Abstract
Background and objectives
Ambulatory BP is increasingly recognized as a better measure of the risk for adverse outcomes related to hypertension, an important comorbidity in patients with CKD. Varying definitions of white-coat and masked hypertension have made it difficult to evaluate differences in prevalence of these BP patterns across CKD cohorts.
Design, setting, participants, & measurements
The International Database of Ambulatory BP in Renal Patients collaborative group established a large database of demographic, clinical, and ambulatory BP data from patients with CKD from cohorts in Italy, Spain, the Chronic Renal Insufficiency Cohort (CRIC) and the African American Study of Kidney Disease and Hypertension Cohort Study (AASK) in the United States, and the CKD Japan Cohort (CKD-JAC). Participants (n=7518) with CKD were included in the present analyses. Cutoffs for defining controlled BP were 140/90 mm Hg for clinic and 130/80 mm Hg for 24-hour ambulatory BP.
Results
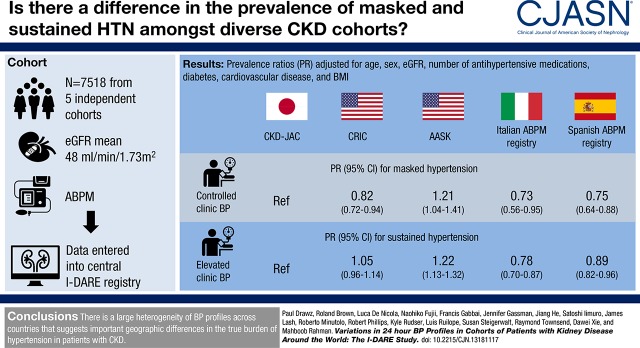
Among those with controlled clinic BP, compared with CKD-JAC, AASK participants were more likely to have masked hypertension (prevalence ratio [PR], 1.21; 95% confidence interval [95% CI], 1.04 to 1.41) whereas CRIC (PR, 0.82; 0.72 to 0.94), Italian (PR, 0.73; 0.56 to 0.95), and Spanish participants (PR, 0.75; 0.64 to 0.88) were less likely. Among those with elevated clinic BP, AASK participants were more likely to have sustained hypertension (PR, 1.22; 95% CI, 1.13 to 1.32) whereas Italian (PR, 0.78; 0.70 to 0.87) and Spanish participants (PR, 0.89; 0.82 to 0.96) were less likely, although CRIC participants had similar prevalence as CKD-JAC. Prevalence of masked and sustained hypertension was elevated in males, patients with diabetes, participants on four or more antihypertensives, and those with moderate-to-severe proteinuria.
Conclusions
In a large, multinational database, the prevalence of masked and sustained hypertension varied across cohorts independent of important comorbidities.
Keywords: hypertension; chronic kidney disease; ethnicity; Masked Hypertension; Antihypertensive Agents; Prevalence; Blood Pressure Monitoring, Ambulatory; Cohort Studies; White Coat Hypertension; blood pressure; Renal Insufficiency, Chronic; kidney; proteinuria; Comorbidity; diabetes mellitus
Introduction
Hypertension is the leading chronic disease risk factor in the world (1). Although BP has traditionally been measured in the clinic, advances in technology over the last 30 years have enabled measurement of BP at home and with ambulatory BP monitoring (2). Ambulatory BP, which provides information on BPs during both the day and night, is increasingly recognized as a better measure of the risk for hypertension-related adverse outcomes (3,4). Compared with clinic BP, ambulatory BP is a stronger and independent risk factor for adverse cardiovascular events and ESKD (5–7). Compared with patients with controlled clinic and ambulatory BP, those with controlled clinic BP but elevated ambulatory BP, known as masked hypertension, are at increased risk for adverse cardiovascular and kidney outcomes; patients with elevated clinic BP but controlled ambulatory BP, known as white-coat hypertension, may have similar low risk for adverse outcomes as those with controlled clinic and ambulatory BP, although a recent report from Spain demonstrated increased risk for mortality with white-coat hypertension (7–14).
CKD is associated with elevated ambulatory BP and increased risk for adverse outcomes (8,12,15). Patients with CKD are more likely to be hypertensive due to multiple factors including sodium retention, endothelial dysfunction, inflammation, and increased sympathetic nervous system activity (16). These same factors also likely contribute to elevated ambulatory BP and increased prevalence of masked and sustained hypertension in this population (12,17,18). Given the proclivity of patients with CKD to elevated BP and adverse cardiovascular outcomes, CKD cohorts are important for studying the pathophysiology and related adverse outcomes associated with abnormal BP patterns.
A number of groups have measured ambulatory BP in cohorts of patients with CKD and reported estimates of the prevalence of white-coat and masked hypertension. However, because of varying definitions of white-coat and masked hypertension, it is difficult to evaluate differences in prevalence across studies (8,12,19). Furthermore, because of their size and homogenous populations, these cohorts are limited in their ability, on their own, to examine relationships between ambulatory BP and outcomes in important subgroups as well as relationships between ambulatory BP and relatively uncommon events such as stroke, for which elevated ambulatory BP is likely an important risk factor.
The International Database of Ambulatory BP in Renal Patients (I-DARE) collaborative group consists of investigators from several different countries interested in ambulatory BP in patients with predialysis CKD. The initial cohorts were provided by the investigators who established the I-DARE collaborative group with the intent to include other CKD cohorts with ambulatory BP data in the future. A large database derived from individual databases in various countries was established to allow for combined analyses of the relationships between clinical and demographic characteristics, CKD, ambulatory BP, and clinical outcomes. The main aims of the research facilitated by this collaboration will be to: (1) evaluate the clinical and demographic characteristics associated with elevated ambulatory BP, (2) evaluate the association between elevated ambulatory BP and adverse cardiovascular and kidney outcomes in patients with CKD and whether there are any ethnic or geographic differences in these associations, and (3) establish a collaborative group to conduct multicenter clinical trials to evaluate whether optimizing ambulatory BP reduces morbidity and mortality. One of the long-term goals of the I-DARE collaboration is to determine whether antihypertensive therapy targeted at ambulatory BP parameters (such as masked hypertension) is superior to management on the basis of conventional office-based pressure. Establishment of this large database is an important step in the process by providing valuable epidemiologic data for design of a future clinical trial. This paper has three objectives: (1) describe the demographics and baseline characteristics for the initial cohorts included in the I-DARE collaborative, (2) describe the methods for measuring clinic and ambulatory BP in each cohort, and (3) report on the prevalence of white-coat, masked, and sustained hypertension across cohorts using uniform definitions and evaluate whether there are differences between cohorts after adjusting for important comorbidities.
Materials and Methods
I-DARE Cohorts
The I-DARE collaborative group includes data from five cohorts which are briefly described below. Ambulatory BP monitoring was conducted over 24 hours in all cohorts; participants were provided with instructions and asked to record sleep and awake times in a diary. Although each cohort had criteria for satisfactory ambulatory blood pressure monitoring (ABPM), for the combined analyses, ABPM was considered satisfactory if 14 or more readings were available during the day and six or more readings were available at night (12). Each cohort was approved by a local Institutional Review Board; this study adhered to the principles of the Declaration of Helsinki.
Italian Ambulatory BP Monitoring Cohort.
This is a prospective cohort study of consecutive hypertensive patients with CKD referred to four kidney clinics in Italy from January 1, 2003 to December 31, 2006 (8,20). Ambulatory BP monitoring was performed in all patients with hypertension as part of the initial work up. Clinic BP was measured in a sitting position three times at 5-minute intervals during the visit by the same physician who was not aware of the results of the ambulatory BP recordings. Clinic BP was reported as the mean of the six values recorded on the two consecutive days in which the ambulatory BP device was installed and removed. Ambulatory BP was obtained in 489 participants on a workday and under regular antihypertensive treatment. ABPM was considered adequate if 14 or more readings were available during the day and seven or more during the night (Table 1).
Table 1.
Cohort characteristics and 24-h ambulatory and clinic BP protocols for each cohort within the International Database of Ambulatory BP in Renal Patients
| Variable | AASK (25) | CRIC (12) | Italian Cohort (8) | CKD-JAC (29) | Spanish Cohort (22) |
|---|---|---|---|---|---|
| Cohort characteristics | |||||
| Setting | Observational study at the conclusion of an RCT | Observational study | Kidney clinic–based observational study | Observational study | PCP clinic–based observational study |
| Total number of participants | 691 | 3939 | 489 | 2977 | 29,319 |
| Participants with ABPM | 648 | 1563 | 489 | 1070 | 29,319 |
| Participants with CKD at time of ABPM | 587 | 1283 | 428 | 1067 | 4153 |
| Ambulatory BP protocol | |||||
| ABPM Device | SpaceLabs 90207 | SpaceLabs 90207/90217 | SpaceLabs 90207 | TM-2421 | SpaceLabs 90207 |
| Frequency | Every 30 min | Every 30 min | Every 15 min day | Every 30 min | Every 20 min |
| Every 30 min night | |||||
| Duration | 24 h | 24 h | 24 h | 24 h | 24 h |
| Diary of sleep/wake times | Recorded by patients | Recorded by patients | Recorded by patients | Recorded by patients | Recorded by patients |
| Clinic BP protocol | |||||
| Device | Tycos classic hand-held aneroid | Aneroid sphygmomanometer | Mercury sphygmomanometer | Automated sphygmomanometer | Mercury sphygmomanometer or oscillometric devices |
| Timing | During a 60-d window of ABPM | At the time of ABPM placement | On two consecutive days when ABPM was placed and removed | At the time of ABPM placement | At the time of ABPM placement |
| Technique | Standardized; three consecutive seated readings. Average of the last two recorded as clinic BP | Standardized; average of three consecutive seated readings | Standardized; average of six readings (three on each day) | Standardized; average of three consecutive seated readings | Average of two readings |
AASK, African American Study of Kidney Disease and Hypertension Cohort Study; CRIC, Chronic Renal Insufficiency Cohort; CKD-JAC, Chronic Kidney Disease Japan Cohort; RCT, randomized controlled trial; PCP, primary care provider; ABPM, ambulatory blood pressure monitoring.
Spanish Ambulatory BP Monitoring Cohort.
The Spanish ABPM Registry was established in 2004 to encourage use of ABPM in primary care. The available dataset included records from 29,319 patients seen in primary care clinics; the subset of patients with CKD were used for the current analyses (21–24). Patients were 18 years of age or older with hypertension and an indication for ABPM (either white-coat hypertension, resistant hypertension, or assessment of dipper status or drug efficacy). Physicians and nurses in primary care obtained ABPM and uploaded relevant clinical information to an internet-based registry. Office BP was measured at the clinic with a calibrated mercury sphygmomanometer or a validated automatic oscillometric device, after 5 minutes of rest in a sitting position, and under standardized conditions. Clinic BP values were calculated as the mean of two readings. Ambulatory BP was measured on working days and the patients were instructed to maintain their usual activities. ABPM was considered adequate if recordings included successful recording of ≥80% of systolic BP and diastolic BP during both the daytime and nocturnal periods, and at least one BP measurement per hour, a threshold that was more stringent than the other cohorts (Table 1).
African American Study of Kidney Disease and Hypertension Cohort Study.
The African American Study of Kidney Disease and Hypertension (AASK) cohort study was a 5-year prospective, observational study that began in 2002 after completion of the AASK trial (25,26). Inclusion in the AASK cohort study required prior participation in the AASK trial, which enrolled hypertensive black participants, aged 18–70 years, and with GFR 20–65 ml/min per 1.73 m2. At the end of the trial phase, participants were followed regularly every 3 months for BP measurement and adjustment of their antihypertensive regimen. Trained and certified coordinators performed all clinic BP measurements using a Tycos classic hand-held aneroid device. Three consecutive seated readings were recorded with the mean of the last two readings documented as the clinic visit value. Twenty-four-hour ABPM was performed in 617 participants. The ABPM was considered adequate if the monitor had been worn for a full 24 hours and if there were at least 14 acceptable reading between 6:00 am and midnight (daytime), and six acceptable readings between midnight and 6:00 am (nighttime) (Table 1).
Chronic Renal Insufficiency Cohort Study.
The Chronic Renal Insufficiency Cohort (CRIC) is an observational cohort study of patients with CKD recruited between 2003 and 2007 in seven clinical centers in the United States (27). Twenty-four-hour ambulatory BP was measured in 1563 participants (12). Clinic BP was measured three times during a clinic visit by trained study staff after a 5-minute rest period, following guideline-recommended protocols using a Tycos classic hand-held aneroid device with appropriately sized cuffs; the average of these measurements was used to define clinic BP. ABPM was conducted during the second phase of CRIC between 2008 and 2012. A recording was considered valid if there were at least 14 readings between 06:00 am and midnight and at least six readings between midnight and 06:00 am (Table 1).
CKD Japan Cohort.
The CKD Japan Cohort (CKD-JAC) is an observational cohort study; participants were Japanese or Asian living in Japan, aged 20–75 years, and had stage 3–5 CKD (28). All of the clinic BP measurements were performed using an automated sphygmomanometer after 5 minutes of rest. Three consecutive seated readings were recorded and the average was used to define clinic BP. ABPM was conducted in a subgroup of 1070 patients from September of 2007 to April of 2010 (Table 1). Each ABPM was manually reviewed for outliers and adequacy (29).
The following data have been sent from each cohort to the I-DARE coordinating center at the University of Minnesota: demographic information; body mass index; laboratory data including serum creatinine, urine albumin, and urine protein; past medical history including diabetes, hypertension, coronary artery disease, heart failure, stroke/transient ischemic attack, and peripheral vascular disease; antihypertensive medication use; and clinic BPs. Cardiovascular disease was defined by a history of coronary artery disease, heart failure, or stroke/transient ischemic attack. Raw ambulatory BP data were available from all sites except the Italian cohort which provided averages (daytime, nighttime, and 24-hour) for each patient. All information provided was from before or at the time of ABPM.
Cutoffs used to define BP categories were <140/90 mm Hg for clinic BP and <130/80 mm Hg for 24-hour ambulatory BP (30). Masked hypertension was defined by controlled clinic BP (systolic BP <140 mm Hg and diastolic BP <90 mm Hg) and elevated ambulatory BP (systolic BP ≥130 mm Hg or diastolic BP ≥80 mm Hg), white-coat hypertension by elevated clinic BP (systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg) and controlled ambulatory BP (systolic BP <130 mm Hg and diastolic BP <80 mm Hg), controlled BP by controlled clinic and ambulatory BP, and sustained hypertension by elevated clinic and ambulatory BP. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation except for the CKD-JAC participants for whom eGFR was calculated using an equation specific to the Japanese population (29,31). Proteinuria was categorized as mild (urine albumin-to-creatinine ratio <30 mg/g [Spanish cohort], 24-hour urine protein <150 mg [AASK, Italian cohort, and CKD-JAC], and urine protein-to-creatinine ratio <150 mg/g [CRIC]), moderate, or severe (urine albumin-to-creatinine ratio >300 mg/g, 24-hour urine protein >500 mg, and urine protein-to-creatinine ratio >500 mg/g). Patients were included if they had an eGFR<60 ml/min per 1.73 m2 or moderate or severe proteinuria. Patients with ESKD were excluded from the present analyses.
Statistical Analyses
Baseline characteristics for each cohort are reported using means and SDs for continuous variables and frequencies and percentages for categoric variables. The primary analysis consisted of two models, one among those with controlled clinic BP (to evaluate prevalence ratios for masked hypertension), and another among those with elevated clinic BP (to evaluate prevalence ratios for sustained hypertension). Poisson regression with robust variance estimation was used to evaluate whether the prevalence of masked (among those with controlled clinic BP) and sustained hypertension (among those with elevated clinic BP) differs between cohorts, adjusting for age, sex, eGFR, number of antihypertensive medications, diabetes, cardiovascular disease, and body mass index. Because of variability of measurement of proteinuria and significant missing data for this variable in the Spanish cohort, proteinuria was only adjusted for in a secondary analysis. All analyses were conducted using R version 3.3.3 (www.R-project.org).
Results
There were 7518 participants from the five cohorts with either eGFR<60 ml/min per 1.73 m2 or moderate or severe proteinuria at the time of ABPM. The majority of the participants (n=4153) came from the Spanish cohort. Characteristics at the time of ABPM are shown in Table 2. Overall, there were substantial differences between cohorts across many patient characteristics. In particular, average (SD) age ranged from 60.6 (10.2) years in the AASK cohort to 67.5 (12.6) years in the Spanish cohort (Table 2). Average (SD) eGFR ranged from 28.1 (12.3) ml/min per 1.73 m2 in CKD-JAC to 58.2 (23.1) ml/min per 1.73 m2 in the Spanish cohort, whereas moderate-to-severe proteinuria was present in 38.6% of participants in the Spanish cohort compared with 87.6% of CKD-JAC participants.
Table 2.
Demographics and clinical characteristics by cohort for the International Database of Ambulatory BP in Renal Patients
| Covariate | Overall (n=7518) | AASK (n=587) | CRIC (n=1283) | Italian Cohort (n=428) | CKD-JAC (n=1067) | Spanish Cohort (n=4153) |
|---|---|---|---|---|---|---|
| Age, yr | 65 (12) | 61 (10) | 64 (10) | 66 (14) | 61 (11) | 68 (13) |
| Men, N (%) | 4080 (54) | 370 (63) | 732 (57) | 252 (59) | 675 (63) | 2051 (49) |
| Race, N (%) | ||||||
| Black | 1092 (14) | 587 (100) | 505 (39) | 0 (0) | 0 (0) | 0 (0) |
| Other | 1229 (16) | 0 (0) | 162 (13) | 0 (0) | 1067 (100) | 0 (0) |
| White | 5197 (69) | 0 (0) | 616 (48) | 428 (100) | 0 (0) | 4153 (100) |
| Body mass index, kg/m2 | 29 (6) | 31 (7) | 32 (7) | 29 (5) | 23 (4) | 29 (5) |
| Current smoker, N (%) | 941 (12) | 104 (18) | 113 (9) | 98 (23) | 138 (13) | 488 (12) |
| Prior smoker, N (%) | 1465 (20) | 345 (59) | 684 (53) | N/A | 436 (41) | N/A |
| Medications, N (%) | ||||||
| β blockers | 2280 (30) | 246 (42) | 692 (54) | 154 (36) | 184 (17) | 1004 (24) |
| Diuretics | 3531 (47) | 498 (85) | 717 (56) | 119 (28) | 306 (29) | 1891 (46) |
| ACEI/ARBs | 4582 (61) | 491 (84) | 870 (68) | 363 (85) | 858 (80) | 2000 (48) |
| Calcium channel blockers | 2949 (39) | 168 (29) | 583 (45) | 207 (48) | 587 (55) | 1404 (34) |
| Vasodilators | 438 (6) | 171 (29) | 187 (15) | 80 (19) | 0 (0) | N/A |
| No. Htn meds | 2.44 (1.48) | 3.63 (1.35) | 2.69 (1.43) | 2.75 (1.41) | 2.25 (1.46) | 2.22 (1.43) |
| Diabetes, N (%) | 2620 (35) | 56 (9.5) | 583 (45) | 161 (38) | 378 (35) | 1442 (35) |
| Stroke/TIA, N (%) | 757 (10) | 106 (18) | 160 (12) | 33 (8) | 125 (12) | 333 (8) |
| Peripheral vascular disease, N (%) | 233 (3) | 35 (6) | 96 (7.5) | 31 (7) | 71 (7) | N/A |
| Coronary artery disease, N (%) | 1119 (15) | 81 (14) | 375 (29) | 82 (19) | 138 (13) | 443 (11) |
| Heart failure, N (%) | 454 (6) | 58 (10) | 134 (10) | 22 (5) | 42 (4) | 198 (5) |
| Cardiovascular disease (w/o PVD), N (%) | 1928 (26) | 203 (35) | 508 (40) | 119 (28) | 248 (23) | 850 (20) |
| Serum creatinine, mg/dl | 1.7 (1.1) | 2.4 (1.4) | 2.1 (1.2) | 2.0 (1.1) | 2.2 (1.2) | 1.3 (0.7) |
| eGFR, ml/min per 1.73 m2 | 48 (23) | 37 (14) | 38 (15) | 40 (19) | 28 (12) | 58 (23) |
| eGFR groups, N (%) | ||||||
| <15 | 418 (6) | 41 (7) | 74 (6) | 37 (9) | 178 (17) | 88 (2) |
| 15–30 | 1253 (17) | 150 (26) | 330 (26) | 107 (25) | 436 (41) | 230 (6) |
| 30–45 | 1908 (25) | 221 (38) | 461 (36) | 121 (28) | 348 (33) | 757 (18) |
| 45–60 | 2621 (35) | 165 (28) | 363 (28) | 116 (27) | 100 (9) | 1877 (45) |
| 60+ | 1318 (18) | 10 (2) | 55 (4) | 47 (11) | 5 (0.5) | 1201 (29) |
| Proteinuria, N (%) | ||||||
| Normal/mild | 1792 (24) | 338 (58) | 464 (36) | 111 (26) | 117 (11) | 762 (18) |
| Moderate | 2199 (29) | 106 (18) | 288 (22) | 133 (31) | 298 (28) | 1374 (33) |
| Severe | 1547 (21) | 139 (24) | 360 (28) | 184 (43) | 637 (60) | 227 (6) |
| Missing | 1980 (26) | 4 (0.7) | 171 (13) | 0 (0) | 15 (1) | 1790 (43) |
Values presented are N (%) or mean (SD) where indicated. Proteinuria was categorized as mild (urine albumin-to-creatinine ratio <30 mg/g [Spanish cohort], 24-h urine protein <150 mg [AASK, Italian cohort, and CKD-JAC], and urine protein-to-creatinine ratio <150 mg/g [CRIC]), moderate, or severe (urine albumin-to-creatinine ratio >300 mg/g, 24-h urine protein >500 mg, and urine protein-to-creatinine ratio >500 mg/g). AASK, African American Study of Kidney Disease and Hypertension Cohort Study; CRIC, Chronic Renal Insufficiency Cohort; CKD-JAC, Chronic Kidney Disease Japan Cohort; N/A, not available; ACEI/ARBs, angiotensin converting enzyme inhibitors/angiotensin receptor blockers; Htn, hypertension; TIA, transient ischemic attack; w/o, without; PVD, peripheral vascular disease.
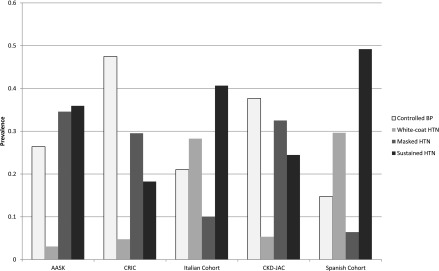
Overall, the average clinic systolic BP was 144/80 mm Hg, ranging from 127/69 mm Hg in CRIC to 153/84 mm Hg in the Spanish cohort. The average 24-hour ambulatory BP was 131/74 mm Hg, ranging from 129/72 mm Hg in CRIC to 137/81 mm Hg in AASK (Table 3). The percentage of participants with elevated ambulatory BP ranged from 48% in CRIC to 71% in AASK. Among all participants in each cohort, prevalence of masked hypertension ranged from 6% (95% confidence interval [95% CI], 6% to 7%) in the Spanish cohort to 35% (95% CI, 31% to 38%) in AASK, whereas the prevalence of sustained hypertension ranged from 18% (95% CI, 27% to 32%) in CRIC to 49% (95% CI, 48% to 51%) in the Spanish cohort (Figure 1, Table 3).
Table 3.
BP measures by cohort for the International Database of Ambulatory BP in Renal Patients
| Covariate | Overall (n=7518) | AASK (n=587) | CRIC (n=1283) | Italian Cohort (n=428) | CKD-JAC (n=1067) | Spanish Cohort (n=4153) |
|---|---|---|---|---|---|---|
| Elevated clinic BP, N (%) | 4411 (59) | 229 (39) | 295 (23) | 295 (69) | 318 (30) | 3274 (79) |
| Elevated ambulatory BP, N (%) | 4162 (55) | 414 (71) | 613 (48) | 217 (51) | 608 (57) | 2310 (56) |
| Hypertension, N (%) | ||||||
| Controlled BP | 1868 (25) | 155 (26) | 609 (48) | 90 (21) | 402 (38) | 612 (15) |
| White coat | 1488 (20) | 18 (3) | 61 (5) | 121 (28) | 57 (5) | 1231 (30) |
| Masked | 1239 (16) | 203 (35) | 379 (30) | 43 (10) | 347 (32) | 267 (6) |
| Sustained | 2923 (39) | 211 (36) | 234 (18) | 174 (41) | 261 (24) | 2043 (49) |
| Clinic systolic BP, mm Hg | 144 (24) | 134 (21) | 127 (21) | 146 (20) | 131 (18) | 153 (22) |
| 24-h systolic BP, mm Hg | 131 (17) | 137 (17) | 129 (16) | 129 (18) | 131 (17) | 132 (16) |
| Day systolic BP, mm Hg | 134 (17) | 137 (17) | 133 (16) | 131 (18) | 135 (17) | 134 (17) |
| Night systolic BP, mm Hg | 124 (20) | 135 (21) | 122 (19) | 123 (20) | 123 (20) | 124 (19) |
| Clinic diastolic BP, mm Hg | 79.8 (14) | 79.7 (12) | 68.7 (12) | 81.2 (12) | 76.7 (11) | 84.0 (13) |
| 24-h diastolic BP, mm Hg | 74.0 (11) | 81.0 (11) | 72.2 (10) | 72.0 (10) | 78.1 (9) | 72.7 (11) |
| Day diastolic BP, mm Hg | 76.4 (11) | 82.5 (11) | 75.3 (10) | 74.7 (11) | 80.7 (10) | 75.0 (11) |
| Night diastolic BP, mm Hg | 67.6 (12) | 77.4 (13) | 66.5 (11) | 66.1 (11) | 72.5 (10) | 65.4 (12) |
Values presented are N (%) or mean (SD) where indicated. AASK, African American Study of Kidney Disease and Hypertension Cohort Study; CRIC, Chronic Renal Insufficiency Cohort; CKD-JAC, Chronic Kidney Disease Japan Cohort.
Figure 1.
Prevalence of hypertension phenotypes varies by cohort in the International Database of Ambulatory BP in Renal Patients cohort. AASK, African American Study of Kidney Disease and Hypertension; CKD-JAC, chronic kidney disease Japan Cohort; CRIC, Chronic Renal Insufficiency Cohort; HTN, hypertension.
Among patients with controlled clinic BP, participants from AASK were more likely to have masked hypertension whereas participants from CRIC, Italy, and Spain were less likely to have masked hypertension compared with participants from CKD-JAC (Table 4, top). The prevalence of masked hypertension was greater in men and patients with diabetes (Table 4, top). After adjusting for proteinuria, the prevalence of masked hypertension was similar among CRIC and CKD-JAC participants (Supplemental Table 1).
Table 4.
Adjusted prevalence ratios for masked hypertension and sustained hypertension among those with controlled clinic BP and elevated clinic BP, respectively
| Covariate | N with Outcome/N at Riska | Unadjusted Proportion (%) | Adjusted PR (95% CI) | P Value |
|---|---|---|---|---|
| Controlled clinic BP (PR for masked hypertension) | ||||
| Cohort | ||||
| CKD-JAC | 318 of 685 | 46 | 1 (reference) | |
| CRIC | 369 of 970 | 38 | 0.82 (0.72 to 0.94) | 0.003 |
| AASK | 196 of 344 | 57 | 1.21 (1.04 to 1.41) | 0.01 |
| Italian cohort | 43 of 133 | 32 | 0.73 (0.56 to 0.95) | 0.02 |
| Spanish cohort | 265 of 877 | 30 | 0.75 (0.64 to 0.88) | <0.001 |
| Age (per 10 yr) | 0.97 (0.93 to 1.01) | 0.11 | ||
| Sex | ||||
| Female | 411 of 1273 | 32 | 1 (reference) | |
| Male | 780 of 1736 | 45 | 1.30 (1.18 to 1.42) | <0.001 |
| eGFR categories | ||||
| ≥45 | 402 of 1192 | 34 | 1 (reference) | |
| 30–45 | 372 of 935 | 40 | 1.04 (0.92 to 1.17) | 0.55 |
| <30 | 417 of 882 | 47 | 1.16 (1.03 to 1.30) | 0.02 |
| Diabetes | ||||
| No | 756 of 2048 | 37 | 1 (reference) | |
| Yes | 435 of 961 | 45 | 1.23 (1.12 to 1.35) | <0.001 |
| BMI (per 5 units) | 1.00 (0.96 to 1.04) | 0.90 | ||
| CVD (w/o PVD) | ||||
| No | 804 of 2130 | 38 | 1 (reference) | |
| Yes | 387 of 879 | 44 | 1.04 (0.95 to 1.15) | 0.40 |
| Hypertension meds | ||||
| 0 | 742 of 2047 | 36 | 0.97 (0.80 to 1.17) | 0.73 |
| 1–3 | 77 of 246 | 31 | 1 (reference) | |
| 4+ | 372 of 716 | 52 | 1.25 (1.14 to 1.38) | <0.001 |
| Elevated clinic BP (PR for sustained hypertension) | ||||
| Cohort | ||||
| CKD-JAC | 238 of 292 | 82 | 1 (reference) | |
| CRIC | 230 of 289 | 80 | 1.05 (0.96 to 1.14) | 0.29 |
| AASK | 203 of 221 | 92 | 1.22 (1.13 to 1.32) | <0.001 |
| Italian cohort | 174 of 295 | 59 | 0.78 (0.70 to 0.87) | <0.001 |
| Spanish cohort | 2036 of 3264 | 62 | 0.89 (0.82 to 0.96) | 0.002 |
| Age (per 10 yr) | 0.97 (0.95 to 0.99) | <0.001 | ||
| Sex | ||||
| Female | 1256 of 2100 | 60 | 1 (reference) | |
| Male | 1625 of 2261 | 72 | 1.15 (1.10 to 1.20) | <0.001 |
| eGFR categories | ||||
| ≥45 | 1694 of 2712 | 62 | 1 (reference) | |
| 30–45 | 625 of 931 | 67 | 1.02 (0.97 to 1.08) | 0.40 |
| <30 | 562 of 718 | 78 | 1.12 (1.06 to 1.19) | <0.001 |
| Diabetes | ||||
| No | 1726 of 2747 | 63 | 1 (reference) | |
| Yes | 1155 of 1614 | 72 | 1.14 (1.09 to 1.19) | <0.001 |
| BMI (per five units) | 0.97 (0.95 to 0.99) | 0.01 | ||
| CVD (w/o PVD) | ||||
| No | 2169 of 3358 | 65 | 1 (reference) | |
| Yes | 712 of 1003 | 71 | 1.04 (0.99 to 1.09) | 0.13 |
| Hypertension meds | ||||
| 0 | 1803 of 2850 | 63 | 1.02 (0.94 to 1.09) | 0.66 |
| 1–3 | 308 of 491 | 63 | 1 (reference) | |
| 4+ | 770 of 1020 | 75 | 1.10 (1.05 to 1.15) | <0.001 |
PR, prevalence ratio; 95% CI, 95% confidence interval; CKD-JAC, Chronic Kidney Disease Japan Cohort; CRIC, Chronic Renal Insufficiency Cohort; AASK, African American Study of Kidney Disease and Hypertension Cohort Study; BMI, body mass index; CVD, cardiovascular disease; w/o, without; PVD, peripheral vascular disease.
Only includes the complete cases included in the model.
Among patients with elevated clinic BP, participants from AASK were more likely to have sustained hypertension whereas participants from Italy and Spain were less likely to have sustained hypertension compared with participants from CKD-JAC (Table 4, bottom). The prevalence of masked hypertension was greater in men, those with severe CKD (eGFR<30 ml/min per 1.73 m2), and patients with diabetes (Table 4, bottom). Results were consistent for Italian and AASK participants after adjusting for proteinuria (Supplemental Table 1).
Discussion
We demonstrate wide variations in 24-hour BP profiles in patients with CKD from different countries; prevalence of masked and sustained hypertension (among those with controlled and uncontrolled clinic BP, respectively) differs between cohorts even after adjusting for potential confounders including age, sex, eGFR, and diabetes. Compared with CKD-JAC participants, AASK participants were more likely to have masked and sustained hypertension whereas those from the Italian and Spanish cohorts were less likely to have masked hypertension.
Although our study documents that 24-hour BP profiles are variable when compared across different countries, the etiology behind these differences remains poorly defined. The prevalence of masked hypertension in previous cohorts of patients with CKD has ranged from 4.7% to 31.3% (32). Higher prevalence of masked hypertension has been demonstrated in blacks compared with other racial ethnic groups (26); it is possible that differences in racial/ethnic composition in the populations studied contribute to the differences seen in our study. Other investigators have reported differences in prevalence of hypertension control across diverse cohorts as well as differences in the effect of CKD on adverse outcomes. The International Network of CKD cohorts reported greater prevalence of uncontrolled BP in India, Europe, and Uruguay compared with North American cohorts (33). The same group also demonstrated that the effect of advanced CKD on CKD progression and all-cause mortality varied across cohorts (34). Recently, the European CKD Burden Consortium reported considerable variation in the prevalence of CKD between countries that was independent of diabetes, hypertension, and obesity (35). These findings are consistent with the present results and support the need for further study to understand the factors that contribute to differences in CKD and hypertension seen across different countries. Potential factors to be studied include lifestyle, diet, genetics, differences in healthcare systems, and access to care. The I-DARE collaborative group provides a platform for further study of the factors mediating the relationship between ambulatory BP and BP patterns (e.g., masked hypertension) and adverse outcomes such as cardiovascular disease, CKD progression, and mortality.
This study also showed that male sex, diabetes, and proteinuria are associated with increased prevalence of masked and sustained hypertension. These factors are associated with increased prevalence of hypertension so it is not surprising that they are associated with elevated ambulatory BP in patients with both controlled and elevated clinic BP. These findings are consistent with prior studies. In an elderly cohort with normal clinic BP, older age and male sex were associated with masked hypertension (36). Other studies have demonstrated associations between masked hypertension and proteinuria (37,38). This study is the largest and most diverse CKD population to demonstrate these associations. The findings that masked hypertension is associated with certain clinical characteristics and target organ damage are consistent in several studies. Therefore, it is important to identify patients who are more likely to have masked hypertension. Development of a tool that can identify patients likely to have masked hypertension on the basis of routinely acquired clinical data is an important next step in this field. This will allow the targeted use of the more expensive and cumbersome ABPM technology in patients at high risk to confirm the diagnosis of masked hypertension. The size and diversity of I-DARE is well suited to this purpose.
We have established a unique multinational database of patients with CKD with ambulatory BP measurements. Strengths of this study include the large number of patients and the diverse cohorts from multiple different countries and settings. The International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) is a good example of how international collaboration can lead to major advances in the field. For example, data from the IDACO collaboration were used to revise the normative criteria for ambulatory BP monitoring (39) and demonstrated that nighttime BP is an independent predictor of adverse outcomes (40). The I-DARE dataset has minimal missing data for important potential confounders such as tobacco use, comorbidities, and antihypertensive medications. Our study is limited in that the BP measurement technique in clinic was not standardized across studies, although the BP measurement technique was adherent to guideline recommendations in AASK, CRIC, CKD-JAC, and the Italian cohort. This discrepancy in how clinic BP was measured may explain part of the effect for the Spanish cohort because it may have led to an overestimation of the prevalence of white-coat hypertension and an underestimation of the prevalence of masked hypertension in that cohort. Patients were selected for the Italian and Spanish cohorts in part due to elevated clinic BP which may lead to an underestimation of the prevalence of masked hypertension in these populations. Additionally, different recruiting strategies across cohorts led to different patient characteristics which likely explains some of the variation in 24-hour BP phenotypes. Clinic BP targets varied over time and across countries. There is only a single assessment of ambulatory BP. In addition, prevalence estimates derived from clinical populations may not fully reflect population estimates, because clinical cohorts represent patients referred for care, and may differ in comorbidity, access to care, and other characteristics from the general CKD population. Finally, assessment of proteinuria varied across cohorts and was missing in 43% of participants in the Spanish cohort.
In conclusion, the I-DARE collaborative group has assembled clinical and demographic data as well as ambulatory BPs on >7500 patients with nondialysis CKD from Italy, Spain, the United States, and Japan. We demonstrated that the prevalence of masked and sustained hypertension varied across cohorts independent of important comorbidities. These results are hypothesis generating. Specifically, on the basis of these novel findings, studies are needed to determine the effects of race, ethnicity, and lifestyle factors on ambulatory BP as well as whether these variables mediate the relationship between abnormal ambulatory BP and adverse outcomes.
Disclosures
None.
Supplementary Material
Acknowledgments
Funding for the Chronic Renal Insufficiency Cohort Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) UL1TR000003, Johns Hopkins University UL1 TR-000424, the University of Maryland General Clinical Research Center M01 RR-16500, the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the NCATS component of the NIH and NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research UL1TR000433, the University of Illinois at Chicago Center for Clinical and Translational Science UL1RR029879, Tulane Center of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, and Kaiser Permanente NIH/National Center for Research Resources University of California, San Francisco-Clinical and Translational Science Institute UL1 RR-024131.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13181117/-/DCSupplemental.
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA: A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staessen JA, Byttebier G, Buntinx F, Celis H, O’Brien ET, Fagard R; Ambulatory Blood Pressure Monitoring and Treatment of Hypertension Investigators : Antihypertensive treatment based on conventional or ambulatory blood pressure measurement. A randomized controlled trial. JAMA 278: 1065–1072, 1997 [PubMed] [Google Scholar]
- 3.Drawz PE, Abdalla M, Rahman M: Blood pressure measurement: Clinic, home, ambulatory, and beyond. Am J Kidney Dis 60: 449–462, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siu AL; US Preventive Services Task Force : Screening for high blood pressure in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 163: 778–786, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J; Systolic Hypertension in Europe Trial Investigators : Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA 282: 539–546, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O’Brien E: Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: The Dublin outcome study. Hypertension 46: 156–161, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R, Andersen MJ: Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 69: 1175–1180, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Minutolo R, Gabbai FB, Agarwal R, Chiodini P, Borrelli S, Bellizzi V, Nappi F, Stanzione G, Conte G, De Nicola L: Assessment of achieved clinic and ambulatory blood pressure recordings and outcomes during treatment in hypertensive patients with CKD: A multicenter prospective cohort study. Am J Kidney Dis 64: 744–752, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Pierdomenico SD, Cuccurullo F: Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: An updated meta analysis. Am J Hypertens 24: 52–58, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Cuspidi C, Sala C, Tadic M, Rescaldani M, Grassi G, Mancia G: Untreated masked hypertension and subclinical cardiac damage: A systematic review and meta-analysis. Am J Hypertens 28: 806–813, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Cuspidi C, Rescaldani M, Tadic M, Sala C, Grassi G, Mancia G: White-coat hypertension, as defined by ambulatory blood pressure monitoring, and subclinical cardiac organ damage: A meta-analysis. J Hypertens 33: 24–32, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, Deo R, Fischer MJ, He J, Hsu CY, Huan Y, Keane MG, Kusek JW, Makos GK, Miller ER 3rd, Soliman EZ, Steigerwalt SP, Taliercio JJ, Townsend RR, Weir MR, Wright JT Jr, Xie D, Rahman M; Chronic Renal Insufficiency Cohort Study Investigators : Masked hypertension and elevated nighttime blood pressure in CKD: Prevalence and association with target organ damage. Clin J Am Soc Nephrol 11: 642–652, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin SS, Thijs L, Asayama K, Li Y, Hansen TW, Boggia J, Jacobs L, Zhang Z, Kikuya M, Björklund-Bodegård K, Ohkubo T, Yang WY, Jeppesen J, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Filipovský J, Imai Y, Wang JG, O’Brien E, Staessen JA; IDACO Investigators : The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol 68: 2033–2043, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, Ruiz-Hurtado G, Segura J, Rodríguez-Artalejo F, Williams B: Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med 378: 1509–1520, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Townsend RR, Taler SJ: Management of hypertension in chronic kidney disease. Nat Rev Nephrol 11: 555–563, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Grassi G, Seravalle G, Trevano FQ, Dell’oro R, Bolla G, Cuspidi C, Arenare F, Mancia G: Neurogenic abnormalities in masked hypertension. Hypertension 50: 537–542, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Veerabhadrappa P, Diaz KM, Feairheller DL, Sturgeon KM, Williamson ST, Crabbe DL, Kashem AM, Brown MD: Endothelial-dependent flow-mediated dilation in African Americans with masked-hypertension. Am J Hypertens 24: 1102–1107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minutolo R, Borrelli S, Scigliano R, Bellizzi V, Chiodini P, Cianciaruso B, Nappi F, Zamboli P, Conte G, De Nicola L: Prevalence and clinical correlates of white coat hypertension in chronic kidney disease. Nephrol Dial Transplant 22: 2217–2223, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Minutolo R, Agarwal R, Borrelli S, Chiodini P, Bellizzi V, Nappi F, Cianciaruso B, Zamboli P, Conte G, Gabbai FB, De Nicola L: Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med 171: 1090–1098, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Gorostidi M, Banegas JR, de la Sierra A, Vinyoles E, Segura J, Ruilope LM: Ambulatory blood pressure monitoring in daily clinical practice - the Spanish ABPM Registry experience. Eur J Clin Invest 46: 92–98, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Gorostidi M, Sarafidis PA, de la Sierra A, Segura J, de la Cruz JJ, Banegas JR, Ruilope LM; Spanish ABPM Registry Investigators : Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: A 5,693-patient cross-sectional analysis from Spain. Am J Kidney Dis 62: 285–294, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Banegas JR, Ruilope LM, de la Sierra A, de la Cruz JJ, Gorostidi M, Segura J, Martell N, García-Puig J, Deanfield J, Williams B: High prevalence of masked uncontrolled hypertension in people with treated hypertension. Eur Heart J 35: 3304–3312, 2014 [DOI] [PubMed] [Google Scholar]
- 24.de la Sierra A, Banegas JR, Segura J, Gorostidi M, Ruilope LM; CARDIORISC Event Investigators : Ambulatory blood pressure monitoring and development of cardiovascular events in high-risk patients included in the Spanish ABPM registry: The CARDIORISC Event study. J Hypertens 30: 713–719, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Gabbai FB, Rahman M, Hu B, Appel LJ, Charleston J, Contreras G, Faulkner ML, Hiremath L, Jamerson KA, Lea JP, Lipkowitz MS, Pogue VA, Rostand SG, Smogorzewski MJ, Wright JT, Greene T, Gassman J, Wang X, Phillips RA; African American Study of Kidney Disease and Hypertension (AASK) Study Group : Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin J Am Soc Nephrol 7: 1770–1776, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, Rostand S, Hiremath L, Sika M, Kendrick C, Hu B, Greene T, Appel L, Phillips RA; African American Study of Kidney Disease and Hypertension Collaborative Research Group : Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 53: 20–27, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai E, Matsuo S, Makino H, Watanabe T, Akizawa T, Nitta K, Iimuro S, Ohashi Y, Hishida A; CKD-JAC Study Group : Chronic Kidney Disease Japan Cohort (CKD-JAC) study: Design and methods. Hypertens Res 31: 1101–1107, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Iimuro S, Imai E, Watanabe T, Nitta K, Akizawa T, Matsuo S, Makino H, Ohashi Y, Hishida A; Chronic Kidney Disease Japan Cohort Study Group : Clinical correlates of ambulatory BP monitoring among patients with CKD. Clin J Am Soc Nephrol 8: 721–730, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y; European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability : European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 32: 1359–1366, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bangash F, Agarwal R: Masked hypertension and white-coat hypertension in chronic kidney disease: A meta-analysis. Clin J Am Soc Nephrol 4: 656–664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Pinho N, Levin A, Fukagawa M, Hoy WE, Robinson BM, Feldman H, Zhang L, Eckardt K-U, Jha V, Oh K-H, Sola L, Mayer GJ, De Borst MH, Taal MW, Stengel B: Global variation in blood pressure control and antihypertensive therapy in CKD patients with hypertension, New Orleans, LA, ASN Kidney Week, 2017 [Google Scholar]
- 34.Orlandi P, Shardlow A, Levin A, Ahn C, Healy H, Oh K-H, Sola L, Nessel L, Taal M, Fukagawa M, Fujii N, Djurdjev O, Rios P, Yang W, Hoy WE, Feldman H: Global variation in rates of ESRD and death among the iNET-CKD studies, New Orleans, LA, ASN Kidney Week, 2017 [Google Scholar]
- 35.Brück K, Stel VS, Gambaro G, Hallan S, Völzke H, Ärnlöv J, Kastarinen M, Guessous I, Vinhas J, Stengel B, Brenner H, Chudek J, Romundstad S, Tomson C, Gonzalez AO, Bello AK, Ferrieres J, Palmieri L, Browne G, Capuano V, Van Biesen W, Zoccali C, Gansevoort R, Navis G, Rothenbacher D, Ferraro PM, Nitsch D, Wanner C, Jager KJ; European CKD Burden Consortium : CKD prevalence varies across the European general population. J Am Soc Nephrol 27: 2135–2147, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mallion JM, Clerson P, Bobrie G, Genes N, Vaisse B, Chatellier G: Predictive factors for masked hypertension within a population of controlled hypertensives. J Hypertens 24: 2365–2370, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa J, Hoshide S, Eguchi K, Schwartz JE, Pickering TG, Shimada K, Kario K: Masked hypertension defined by ambulatory blood pressure monitoring is associated with an increased serum glucose level and urinary albumin-creatinine ratio. J Clin Hypertens (Greenwich) 12: 578–587, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leitão CB, Canani LH, Kramer CK, Boza JC, Pinotti AF, Gross JL: Masked hypertension, urinary albumin excretion rate, and echocardiographic parameters in putatively normotensive type 2 diabetic patients. Diabetes Care 30: 1255–1260, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Hansen TW, Kikuya M, Thijs L, Li Y, Boggia J, Björklund-Bodegârd K, Torp-Pedersen C, Jeppesen J, Ibsen H, Staessen JA: Diagnostic thresholds for ambulatory blood pressure moving lower: A review based on a meta-analysis-clinical implications. J Clin Hypertens (Greenwich) 10: 377–381, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Wang J, Sandoya E, O’Brien E, Staessen JA; International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators : Prognostic accuracy of day versus night ambulatory blood pressure: A cohort study. Lancet 370: 1219–1229, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.