animals (p < 0.001), while IL-12 at this dose caused a transient reduction of tumors at early time (between 4 and 11 days post treatment), and after this period most tumors regained exponential growth except one mouse whose tumor became undetectable. Significantly, RT/IL-12 showed a pronounced synergistic antitumor effect, leading to sustained tumor regression and suppression in most animals (complete regression in 4 of 10 mice [40%], and partial regression in 5 of 10 mice [50%]), with the mean tumor volume being 20 ± 11 mm3 on day 35, which was dramatically smaller than that of either of the monotherapy group (both p < 0.001). We further investigated whether RT/IL-12 can confer beneficial survival in mice with established HCC. As shown in Figure 1(b), monotherapy of radiation or IL-12 significantly prolonged the mean survival time from 46 ± 1 days in the untreated group to 61 ± 2 days in the radiation group and 61 ± 3 days in the IL-12 group (p < 0.001 in both). As expected, the best therapeutic result was achieved by RT/IL-12 treatment, which further prolonged the mean survival time to 96 ± 3 days (p < 0.001 compared with each of the three other groups). We further investigated whether RT/IL-12 has similar antitumor efficacy in another cancer type, the CT26 colorectal cancer. Mice bearing large s.c. CT26 tumors (volumes between 150 and 200 mm3) were treated with radiation, Ad/IL-12 or a combination of both. RT/IL-12 also showed a pronounced synergistic antitumor effect, leading to sustained tumor regression and suppression in most animals (complete regression in 1 of 10 mice [10%], and partial regression in 5 of 10 mice [50%]), while either monotherapy alone only delayed tumor growth but did not induce tumor regression (Supplementary Figure. S2).
Figure 1.
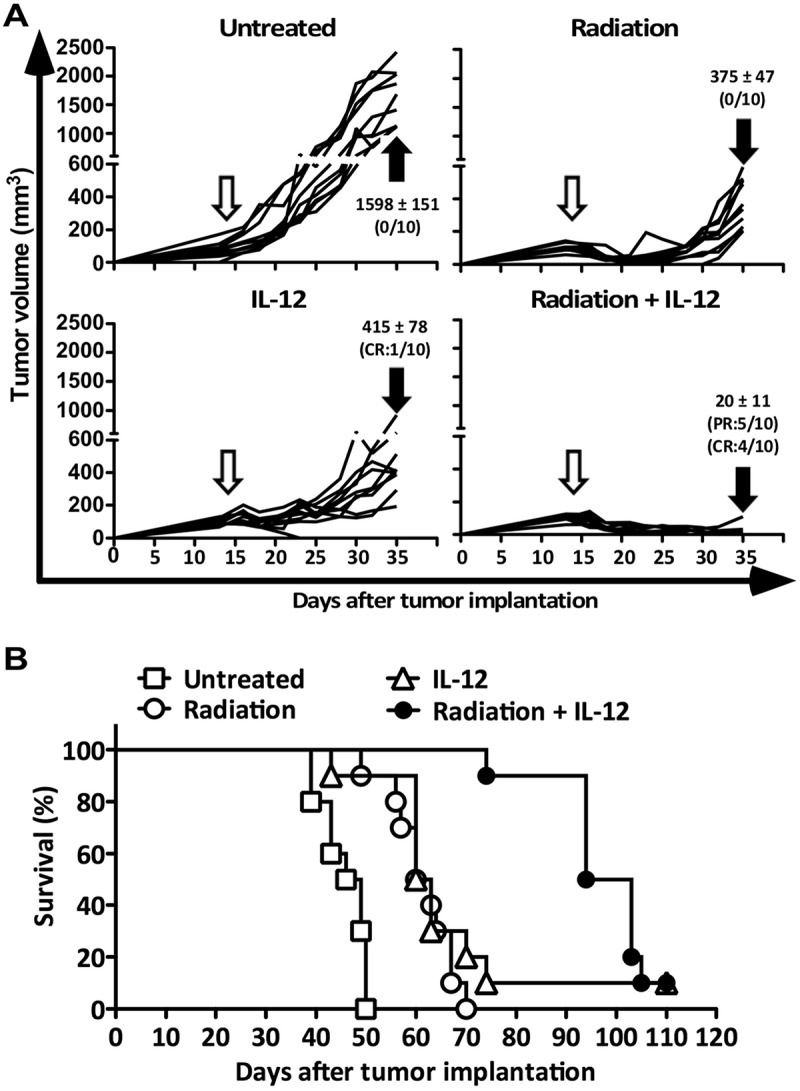
Antitumor activity of combined radiation and IL-12 on subcutaneous HCC. BALB/c mice (n = 10 in each group) were injected into the right flank with 1 × 106 BNL HCC cells. Fourteen days later, these mice were randomly grouped and treated with 10 Gy radiation, 1 × 108 pfu of Ad/IL-12 by intratumoral injection, combination of both or remained untreated. (A) Tumor volume was measured 3 times/week for three weeks and (B) mice were kept for long-term survival observation. The data are presented as mean ± SEM. CR: complete regression; PR: partial regression. Unfilled arrow: day of treatment. Filled arrow: 3 weeks post treatment for tumor volume measurement. Panels represent data from 2 independent experiments.
We further examined whether RT/IL-12 could elicit systemic antitumor responses against distant tumors. To test this, mice were subcutaneously implanted with CT26 cells in the both flanks. After 14 days, primary tumors in the right flank (volumes between 200 and 250 mm3) were treated with radiation, Ad/IL-12 or a combination of both. Distant tumors in the left flank remained untreated and their growth was monitored. As shown in Figure 2, RT/IL-12 showed a pronounced systemic effect, completely suppressing growth of distant tumors in all animals. IL-12 monotherapy showed some minor effect on the growth of distant tumors, while radiation monotherapy did not show suppressive effect on distant tumors. These results demonstrate that RT/IL-12 not only showed a pronounced synergistic antitumor effect on the locally treated tumors, but also had a systemic effect on distant tumors.
Figure 2.

Systemic antitumor effect of radiation and IL-12 combination therapy. Groups of BALB/c mice (n = 5) were injected subcutaneously with 5 × 105 CT26 cells in the right flank and 1 × 105 CT26 cells in the left flank. Tumors on right flank were treated with radiation, Ad/IL-12, combination of both or remained untreated as described above, and the left flank tumor remained untreated. Tumor growth on the left flank was monitored at the indicated times. The data are presented as mean ± SEM.
We then investigated whether RT/IL-12 has similar antitumor efficacy in more clinically relevant orthotopic HCC. BALB/c mice (n = 10) were injected with BNL-P2 HCC cells into the left liver lobe and treated on day 10 when the average tumor size was between 6–8 mm in diameter. Mice were sacrificed 21 days after treatment and tumor volumes were measured. As shown in Figure 3, monotherapy of radiation or IL-12 significantly suppressed tumor growth, the mean tumor volumes being 367 ± 203 mm3 (p < 0.001) in the radiation group and 241 ± 204 mm3 in the IL-12 group (p < 0.001), compared with 2,797 ± 304 mm3 in the untreated group. We did not see tumor regression in either of the groups receiving single treatment. Remarkably, RT/IL-12 treatment showed profound tumor regression in most animals (complete regression in 3 of 10 mice [30%], and partial regression in 5 of 10 mice [50%]), with the mean tumor volume being 53 ± 120 mm3, which was significantly smaller than that of the other groups (p < 0.001, versus untreated; p < 0.01, versus radiation; p < 0.05, versus IL-12). To further assess the therapeutic efficacy of RT/IL-12 for large orthotopic HCC, we also treated tumor-bearing mice on day 14 when the average tumor size was between 8–10 mm in diameter. Impressively, RT/IL-12 treatment led to sustained tumor regression of these large orthotopic HCC (complete regression in 3 of 10 mice [30%], and partial regression in 7 of 10 mice [70%]), with the mean tumor volume being 12 ± 5 mm3. These results demonstrate that combination of radiation and IL-12 greatly improved the antitumor activity of either single therapy against large established hepatic tumors.
Figure 3.

Antitumor activity of combined radiation and IL-12 on large orthotopic HCC. BALB/c mice (n = 6–10 in different groups) were injected into the left liver lobe with 3 × 105 BNL HCC cells. Ten days later, these mice were randomly grouped and treated with 10 Gy radiation, 1 × 108 pfu of Ad/IL-12 by intratumoral injection, combination of both or remained untreated. Mice were sacrificed at day 31. For large orthotopic model, mice were treated on day 14 and sacrificed on day 35 for tumor volume measurement. (A) Tumor volumes were presented as before (day 10 or 14) and after (21 days after) treatment. (B) Representative photographs of the liver of each group. The data are presented as mean ± SEM.
IFN-γ is the major down-stream cytokine induced by IL-12 and is critical for the development of cell-mediated antitumor immune responses. The serum levels of IL-12 and IFN-γ in the different groups were determined by cytokine bead array 3 and 7 days post treatment (Supplementary Figure. S3). The results showed that radiation alone did not increase serum IL-12 and IFN-γ as compared with that in the untreated group, while both IL-12 and the RT/IL-12 treatments induced low but significantly higher levels of IL-12 and IFN-γ in the circulation.
RT/IL-12 decreased the number of MDSCs and increased CD8+ T cells in tumor tissues
Liver is generally considered as an immune tolerant organ, favoring induction of immune privilege rather than immunity.9 Tumors are also known to employ several immunosuppressive mechanisms to prevent antitumor immune responses.4 Under this stringent immune tolerant environment, we were interested to know whether tumor-infiltrating immune cells of orthotopic liver tumors could be appropriately activated to gain the ability to eliminate tumors. To address this question, we applied the orthotopic BNL-P2 HCC model to investigate the effect of different treatments on local (liver) immune cells. We first analyzed whether there were quantitative differences in the populations of tumor-infiltrating immune cells among the different treatment groups. We established a multicolor flow cytometric method to analyze different myeloid and lymphoid cells infiltrating the tumors in a detailed manner (Supplementary Figure. S1). As shown in Figure 4(a), the percentage of total leukocytes (CD45+) was significantly increased from 38.1 ± 1.7% in the untreated group to 52.2 ± 4.9% in the IL-12 group (p < 0.05) and 59.1 ± 4.7% in the RT/IL-12 group (p < 0.01), whereas the percentage of leukocytes was slightly decreased in the radiation group (28.0 ± 2.6%). Among these tumor-infiltrating leukocytes, the percentage of myeloid cells (CD45+CD11b+) was unaffected by radiation, but decreased from 60.4 ± 3.6% in the untreated group to 50.3 ± 3.1% in the IL-12 group and 43.9 ± 2.7% in the RT/IL-12 group (p < 0.01). We further analyzed the subpopulations of these tumor-infiltrating myeloid cells and found that both IL-12 and RT/IL-12 reduced the percentage of MDSCs (CD45+CD11b+Gr1high) from 19.1 ± 2.0% in the untreated group to 13.3 ± 2.2% and 8.2 ± 1.1%, respectively, with RT/IL-12 having greater impact on MDSCs and reaching statistical significance (p < 0.01). We also analyzed dendritic cells (defined as CD45+CD11b+CD11c+Gr1lo) and found that both IL-12 and RT/IL-12 increased the percentage of DCs from 5.5 ± 0.5% in the untreated group to 10.8 ± 1.2% and 7.5 ± 1.2% for IL-12 and RT/IL-12, respectively. IL-12 therapy tended to have a greater impact on DCs than the RT/IL-12 but did not reach statistical significance. In contrast to IL-12 and RT/IL-12, radiation alone had little effect on the different populations of myeloid cells within the tumors.
Figure 4.
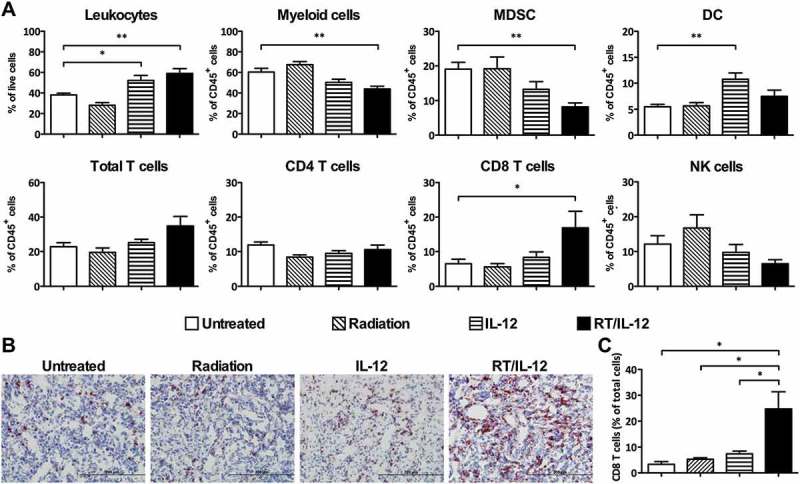
The change of cell population after combination of radiation and IL-12 treatment in tumor microenvironment. BALB/c mice (n = 4–11 in different groups) were injected with 3 × 105 BNL HCC cells into the left liver lobe. Fourteen days later, these mice treated with 10 Gy radiation, 1 × 108 pfu of Ad/IL-12 by intratumoral injection, combination of both or remained non-treated. (A) Mice were sacrificed 7 days after treatment and the leukocyte subpopulations within the tumor were analyzed by flow cytometer. (B) Tumor tissues were removed 7 days after treatment for immunohistochemical (IHC) analysis. Cryostat sections were immunohistochemically stained with anti-CD8 and developed with AEC, then counterstained with hematoxylin. Original magnification, ×200. (C) The percentage of CD8+ cells in tumor tissues. The IHC stained slides were scanned and analyzed using a Panoramic 250 slide scanner. The data are presented as mean ± SEM. *, p < 0.05; **, p < 0.01. Representative data are shown from three experiments. RT/IL-12: radiation plus IL-12.
We next assessed the effect of the different treatments on lymphoid cells within the tumor. The percentage of total T cells (CD45+TCR-β+) was not significantly affected by either radiation or IL-12 single treatment. RT/IL-12 increased the percentage of tumor-infiltrating T cells by about 1.5-fold to 34.9 ± 5.5% as compared with 22.8 ± 2.3% in the untreated group but did not reach statistical significance. Importantly, RT/IL-12 exhibited the greatest effect on CD8+ T cells, the percentage of which was increased 2.6-fold as compared with that of the untreated group (16.9 ± 4.8% versus 6.5 ± 1.3%, p < 0.05). Neither radiation nor IL-12 single treatment had any effect on the percentage of tumor-infiltrating CD8+ T cells. This result was further confirmed by immunohistochemical (IHC) staining of tumor tissues and quantitatively analyzed by a digital whole slide imaging and analysis system. Consistent with what was found in flow cytometric analyses, IHC revealed that RT/IL-12 induced a 7.5-fold increase in infiltrating CD8+ T cells (from 3.3% to 24.7%) in the tumor tissues, while radiation and IL-12 single treatments had much less effect (Figure 4(b,c)). As for NK cells and CD4+ T cells, we found by flow cytometric analyses that the percentage of these two lymphoid cell populations were insignificantly affected by different treatments, with radiation slightly increasing and RT/IL-12 decreasing the percentage of NK cells. Taken together, these results clearly demonstrate that combination therapy of RT/IL-12 had a much greater impact on tumor-infiltrating leukocytes than radiation or IL-12 single treatments, in particular for CD8+ T cells and MDSCs.
RT/IL-12 treatment enhanced DC maturation
Previous studies have demonstrated that IL-12 treatment enhanced functions of antigen-presenting cells.21 To investigate whether RT/IL-12 can enhance the maturation status of tumor-infiltrating myeloid cells, especially DCs, we analyzed the expression of three classic maturation markers, MHC class II, CD40 and CD86, in the different treatment groups. Figure 5(a) shows a representative histogram; the summarized results are shown in Figure 5(b). Most tumor-infiltrating DCs with or without treatment were MHC class II+ and CD86+. IL-12 and RT/IL-12 treatment increased the percentage of CD40+ DCs about 2-fold as compared with the untreated and radiation groups. Significantly, DCs from IL-12 and RT/IL-12 groups increased mean fluorescence intensity (MFI) for these three maturation markers, especially for MHC class II and CD86. In contrast, radiation alone had little effect on these three myeloid cell maturation markers. Collectively, these data demonstrate that IL-12 and RT/IL-12 treatments caused the elevation of DC numbers within the tumor, but also stimulated the expression of maturation markers on these cells.
Figure 5.
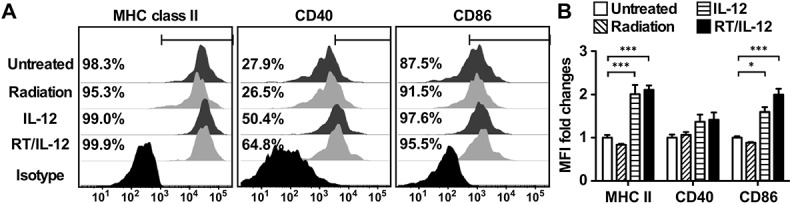
Combination of radiation and IL-12 increased the frequency and maturation of tumor-infiltrated DCs. BALB/c mice (n = 4–7 in different groups) were injected into the left liver lobe with BNL HCC cells and treated as described in Figure 3. (A) Mice were sacrificed 7 days post-treatment. CD11c+ DCs from TILs were gated and stained with anti-MHC class II CD40, and CD86 Abs, or appropriate isotype-control Abs, as described in Materials and Methods. The percentages of positive cells for surface proteins are shown. The results shown were from one representative mouse of each group. (B) Summarized MFI fold changes for surface proteins were presented as mean ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001. RT/IL-12: radiation plus IL-12.
Tumor-infiltrating CD8+ T cells and NK cells were functionally activated by RT/IL-12 treatment
Previous studies of IL-12-based immunotherapies demonstrated that CD8+ T cells and NK cells are the major effector populations that control tumor growth.18,22 Therefore, we determined how RT/IL-12 influenced effector functions of tumor-infiltrating CD8+ T cells and NK cells by examining their production of intracellular IFN-γ and TNF-α and membrane expression of CD107a, a measure of effector cell degranulation associated with perforin/granzyme-dependent cytotoxicity. To obtain cell status that reflects their in vivo activities at the time of tumor harvest, these effector lymphocytes were directly analyzed by flow cytometry without ex vivo restimulation. Compared with the untreated group, RT/IL-12 increased the percentage of CD107a+ CD8+ T cells by 2.6-fold (from 17.1% to 44.0%, p < 0.05, Figure 6(a)) and CD107a+ NK cells by 3.3-fold (from 24.6% to 81.5%, p < 0.001, Figure 6(b)). Significantly, RT/IL-12 also greatly increased the CD107a expression levels in these killer cells, as indicated by MFI increases of 5.3-fold on CD8+ T cells and 35.8-fold on NK cells, suggesting that these tumor-infiltrating killer cells were highly potent in cytotoxic activities within the tumors, even in the absence of additional in vitro stimulation. Similarly, RT/IL-12 treatment also increased the percentages and/or the expression levels of IFN-γ and TNF-α on CD8+ T cells and NK cells. IL-12 alone treatment increased the percentage and the expression levels of CD107a, TNF-α and IFN-γ on CD8+ T cells and NK cells, although in general the magnitude of responses were weaker than mice treated with RT/IL-12 combination therapy. In contrast, radiation alone did not mediate significant changes on these three activation markers on CD8+ T cells or NK cells. Taken together, these results demonstrate that RT/IL-12 treatment resulted in activation and degranulation of CD8+ T cells and NK cells in the tumor, strongly suggesting that these cells most likely contribute to tumor regression and may be directly involved in cancer cell killing within tumor microenvironment.
Figure 6.
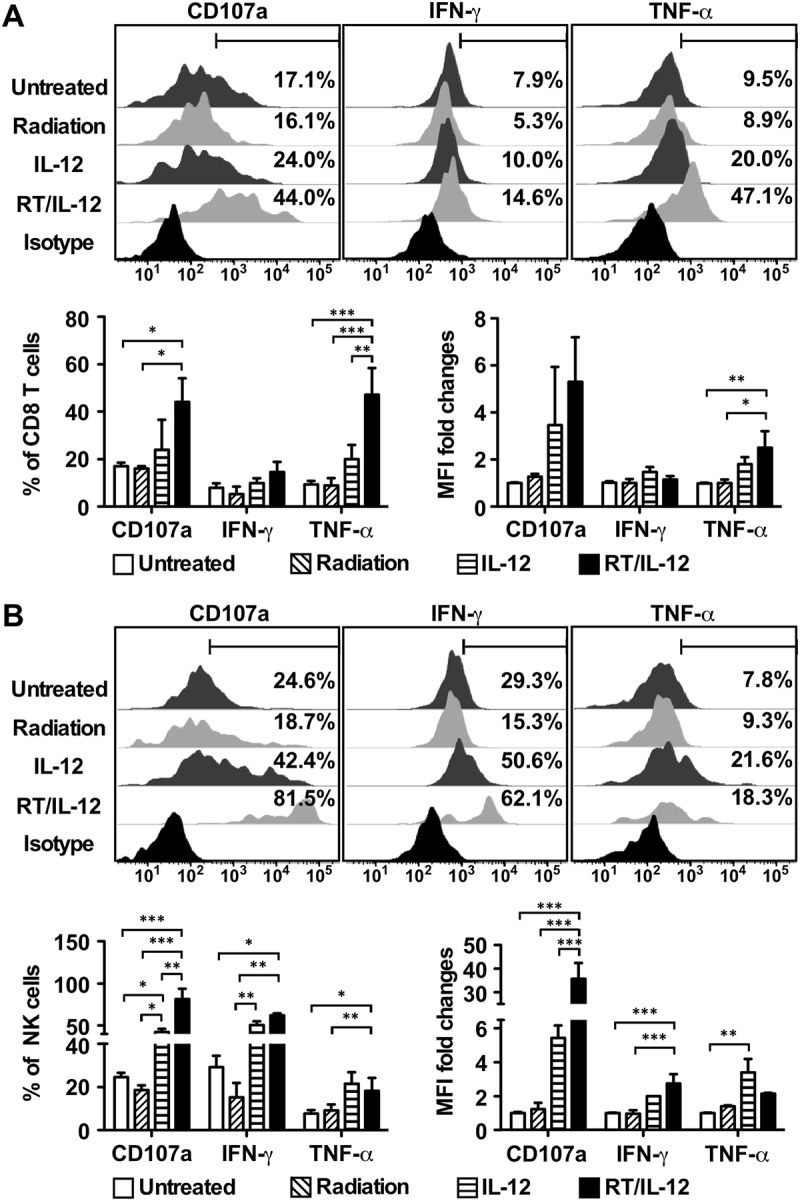
Combination of radiation and IL-12 significantly increased CD107a expression on CD8 T cells and NK cells. BALB/c mice (n = 4–10 in different groups) were injected into the left liver lobe with 3 × 105 BNL HCC cells, and 14 days later received radiation, 1 × 108 pfu of Ad/IL-12 by intratumoral injection, combination of both or remained non-treated. The percentage and MFI fold changes of CD107a, IFN-γ and TNF-α on tumor-infiltrating (A) CD8 T cells and (B) NK cells were analyzed 7 days later. The summarized results were presented as mean ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001. RT/IL-12: radiation plus IL-12.
RT/IL-12 decreased the suppressive activity of tumor-infiltrating MDSCs
Accumulation of MDSC in tumor microenvironment promoted tumor cell survival and suppressed proliferation and functional activity of T cells.23 We have demonstrated that RT/IL-12 combination treatment reduced MDSC accumulation in the tumor microenvironment (Figure 4(a)). Moreover, we demonstrated that these RT/IL-12-treated MDSCs express higher levels of MHC class II and CD86 (Supplementary Figure. S4), suggesting that their functional activities may also be modulated. To directly investigate whether RT/IL-12 treatment affected the suppressive capacity of MDSCs, we sorted tumor derived MDSCs from the different treatment groups and co-cultured with naïve splenocytes previously stimulated with CD3/CD28 antibodies. As shown in Figure 7(a), T cell proliferation was reduced by more than 90% when co-cultured with MDSCs from the untreated group at different splenocyte to MDSC ratios (10:5 and 10:2.5). MDSCs from the radiation or IL-12 single treatment groups showed less suppressive activity, where T cell proliferation was reduced between 67% and 78%. RT/IL-12 treatment had the greatest impact on the inhibitory effect of MDSC, and decreased T cell proliferation between 38% and 56%. The impact of radiation and IL-12 on the suppressive functions was limited at the local treatment site, since MDSCs isolated from the spleens of animals in the four different groups showed similar suppressive activity (Supplementary Figure. S5). It has been previously reported that reactive oxygen species (ROS) contribute to differentiation of M2 macrophage and formation of tumor-associated macrophages,24 along with MDSC-mediated T cell suppression.25 We investigated whether RT/IL-12 treatment has an effect on ROS production of MDSCs. Figure 7(b) shows a representative histogram and the summary results are shown in Figure 7(c). Single treatment of radiation or IL-12 only slightly decreased ROS production of MDSCs, while RT/IL-12 combination treatment greatly decreased the percentage of ROS+ cells and the MFI of ROS in MDSCs compared with that of the untreated group (p < 0.001).
Figure 7.
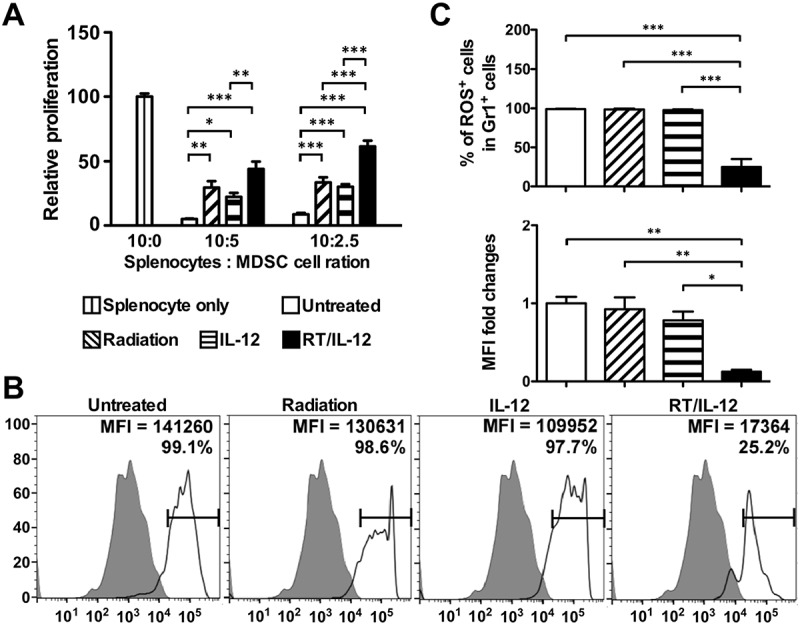
Combination of radiation and IL-12 subdued the suppressive activity and ROS production of tumor-infiltrated MDSC. BALB/c mice (n = 6–10 in different groups) were injected into the left liver lobe with BNL HCC cells and treated as described in Figure 3. Mice were sacrificed 7 days post-treatment. (A) The suppressive activity of MDSC obtained from TILs. Gr1± cells were purified with magnetic beads. Splenocytes from naïve mice were stimulated with αCD3/αCD28 Abs in the presence of purified Gr1± cells at a ratio of 10:5 or 10:2.5. Three days later, 1 μCi 3H-thymidine was added and cultured for additional 16 h. Cells were harvested and data were calculated by a TopCount instrument. (B) The levels of ROS in purified MDSC were measured by fluorescence intensity of 2ʹ,7ʹ-dichlorofluorescin diacetate labeling after PMA stimulation for 30 minutes. (C) Summarized results presented as means ± SEM. **, p < 0.01; ***, p < 0.001. RT/IL-12: radiation plus IL-12.
Discussion
In this study, we demonstrated that combining radiotherapy and an adenoviral vector encoding IL-12 led to tumor regression in animals bearing large subcutaneous and orthotopic HCC tumors, induced systemic effect on distant tumor and significantly prolonged their survival. We also observed that multiple cellular mechanisms contribute to the robust antitumor responses observed in the combination therapy (RT/IL-12). First, we found that RT/IL-12 reduced the number of tumor-infiltrating myeloid-derived suppressor cells (MDSCs) and also subdued their immune-suppressive properties. Secondly, RT/IL-12 treatment activated intra-tumoral dendritic cells (DCs) and may enhance Ag presenting functions, as indicated by increased surface expression of MHC class II, CD40 and CD86. Thirdly, RT/IL-12 treatment increased the accumulation of tumor-infiltrating CD8+ T cells and elevated cell markers correlated with cytotoxic functions of tumor-infiltrating CD8+ T cells and NK cells. This cytotoxic marker, CD107a, is a surrogate metric for T-cell cytolytic potential and cytokine expression,26 and our observed elevated expression correlated with the presence of increased intracellular levels of IFN-γ and TNF-α in intra-tumoral CD8+ T cells and NK cells. Together, these results strongly suggest that RT/IL-12 treatment can defeat the intratumoral suppressive microenvironment within large established hepatic tumors, and stimulate powerful cytokine and cellular anti-tumor immune mechanisms. We observed almost complete regression of large established orthotopic HCC tumors by this protocol.
Orthotopic HCC tumor is an important murine model to investigate the complex tumor-host interactions in the liver. This model has been reported to recapitulate key pathological features in human HCC.27 The antitumor effects of Ad/IL-12 have been demonstrated in several orthotopic HCC animal models, including mouse, rat and woodchuck.28–30 However, Ad/IL-12 alone failed in clinical trials of patients with hepatic malignancy.31 One of the challenges in treating established hepatic tumor with immunotherapy is to induce anti-tumor immune responses within the tolerogenic microenvironment of the liver and in the hepatic tumor. There is growing evidence suggesting that RT may be a practical solution in overcoming his hurdle, and may enhance antitumor efficacy of immunotherapy.32 Our data presented here demonstrate that RT/IL-12 combination therapy is sufficient to reverse the tolerogenic microenvironment of the liver and hepatic tumors to induce strong antitumor responses in an orthotopic HCC tumor model in mice. This is consistent with the observation that RT synergizes with the antitumor effects of IL-12 in various experimental animal models.15–18,22,33 Our study further extends this combination strategy to treat HCC.
Increased accumulation of immature or regulatory DCs in tumors may result in antigen-specific anergy instead of immunity, and is sufficient to promote tumor growth.5 Recently, immune suppressive regulatory DCs have also been identified in HCC patients.34 DCs differentiate into mature DCs through microenvironmental stimuli that can efficiently launch immune responses. Kerkar and colleagues found that IL-12 could reprogram immunosuppressive DCs to regain immunostimulating functions that promote anti-tumor T cells.35 It is known that maturation of DCs is associated with increased expression of MHC class II and co-stimulatory molecules, such as CD40, CD80 and CD86 on the cell surface,36 and that interaction of CD40 and CD40L is essential for the differentiation of DCs from immature into fully mature phenotype that are able to prime adaptive T cell responses.37 In agreement with these reports, our results showed that RT/IL-12 treatment greatly increased the levels of MHC class II and co-stimulatory molecules CD40 and CD86 on DCs (Figure 5), suggesting that these DCs may gain antigen-presentation functions to promote antitumor T cell responses.
MDSCs, one of the major immune suppressor cells in the tumor microenvironment, can suppress immune responses and facilitate tumor progression.6 MDSCs can also enhance cancer stem cell gene expression, resulting in increased cancer cell stemness and increasing metastatic and tumorigenic potential 38. MDSCs are found in both tumor tissues and peripheral blood from patients with HCC, and elevated MDSC numbers correlated with clinical tumor progression.39,40 Reducing the numbers of MDSCs in murine tumors markedly improved the antitumor responses of chemotherapy.8 In human, myeloid-derived suppressor cells as an immune parameter in patients with concurrent sunitinib and stereotactic body radiotherapy.41 In agreement with these studies, our results showed that RT/IL-12 significantly reduced the numbers of MDSCs from tumor-infiltrating leukocytes (TILs), (. 4A). It should be emphasized that the effect of RT/IL-12 was limited to the tumor microenvironment since MDSC numbers in the periphery (spleen) remained unchanged (data not shown). The suppressive activity of MDSCs from RT/IL-12 treated tumors was significantly impaired (Figure 7(a)), which was not seen in MDSCs from spleen (Supplementary Figure. S5). ROS production disrupts TCR signaling and it has been reported that reducing ROS releases CD8 T cell suppression by MDSC.42 This mechanism appears to underlie the effects of RT/IL-12 on MDSC-mediated T cell suppression, as ROS production from tumor-infiltrating MDSCs only decreased when the tumor was treated with RT in combination with IL-12, but not with RT or with IL-12 alone (Figure 7(b,c). Another potential effect of combination therapy on MDSCs is to promote their further differentiation towards macrophages and DCs, as observed in TLR-7/8 agonist-stimulated MDSCs.43 In agreement with this possibility, our results showed that RT/IL-12 treatment significantly increased expression of MHC class II and CD86 (Supplementary Figure. S4).
Radiation is well known for its direct tumoricidal effect via induction of double-strand breaks or apoptosis. Recently, it has been shown that radiation can also enhance the cross-priming capacity of tumor-infiltrating dendritic cells and subsequently activate CD8+ T cells 44. In our study, radiation at a single dose of 10 Gy can partially delayed tumor growth (Figure 1(a) and Supplementary Figure S2), likely through a direct cytocidal effect and induction of cytotoxic CD8+ T cells. Combination of a local IL-12 treatment provides a strategy to further enhanced these CD8+ T cell responses and thus achieves a much better antitumor effect than either monotherapy alone. It has been shown that the antitumor efficacy of RT/IL-12 depends on the presence of T and NK cells.17,18,22 In this study, our data showed that RT/IL-12 treatment significantly increased the numbers of tumor-infiltrating CD8+ T cells with potent cytotoxic activities (Figure 6(a)), as demonstrated by their increased surface expression of CD107a.26 For tumor-infiltrating NK cells, although the number was not significantly changed post RT/IL-12 treatment, these cells were highly active in the cytotoxic phenotype as demonstrated by their up to 30-fold increase in CD107a expression (Figure 6(b)). These results strongly suggest that RT/IL-12 combination therapy was likely able to eliminate large established orthotopic HCC through activation of CD8+ cytotoxic T cells and NK cells.
RT is an established treatment option for patients with advanced HCC. Stereotactic body radiotherapy (SBRT) is an emerging radiotherapy technique that safely and effectively treats HCC by delivering large dose of radiation (6–15 Gy per fraction, as used in our RT/IL-12 protocol) to liver tumors in 1–6 fractions.45 SBRT is believed to achieve tumor control via its effects on vascular, stromal and immunological aspects of the tumor microenvironment.46 The early clinical success of combining RT with systemic therapy to treat advanced HCC marks the promise of RT-containing multimodality protocols for this dismal disease.47 Currently, cancer immunotherapy is being rigorously tested for its synergism with RT. An outstanding example is the success in controlling metastatic melanoma with combination of SBRT and ipilimumab, an anti-CTLA-4 antibody.48 The power of such an approach is reproducibly demonstrated in several clinical studies on patients with metastatic melanoma, non-small-cell lung cancer (NSCLC) and brain metastases.49–51 It is also possible to induce antitumor responses in the tolerogenic microenvironment in liver, as combining ipilimumab with SBRT to a liver lesion is reported to result in durable disease suppression in a patient with metastatic non-small cell lung cancer.50 These human studies not only demonstrate the safety in combination of SBRT with immunotherapy, but also point out the potential for treating liver malignancy with such an approach. In this context, RT/IL-12 appears to be a very promising treatment protocol to be further tested in early phase clinical trials on patients with advanced HCC.
Collectively, RT/IL-12 induces strong antitumor responses in a murine model of orthotopic HCC tumor through multiple mechanisms. These mechanisms include: reducing numbers of MDSCs and their suppressive activity, enhancing antigen presentation functions of DCs and subsequently inducing activation and expansion of cytotoxic T cells, and enhancing cytotoxic activity of NK cells. The translational potential of RT/IL-12 warrants further clinical investigation given the efficacy seen in our data and the safety profile.
Materials and methods
Animals and cell lines
Female BALB/c mice (6- to 8-week-old) were purchased from the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan). All animal studies were conducted in specific pathogen-free conditions and in accordance with guidelines approved by the Animal Care and Usage Committee of Academia Sinica (Taipei, Taiwan). Murine BNL 1ME A.7R.1 (BNL; American Type Culture Collection, Manassas, VA) is a methylcholanthrene transformed hepatocellular carcinoma cell line derived from BALB/c mice. A highly liver-metastatic subline of BNL (denoted hereafter BNL-P2) was generated by in vivo passage and recovered tumors from hepatic metastases after intrasplenic inoculation of BNL cells in BALB/c mice. Cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St Louis, MO) supplemented with heat-inactivated 10% bovine fetal serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 humidified incubator.
Subcutaneous and orthotopic HCC tumor models
Exponentially growing BNL-P2 cells were harvested and used for experiments only if their viability exceeded 80%, as determined by Trypan blue staining. For the subcutaneous (s.c.) HCC model, 1 × 106 BNL-P2 cells were injected into the right flank of mice on day 0. Mice were treated when the tumor volume reached between 150 and 200 mm3 (≥ 10 mm in diameter, about 14 days post injection) with radiation (10 Gy), intratumoral injection of a single dose of adenoviral vector encoding a single-chain murine IL-12 (Ad/IL-12, 1 × 108 p.f.u.) in 30 ·l of saline,52 or a combination therapy with radiation (10 Gy) followed immediately with intratumoral Ad/IL-12 (1 × 108 p.f.u.). For radiation treatment, mice were restrained in a customized acrylic holder and protected by a 5-mm thick lead shield containing an opening of 1-cm diameter that exposed only the tumor to radiation. Tumors were exposed to a single dose of radiation of 10 Gy delivered at a mean dose rate of 6.72 Gy/min using RS 2000 biological irradiator (Rad Source Technologies, Suwanee, GA). Control mice were untreated. To study the systemic antitumor effect, mice were injected subcutaneously at day 0 with 5 × 105 CT26 cells in the right flank and 1 × 105 CT26 cells in the left flank. The right flank tumors at day 14 received the different treatment as described above, and the left flank tumor remained untreated. Tumor volumes were measured 3 times per week along three orthogonal axes (x, y, and z) and tumor volumes calculated as xyz × 0.52. Mice were monitored for 21 days.
For the orthotopic HCC model, 3 × 105 BNL-P2 cells were injected into the left lobe of the liver in mice through an incision on day 0. At day 10 and 14 post tumor transplantation, an abdominal incision was made to expose the tumor for radiation and/or Ad/IL-12 injection, with the rest of the liver and body protected by a lead shield as described above. These mice were sacrificed 21 days post treatment and tumor volumes were measured as described above.
For the s.c. colorectal carcinoma model, 5 × 105 CT26 cells were injected into the right flank of mice on day 0. Mice were treated when the tumor volume reached between 150 and 200 mm3 (≥ 10 mm in diameter, about 14 days post injection) as described above. These mice were sacrificed 21 days post treatment and tumor volumes were measured as described above.
Cytokine bead array
Serum samples were collected 3 and 7 days post-treatment. The concentrations of serum IL-12 and IFN-γ were measured using multiplex FlowCytomix kit (eBioscience) according to manufacturer’s directions. In brief, serum samples were added to the bead mixtures coated with antibodies specific to mouse cytokines and the biotin-conjugated mixture was added to the mixture complex. After incubation, the streptavidin-phycoerythrin (PE) solution was added to bind the biotin-conjugate. The standard curve was prepared from serial dilutions of a standard mixture. The concentrations of cytokines were analyzed using an LSR II cytometer (BD Pharmingen, San Jose, CA) and the data were processed using the FlowJo V10 software (Treestar, Ashland, OR).
Flow cytometric analysis
Single-cell suspensions of tumor-infiltrating leukocytes (TILs) and splenocytes were processed and collected as previously described.53 A multicolor flow cytometric analysis and the gating strategy were developed for phenotyping tumor-infiltrating immune cells as shown in Supplementary Figure. S1. For lymphoid lineages, cells were stained with efluor506 viability dye, APC-CD45, Pe-Cy7-CD3, FITC-CD4, APC-Cy7-CD8 and PE-PanNK. For myeloid lineages, cells were stained with efluor506 viability dye, Pacific blue-CD45, APC-Gr1, PE-Cy7 CD11c, PE-F4/80 and Alexa700-CD11b. For myeloid cell activation markers, cells were stained with APC-eFluor780-MHC class II, PerCP-Cy5.5-CD40 and APC-Cy7-CD86.
To analyze cytotoxicity of TILs and splenocytes, cells were stained with PE-CD107a for 5 h at 37°C and monensin (1 μM, Sigma Chem. Co., St. Louis, MO) was added during the last 4 h of incubation. Then, cells were stained with Alexa 700-CD4, eFluor 450-CD8, and FITC-PanNK for determination of CD107a expression on different lymphocyte subpopulations. These cells were then fixed and permeabalized with 4% paraformaldehyde and stained with PerCP-Cy5.5-IFN-γ and APC-TNF-α for measuring intracellular cytokines. All mAbs were purchased from either BD Pharmingen, eBioscience (San Diego, CA) or BioLegend (San Diego, CA). The stained cells were analyzed using an LSR II cytometer and data were processed using FlowJo V10 software.
Immunohistochemistry staining
Immunohistochemical analysis of tumor samples was performed as described previously.13 Frozen sections of tumor tissues (10 μm) were stained with rat antibodies against mouse CD8 (KT-15; Santa Cruz biotechnology, Dallas, TX), followed by incubation with Histofine Simple Mouse MAXPO for Rat (Nichirei Biosciences, Tokyo, Japan), and visualized with the Vectastain ABC Elite Kit (Vector Laboratories, Burlingame, CA) and AEC+ substrate-chromogen (Dako), and counterstained with hematoxylin. Positive cells were quantified using a Pannoramic 250 digital slide scanner (3DHISTECH, Budapest, Hungary) at × 200 magnification.
MDSC suppressive assay
Gr1+ MDSC cells from each group were purified 7 days post treatment from tumor tissues and splenocytes using magnetic beads (Miltenyi Biotec, San Diego, CA). Splenocytes (1 x 105 cells) from naïve BALB/c mice were stimulated with ·CD3/·CD28 Abs (splenocytes: Dynabeads = 1: 0.6) in the presence of purified Gr1+ cells from different groups at a ratio of 10:5 or 10:2.5. Three days later, 1 μCi 3H-thymidine (Amersham Pharmacia, New Territories, Hong Kong) was added to each well and cultured for additional 16 h. Cells were harvested and incorporated radioactivity was measured on a TopCount microplate scintillation and luminescence counter (PerkinElmer, Wellesley, MA).
ROS detection
The oxidation-sensitive dye 2ʹ, 7ʹ-dichlorofluorescin diacetate (DCFDA; Thermo Fisher Scientific, Waltham, MA) was used to measure ROS production by MDSCs. Purified MDSCs were incubated at 37°C in RPMI in the presence of 2.5 μM DCFDA and 30 ng/ml phorbol myristate acetate (PMA; Sigma- Aldrich) for 30 min. Cells were then labeled with anti–Gr1 and anti-CD11b antibodies on ice and analyzed by LSR II.
Statistics
Results were presented as mean ± SEM. One-way ANOVA with Tukey’s comparison was used to analyze statistical differences across multiple groups of animals. p < 0.05 was considered statistically significant.
Funding Statement
This work was supported by Academia Sinica and Ministry of Science and Technology under grants MOST103-2320-B-001-009, MOST 104-0210-01-09-02 and MOST 105-0210-01-13-01.
Acknowledgments
We thank Dr. An Chen for technical help with immunohistochemical analysis.
Disclosure statement
No potential conflicts of interest were disclosed.
Supplementary Material
Supplemental data for this article can be accessed here.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D.. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.doi: 10.3322/caac.20107 PMID:21296855 [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol 2014;11:525–535.doi: 10.1038/nrclinonc.2014.122 PMID:25091611 [DOI] [PubMed] [Google Scholar]
- 3.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2015;12:681–700.doi: 10.1038/nrgastro.2015.173 PMID:26484443 [DOI] [PubMed] [Google Scholar]
- 4.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298–306.doi: 10.1038/nrc3245 PMID:22419253 [DOI] [PubMed] [Google Scholar]
- 5.Shurin GV, Ma Y, Shurin MR. Immunosuppressive mechanisms of regulatory dendritic cells in cancer. Cancer Microenviron 2013;6:159–167.doi: 10.1007/s12307-013-0133-3 PMID:23749739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012;12:253–268.doi: 10.1038/nri3175 PMID:22437938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016;7:12150.doi: 10.1038/ncomms12150 PMID:27381735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res 2009;69:2506–2513.doi: 10.1158/0008-5472.CAN-08-4323 PMID:19244102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crispe IN.Immune tolerance in liver disease. Hepatology 2014;60:2109–2117.doi: 10.1002/hep.27254 PMID:24913836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol 2016;13:516–524.doi: 10.1038/nrclinonc.2016.30 PMID:26951040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer 2016;4:51.doi: 10.1186/s40425-016-0156-7 PMID:27660705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavallo F, Signorelli P, Giovarelli M, Musiani P, Modesti A, Brunda MJ, Colombo MP, Forni G. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J Natl Cancer Inst 1997;89:1049–1058.doi: 10.1093/jnci/89.14.1049 PMID:9230887 [DOI] [PubMed] [Google Scholar]
- 13.Lo CH, Chang CM, Tang SW, Pan WY, Fang CC, Chen Y, Wu PY, Chen KY, Ma HI, Xiao X, et al. Differential antitumor effect of interleukin-12 family cytokines on orthotopic hepatocellular carcinoma. J Gene Med 2010;12:423–434.doi: 10.1002/jgm.1452 PMID:20440753 [DOI] [PubMed] [Google Scholar]
- 14.Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J, Kulig P, Becher B. New insights into IL-12-mediated tumor suppression. Cell Death Differ 2015;22:237–246.doi: 10.1038/cdd.2014.134 PMID:25190142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teicher BA, Ara G, Menon K, Schaub RG. In vivo studies with interleukin-12 alone and in combination with monocyte colony-stimulating factor and/or fractionated radiation treatment. Int J Cancer 1996;65:80–84.doi: PMID:8543401 [DOI] [PubMed] [Google Scholar]
- 16.Seetharam S, Staba MJ, Schumm LP, Schreiber K, Schreiber H, Kufe DW, Weichselbaum RR. Enhanced eradication of local and distant tumors by genetically produced interleukin-12 and radiation. Int J Oncol 1999;15:769–773. PMID:10493960 [DOI] [PubMed] [Google Scholar]
- 17.Kim W, Seong J, Oh HJ, Koom WS, Choi KJ, Yun CO. A novel combination treatment of armed oncolytic adenovirus expressing IL-12 and GM-CSF with radiotherapy in murine hepatocarcinoma. J Radiat Res 2011;52:646–654.doi: 10.1269/jrr.10185 PMID:21952320 [DOI] [PubMed] [Google Scholar]
- 18.Lohr F, Hu K, Haroon Z, Samulski TV, Huang Q, Beaty J, Dewhirst MW, Li CY. Combination treatment of murine tumors by adenovirus-mediated local B7/IL12 immunotherapy and radiotherapy. Mol Ther 2000;2:195–203.doi: 10.1006/mthe.2000.0114 PMID:10985949 [DOI] [PubMed] [Google Scholar]
- 19.Jeanbart L, Swartz MA. Engineering opportunities in cancer immunotherapy. Proc Natl Acad Sci U S A 2015;112:14467–14472.doi: 10.1073/pnas.1508516112 PMID:26598681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waehler R, Ittrich H, Mueller L, Krupski G, Ameis D, Schnieders F. Low-dose adenoviral immunotherapy of rat hepatocellular carcinoma using single-chain interleukin-12. Hum Gene Ther 2005;16:307–317.doi: 10.1089/hum.2005.16.307 PMID:15812226 [DOI] [PubMed] [Google Scholar]
- 21.Stober D, Schirmbeck R, Reimann J. IL-12/IL-18-dependent IFN-gamma release by murine dendritic cells. J Immunol 2001;167:957–965.doi: 10.4049/jimmunol.167.2.957 PMID:11441104 [DOI] [PubMed] [Google Scholar]
- 22.Fujita T, Timme TL, Tabata K, Naruishi K, Kusaka N, Watanabe M, Abdelfattah E, Zhu JX, Ren C, Yang G, et al. Cooperative effects of adenoviral vector-mediated interleukin 12 gene therapy with radiotherapy in a preclinical model of metastatic prostate cancer. Gene Ther 2007;14:227–236.doi: 10.1038/sj.gt.3302788 PMID:17024109 [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res 2013;73:2782–2794.doi: 10.1158/0008-5472.CAN-12-3981 PMID:23418320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res 2013;23:898–914.doi: 10.1038/cr.2013.75 PMID:23752925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008;181:5791–5802.doi: 10.4049/jimmunol.181.8.5791 PMID:18832739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell Immunol 2009;254:149–154.doi: 10.1016/j.cellimm.2008.08.007 PMID:18835598 [DOI] [PubMed] [Google Scholar]
- 27.Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol 2009;90:367–386.doi: 10.1111/j.1365-2613.2009.00656.x PMID:19659896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barajas M, Mazzolini G, Genove G, Bilbao R, Narvaiza I, Schmitz V, Sangro B, Melero I, Qian C, Prieto J. Gene therapy of orthotopic hepatocellular carcinoma in rats using adenovirus coding for interleukin 12. Hepatology 2001;33:52–61.doi: 10.1053/jhep.2001.20796 PMID:11124820 [DOI] [PubMed] [Google Scholar]
- 29.Chang CJ, Chen YH, Huang KW, Cheng HW, Chan SF, Tai KF, Hwang LH. Combined GM-CSF and IL-12 gene therapy synergistically suppresses the growth of orthotopic liver tumors. Hepatology 2007;45:746–754.doi: 10.1002/hep.21560 PMID:17326190 [DOI] [PubMed] [Google Scholar]
- 30.Huang KW, Wu HL, Lin HL, Liang PC, Chen PJ, Chen SH, Lee HI, Su PY, Wu WH, Lee PH, et al. Combining antiangiogenic therapy with immunotherapy exerts better therapeutical effects on large tumors in a woodchuck hepatoma model. Proc Natl Acad Sci U S A 2010;107:14769–14774.doi: 10.1073/pnas.1009534107 PMID:20679198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I, Benito A, Larrache J, Pueyo J, Subtil JC, et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol 2004;22:1389–1397.doi: 10.1200/JCO.2004.04.059 PMID:15084613 [DOI] [PubMed] [Google Scholar]
- 32.Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol 2014;4:325.doi: 10.3389/fonc.2014.00325 PMID:25506582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohr F, Hu K, Huang Q, Zhang L, Samulski TV, Dewhirst MW, Li CY. Enhancement of radiotherapy by hyperthermia-regulated gene therapy. Int J Radiat Oncol Biol Phys 2000;48:1513–1518.doi: 10.1016/S0360-3016(00)00788-4 PMID:11121657 [DOI] [PubMed] [Google Scholar]
- 34.Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y, Lin C, Pan Z, Yu Y, Jiang M, et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 2014;59:567–579.doi: 10.1002/hep.26694 PMID:23960017 [DOI] [PubMed] [Google Scholar]
- 35.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest 2011;121:4746–4757.doi: 10.1172/jci58814 PMID:22056381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012;12:265–277.doi: 10.1038/nrc3258 PMID:22437871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med 2004;199:1607–1618.doi: 10.1084/jem.20040317 PMID:15197224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity 2013;39:611–621.doi: 10.1016/j.immuni.2013.08.025 PMID:24012420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008;135:234–243.doi: 10.1053/j.gastro.2008.03.020 PMID:18485901 [DOI] [PubMed] [Google Scholar]
- 40.Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother 2013;62:1421–1430.doi: 10.1007/s00262-013-1447-1 PMID:23764929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen HM, Ma G, Gildener-Leapman N, Eisenstein S, Coakley BA, Ozao J, Mandeli J, Divino C, Schwartz M, Sung M, et al. Myeloid-derived suppressor cells as an immune parameter in patients with concurrent sunitinib and stereotactic body radiotherapy. Clin Cancer Res 2015;21:4073–4085.doi: 10.1158/1078-0432.CCR-14-2742 PMID:25922428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol 2004;172:989–999.doi: 10.4049/jimmunol.172.2.989 PMID:14707072 [DOI] [PubMed] [Google Scholar]
- 43.Lee M, Park CS, Lee YR, Im SA, Song S, Lee CK. Resiquimod, a TLR7/8 agonist, promotes differentiation of myeloid-derived suppressor cells into macrophages and dendritic cells. Arch Pharm Res 2014;37:1234–1240.doi: 10.1007/s12272-014-0379-4 PMID:24748512 [DOI] [PubMed] [Google Scholar]
- 44.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res 2011;71:2488–2496.doi: 10.1158/0008-5472.CAN-10-2820 PMID:21300764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, Schipper MJ, Feng M. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol 2016;34:452–459.doi: 10.1200/JCO.2015.61.4925 PMID:26628466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 2015;15:409–425.doi: 10.1038/nrc3958 PMID:26105538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen SW, Lin LC, Kuo YC, Liang JA, Kuo CC, Chiou JF. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2014;88:1041– 1047.doi: 10.1016/j.ijrobp.2014.01.017 PMID:24661657 [DOI] [PubMed] [Google Scholar]
- 48.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–931.doi: 10.1056/NEJMoa1112824 PMID:22397654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, Swetter SM, Knox SJ. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Trans Oncol 2012;5:404–407.doi: 10.1593/tlo.12280 PMID:23323154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013;1:365–372.doi: 10.1158/2326-6066.CIR-13-0115 PMID:24563870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013;2:899–906.doi: 10.1002/cam4.140 PMID:24403263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan WY, Lo CH, Chen CC, Wu PY, Roffler SR, Shyue SK, Tao MH. Cancer immunotherapy using a membrane-bound interleukin-12 with B7-1 transmembrane and cytoplasmic domains. Mol Ther 2012;20:927–937.doi: 10.1038/mt.2012.10 PMID:22334018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang CM, Lo CH, Shih YM, Chen Y, Wu PY, Tsuneyama K, Roffler SR, Tao MH. Treatment of hepatocellular carcinoma with adeno-associated virus encoding interleukin-15 superagonist. Human Gene Ther 2010;21:611–621.doi: 10.1089/hum.2009.187 PMID:20064014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


