ABSTRACT
Surgical resection of the primary tumor provides the best chance of cure for patients with colorectal carcinoma (CRC). However, bacterial translocation during intestinal surgery has been correlated with poor long-term oncological outcome. Therefore, we investigated the influence of bacterial contamination during colon surgery on CRC liver metastases development. Blood and liver samples of patients undergoing resection of primary CRC or liver metastases were collected. Cell numbers, activation markers and inflammatory mediators were determined. Tumor cell adhesion and outgrowth after sham- or colectomy operations were determined in a rat model, in which tumor cells had been injected into the portal vein.
White blood cells and granulocytes were increased in per- and post-operative patient blood samples. IL-6 was also increased post-operatively compared to the preoperative level. Expression of NOX-2, NOX-4 and polymorphonuclear cells (PMNs) numbers were elevated in post-operative human liver samples. In vitro stimulation of macrophages with plasma of rats after colectomy resulted in production of reactive oxygen species (ROS). Colectomy in rats increased D-lactate levels in plasma, supporting bacterial translocation. Decreased expression of tight junction molecules and increased tumor cell adhesion and outgrowth was observed. Treatment with a selective decontamination of the digestive tract (SDD) cocktail decreased tumor cell adherence after colectomy.
In conclusion, postoperative bacterial translocation may activate liver macrophages and PMNs, resulting in ROS production. As we previously showed that ROS release led to liver vasculature damage, circulating tumor cells may adhere to exposed extracellular matrix and grow out into liver metastases. This knowledge is pivotal for development of therapeutic strategies to prevent surgery-induced liver metastases development.
keywords: colorectal tumor surgery, bacterial translocation, macrophages, liver metastases, reactive oxygen species
Introduction
Tumors of the colon and rectum (colorectal carcinoma; CRC) are the most frequent tumors of the digestive tract, and continues to be one of the leading causes of cancer-related death globally. Annually ~1.4 million cases of CRC are diagnosed worldwide, causing the death of around 700.000 patients.1 Surgical resection of the primary tumor is the cornerstone of treatment. Unfortunately, patients frequently develop distant metastases within a short period after surgery, which is accompanied by high morbidity and mortality.2 The liver is the main target organ for metastases development. Synchronous liver metastases are found in ~20% of patients with CRC at the time of surgical resection of the primary tumor.2 Additionally, ~10-25% of the patients who do not have evidence of metastatic disease at the time of resection and as such are eligible for curative surgery, will nonetheless develop colorectal liver metastases within five years.3, 4
Paradoxically, a growing body of evidence suggests that surgical trauma may promote the risk of metastases development.5-7 There is no consensus yet on whether laparoscopic colectomy is superior to open approach with respect to oncological outcome of cancer patients, and conflicting data has been described. In one study it was reported that patients undergoing conventional open colectomy for removal of the colorectal tumor had poor long-term cancer related survival compared to patients undergoing laparoscopy-assisted surgery.8 Two other studies confirmed the oncological safety of laparoscopic surgery for colorectal cancer, but did not show differences in long term patient outcome.9, 10 A recent study in which laparoscopic versus open surgery for rectal cancer was compared, showed similar overall rates of locoregional recurrence, disease-free and overall survival.11 However, a small benefit in disease-free survival after laparoscopy of patients with stage III disease was suggested. Further studies are required to establish if reducing surgical trauma during cancer surgery is correlated with improved survival.
A correlation between surgical trauma and tumor outgrowth was however strongly supported by multiple experimental studies, as it was demonstrated in animal models that surgery increased both locoregional and distant tumor development.12-16 We previously demonstrated in a rat model that laparotomy led to activation of macrophages in the liver (also referred to as Kupffer cells; KCs), which subsequently produced ROS.17 The major source of ROS in immune cells is a nicotinamide adenine dinucleotide phosphate oxidase (NOX) enzyme complex, which is composed of multiple membrane associated and cytosolic components.18 After exposure to bacterial products such as lipopolysaccharide (LPS), macrophages as well as PMNs get activated and generate ROS via NOX enzymes. ROS production had a destructive effect on the integrity of the liver vasculature, resulting in exposure of sub-cellular extracellular matrix (ECM) components to which circulating tumor cells adhered in an experimental rat model.16
Importantly, circulating tumor cells are present in peripheral blood of patients with CRC prior to surgery.19-21 Moreover, it was shown that the number of circulating tumor cells increased during, or shortly after resection in both peripheral and portal blood, which suggest that manipulation of the primary CRC may lead to dissemination of tumor cells.21-23 Post-operative detection of circulating tumor cells was associated with tumor recurrence and poor long-term survival of patients.24-29 Thus, surgery-induced alterations in the liver vasculature in patients may contribute to the development of liver metastases by allowing increased adhesion of circulating tumor cells.
Additionally, several studies linked per-operative bowel perforation or anastomotic leakage after resection of CRC with increased tumor recurrence and higher cancer-specific mortality.30-38 Since the colon contains the largest bacterial load in the body, surgical resection may lead to bacterial translocation into the abdominal cavity or blood circulation. Endotoxin, which is an important component of the outer membrane of Gram-negative bacteria, was detected in post-operative plasma of patients.39 Elevation of endotoxin levels in blood was accompanied by intestinal permeability, which suggested that the epithelial barrier was impaired after surgery.39, 40 Since the liver is the first organ receiving blood from the intestines, bacterial components may activate KCs and PMNs and induce ROS production. Patients with positive bacterial translocation after surgery had significantly shorter disease-free survival,41 supporting the negative impact of bacterial contamination after surgery on oncological outcome. However, the exact role of bacterial translocation in liver metastases development is unknown.
We therefore investigated the effects of surgical resection of CRC in blood or plasma samples of patients on immune cells counts and the levels of inflammatory markers. Additionally, we determined the effect of colectomy on the expression of NOX enzymes in pre- and post-operative liver samples. To investigate bacterial translocation during colon resection and the effects on tumor cell adherence and liver metastases outgrowth, we established an experimental colectomy model in which a part of the colon was removed surgically with or without additional selective decontamination of the digestive tract (SDD). Our study demonstrates that colon resection-induced bacterial translocation may play a pivotal role in development of liver metastases. This support that prevention of bacterial translocation during surgery may improve patient long term survival.
Results
Surgical resection of colorectal tumors induces changes in white blood cell (WBC) composition and release of inflammatory mediators
To investigate the impact of CRC surgery on the numbers and activation of circulating immune cells as well as on release of inflammatory markers in plasma we collected peripheral blood samples from patients who underwent surgical resection of primary colorectal carcinoma. In total 9 patients were included for this study (see supplemental table 1 for patient characteristics). Six out of nine patients were operated with minimal invasive surgery. Due to the small patient numbers we were unable to address potential differences between laparotomy versus minimal invasive surgery. Increased numbers of WBCs were found in per- or post-operative blood samples of 8 out of 9 patients compared to pre-operative blood samples (Table 1, and Figure 1A). Microscopic analyses of blood smear samples demonstrated that the percentages of lymphocytes and monocytes were significantly decreased in post-operative blood samples of the majority of patients, but no differences were observed in absolute numbers. Increased granulocyte numbers were present in per- or post-operative blood samples. In comparison to the pre-operative blood samples, expression of MHCII on monocytes was decreased post-operatively in 6 patients.
Table 1.
cell numbers and expression of MHCII by monocyte cells; as well as levels of IL-6, TNFα and CRP in plasma samples of patients. N.D.: not determined. Two tailed Wilcoxon signed-rank tests were used to test the differences in patients’ blood or plasma samples. P values <0.05 (indicated in bold) were considered significantly different.
| Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | Pt 6 | Pt 7 | Pt 8 | Pt 9 | pre-per | pre-post | per-post | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (x106 cell/ml) | ||||||||||||
| pre- | 7 | 5.2 | 6.6 | 5.4 | 3.6 | 5.7 | 7.2 | 6.5 | 3.2 | p = 0.025 | p = 0.033 | p = 0.67 |
| per- | 19.3 | 8.3 | ND | 4.9 | 10.4 | 9.9 | 8.8 | 6.7 | 5.2 | |||
| post- | 23.7 | 60 | 12.8 | 21.6 | 6.5 | 9.6 | 7.3 | 6.4 | 2.3 | |||
| Lymphocytes (x106 cell/ml) (%) | ||||||||||||
| pre- | ND | ND | 1.78 (27) | 1.62 (30) | 0.72 (20) | 0.63 (11) | 0.29 (4) | 1.37 (21) | 0.22 (7) | p = 0.25 | p = 0.18 | p = 0.46 |
| per- | ND | ND | ND | 1.03 (21) | 0.73 (7) | 0.60 (6) | 0.26 (3) | 0.94 (14) | 0.57 (11) | |||
| post- | ND | ND | 1.79 (14) | 1.94 (9) | 0.52 (8) | 0.10 (1) | 0.22 (3) | 0.96 (15) | 0.21 (9) | |||
| Monocytes (x106 cell/ml) (%) | ||||||||||||
| pre- | ND | ND | 0.66 (10) | 0.65 (12) | 0.29 (8) | 0.74 (13) | 1.01 (14) | 1.76 (27) | 0.67 (21) | p = 0.75 | p = 1.00 | p = 0.46 |
| per- | ND | ND | ND | 0.64 (13) | 0.94 (9) | 0.50 (5) | 1.06 (12) | 1.47 (22) | 0.94 (18) | |||
| post- | ND | ND | 0.90 (7) | 1.94 (9) | 0.65 (10) | 0.58 (6) | 0.73 (10) | 1.34 (21) | 0.37 (16) | |||
| Granulocytes (x106 cell/ml) (%) | ||||||||||||
| pre- | ND | ND | 4.16 (63) | 3.13 (58) | 2.60 (72) | 4.33 (76) | 5.90 (82) | 3.45 (53) | 2.30 (72) | p = 0.028 | p = 0.043 | p = 0.46 |
| per- | ND | ND | ND | 3.19 (65) | 8.74 (84) | 8.81 (89) | 7.66 (87) | 4.29 (64) | 3.69 (71) | |||
| post- | ND | ND | 10.11 (79) | 17.7 (82) | 5.33 (82) | 8.93 (93) | 6.35 (87) | 4.10 (64) | 0.37 (75) | |||
| MHCII (geomean) | ||||||||||||
| pre- | 222 | 1010 | 837 | 7 | 444 | 21 | 179 | 357 | 278 | p = 0.092 | p = 0.066 | p = 0.25 |
| per- | 139 | 845 | ND | 9 | 219 | 7 | 165 | 420 | 232 | |||
| post- | 153 | 537 | 612 | 9 | 211 | 7 | 181 | 390 | 201 | |||
| IL-6 (pg/ml) | ||||||||||||
| pre- | 0 | 0 | 0 | 0 | 5.9 | 9.2 | 0 | 0 | 0 | p = 0.11 | p = 0.012 | p = 0.018 |
| per- | 0 | 14.4 | ND | 0 | 116.8 | 13.9 | 0 | 0 | 0 | |||
| post- | 40.8 | 187.2 | 22.6 | 57.9 | 351.4 | 105.8 | 4.6 | 0 | 4.6 | |||
| TNFα (pg/ml) | ||||||||||||
| pre- | 0 | 3.91 | 38.79 | 0.93 | 4.9 | 23.23 | 3.66 | 4.4 | 12.98 | p = 0.61 | p = 0.050 | p = 0.091 |
| per- | 0 | 7.49 | ND | 0.53 | 7.49 | 16.7 | 5.15 | 2.71 | 7.75 | |||
| post- | 0 | 3.66 | 32.47 | 0.02 | 4.15 | 17.87 | 4.65 | 2.02 | 6.44 | |||
| CRP (μg/ml) | ||||||||||||
| pre- | >5.50 | 0.28 | >5.50 | 0.23 | 0.61 | 4.00 | 1.56 | 0.29 | 0.21 | p = 1.00 | p = 0.89 | p = 0.89 |
| per- | 2.36 | 0.22 | ND | 0.32 | 0.73 | 2.27 | 1.78 | 0.27 | 0.45 | |||
| post- | 2.32 | 0.39 | >5.50 | 0.20 | 0.124 | 4.23 | 1.59 | 0.74 | 0.24 |
Figure 1.
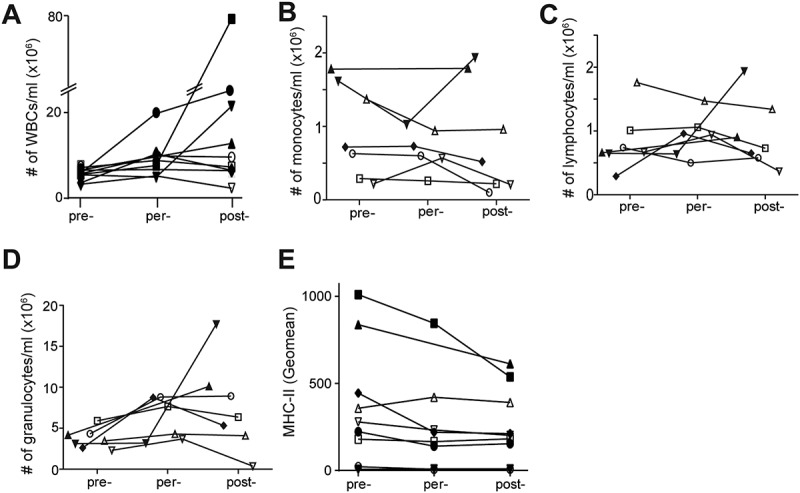
Rapid induction of inflammatory response during surgical resection of CRC. A: WBCs counts (pre-per p = 0.025, pre-post p = 0.033). B: absolute numbers of granulocytes. C: absolute numbers of monocytes. D: absolute numbers of lymphocytes (pre-per p = 0.028, pre-post p = 0.043). E: Expression of MHCII by CD14+ monocytes.
We also investigated the effects of resection of colorectal carcinoma on the levels of inflammatory mediators in plasma samples. The level of IL-6 was increased in post-operative plasma samples of 8 out of 9 patients compared to pre- or per-operative samples (Figure 2A). In contrast, the level of TNFα was decreased post-operatively in plasma samples of 5 patients compared to pre-operative samples (Figure 2B). The level of C-reactive protein (CRP) in plasma samples was not affected during surgery (Figure 2C).
Figure 2.
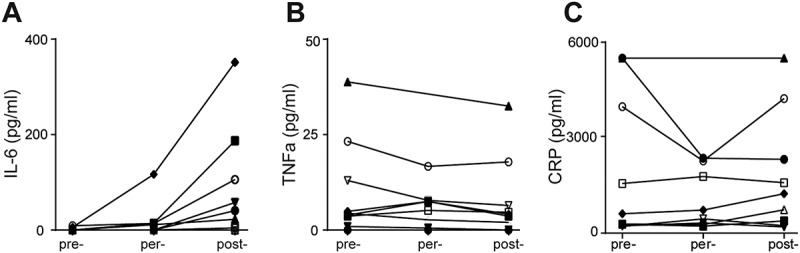
Surgical procedure stimulate rapid alterations of inflammatory markers levels during surgical resection of CRC. A-C: Levels of IL-6 (A) (pre-post p = 0.012, per-post p = 0.018), TNFα (B) and CRP (C) in pre-, per- and post-operative plasma samples.
Surgery enhances the expression of ROS producing enzymes in the liver
To investigate the effects of surgery on the liver we collected liver biopsies from patients undergoing surgical resection of CRC liver metastases. Four patients were included. Liver biopsies were taken at the start and end of the operation. Since our previous experiments in rats indicated that surgery led to production of ROS in the liver,17 we analyzed the expression of ROS producing NOX enzymes in patient liver samples. Immunohistochemical analysis demonstrated that the total area of NOX-2 positivity was increased in post-operative samples (Figure 3A). Furthermore, we observed enhanced expression of NOX-4 in post- operative liver samples, which was due to higher numbers of NOX-4 positive cells per field of view (Figure 3B). Double staining in liver samples showed that NOX-2 expression was restricted to CD68-positive cells in the liver, which were likely KCs based on their cellular shape and characteristic distribution around the portal triad (Figure 3C). The number of CD68 positive cells in per- and post-operative liver samples was unaffected (Supplemental Figure 1). The larger area of NOX-2 expression supported that the expression of NOX-2 per cell was increased in post-operative liver samples (Figure 3A). NOX-4+ cells were PMNs as evidenced by the expression of granulocytes activation marker CD66b (Figure 3D).
Figure 3.
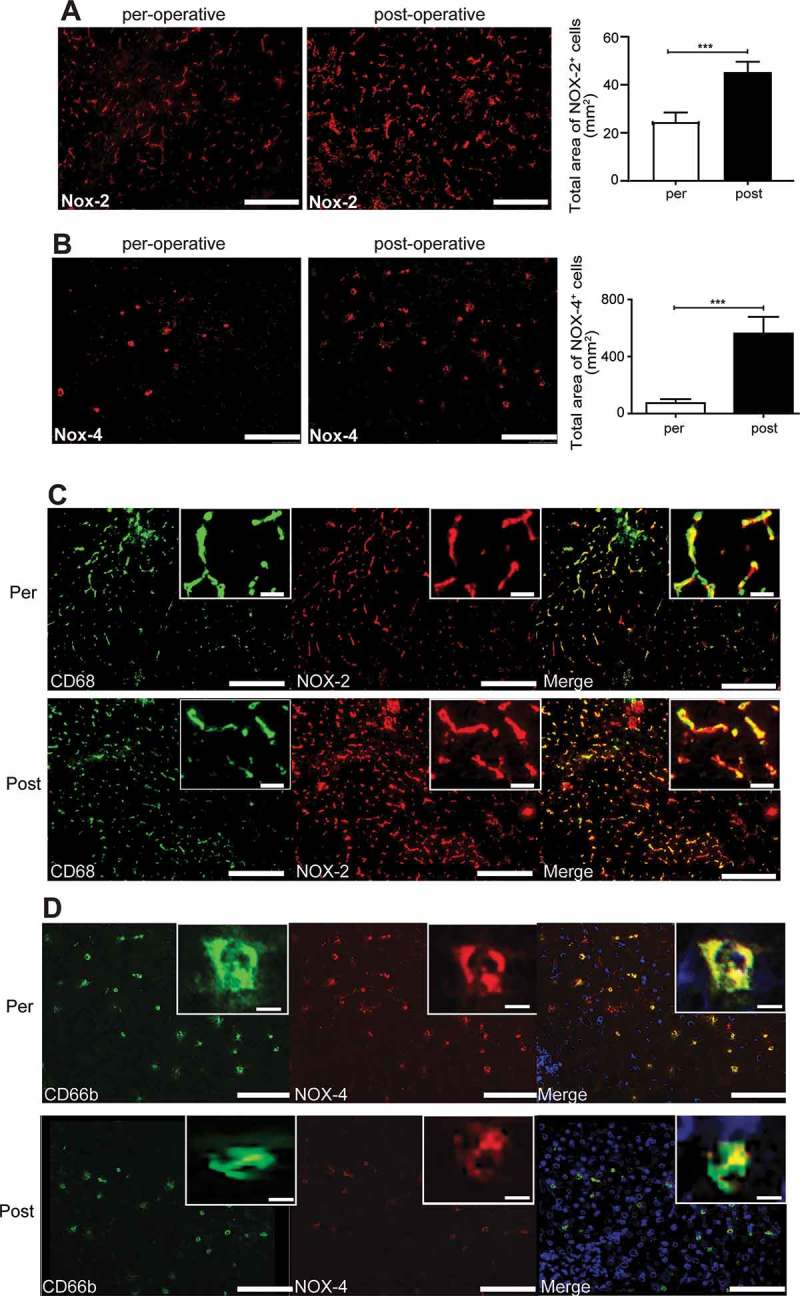
Surgery enhances the expression of ROS producing enzymes in liver samples of patients. A: representative images and quantification of NOX-2 expression in per- or post-operative liver samples of patients. (n = 4) B: representative images and quantification of NOX-4 expression in per- or post-operative liver samples of patients, fov = field of view, (n = 4). C: representative images of CD68 and NOX2 expression in per- (upper panels) or post-operative (lower panels) liver samples. Green: CD68, Red: NOX-2, blue: cell nuclei, scale bar is 100 μm. D: representative images of CD66b and NOX4 expression in per- (upper panels) or post-operative (lower panels) liver samples. Green: CD66b, Red: NOX-4, blue: cell nuclei, scale bar is 100 μm. *p<0.05, ***p<0.001.
Colectomy augmented tumor cell adhesion in the liver and metastases outgrowth in rats
To determine the effects of surgery on tumor cell adherence and tumor development in the liver we established an experimental model, in which rats underwent partial resection of the colon (partial colectomy). We investigated whether colectomy influenced tumor cell adherence in the liver compared to laparotomy (sham operation). A group that received anesthesia only served as control group. To avoid intra-operative contamination (or other differences) due to a longer operative time, al procedures (anesthesia only, laparotomy, or colectomy) lasted 45 minutes. We observed an increase in tumor cell adhesion after laparotomy, which was further augmented after a partial colectomy (Figure 4A and B). Laparotomy increased the development of liver metastases, compared to controls rats (Figure 4C). However, the highest numbers of tumor nodules were found in the group of rats that underwent partial resection of the colon, compared to rats from control or laparotomy group. No metastases were visible in other organs at the time of sacrifice in all groups.
Figure 4.
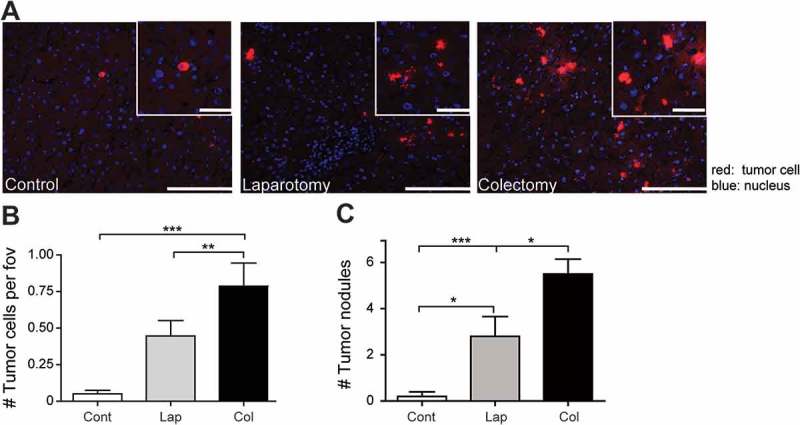
Colorectal cancer surgery augments tumor cell adhesion. A: Representative images of tumor cell numbers in the livers of control rats, after laparotomy or partial colectomy. (n = 6 per group). Red: DiI-labeled CC531s cells, blue: cell nuclei. Scale bar is 100 μm. Quantification of tumor cells (B) or tumor nodules (C) in the livers of rats from control, laparotomy or colectomy groups. (n = 12/group). *p<0.05, **p<0.01, ***p<0.001. Cont: control, Lap: laparotomy, Col: colectomy.
To investigate whether colectomy may result in bacterial contamination, we determined bacterial outgrowth from smear samples that were collected during partial colectomy in rats. Smear samples were taken from the exterior of the colon wall at the beginning of the operation (pre-operative), during the operation (per-operative) and after the colon segment had been removed and an anastomosis was made (post-operative). Smear samples were plated and bacterial outgrowth was allowed overnight. No bacterial colonies were seen after plating pre-operative smears (Figure 5A and B). However, we observed a significantly increased bacterial outgrowth from per-operative smear samples that were taken after the first incision in the colon was made. Furthermore, the highest numbers of bacterial colonies were observed when smear samples were taken of the suture after closure of the anastomosis. This indicated that a partial colectomy led to bacterial contamination of the peritoneal cavity. To investigate whether bacterial translocation resulted in presence of bacterial products in peripheral blood we measured the levels of D-lactate, which is a product of bacterial fermentation in the gastrointestinal tract,42 in plasma samples of animals that had been taken 2 hours post-operatively. The level of D-lactate was significantly enhanced in plasma samples of animals that underwent partial colon resection, compared to the plasma samples of rats from control rats or laparotomy group (Figure 5C), supporting systemic contamination. Furthermore, incubation of bone marrow-derived macrophages with plasma samples from rats that had been collected after colon resection induced higher production of ROS, compared to plasma samples of rats from control or laparotomy groups (Figure 5D). The IS-pro results of fecal samples demonstrated that all rats had a highly individualized and diverse microbiota composition with a diverse array of Bacteroidetes, Firmicutes and Proteobacteria (Figure 5E).
Figure 5.
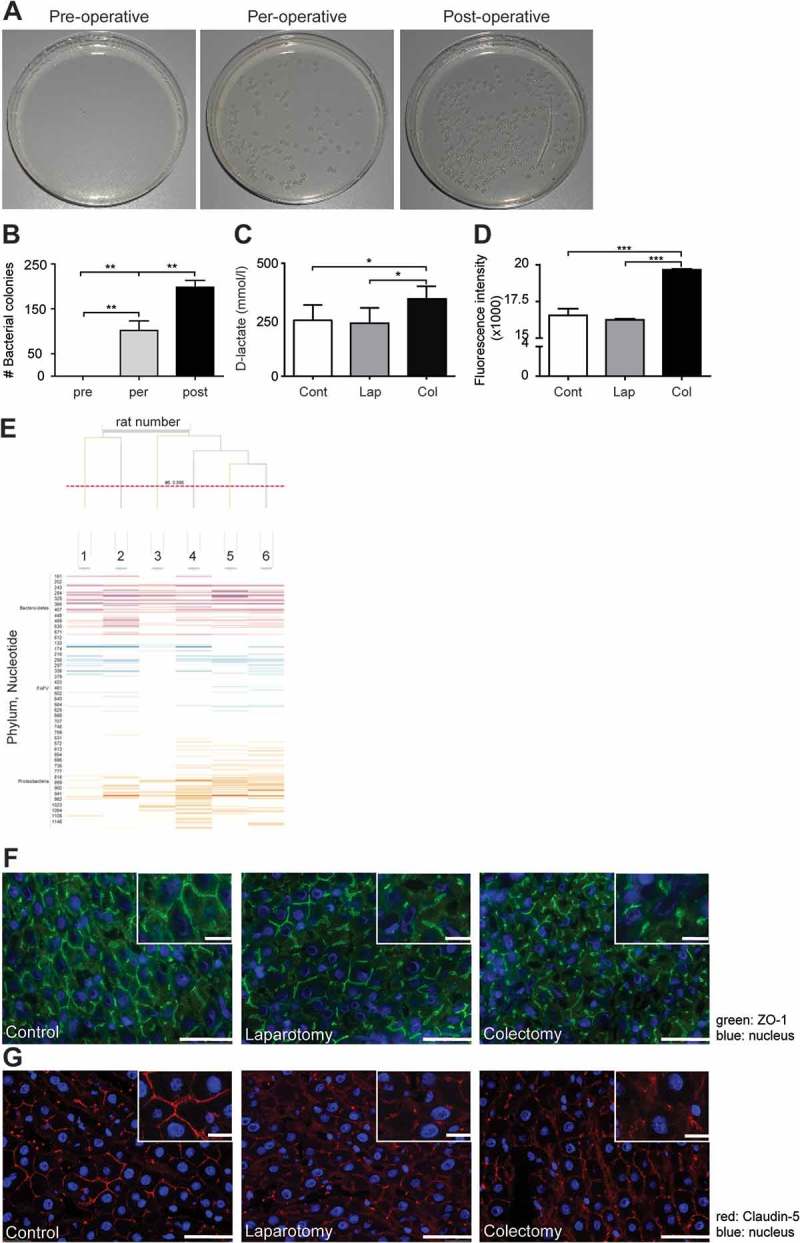
Partial colon resection facilitates bacterial translocation into the peritoneal cavity. A. Representative images of bacterial culture plates from smear samples that were taken from the exterior of the colon wall at begin of surgery (pre-operative), during the operation (per-operative) and after the colon segment had been removed and an anastomosis was made (post-operative). B: Numbers of bacterial colonies from swaps that were taken from the exterior of the colon wall pre-, per- or post-operatively. (n = 6 per group). C: Levels of D-lactate in peripheral blood samples of rats from the control, laparotomy or colectomy group, which had been sacrificed 2 hours post-operatively. D: Intracellular ROS levels in bone marrow-derived macrophages, which had been incubated with plasma samples of rats from control, laparotomy or colectomy group that had been sacrificed 2 hours post-operatively. **p<0.05, **p<0.01, ***p<0.001. Expression of tight junction proteins (E) Characterization of the microbiota composition of rat feces (F) ZO-1 (green) or (G) Claudin-5 (red) in the livers of rats from control, laparotomy or colectomy group. Scale bar is 100 μm. Blue: cell nuclei. Cont: control, Lap: laparotomy, Col: colectomy.
Since abdominal surgery affected the vascular integrity of the liver and facilitated distant adherence of tumor cells17 we next investigated the effect of colon resection on the vascular lining of the liver. We found decreased expression of the tight junction molecules ZO-1 and Claudin-5 in liver samples of animals from either laparotomy or colectomy group, indicating loss of cell-cell contact (Figure 5F and G).
Immunohistological staining of rat liver samples demonstrated that the numbers of ED-1+ monocytes/macrophages in control, laparotomy or colectomy groups (Figure 6A) were comparable, indicating that surgery had no effect on accumulation of monocytes. However, we observed a significantly increased numbers of PMNs in the liver postoperatively, especially in rats that underwent a partial colectomy (Figure 6B). This supports that colectomy leads to sequestration of PMNs in the liver and is comparable to our observations in the post-operative liver samples of patients as shown in Figure 3B.
Figure 6.
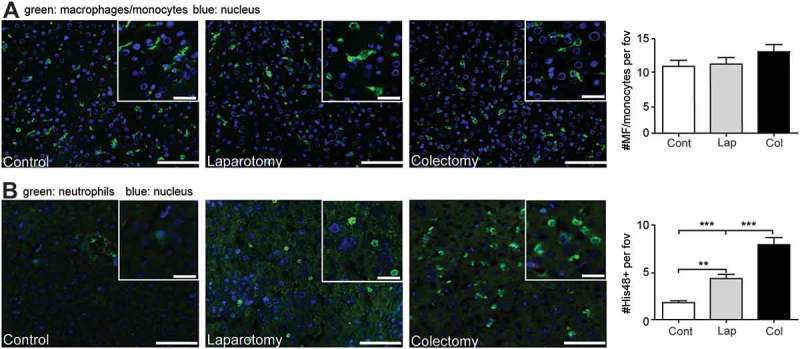
partial colon resection results in accumulation of granulocytes in the liver. A: representative images and quantification of the amount of ED1+ (monocytes/macrophages) cells in liver samples from the control rats or rats that underwent either laparotomy or colectomy. Green: ED1+ cells, blue: cell nuclei. B: representative images and numbers of granulocytes in liver samples of rats after anesthesia, laparotomy or colectomy. Green: His48+ cells, blue: cell nuclei. Scale bar is 100 μm. **p<0.01, ***p<0.001. Cont: control, Lap: laparotomy, Col: colectomy.
A SDD regime reduced tumor cell adhesion in the liver
To further investigate the influence of bacterial contamination after colectomy on tumor cell adherence, rats were treated with a selective decontamination of the digestive tract (SDD) regime. Pre-operative treatment with this antibiotic cocktail significantly decreased the adherence of tumor cells in the liver of rats undergoing colectomy compared to rats, which had not been treated with antibiotics (colectomy+SDD vs colectomy–SDD) (Figure 7). The SDD regime had no influence on tumor cell adherence in rats receiving anesthesia only, or on rats that underwent the sham operation, supporting that bacterial spillage during colon resection facilitates tumor cell adhesion in the liver.
Figure 7.
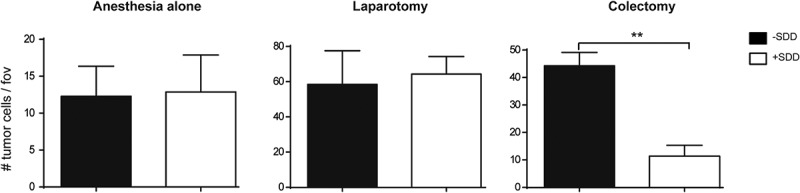
Numbers of tumor cells per field of view (fov) in the liver of rats that were treated without (black bars) or with SDD (white bars) regime and underwent anesthesia alone, laparotomy or colectomy. ** p < 0.01.
Discussion
Resection of primary CRC is a necessary procedure, and provides the best chance of cure for patients. Unfortunately, in a sub-population of patients, who do not have evidence of metastatic disease at the time of resection, liver metastases will develop post-operatively.3, 4 This supports that these patients had either undetectable micro-metastases or circulating tumor cells, which can grow out into distant metastases after successful removal of the primary tumor. It has been suggested that surgery increase the risk of liver metastases development.15, 16, 43
In response to surgery-induced tissue injury immune cells like monocytes and PMNs are activated. In the current study we observed an increase in WBCs and granulocytes cell counts in per- and/or post-operative blood samples. We also found enhanced levels of IL-6 in post-operative plasma samples. A trend towards lower MHC class II expression on monocytes (p = 0.066) was observed. Previous studies also demonstrated a decrease in MHCII expression after surgery.44, 45 Furthermore, an increase in levels of IL-6, CRP and D-lactate was reported.44–50 In all of these studies patient blood samples were collected several hours to days after closure of the abdominal cavity. In our study we collected blood samples from the induction of anesthesia to immediately after closure of the abdominal cavity by suture. As reflected by our data, the observed alterations in blood cell counts as well as inflammatory markers are already measurable during the procedure. In the current study the levels of CRP were unaltered. This may be due to delayed release of CRP after post-operative day 1, as demonstrated by previous studies.45-50 The small number of patients is a limiting factor of the study, and it would be interesting to investigate more patients and/or time points after surgery.
In our experimental model we observed that colon resection caused bacterial translocation into the abdominal cavity. Additionally, the levels of D-lactate were significantly elevated in plasma samples of rats after colectomy, which suggested that bacterial components were diffused into the blood circulation. Furthermore, the numbers of adhered tumor cells as well subsequent tumor outgrowth in the liver after colon resection was significantly increased. It has been demonstrated that injection of endotoxin in an experimental model enhanced colon carcinoma cell adhesion in the liver.51, 52 Importantly, a recent study demonstrated that bowel mobilization already led to bacterial translocation in patients.40 Furthermore, patients with anastomotic leakage or who encountered bacterial translocation after a colectomy had poor disease-free survival,41 indicating that bacterial contamination had a negative impact on long-term patient outcome. Even though this simplified surgical rat model does not completely reflect the human situation, as multiple peri- and post-operative factors (e.g. complete mesocolic excision)53 may influence patient outcome, collectively our data support that bacterial spillage during CRC resection may contribute to liver metastases outgrowth. Fecal sample analysis demonstrated that all rats had a highly individualized microbiota composition with a highly diverse array of Bacteroidetes, Firmicutes and Proteobacteria. Due to this highly individualized microbiota, it was not possible to pinpoint individual bacterial species that we would expect to translocate to the bloodstream after surgery. However, on a higher taxonomic level, it could be seen that the rat microbiota was dominated by the phyla Bacteroidetes, Firmicutes and Proteobacteria. Microbial products are potent activators of macrophages by robust stimulation of ROS production and release.18 Because KCs are in vivo in close contact with sinusoidal endothelial cells, activation of KCs by bacterial components may lead to production and release of ROS and damage the liver vascular integrity. We previously demonstrated that incubation of macrophage-endothelial co-culture with bacterial endotoxin led to damage of endothelial monolayer, which was prevented by addition of ROS scavenger.51 Damaged endothelial monolayers exposed the underlying extracellular matrix (ECM) and enabled adhesion of tumor cells to the exposed ECM.17 In the current study we demonstrated that the expression of the ROS producing enzymes NOX-2 and NOX-4 was increased in post-surgical liver samples of patients. Expression of NOX-2 or NOX-4 was restricted to KCs or PMNs, respectively, supporting that surgery results to ROS production in the liver of patients. Furthermore, we previously demonstrated decreased expression of tight junction molecule Claudin-5 in post-surgical liver samples17 indicating that ROS production may lead to liver damage. Importantly, tumor cells can adhere to exposed ECM, indicating that damaged vascular lining may facilitate tumor cell adhesion.
Experimental therapy using an anti-oxidant to prevent metastases formation proved unsuccessful17 indicating that ROS production may lead to liver damage. This was likely due to interference with tumor cell killing by macrophages, which is a ROS-dependent process. A more successful approach may therefore be the prevention of immune cell activation by inflammatory mediators and/or bacterial components. Recently, in a mouse model LPS was shown to stimulate metastases outgrowth in the liver.44 Therefore, blockade of the interaction between LPS and its receptor TLR4 on immune cells by either LPS scavengers or TLR4 antagonists may be used to prevent tumor development. Alternatively, it has been demonstrated that SDD with antibiotics prior to resection of colorectal carcinoma reduced post-surgical infectious complications and anastomotic leakage.54 We observed that treatment of rats with a SDD regime prior to colectomy resulted in diminished adherence of tumor cells in the liver, compared to untreated animals. Taken together, it is likely that the bacterial load as well as blood endotoxin concentrations are reduced after SDD, which may lead to a decreased risk of developing liver metastases and improved oncological outcome for patients. Designing optimal pre- or per-operative therapeutic strategies will ultimately greatly improve patient outcome.
Materials and Methods
All patients gave informed consent according to the guidelines of the medical ethics committee (METC) of the VUmc. Peripheral blood samples (10 ml) were collected pre-, per- and post-operatively in Heparin Vacutainer (BD Falcon, Bedford, MA) during surgical removal of colorectal tumor (METC 2011/101, see supplemental table 1A for patient characteristics). Pre-operative samples were taken directly after induction of anesthesia. Per-operative samples were taken 1 hour after the first incision and post-operative samples were obtained immediately after closure of the abdominal cavity by suture.
Additionally, liver samples from patients undergoing resection of liver metastases from colorectal cancer (METC 2011/100) were taken at the beginning and end of the of resection of colorectal liver metastases (per- and post-operative) and snap frozen in liquid nitrogen for microscopic analysis.
Patient blood samples
The numbers of white blood cells (WBCs) were determined with tryphan blue exclusion. The Diff-Quick Staining Kit (Cruinn Diagnostics Limited, Dublin, Ireland) was used to differentiate between granulocytes, monocytes and lymphocytes. The numbers of cells were determined microscopically.
Blood samples were incubated with fluorescently labeled antibodies against CD14 (Biolegend, San Diego, CA), CD15 (Biolegend) or HLA-DR (Biolegend) for 30 minutes on ice. Red blood cells were lysed by using lysis buffer (BD Falcon) and samples were centrifuged. After washing with PBS which was supplemented with 0.5% BSA samples were measured by flow cytometry with a Cyan ADP High Performance Research Flow Cytometer (Beckman Coulter Inc., Brea, CA). Data analysis was performed with the software FlowJo10 (FlowJo, LLC, Ashland, OR).
Alternatively, blood samples were centrifuged for 7 minutes at 1300 rpm and after separation of cells, plasma was centrifuged for 5 minutes at 2500 rpm. Samples were aliquoted and stored at -80°C for further analysis.
The levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNFα and C-reactive protein (CRP) were determined in plasma samples with a human IL-6 ELISA kit (Sanquin, Amsterdam, the Netherlands), human TNFα ELISA kit (Sanquin) or CRP instant ELISA kit (eBioscience, Vienna, Austria), according to the instructions of the manufacturers.
Tumor cell culture
The CC531s tumor cell line is a moderately differentiated colonic adenocarcinoma, which is transplantable in Wag/Rij rats.7, 16 CC531s tumor cells were cultured under standard incubator conditions in DMEM (Gibco, Irvine, UK) supplemented with 10% heat-inactivated fetal calf serum (FCS, Gibco), 100 U/ml penicillin, 100m μg/ml streptomycin and 200mM L-glutamine (hereafter referred to as complete DMEM). Cell suspensions were prepared by enzymatic detachment with trypsin-EDTA solution, and contained both single tumor cells and small cell clusters (2-8 cells). Viability was assessed by a tryphan blue exclusion staining and was always >95%. CC531s tumor cells were fluorescently labeled for short-term experiments, by incubation in complete DMEM containing 5 μg/ml 1,1’dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiI, Sigma-Aldrich, St Louis, MO) for 30 minutes at 37ºC. Cells were subsequently washed with HBSS, according to the manufacturer’s instructions, and suspended in HBSS for further use in experiments
Animals
Male inbred Wag/Rij rats (200-220g, Charles River, Maastricht, the Netherlands) were kept under standard laboratory conditions and had access to food and water ad libitum. The Committee for Animal Research of the VUmc approved all experiments, according to institutional and national guidelines.
Animal models
In order to study the adherence of tumor cells and outgrowth of liver metastases in the absence of trauma, or presence of surgical trauma and/or bacterial products, portal veins of rats were first catheterized as described previously.16 Briefly, rats received a midline incision under isoflurane anesthesia, after which the portal vein was exposed. A 2-french silicon catheter was secured after insertion in the portal vein with a purse-string suture. The catheter was passed through the muscle wall and tunneled subcutaneously towards the subcutaneous pocket between the scapulae of the animal, where an attached mini vascular access port was positioned. The catheter was flushed every other day with glycerol containing heparin (50 IU/ml) to prevent clotting of the catheter.
Animals were allowed a 14-day recovery period, after which they were divided into 3 groups. Group 1 was a control group, which received isoflurane anesthesia only for 45 minutes (= maximum duration of surgery in other groups). Rats in group 2 underwent a sham operation, which was defined as midline laparotomy (laparotomy group). Briefly, the intestines were transferred out of the abdominal cavity, covered with moist gauze for 45 minutes, after which intestines were replaced in the abdominal cavity and the wound sutured. In group 3, rats underwent a partial colectomy with an end-to-end anastomosis, in which a segment of the colon was removed and both ends sutured (colectomy group). This procedure lasted 45 minutes in total. Smear samples from the colon wall were taken with a cotton swab at the beginning of the operation (pre-operative), during the operation (per-operative) and after the partial resection at the anastomosis site (post-operative). Tryptone (10gr/L), yeast extract (5gr/L) and agar (15gr/L) was dissolved in 1L distilled water, autoclaved, cooled and subsequently poured into culture plates. Smear samples were plated and cultured for 24 hours under aerobic conditions in an incubator at 37ºC, after which bacterial colonies were determined. Furthermore, fecal samples obtained at the site of the resection and anastomosis were analysed with IS-PRO. The IS-pPRO technique is a standard designed procedure for routine microbiota analysis, which was used for microbiota composition characterization, as described previously.55
All animals (control-, laparotomy- and colectomy groups; n = 6/group) received 2 × 106 DiI-labeled CC531s tumor cells in 500 μl HBSS through the catheter in short-term experiments. Animals were sacrificed 2 hours post-operatively, after which liver samples were snap frozen for further analysis. Additionally, blood samples were taken via a cardiac puncture, and centrifuged for 5 minutes at 300 g. Plasma samples were collected and stored at -80°C for further analysis. Alternatively, for long-term experiments, 2 × 105 CC531s tumor cells were injected through the catheter. Animals were sacrificed 14 days after tumor cell injection (n = 12/group). Tumor nodules were scored macroscopically by two blinded independent investigators. Experiments were repeated three times.
In an additional set of experiments, rats received a selective decontamination of the digestive tract (SDD) regime, prior to surgery. The SDD prophylaxis antibiotic cocktail consisted of 20mg/ml polymyxine E sulfate, 16mg/ml tobramycin (narrow band gram-negative antibiotic) and 5mg/ml amphotericin B (a broad spectrum anti-fungal). Rats received either normal drinking water or drinking water supplemented with the SDD cocktail for 5 days.
At 6 days the rats were divided in different experimental groups. Groups 1 and 2 received anesthesia only with or without SDD regime (anesthesiaSDD– or anesthesiaSDD+), whereas groups 3 and 4 underwent a sham operation, which was defined as midline laparotomy (laparotomySDD– or laparotomySDD+). Groups 5 and 6 underwent a subtotal colectomy (colectomySDD– or colectomySDD+). Surgical procedures are described above. All animals (n = 6/group) received 2 × 106 DiI-labeled CC531s tumor cells in 500 μl HBSS through the catheter.
Bone marrow-derived macrophages culture
Bone marrow was harvested from freshly isolated femur, tibia and humerus from healthy Wag/Rij rats. After removal of connective tissues and muscles, bone marrow was flushed and single cell suspensions were made by passing bone marrow through sterile 70 µm filters (BD Falcon). Cells were allowed to differentiate into macrophages incubation for 7 days with complete DMEM supplemented with 15% L929 conditioned medium (containing macrophage-colony stimulating factor). Macrophages were harvested by 15 minutes incubation with trypsin-EDTA and subsequent scraping with a cell scraper. Macrophages were seeded in black clear-bottom 96-well plates (1 × 105 cells/well).
D-lactate and ROS measurement
D-lactate was detected with a D-lactate colorimetric assay kit (Biovision). Plasma samples from control rats, or from the laparotomy or colectomy groups were diluted to 6.25% in HBSS. Macrophages were incubated with plasma samples for 3 hours. After washing, 10 μM CM-H2DCFDA (Molecular Probes, Eugene, OR), reflecting intracellular ROS levels, was added and cells were incubated for 1 hour in the incubator. Fluorescence was measured (485 nm excitation/520 nm emission filters; Fluostar Galaxy, BMG Lab technologies, Offenburg, Germany).
Fluorescence microscopy
Cryostat liver sections from human or rat samples (5 μm) were fixed for 10 minutes in acetone and air-dried. After blocking with 10% normal goat serum or donkey serum for 15 minutes, human liver slides were incubated for 1 hour with primary antibodies against CD66b (granulocytic cell activation marker, BD Pharmingen, Franklin Lakes, NJ), NOX-2 (gp91phox)51 NOX-4 (Santa Cruz Biotechnology, Inc, Dallas, TX) and CD68 (LabNed, Amstelveen, The Netherlands). Rat liver slides were incubated with primary antibodies ED1 (marker for monocytes and macrophages, Serotec, Oxford, UK), His48 (marker for granulocytes, BD Pharmingen, Germany), claudin-5 or zona occludens 1 (ZO-1, Zymed Laboratories, San Francisco, CA), at room temperature in a humidified tissue chamber. After washing, visualization was achieved by incubation with Alexa-488, -555, or -647, labeled secondary goat anti- mouse antibodies (Molecular Probes, Eugene, Oregon). Cell nuclei were stained with Hoechst (10 μg/ml, Molecular Probes). Sections were washed, mounted and examined with a Leica DM6000 fluorescent microscope (Leica Microsystems BV, Rijswijk, the Netherlands). The numbers of DiI-labeled tumor cells in rat liver samples were quantified in 20 stitched fields per liver sample with the digital image analysis program AnalySIS (Soft Imaging System GmbH, Munster, Germany). Through a constant predefined threshold for color components, expression of Claudin-5 and ZO-1 tight junction molecules was examined. Also expression of Nox-2 and Nox-4 were examined through a constant predefined threshold for color component per area.
Two tailed Wilcoxon signed-rank tests were used to test the differences in patients’ blood or plasma samples. ANOVA tests were used for comparison between three animal groups (control, laparotomy and colectomy). Statistical significance was accepted at p<0.05.
Funding Statement
Nederlandse Organisatie voor Wetenschappelijk Onderzoek
Abbreviations
- CRC
colorectal cancer
- CRP
C-reactive protein
- DiI
1,1’dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate
- ECM
extracellular matrix
- FCS
fetal calf serum
- IL-6
interleukin-6
- KCs
Kupffer cells
- LPS
lipopolysaccharide
- METC
medical ethics committee
- PMN
polymorphonuclear cells
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- ROS
reactive oxygen species
- SDD
selective decontamination of the digestive tract
- TNFα
tumor necrosis factor alpha
- WBCs
white blood cells
- ZO-1
zona occludens 1
Acknowledgements
The authors thank Jamie L. Lim (department. of Molecular Cell Biology and Immunology, VU University Medical Center, Amsterdam, the Netherlands) for technical support.
Disclosure statement
The authors have no conflicting, professional or personal interests.
Grant support
This project was financially supported by the Dutch Cancer Foundation under grant number (KWF VU2011–4931) and the Netherlands Organization for Scientific Research under grant number NWO Mosaic Bursary 017.008.057.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A.. Global cancer statistics, 2012. CA: a cancer journal for clinicians 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Qiu M, Hu J, Yang D, Cosgrove DP, Xu R.. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget. 2015;6:38658–66. doi: 10.18632/oncotarget.6130 PMID:26484417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akgul O, Cetinkaya E, Ersoz S, Tez M.. Role of surgery in colorectal cancer liver metastases. World journal of gastroenterology. 2014;20:6113–22. doi: 10.3748/wjg.v20.i20.6113 PMID:24876733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augestad KM, Bakaki PM, Rose J, Crawshaw BP, Lindsetmo RO, Dorum LM, Koroukian SM, Delaney CP.. Metastatic spread pattern after curative colorectal cancer surgery. A retrospective, longitudinal analysis. Cancer epidemiology. 2015;39:734–44. doi: 10.1016/j.canep.2015.07.009 PMID:26277328 [DOI] [PubMed] [Google Scholar]
- 5.Coffey JC, Smith MJ, Wang JH, Bouchier-Hayes D, Cotter TG, Redmond HP.. Cancer surgery: risks and opportunities. BioEssays: news and reviews in molecular, cellular and developmental biology. 2006;28:433–7. doi: 10.1002/bies.20381 PMID:16547958 [DOI] [PubMed] [Google Scholar]
- 6.Ceelen W, Pattyn P, Mareel M.. Surgery, wound healing, and metastasis: recent insights and clinical implications. Crit Rev Oncol Hematol. 2014;89:16–26. doi: 10.1016/j.critrevonc.2013.07.008 PMID:23958676 [DOI] [PubMed] [Google Scholar]
- 7.Tohme S, Simmons RL, Tsung A.. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017;77:1548–52. doi: 10.1158/0008-5472.CAN-16-1536 PMID:28330928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM.. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248:1–7. doi: 10.1097/SLA.0b013e31816a9d65 PMID:18580199 [DOI] [PubMed] [Google Scholar]
- 9.Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ.. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–45. doi: 10.1002/bjs.7160 PMID:20629110 [DOI] [PubMed] [Google Scholar]
- 10.Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3 PMID:19071061 [DOI] [PubMed] [Google Scholar]
- 11.Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–32. doi: 10.1056/NEJMoa1414882 PMID:25830422 [DOI] [PubMed] [Google Scholar]
- 12.Weese JL, Ottery FD, Emoto SE.. Do operations facilitate tumor growth? An experimental model in rats. Surgery. 1986;100:273–7.PMID:3738755 [PubMed] [Google Scholar]
- 13.Mutter D, Hajri A, Tassetti V, Solis-Caxaj C, Aprahamian M, Marescaux J.. Increased tumor growth and spread after laparoscopy vs laparotomy: influence of tumor manipulation in a rat model. Endosc Surg. 1999;13:365–70. doi: 10.1007/s004649900991 PMID:10094749 [DOI] [PubMed] [Google Scholar]
- 14.Georges C, Lo T, Alkofer B, Whelan R, Allendorf J.. The effects of surgical trauma on colorectal liver metastasis. Surg Endosc. 2007;21:1817–9. doi: 10.1007/s00464-007-9290-0 PMID:17522938 [DOI] [PubMed] [Google Scholar]
- 15.Oosterling SJ, van der Bij GJ, Bogels M, ten Raa S, Post JA, Meijer GA, Beelen RH, van Egmond M.. Anti-beta1 integrin antibody reduces surgery-induced adhesion of colon carcinoma cells to traumatized peritoneal surfaces. Ann Surg. 2008;247:85–94. doi: 10.1097/SLA.0b013e3181588583 PMID:18156927 [DOI] [PubMed] [Google Scholar]
- 16.van der Bij GJ, Oosterling SJ, Bogels M, Bhoelan F, Fluitsma DM, Beelen RH, Meijer S, van Egmond M.. Blocking alpha2 integrins on rat CC531s colon carcinoma cells prevents operation-induced augmentation of liver metastases outgrowth. Hepatology (Baltimore, Md). 2008;47:532–43. doi: 10.1002/hep.22013 PMID:18098323 [DOI] [PubMed] [Google Scholar]
- 17.Gul N, Bogels M, Grewal S, van der Meer AJ, Rojas LB, Fluitsma DM, van den Tol MP, Hoeben KA, van Marle J, de Vries HE, et al. Surgery-induced reactive oxygen species enhance colon carcinoma cell binding by disrupting the liver endothelial cell lining. Gut. 2011;60:1076–86. doi: 10.1136/gut.2010.224717 PMID:21278144 [DOI] [PubMed] [Google Scholar]
- 18.Lambeth JD, Neish AS.. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annual review of pathology. 2014;9:119–45. doi: 10.1146/annurev-pathol-012513-104651 PMID:24050626 [DOI] [PubMed] [Google Scholar]
- 19.Sastre J, Maestro ML, Puente J, Veganzones S, Alfonso R, Rafael S, García-Saenz JA, Vidaurreta M, Martín M, Arroyo M.. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2008;19:935–8. doi: 10.1093/annonc/mdm583. [DOI] [PubMed] [Google Scholar]
- 20.Bork U, Rahbari NN, Scholch S, Reissfelder C, Kahlert C, Buchler MW, Weitz J, Koch M.. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br J Cancer. 2015;112:1306–13. doi: 10.1038/bjc.2015.88 PMID:25867263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wind J, Tuynman JB, Tibbe AG, Swennenhuis JF, Richel DJ, van Berge Henegouwen MI, et al. Circulating tumour cells during laparoscopic and open surgery for primary colonic cancer in portal and peripheral blood. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2009;35:942–50. doi: 10.1016/j.ejso.2008.12.003 PMID:19153024 [DOI] [PubMed] [Google Scholar]
- 22.Allen-Mersh TG, McCullough TK, Patel H, Wharton RQ, Glover C, Jonas SK.. Role of circulating tumour cells in predicting recurrence after excision of primary colorectal carcinoma. Surg Br J. 2007;94:96–105. doi: 10.1002/bjs.5526 PMID:17058316 [DOI] [PubMed] [Google Scholar]
- 23.Koch M, Kienle P, Sauer P, Willeke F, Buhl K, Benner A, Lehnert T, Herfarth C, von Knebel Doeberitz M, et al. Hematogenous tumor cell dissemination during colonoscopy for colorectal cancer. Surg Endosc. 2004;18:587–91. doi: 10.1007/s00464-003-9066-0 PMID:14735340 [DOI] [PubMed] [Google Scholar]
- 24.Groot Koerkamp B, Rahbari NN, Buchler MW, Koch M, Weitz J.. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann Surg Oncol. 2013;20:2156–65. doi: 10.1245/s10434-013-2907-8 PMID:23456317 [DOI] [PubMed] [Google Scholar]
- 25.Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M, Weitz J.. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138:1714–26. doi: 10.1053/j.gastro.2010.01.008 PMID:20100481 [DOI] [PubMed] [Google Scholar]
- 26.Thorsteinsson M, Jess P.. The clinical significance of circulating tumor cells in non-metastatic colorectal cancer–a review. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2011;37:459–65. doi: 10.1016/j.ejso.2011.01.025 PMID:21324632 [DOI] [PubMed] [Google Scholar]
- 27.Galizia G, Gemei M, Orditura M, Romano C, Zamboli A, Castellano P, et al. Postoperative detection of circulating tumor cells predicts tumor recurrence in colorectal cancer patients. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2013;17:1809–18. doi: 10.1007/s11605-013-2258-6 PMID:23813048 [DOI] [PubMed] [Google Scholar]
- 28.Flatmark K, Borgen E, Nesland JM, Rasmussen H, Johannessen HO, Bukholm I, Rosales R, Hårklau L, Jacobsen HJ, Sandstad B, et al. Disseminated tumour cells as a prognostic biomarker in colorectal cancer. Br J Cancer. 2011;104:1434–9. doi: 10.1038/bjc.2011.97 PMID:21448171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uen YH, Lu CY, Tsai HL, Yu FJ, Huang MY, Cheng TL, Lin SR, Wang JY.. Persistent presence of postoperative circulating tumor cells is a poor prognostic factor for patients with stage I-III colorectal cancer after curative resection. Ann Surg Oncol. 2008;15:2120–8. doi: 10.1245/s10434-008-9961-7 PMID:18481151 [DOI] [PubMed] [Google Scholar]
- 30.Belt EJ, Stockmann HB, Abis GS, de Boer JM, de Lange-de Klerk ES, van Egmond M, et al. Peri-operative bowel perforation in early stage colon cancer is associated with an adverse oncological outcome. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2012; 16:2260–6. doi: 10.1007/s11605-012-2053-9 PMID:23093449 [DOI] [PubMed] [Google Scholar]
- 31.Law WL, Choi HK, Lee YM, Ho JW, Seto CL.. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2007;11:8–15. doi: 10.1007/s11605-006-0049-z PMID:17390180 [DOI] [PubMed] [Google Scholar]
- 32.Marra F, Steffen T, Kalak N, Warschkow R, Tarantino I, Lange J, et al. Anastomotic leakage as a risk factor for the long-term outcome after curative resection of colon cancer. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2009;35:1060–4. doi: 10.1016/j.ejso.2009.02.011 PMID:19303243 [DOI] [PubMed] [Google Scholar]
- 33.McArdle CS, McMillan DC, Hole DJ.. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg. 2005;92:1150–4. doi: 10.1002/bjs.5054 PMID:16035134 [DOI] [PubMed] [Google Scholar]
- 34.Ptok H, Marusch F, Meyer F, Schubert D, Gastinger I, Lippert H.. Impact of anastomotic leakage on oncological outcome after rectal cancer resection. Br J Surg. 2007;94:1548–54. doi: 10.1002/bjs.5707 PMID:17668888 [DOI] [PubMed] [Google Scholar]
- 35.Lu ZR, Rajendran N, Lynch AC, Heriot AG, Warrier SK.. Anastomotic Leaks After Restorative Resections for Rectal Cancer Compromise Cancer Outcomes and Survival. Dis Colon Rectum. 2016;59:236–44. doi: 10.1097/DCR.0000000000000554 PMID:26855399 [DOI] [PubMed] [Google Scholar]
- 36.Katoh H, Yamashita K, Wang G, Sato T, Nakamura T, Watanabe M.. Anastomotic leakage contributes to the risk for systemic recurrence in stage II colorectal cancer. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2011;15:120–9. doi: 10.1007/s11605-010-1379-4 PMID:21086058 [DOI] [PubMed] [Google Scholar]
- 37.Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P.. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253:890–9. doi: 10.1097/SLA.0b013e3182128929 PMID:21394013 [DOI] [PubMed] [Google Scholar]
- 38.Kube R, Mroczkowski P, Granowski D, Benedix F, Sahm M, Schmidt U, et al. Anastomotic leakage after colon cancer surgery: a predictor of significant morbidity and hospital mortality, and diminished tumour-free survival. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2010;36:120–4. doi: 10.1016/j.ejso.2009.08.011 PMID:19775850 [DOI] [PubMed] [Google Scholar]
- 39.Schietroma M, Pessia B, Carlei F, Cecilia EM, Amicucci G.. Intestinal permeability, systemic endotoxemia, and bacterial translocation after open or laparoscopic resection for colon cancer: a prospective randomized study. Int J Colorectal Dis. 2013;28:1651–60. doi: 10.1007/s00384-013-1751-4 PMID:23917392 [DOI] [PubMed] [Google Scholar]
- 40.Schietroma M, Pessia B, Carlei F, Cecilia EM, De Santis G, Amicucci G.. Laparoscopic versus open colorectal surgery for colon cancer: the effect of surgical trauma on the bacterial translocation. A prospective randomized study. American journal of surgery. 2015;210:263–9. [DOI] [PubMed] [Google Scholar]
- 41.Chin KF, Kallam R, O’Boyle C, MacFie J.. Bacterial translocation may influence the long-term survival in colorectal cancer patients. Dis Colon Rectum. 2007;50:323–30. doi: 10.1007/s10350-006-0827-4 PMID:17237910 [DOI] [PubMed] [Google Scholar]
- 42.van der Voort PH, Westra B, Wester JP, Bosman RJ, van Stijn I, Haagen IA, Loupatty FJ, Rijkenberg S.. Can serum L-lactate, D-lactate, creatine kinase and I-FABP be used as diagnostic markers in critically ill patients suspected for bowel ischemia. BMC anesthesiology. 2014;14:111. doi: 10.1186/1471-2253-14-111 PMID:25844063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP.. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4:760–8. doi: 10.1016/S1470-2045(03)01282-8 PMID:14662433 [DOI] [PubMed] [Google Scholar]
- 44.Liu C, Liu J, Zhang S.. Laparoscopic versus conventional open surgery for immune function in patients with colorectal cancer. Int Colorectal Dis J. 2011;26:1375–85. doi: 10.1007/s00384-011-1281-x PMID:21822596 [DOI] [PubMed] [Google Scholar]
- 45.Veenhof AA, Sietses C, von Blomberg BM, van Hoogstraten IM, MH vd Pas, WJ Meijerink, et al. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: a randomized trial. Int J Colorectal Dis. 2011;26:53–9. doi: 10.1007/s00384-010-1056-9 PMID:20922542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veenhof AA, Vlug MS, van der Pas MH, Sietses C, van der Peet DL, de Lange-de Klerk ES, et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg. 2012;255:216–21. doi: 10.1097/SLA.0b013e31824336e2 PMID:22241289 [DOI] [PubMed] [Google Scholar]
- 47.Hyspler R, Ticha A, Kaska M, Zaloudkova L, Pliskova L, Havel E, Zadák Z.. Markers of Perioperative Bowel Complications in Colorectal Surgery Patients. Dis Markers. 2015;2015:428535. doi: 10.1155/2015/428535 PMID:26788017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiao Z, Li Z, Li J, Lu L, Lv Y, Li J.. Bacterial translocation and change in intestinal permeability in patients after abdominal surgery. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2009; 29:486–91. doi: 10.1007/s11596-009-0419-3. [DOI] [PubMed] [Google Scholar]
- 49.Franke A, Lante W, Kollig E, Markewitz A.. A comparison of monocyte counts and ex vivo and in vitro monocyte cytokine production after major surgical trauma. Surg Res J. 2009;154:91–8. doi: 10.1016/j.jss.2008.06.003 PMID:18952234 [DOI] [PubMed] [Google Scholar]
- 50.McSorley ST, Watt DG, Horgan PG, McMillan DC.. Postoperative Systemic Inflammatory Response, Complication Severity, and Survival Following Surgery for Colorectal Cancer. Ann Surg Oncol. 2016;23:2832–40. doi: 10.1245/s10434-016-5204-5 PMID:27016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gul N, Babes L, Siegmund K, Korthouwer R, Bogels M, Braster R, Vidarsson G, TL ten Hagen, Kubes P, van Egmond M.. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J Clin Invest. 2014;124:812–23. doi: 10.1172/JCI66776 PMID:24430180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S, Ferri LE.. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011;71:1989–98. doi: 10.1158/0008-5472.CAN-10-2833 PMID:21363926 [DOI] [PubMed] [Google Scholar]
- 53.Bertelsen CA, Neuenschwander AU, Jansen JE, Wilhelmsen M, Kirkegaard-Klitbo A, Tenma JR, et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015;16:161–8. doi: 10.1016/S1470-2045(14)71168-4 PMID:25555421 [DOI] [PubMed] [Google Scholar]
- 54.Roos D, Dijksman LM, Sondermeijer BM, Oudemans-van Straaten HM, TdW L, Gerhards MF.. Perioperative selective decontamination of the digestive tract (SDD) in elective colorectal surgery. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2009;13:1839–44. doi: 10.1007/s11605-009-0970-z PMID:19636641 [DOI] [PubMed] [Google Scholar]
- 55.Budding AE, Grasman ME, Lin F, Bogaards JA, Soeltan-Kaersenhout DJ, Vandenbroucke-Grauls CM, et al IS-pro: high-throughput molecular fingerprinting of the intestinal microbiota. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24:4556–64. doi: 10.1096/fj.10-156190 PMID:20643909 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


