Abstract
Pharmacokinetics and pharmacodynamics follow the logic of cause and consequence. Receptor-mediated and reversible effects can be distinguished from direct and irreversible effects. Reversible effects are capacity-limited and saturable whereas irreversible effects are limited only by the number of viable targets. In the case of receptor-mediated and reversible effects a threshold and a ceiling concentration can be defined. Antimicrobial drugs with concentration-dependent action are distinguished from drugs with time-dependent action. Concentration-dependent effects are associated with a high ceiling concentration and the target is the high peak. Time-dependent effects are associated with a high threshold concentration and the target is the high trough. During kidney dysfunction, alterations of drug response are usually attributed to pharmacokinetic but rarely to pharmacodynamic changes. Dose adjustment calculations, therefore, tacitly presume that pharmacodynamic parameters remain unchanged while only pharmacokinetic parameters are altered in kidney failure. Kidney dysfunction influences the pharmacokinetic parameters of at least 50% of all essential drugs. Clinicians usually consider pharmacokinetics when kidney disease is found, but pharmacodynamics is as important. Alterations of pharmacodynamic parameters are conceivable but only rarely reported in kidney failure. Sometimes surprising dosing adjustments are needed when pharmacodynamic concepts are brought into the decision process of which dose to choose. Pharmacokinetics and pharmacodynamics should both be considered when any dosing regimen is determined.
Keywords: Tacrolimus, pharmacokinetics, kidney failure, chemotherapy
Background and Introduction
Similar to the fact that every patient behaves differently, individual drugs also behave in different ways compared with each other. However, in nature and thus in medicine, basic laws can be identified that apply not only to every drug but also to every patient. Pharmacokinetics and pharmacodynamics present such mathematic laws (Figure 1). Drug concentrations produce the drug effects. Pharmacokinetics are the cause and pharmacodynamics the consequence. Pharmacokinetics allow us to calculate the dose adjustment in kidney disease where sometimes dramatic alterations can be found in roughly half the drugs. Pharmacodynamics allow for a quantitative description of the individual drug response. Additionally, they aid in modeling activation, inhibition, and interaction at the target receptor side (Table 1).
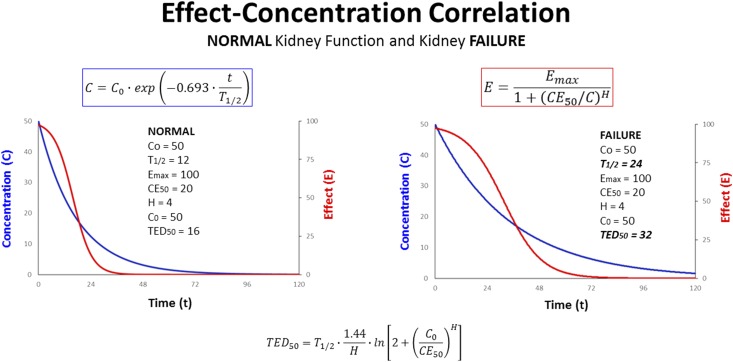
Figure 1.
Pharmacodynamics (PD) follow pharmacokinetics (PK). The time-dependent course of the effect can be modeled by inserting the time-dependent concentration decline (C) into the equation for the pharmacodynamic Emax model (E). In contrast to an irreversible effect, reversible effects (E) concomitantly diminish when concentrations (C) decline with time (t). The effect bisection time will rise in proportion to the T1/2 (T1/2→TED50). This suggests to extend the administration interval when the elimination is impaired in kidney failure (T1/2: 12→24). Emax, maximum effect; CE50, concentration producing half-maximum effect; TED50, effect bisection time.
Table 1.
Pharmacodynamics of medications
| Factors that influence the clinical pharmacologic drug response as measured by onset, intensity, and duration of effect. These factors act primarily by affecting the drug concentration at the receptor site |
| Drug dose |
| Drug pharmacokinetics |
| Receptor number |
| Organ response to receptor activation |
| Counter-regulatory (competing) influences at the receptor |
| Pathologic processes (aging, acute and chronic illness, and kidney disease) can affect pharmacodynamics (clinical response) |
| Decreased receptor number (Emax) and sensitivity (CE50) |
| Decreased receptor binding |
| Altered signal transduction |
| Drugs can interact and compete for similar receptors having multiple effects |
| Synergistic effects |
| Antagonistic effects |
| Drug toxicity (S-shaped effect-concentration correlation and Hill coefficient) |
Emax, maximum effect; CE50, concentration producing half-maximum effect.
Two different mechanisms can be distinguished in pharmacodynamics. The reversible effects, which are usually receptor-mediated and saturable, and the irreversible effects, which are direct and proportional to rising concentrations. The reversible effects are observed with both increasing and decreasing concentrations. The irreversible effects, however, can only be produced by increasing concentrations.
In this paper, we will describe how pharmacodynamic parameters can be extracted from published data and subsequently how these parameters can be used to modify drug dosage in the state of kidney failure. As illustrated by apixaban with a reversible effect, the effect duration will last longer and can be predicted exactly from pharmacodynamic parameters when elimination is impaired in kidney failure. As an example with an irreversible effect, we will discuss how to make pharmacodynamics instrumental for carboplatin dose adjustment to kidney dysfunction. Further drugs will be discussed in the Supplemental Material where pharmacodynamics matter for medication in kidney patients.
Reversible Effect
Pharmacodynamics of reversible effects are capacity-limited and described by the saturable maximum effect (Emax) model (1). The effect cannot grow higher than the Emax, meaning that no more than a 100% response can be elicited due to the biologically limited number of molecular binding sites. An extension of the Emax model yields the sigmoid Hill equation (Supplemental Material). The Hill coefficient (H), sometimes referred to as the “γ” coefficient, represents a purely empiric parameter that determines the sigmoidicity of the effect-concentration correlation. The higher the H, the more the effect-concentration correlation looks S-shaped (Supplemental Figure 1). The usual form of the sigmoid Emax model can be rearranged into a true hyperbolic form.
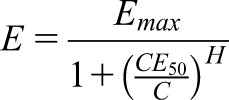 |
Dividing by a fraction is done by multiplication with the reverse fraction: When neglecting the 1+ term in the denominator, one can immediately see that high concentrations (C) produce a strong effect (E). One can also see the effect of the concentration producing the half-maximum effect (CE50). Because the CE50 corresponds to the Kd, a high CE50 is associated with only a weak effect (Supplemental Material). If the CE50 needed to produce 50% of Emax is low, however, the affinity to the receptor is high and this drug has a strong potency.
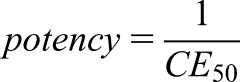 |
The Emax corresponds to the number of receptors or enzyme molecules. Thus, it is primarily a patient-related, not a drug-related, parameter. Emax can also explain how polypharmacy might work: in the case of synergistically acting drugs such as losartan and a thiazide, the receptor-mediated effects and thus the Emax values are additive on BP (E=Emax1+Emax2). Antagonistic combinations such as a typical antipsychotic with a dopaminergic anti-Parkinson drug are unfavorable because the Emax is mutually minimized (E=Emax1−Emax2).
On the other side, the CE50 parameter reflects the intrinsic power of the drug. It is a drug-related parameter. Alterations of the receptor affinity, however, result in CE50 changes and thus the individual response can be influenced by drug interaction, activation, or competition. Alterations of both Emax and CE50 can further occur as disease-related phenomena. In the elderly, changes in drug response often have been explained by an impaired kidney function. An increased sensitivity and a higher drug potency also can be due to a decrease in CE50 values in the elderly.
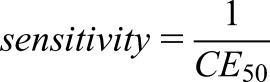 |
An increased sensitivity has been reported for midazolam (CE50: 522→223 ng/ml), nifedipine, morphine, phenytoin, and warfarin, but more resistance with a higher CE50 has been observed for albuterol and metoprolol (2). In the former five examples, a dose reduction might be needed due to pharmacodynamic alterations. The opposite pharmacodynamic change is seen with albuterol and metoprolol that require a higher dose or a change to an alternative drug.
Furosemide and Canagliflozin
In contrast to tacit presumptions, a change in the CE50 can be predicted for furosemide and canagliflozin in CKD. Less potency and a decreased sensitivity with a higher CE50 will be predicted for furosemide or canagliflozin because a higher than normal dose with higher intratubular concentrations is needed to produce the diuretic effect: Although furosemide T1/2 rises from 2 to 10 hours, nephrologists in general experienced that the dose should not be reduced, but instead be increased from 40 to >500 mg to obtain the diuretic effect in kidney failure (3). Canagliflozin, likewise, has been shown to require a higher dose of 300 mg in patients with kidney dysfunction because 100 mg results in underdosing, irrespective of the fact that the T1/2 rises from 13 to 17 hours (4). The observations with furosemide and canagliflozin can be explained by pharmacodynamic not by pharmacokinetic changes in kidney failure.
The H is the exponent of a power function. A high H will result in an augmented CE50/C ratio value if the ratio is >1.0 (>>1.0). In contrast, values of the CE50/C ratio <1.0 will decrease dramatically (<<1.0). In the case of the special condition where the concentration, C, equals the CE50, the ratio of CE50 over C is 1.0 with any H. Without knowing the H, therefore, the CE50 can be read off directly from simultaneous measurements of concentrations and corresponding effects (Figure 2).
Great progress has been made by distinguishing antimicrobial drugs with a concentration-dependent effect from drugs with a time-dependent effect. This difference can be explained by the H: Anti-infective drugs classified as concentration-dependent have a low H<2.0, whereas anti-infective drugs with a time-dependent effect have a high H>2.0 (5). Using the sigmoid Hill equation, the threshold (CE05) and the ceiling concentration (CE95) can be derived (Supplemental Material). For a high H, the CE95 will be low but, simultaneously, the CE05 is high (Supplemental Figure 1).
Drugs with a concentration-dependent effect and a low H have a high CE95, such as gentamicin, levofloxacin, linezolide, daptomycin, colistin, and voriconazole (6). In this case a higher dose results in a stronger effect (Supplemental Figure 1). Conversely, drugs with a time-dependent effect and a high H have a low CE95, but the CE05 will be high such as with piperacillin, ceftazidime, meropenem, vancomycin, clarithromycin, doxycycline, and antiviral drugs (6): In cases with a high threshold, low trough levels might miss the therapeutic target (Ctrough<CE05), because they presumably could fall below the microbiologically minimal inhibitory concentration (MIC).
The dosing regimen can be adjusted to the individual condition by changing either the dose or the length of the administration interval (τ). The decision depends on whether the peak or the trough is the target with repetitive dosing. A high peak is aimed at for the concentration-dependent pharmacodynamics (Ctarget=Cpeak) and a high trough for the time-dependent pharmacodynamics (Ctarget=Ctrough). To increase efficiency, drugs with a concentration-dependent action require the application of a higher dose (e.g., apixaban). In contrast, drugs with a time-dependent action need a higher frequency of their dosing schedule, similar to a continuous infusion (e.g., vancomycin).
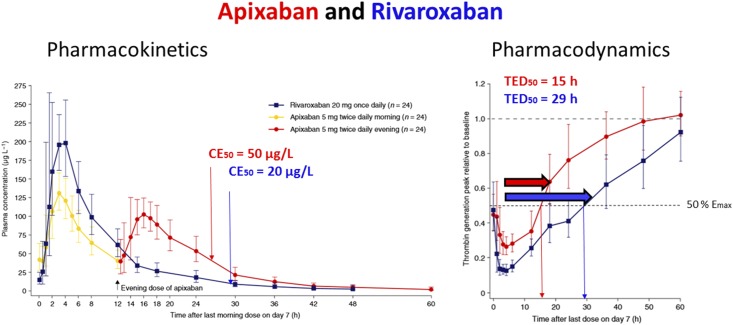
Figure 2.
Pharmacokinetics and pharmacodynamics of apixaban and rivaroxaban (12). Pharmacodynamics (right): The effect bisection time (TED50) can be read off at approximately 15 hours for apixaban (red arrow) and at 29 hours for rivaroxaban (blue arrow). The time after dosing when 50% of maximum effect (Emax) is produced is 15 hours with apixaban but 29 hours with rivaroxaban. Pharmacokinetics (left): At 27 hours (=12+15), the concentration producing 50% of Emax (CE50) can visually be determined with CE50=50 µg/L for apixaban (red) but at 29 hours with CE50=20 µg/L for rivaroxaban (blue).
Vancomycin, Meropenem, and Piperacillin
According to pharmacodynamically based regimens, vancomycin with a time-dependent action should be administered by continuous infusion to increase efficacy but decrease toxicity (7). The target concentration is no longer regarded as a trough level of 10 mg/L. Instead, the target is the average steady state serum concentration Css of up to 25 mg/L (Css×24 hours=area under the curve [AUC]) which is equivalent to an AUC of 400–600 hours×mg/L (7). To meet this target a bolus loading dose of 1500 mg (20 mg/kg) with a subsequently administered continuous infusion might be needed. The subsequent infusion rate must be adjusted to the kidney function or to the RRT according to pharmacokinetic principles. Analogous to vancomycin, meropenem should also be administered as a continuous infusion with the steady state concentration as the therapeutic target (Css) of four times the MIC and up to 32 mg/L (8). This target is in agreement with the time-dependent meropenem action because for H>2.0 the CE50 value roughly corresponds to four times the CE05 (4×CE05=CE50) and the CE05 relates to the MIC (CE05∼MIC). In agreement with these predictions, the mortality was less when β-lactam antibiotics were administered by prolonged infusion (>3 hours) versus short-term infusion (<60 minute); this difference was significant with meropenem and piperacillin but not with ceftazidime and not when just the T1/2 values are prolonged due to impaired kidney function (9).
Apixaban
Pharmacokinetics and pharmacodynamics are closely correlated (Supplemental Material). When concentrations decrease with time, the reversible, receptor-mediated effect will also decrease with time (Figure 1). Analogous to the elimination T1/2 in pharmacokinetics, the effect bisection time (TED50) can be stated as a measure of the effect duration in pharmacodynamics (10). The TED50 indicates the time needed to decrease the effect by 50% (E2=0.50×E1); thus, the TED50 depends on the concentration (C1) producing the initial effect (E1).
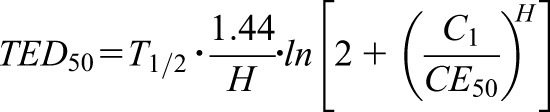 |
The pharmacokinetics of apixaban and rivaroxaban are comparable regarding their normal T1/2 of 8 hours for both direct-acting oral anticoagulants (11). However, the recommended administration interval differs with 12 hours for apixaban and 24 hours for rivaroxaban. It has been demonstrated that plasma levels after a regular dose of 5 mg apixaban reach a maximum concentration of 139 µg/L, in comparison with 20 mg rivaroxaban reaching a higher maximum plasma concentration of 227 µg/L (12). From the published diagram depicting the effect on thrombin generation, the TED50 can be read off with TED50=15 hours for apixaban and with TED50=29 hours for rivaroxaban (Figure 2). In addition, the concentration producing the CE50 can visually be determined with CE50=50 µg/L for apixaban and with CE50=20 µg/L for rivaroxaban just at the time where 50% of the maximum effect is produced (Figure 2).
Using the equation for the TED50 and on the basis of these pharmacokinetic and pharmacodynamic parameter values, the H can be estimated by an iterative numeric solution (Supplemental Figure 2). Thus, the still missing pharmacodynamic parameter can be derived and the estimates are quite comparable with H=1.4 for apixaban and H=1.2 for rivaroxaban. Accordingly, the pharmacodynamics of both apixaban and rivaroxaban can be stated to be concentration-dependent, allowing for a long administration interval. Such inferences can explain why rivaroxaban is applied with an administration interval of 24 hours, although its T1/2 is only 8 hours.
The insight into the pharmacodynamics might affect clinical dosing practice in kidney failure where the T1/2 of apixaban rises to 17 hours, whereas the rivaroxaban T1/2 increases to only 10 hours (11). The antithrombotic efficacy and the bleeding risk were not different for apixaban and rivaroxaban even in CKD (13). The recommended dose of apixaban is 2.5 mg twice a day in advanced kidney disease (13). With 2.5 mg apixaban twice a day the trough concentrations were measured as low as 50 µg/L (14). Because the T1/2 is 17 hours, the corresponding peak concentration will be estimated with 82 µg/L. Because apixaban has a concentration-dependent effect, the aim should be the normal peak of 139 µg/L.
Instead of dosing 2.5 mg every 12 hours, the pharmacodynamic dose adjustment of apixaban to kidney failure would suggest 5 mg once a day because the T1/2 is 17 hours and the TED50 will be estimated with TED50=29 hours. Thus, the suggested apixaban administration interval is just equal to the 24-hour interval of rivaroxaban in kidney failure not requiring dialysis. Otherwise, the trend for apixaban underdosing becomes apparent when the dose for patients receiving dialysis is recommended as 5 mg twice daily, whereas rivaroxaban could still be dosed at 15 mg once a day (13).
Anticancer Therapy
In pharmacokinetics the drug dose results in one concentration but in pharmacodynamics one and the same concentration results in at least two effects—the beneficial and the adverse effect (Supplemental Figure 3). Conventional drugs with a beneficial effect will also have adverse effects. The adverse drug reaction can even be used for a pharmacodynamic monitoring of the therapeutic effect. The therapeutic response to the tyrosine kinase inhibitor erlotinib, likewise, can only be expected if there is a skin rash. Mild myelosuppression—not myelotoxicity, not aplasia—but still tolerable grade 3 anemia, thrombocytopenia, neutropenia, or lymphocytopenia are easy measurable drug effects that might indicate sufficiently high dosing to guarantee the therapeutic target in oncology and immunosuppression (Table 2). Therefore, a grade 3 neutropenia should not give reason to reduce the dose: Some toxicity is needed for anticancer chemotherapy to meet the therapeutic target.
Table 2.
Adverse drug events as surrogate markers for the pharmacodynamic monitoring of therapeutic targets
| Class | Drug | Pharmacodynamic Target | Reference |
|---|---|---|---|
| Anticancer | Adriamycin=doxorubicin | Neutrophil count 1.5/nl | (27) |
| Carboplatin | Grade 2 and 3 neutropenia 1.5–1.0/nl | (28) | |
| Cisplatin | Neutrophil count 1.5/nl | (27) | |
| Cyclophosphamide | Neutrophil count 1.5/nl | (27) | |
| Docetaxel | Grade 3–4 neutropenia <1.0/nl | (29) | |
| Doxorubicin | Neutrophil count 1.5/nl | (27) | |
| Fluorouracil (5FU) | Neutrophil count 1.5/nl | (27) | |
| Paclitaxel escalated | Grade 2–3 neutropenia 1.0–1.5/nl | (30) | |
| Anti-infective | Celgosivir | Δ platelet nadir −∆ 60/nl | (31) |
| Δ hematocrit −∆ 6% | |||
| Valganciclovir | White blood cell count <3/nl | (32) | |
| Hematology | Imatinib | White blood cell count <4.0/nl | (33) |
| Lenalidomide | Δ platelet −∆ 50% | (34) | |
| Grade 3–4 neutropenia and thrombocytopenia | |||
| Trifluridine/tipiracil | Neutropenia or leukopenia or anemia or thrombocytopenia grade 3–4 | (35) | |
| Immunosuppression | Azathioprine | White blood cell count <3.0/nl | (36) |
| Neutropenia <1.0/nl | |||
| Thrombocytopenia <100/nl | |||
| Cyclophosphamide | Neutrophil count <4/nl, | (37) | |
| White blood cell count <4/nl, | (38) | ||
| Lymphopenia threshold 1.0/nl | (39) | ||
| Mycophenolate | Leukopenia <4/nl | (40) | |
| Rituximab | CD19+ B cells <10/mm3=10/µl=0.010/nl | (22) | |
| CD4+ T cells <200/µl=0.2/nl | (41–50) |
Mild myelosuppression with anemia, neutropenia, lymphocytopenia, and thrombocytopenia might indicate a sufficiently high dose of anticancer, anti-infective, or hematologic and immunosuppressive drugs.
Furosemide Infusion
Ototoxicity is considered to be a serious side effect of furosemide. This side effect must be classified as concentration-dependent because the H for the hearing loss under furosemide is reportedly low at H=1.5 (15). Accordingly, the occurrence of ototoxicity is less likely when furosemide is administered by a continuous infusion instead of bolus injection, the latter resulting in a high serum peak level. High serum peak levels and ototoxicity will be avoided by a continuous infusion. Continuous infusion also has been shown to increase the diuretic response and will be advantageous regarding the higher dosage of furosemide usually needed in kidney failure (16).
Irreversible Effect
In contrast to the reversible effects, irreversible effects rarely have been modeled in the literature. Published examples for irreversible effects include the drugs ibrutinib (17), cisplatin (18), clopidogrel (19), and pantoprazole (20). An irreversible effect might be produced by one single drug administration. Irreversible effects persist much longer than concentrations of the drug will remain measurable in the body (Supplemental Material). Whereas reversible effects target a receptor or enzyme molecule, irreversible effects target an on-off mechanism or an active cell (bacteria, cancer cell, immune cell).
Rituximab
The normal dose of the CD20+ B cell antibody rituximab is 375 mg/sqm weekly for 4 weeks. However, two doses of 1000 mg within 2 weeks became the preferred regimen published in the Membranous Nephropathy Trial of Rituximab on the nephrotic syndrome due to membranous GN (21). Although shorter and less frequently dosed (time of treatment [T]=2 weeks), this protocol produces a long-lasting effect as does the standard 4 weeks regimen (21). The irreversible effect can be modeled as depending on dose (D), volume (Vd), and the time of infusion (4 hours) but also as depending on the T.
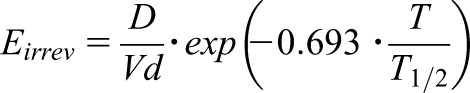 |
As quantitated here for the irreversible effect, the total D (D) (2×1000 mg=2000 mg) given within a shorter time of 2 weeks (T=14 days) will induce a 2.3-fold stronger response (E irrev) than the same dose (4×500 mg=2000 mg) given within the usual 4 weeks (T=28 days). With T1/2=11.5 days, rituximab concentrations are negligible after 50 days but the effect on B cells will persist for 200 days or even up to 500 days (22). Thus, the effect on the initial B cell population can be stated as irreversible. The irreversible effect will persist until the bone marrow regenerates and the immune system will be able to produce a new B cell generation.
Carboplatin
Pharmacodynamics of an irreversible effect will have considerable implications for drug dose adjustment in kidney failure. The difference between reversible and irreversible effects can be illustrated with carboplatin, where the T1/2 increases four-fold from 4.5 to 17 hours in kidney failure (23). With the traditional Calvert formula, the pharmacodynamics of a reversible effect are presumed and the area under the curve is kept unchanged when the kidney function changes (AUC=constant [const.]). With the target AUC of 7 minutes×mg/ml the normal dose of 1000 mg would have to be reduced to 210 mg for kidney failure with a GFR of 5 ml/min (24) when using the Calvert equation (210=7×[5+25]).
In the case of kidney failure, however, a less rigorous dose reduction is required than proportionate to the rise of the T1/2 because only the higher dose can here produce the same irreversible effect as with normal conditions (E irrev=const.): Conveniently, the recommended time of infusion is unchanged with T=2 hours (25). When keeping the infusion time const. (T=Tnorm=const.), the carboplatin dose must be reduced (Supplemental Material).
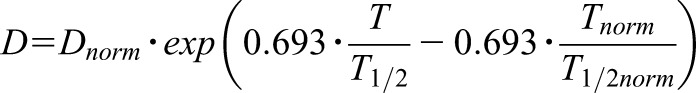 |
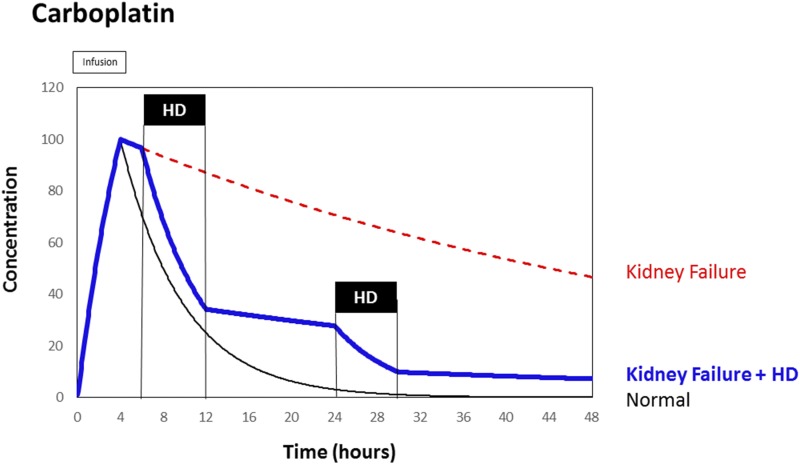
But the dose adjustment on the basis of the pharmacodynamics of an irreversible effect suggests that a dose of 770 mg not 210 mg will be needed to stop the cancer cells: Thus the normal standard dose will be reduced by 23% when giving the 770 mg (1.0–770/1000=0.23) but not by 79% as when reducing to 210 mg (1.0–210/1000=0.79). To limit the risk for severe and intolerable adverse events it will be necessary and has frequently been recommended to perform an immediate hemodialysis session just 2 hours after carboplatin administration (23,25,26). The hemodialysis can be seen as an artificial substitute of normal kidney function (Figure 3).
Figure 3.
Carboplatin and kidney failure: Near-normal elimination kinetics can be established by hemodialysis (HD) initiated 2 hours after carboplatin infusion.
No dose reduction would be needed if the infusion time could be extended in parallel with the four-fold prolongation in the elimination T1/2. But such a four-times-longer infusion time (T=8 hours) would be unfeasible with carboplatin in clinical practice and this regimen does not solve the problem of an increased risk for toxicity due to a rise in the AUC.
Conclusions
Values for pharmacodynamic parameters can be extracted from the published literature using respective key words. With the keywords “EC50+pharmacodynamics” a total of 16,051 publications, with “Hill+pharmacodynamics” 2412 publications, with “E-max model+pharmacodynamics” 436 publications, and even for the keywords “CE50+pharmacodynamics” a total of 41 publications can be identified in PubMed (December 2017). In kidney failure, pharmacokinetics can dramatically change leading to alterations in drug action; but alterations in pharmacodynamic parameters have rarely been considered (Figure 4). Sometimes the values for the CE50 concentration and the H can be extracted and exploited to derive dose modifications appropriate for target attainment. The target is the peak level close to the CE95 for a life-saving induction therapy. The target is the trough level above the CE05 with a long-term maintenance therapy. However, for irreversible effects the target should be the normal maximum concentration.
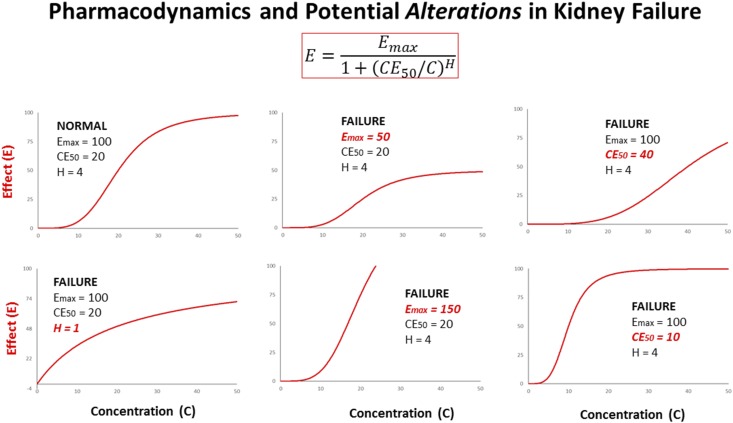
Figure 4.
The effect (E) depends on concentrations (C) according to the sigmoid Emax model. Pharmacodynamic parameters as determined for normal kidney function potentially might change due to kidney failure: When the Hill coefficient decreases (H: →1.0) the dose must be increased. When the maximum effect is diminished (Emax: →50) more of the drug or another drug should be given. When the effects of two drugs are additive (Emax: →150) the combination has advantages. When the concentration producing the half-maximum effect increases (CE50: →40) a higher dose will be needed. Conversely, when the sensitivity increases (CE50: →10) the dose must be reduced. Most frequently, but not exclusively so, the dosage should be reduced in kidney failure.
Disclosures
F.K. received honoraria from Novartis, Hexal, TEWA, Astellas, Roche, Alexion, Medice, Beyer, Pfizer, MSD, Aspen, Shield, and Akademie Niere; he is an employee of Universitätsklinikum Ulm and KfH. A.H. has no conflict of interest.
Supplementary Material
Footnotes
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10960917/-/DCSupplemental.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Wagner JG: Kinetics of pharmacologic response. I. Proposed relationships between response and drug concentration in the intact animal and man. J Theor Biol 20: 173–201, 1968 [DOI] [PubMed] [Google Scholar]
- 2.Aymanns C, Keller F, Maus S, Hartmann B, Czock D: Review on pharmacokinetics and pharmacodynamics and the aging kidney. Clin J Am Soc Nephrol 5: 314–327, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Huang CM, Atkinson AJ Jr, Levin M, Levin NW, Quintanilla A: Pharmacokinetics of furosemide in advanced renal failure. Clin Pharmacol Ther 16: 659–666, 1974 [DOI] [PubMed] [Google Scholar]
- 4.Yamout H, Perkovic V, Davies M, Woo V, de Zeeuw D, Mayer C, Vijapurkar U, Kline I, Usiskin K, Meininger G, Bakris G: Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol 40: 64–74, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Czock D, Keller F: Mechanism-based pharmacokinetic-pharmacodynamic modeling of antimicrobial drug effects. J Pharmacokinet Pharmacodyn 34: 727–751, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Martinez MN, Papich MG, Drusano GL: Dosing regimen matters: The importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob Agents Chemother 56: 2795–2805, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saugel B, Gramm C, Wagner JY, Messer M, Lahmer T, Meidert AS, Schmid RM, Huber W: Evaluation of a dosing regimen for continuous vancomycin infusion in critically ill patients: An observational study in intensive care unit patients. J Crit Care 29: 351–355, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Jamal JA, Mat-Nor MB, Mohamad-Nor FS, Udy AA, Wallis SC, Lipman J, Roberts JA: Pharmacokinetics of meropenem in critically ill patients receiving continuous venovenous haemofiltration: A randomised controlled trial of continuous infusion versus intermittent bolus administration. Int J Antimicrob Agents 45: 41–45, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME: Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: A systematic review and meta-analysis of randomised trials. Lancet Infect Dis 18: 108–120, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Keller F, Schröppel B, Ludwig U: Pharmacokinetic and pharmacodynamic considerations of antimicrobial drug therapy in cancer patients with kidney dysfunction. World J Nephrol 4: 330–344, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough PA, Ball T, Cox KM, Assar MD: Use of oral anticoagulation in the management of atrial fibrillation in patients with ESRD: Pro. Clin J Am Soc Nephrol 11: 2079–2084, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreutz R, Persson PB, Kubitza D, Thelen K, Heitmeier S, Schwers S, Becka M, Hemmrich M: Dissociation between the pharmacokinetics and pharmacodynamics of once-daily rivaroxaban and twice-daily apixaban: A randomized crossover study. J Thromb Haemost 15: 2017–2028, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Fanikos J, Burnett AE, Mahan CE, Dobesh PP: Renal function considerations for stroke prevention in atrial fibrillation. Am J Med 130: 1015–1023, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML: Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol 28: 2241–2248, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos-Sacchi J, Wu M, Kakehata S: Furosemide alters nonlinear capacitance in isolated outer hair cells. Hear Res 159: 69–73, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Palazzuoli A, Ruocco G, Vescovo G, Valle R, Di Somma S, Nuti R: Rationale and study design of intravenous loop diuretic administration in acute heart failure: DIUR-AHF. ESC Heart Fail 4: 479–486, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeks ED: Ibrutinib: A Review in Chronic Lymphocytic Leukaemia. Drugs 77: 225–236, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Qu K, Lin T, Wei J, Meng F, Wang Z, Huang Z, Wan Y, Song S, Liu S, Chang H, Dong Y, Liu C: Cisplatin induces cell cycle arrest and senescence via upregulating P53 and P21 expression in HepG2 cells. Nan Fang Yi Ke Da Xue Xue Bao 33: 1253–1259, 2013 [PubMed] [Google Scholar]
- 19.Dovlatova NL, Jakubowski JA, Sugidachi A, Heptinstall S: The reversible P2Y12 antagonist cangrelor influences the ability of the active metabolites of clopidogrel and prasugrel to produce irreversible inhibition of platelet function. J Thromb Haemost 6: 1153–1159, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Ferron GM, McKeand W, Mayer PR: Pharmacodynamic modeling of pantoprazole's irreversible effect on gastric acid secretion in humans and rats. J Clin Pharmacol 41: 149–156, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, Hogan MC, Dillon JJ, Hickson LJ, Li X, Cattran DC; Mayo Nephrology Collaborative Group: Rituximab therapy in idiopathic membranous nephropathy: A 2-year study. Clin J Am Soc Nephrol 5: 2188–2198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sellier-Leclerc AL, Baudouin V, Kwon T, Macher MA, Guérin V, Lapillonne H, Deschênes G, Ulinski T: Rituximab in steroid-dependent idiopathic nephrotic syndrome in childhood--follow-up after CD19 recovery. Nephrol Dial Transplant 27: 1083–1089, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Oguri T, Shimokata T, Inada M, Ito I, Ando Y, Sasaki Y, Hasegawa Y: Pharmacokinetic analysis of carboplatin in patients with cancer who are undergoing hemodialysis. Cancer Chemother Pharmacol 66: 813–817, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E: Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin Oncol 7: 1748–1756, 1989 [DOI] [PubMed] [Google Scholar]
- 25.Kamei K, Sako M, Ishikawa T, Sato M, Ogura M, Uno T, Kiyotani C, Mori T, Tanaka H, Ito S, Nakamura H: Pharmacokinetics of carboplatin in a one-year-old anuric boy undergoing hemodialysis and a review of the literature. Ther Apher Dial 19: 491–496, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Hirakawa H, Nakashima C, Nakamura T, Masuda M, Funakoshi T, Nakagawa S, Horimatsu T, Matsubara K, Muto M, Kimura S, Sueoka-Aragane N: Chemotherapy for primary mediastinal yolk sac tumor in a patient undergoing chronic hemodialysis: A case report. J Med Case Reports 11: 43, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurney H: How to calculate the dose of chemotherapy. Br J Cancer 86: 1297–1302, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carus A, Gurney H, Gebski V, Harnett P, Hui R, Kefford R, Wilcken N, Ladekarl M, von der Maase H, Donskov F: Impact of baseline and nadir neutrophil index in non-small cell lung cancer and ovarian cancer patients: Assessment of chemotherapy for resolution of unfavourable neutrophilia. J Transl Med 11: 189, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pond GR, Berry WR, Galsky MD, Wood BA, Leopold L, Sonpavde G: Neutropenia as a potential pharmacodynamic marker for docetaxel-based chemotherapy in men with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer 10: 239–245, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Shitara K, Yuki S, Tahahari D, Nakamura M, Kondo C, Tsuda T, Kii T, Tsuji Y, Utsunomiya S, Ichikawa D, Hosokawa A, Ishiguro A, Sakai D, Hironaka S, Oze I, Matsuo K, Muro K: Randomised phase II study comparing dose-escalated weekly paclitaxel vs standard-dose weekly paclitaxel for patients with previously treated advanced gastric cancer. Br J Cancer 110: 271–277, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung C, Wei Y, Watanabe S, Lee HS, Khoo YM, Fan L, Rathore AP, Chan KW, Choy MM, Kamaraj US, Sessions OM, Aw P, de Sessions PF, Lee B, Connolly JE, Hibberd ML, Vijaykrishna D, Wijaya L, Ooi EE, Low JG, Vasudevan SG: Extended evaluation of virological, immunological and pharmacokinetic endpoints of CELADEN: A randomized, placebo-controlled trial of celgosivir in dengue fever patients. PLoS Negl Trop Dis 10: e0004851, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens DR, Sawinski D, Blumberg E, Galanakis N, Bloom RD, Trofe-Clark J: Increased risk of breakthrough infection among cytomegalovirus donor-positive/recipient-negative kidney transplant recipients receiving lower-dose valganciclovir prophylaxis. Transpl Infect Dis 17: 163–173, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Stegmeier F, Warmuth M, Sellers WR, Dorsch M: Targeted cancer therapies in the twenty-first century: Lessons from imatinib. Clin Pharmacol Ther 87: 543–552, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Le Bras F, Sebert M, Kelaidi C, Lamy T, Dreyfus F, Delaunay J, Banos A, Blanc M, Vey N, Schmidt A, Visanica S, Eclache V, Turlure P, Beyne-Rauzy O, Guerci A, Delmer A, de Botton S, Rea D, Fenaux P, Adès L: Treatment by lenalidomide in lower risk myelodysplastic syndrome with 5q deletion--the GFM experience. Leuk Res 35: 1444–1448, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni A, Hochster H, Cleary JM, Prenen H, Benedetti F, Mizuguchi H, Makris L, Ito M, Ohtsu A; RECOURSE Study Group: Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 372: 1909–1919, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Lewis JD, Abramson O, Pascua M, Liu L, Asakura LM, Velayos FS, Hutfless SM, Alison JE, Herrinton LJ: Timing of myelosuppression during thiopurine therapy for inflammatory bowel disease: Implications for monitoring recommendations. Clin Gastroenterol Hepatol 7: 1195–1201, quiz 1141–1142, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fauci AS, Katz P, Haynes BF, Wolff SM: Cyclophosphamide therapy of severe systemic necrotizing vasculitis. N Engl J Med 301: 235–238, 1979 [DOI] [PubMed] [Google Scholar]
- 38.Rasche FM, Klotz CH, Czock D, Karges W, Muche R, Jehle PM, Mertz A, Keller F: Cyclophosphamide pulse therapy in advanced progressive IgA nephropathy. Nephron Clin Pract 93: c131–c136, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Goupil R, Brachemi S, Nadeau-Fredette AC, Déziel C, Troyanov Y, Lavergne V, Troyanov S: Lymphopenia and treatment-related infectious complications in ANCA-associated vasculitis. Clin J Am Soc Nephrol 8: 416–423, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreso F, Serón D, Morales JM, Cruzado JM, Gil-Vernet S, Pérez JL, Fulladosa X, Andrés A, Grinyó JM: Incidence of leukopenia and cytomegalovirus disease in kidney transplants treated with mycophenolate mofetil combined with low cyclosporine and steroid doses. Clin Transplant 12: 198–205, 1998 [PubMed] [Google Scholar]
- 41.Mélet J, Mulleman D, Goupille P, Ribourtout B, Watier H, Thibault G: Rituximab-induced T cell depletion in patients with rheumatoid arthritis: Association with clinical response. Arthritis Rheum 65: 2783–2790, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Hartmann B, Czock D, Keller F: Drug therapy in patients with chronic renal failure. Dtsch Arztebl Int 107: 647–655, quiz 655–656, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doshi S, Chow A, Pérez Ruixo JJ: Exposure-response modeling of darbepoetin alfa in anemic patients with chronic kidney disease not receiving dialysis. J Clin Pharmacol 50[Suppl]: 75S–90S, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Mendes M, Ferreira AC, Navarro D, Pinto B, Gomes F, Matias P, Jorge C, Aires I, Ferreira A: Optimizing the use of darbepoetin-α with a split strategy: A concept change. Clin Nephrol 89: 113–119, 2018 [DOI] [PubMed] [Google Scholar]
- 45.Keller F, Sommerer C, Giese T, Zeier M, Schröppel B: Correlation between pharmacokinetics of tacrolimus and pharmacodynamics on NFAT-regulated gene expression in stable kidney transplant recipients. Clin Nephrol 87 (2017) (2):93–99, 2017 [DOI] [PubMed] [Google Scholar]
- 46.Cleton A, de Greef HJ, Edelbroek PM, Voskuyl RA, Danhof M: Application of a combined “effect compartment/indirect response model” to the central nervous system effects of tiagabine in the rat. J Pharmacokinet Biopharm 27: 301–323, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Czock D, Rasche FM, Carius A, Glander P, Budde K, Bauer S, Keller F, von Müller L: Pharmacokinetics and pharmacodynamics of mycophenolic acid after enteric-coated mycophenolate versus mycophenolate mofetil in patients with progressive IgA nephritis. J Clin Pharmacol 47: 850–859, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Keller F, Hartmann B, Czock D: Time of effect duration and administration interval for sitagliptin in patients with kidney failure. Eur J Drug Metab Pharmacokinet 39: 77–85, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Rolling KE, Jorgenson MR, Descourouez JL, Mandelbrot DA, Redfield RR, Smith JA: Ganciclovir-resistant cytomegalovirus infection in abdominal solid organ transplant recipients: Case series and review of the literature. Pharmacotherapy 37: 1258–1271, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Gudin JA: Assessment of extended-release opioid analgesics for the treatment of chronic pain. J Pain Palliat Care Pharmacother 27: 49–61, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.