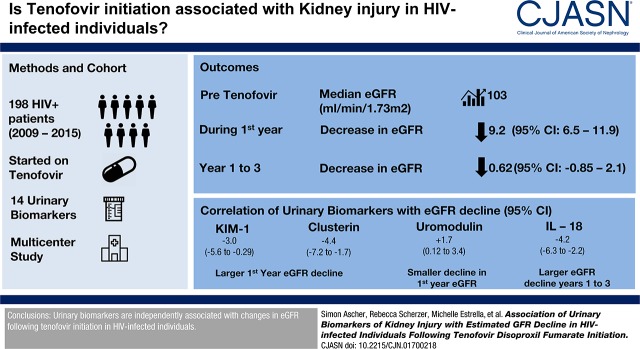
Abstract
Background and objectives
Tenofovir disoproxil fumarate (tenofovir) is associated with elevated concentrations of biomarkers of kidney damage and dysfunction in individuals with HIV. The relationship of these kidney biomarkers with longitudinal kidney function decline is unknown.
Design, setting, participants, & measurements
We evaluated associations of 14 urinary biomarkers of kidney injury with changes in eGFR among 198 men and women with HIV who initiated tenofovir between 2009 and 2015 in the Multicenter AIDS Cohort Study and Women’s Interagency HIV Study. Urinary biomarkers included albumin-to-creatinine ratio, α-1-microglobulin, β-2-microglobulin, cystatin C, kidney injury molecule-1 (KIM-1), IL-18, neutrophil gelatinase–associated lipocalin (NGAL), clusterin, osteopontin, uromodulin, monocyte chemoattractant protein-1, EGF, trefoil factor 3, and chitinase 3-like protein 1. We used multivariable linear mixed-effect models controlling for demographics, traditional kidney disease risk factors, and HIV-related risk factors to evaluate associations of baseline biomarkers with first-year changes in eGFR, and associations of year 1 and first-year change in biomarkers with changes in eGFR from year 1 to year 3. We used the least absolute shrinkage and selection operator method to identify a parsimonious set of biomarkers jointly associated with changes in eGFR.
Results
Median eGFR before tenofovir initiation was 103 (interquartile range, 88–116) ml/min per 1.73 m2. During the first year of tenofovir use, eGFR decreased on average by 9.2 (95% confidence interval, 6.5 to 11.9) ml/min per 1.73 m2 and was stable afterward (decrease of 0.62; 95% confidence interval, −0.85 to 2.1 ml/min per 1.73 m2 per year). After multivariable adjustment, higher baseline β-2-microglobulin, KIM-1, and clusterin were associated with larger first-year eGFR declines, whereas higher baseline uromodulin was associated with a smaller eGFR decline. First-year increase in urinary cystatin C and higher year 1 IL-18 were associated with larger annual eGFR declines from year 1 to year 3. The parsimonious models identified higher pre-tenofovir clusterin and KIM-1, lower pre-tenofovir uromodulin, and higher year 1 IL-18 as jointly associated with larger eGFR declines.
Conclusions
Urinary biomarkers of kidney injury measured before and after tenofovir initiation are associated with subsequent changes in eGFR in individuals with HIV.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2018_08_28_CJASNPodcast_18_9_S.mp3
Keywords: HIV; Kidney injury; Tenofovir disoproxil fumarate; Tenofovir; LCN2 protein, human; Lipocalin-2; Cystatin C; Uromodulin; interleukin 18 protein, human; creatinine; risk factors; Chemokine CCL2; Cohort Studies; epidermal growth factor; Acquired Immunodeficiency Syndrome; Hepatitis A Virus Cellular Receptor 1; glomerular filtration rate; Biomarkers; Demography; Albumins
Introduction
Tenofovir disoproxil fumarate (tenofovir) is a non-nucleoside reverse transcription inhibitor widely used for the treatment and pre-exposure prophylaxis of HIV infection. International guidelines recommend tenofovir in first-line antiretroviral therapy regimens, and the majority of single-tablet antiretroviral therapy regimens include tenofovir (1,2). Although early randomized trials suggested that tenofovir had no significant nephrotoxicity (3,4), subsequent studies demonstrated that tenofovir exposure is associated with increased risk of AKI, proteinuria, Fanconi syndrome, and CKD (5–7). Tenofovir-associated nephrotoxicity is hypothesized to occur primarily in the proximal tubule, where excessive intracellular tenofovir accumulation can cause mitochondrial toxicity and acute tubular necrosis (8,9). Detection of kidney function decline during tenofovir use can prompt changing antiretroviral therapy regimens, but recognition is often late and losses in kidney function may be irreversible (10,11).
Despite these concerns, current methods for monitoring tenofovir-associated nephrotoxicity in individuals with HIV using urinary albumin and serum creatinine have limitations. Because urinary albumin primarily reflects glomerular rather than tubular injury, tenofovir use is associated with a nonalbumin pattern of proteinuria (12). Tenofovir use is also associated with modest reductions in eGFR by serum creatinine (5), but substantial kidney injury can occur before a measurable loss in kidney function. However, data are limited from studies utilizing kidney injury biomarkers to prognosticate kidney outcomes in tenofovir users with HIV.
In this study, we evaluated associations of 14 urinary biomarkers with longitudinal changes in kidney function in men and women with HIV initiating tenofovir. The urinary biomarker panel included albumin-to-creatinine ratio (ACR), α-1-microglobulin (α1m), β-2-microglobulin (β2m), cystatin C, kidney injury molecule-1 (KIM-1), IL-18, neutrophil gelatinase–associated lipocalin, clusterin, osteopontin, uromodulin, monocyte chemoattractant protein-1, epidermal growth factor (EGF), trefoil factor 3, and chitinase 3-like protein 1. The panel consists of biomarkers previously studied in tenofovir users with HIV, in addition to biomarkers approved by the US Food and Drug Administration, European Medicines Agency, and the Japanese Pharmaceutical and Medical Devices Agency for use in preclinical trials of drug-induced nephrotoxicity (13). We hypothesized that changes in urinary biomarkers would be independently associated with changes in eGFR after tenofovir initiation in this ambulatory cohort of men and women with HIV.
Materials and Methods
Study Design
The Multicenter AIDS Cohort Study (MACS) and Women’s Interagency HIV Study (WIHS) are two of the largest prospective, multicenter cohort studies of individuals with and without HIV in the United States. The design and methods of each study have been described previously (14,15). MACS consists of 6972 men with and without HIV, with similar backgrounds, who were enrolled in 1984–1985, 1987–1990, or 2001–2003 from four sites: Baltimore, Maryland/Washington, DC; Chicago, Illinois; Los Angeles, California; and Pittsburgh, Pennsylvania. WIHS consists of 4982 women with and without HIV, with similar backgrounds, who were enrolled in 1994–1995 and 2001–2002 from Bronx/Manhattan, New York; Brooklyn, New York; Chicago, Illinois; Los Angeles, California; San Francisco, California; and Washington DC, and between 2011 and 2015 from Atlanta, Georgia; Chapel Hill, North Carolina; Miami, Florida; and Birmingham, Alabama/Jackson, Mississippi. MACS and WIHS participants attend semiannual visits that include a standardized questionnaire, a physical examination, and collection of laboratory specimens.
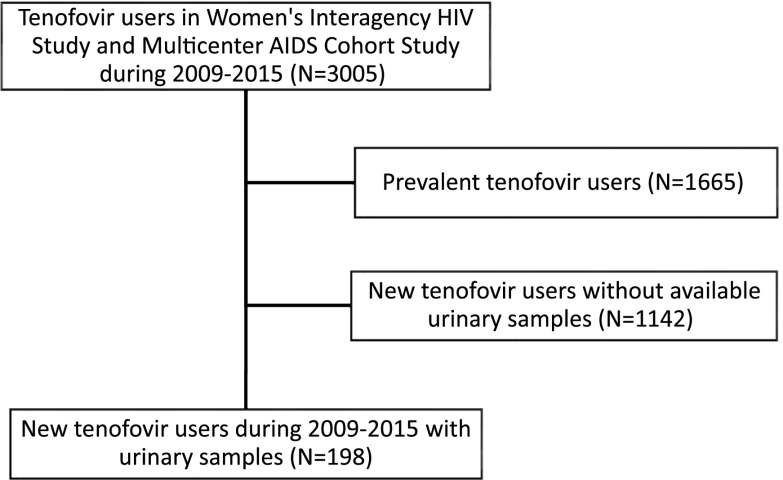
We designed a nested study within MACS and WIHS to investigate the trajectory of kidney injury and kidney function after tenofovir initiation in participants with HIV. Participants were included in our analysis on the basis of availability of both urine measures and eGFR measures. The study included 198 individuals with HIV (87 men and 111 women) who initiated a tenofovir-based antiretroviral therapy regimen between 2009 and 2015 and had available urine and serum specimens collected within 12 months both before and after initiating tenofovir (Figure 1). Biomarkers were measured using stored urine specimens that were collected on average 6 months before participants initiated tenofovir, 12 months after participants initiated tenofovir, and at up to two subsequent annual follow-up visits.
Figure 1.
The study population comprised of HIV-infected persons who initiated TDF during the periods of urine sample collection in the WIHS and MACS cohorts.
The institutional review boards of participating institutions approved the study protocol, and informed consent was obtained from all study participants. The University of California, San Francisco and San Francisco Veterans Affairs Medical Center Institutional Review Board approved the present analysis (IRB approval number 14-14671).
Exposures
For each of the 14 urinary biomarkers, we modeled associations with eGFR changes in three ways: (1) using the baseline concentration (pre-tenofovir), (2) using the estimated first-year biomarker change, and (3) using the biomarker concentration at year 1 (i.e., 1 year after tenofovir initiation). The median time from pre-tenofovir urinary biomarker samples to tenofovir initiation was 1.0 years (interquartile range [IQR], 0.90–1.20), and the median time from pre-tenofovir to first-year urinary biomarker samples was 1.7 years (IQR, 1.5–2.0). Because samples may not have been collected exactly at yearly intervals after tenofovir initiation, we used linear mixed-effect models with random intercepts and slopes to estimate the first-year change in biomarker concentrations. All urinary biomarkers were measured at the University of Vermont Biomarker Laboratory. Details regarding assay brands, biomarker ranges, and interassay coefficients of variation are shown in Supplemental Table 1. Urinary biomarker correlations are shown in Supplemental Table 2. In accordance with MACS and WIHS protocols, urine specimens were refrigerated immediately after collection and centrifuged at 1000×g to remove cellular debris. The supernatant was aliquoted into 1-ml vials and then stored at −80°C until biomarker measurement, without prior freeze-thaw. Laboratory personnel performing the biomarker assays were blinded to participants’ clinical information.
Outcome
Our primary outcomes were the estimated first-year change in eGFR after tenofovir initiation, and the estimated annual change in eGFR from year 1 to year 3 after tenofovir initiation. We calculated eGFR using the 2009 CKD Epidemiology Collaboration creatinine equation (16). Serum creatinine was measured semiannually in the clinical laboratories of each MACS and WIHS site.
Covariates
Demographics, kidney disease risk factors, and HIV-related characteristics were collected at each study visit. The following baseline covariates were analyzed in all multivariable models: age, sex, race/ethnicity, diabetes mellitus (defined as fasting glucose ≥126 mg/dl, hemoglobin A1c ≥6.5%, or self-reported history of diabetes and diabetes medication use), systolic and diastolic BP, hypertension (defined as systolic BP >140 mm Hg, diastolic BP >90 mm Hg, or self-reported history of hypertension and antihypertensive medication use), self-reported history of cardiovascular disease, statin use, LDL and HDL cholesterol levels, body mass index, cigarette smoking status (current, past, or never), serum albumin level, self-reported current heroin use, hepatitis C virus (HCV) infection (confirmed by detectable HCV RNA after a positive HCV antibody result), current and nadir CD4 lymphocyte count, current and peak plasma HIV-1 RNA level, history of clinical AIDS diagnosis, duration of HIV infection, and current antiretroviral therapy use. The percentage of missing observations for each covariate ranged from 0% to 10%. Multiple imputation with the Markov chain Monte Carlo method was used to impute missing covariates, with 15 imputations to yield approximately 95% relative efficiency.
Statistical Analyses
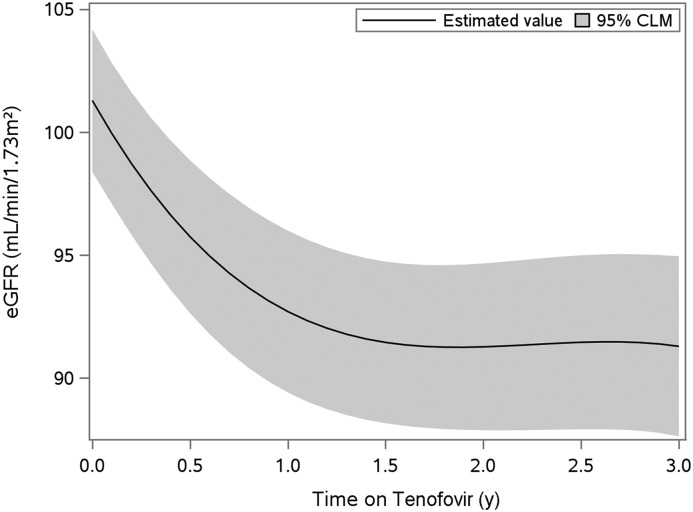
We summarized baseline demographics and clinical characteristics overall and stratified by WIHS and MACS cohorts. For each urinary biomarker, we used linear mixed-effects models with random intercepts and slopes to estimate biomarker associations with changes in eGFR. An advantage of using linear mixed models is that they use all of the observed data for analysis, with the assumption that missing data are missing at random. Graphical examination of the eGFR trajectories found curvilinear changes in eGFR (Figure 2). Therefore, we modeled eGFR using linear splines, with potentially different slopes during the first year and subsequent follow-up (0–1 and 1–3 years). Because the eGFR trajectory showed a large decline in the first year followed by a plateau, we evaluated baseline urinary biomarker associations with first-year eGFR changes, and we separately evaluated year 1 and first-year change in urinary biomarker associations with eGFR changes from year 1 to year 3. We analyzed all urinary biomarkers as log-transformed continuous variables because of their right-skewed distribution. The detectable limit of the α1m assay was 0.5 mg/dl, and approximately 15% of urine α1m values were undetectable. As a result, we used a left-censored linear mixed-effect model to impute values that were below the limit of detection and to estimate the first-year change in α1m for each participant. Interaction terms between each biomarker and time on tenofovir were used to estimate changes in eGFR attributable to a 1 SD change in the biomarker. To determine whether individual biomarkers were independently associated with changes in eGFR, multivariable models were adjusted for urine creatinine, demographics, traditional kidney disease risk factors, HIV-related risk factors (as listed above in the Covariates section), and covariate-by-time interactions.
Figure 2.
Estimated GFR by creatinine declined rapidly during the first year after TDF initiation, but then was stable over the following two years. Estimates are from linear mixed-effects models adjusting for urine creatinine and using smoothed splines. CLM, confidence interval for the mean.
Although this study evaluated several different intercorrelated biomarkers, we did not include formal adjustments for multiple comparisons. Rather than using traditional multiple comparison adjustments to control the type 1 error rate, we modeled biomarkers in combination using the least absolute shrinkage and selection operator (LASSO) procedure, which shrinks regression coefficients and selects predictors by imposing a penalty on their size (17). This is because we hypothesized a priori that all of the urinary biomarkers, with the exception of uromodulin and EGF, would be associated with worsening kidney function decline. This biologically coherent pattern dictates that results should be mutually reinforcing, rather than a series of independent tests. We modeled biomarkers in combination using an extension of LASSO for linear mixed-effects models (18). We allowed the regularization parameter λ to vary over a wide range of values (so that all penalized coefficients were zero at the largest λ) and used the Bayesian information criterion to guide the selection of the optimal λ and number of included biomarkers. Our final model was estimated for the combination of LASSO-selected markers using linear mixed-effects models, retaining only markers that remained statistically significant.
The LASSO analysis was implemented using the R package lmmlasso. All other analyses were performed using the SAS system, version 9.4 (SAS Institute, Inc., Cary, NC).
Results
The median duration of follow-up starting from the pre-tenofovir baseline visit was 3.0 (IQR, 2.0–3.5) years. At the pre-tenofovir visit, the mean age was 48 years, the median eGFR was 103 (IQR, 88–116) ml/min per 1.73 m2, and 70% of persons had detectable HIV RNA (Table 1). At the time of tenofovir initiation, 33% were receiving highly active antiretroviral therapy, 52% had never received it, and 15% were not receiving highly active antiretroviral therapy but had previously used it. During the first year after tenofovir initiation, there were statistically significant increases in α1m, clusterin, trefoil factor 3, KIM-1, and uromodulin, and a significant decrease in IL-18 (Supplemental Table 3). In addition, eGFR decreased on average by 9.2 (95% confidence interval [95% CI], 6.5 to 11.9) ml/min per 1.73 m2 after the first year and 0.62 (95% CI, −0.85 to 2.1) ml/min per 1.73 m2 annually thereafter (Figure 2). Twelve out of 198 (6.1%) participants were lost to follow-up. However, we found no statistically significant differences in the baseline characteristics of participants who were lost to follow-up compared with those who remained in the study.
Table 1.
Summary of baseline (pre-tenofovir) demographic and clinical characteristics of Women’s Interagency HIV Study (WIHS) and Multicenter AIDS Cohort Study (MACS) participants with HIV
| Parameter | Overall, n=198 | WIHS: Women, n=111 | MACS: Men, n=87 |
|---|---|---|---|
| Age, yr | 48 (41–54) | 46 (40–53) | 49 (44–56) |
| Race | |||
| Black | 126 (64%) | 87 (78%) | 39 (45%) |
| Other | 13 (7%) | 9 (8%) | 4 (5%) |
| White | 59 (30%) | 15 (14%) | 44 (51%) |
| Hispanic | 29 (15%) | 20 (18%) | 9 (10%) |
| Smoking | |||
| Current | 73 (37%) | 46 (41%) | 27 (31%) |
| Past | 62 (31%) | 25 (23%) | 37 (43%) |
| Never | 62 (31%) | 40 (36%) | 22 (26%) |
| Diabetes mellitus | 32 (17%) | 20 (18%) | 12 (17%) |
| Systolic BP, mm Hg | 126 (114–137) | 121 (110–134) | 129 (117–137) |
| Diastolic BP, mm Hg | 77 (71–86) | 74 (69–85) | 80 (72–87) |
| Hypertension | 93 (48%) | 56 (50%) | 37 (44%) |
| LDL Cholesterol, mg/dl | 101 (79–121) | 98 (77–117) | 105 (81–126) |
| HDL Cholesterol, mg/dl | 46 (38–57) | 48 (39–58) | 43 (36–53) |
| Statin use | 31 (16%) | 17 (15%) | 14 (17%) |
| History of cardiovascular disease | 13 (7%) | 4 (4%) | 9 (10%) |
| BMI, kg/m2 | 27 (23–32) | 29 (24–34) | 25 (23–28) |
| Duration of HIV infection, yr | 9 (7–15) | 10 (8–15) | 8 (5–20) |
| Current HAART | |||
| HAART use | 66 (33%) | 31 (28%) | 35 (40%) |
| NRTI use | 67 (34%) | 31 (28%) | 36 (41%) |
| NNRTI use | 34 (17%) | 13 (12%) | 21 (24%) |
| PI use | 30 (15%) | 14 (13%) | 16 (18%) |
| Current CD4, cells/mm3 | 483 (338–682) | 485 (314–667) | 465 (387–716) |
| Nadir CD4, cells/mm3 | 347 (223–471) | 314 (191–477) | 382 (272–464) |
| History of AIDS | 26 (13%) | 23 (21%) | 3 (3%) |
| Current HIV RNA <80 copies/ml | 56 (29%) | 24 (22%) | 32 (37%) |
| Hepatitis C virus infection | 33 (17%) | 20 (18%) | 13 (15%) |
| Ever heroin use | 28 (16%) | 18 (20%) | 10 (11%) |
| Serum albumin, g/dl | 4.2 (3.8–4.4) | 4.1 (3.8–4.3) | 4.3 (4.1–4.6) |
| eGFR, ml/min per 1.73 m2 | 103 (88–116) | 103 (85–117) | 104 (92–116) |
| Albumin-to-creatinine ratio, mg/g | 3.2 (1.9–7.1) | 3.5 (2.0–12.0) | 3.0 (1.8–5.7) |
| Albumin-to-creatinine ratio>30 mg/g | 15 (8%) | 6 (5%) | 9 (10%) |
Data are presented as median (interquartile range) or n (percent). BMI, body mass index; HAART, highly active antiretroviral therapy; NRTI, nucleoside reverse transcription inhibitor; NNRTI, nonnucleoside reverse transcription inhibitor; PI, protease inhibitor.
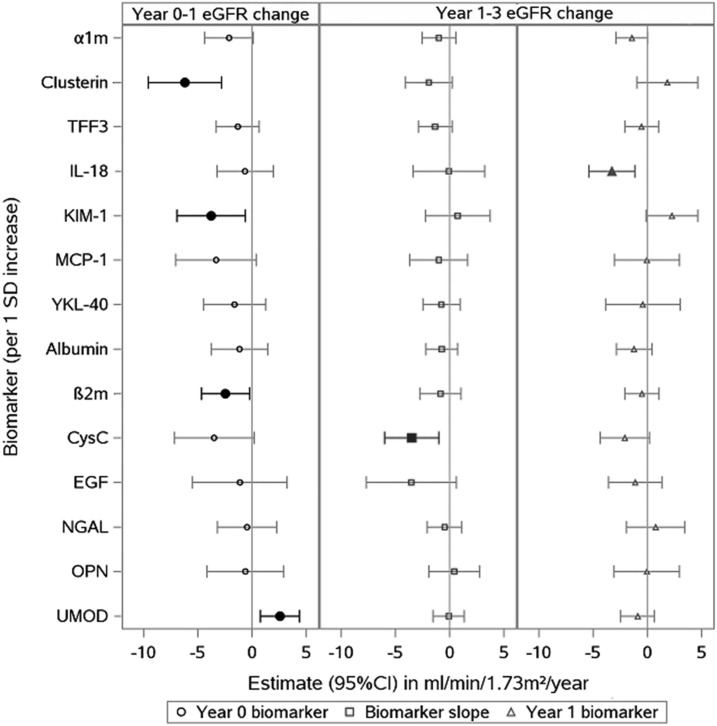
In unadjusted analyses, higher baseline β2m, KIM-1, and clusterin concentrations and a lower baseline uromodulin concentration were associated with larger first-year decreases in eGFR after tenofovir initiation (Table 2). There was minimal attenuation of these associations after multivariable adjustment for traditional and HIV-related kidney disease risk factors (Figure 3).
Table 2.
Associations of baseline urinary biomarkers with first-year change in eGFR after tenofovir initiation in Women’s Interagency HIV Study (WIHS) and Multicenter AIDS Cohort Study (MACS) participants with HIV
| Baseline Biomarker, per 1 SD | Estimated Change in eGFR in ml/min per 1.73 m2 (95% Confidence Interval) | |
|---|---|---|
| Unadjusteda | Multivariable Adjustedb | |
| α-1-Microglobulin | −1.83 (−4.09 to 0.44) | −2.12 (−4.36 to 0.13) |
| Clusterin | −6.30 (−9.76 to −2.84) | −6.20 (−9.57 to −2.82) |
| Trefoil factor 3 | −1.78 (−3.72 to 0.16) | −1.32 (−3.30 to 0.65) |
| IL-18 | −0.14 (−2.78 to 2.50) | −0.64 (−3.22 to 1.94) |
| Kidney injury molecule-1 | −4.08 (−7.16 to −0.99) | −3.77 (−6.91 to −0.63) |
| Monocyte chemoattractant protein-1 | −3.69 (−7.40 to 0.03) | −3.32 (−7.04 to 0.40) |
| Chitinase 3-like protein 1 | −1.75 (−4.65 to 1.14) | −1.60 (−4.46 to 1.26) |
| Albumin-to-creatinine ratio | −0.87 (−3.44 to 1.70) | −1.16 (−3.77 to 1.46) |
| β-2-Microglobulin | −2.77 (−4.97 to −0.57) | −2.44 (−4.64 to −0.24) |
| Cystatin C | −3.50 (−7.39 to 0.38) | −3.48 (−7.16 to 0.19) |
| Epidermal growth factor | −0.64 (−5.07 to 3.78) | −1.13 (−5.48 to 3.23) |
| Neutrophil gelatinase–associated lipocalin | −1.22 (−3.95 to 1.51) | −0.45 (−3.19 to 2.28) |
| Osteopontin | −0.78 (−4.70 to 3.14) | −0.62 (−4.15 to 2.91) |
| Uromodulin | 2.85 (1.10 to 4.60) | 2.57 (0.75 to 4.38) |
Estimates are in ml/min per 1.73 m2 per 1 SD increment in biomarker. Estimates from linear mixed-effects models incorporating eGFR values starting at tenofovir initiation (year 0). Models control for each biomarker separately. Time on tenofovir is modeled using a linear spline with cut-off points at year 1 and year 3. Estimates in bold are statistically significant associations.
Unadjusted models include biomarker, time, biomarker by time interaction, and urine creatinine.
Adjusted models additionally control for demographics, diabetes mellitus, smoking, BP, hypertension, HDL, LDL, cardiovascular disease, statin, body mass index, serum albumin, heroin use, current and nadir CD4, current and peak HIV RNA, HIV duration at baseline, history of AIDS, hepatitis C virus, and baseline antiretroviral therapy use.
Figure 3.
Among 14 urine biomarkers evaluated, the baseline values of 4 biomarkers (clusterin, KIM-1, b2m, and UMOD) were associated with year 1 eGFR change; first-year change in CysC and year 1 IL-18 values were associated with eGFR change from year 1-3. Estimates are in ml/min per 1.73 m2 per 1 SD increment in biomarker. Associations are of baseline urinary biomarkers (left) with year 0–1 eGFR change and change in urinary biomarkers from year 0–1 (middle) and year 1 biomarkers (right) with annual eGFR change from year 1 to year 3. Estimates from linear mixed-effects models incorporating eGFR values starting at tenofovir initiation (year 0). Each association estimated from models that included biomarker, time, biomarker-by-time interaction, urine creatinine, demographics, diabetes mellitus, smoking, BP, hypertension, HDL, LDL, cardiovascular disease, statin, body mass index, serum albumin, heroin use, current and nadir CD4, current and peak HIV RNA, HIV duration at baseline, history of AIDS, HCV, and baseline antiretroviral therapy use. Models control for each biomarker separately. Time on tenofovir is modeled using a linear spline with cut-off points at year 1 and year 3. Filled symbols denote statistically significant associations. a1m, a-1-microglobulin; b2m, b-2-microglobulin; CysC, cystatin C; EGF, epidermal growth factor; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1; NGAL, neutrophil gelatinase–associated lipocalin; OPN, osteopontin; TFF3, trefoil factor 3; UMOD, uromodulin; YKL-40, chitinase 3-like protein 1.
We also modeled associations of first-year changes in biomarker concentrations and year 1 biomarker concentrations with annual changes in eGFR from year 1 to year 3 after tenofovir initiation. In unadjusted analyses, we found that a larger first-year increase in urinary cystatin C concentration and a higher IL-18 concentration at year 1 were each associated with larger annual decreases in eGFR from year 1 to year 3 (Table 3). There was no apparent attenuation of these associations after multivariable adjustment for traditional and HIV-related kidney disease risk factors (Figure 3).
Table 3.
Associations of urinary biomarkers with annual change in eGFR during years 1–3 after tenofovir initiation in Women’s Interagency HIV Study and Multicenter AIDS Cohort Study participants with HIV
| Biomarker, per 1 SD | Estimated Annual Change in eGFR in ml/min per 1.73 m2 (95% Confidence Interval) | |||
|---|---|---|---|---|
| First-Year Change in Biomarker | Year 1 Biomarker | |||
| Unadjusteda | Multivariable Adjustedb | Unadjusteda | Multivariable Adjustedb | |
| α-1-Microglobulin | −0.93 (−2.17 to 0.30) | −0.98 (−2.53 to 0.56) | −0.97 (−2.17 to 0.23) | −1.41 (−2.88 to 0.05) |
| Clusterin | −1.57 (−3.68 to 0.54) | −1.91 (−4.07 to 0.25) | 0.96 (−1.70 to 3.63) | 1.84 (−0.97 to 4.65) |
| Trefoil factor 3 | −0.43 (−1.81 to 0.95) | −1.33 (−2.90 to 0.23) | 0.21 (−1.25 to 1.66) | −0.53 (−2.08 to 1.02) |
| IL-18 | 0.62 (−2.51 to 3.75) | −0.06 (−3.37 to 3.24) | −3.12 (−5.18 to −1.06) | −3.26 (−5.39 to −1.14) |
| Kidney injury molecule-1 | 0.50 (−2.67 to 3.67) | 0.75 (−2.25 to 3.74) | 1.47 (−0.74 to 3.69) | 2.27 (−0.12 to 4.65) |
| Monocyte chemoattractant protein-1 | 0.29 (−2.86 to 3.44) | −1.01 (−3.67 to 1.66) | 0.55 (−2.67 to 3.77) | −0.04 (−3.05 to 2.96) |
| Chitinase 3-like protein 1 | −0.11 (−1.74 to 1.52) | −0.75 (−2.47 to 0.96) | −0.05 (−3.12 to 3.03) | −0.40 (−3.86 to 3.05) |
| Albumin-to-creatinine ratio | −0.27 (−1.74 to 1.21) | −0.74 (−2.20 to 0.73) | −0.77 (−2.38 to 0.83) | −1.22 (−2.85 to 0.42) |
| β-2-Microglobulin | −0.25 (−2.17 to 1.66) | −0.84 (−2.74 to 1.06) | 0.28 (−1.21 to 1.78) | −0.50 (−2.06 to 1.06) |
| Cystatin C | −2.70 (−5.25 to −0.15) | −3.50 (−5.98 to −1.02) | −0.76 (−2.81 to 1.29) | −2.07 (−4.34 to 0.21) |
| Epidermal growth factor | −4.38 (−8.79 to 0.02) | −3.55 (−7.70 to 0.60) | −1.24 (−3.63 to 1.15) | −1.11 (−3.56 to 1.34) |
| Neutrophil gelatinase–associated lipocalin | −0.10 (−1.75 to 1.55) | −0.47 (−2.06 to 1.13) | −0.14 (−2.98 to 2.70) | 0.78 (−1.91 to 3.47) |
| Osteopontin | 0.65 (−1.74 to 3.05) | 0.42 (−1.93 to 2.77) | 0.24 (−2.58 to 3.06) | −0.05 (−3.07 to 2.97) |
| Uromodulin | 0.16 (−1.19 to 1.50) | −0.10 (−1.55 to 1.36) | 0.05 (−1.53 to 1.63) | −0.90 (−2.46 to 0.66) |
Estimates are in ml/min per 1.73 m2 per 1 SD increment in biomarker. Estimates from linear mixed models incorporating eGFR values starting at tenofovir initiation (year 0). Models control for each biomarker separately. Time on tenofovir is modeled using a linear spline with cut-off points at year 1 and year 3. Estimates in bold are statistically significant associations.
Unadjusted models include only biomarker, time, biomarker-by-time interaction, and urine creatinine.
Adjusted models additionally control for demographics, diabetes mellitus, smoking, BP, hypertension, HDL, LDL, cardiovascular disease, statin, body mass index, serum albumin, heroin use, current and nadir CD4, current and peak HIV RNA, HIV duration at baseline, history of AIDS, hepatitis C virus, and baseline antiretroviral therapy use.
Finally, we used the LASSO method to identify parsimonious combinations of biomarkers that associated with changes in eGFR during the first year after tenofovir initiation, and during the subsequent year 1–3 period. For first-year eGFR change, the best-fitting LASSO model retained a total of four baseline biomarkers out of the 14 candidates: clusterin, KIM-1, β2m, and uromodulin. For changes in eGFR during the year 1–3 period, in which we considered both first-year biomarker changes and year 1 biomarker concentrations, the LASSO method retained only one biomarker out of the 28 candidates: year 1 IL-18. We then modeled all five LASSO-selected biomarkers in combination using linear mixed models, controlling for HIV-related factors and traditional kidney risk factors. We found that higher baseline clusterin and KIM-1 were independently associated with larger decreases in first-year eGFR (clusterin: −4.4; 95% CI, −7.2 to −1.7; KIM-1: −3.0; 95% CI, −5.6 to −0.29; all estimates in ml/min per 1.73 m2 per 1 SD increment in biomarker), whereas higher baseline uromodulin was associated with a smaller decrease in first-year eGFR (+1.7; 95% CI, 0.12 to 3.4). Higher baseline β2m was also associated with a larger decrease in eGFR, but did not remain statistically significant (−2.1; 95% CI, −4.2 to 0.08). In addition, higher levels of IL-18 at year 1 were associated with larger decreases in eGFR from year 1 to year 3 in a model that controlled for all LASSO-selected markers in addition to other risk factors (−4.2; 95% CI, −6.3 to −2.2).
Discussion
Monitoring for tenofovir-associated nephrotoxicity among individuals with HIV has become increasingly important because of the growing burden of CKD in this population, the widespread use of tenofovir for the treatment of HIV infection, and the potential irreversibility of tenofovir-associated kidney function decline (10,11,19,20). In this study of men and women with HIV, we found that higher pre-tenofovir β2m, clusterin, and KIM-1 were associated with subsequently larger first-year decreases in eGFR independent of traditional and HIV-related kidney disease risk factors, whereas higher pre-tenofovir uromodulin was associated with smaller first-year decreases in eGFR. After tenofovir initiation, we found that a larger first-year increase in urinary cystatin C and a higher year 1 IL-18 were individually associated with larger annual decreases in eGFR from year 1 to year 3. When modeling biomarkers in combination, we found that higher baseline clusterin and KIM-1 and lower baseline uromodulin were jointly and independently associated with larger first-year decreases in eGFR, and higher year 1 IL-18 remained associated with larger annual decreases in eGFR from year 1 to year 3. Overall, our findings suggest that a panel of diverse urinary biomarkers representing different domains of kidney injury may be useful for predicting kidney outcomes in tenofovir initiators with HIV.
To our knowledge, this is the first study in tenofovir initiators with HIV to evaluate associations of a comprehensive panel of urinary biomarkers that reflect kidney tubular dysfunction, injury, fibrosis, and repair with changes in kidney function. Consistent with our findings, a study of 655 tenofovir initiators with HIV found that higher urinary β2m measured before and after tenofovir initiation was associated with greater longitudinal eGFR decline over an average of 3 years (21). Another study of 40 current tenofovir users with HIV showed an association between early increases in urinary β2m and subsequent eGFR decline over a 96-week period (22). In a study of 424 current antiretroviral therapy users with HIV, individuals with kidney tubular damage (defined using urinary α1m, β2m, N-acetyl-β-d-glucosaminidase, and γ-glutamyl transpeptidase) had an unadjusted average decrease in eGFR of approximately 10 ml/min per 1.73 m2 over a 1-year follow-up period, whereas individuals without kidney tubular damage had no change in eGFR (23).
The associations of higher pre-tenofovir β2m with first-year eGFR decline and larger first-year increase in urinary cystatin C with annual eGFR decline from year 1 to year 3 suggest that proximal tubular dysfunction may be involved in tenofovir-associated kidney function decline. β2m and cystatin C are freely filtered, low-molecular-weight proteins that should be fully reabsorbed in the proximal tubule and thus reflect proximal tubular dysfunction when detected in the urine (24). Tenofovir-associated nephrotoxicity is thought to be caused by mitochondrial toxicity localized to the proximal tubule, but the pathophysiology linking tenofovir exposure to kidney function decline has not been fully characterized (8,9). Proximal tubular dysfunction is most likely an early manifestation of proximal tubular injury, but also may directly lead to loss of kidney function by increasing downstream tubular concentrations of potentially nephrotoxic proteins (25). Alternatively, our observations could be explained by proximal tubular dysfunction causing impaired tubular creatinine secretion that might lead to decreases in creatinine-based eGFR.
Urinary biomarkers of kidney injury, such as clusterin, IL-18, KIM-1, neutrophil gelatinase–associated lipocalin, and osteopontin, have predictive value in the early detection of AKI, but their prognostic value with respect to long-term kidney outcomes is less clear. The associations of higher pre-tenofovir clusterin and KIM-1 with first-year eGFR decline and higher year 1 IL-18 with annual eGFR decline from year 1 to year 3 suggest preexisting and continued proximal tubular injury may predispose individuals with HIV who initiate tenofovir to more substantial losses in kidney function. Clusterin and KIM-1 are upregulated and overexpressed by dedifferentiated proximal tubular cells after injury, and IL-18 is a proinflammatory cytokine released by proximal tubular cells in response to injury or inflammation (26–28). Studies using murine models of selective proximal tubular injury with diphtheria toxin show that repeated injury causes maladaptive kidney repair mechanisms that give rise to histologic findings characteristically seen in CKD (29,30). Other experimental models of drug-induced proximal tubular injury using folic acid and cisplatin demonstrate similar findings (31,32).
We also found that higher baseline uromodulin had a protective association with first-year eGFR. Uromodulin (also known as Tamm–Horsfall protein) is a glycoprotein exclusively produced in the kidney by kidney tubular epithelial cells lining the thick ascending limb of the loop of Henle (33). Although the physiologic function of uromodulin remains unclear, higher uromodulin concentrations have been associated with higher eGFR and increased kidney mass, suggesting that uromodulin may be a marker of overall kidney tubular function and reserve (34).
Strengths of our study include a contemporary and diverse cohort of men and women with HIV, serial biomarker measurements pre- and post-tenofovir initiation, and adjustment for multiple traditional and HIV-related kidney disease risk factors. The 14 urinary biomarkers in our panel localize to different regions of the nephron and reflect distinct pathophysiologic mechanisms, which together provide a more comprehensive assessment of drug-induced nephrotoxicity than any single biomarker. Our study also has several limitations. First, urinary biomarkers were measured once before tenofovir initiation and annually after tenofovir initiation, which may have led to exposure misclassification from missing biomarker trajectory changes between measurements. Second, we used creatinine-based eGFR instead of using cystatin C or measured GFR, which may have led to nondifferential outcome misclassification if tenofovir biases creatinine-based eGFR by impairing proximal tubular creatinine secretion. Third, our study may not have accounted for all potential confounders, including exposure to other nephrotoxic medications. Fourth, tenofovir initiators with HIV in our more contemporary study period are likely healthier with less kidney disease than prevalent tenofovir users, which may have led to smaller changes in eGFR after tenofovir initiation in our cohort. To reduce the possibility of false discovery, we used LASSO, a penalized regression method for variable selection that is designed to produce parsimonious models in the setting of high-dimensional data. However, chance findings are still possible, and therefore our findings will need to be validated in other cohorts. Finally, our results may not be generalizable to individuals with HIV initiating nontenofovir-based antiretroviral therapy regimens or to individuals without HIV initiating tenofovir for pre-exposure prophylaxis and treatment of chronic hepatitis B infection.
In summary, urinary biomarkers reflecting pre-existing and tenofovir-associated kidney injury are independently associated with changes in eGFR after tenofovir initiation in individuals with HIV. Our study has several important implications. Compared with prior single biomarker studies, our urinary biomarker panel represents a novel approach to tenofovir-associated nephrotoxicity monitoring that may allow for prediction of kidney outcomes at the time of tenofovir initiation before irreversible losses to kidney function occur. However, for a urinary biomarker panel to be integrated into a clinical decision-making tool, future research should validate our findings in other cohorts of tenofovir users with HIV, determine whether a more parsimonious set of biomarkers can be selected from our panel, evaluate biomarker thresholds for classifying individuals into different risk groups, and evaluate whether biomarkers accurately predict which tenofovir users with HIV would benefit from switching to alternative antiretrovirals. For example, a urinary biomarker panel may help inform whether individuals with HIV should consider switching to the newer tenofovir prodrug, tenofovir alafenamide fumarate, which may be less nephrotoxic (35). Our work using a urinary biomarker panel may also serve as a model for additional drug safety evaluations.
Disclosures
M.G.S. is a Scientific Advisor and holds stock options in TAI Diagnostics and Cricket Health, Inc.
Supplementary Material
Acknowledgments
Data in this article were collected by the Multicenter AIDS Cohort Study (MACS; http://aidscohortstudy.org) and the Women’s Interagency HIV Study (WIHS).
The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional cofunding from the National Cancer Institute (NCI) (U01-AI35039 to S.W., U01-AI35040 to R.D. and O.M.M., U01-AI35041 to C.R., U01-AI35042 to J.M., and U01-AI35043 to L.J. and G.D.), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute, and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by grant UL1-TR001079 (Johns Hopkins University Institute for Clinical and Translational Research) from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The WIHS is funded primarily by the NIAID (U01 AI103401 to M.C.K., U01 AI103408 to G.W. and I.O., U01 AI035004 to K.A., U01 AI031834 to H.M., U01 AI034993 to M.C., U01 AI034994 to S.G.K., U01 AI103397 to M.A.F., U01 AI103390 to A.A.A., U01 AI034989 to R.M.G. and P.C.T., U01 AI042590 to S.G, U01-HD-032632 to J.M.)., with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the NCI, the NIDA, and the NIMH. Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by grants UL1-TR000004 (University of California, San Francisco, Clinical and Translational Science Award), UL1-TR000454 (Atlanta Clinical and Translational Science Award), and P30-AI-050410 (Univeristy of North Carolina at Chapel Hill Center for AIDS Research). M.G.S. and R.S. are funded by grant R01AG034853-08 (National Institute of Aging).
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the NIH, Johns Hopkins Institute for Clinical and Translational Research, or NCATS.
MACS (Principal Investigators): Joseph Margolick (Johns Hopkins University Bloomberg School of Public Health); Steven Wolinsky (Northwestern University); Roger Detels, Oto Martinez-Maza (University of California, Los Angeles); Charles Rinaldo (University of Pittsburgh); Lisa Jacobson, Gypsyamber D’Souza (the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health). WIHS (Principal Investigators): Mirjam-Colette Kempf and Deborah Konkle-Parker University of Alabama at Birmingham WIHS; Ighovwerha Ofotokun and Gina Wingood Atlanta WIHS; Kathryn Anastos Bronx WIHS; Howard Minkoff and D.G. Brooklyn WIHS; M.C. and Audrey French Chicago WIHS; Seble Kassaye Metropolitan Washington WIHS; Margaret Fischl and Lisa Metsch Miami WIHS; Adaora Adimora University of North Carolina at Chapel Hill WIHS; Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien ConnieWofsyWomen’s HIVStudy, Northern California; Stephen Gange and Elizabeth Golub WIHS Data Management and Analysis Center; Joel Milam Southern California WIHS.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01700218/-/DCSupplemental.
References
- 1.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA; Infectious Diseases Society of America : Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis 58: e1–e34, 2014 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization : Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach, Geneva, World Health Organization, 2016 [PubMed] [Google Scholar]
- 3.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, Coakley DF, Lu B, Toole JJ, Cheng AK; 903 Study Group : Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: A 3-year randomized trial. JAMA 292: 191–201, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Izzedine H, Hulot JS, Vittecoq D, Gallant JE, Staszewski S, Launay-Vacher V, Cheng A, Deray G; Study 903 Team : Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients. Data from a double-blind randomized active-controlled multicentre study. Nephrol Dial Transplant 20: 743–746, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M: Systematic review and meta-analysis: Renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis 51: 496–505, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, Shlipak MG: Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS 26: 867–875, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryom L, Mocroft A, Kirk O, Worm SW, Kamara DA, Reiss P, Ross M, Fux CA, Morlat P, Moranne O, Smith C, Lundgren JD; D:A:D Study Group : Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: The D:A:D study. J Infect Dis 207: 1359–1369, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohler JJ, Hosseini SH, Hoying-Brandt A, Green E, Johnson DM, Russ R, Tran D, Raper CM, Santoianni R, Lewis W: Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Invest 89: 513–519, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D’Agati VD, Markowitz GS: Tenofovir nephrotoxicity: Acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int 78: 1171–1177, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Wever K, van Agtmael MA, Carr A: Incomplete reversibility of tenofovir-related renal toxicity in HIV-infected men. J Acquir Immune Defic Syndr 55: 78–81, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Jose S, Hamzah L, Campbell LJ, Hill T, Fisher M, Leen C, Gilson R, Walsh J, Nelson M, Hay P, Johnson M, Chadwick D, Nitsch D, Jones R, Sabin CA, Post FA; UK Collaborative HIV Cohort Study Steering Committee : Incomplete reversibility of estimated glomerular filtration rate decline following tenofovir disoproxil fumarate exposure. J Infect Dis 210: 363–373, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sise ME, Hirsch JS, Canetta PA, Herlitz L, Mohan S: Nonalbumin proteinuria predominates in biopsy-proven tenofovir nephrotoxicity. AIDS 29: 941–946, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Coons SJ: The FDA’s critical path initiative: A brief introduction. Clin Ther 31: 2572–2573, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr : The Multicenter AIDS Cohort Study: Rationale, organization, and selected characteristics of the participants. Am J Epidemiol 126: 310–318, 1987 [DOI] [PubMed] [Google Scholar]
- 15.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J; WIHS Collaborative Study Group : The Women’s Interagency HIV study. Epidemiology 9: 117–125, 1998 [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibshirani R: Regression shrinkage and selection via the lasso. J R Stat Soc B 58: 267–288, 1996 [Google Scholar]
- 18.Schelldorfer J, Bühlmann P, van de Geer S: Estimation for high-dimensional linear mixed-effects models using $\ell_1$-penalization. Scand J Stat 38: 197–214, 2011 [Google Scholar]
- 19.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, Berti A, Rossi E, Roverato A, Palella F: Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 53: 1120–1126, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Wong C, Gange SJ, Buchacz K, Moore RD, Justice AC, Horberg MA, Gill MJ, Koethe JR, Rebeiro PF, Silverberg MJ, Palella FJ, Patel P, Kitahata MM, Crane HM, Abraham AG, Samji H, Napravnik S, Ahmed T, Thorne JE, Bosch RJ, Mayor AM, Althoff KN; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) : First occurrence of diabetes, chronic kidney disease, and hypertension among North American HIV-infected adults, 2000-2013. Clin Infect Dis 64: 459–467, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishijima T, Kurosawa T, Tanaka N, Kawasaki Y, Kikuchi Y, Oka S, Gatanaga H: Urinary β2 microglobulin can predict tenofovir disoproxil fumarate-related renal dysfunction in HIV-1-infected patients who initiate tenofovir disoproxil fumarate-containing antiretroviral therapy. AIDS 30: 1563–1571, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Kinai E, Hanabusa H: Progressive renal tubular dysfunction associated with long-term use of tenofovir DF. AIDS Res Hum Retroviruses 25: 387–394, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Ando M, Yanagisawa N, Ajisawa A, Tsuchiya K, Nitta K: Kidney tubular damage in the absence of glomerular defects in HIV-infected patients on highly active antiretroviral therapy. Nephrol Dial Transplant 26: 3224–3229, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, Grenet O, Pantano S, Moulin P, Wahl D, Mahl A, End P, Staedtler F, Legay F, Carl K, Laurie D, Chibout SD, Vonderscher J, Maurer G: Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol 28: 463–469, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Norden AG, Lapsley M, Lee PJ, Pusey CD, Scheinman SJ, Tam FW, Thakker RV, Unwin RJ, Wrong O: Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int 60: 1885–1892, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Jones SE, Jomary C: Clusterin. Int J Biochem Cell Biol 34: 427–431, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL: Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 43: 405–414, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV: Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82: 172–183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takaori K, Nakamura J, Yamamoto S, Nakata H, Sato Y, Takase M, Nameta M, Yamamoto T, Economides AN, Kohno K, Haga H, Sharma K, Yanagita M: Severity and frequency of proximal tubule injury determines renal prognosis. J Am Soc Nephrol 27: 2393–2406, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan H-T, Li X-Z, Pitera JE, Long DA, Woolf AS: Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 α. Am J Pathol 163: 2289–2301, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharp CN, Doll MA, Dupre TV, Shah PP, Subathra M, Siow D, Arteel GE, Megyesi J, Beverly LJ, Siskind LJ: Repeated administration of low-dose cisplatin in mice induces fibrosis. Am J Physiol Renal Physiol 310: F560–F568, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachmann S, Koeppen-Hagemann I, Kriz W: Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry 83: 531–538, 1985 [DOI] [PubMed] [Google Scholar]
- 34.Pruijm M, Ponte B, Ackermann D, Paccaud F, Guessous I, Ehret G, Pechère-Bertschi A, Vogt B, Mohaupt MG, Martin PY, Youhanna SC, Nägele N, Vollenweider P, Waeber G, Burnier M, Devuyst O, Bochud M: Associations of urinary uromodulin with clinical characteristics and markers of tubular function in the general population. Clin J Am Soc Nephrol 11: 70–80, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, Pozniak A, Thompson M, Podzamczer D, Molina JM, Oka S, Koenig E, Trottier B, Andrade-Villanueva J, Crofoot G, Custodio JM, Plummer A, Zhong L, Cao H, Martin H, Callebaut C, Cheng AK, Fordyce MW, McCallister S; GS-US-292-0104/0111 Study Team : Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: Two randomised, double-blind, phase 3, non-inferiority trials. Lancet 385: 2606–2615, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.