Abstract
Background and objectives
Postoperative ultrasound is commonly used to assess arteriovenous fistula (AVF) maturation for hemodialysis, but its utility for predicting unassisted AVF maturation or primary AVF patency for hemodialysis has not been well defined. This study assessed the predictive value of postoperative AVF ultrasound measurements for these clinical AVF outcomes.
Design, setting, participants, & measurements
We queried a prospective vascular access database to identify 246 patients on catheter-dependent hemodialysis who underwent AVF creation between 2010 and 2016 and obtained a postoperative ultrasound within 90 days. Multivariable logistic regression was used to evaluate the association of clinical characteristics and postoperative ultrasound measurements with unassisted AVF maturation. A receiver operating characteristic curve estimated the predictive value of these factors for unassisted AVF maturation. Finally, multivariable survival analysis was used to identify factors associated with primary AVF patency in patients with unassisted AVF maturation.
Results
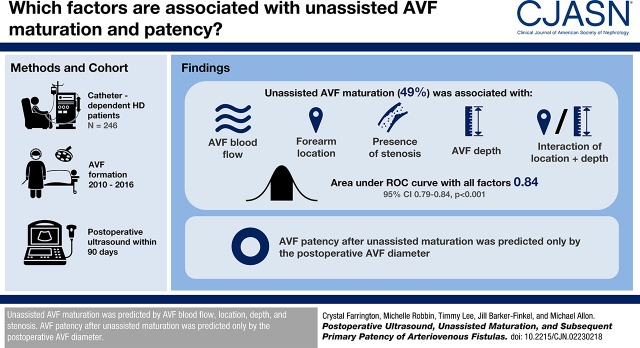
Unassisted AVF maturation occurred in 121 out of 246 patients (49%), assisted maturation in 55 patients (22%), and failure to mature in 70 patients (28%). Using multivariable logistic regression, unassisted AVF maturation was associated with AVF blood flow (odds ratio [OR], 1.30; 95% confidence interval [95% CI], 1.18 to 1.45 per 100 ml/min increase; P<0.001), forearm location (OR, 0.37; 95% CI, 0.08 to 1.78; P=0.21), presence of stenosis (OR, 0.45; 95% CI, 0.23 to 0.88; P=0.02); AVF depth (OR, 0.88; 95% CI, 0.77 to 1.00 per 1 mm increase; P=0.05), and AVF location interaction with depth (OR, 0.50; 95% CI, 0.28 to 0.84; P=0.02). The area under the receiver operating characteristic curve, using all these factors, was 0.84 (95% CI, 0.79 to 0.89; P<0.001). Primary AVF patency in patients with unassisted maturation was associated only with AVF diameter (hazard ratio, 0.84; 95% CI, 0.76 to 0.94 per 1 mm increase; P=0.002).
Conclusions
Unassisted AVF maturation is predicted by AVF blood flow, location, depth, and stenosis. AVF patency after unassisted maturation is predicted only by the postoperative AVF diameter.
Keywords: vascular access; arteriovenous fistula; hemodialysis access; Humans, ROC Curve; Forearm; Logistic Models; Constriction, Pathologic; Prospective Studies; renal dialysis; Survival Analysis
Introduction
Arteriovenous fistulas (AVFs) often fail to mature or require surgical or endovascular intervention to achieve maturation (1,2). Even after successful AVF maturation, interventions are frequently necessary to maintain long-term patency for hemodialysis (3,4). Prior studies have evaluated postoperative ultrasound criteria to assess AVF maturation and identify the need for an intervention to promote maturation. These studies have reported fairly high predictive values for AVF maturation using a threshold postoperative blood flow of 420–800 ml/min or diameter of 3.6–5.4 mm. However, the generalizability of these studies has been limited by the relatively small number of patients, the preponderance of forearm AVFs, and variable definitions of AVF maturation (5–13). Two small studies from our center observed that the combination of an AVF blood flow ≥500 ml/min and draining vein diameter ≥4 mm measured during a 6-week postoperative ultrasound was highly predictive of overall AVF maturation (unassisted or assisted) (6,14). Although published studies have focused on the value of postoperative AVF ultrasound measurements for predicting AVF maturation, none have assessed their predictive value for determining primary patency (time to first intervention once an AVF has achieved unassisted maturation).
We retrospectively analyzed a large cohort of patients dependent on hemodialysis, at the University of Alabama at Birmingham (UAB) medical center, in whom an AVF was created to (1) assess the predictive value of postoperative ultrasound for unassisted AVF maturation, and (2) evaluate whether postoperative ultrasound measurements predicted primary AVF patency in patients with unassisted AVF maturation.
Materials and Methods
Study Population
The UAB is a large dialysis center with approximately 550 patients with ESKD who receive treatment at ten hemodialysis units in the greater Birmingham area. The medical directors of these dialysis units are nephrologists on the UAB faculty. Preoperative and postoperative ultrasounds are performed by radiologists and all vascular access surgeries are performed by four experienced transplant surgeons at UAB. Interventional radiologists and nephrologists perform nearly all percutaneous interventions on patients with vascular accesses, with surgeons performing only a small number of endovascular procedures.
Clinical Management of Vascular Access
Before vascular access surgery, all patients underwent standardized vascular mapping using ultrasound (15,16). Preexisting thrombosis or stenosis in the vasculature disqualified patients from receiving an AVF at that anatomic site. Minimum vessel criteria to be considered for AVF creation included arterial diameter ≥2 mm and vein diameter ≥2.5 mm (16). The surgeons used the results of preoperative ultrasound vein mapping along with their clinical judgment to determine the best type and location of vascular access for each patient. AVFs were created in either the forearm (radiocephalic) or the upper arm (brachiocephalic or transposed brachiobasilic).
Ultrasound findings and clinical evaluation were used to assess AVF maturity and to identify the need for additional interventions to salvage immature AVFs (6), such as revision of the anastomosis or ligation of large accessory veins. If an AVF was deemed too deep for cannulation on postoperative ultrasound or physical examination, it underwent surgical transposition (superficialization). For the purpose of study analysis, standalone surgical transposition of an AVF (without a concurrent surgical intervention such as revision of the anastomosis) was considered to be a procedure to facilitate cannulation rather than an intervention to assist AVF maturation (17,18).
Postoperative Ultrasound Procedure
Patients underwent a postoperative ultrasound, usually within 6 weeks after AVF creation (15). Ultrasound was performed by experienced sonographers with Registered Diagnostic Medical Sonographer certification and read by radiologists with special expertise in hemodialysis ultrasound in a radiology department accredited by the American College of Radiology, using a standardized protocol previously described (6). After an initial overview of the AVF, draining vein diameter was measured at four prescribed locations (2, 5, 10, and 15 cm proximal to the anastomosis) and averaged, and areas of focal stenosis recorded and evaluated with color and spectral Doppler ultrasound. A stenotic lesion was considered hemodynamically significant if the ratio of peak systolic velocity at the stenosis compared with upstream was ≥3:1 within 2 cm of the anastomosis or ≥2:1 in the feeding artery or draining vein (19). AVF depth was measured as the distance from the skin surface to the anterior AVF wall and averaged at the four locations vein diameter was measured. Three to five AVF blood flow measurements were obtained in the draining vein at approximately 10 cm from the anastomosis, and the results were averaged. Clinicians were provided with the ultrasound measurements.
Postmaturation Management of AVFs
Once the AVF was successfully used for dialysis, it was assessed clinically by hemodialysis nurses and nephrologists, specifically focusing on abnormalities detected by physical examination, technical issues related to AVF cannulation, or an unexplained decrease in the Kt/V (20,21). We did not utilize flow monitoring to screen for AVF stenosis. If clinical evaluation suggested the presence of hemodynamically significant stenosis, the patient was referred to interventional radiology/nephrology for a fistulogram. Angioplasty was performed if imaging studies showed >50% stenosis.
Data Collection
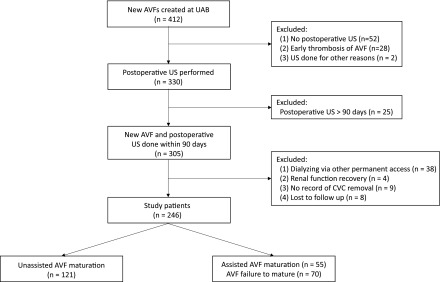
Two full-time dialysis access coordinators in the Division of Nephrology at UAB maintained a comprehensive, prospective, computerized database of all dialysis access-related procedures performed at UAB (22). The UAB Institutional Review Board approved this retrospective study and provided a waiver of consent (approval #X-980813005). We queried this database to identify 412 patients on maintenance hemodialysis undergoing a new AVF creation at UAB between January 1, 2010 and December 31, 2016. Patients were excluded if no postoperative ultrasound was recorded in our database (13%), if the AVF thrombosed before the ultrasound (7%), or if the ultrasound was done for reasons other than assessment of maturity, such as hematoma evaluation or arm swelling (<1%) (Figure 1). Among the 330 patients with a postoperative ultrasound, we excluded 25 patients (8%) whose ultrasound was done ≥90 days after AVF creation. Of the remaining 305 patients with new AVF creation and postoperative ultrasound performed within 90 days, an additional 38 were excluded because of a concurrent functional permanent access (AVF, arteriovenous graft, or peritoneal dialysis catheter). Four patients were excluded because they recovered kidney function shortly after AVF creation. An additional nine patients were excluded because the initial date of first successful AVF use was unclear. Finally, eight patients were lost to follow-up and were not included in the analysis.
Figure 1.
Patient inclusion and exclusion criteria. CVC, central venous catheter; US, ultrasound.
The remaining 246 patients were divided into two subgroups: unassisted AVF maturation and assisted AVF maturation or AVF failure to mature. An AVF was considered mature if the central venous catheter was removed within 180 days of the postoperative ultrasound, such that the AVF was the sole vascular access in use. It was considered to have assisted maturation if there were percutaneous or surgical interventions before maturation (other than an isolated superficialization procedure), and to have unassisted maturation if no such interventions were required. Finally, an AVF was deemed to have failed to mature if the patient remained dependent on a central venous catheter for >180 days after the postoperative ultrasound. The study population of 246 patients included 41 patients enrolled at our medical center in the observational Hemodialysis Fistula Maturation (HFM) study (23). The 6-week postoperative ultrasound procedure used in the HFM cohort was identical to that used in the nonstudy patient, and the results were made available to the clinicians. The HFM study did not stipulate any AVF interventions or cannulation practices; these were completely at the discretion of the nephrologist or surgeon.
The electronic medical records of the patients who met the study criteria were reviewed for demographic and clinical information. Ultrasound data, including mean blood flow, mean draining vein diameter, mean AVF depth, and presence of stenosis, were extracted. Patients with unassisted AVF maturation were followed until the time of first endovascular or surgical intervention (angioplasty, thrombectomy, or surgical revision). For those patients who did not require an intervention after AVF maturation, follow-up was censored at the time of death, kidney transplant, relocation to a non-UAB dialysis unit, or end of study follow-up.
Statistical Analyses
There were no missing data for demographic, clinical, or ultrasound variables for the study cohort. Baseline demographic and clinical characteristics of patients with the two categories of AVF outcomes (unassisted AVF maturation versus assisted AVF maturation of failure to mature) were compared by a chi-squared test for categorical variables and ANOVA or nonparametric tests for continuous variables. We used univariable analysis looking at P values and clinical importance to explore the unadjusted association between the variables and the clinical outcome. We then used stepwise selection process with a significance level for entry of 0.05 and a significance level for stay of 0.10 to build our multivariable model. We also compared several multivariable models by partial likelihood ratio tests to ensure important variables were not dropped by stepwise selection.
Multivariable logistic regression was used to evaluate the association of clinical patient characteristics and postoperative ultrasound measurements with unassisted AVF maturation. Receiver operating characteristic (ROC) curves were generated to determine the predictive value of ultrasound characteristics on unassisted AVF maturation. An area under the curve (AUC) >0.7 was considered clinically significant. Kaplan–Meier analysis was used to evaluate the survival of AVFs with unassisted maturation. Log-rank test was used to compare survival curves. Finally, multivariable Cox proportional hazard models were done, with resulting hazard ratios (HRs) and 95% confidence intervals (95% CIs) determined. P values <0.05 were considered statistically significant.
Results
Clinical Characteristics and Postoperative Ultrasound Measurements of the Study Population
A total of 246 new AVFs created in patients dependent on catheter hemodialysis with a postoperative ultrasound obtained within 90 days were included in the study (Table 1). The mean age of the study population was 53±14 years; there were 62% men and 82% were black. Slightly over half of the patients had diabetes, and nearly half were obese. A substantial proportion of patients had vascular disease or heart failure. Finally, 63% of the AVFs were placed in the upper arm and 37% in the forearm. Compared with the patients excluded from the study, those included in the analysis were more likely to have diabetes, coronary artery disease, peripheral artery disease, and heart failure, and were more likely to be taking antiplatelet agents (Table 1).
Table 1.
Comparison of patients with AVFs included in and excluded from the study
| Variable | Study Patients | Excluded Patients | P Value |
|---|---|---|---|
| No. of AVFs | 246a | 166 | |
| Age in yr, mean±SD | 53±14 | 51±15 | 0.11 |
| Men, n (%) | 153 (62) | 107 (64) | 0.64 |
| Black, n (%) | 202 (82) | 124 (75) | 0.07 |
| Comorbidities, n (%) | |||
| Hypertension | 234 (95) | 152 (92) | 0.14 |
| Diabetes | 139 (56) | 65 (39) | <0.001 |
| Coronary artery disease | 69 (28) | 24 (14) | 0.001 |
| Peripheral vascular disease | 38 (15) | 10 (6) | 0.004 |
| Cerebrovascular disease | 51 (21) | 22 (13) | 0.05 |
| Heart failure | 85 (34) | 29 (17) | <0.001 |
| Obesity, BMI≥30 kg/m2 | 114 (46) | 66 (40) | 0.19 |
| AVF location, upper arm | 155 (63) | 118 (71) | 0.09 |
| Antiplatelet agent | 117 (48) | 62 (37) | 0.04 |
AVF, arteriovenous fistula; BMI, body mass index.
In 223 patients.
Of the study patients, 121 (49%) had unassisted AVF maturation. Of the remaining patients, 55 (22%) had assisted AVF maturation and 70 (28%) had AVFs that failed to mature. There were no significant statistical differences in patient demographics or comorbidities between patients within the two AVF outcome groups (unassisted AVF maturation versus AVF failure to mature or assisted maturation) (Table 2). However, a forearm location was associated with lower likelihood of unassisted AVF maturation (P<0.001).
Table 2.
Association of baseline demographic, clinical, and postoperative ultrasound characteristics of patients with unassisted AVF maturation versus assisted AVF maturation or failure to mature
| Variable | All Patients | Unassisted Maturation | Assisted Maturation or Failure to Mature | P Value |
|---|---|---|---|---|
| No. of patients | 246 | 121 | 125 | |
| Age in yr, mean±SD | 53±14 | 51±12 | 55±15 | 0.07 |
| Men, n (%) | 153 (62) | 82 (68) | 71 (57) | 0.08 |
| Black, n (%) | 202 (82) | 103 (85) | 99 (79) | 0.22 |
| Comorbidities, n (%) | ||||
| Hypertension | 234 (95) | 114 (94) | 120 (96) | 0.52 |
| Diabetes mellitus | 139 (56) | 69 (57) | 70 (56) | 0.87 |
| Coronary artery disease | 69 (28) | 32 (26) | 37 (30) | 0.58 |
| Peripheral vascular disease | 38 (15) | 20 (17) | 18 (14) | 0.64 |
| Cerebrovascular disease | 51 (21) | 22 (18) | 29 (23) | 0.33 |
| Heart failure | 85 (34) | 44 (36) | 41 (33) | 0.56 |
| Obesity, BMI≥30 kg/m2 | 114 (46) | 59 (49) | 55 (44) | 0.45 |
| Antiplatelet drugs | 117 (48) | 56 (46) | 61 (49) | 0.69 |
| AVF location, upper arm | 155 (63) | 91 (75) | 64 (51) | <0.001 |
| Postoperative ultrasound | ||||
| Draining vein diameter in mm, mean±SD | 7.4±2.2 | 8.0±2.4 | 6.8±2.1 | <0.001 |
| Blood flow, ml/min, median [IQR] | 665 [424–926] | 860 [643–1142] | 486 [227–714] | <0.001 |
| Depth in mm, mean±SD | 3.5±2.8 | 3.0±2.4 | 4.1±3.2 | 0.002 |
| Stenosis, n (%) | 133 (54) | 47 (39) | 86 (69) | <0.001 |
AVF, arteriovenous fistula; BMI, body mass index; IQR, interquartile range.
The median time from AVF creation to the postoperative ultrasound was 36 days (interquartile range [IQR], 31–46 days). The range was 6–90 days, and 24 ultrasounds (10%) were performed more than 8 weeks after AVF creation. Compared with patients whose AVFs failed to mature or had assisted maturation, those with unassisted AVF maturation had a greater draining vein diameter, a higher AVF blood flow, a smaller AVF depth, and a lower frequency of AVF stenosis (Table 2).
Association of Clinical Characteristics and Postoperative Ultrasound Measurements with Unassisted AVF Maturation
We evaluated the association of clinical features and ultrasound measurements with unassisted AVF maturation compared with assisted AVF maturation or AVF failure to mature (Table 3). None of the demographic or clinical characteristics were associated with unassisted AVF maturation. A forearm AVF location was associated with a lower likelihood of unassisted AVF maturation, as previously reported (2). In addition, several postoperative ultrasound measurements were associated with unassisted AVF maturation. Specifically, a greater AVF diameter and blood flow were associated with a higher likelihood of unassisted AVF maturation. Conversely, a deeper AVF and presence of stenosis were each associated with a lower likelihood of unassisted AVF maturation. The likelihood of unassisted AVF maturation was 52% in patients with a postoperative blood flow of 500–800 ml/min versus 75% in those with a postoperative blood flow >800 ml/min (odds ratio [OR], 0.36; 95% CI, 0.19 to 0.69; P=0.002). Likewise, the likelihood of unassisted AVF maturation was 43% in patients with a postoperative AVF diameter of 4–7 mm versus 56% in those with a postoperative diameter >7 mm (OR, 0.60; 95% CI, 0.36 to 0.99; P=0.05). Finally, the positive predictive value of the combination of a postoperative blood flow ≥500 ml/min and diameter ≥4 mm was 64% for unassisted AVF maturation. We also evaluated the interactions between AVF location and the ultrasound measurements with respect to unassisted AVF maturation. There was a significant interaction of AVF location with depth (P=0.02), but not with blood flow (P=0.42) or stenosis (P=0.23). Specifically, a deep AVF was more likely to have an unassisted maturation if it was in the upper arm than in the forearm.
Table 3.
Odds ratios for unassisted AVF maturation versus assisted maturation and nonmaturation: univariable analysis
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Age (per 10 yr) | 0.87 | 0.72 to 1.05 | 0.16 |
| Sex, female versus male | 0.63 | 0.37 to 1.05 | 0.08 |
| Race, nonblack versus black | 0.67 | 0.34 to 1.28 | 0.23 |
| Hypertension | 0.68 | 0.21 to 2.22 | 0.52 |
| Diabetes mellitus | 1.04 | 0.63 to 1.72 | 0.87 |
| Coronary artery disease | 0.85 | 0.49 to 1.49 | 0.58 |
| Peripheral vascular disease | 1.18 | 0.59 to 2.32 | 0.64 |
| Cerebrovascular disease | 0.74 | 0.39 to 1.37 | 0.33 |
| Heart failure | 1.18 | 0.69 to 2.00 | 0.56 |
| Obesity, BMI≥30 kg/m2 | 1.20 | 0.74 to 2.00 | 0.45 |
| AVF location, forearm versus upper arm | 0.34 | 0.20 to 0.59 | <0.001 |
| Postoperative ultrasound | |||
| Draining vein diameter, per 1 mm | 1.30 | 1.15 to 1.49 | <0.001 |
| Blood flow, ml/min per 100 ml/min | 1.33 | 1.22 to 1.45 | <0.001 |
| Depth, per 1 mm | 0.83 | 0.59 to 0.91 | 0.003 |
| Stenosis | 0.29 | 0.17 to 0.49 | <0.001 |
AVF, arteriovenous fistula; 95% CI, 95% confidence interval; BMI, body mass index.
Using multivariable logistic regression, unassisted AVF maturation was associated with the following factors: AVF blood flow (OR, 1.30; 95% CI, 1.18 to 1.45 per 100 ml/min increase; P<0.001), forearm location (OR, 0.37; 95% CI, 0.08 to 1.78; P=0.21), presence of stenosis (OR, 0.45; 95% CI, 0.23 to 0.88; P=0.02), AVF depth (OR, 0.88; 95% CI, 0.77 to 1.00 per 1 mm increase; P=0.05), and depth interaction with AVF location (OR, 0.50; 95% CI, 0.28 to 0.84; P=0.02) (Table 4). The overall P value for the multivariable model incorporating all four variables was <0.001.
Table 4.
Odds ratios for unassisted AVF maturation versus assisted maturation and nonmaturation: multivariable analysis
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Blood flow, ml/min, per 100 ml/min | 1.30 | 1.18 to 1.45 | <0.001 |
| AVF location, forearm versus upper arm | 0.37 | 0.08 to 1.78 | 0.21 |
| Stenosis | 0.45 | 0.23 to 0.88 | 0.02 |
| Depth, per 1 mm | 0.88 | 0.77 to 1.00 | 0.05 |
| Depth interaction with AVF location | 0.50 | 0.28 to 0.84 | 0.02 |
Overall P value for the model <0.001. AVF, arteriovenous fistula; 95% CI, 95% confidence interval.
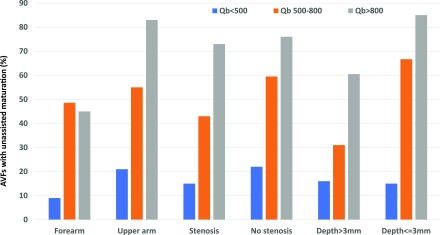
The interaction of AVF blood flow with the other three variables in the model in predicting the likelihood of unassisted AVF maturation is depicted in Figure 2. Overall, the likelihood of unassisted AVF maturation became progressively higher in patients in the postoperative AVF blood flow categories of <500, 500–800, and >800 ml/min, respectively. However, for each postoperative blood flow subgroup, the likelihood of unassisted AVF maturation was smaller for forearm versus upper arm AVFs, smaller if the AVF had a stenosis versus no stenosis, and smaller if the AVF depth was >3 mm versus ≤3 mm. All interactions underlying these figures were nonsignificant (P>0.2).
Figure 2.
Progressive increases in AVF blood flow are associated with higher rates of unassisted AVF maturation for multiple subgroups. However, at any given blood flow, forearm location, stenosis, and greater depth were each associated with a decreased likelihood of unassisted AVF maturation. Qb, AVF blood flow.
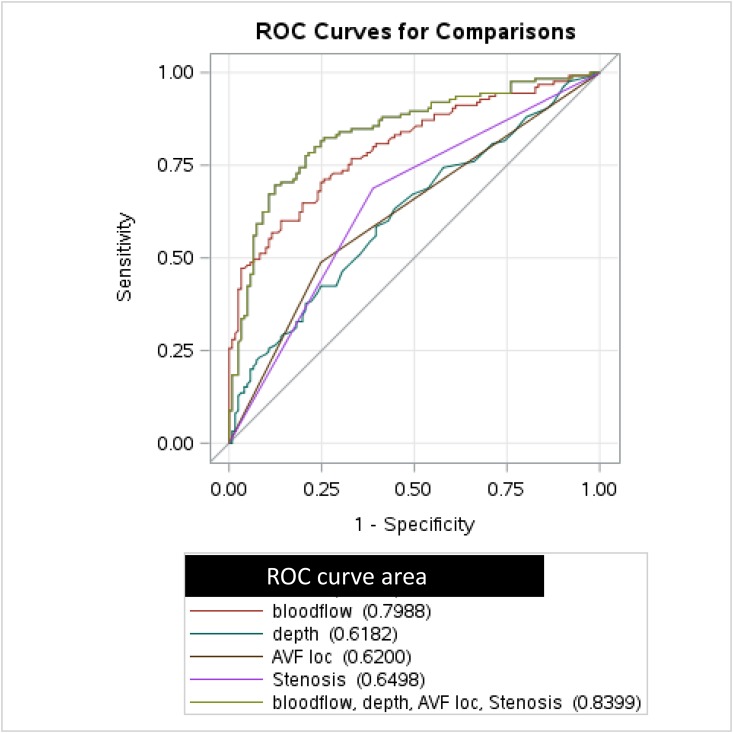
Finally, we generated ROC curves to quantify the predictive value of these four clinical and postoperative ultrasound AVF measurements for the likelihood of unassisted AVF maturation (Figure 3). The ROC curve that included only blood flow had a fairly high predictive value (AUC=0.80; 95% CI, 0.75 to 0.86; P<0.001). When all four factors (AVF blood flow, AVF location, AVF stenosis, and AVF depth) were included in the model, the AUC increased further, to 0.84 (95% CI, 0.79 to 0.89; P<0.001). We used ROC contrast estimation comparing the ROC curve for blood flow alone with the full model with the addition of stenosis, AVF location, and depth. The ROC curve for the full model was significantly different from the ROC for blood flow alone (95% CI, 0.01 to 0.08; P=0.03). Finally, separate ROC analyses for forearm and upper arm AVFs using the same factors yielded AUCs of 0.85 and 0.84, respectively.
Figure 3.
ROC curve showing that unassisted AVF maturation is predicted by postoperative blood flow, AVF depth, AVF location, and postoperative stenosis. loc, location.
Association of Clinical and Postoperative Ultrasound Measurements with Primary AVF Patency in Patients with Unassisted AVF Maturation
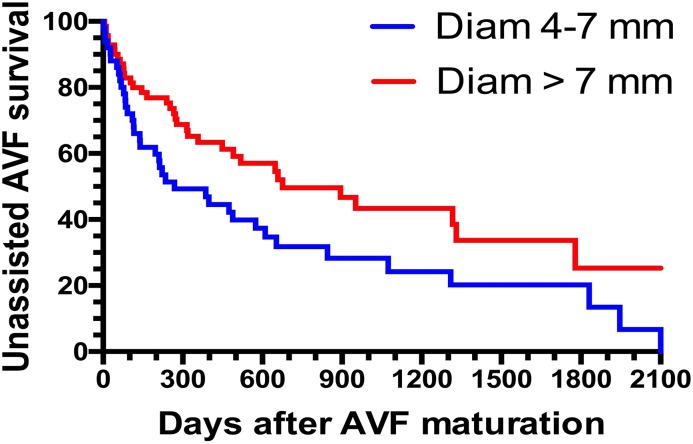
Among patients with unassisted AVF maturation, the median time from the postoperative ultrasound to successful use was 61 days (IQR, 42–81 days). Median patient follow-up after AVF maturation was 317 days (IQR, 110–617 days). We evaluated whether clinical characteristics or postoperative ultrasound measurements were associated with the likelihood of a subsequent AVF intervention in the subset of 121 patients with unassisted AVF maturation. The primary AVF patency (time from AVF maturation to the first intervention) was not significantly associated with patient age, sex, race, or baseline comorbidities (Table 5). It was also not different between forearm and upper arm AVFs. Among the four postoperative ultrasound measurements, only AVF diameter was associated with primary AVF patency. Specifically, each 1-mm increase in AVF diameter was associated with a 16% lower likelihood of an AVF intervention after maturation. Compared with AVFs with a postoperative diameter >7 mm, those with a diameter of 4–7 mm were significantly more likely to lose primary patency (HR, 1.72; 95% CI, 1.10 to 2.83; P=0.001) (Figure 4). The median time to loss of primary patency was 269 (IQR, 83–1074) days in patients with a postoperative AVF diameter 4–7 mm, compared with 676 (IQR, 251–1778) days in those with an AVF diameter >7 mm. Primary AVF patency was not associated with other postoperative AVF measurements, including blood flow, depth, or stenosis. Finally, using a multivariable model, the only factor associated with postmaturation intervention was AVF diameter (HR per 1 mm increase in diameter, 0.84; 95% CI, 0.76 to 0.94; P=0.002).
Table 5.
Postmaturation AVF survival without intervention for patients with unassisted maturation: univariable analysis
| Variable | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| No. of patients | |||
| Age, per 10 yr | 1.10 | 0.91 to 1.36 | 0.30 |
| Sex, female versus male | 1.01 | 0.62 to 1.66 | 0.96 |
| Race, black versus nonblack | 1.26 | 0.64 to 2.47 | 0.50 |
| Hypertension | 1.02 | 0.43 to 2.41 | 0.96 |
| Diabetes mellitus | 1.18 | 0.74 to 1.87 | 0.49 |
| Coronary artery disease | 1.34 | 0.81 to 2.21 | 0.27 |
| Peripheral vascular disease | 1.21 | 0.67 to 2.17 | 0.54 |
| Cerebrovascular disease | 1.04 | 0.58 to 1.86 | 0.91 |
| Heart failure | 1.05 | 0.64 to 1.72 | 0.84 |
| Obesity, BMI≥30 kg/m2 | 0.97 | 0.61 to 1.54 | 0.90 |
| AVF location, upper arm versus forearm | 1.12 | 0.66 to 1.90 | 0.67 |
| Postoperative ultrasound | |||
| Stenosis | 1.06 | 0.66 to 1.68 | 0.82 |
| Diameter, per mm | 0.84 | 0.76 to 0.94 | 0.001 |
| Blood flow, per 100 ml/min | 0.95 | 0.90 to 1.00 | 0.05 |
| Depth, per mm | 1.06 | 0.97 to 1.15 | 0.24 |
AVF, arteriovenous fistula; 95% CI, 95% confidence interval; BMI, body mass index.
Figure 4.
Larger postoperative diameter is associated with greater primary AVF patency after unassisted maturation. Diam, diameter.
Discussion
This study evaluated the predictive value of a postoperative ultrasound for both unassisted AVF maturation and primary AVF patency in patients who achieved unassisted AVF maturation. Our analysis identified four factors that predicted unassisted maturation: AVF blood flow, stenosis, depth, and location. Interestingly, although stenosis and AVF location predicted unassisted AVF maturation, neither factor was associated with primary AVF patency. It is notable that a substantial proportion (39%) of AVFs with unassisted maturation had a postoperative stenosis, confirming a previous observation that many AVFs mature without correcting an underlying stenosis (19).
Several previous studies have assessed the predictive value of postoperative ultrasound measurements for AVF maturation (5–13). These studies had some limitations affecting their generalizability. First, four of the studies enrolled only patients with forearm AVFs (7–9,11), so the results could not be extrapolated to upper arm AVFs. Even among those studies that included upper arm AVFs (5,6,10,12,13), the numbers were quite modest (21–79 patients), compared with 155 upper arm AVFs in this study. Second, the definition of AVF maturation differed among the studies, making it difficult to compare their findings. Thus, for example, the two previous studies from our center focused on the predictive value of postoperative ultrasound measurements for overall AVF maturation, both assisted and unassisted (6,12). By contrast, our study assessed prediction of only unassisted maturation. Third, all of these studies considered the predictive value of only two ultrasound factors, blood flow and diameter, whereas our study also assessed AVF depth and stenosis. Finally, our study was unique in that it performed multivariable logistic regression to assess the interaction among the ultrasound measurements, whereas the previous studies either analyzed each parameter separately, or at most, evaluated only the interaction of AVF diameter and blood flow.
In contrast to the multiple factors associated with unassisted AVF maturation, only one factor (AVF diameter) predicted primary patency of AVFs achieving unassisted maturation. Why might AVFs with a greater diameter have more prolonged patency before requiring an intervention? The major etiology of AVF failure is the development of critical, flow-limiting stenosis, as a consequence of aggressive neointimal hyperplasia (24). It is plausible that AVFs with a greater initial average diameter require a longer period of progressive intimal hyperplasia before the stenosis becomes flow-limiting. Importantly, we did not perform flow monitoring of AVFs, so the decision to intervene was dictated by abnormalities in clinical monitoring rather than arbitrary blood flow thresholds.
The strengths of this study include standardized postoperative imaging techniques performed by experienced sonographers and radiologists at our institution. Prospective collection of vascular access data along with the vast majority of patients in the study receiving all of their medical care within the UAB health system allowed for comprehensive data and greater confidence in the accuracy and completeness of access outcomes. The relatively large study size (246 patients), compared with <100 patients in most previous publications (25), increased the likelihood of finding statistically significant associations. Third, we analyzed ultrasound measurements (diameter, blood flow, and depth) as continuous variables, whereas previous studies simply evaluated threshold values.
Our study also has a number of limitations. First, although AVF outcomes were collected prospectively, they were analyzed retrospectively, which may lead to selection bias. Second, these findings represent the experience of a single center, and the results may not generalize to hemodialysis populations at all centers. In fact, analysis of the seven-center HFM study reported large intercenter variability in the frequency of unassisted AVF maturation, from 38% to 77%, that could not be explained by the postoperative ultrasound measurements (26). Third, our study population reflects the predominance of black patients, and it is unknown whether our findings generalize to white patients undergoing AVF creation. Fourth, 20% of the patients in our initial cohort did not have postoperative ultrasound, and the excluded patients had fewer comorbidities than the study population. Thus, our results are applicable to patients with higher comorbidity, in whom the postoperative ultrasound is likely more valuable. Finally, the clinicians were aware of the results of the postoperative ultrasound. To the extent that the ultrasound influenced their management of the AVFs, this may have confounded the interpretation of the predictive value of the ultrasound for AVF outcomes.
In conclusion, four findings on postoperative ultrasound are predictive of better unassisted AVF maturation: greater AVF blood flow, smaller depth, upper arm location, and absence of stenosis. Once an AVF has achieved unassisted maturation, however, only diameter is predictive of the subsequent primary patency. AVFs that achieve unassisted maturation but have smaller postoperative diameters may require closer monitoring to identify those AVFs possibly needing earlier intervention. Finally, a multicenter study looking at the predictive value of postoperative ultrasound measurements for unassisted AVF maturation and primary AVF patency after maturation is needed to confirm the observations from the current single-center study.
Disclosures
T.L. is a consultant for Proteon Therapeutics, Merck, and Boston Scientific. M.A. is a consultant for CorMedix.
Acknowledgments
C.A.F. is supported by a postdoctoral institutional T-32 grant from the National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) (T32 DK007545-30). T.L. is supported by grant 2R44 DK109789-02 from the NIDDK, 1R01HL139692-01 from the National Heart, Lung, and Blood Institute, and grant 1I01BX003387-02 from a Veterans Affairs Merit Award. M.A. is supported by grant R21-DK104248 from the NIDDK.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Harms JC, Rangarajan S, Young CJ, Barker-Finkel J, Allon M: Outcomes of arteriovenous fistulas and grafts with or without intervention before successful use. J Vasc Surg 64: 155–162, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Stolic R: Most important chronic complications of arteriovenous fistulas for hemodialysis. Med Princ Pract 22: 220–228, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu H, Patel S, Hanisch JJ, Santana JM, Hashimoto T, Bai H, Kudze T, Foster TR, Guo J, Yatsula B, Tsui J, Dardik A: Future research directions to improve fistula maturation and reduce access failure. Semin Vasc Surg 29: 153–171, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferring M, Henderson J, Wilmink T: Accuracy of early postoperative clinical and ultrasound examination of arteriovenous fistulae to predict dialysis use. J Vasc Access 15: 291–297, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Robbin ML, Chamberlain NE, Lockhart ME, Gallichio MH, Young CJ, Deierhoi MH, Allon M: Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 225: 59–64, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Wong V, Ward R, Taylor J, Selvakumar S, How TV, Bakran A: Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg 12: 207–213, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Lin SL, Huang CH, Chen HS, Hsu WA, Yen CJ, Yen TS: Effects of age and diabetes on blood flow rate and primary outcome of newly created hemodialysis arteriovenous fistulas. Am J Nephrol 18: 96–100, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Malovrh M: Native arteriovenous fistula: Preoperative evaluation. Am J Kidney Dis 39: 1218–1225, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Back MR, Maynard M, Winkler A, Bandyk DF: Expected flow parameters within hemodialysis access and selection for remedial intervention of nonmaturing conduits. Vasc Endovascular Surg 42: 150–158, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Zhu YL, Ding H, Fan PL, Gu QL, Teng J, Wang WP: Predicting the maturity of haemodialysis arteriovenous fistulas with colour Doppler ultrasound: A single-centre study from China. Clin Radiol 71: 576–582, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Singh P, Robbin ML, Lockhart ME, Allon M: Clinically immature arteriovenous hemodialysis fistulas: Effect of US on salvage. Radiology 246: 299–305, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Ives CL, Akoh JA, George J, Vaughan-Huxley E, Lawson H: Pre-operative vessel mapping and early post-operative surveillance duplex scanning of arteriovenous fistulae. J Vasc Access 10: 37–42, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Lee T, Magill M, Burke SK, Blair AT, Robbin ML, Allon M: Comparison of postoperative ultrasound criteria to predict unassisted use of arteriovenous fistulas for hemodialysis. J Vasc Access 19: 167–171, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Robbin ML, Gallichio MH, Deierhoi MH, Young CJ, Weber TM, Allon M: US vascular mapping before hemodialysis access placement. Radiology 217: 83–88, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML: Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60: 2013–2020, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Rego D, Nogueira C, Matos A, Almeida P, Queiros J, Silva F, Sousa C, Almeida R: Two-stage basilic vein transposition: Second stage results. Ther Apher Dial 22: 73–78, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Cooper J, Power AH, DeRose G, Forbes TL, Dubois L: Similar failure and patency rates when comparing one- and two-stage basilic vein transposition. J Vasc Surg 61: 809–816, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Allon M, Robbin ML, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Litovsky S: Preoperative venous intimal hyperplasia, postoperative arteriovenous fistula stenosis, and clinical fistula outcomes. Clin J Am Soc Nephrol 8: 1750–1755, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maya ID, Oser R, Saddekni S, Barker J, Allon M: Vascular access stenosis: Comparison of arteriovenous grafts and fistulas. Am J Kidney Dis 44: 859–865, 2004 [PubMed] [Google Scholar]
- 21.Robbin ML, Oser RF, Lee JY, Heudebert GR, Mennemeyer ST, Allon M: Randomized comparison of ultrasound surveillance and clinical monitoring on arteriovenous graft outcomes. Kidney Int 69: 730–735, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Allon M, Bailey R, Ballard R, Deierhoi MH, Hamrick K, Oser R, Rhynes VK, Robbin ML, Saddekni S, Zeigler ST: A multidisciplinary approach to hemodialysis access: Prospective evaluation. Kidney Int 53: 473–479, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Robbin ML, Greene T, Cheung AK, Allon M, Berceli SA, Kaufman JS, Allen M, Imrey PB, Radeva MK, Shiu YT, Umphrey HR, Young CJ; Hemodialysis Fistula Maturation Study Group : Arteriovenous fistula development in the first 6 weeks after creation. Radiology 279: 620–629, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang CJ, Ko PJ, Hsu LA, Ko YS, Ko YL, Chen CF, Huang CC, Hsu TS, Lee YS, Pang JH: Highly increased cell proliferation activity in the restenotic hemodialysis vascular access after percutaneous transluminal angioplasty: Implication in prevention of restenosis. Am J Kidney Dis 43: 74–84, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Beathard GA, Lok CE, Glickman MH, Al-Jaishi AA, Bednarski D, Cull DL, Lawson JH, Lee TC, Niyyar VD, Syracuse D, Trerotola SO, Roy-Chaudhury P, Shenoy S, Underwood M, Wasse H, Woo K, Yuo TH, Huber TS: Definitions and end points for interventional studies for arteriovenous dialysis access. Clin J Am Soc Nephrol 13: 501–512, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allon M, Imrey PB, Cheung AK, Radeva M, Alpers CE, Beck GJ, Dember LM, Farber A, Greene T, Himmelfarb J, Huber TS, Kaufman JS, Kusek JW, Roy-Chaudhury P, Robbin ML, Vazquez MA, Feldman HI; Hemodialysis Fistula Maturation (HFM) Study Group : Relationships between clinical processes and arteriovenous fistula cannulation and maturation: A multicenter prospective cohort study. Am J Kidney Dis 71: 677–689, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]