Abstract
Background and objectives
Patients with CKD have a high risk of atrial fibrillation. Both CKD and atrial fibrillation are associated with higher risk of stroke and death. However, the effect of incident atrial fibrillation on stroke risk among patients with CKD is unknown.
Design, setting, participants, & measurements
Our study included adults with CKD (eGFR<60 ml/min per 1.73 m2) without previously documented atrial fibrillation who had been in contact with health care in Stockholm, Sweden during 2006–2011. Incident atrial fibrillation was identified by administrative diagnostic codes in outpatient or inpatient care and treated as a time-updated exposure in the analysis of stroke and death risk. Stroke events and deaths were ascertained from regional and national registers with complete coverage. Covariates included demographics, comorbidities, therapeutic procedures, and medications. Multivariable Cox regression analysis and competing risk analysis (accounting for death) were used to estimate the association between incident atrial fibrillation and stroke.
Results
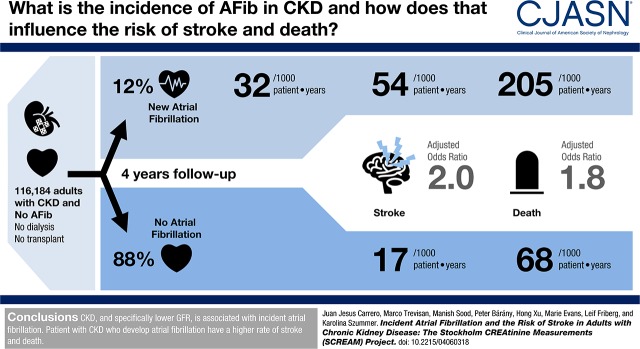
Among 116,184 adults with CKD, 13,412 (12%) developed clinically recognized atrial fibrillation during a mean follow-up of 3.9 years (interquartile range, 2.3–5.7 years). Incidence of atrial fibrillation increased across lower eGFR strata: from 29.4 to 46.3 atrial fibrillations per 1000 person-years in subjects with eGFR=45–60 and <30 ml/min per 1.73 m2, respectively; 1388 (53.8 per 1000 person-years) cases of stroke and 5592 (205.1 per 1000 person-years) deaths occurred after incident atrial fibrillation compared with 6850 (16.6 per 1000 person-years) cases of stroke and 28,613 (67.5 per 1000 person-years) deaths during periods without atrial fibrillation. After adjustment, incident atrial fibrillation was associated with higher risk of stroke (hazard ratio, 2.00; 95% confidence interval, 1.88 to 2.14) and death (hazard ratio, 1.76; 95% confidence interval, 1.71 to 1.82). This was attributed to both ischemic stroke (hazard ratio, 2.11; 95% confidence interval, 1.96 to 2.28) and intracranial bleeds (hazard ratio, 1.64; 95% confidence interval, 1.42 to 1.90). Stroke risk was similar across all eGFR strata. In competing risk analyses accounting for death, the association between incident atrial fibrillation and stroke was attenuated but remained higher (subhazard ratio, 1.49; 95% confidence interval, 1.39 to 1.60).
Conclusions
Patients with CKD who develop atrial fibrillation are at higher risk of stroke and death.
Keywords: chronic kidney disease; cardiovascular disease; Atrial Fibrillation; Incidence; Stroke; Follow-Up Studies; Brain Ischemia; Intracranial Hemorrhages; glomerular filtration rate; Renal Insufficiency, Chronic; Risk; Patient Care
Introduction
CKD is a rapidly growing public health problem, with a population prevalence of 5%–15% (1,2). CKD is associated with a substantially higher risk of cardiovascular complications and death (3). Atrial fibrillation is an important and frequent cardiovascular complication of patients with CKD, with an incidence two- to threefold higher than that in the general population (4–7). CKD and atrial fibrillation share many risk factors, making it difficult to discern the contributions of individual factors to either condition or associated outcomes (8). It has been suggested that the association between CKD and atrial fibrillation may be bidirectional (9,10).
Separately, both CKD (4,6,11) and atrial fibrillation (12–14) are established risk factors for stroke. A recent study suggested that incident atrial fibrillation is associated with a higher risk of death but not with a higher risk of stroke in patients undergoing hemodialysis when death was accounted for as a competing risk (15). The contribution of atrial fibrillation as a mediator of stroke (as well as the stroke subtypes) in the greater community with CKD is presently unknown.
The aim of this study was to estimate the risk of stroke and death that the development of atrial fibrillation may convey to patients with CKD with moderate to advanced kidney disease not requiring dialysis. The question is relevant for improving risk stratification and implementation of more stringent stroke prevention therapies in high-risk individuals, being identified as a research priority at a recent Kidney Disease Improving Global Outcomes (KDIGO) controversies conference (16). However, because many individuals with CKD may die before the initial stroke, traditional analytic approaches may overestimate the true excess stroke risk. Therefore, as a secondary objective, we evaluated death as a competing risk of stroke.
Materials and Methods
Data Sources
This study uses the Stockholm CREAtinine Measurements (SCREAM) health care utilization cohort (17), which includes all residents in the region of Stockholm, Sweden who have had at least one measurement of serum creatinine in ambulatory or hospital care during 2006–2011. The SCREAM covers 98% of all patients with cardiovascular disease registered in the region during this period (17). Laboratory data were linked with regional and national administrative databases for complete information on health care utilization, including diagnoses and procedures, dispensed drugs, validated RRT end points, and follow-up for death, with minimal loss to follow-up. The study used only deidentified data, and thus, individual informed consent was not required. The study was approved by regional institutional review boards and adheres to the Declaration of Helsinki.
Patient Selection
Eligible participants for this study were adults (≥18 years old) with CKD (eGFR<60 ml/min per 1.73 m2) not undergoing dialysis with no history of kidney transplantation and with no prior diagnosis of atrial fibrillation (Supplemental Figure 1). The date of the first available creatinine measured at an outpatient consultation was used to estimate GFR, and if it qualified for CKD, this was considered the index date. eGFR was calculated with the Chronic Kidney Disease Epidemiology Collaboration equation (18) using isotope dilution mass spectrometry standardized serum creatinine tests performed in connection with an ambulatory health care visit. Creatinine values <0.3 and >17.0 mg/dl were considered implausible and discarded. In addition, patients undergoing RRT (dialysis or kidney transplantation) were identified (and excluded from the study) via linkage with the national Swedish Renal Register. CKD severity was then categorized according to the KDIGO staging as follows (19): eGFR=60–45 ml/min per 1.73 m2, eGFR=44–30 ml/min per 1.73 m2, eGFR=29–15 ml/min per 1.73 m2, and eGFR<15 ml/min per 1.73 m2. In the statistical calculations, eGFR=29−15 ml/min per 1.73 m2 and eGFR<15 ml/min per 1.73 m2 were merged to one group labeled eGFR<30 ml/min per 1.73 m2. Previous history of atrial fibrillation was defined as having an International Classification of Diseases, Tenth Edition (ICD-10) I48 diagnosis in primary care, outpatient specialist care, or hospital care (Supplemental Table 1) since the inception of the ICD-10 system in Sweden (1997). Among (total number of individuals with eGFR<60 ml/min per 1.73 m2) 144,109 participants who met initial inclusion criteria, we excluded 1251 subjects undergoing RRT and 26,469 with previously documented atrial fibrillation.
Study Exposure and Covariates
The study exposure is incident atrial fibrillation, which was defined as the first ICD-10 I48 diagnosis after index date. The diagnosis of atrial fibrillation by ICD-10 code has a high diagnostic validity of 95% compared with medical records, including electrocardiograms (20). Study covariates included age, sex, comorbid history, therapeutic procedures undertaken, and medications, and all definitions and algorithms used are detailed in Supplemental Table 1. Comorbidities identified for this study used established algorithms of 85%–95% validity (21). Comorbid history considered ICD-10 codes since 1997 in any position, with the exception of cancer history (within the previous 3 years). Therapeutic procedures were identified from Nordic Medico-Statistical Committee classification codes (22). The congestive heart failure, hypertension, age≥75 years, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, vascular disease including myocardial infarction, age 65–74 years, sex category-females (CHA2DS2-VASc) score (23) was estimated by ICD-10 codes (Supplemental Table 1). Information on pharmacy-dispensed medications was obtained from the Dispensed Drug Registry, a nationwide register that records complete information on all prescription drugs dispensed at Swedish pharmacies. Medications were assumed to be concomitant if there was a pharmacy dispensation within the previous 6 months or 15 days after index date.
Study Outcomes
The primary study outcomes were stroke and all-cause death. Deaths were retrieved from the Swedish death registry, which is updated monthly and has complete national coverage. The stroke outcome included the composite of fatal and nonfatal ischemic strokes and intracranial bleeding. Nonfatal events were identified from diagnostic codes (Supplemental Table 1) issued in primary or secondary position at discharge. Fatal events were identified by diagnostic codes issued as primary or secondary cause of death in the Swedish death registry. Secondary outcomes considered ischemic stroke and intracranial bleeding events separately. Follow-up occurred until an outcome event was reached, the patient died from other causes, or the end of the study period on December 31, 2012.
Statistical Analyses
Baseline characteristics are presented for continuous data as mean (SD) or median (interquartile range). Categorical data are presented as number (percentage). We estimated incidence rates of atrial fibrillation overall and across eGFR strata.
We performed multivariable Cox proportional hazards regression models to examine the independent association between development of atrial fibrillation during follow-up and incident stroke. For this analysis, atrial fibrillation was considered as a time-updated exposure. Thus, a patient who developed atrial fibrillation during follow-up contributed time to the no atrial fibrillation exposure group during the time before atrial fibrillation had been diagnosed and thereafter, to atrial fibrillation–exposed group.
Covariates were also time updated at the time of incident atrial fibrillation and included age, sex, eGFR, history of intracranial bleeding, heart failure, anemia, hypertension, diabetes, vascular disease, prior stroke/transient ischemic attack/systemic embolism, percutaneous coronary intervention, coronary artery bypass graft surgery, peripheral arterial disease, pulmonary embolism, deep venous thrombosis, valvular disease, liver disease, thyroid disease, chronic obstructive pulmonary disease, cancer, alcohol abuse, dementia, and concurrent use of warfarin, aspirin, clopidogrel, selective serotonin reuptake inhibitors (which may increase the risk of bleeding by acting on the platelets [24]), and proton pump inhibitors (a marker for prior gastric/duodenal ulcer). Analyses were run for all study outcomes in the complete dataset and the prespecified subpopulations with differing eGFR category at entry (60–45, <45–30, and <30 ml/min per 1.73 m2).
Because of the high mortality in patients with CKD, competing risk analyses using Fine and Gray regression models were also performed (25). Death due to other causes than the study outcomes was the competing risk, and results are provided as subhazard ratios with 95% confidence intervals (95% CIs). All analyses were performed using R (www.r-project.org) and Stata version 14 (www.stata.com).
Results
Baseline Characteristics
The total study population included 116,184 individuals with CKD (Supplemental Figure 1). At cohort entry, the mean age was 75 (SD=12) years old, 60% were women, 18% had diabetes, 9% had a prior myocardial infarction history, and 11% had a prior stroke/transient ischemic attack or systemic embolism (Table 1). The majority had eGFR=30–60 ml/min per 1.73 m2 (95%), and the remaining 5% had an eGFR<30 ml/min per 1.73 m2. During follow-up, 13,412 subjects (12%) developed atrial fibrillation. The overall incidence was 31.7 per 1000 person-years (95% CI, 31.1 to 32.2). There was an inverse relation between incidence of atrial fibrillation and kidney function. Thus, the incidence was 29.4 per 1000 person-years (95% CI, 28.8 to 29.9) with eGFR=60–45 ml/min per 1.73 m2, 40.8 per 1000 person-years (95% CI, 39.2 to 42.4) with eGFR<45–30 ml/min per 1.73 m2, and 46.3 per 1000 person-years (95% CI, 43.0 to 50.0) with eGFR<30 ml/min per 1.73 m2.
Table 1.
Baseline characteristics
| Covariate | n=116,184a |
| Age, yr, mean (SD) | 75 (12) |
| Women, n (%) | 70,241 (60) |
| eGFR, ml/min per 1.73 m2, mean (SD) | 51 (9) |
| eGFR category, ml/min per 1.73 m2, n (%) | |
| 45–60 | 93,192 (80) |
| 30–44 | 17,796 (15) |
| <30 | 5196 (5) |
| CHA2DS2-VASc, mean (SD) | 2.00 (1.61) |
| Hypertension, n (%) | 39,004 (34) |
| Diabetes mellitus, n (%) | 21,223 (18) |
| Vascular disease, n (%) | 15,766 (14) |
| Myocardial infarction, n (%) | 10,663 (9) |
| Ischemic heart disease, n (%) | 20,797 (18) |
| Transient ischemic stroke, n (%) | 4616 (4) |
| Stroke/transient ischemic attack/systemic embolism, n (%) | 13,081 (11) |
| Intracranial bleeding, n (%) | 2158 (2) |
| Heart failure, n (%) | 15,058 (13) |
| Percutaneous coronary intervention, n (%) | 2959 (2) |
| Coronary artery bypass grafting, n (%) | 1233 (1) |
| Peripheral arterial disease, n (%) | 6624 (6) |
| Pulmonary embolism, n (%) | 1879 (2) |
| Deep venous thromboembolism, n (%) | 3041 (3) |
| Valvular atrial fibrillation, n (%) | 953 (0.8) |
| Other valvular disease, n (%) | 3192 (3) |
| Prosthetic heart valve (biologic), n (%) | 477 (0.4) |
| Prosthetic heart valve (mechanical), n (%) | 636 (0.5) |
| Pacemaker or intracardiac defibrillator, n (%) | 2446 (2) |
| Liver disease, n (%) | 2251 (2) |
| Thyroid disease, n (%) | 7357 (6) |
| Chronic obstructive pulmonary disease, n (%) | 6117 (5) |
| Anemia, n (%) | 12,820 (11) |
| Cancer diagnosis in the last 3 yr, n (%) | 17,564 (15) |
| Alcohol abuse, n (%) | 3155 (3) |
| Dementia, n (%) | 5774 (5) |
| Concurrent medication, n (%) | |
| Aspirin | 36,608 (32) |
| Warfarin | 2760 (2) |
| Clopidogrel | 2886 (2) |
| Statin | 29,977 (26) |
| Selective serotonin reuptake inhibitors | 10,232 (9) |
| Proton pump inhibitors | 21,150 (18) |
CHA2DS2-VASc, congestive heart failure, hypertension, age≥75 years, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, vascular disease including myocardial infarction, age 65–74 years, sex category-females.
At baseline, all individuals were free from atrial fibrillation.
Incident Atrial Fibrillation and the Risk of Stroke and Death
The median follow-up time was 3.9 years (interquartile range, 2.3–5.7). During this period, 8238 subjects had a stroke (Table 2). The majority of these were ischemic (n=6355), but there were also intracranial bleeding events (n=2054; nonmutually exclusive). A total of 1388 strokes occurred after development of atrial fibrillation (53.8 per 1000 person-years) in comparison with a total of 6850 strokes that occurred during periods without atrial fibrillation (16.6 per 1000 person-years). A higher crude stroke incidence after incident atrial fibrillation was observed for both ischemic stroke and intracranial bleeds. A total of 34,205 deaths were recorded, with higher incidence after development of atrial fibrillation (205.1 per 1000 person years) than during nonatrial fibrillation periods (67.5 per 1000 person years).
Table 2.
Association between incident atrial fibrillation and subsequent risk of death and stroke among adults with nondialysis-dependent CKD
| Total No. of Events | Nonatrial Fibrillation N Event (IR per 1000 person-yr)a | Incident Atrial Fibrillation N Event (IR per 1000 person-yr)a | Adjusted Hazard Ratio (95% CI)b | P Value | |
|---|---|---|---|---|---|
| All-cause death | 34,205 | 28,613 (67.5) | 5592 (205.1) | 1.76 (1.71 to 1.82) | <0.001 |
| All-cause stroke | 8238 | 6850 (16.6) | 1388 (53.8) | 2.00 (1.88 to 2.14) | <0.001 |
| Ischemic stroke | 6355 | 5251 (12.6) | 1104 (42.5) | 2.11 (1.96 to 2.28) | <0.001 |
| Intracranial bleed | 2054 | 1762 (4.2) | 292 (10.8) | 1.64 (1.42 to 1.91) | <0.001 |
IR, incidence rate; 95% CI, 95% confidence interval.
IRs per person-years for study outcomes after incident atrial fibrillation and during nonatrial fibrillation periods.
Adjusted hazard ratios for study outcomes associated with the development of incident atrial fibrillation. Multivariable adjustment included age, sex, eGFR, history of intracranial bleeding, history of heart failure, anemia, hypertension, diabetes, vascular disease, prior stroke, transient ischemic attack, percutaneous coronary intervention, coronary artery bypass grafting, peripheral arterial disease, pulmonary embolism, deep venous thrombosis, valvular disease, liver disease, thyroid disease, chronic obstructive pulmonary disease, cancer, alcohol abuse, and dementia as well as use of warfarin, aspirin, clopidogrel, selective serotonin reuptake inhibitors, and proton pump inhibitors.
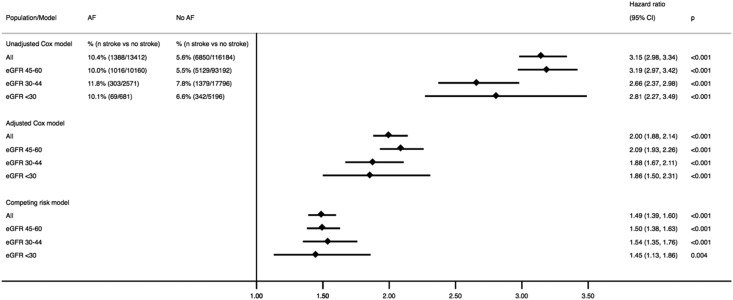
In fully adjusted analyses, the development of atrial fibrillation associated with a hazard ratio of 2.00 (95% CI, 1.88 to 2.14) higher risk of stroke in adults with CKD (Figure 1, Table 2). This was explained by a higher risk for both ischemic stroke and intracranial bleeds. The magnitude was comparable for all eGFR categories (Figure 1), and no statistically significant interactions were noted between incident atrial fibrillation and eGFR categories (P>0.10). When warfarin was added last in the adjusted model, this did not change the estimates across the eGFR categories (Supplemental Table 2).
Figure 1.
The risk of all-cause stroke associated with incident atrial fibrillation (AF) in adults with nondialysis-dependent CKD overall and stratified by eGFR categories. Shown are the crude and adjusted Cox models as well as a Fine and Gray competing risk model with death due to other causes as the competing event. The interaction effect of eGFR (milliliters per minute per 1.73 m2) categories and AF was not statistically significant in the three models (P>0.10). The hazard ratio (HR) was adjusted for age, sex, eGFR (milliliters per minute per 1.73 m2), history of intracranial bleeding, history of heart failure, anemia, hypertension, diabetes, vascular disease, prior history of stroke, percutaneous coronary intervention, coronary artery bypass grafting, peripheral arterial disease, pulmonary embolism, deep venous thrombosis, valvular disease, liver disease, thyroid disease, chronic obstructive pulmonary disease, cancer, alcohol abuse, and dementia as well as use of warfarin, aspirin, clopidogrel, selective serotonin reuptake inhibitors, and proton pump inhibitors. 95% CI, 95% confidence interval.
Incident atrial fibrillation was also associated with hazard ratio of 1.76 (95% CI, 1.71 to 1.82) higher risk of death. When death by other causes was considered as a competing risk (Figure 1), the overall stroke risk was considerably reduced in magnitude, but it was still 49% higher after incident atrial fibrillation (subhazard ratio, 1.49; 95% CI, 1.39 to 1.60) compared with nonatrial fibrillation. No major differences across eGFR strata were observed (Figure 1), with a P for interaction >0.10. The risks for the two subtypes of stroke were similar across eGFR strata, but the magnitude of the risk was higher for ischemic stroke (subhazard ratio, 1.56; 95% CI, 1.44 to 1.68) than for intracranial bleeds (subhazard ratio, 1.22; 95% CI, 1.06 to 1.42).
Discussion
In this region-representative study, we assessed the incidence of atrial fibrillation and the risk of stroke and death that incident atrial fibrillation conveys to adults with CKD. We observed that the incidence of atrial fibrillation increases with lower eGFR. The development of atrial fibrillation was associated with higher risks of stroke and death in these individuals. The association between incident atrial fibrillation and stroke was attenuated when analyzed with death as a competing risk, but it still remained elevated. The stroke risk was similar across different stages of CKD.
The incidence of atrial fibrillation was high among patients with CKD and increased with lower eGFR categories, which supports and extends previous results. Our estimates agree with previous studies reporting atrial fibrillation prevalence among adults with nondialysis CKD ranging between 16% and 21%, which is two to three times higher than that in the general population (26–28). In the United States, the risk of incident atrial fibrillation was estimated to be 16% higher for every 20-ml/min per 1.73 m2 lower eGFR (9). This higher risk of atrial fibrillation has been found to be independent of other known atrial fibrillation risk factors, such as age, sex, hypertension, and other underlying cardiovascular diseases (9).
Our observation that incident atrial fibrillation doubles the mortality risk in adults with CKD is also in agreement with previous studies (10,15). In the study by Bansal et al. (10), there were 81,088 patients with CKD and without prior atrial fibrillation diagnosis. During a mean of 4.8 years, 7.7% of patients developed atrial fibrillation, which was associated with a 66% higher risk of death adjusted for other covariates. This higher risk is likely to be explained by underlying cardiac conditions (e.g., such as heart failure), because only 6%–8% of the deaths in atrial fibrillation are typically related to stroke (29,30).
In addition, we present a novel association between incident atrial fibrillation and the risk of stroke in the setting of CKD. Previous cross-sectional studies exploring populations with prevalent atrial fibrillation have reported that the concomitant presence of CKD is associated with a higher risk of stroke (5,7,11,31) and that worse CKD stages are associated with higher stroke risk (5). These studies quantify the risk that one baseline condition (CKD) confers to patients with atrial fibrillation. Our results are not contradictory, because we address a different question: we are estimating the stroke risk that the development of atrial fibrillation adds to a subject with CKD, acknowledging that CKD per se is a risk factor for stroke (6). We found that the risks associated with atrial fibrillation were consistent across the CKD strata. Thus, it did not increase with lower kidney function. It is unknown why there was no further increased stroke risk associated with atrial fibrillation in the lower CKD strata. Possibilities include the high risk of stroke among patients with CKD being explained by other factors specific for this patient group, such as hypertensive disease. It is also possible that, whereas the stroke risk attributable to atrial fibrillation would be thromboembolic, other stroke types, such as lacunar infarcts, will be higher with lower eGFR.
Atrial fibrillation is the most common sustained arrhythmia. Atrial fibrillation conveys an elevated risk of stroke, and evidence-based guidelines strongly recommend therapeutic interventions to reduce this risk (32,33). There is currently uncertainty as to whether to treat and how to treat atrial fibrillation in patients with CKD, and this study serves to increase awareness of the severity of this condition and the need to provide stroke prevention treatment for these patients. Several randomized controlled clinical trials for stroke prevention with anticoagulants have included patients with moderate CKD and found these therapies to be effective (34–36). Nonetheless, patients with advanced and severe CKD remain under-represented in interventional studies, and clinicians still face a lack of guidance for this non-negligible segment of the population. Our findings illustrate the importance of incident atrial fibrillation in the occurrence of adverse clinical outcomes in patients with CKD. Given the elevated incidence and adverse clinical events of atrial fibrillation in CKD, future studies are needed to elucidate the contributing factors leading to the development of atrial fibrillation in the setting of CKD and potentially therapeutic means of prevention of atrial fibrillation and its associated risks.
Our study has several strengths. We examined a large and region-representative sample of well characterized adults with CKD. Sweden has universal and publicly funded health care, which may reduce access to health care/reimbursement bias. We were able to capture documented incident atrial fibrillation in both the inpatient and outpatient settings through validated diagnosis codes, and we had isotope dilution mass spectrometry–calibrated outpatient serum creatinine measurements to calculate the presence and severity of CKD. We studied the risks associated with the development of atrial fibrillation and accounting for the competing risk of death, which offers more unbiased associations compared with prevalent atrial fibrillation cohorts and traditional Cox regression models. Our study also has limitations. This study included a small number of patients with very advanced CKD or stage 4 CKD and hence, a small number of events, which could decrease our ability to detect a significant difference in outcomes. We cannot rule out residual confounding, although we were able to adjust for a wide range of potential explanatory factors, including differential exposure to relevant medications. We acknowledge the lack of information about measurements of BP and body mass index. We used validated algorithms to define both exposure and outcomes, but we acknowledge that misclassification is possible, especially when atrial fibrillation is defined by administrative codes. Furthermore, atrial fibrillation can be asymptomatic and therefore, undiagnosed. Our definition of CKD only included one measure of eGFR and did not include measures of proteinuria. Being an observational study, it cannot determine causality or mechanisms behind the association between atrial fibrillation and stroke in CKD. Finally, our findings may not be generalizable to other health care settings.
In conclusion, we observe that the development of atrial fibrillation was associated with a twofold higher risk of stroke in adults with CKD, which remained high after adjustment for the competing risk of death. This increase was due to both ischemic stroke and intracranial bleedings, and it was similar regardless of CKD severity.
Disclosures
J.J.C. received lecture honoraria, received research funding, or was a consultant for AstraZeneca, Abbott Laboratories, ViforPharma, Baxter Healthcare, Astellas, and Novartis. L.F. received lecture honoraria or was a consultant for Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Pfizer, Sanofi, and St. Jude. K.S. received lecture honoraria from AstraZeneca and ViforPharma. The rest of coauthors report no conflicts of interest.
Supplementary Material
Acknowledgments
This study was funded by The Swedish Society of Medicine (Svenska Läkaresällskapet), the Swedish Heart and Lung Foundation, the Stockholm County Council (ALF project), and Martin Rind and Westman Foundations. K.S. was supported by the Stockholm County Council (clinical research appointment).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04060318/-/DCSupplemental.
References
- 1.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, Levin A: Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 382: 158–169, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Gasparini A, Evans M, Coresh J, Grams ME, Norin O, Qureshi AR, Runesson B, Barany P, Ärnlöv J, Jernberg T, Wettermark B, Elinder CG, Carrero JJ: Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant 31: 2086–2094, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators : Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 385: 117–171, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonde AN, Lip GY, Kamper AL, Fosbøl EL, Staerk L, Carlson N, Torp-Pedersen C, Gislason G, Olesen JB: Renal function and the risk of stroke and bleeding in patients with atrial fibrillation: An observational cohort study. Stroke 47: 2707–2713, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Fang MC, Udaltsova N, Chang Y, Pomernacki NK, Borowsky L, Singer DE; ATRIA Study Investigators : Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: The anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 119: 1363–1369, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B: Low glomerular filtration rate and risk of stroke: Meta-analysis. BMJ 341: c4249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, Patel MR, Mahaffey KW, Halperin JL, Breithardt G, Hankey GJ, Hacke W, Becker RC, Nessel CC, Fox KA, Califf RM; ROCKET AF Steering Committee and Investigators : Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: Validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation 127: 224–232, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Bansal N, Zelnick LR, Alonso A, Benjamin EJ, de Boer IH, Deo R, Katz R, Kestenbaum B, Mathew J, Robinson-Cohen C, Sarnak MJ, Shlipak MG, Sotoodehnia N, Young B, Heckbert SR: eGFR and albuminuria in relation to risk of incident atrial fibrillation: A meta-analysis of the jackson heart study, the multi-ethnic study of atherosclerosis, and the cardiovascular health study. Clin J Am Soc Nephrol 12: 1386–1398, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS: Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation 127: 569–574, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olesen JB, Lip GY, Kamper AL, Hommel K, Køber L, Lane DA, Lindhardsen J, Gislason GH, Torp-Pedersen C: Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 367: 625–635, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Wolf PA, Dawber TR, Thomas HE Jr ., Kannel WB: Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: The Framingham study. Neurology 28: 973–977, 1978 [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, Wolf PA, Benjamin EJ, Levy D: Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am J Cardiol 82[8A]: 2N–9N, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Wolf PA, Abbott RD, Kannel WB: Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 22: 983–988, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Shih CJ, Ou SM, Chao PW, Kuo SC, Lee YJ, Yang CY, Tarng DC, Lin CC, Huang PH, Li SY, Chen YT: Risks of death and stroke in patients undergoing hemodialysis with new-onset atrial fibrillation: A competing-risk analysis of a nationwide cohort. Circulation 133: 265–272, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Turakhia MP, Blankestijn PJ, Carrero JJ, Clase CM, Deo R, Herzog CA, Kasner SE, Passman RS, Pecoits-Filho R, Reinecke H, Shroff GR, Zareba W, Cheung M, Wheeler DC, Winkelmayer WC, Wanner C; Conference Participants : Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J 39: 2314–2325, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Runesson B, Gasparini A, Qureshi AR, Norin O, Evans M, Barany P, Wettermark B, Elinder CG, Carrero JJ: The Stockholm CREAtinine Measurements (SCREAM) project: Protocol overview and regional representativeness. Clin Kidney J 9: 119–127, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, Hamm LL, Lewis JB, Mauer M, Navis GJ, Steffes MW, Eggers PW, Coresh J, Levey AS: Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 56: 486–495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Smith JG, Platonov PG, Hedblad B, Engström G, Melander O: Atrial fibrillation in the Malmö Diet and Cancer study: A study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol 25: 95–102, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO: External review and validation of the Swedish national inpatient register. BMC Public Health 11: 450, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couser WG, Remuzzi G, Mendis S, Tonelli M: The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80: 1258–1270, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ: Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 137: 263–272, 2010 [DOI] [PubMed] [Google Scholar]
- 24.de Abajo FJ: Effects of selective serotonin reuptake inhibitors on platelet function: Mechanisms, clinical outcomes and implications for use in elderly patients. Drugs Aging 28: 345–367, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 26.Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, Ojo A, Teal VL, Jensvold NG, Robinson NL, Dries DL, Bazzano L, Mohler ER, Wright JT, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic kidney disease and prevalent atrial fibrillation: The Chronic Renal Insufficiency Cohort (CRIC). Am Heart J 159: 1102–1107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ananthapanyasut W, Napan S, Rudolph EH, Harindhanavudhi T, Ayash H, Guglielmi KE, Lerma EV: Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 173–181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McManus DD, Corteville DC, Shlipak MG, Whooley MA, Ix JH: Relation of kidney function and albuminuria with atrial fibrillation (from the Heart and Soul Study). Am J Cardiol 104: 1551–1555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gómez-Outes A, Lagunar-Ruíz J, Terleira-Fernández AI, Calvo-Rojas G, Suárez-Gea ML, Vargas-Castrillón E: Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 68: 2508–2521, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J, Commerford P, Jansky P, Avezum A, Sigamani A, Damasceno A, Reilly P, Grinvalds A, Nakamya J, Aje A, Almahmeed W, Moriarty A, Wallentin L, Yusuf S, Connolly SJ; RE-LY Atrial Fibrillation Registry and Cohort Study Investigators : Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: A cohort study. Lancet 388: 1161–1169, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Providência R, Marijon E, Boveda S, Barra S, Narayanan K, Le Heuzey JY, Gersh BJ, Gonçalves L: Meta-analysis of the influence of chronic kidney disease on the risk of thromboembolism among patients with nonvalvular atrial fibrillation. Am J Cardiol 114: 646–653, 2014 [DOI] [PubMed] [Google Scholar]
- 32.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr ., Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines : 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 64: e1–e76, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; ESC Scientific Document Group : 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37: 2893–2962, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, Reilly PA, Siegbahn A, Yusuf S, Wallentin L: Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: A RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation 129: 961–970, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Hijazi Z, Hohnloser SH, Andersson U, Alexander JH, Hanna M, Keltai M, Parkhomenko A, López-Sendón JL, Lopes RD, Siegbahn A, Granger CB, Wallentin L: Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: Insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol 1: 451–460, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, Braunwald E: Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation 134: 24–36, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.