Abstract
Background and objectives
At least 20 susceptibility loci of IgA nephropathy have been identified by genome-wide association studies to date. Whether these loci were associated with disease progression is unclear.
Design, setting, participants, & measurements
We enrolled 613 adult patients with IgA nephropathy for a follow-up of ≥12 months. All 20 IgA nephropathy susceptibility loci were selected and their tag single nucleotide polymorphisms (SNPs) were genotyped. After strict quality control, 16 SNPs and 517 patients with IgA nephropathy were eligible for subsequent analysis. Progression was defined as ESKD or 50% decrease in eGFR. A stepwise Cox regression analysis of all SNPs on Akaike information criterion was performed to select the best model.
Results
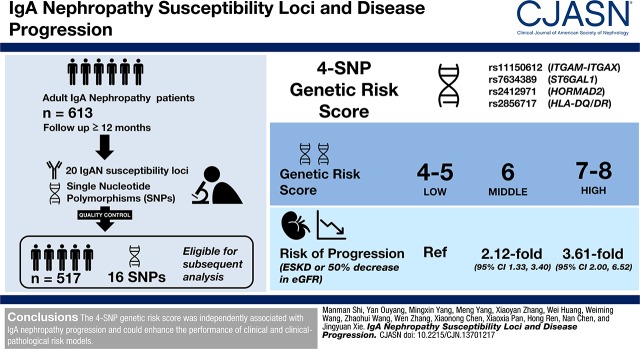
A four-SNP model, rs11150612 (ITGAM-ITGAX), rs7634389 (ST6GAL1), rs2412971 (HORMAD2), and rs2856717 (HLA-DQ/DR), was selected as the best predictive model. The genetic risk score calculated on the basis of the four SNPs was independently associated with disease progression before (hazard ratio [HR], 1.65; 95% confidence interval [95% CI], 1.29 to 2.12) and after adjustment by a recently reported clinical model (HR, 1.29; 95% CI, 1.03 to 1.62) or clinical–pathologic model (HR, 1.35; 95% CI, 1.03 to 1.77). Compared with low genetic risk, patients with middle genetic risk had a 2.12-fold (95% CI, 1.33 to 3.40) increase of progression risk, whereas patients with high genetic risk had 3.61-fold (95% CI, 2.00 to 6.52) progression risk increase. In addition, incorporation of genetic risk score could potentially increase discrimination of the clinical model (c-statistic increase from 0.83 to 0.86) or the clinical–pathologic model (c-statistic increase from 0.82 to 0.85) in predicting 5-year progression risk.
Conclusions
The four-SNP genetic risk score was independently associated with IgA nephropathy progression and could enhance the performance of clinical and clinical–pathologic risk models.
Keywords: IgA nephropathy; progression; genetic risk score; Glomerulonephritis, IGA; Genome-Wide Association Study; Polymorphism, Single Nucleotide; glomerular filtration rate; Follow-Up Studies; Regression Analysis; Kidney Failure, Chronic; Risk; Disease Progression; Quality Control; HLA-DQ Antigens
Introduction
IgA nephropathy is the most common primary GN worldwide. The formation of galactose-deficient IgA1 and antibodies specific for its hinge-region glycans were thought to be key steps of IgA nephropathy development (1–3). Familial aggregation and various reports on prevalence and prognosis in patients with different origins suggested a genetic contribution (4–6). To date, there have been five genome-wide association studies (GWAS) carried out in patients with IgA nephropathy with different ancestries, and at least 20 susceptibility loci have been identified (7–11).
The prognosis of IgA nephropathy is highly variable and it is challenging for clinicians to accurately predict kidney outcome at the time of biopsy. Studies on risk factors were carried out and risk models were established by combining independent clinical or pathologic risk factors together to enhance the predictable power of models (12–19). Most recently, an extended multicenter study developed and validated two risk models by using clinical and Oxford predictors on the basis of Chinese patients with IgA nephropathy. The clinical model from this study contained age, sex, eGFR, hemoglobin, and urine protein excretion. The combined model (clinical–pathologic model) included age, eGFR, and Oxford M and T scores (19). However, no risk prediction models tested the contributions of genetic variants. It is possible that incorporating genetic risk factors such as susceptibility loci identified in GWAS to current risk models could enhance their performance because the multihit pathogenesis model suggests these loci may potentially influence the pathogenesis and severity of IgA nephropathy (3). A post-GWAS revealed that △CFHR3,1 (a deletion variant encompassing CFHR1 and CFHR3) underlining the top signal on chromosome 1 might reduce tubulointerstitial injury (20), and tag single nucleotide polymorphisms (SNPs) in HLA-DQ/DR region might accelerate IgA nephropathy progression (10,21). Further, a genetic risk score including nine susceptibility loci identified in GWAS could predict ESKD in IgA nephropathy (22). However, these results need to be validated and whether genetic risk factors could add predictive value to clinical–pathologic risk equations needs to be further evaluated. Thus, we genotyped 20 SNPs representing all IgA nephropathy susceptibility loci from previous GWAS and select the best genetic model by using a stepwise Cox regression analysis of SNPs passing quality control process on Akaike information criterion (AIC). The combined effect of candidate SNPs was tested and a genetic risk score was established. We then compared the genetic risk score derived from this study and two previously reported genetic risk scores (10,22). Finally, we tested whether the genetic risk score in this study could add predictable value to the most recently published IgA nephropathy risk prediction models (19).
Materials and Methods
Study Design
The enrolled patients were Chinese Han and were biopsied in Ruijin Hospital between 1989 and 2014. IgA nephropathy was defined by kidney biopsy (23). Inclusion criteria were age at biopsy of ≥18 years old, a follow-up time of ≥12 months, and available baseline data. Exclusion criteria were eGFR<15 ml/min per 1.73 m2 at the time of biopsy or with any evidence of Alport syndrome, thin basement membrane disease, SLE, and Henoch–Schonlein purpura or other systemic diseases. A total of 613 patients with IgA nephropathy were enrolled for genotyping 20 candidate SNPs. A total of 517 patients and 16 SNPs were recruited for the subsequent analysis after quality control. Among all patients, Oxford-MESTC (M, mesangial hypercellularity; E, presence of endocapillary proliferation; S, segmental glomerulosclerosis/adhesion; T, severity of tubular atrophy/interstitial fibrosis; C, presence of crescent) score was available in 401 patients.
Detailed baseline data were collected from all patients at the time of kidney biopsy. eGFR was calculated using the CKD Epidemiology Collaboration equation (24). Histologic changes were scored using the Oxford-MESTC score (23,25,26). The start of follow-up was taken as kidney biopsy. The primary outcome was defined as a combination of ESKD and a 50% reduction of eGFR from baseline. In addition to supportive therapy, patients with hypertension and/or proteinuria >0.5 g/24 h were treated with renin-angiotensin system blocker. Immunosuppressive agents were added if patients had massive proteinuria or no response to renin-angiotensin system blocker, presented with rapidly progressive GN, or pathology showed massive crescents, severe inflammatory cell infiltration, or vascular necrosis.
This study was approved by the Institutional Review Board of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine and was conducted in accordance with the principle of the Helsinki Declaration. Written informed consent was obtained from all involved participants.
SNP Genotyping
Twenty SNPs independently associated with IgA nephropathy in previous GWAS (7–11) were selected. Genomic DNA was isolated from peripheral blood sample using the Fujifilm Blood DNA Kit and stored at −80°C for genotyping. All SNPs were genotyped using Sequenom MassArray platform, which combined multiple PCR, Mass Array iPLEX single base extension chemistry, and matrix-assisted laser desorption/ionization-time of flight mass spectrometry. PCR primers and extension probes were designed with the Sequenom online primer design system. The Spectro-CHIP bioarray with purified products was detected using a matrix-assisted laser desorption/ionization-time of flight mass spectrometer. The final results were analyzed using MassArray Workstation software (Typer 4.0).
Statistical Analyses
Continuous variables were summarized as means±SDs (normally distributed) or median (range) (non-normally distributed). Categorical variables were presented as frequencies (percent). We tested for Hardy–Weinberg equilibrium and pairwise linkage disequilibrium (LD) for all SNPs. Haplotypes were phased using PLINK v1.07 and their frequencies were estimated between progressive group and nonprogressive group on the basis of whether the patient progressed to combined outcome within 5 years. Associations between SNPs and kidney function progression were estimated by Cox proportional hazard model, assuming underlying dominant, recessive, and log-additive models. A backward stepdown selection process using AIC was used to select the best outcome prediction model. The selection was stopped with AIC not decreasing after removing the last SNP and we finally chose the model with the lowest AIC. A genetic risk score was calculated based on genotypes of each SNP, which was simply scored using the risk genotypes with a dominant model of SNPs included in the best model. A genetic risk stratification was established on the basis of the tertiles of genetic risk score and separated all patients to low genetic risk group (genetic risk score 4–5), middle genetic risk group (genetic risk score 6), and high genetic risk group (genetic risk score 7–8). Progression rates among different genetic risk groups were compared by Kaplan–Meier analysis. The contributions of genetic risk score to a clinical risk model and a clinical–pathologic risk model (19) were evaluated. Reclassification improvement was assessed using net reclassification improvement (NRI) according to the 5-year risk of combined outcome. The NRI was defined as ([number of events with increased predicted risk−number of events with decreased predicted risk]/number of events+[number of nonevents with decreased predicted risk−number of nonevents with increased predicted risk]/number of nonevents) and estimated according to the method by Pencina et al. (27,28), which was calculated by R package “PredictABEL” (29) with our specific data in this study. We compared this genetic risk score with two established genetic risk scores from prior studies on 5-year progression risk. The first was the standardized genetic risk equation (standardized genetic risk score) by Kiryluk et al. (10), using β-coefficients of the allelic risk. The second used an unweighted approach of nine SNPs (unweighted nine-SNP genetic risk score) by Zhou et al. (22), which was the simple counts of the total number of risk alleles. Four SNPs failed to pass our quality control and were removed from the above two equations, and rs9357155 was replaced by rs2071543, which is in its LD segment (r2=1.00, calculated by SNAP server). (30) The c-statistic was estimated as an area under the receiver operating characteristic curve to predict the 5-year progression. All statistical analyses were performed using SNPassoc package version 1.9–2, survival package version 2.40–1, ggkm package version 1.0, PredictABEL package version 1.2–2, and pROC package version 1.11.0, with R version 3.2.2.
Results
Study Cohort and SNPs Genotyping
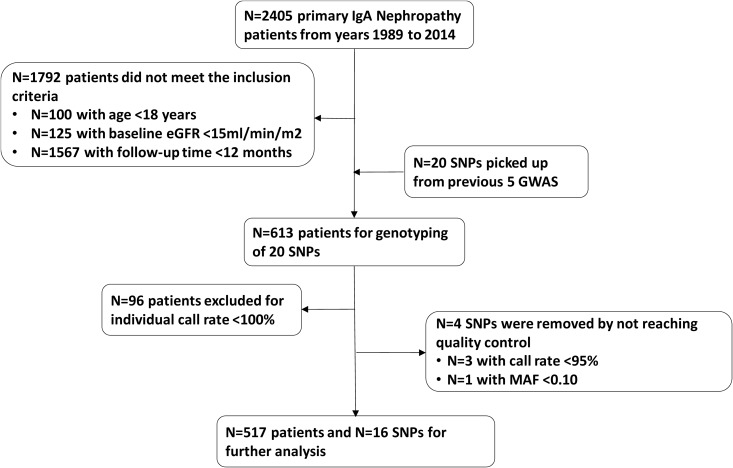
Twenty susceptibility loci from five GWAS were genotyped in 613 patients with IgA nephropathy (7–11). Four SNPs and 96 patients were excluded from this study because they did not pass the quality control filters, which included a per-SNP call rate >95%, per-individual call rate of 100%, and minor allele frequency >0.10. In detail, call rates of rs6677604 (CFH c.1696+2019G>A), rs4077515 (CARD9 p.Ser12Asn), and rs10086568 (DEFA3A/DEFA5A intergene region) were <95%, and the minor allele frequency of rs11574637 (ITGAX c.430+189T>C) was <10%. Individual call rates of 96 patients were <100%. Finally, 16 SNPs and 517 patients with IgA nephropathy were eligible for further analysis (Figure 1). Of these patients, 266 were men and 251 were women. The mean age at biopsy was 38±12 years old, and mean eGFR was 72±32 ml/min per 1.73 m2. Median baseline proteinuria was 1.27 (range, 0.02–12.70) g/24 h. Ninety-six (19%) patients reached the combined outcome during a median follow-up time of 50 (range, 12–238) months (Table 1). Information of the 16 SNPs were shown in Table 2.
Figure 1.
Derivation of the study cohort. MAF, minor allele frequency.
Table 1.
Description of the study cohort
| Characteristic | Patients with IgA Nephropathy |
|---|---|
| Follow-up time, moa | 50 (12–238) |
| Age at biopsy, yrb | 38±12 |
| Men, %c | 266 (52%) |
| eGFR at biopsy, ml/min per 1.73 m2b | 72±32 |
| Mean arterial pressure at biopsy, mm Hgb | 97±13 |
| Proteinuria at biopsy, g/24 ha | 1.27 (0.02–12.70) |
| Serum albumin at biopsy, g/dlb | 3.5±0.7 |
| Hemoglobin at biopsy, g/dlb | 12.9±2.1 |
| Serum IgA at biopsy, mg/dlb | 336±125 |
| Serum IgM at biopsy, mg/dlb | 137±73 |
| Serum IgG at biopsy, mg/dlb | 1106±340 |
| Serum C3 at biopsy, mg/dlb | 106±27 |
| Serum C4 at biopsy, mg/dlb | 25±8 |
| Use of renin-angiotensin system blocker, %c | 351 (76%) |
| Use of steroids, %c | 271 (59%) |
| Pathology (Oxford-MESTC score), %c | |
| M1 | 151 (38%) |
| E1 | 131 (33%) |
| S1 | 339 (85%) |
| T1 | 93 (23%) |
| T2 | 52 (13%) |
| C1 | 140 (35%) |
| C2 | 36 (9%) |
| Kidney IgA deposit 3+ | 208 (53%) |
| Kidney IgG deposit positive | 18 (5%)) |
| Kidney IgM deposit positive | 106 (28%) |
| Kidney C3 deposit positive | 301 (78.4%) |
| Kidney C4 deposit positive | 4 (1%) |
| Combined outcome | 96 (19%) |
MAP, mean arterial blood pressure; Oxford-MESTC score, M (mesangial hypercellularity), E (presence of endocapillary proliferation), S (segmental glomerulosclerosis/adhesion), T (severity of tubular atrophy/interstitial fibrosis), C (presence of crescent).
Non-normally distributed numerical data presented as median (range).
Normally distributed numerical data presented as mean±SD.
Data presented as frequency (%).
Table 2.
SNPs included in this study cohort and their basic information
| Gene | SNP (Minor Allele) | Minor Allele Frequency | N | Call Rate, % | P Value of Hardy–Weinberg Equilibrium | |
|---|---|---|---|---|---|---|
| This Study | HapMap3 (CHB) | |||||
| HLA-DP | rs1883414 (T) | 0.186 | 0.200 | 558 | 95.8 | 0.67 |
| HLA-DQ | rs7763262 (T) | 0.200 | 0.282 | 556 | 96.9 | 0.79 |
| HLA-DQ | rs9275224 (A) | 0.303 | 0.375 | 562 | 95.9 | 1 |
| HLA-DQ/DR | rs2856717 (T) | 0.142 | 0.233 | 570 | 96.9 | 0.17 |
| HLA-DR | rs9275596 (C) | 0.136 | 0.144 | 567 | 96.7 | <0.001 |
| DEFA | rs2738048 (C) | 0.281 | 0.296 | 569 | 96.9 | 0.01 |
| DEFA | rs12716641 (C) | 0.237 | 0.204 | 568 | 96.6 | 1 |
| DEFA | rs9314614 (G) | 0.385 | 0.369 | 564 | 95.9 | 1 |
| TAP2-PSMB9 | rs2071543 (A) | 0.151 | 0.131 | 569 | 97.0 | 0.25 |
| HORMAD2 | rs2412971 (A) | 0.248 | 0.369 | 567 | 96.7 | 0.65 |
| TNFSF13 | rs3803800 (A) | 0.36 | 0.289 | 566 | 96.6 | 0.32 |
| VAV3 | rs17019602 (C) | 0.199 | 0.214 | 562 | 95.8 | 0.35 |
| ITGAM-ITGAX | rs11150612 (G) | 0.260 | 0.228 | 568 | 97.7 | 1 |
| ACCS | rs2074038 (T) | 0.342 | 0.335 | 558 | 95.3 | 0.4 |
| KLF10/ODF1 | rs2033562 (G) | 0.489 | 0.456 | 566 | 96.4 | 0.09 |
| ST6GAL1 | rs7634389 (C) | 0.452 | 0.393 | 565 | 96.2 | 0.45 |
CHB, Han Chinese in Beijing.
Pairwise Interaction Screen among Enrolled SNPs
All 16 SNPs were tested for Hardy–Weinberg equilibrium and inter-SNPs LD. Most of the SNPs agreed with the Hardy–Weinberg equilibrium (Table 2). LD analysis showed that HLA-DQ/DR rs2856717 was in high LD with rs9275224 and rs9275596 (r2=0.38 and r2=0.53, respectively). Two variants around DEFA region rs12716641 and rs2738048 were also in partial LD (r2=0.21) (Supplemental Table 1).
Establish the Best Genetic Predictive Model and Genetic Risk Score
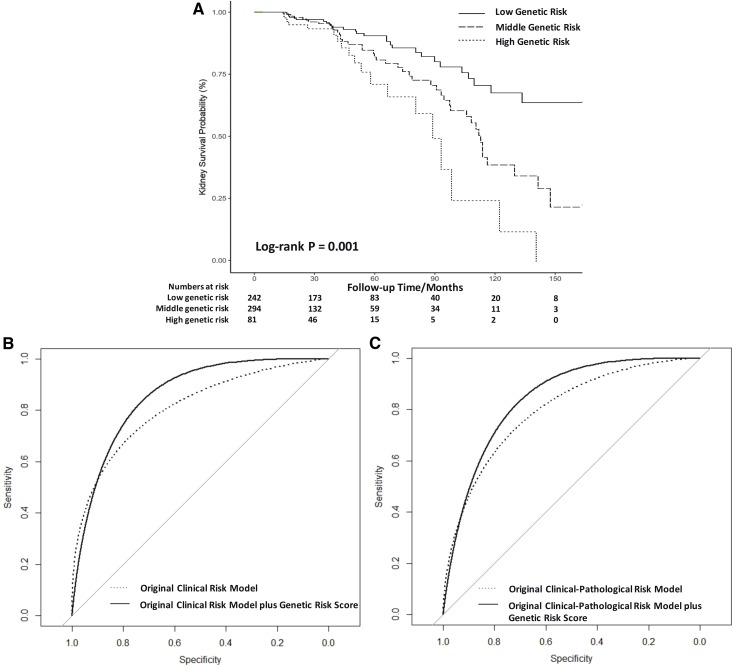
Backward stepwise Cox regression analysis were performed to choose the best genetic predictive model. The best model contained rs11150612, rs7634389, rs2412971, and rs2856717 (Table 3). The genetic risk score using the four SNPs was associated with disease progression before (hazard ratio [HR], 1.65; 95% confidence interval [95% CI], 1.29 to 2.12) and after adjustment by the clinical risk model (HR, 1.29; 95% CI, 1.03 to 1.62) and the clinical–pathologic risk model (HR, 1.35; 95% CI, 1.03 to 1.77) (Table 3). Then, we stratified all patients with IgA nephropathy into three groups by the tertile of genetic risk score. The median outcome-free time in low-, middle-, and high-risk groups were 191, 111, and 87 months, respectively (log-rank test, P=0.001) (Figure 2A). Compared with the low-risk group, patients in the middle-risk group had a 2.12-fold (95% CI, 1.33 to 3.40) increase in the risk of disease progression, whereas patients in the high-risk group had a 3.61-fold (95% CI, 2.00 to 6.52) risk increase (Table 3).
Table 3.
Cox regression analyses for combined outcome with best genetic model and genetic risk score
| Variable | Value | N | HR (95% CI) | P Value |
|---|---|---|---|---|
| Best genetic model | ||||
| rs11150612 | AA | 279 | Reference | — |
| AG or GG | 238 | 1.48 (0.97 to 2.25) | 0.07 | |
| rs7634389 | TT | 158 | Reference | — |
| TC or CC | 359 | 0.70 (0.46 to 1.07) | 0.10 | |
| rs2412971 | GG | 292 | Reference | — |
| GA or AA | 225 | 0.64 (0.42 to 0.99) | 0.04 | |
| rs2856717 | CC | 383 | Reference | — |
| CT or TT | 134 | 2.40 (1.53 to 3.57) | <0.001 | |
| Genetic risk scorea | Per-unit increase | — | 1.65 (1.29 to 2.12) | <0.001 |
| Genetic risk scoreb | Per-unit increase | — | 1.29 (1.03 to 1.62) | 0.03 |
| Genetic risk scorec | Per-unit increase | — | 1.35 (1.03 to 1.77) | 0.03 |
| Genetic risk score groups | ||||
| Low | Genetic risk score 4–5 | 242 | Reference | — |
| Middle | Genetic risk score 6 | 194 | 2.12 (1.33 to 3.40) | 0.002 |
| High | Genetic risk score 7–8 | 81 | 3.61 (2.00 to 6.52) | <0.001 |
Genetic risk score for each SNP was calculated based on its genotype, i.e. risk genotypes (rs11150612 AG or GG, rs7634389 TT, rs2412971 GG, rs2856717 CT or TT) were scored 2 and protective genotypes (rs11150612 AA, rs7634389 TC or CC, rs2412971 GA or AA, rs2856717 CC) were scored 1, and the total genetic risk score for each individual was calculated by the sum of the above four SNPs. Thus, the range of total genetic risk score was 4–8. HRs and 95% CIs were derived from Cox regression analysis. Combined outcome was defined as ESKD or 50% eGFR decline. HR, hazard ratio; 95% CI, 95% confidential interval.
Genetic risk score before adjustment.
Genetic risk score adjusted by clinical model from Xie et al. (19).
Genetic risk score adjusted by clinical and pathologic model from Xie et al. (19).
Figure 2.
Performance of genetic risk classification based on genetic risk score in predicting combined outcome (end-stage kidney disease or 50% eGFR decline). (A) Survival analysis of time without combined outcome according to the tertile of genetic risk score. (B) Receiver operating curves for models predicting the 5-year risk of combined outcome using original clinical risk model and original clinical risk model plus genetic risk score. (C) Receiver operating curves for models predicting the 5-year risk of combined outcome using original clinical-pathologic risk model and the original clinical-pathologic risk model plus genetic risk score. Low genetic risk, genetic risk, score 4-5: middle genetic risk, genetic risk score 6: high genetic risk, genetic risk score 7-8. Original clinical risk model: clinical model from Xie et al. (19). Original clinical–pathologic risk model: clinical and pathologic model from Xie et al. (19).
Cox Regression Analysis on Haplotypes
We carried out haplotype analysis of four SNPs (rs7763262, rs9275224, rs2856717, and rs9275596) in the HLA-DQ/DR region. We used the haplotype CGCT (0.65 in progressive group and 0.66 in nonprogressive group; P=0.78), which was most common and equally distributed in two groups as a reference haplotype. The result showed no specific haplotype had increasing progression risks (Supplemental Table 2).
Association between Different Genetic Risk Score Equations and IgA Nephropathy Progression
We compared genetic risk score established in our study with a previously published standardized genetic risk score and unweighted nine-SNP genetic risk score on progression prediction. No significant association was seen between combined outcome and the two published equations (Supplemental Table 3).
Adding Genetic Risk Score to Clinical or Clinical–Pathologic Risk Equations
To test if the performance could be improved, we added genetic risk score to the clinical risk model and the clinical–pathologic risk model to predict 5-year progression. An increase in r2 (from 17.4% to 18.5% in the clinical model and from 15.1% to 16.8% in the clinical–pathologic model) and c-statistic (from 0.83 to 0.86 in the clinical model and from 0.82 to 0.85 in the clinical–pathologic model) showed a better model fit (Figure 2, B and C, Table 4). The significant increase of continuous NRI (0.42; 95% CI, 0.12 to 0.72) and categorical NRI (0.14; 95% CI 0.05 to 0.22) after adding genetic risk score to the clinical risk model indicated improvement in predictive discrimination (Supplemental Table 4, Table 4).
Table 4.
The discrimination performance of different models predicting the risk of combined outcome at 60 months of follow-up
| Model | c-Statistic (95% CI) | Continuous Net Reclassification Improvement (95% CI) |
|---|---|---|
| Original clinical modela | 0.83 (0.76 to 0.90) | — |
| Original clinical model plus genetic risk scoreb | 0.86 (0.81 to 0.91) | 0.42 (0.12 to 0.72) |
| Original clinical–pathologic modelc | 0.82 (0.74 to 0.89) | — |
| Original clinical–pathologic model plus genetic risk scored | 0.85 (0.78 to 0.91) | 0.30 (−0.05 to 0.64) |
The sex variable is defined as 2 for females and 1 for males. The age variable is defined as age in years at kidney biopsy. eGFR is calculated using the CKD-EPI creatinine equation (in mL/min/1.73 m2). UP, in g/d; Hb, in g/dL; M, as the M score (0, 1) in the Oxford classification; T, as the T score (0, 1, 2) in the Oxford classification. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; Ln, natural logarithm transformed data; Hb, hemoglobin; UP, urine protein.
Original clinical model by Xie et al.: 1−0.9515^exp{−0.5364×(Sex−1.5)−0.0382×(Age−36.5)−0.0459×(eGFR−74.7)+0.1913×[ln(UP)−0.12]−0.1736×(Hb−12.9)}.
Original clinical model plus genetic risk score: 0.256×genetic risk score+4.381×{1−0.9515^exp{−0.5364×(Sex−1.5)−0.0382×(Age−36.5)−0.0459×(eGFR−74.7)+0.1913×[ln(UP)−0.12]−0.1736×(Hb−12.9)}}.
Original clinical–pathologic model by Xie et al.: 1−0.9725^exp{−0.0323×(Age−37.3)−0.0567×(eGFR−72.5)+0.6351×(M−0.39)+0.7452×(T−0.53)}.
Original clinical–pathologic model plus genetic risk score: 0.300×genetic risk score+3.800×{1−0.9725^exp{−0.0323×(Age−37.3)−0.0567×(eGFR−72.5)+0.6351×(M−0.39)+0.7452×(T−0.53)}}.
Discussion
The high heterogeneity of prognosis in patients with IgA nephropathy brings difficulties to patient management and treatment. Risk factors were identified, including baseline kidney function, BP, proteinuria, serum albumin, hemoglobin, blood type, body weight, Oxford-MESTC score, and follow-up proteinuria and BP (13–19). To enhance the predicting accuracy, risk equations were established that showed better performance in predicting prognosis (12–15,17,19,31). Xie et al. (19) developed and validated two risk equations on the basis of a multicenter study: the clinical risk model used five clinical variables, including age at biopsy, sex, baseline eGFR, hemoglobin, and proteinuria; the clinical–pathologic risk model used two clinical and two pathologic variables, including age, baseline eGFR, and Oxford M and T score. The two risk equations showed a good performance for predicting disease progression, but the accuracy still needs to be further enhanced. Variants on candidate genes including HLA-DQB, ACE, GPIa, ICAM-1, MYH9, TNFSF13, DEFA, CFHR, C1GALT1, and ST6GALNAC2 were associated with phenotypes and progression of patients with IgA nephropathy (20,32–36). Here we systemically studied 20 SNPs derived from all five GWAS in an extended Chinese cohort and investigated associations of these loci with IgA nephropathy progression. We found the best genetic model, using rs11150612, rs7634389, rs2412971, and rs2856717, was independently associated with IgA nephropathy progression after adjusted by clinical and pathologic variables. Notably, we determined that a genetic risk score using the four-SNP model could increase the performance of the most recently reported clinical and clinical–pathologic risk models of IgA nephropathy from a large-scale risk assessment study (19).
Zhou et al. (22) established the unweighted nine-SNP genetic risk score by nine SNPs to predict ESKD in a Chinese cohort of 297 patients with IgA nephropathy. Kaplan–Meier plots showed patients with the unweighted nine-SNP genetic risk score ≥16 had a poorer prognosis than patients with the unweighted nine-SNP genetic risk score <16. Kiryluk et al. (10) developed a 15-SNP IgA nephropathy genetic risk calculator, a good tool to predict IgA nephropathy onset risk. The above two equations were validated in our cohort, yet showed no significant association with progression. This may be because of racial differences and the four removed SNPs. Besides, risk alleles of disease onset and progression may be different.
We identified a four-SNP risk model, rs11150612 (ITGAM-ITGAX), rs7634389 (ST6GAL1), rs2412971 (HORMAD2), and rs2856717 (HLA-DQ/DR), that is associated with IgA nephropathy progression. The underlying pathogenic mechanism might be autoantigen formation, antigen-presenting, and thereafter formation and modification of autoantibody regulated by ITGAM-ITGAX, HORMAD2, HLA-DQ/DR, and ST6GAL1 taking part in IgA nephropathy development and progression. HLA-DR/DQ, coding for MHC II molecules and involved in antigen presentation, was detected by all five GWAS regardless of racial origins (7–11). HLA-DRB1 classic alleles have been reported to be related to disease progression in a Chinese IgA nephropathy cohort (37). In this study, we determined patients carrying rs2856717-T had an increased risk for disease progression. The result replicated a recent study (21) from southwest China showing associations between rs2856717/TT or TC and increased risk for IgA nephropathy progression. To further dissect the HLA region, we next phased four SNPs (rs7763262, rs9275224, rs2856717, and rs9275596) in the HLA-DQ/DR region and tested their associations with disease progression. No specific haplotype showed an increasing risk for disease progression, which might be because of the limited sample size of our study. ST6GAL1 encodes ST6Gal-1 sialyltransferase, which involves adding terminal α2,6-linked sialic acids to N-glycans within the Fc domain of IgG (38). Crosslinking reaction between IgG and Fc receptors on the surface of immune cells is the prominent factor in autoimmune diseases like SLE, ANCA-associated vasculitis, and rheumatoid arthritis (39,40). Data showed that the mRNA level of ST6GAL1 increases significantly in the glomeruli of patients with IgA nephropathy compared with normal kidney tissue. Notably, a higher mRNA level of ST6GAL1 in patients with IgA nephropathy with normal kidney function (eGFR>90 ml/min per 1.73 m2) than that in patients with IgA nephropathy with mild kidney dysfunction (eGFR 60–90 ml/min per 1.73 m2) was reported (https://www.nephroseq.org/resource/main.html). Rs2412971 is located in the intronic region of HORMAD2, which belongs to a HORMA domain-containing gene family and plays a role in DNA repair (41). The HORMAD2 locus also encodes several cytokines that interact with complement pathways (42). Kiryluk et al. (6) detected a multiplicative interaction between rs6677604 (CFHR3/R1) and rs2412971 (HORMAD2) loci. Rs2412971-A allele is reversed among CFHR3,1-del homozygotes. The biologic basis of this statistical finding remains unknown. ITGAM-ITGAX encodes integrin αM and αX, which mark intestinal dendritic cells that maintain the balance between inflammation and tolerance. ITGAM and ITGAX also combine with the integrin β2 chain to form leukocyte-specific complement receptors 3 and 4. ITGAM was found to take part in the regulation of intestinal IgA-producing plasma cells in mice (43).
As we know, impaired kidney function, increased BP, proteinuria, and MESTC score are risk factors for a poor outcome among patients with IgA nephropathy (12–15,17,25,26,30,44,45). We proposed that the genetic risk score could add predictive value to the current clinical and pathologic equations. After incorporating the genetic risk score to Xie et al.’s clinical and clinical–pathologic equations, the new equations showed an increased discrimination in predicting IgA nephropathy prognosis. Our study determined that genetic factors could help clinicians to predict kidney outcomes of IgA nephropathy more precisely in addition to clinical and pathologic risk models.
There were some limitations in our study. First, it was a retrospective study from a single center. Second, because of the sample size of our cohort, we did not have enough power to analyze rare SNPs or SNPs with low effect size. Third, we only included SNPs identified by GWAS, other genetic variants might contribute to disease progression and need to be further evaluated. At last, the four SNPs (rs6677604, rs4077515, rs10086568, and rs11574637) excluded from this study during quality control were still possibly associated with IgA nephropathy progression. Larger prospective designed study with different ethnic populations will be helpful to overcome these limitations.
In conclusion, we established a genetic risk score using rs11150612 (ITGAM-ITGAX), rs7634389 (ST6GAL1), rs2412971 (HORMAD2), and rs2856717 (HLA-DQ/DR), which was independently associated with IgA nephropathy progression. The genetic risk score could enhance the performance of clinical or clinical–pathologic risk models of IgA nephropathy.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Key Research and Development Program of China (2016YFC0904100), National Natural Science Foundation of China (81570598, 81370015, and 81000295), Science and Technology Innovation Action Plan of Shanghai Science and Technology Committee (17441902200), Shanghai Municipal Education Commission, Gaofeng Clinical Medicine grant (20152207), the Chinese Medical Association clinical research special fund (13030280413), and Shanghai Jiao Tong University School of Medicine multicenter clinical research project (DLY201510).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13701217/-/DCSupplemental.
References
- 1.Kiryluk K, Novak J: The genetics and immunobiology of IgA nephropathy. J Clin Invest 124: 2325–2332, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novak J, Julian BA, Tomana M, Mestecky J: IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol 28: 78–87, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour SJ, Cattran DC, Kim SJ, Levin A, Wald R, Hladunewich MA, Reich HN: Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int 84: 1017–1024, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Julian BA, Quiggins PA, Thompson JS, Woodford SY, Gleason K, Wyatt RJ: Familial IgA nephropathy. Evidence of an inherited mechanism of disease. N Engl J Med 312: 202–208, 1985 [DOI] [PubMed] [Google Scholar]
- 6.Kiryluk K, Li Y, Sanna-Cherchi S, Rohanizadegan M, Suzuki H, Eitner F, Snyder HJ, Choi M, Hou P, Scolari F, Izzi C, Gigante M, Gesualdo L, Savoldi S, Amoroso A, Cusi D, Zamboli P, Julian BA, Novak J, Wyatt RJ, Mucha K, Perola M, Kristiansson K, Viktorin A, Magnusson PK, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Boland A, Metzger M, Thibaudin L, Wanner C, Jager KJ, Goto S, Maixnerova D, Karnib HH, Nagy J, Panzer U, Xie J, Chen N, Tesar V, Narita I, Berthoux F, Floege J, Stengel B, Zhang H, Lifton RP, Gharavi AG: Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet 8: e1002765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feehally J, Farrall M, Boland A, Gale DP, Gut I, Heath S, Kumar A, Peden JF, Maxwell PH, Morris DL, Padmanabhan S, Vyse TJ, Zawadzka A, Rees AJ, Lathrop M, Ratcliffe PJ: HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol 21: 1791–1797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, Sanna-Cherchi S, Men CJ, Julian BA, Wyatt RJ, Novak J, He JC, Wang H, Lv J, Zhu L, Wang W, Wang Z, Yasuno K, Gunel M, Mane S, Umlauf S, Tikhonova I, Beerman I, Savoldi S, Magistroni R, Ghiggeri GM, Bodria M, Lugani F, Ravani P, Ponticelli C, Allegri L, Boscutti G, Frasca G, Amore A, Peruzzi L, Coppo R, Izzi C, Viola BF, Prati E, Salvadori M, Mignani R, Gesualdo L, Bertinetto F, Mesiano P, Amoroso A, Scolari F, Chen N, Zhang H, Lifton RP: Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 43: 321–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu XQ, Li M, Zhang H, Low HQ, Wei X, Wang JQ, Sun LD, Sim KS, Li Y, Foo JN, Wang W, Li ZJ, Yin XY, Tang XQ, Fan L, Chen J, Li RS, Wan JX, Liu ZS, Lou TQ, Zhu L, Huang XJ, Zhang XJ, Liu ZH, Liu JJ: A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat Genet 44: 178–182, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, Fasel D, Lata S, Prakash S, Shapiro S, Fischman C, Snyder HJ, Appel G, Izzi C, Viola BF, Dallera N, Del Vecchio L, Barlassina C, Salvi E, Bertinetto FE, Amoroso A, Savoldi S, Rocchietti M, Amore A, Peruzzi L, Coppo R, Salvadori M, Ravani P, Magistroni R, Ghiggeri GM, Caridi G, Bodria M, Lugani F, Allegri L, Delsante M, Maiorana M, Magnano A, Frasca G, Boer E, Boscutti G, Ponticelli C, Mignani R, Marcantoni C, Di Landro D, Santoro D, Pani A, Polci R, Feriozzi S, Chicca S, Galliani M, Gigante M, Gesualdo L, Zamboli P, Battaglia GG, Garozzo M, Maixnerová D, Tesar V, Eitner F, Rauen T, Floege J, Kovacs T, Nagy J, Mucha K, Pączek L, Zaniew M, Mizerska-Wasiak M, Roszkowska-Blaim M, Pawlaczyk K, Gale D, Barratt J, Thibaudin L, Berthoux F, Canaud G, Boland A, Metzger M, Panzer U, Suzuki H, Goto S, Narita I, Caliskan Y, Xie J, Hou P, Chen N, Zhang H, Wyatt RJ, Novak J, Julian BA, Feehally J, Stengel B, Cusi D, Lifton RP, Gharavi AG: Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet 46: 1187–1196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Foo JN, Wang JQ, Low HQ, Tang XQ, Toh KY, Yin PR, Khor CC, Goh YF, Irwan ID, Xu RC, Andiappan AK, Bei JX, Rotzschke O, Chen MH, Cheng CY, Sun LD, Jiang GR, Wong TY, Lin HL, Aung T, Liao YH, Saw SM, Ye K, Ebstein RP, Chen QK, Shi W, Chew SH, Chen J, Zhang FR, Li SP, Xu G, Tai ES, Wang L, Chen N, Zhang XJ, Zeng YX, Zhang H, Liu ZH, Yu XQ, Liu JJ: Identification of new susceptibility loci for IgA nephropathy in Han Chinese. Nat Commun 6: 7270, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbour SJ, Espino-Hernandez G, Reich HN, Coppo R, Roberts ISD, Feehally J, Herzenberg AM, Cattran DC; Oxford Derivation, North American Validation and VALIGA Consortia; Oxford Derivation North American Validation and VALIGA Consortia : The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int 89: 167–175, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Bartosik LP, Lajoie G, Sugar L, Cattran DC: Predicting progression in IgA nephropathy. Am J Kidney Dis 38: 728–735, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L: Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 22: 752–761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y: A scoring system to predict renal outcome in IgA nephropathy: A nationwide 10-year prospective cohort study. Nephrol Dial Transplant 24: 3068–3074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouyang Y, Xie J, Yang M, Zhang X, Ren H, Wang W, Chen N: Underweight is an independent risk factor for renal function deterioration in patients with IgA nephropathy. PLoS One 11: e0162044, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J, Kiryluk K, Wang W, Wang Z, Guo S, Shen P, Ren H, Pan X, Chen X, Zhang W, Li X, Shi H, Li Y, Gharavi AG, Chen N: Predicting progression of IgA nephropathy: New clinical progression risk score. PLoS One 7: e38904, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang M, Xie J, Ouyang Y, Zhang X, Shi M, Li X, Wang Z, Shen P, Ren H, Zhang W, Wang W, Chen N: ABO blood type is associated with renal outcomes in patients with IgA nephropathy. Oncotarget 8: 73603–73612, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie J, Lv J, Wang W, Li G, Liu Z, Chen H, Xu F, Sun J, Ouyang Y, Zhang X, Yang M, Shi M, Zhang W, Ren H, Kiryluk K, Zhang H, Chen N: Kidney failure risk prediction equations in IgA nephropathy: A multicenter risk assessment study in Chinese patients [published online ahead of print March 17, 2018]. Am J Kidney Dis 10.1053/j.ajkd.2018.01.043 [DOI] [PubMed] [Google Scholar]
- 20.Xie J, Kiryluk K, Li Y, Mladkova N, Zhu L, Hou P, Ren H, Wang W, Zhang H, Chen N, Gharavi AG: Fine mapping implicates a deletion of CFHR1 and CFHR3 in protection from IgA nephropathy in Han Chinese. J Am Soc Nephrol 27: 3187–3194, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Li G, Hong D, Zou Y, Fei D, Wang L: Replication of genome-wide association study identified seven susceptibility genes, affirming the effect of rs2856717 on renal function and poor outcome of IgA nephropathy. Nephrology (Carlton) 22: 811–817, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Zhou XJ, Qi YY, Hou P, Lv JC, Shi SF, Liu LJ, Zhao N, Zhang H: Cumulative effects of variants identified by genome-wide association studies in IgA nephropathy. Sci Rep 4: 4904, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, Liu ZH, Roberts IS, Yuzawa Y, Zhang H, Feehally J; IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants : Oxford classification of IgA nephropathy 2016: An update from the IgA nephropathy classification working group. Kidney Int 91: 1014–1021, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Pencina MJ, D'Agostino RB Sr ., D'Agostino RB Jr ., Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27(2): 157–172; discussion 207–112, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Pencina MJ, D’Agostino RB Sr ., Demler OV: Novel metrics for evaluating improvement in discrimination: Net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med 31: 101–113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kundu S, Aulchenko YS, van Duijn CM, Janssens AC: PredictABEL: An R package for the assessment of risk prediction models. Eur J Epidemiol 26: 261–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O'Donnell CJ, de Bakker PI: SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics (Oxford, England) 24: 2938–2939, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka S, Ninomiya T, Katafuchi R, Masutani K, Tsuchimoto A, Noguchi H, Hirakata H, Tsuruya K, Kitazono T: Development and validation of a prediction rule using the Oxford classification in IgA nephropathy. Clin J Am Soc Nephrol 8: 2082–2090, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng W, Zhou X, Zhu L, Shi S, Lv J, Liu L, Zhang H: Polymorphisms in the nonmuscle myosin heavy chain 9 gene (MYH9) are associated with the progression of IgA nephropathy in Chinese. Nephrol Dial Transplant 26: 2544–2549, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Han SS, Yang SH, Choi M, Kim HR, Kim K, Lee S, Moon KC, Kim JY, Lee H, Lee JP, Jung JY, Kim S, Joo KW, Lim CS, Kang SW, Kim YS, Kim DK: The role of TNF superfamily member 13 in the progression of IgA nephropathy. J Am Soc Nephrol 27: 3430–3439, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunley TE, Julian BA, Phillips JA 3rd, Summar ML, Yoshida H, Horn RG, Brown NJ, Fogo A, Ichikawa I, Kon V: Angiotensin converting enzyme gene polymorphism: Potential silencer motif and impact on progression in IgA nephropathy. Kidney Int 49: 571–577, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Niu D, Ren Y, Xie L, Sun J, Lu W, Hao Y, Zhang Y, Yin A, Li H, Lv J, Li S: Association between CCDC132, FDX1 and TNFSF13 gene polymorphisms and the risk of IgA nephropathy. Nephrology (Carlton) 20: 908–915, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto R, Nagasawa Y, Shoji T, Inoue K, Uehata T, Kaneko T, Okada T, Yamauchi A, Tsubakihara Y, Imai E, Isaka Y, Rakugi H: A candidate gene approach to genetic prognostic factors of IgA nephropathy--a result of Polymorphism REsearch to DIstinguish genetic factors Contributing To progression of IgA Nephropathy (PREDICT-IgAN). Nephrol Dial Transplant 24: 3686–3694, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Cao HX, Li M, Nie J, Wang W, Zhou SF, Yu XQ: Human leukocyte antigen DRB1 alleles predict risk and disease progression of immunoglobulin A nephropathy in Han Chinese. Am J Nephrol 28: 684–691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaveri SV, Lacroix-Desmazes S, Bayry J: The antiinflammatory IgG. N Engl J Med 359: 307–309, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F: In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc Natl Acad Sci U S A 105: 15005–15009, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baudino L, Azeredo da Silveira S, Nakata M, Izui S: Molecular and cellular basis for pathogenicity of autoantibodies: Lessons from murine monoclonal autoantibodies. Springer Semin Immunopathol 28: 175–184, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Wojtasz L, Daniel K, Roig I, Bolcun-Filas E, Xu H, Boonsanay V, Eckmann CR, Cooke HJ, Jasin M, Keeney S, McKay MJ, Toth A: Mouse HORMAD1 and HORMAD2, two conserved meiotic chromosomal proteins, are depleted from synapsed chromosome axes with the help of TRIP13 AAA-ATPase. PLoS Genet 5: e1000702, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kastl SP, Speidl WS, Kaun C, Katsaros KM, Rega G, Afonyushkin T, Bochkov VN, Valent P, Assadian A, Hagmueller GW, Hoeth M, de Martin R, Ma Y, Maurer G, Huber K, Wojta J: In human macrophages the complement component C5a induces the expression of oncostatin M via AP-1 activation. Arterioscler Thromb Vasc Biol 28: 498–503, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Kunisawa J, Gohda M, Hashimoto E, Ishikawa I, Higuchi M, Suzuki Y, Goto Y, Panea C, Ivanov II, Sumiya R, Aayam L, Wake T, Tajiri S, Kurashima Y, Shikata S, Akira S, Takeda K, Kiyono H: Microbe-dependent CD11b+ IgA+ plasma cells mediate robust early-phase intestinal IgA responses in mice. Nat Commun 4: 1772, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, Liu Z: Long-term renal survival and related risk factors in patients with IgA nephropathy: Results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 27: 1479–1485, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Radford MG Jr ., Donadio JV Jr ., Bergstralh EJ, Grande JP: Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol 8: 199–207, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.