ABSTRACT
The high expression of inducible nitric oxide synthase (NOS2) by myeloid-derived suppressor cells (MDSCs) is a key mechanism of immune evasion in cancer. Recently we reported that NOS2 is also expressed by γδ T cells in melanoma, contributing to their polarization towards a pro-tumor phenotype. The molecular mechanisms underlying regulation of NOS2 expression in tumor-induced γδ T cells remain unexplored. By using the model of mice transgenic for the ret oncogene (Ret mice) that develops a spontaneous metastatic melanoma, we evidence that interleukin (IL)-1β and IL-6 drive NOS2 expression in γδ T cells. Indeed, their in vivo neutralization lessens the γδ T cell capacity to produce not only NOS2, but also IL-17 involved in the recruitment of MDSCs at the primary tumor site. The treatment also delayed tumor cell dissemination and induced vitiligo in a significant proportion of Ret mice. Interestingly, Ret mice developing a less aggressive melanoma, characterized by the spontaneous development of a concomitant autoimmune vitiligo, exhibit a weaker concentration of inflammatory cytokines and a reduction of tumor infiltrating γδ T cells expressing NOS2, when compared to Ret mice without any signs of vitiligo. Overall our results support that the level of inflammation at the tumor site regulates NOS2 expression by γδ T cells and the development of vitiligo associated melanoma.
KEYWORDS: pro-tumorogenic γδ T cells, NOS2, interleukin 1β, interleukin 6, melanoma
Introduction
Melanoma represents 5% of skin cancer and is the deadliest form due to its high metastatic potential. Metastasis formation is a multistep process starting by tumor cell dissemination, an event that occurs early, even before the clinical diagnosis of the primary tumor.1 Metastasis is not only directed by cancer-cell-intrinsic mechanisms that allow tumor cells to invade the local microenvironment, reach the circulation, and colonize distant sites but also by the tumor microenvironment within myeloid derived suppressor cells (MDSCs) play a key role.
Polymorphonuclear (PMN-) MDSCs are the major subset of MDSCs that accumulates at the primary tumor site.2 These cells favor tumor progression by inhibiting anti-tumor responses, by inducing angiogenesis or by initiating a pre-metastatic niche.3 Furthermore, tumor infiltrating PMN-MDSCs contribute to cancer cell dissemination via epithelial-mesenchymal transition of melanoma cells.4 Many reports indicated that these cells are recruited by gamma delta (γδ) T cells.
The cellular and cytokine composition of the tumor microenvironment can affect the γδ T cell plasticity and subsequently their anti-tumor properties.5 This may explain in part the dual role of these T cells in both human cancers and mouse models.6 Indeed, protective role of γδ T cells has been established in mouse models (reviewed in 7). A meta-analysis evidenced an association between their proportion within the microenvironment in a multitude of human malignancies and a favorable prognosis.8 However, γδ T cells can also promote tumor development and metastasis, for instance via expressing PDL1 and galectin 99 or producing IL-17 in both human and rodent cancers.10–12 Recently, we identified nitric oxide synthase 2 (NOS2) that catalyzes the production of nitric oxide (NO) and L-citrulline from L- arginine, as a major driver of a pro-tumor profile in γδ T cells by favoring their IL-17 production.13 Indeed, NOS2 leads to the recruitment of PMN-MDSCs at the primary tumor site and subsequently to metastasis formation in melanoma. In this model, mice are transgenic for the activated ret oncogene (Ret mice) and develop a metastatic melanoma.14,15 A spontaneous vitiligo occurs in nearly forty percent of cases resulting in a delayed incidence of cutaneous and distant metastasis.15
Melanoma patients developing a vitiligo-like depigmentation, either spontaneously or in response to immunotherapy 16 are partially protected against tumor progression. This autoimmune skin disease is associated with more favourable clinical outcome.17,18 Vitiligo results from a robust anti-melanoma immunity mainly mediated by CD8+ T cells that also recognize normal melanocytes sharing expression of melanocyte differentiation antigens.19–21
In the present study, we pursued the investigation that autocrine NOS2 expression in tumor infiltrating γδ T cells plays a role in the tumor aggressiveness. By using the Ret model, we further show that IL-1β and IL-6 promote NOS2 expression in γδ T cells and control the balance between melanoma and vitiligo associated melanoma.
Results
Vitiligo development delays melanoma progression less efficiently than genetic Nos2 inactivation
Vitiligo occurs spontaneously in 35 to 40% of Ret mice and the metastatic melanoma progression is significantly delayed in mice exhibiting vitiligo (RetV) compared to mice with normal skin (RetNV) (15, and Figure 1). Recently, we have shown a delay of tumor onset in Ret mice deficient for NOS2 (RetNos2KO).13 Interestingly we show that the protection of these mice is not due to a higher incidence of vitiligo because only 35% of 6 month old RetNos2KO mice display skin depigmentation (Figure 1A). Regarding the impact on tumor outgrowth, the kinetics of primary tumor incidence are similar in RetV and RetNos2KO mice (Figure 1B), but the spontaneous vitiligo development is less efficient than the genetic Nos2 inactivation to limit metastasis formation (Figure 1C). These results suggest the involvement of NOS2 in the vitiligo development.
Figure 1.
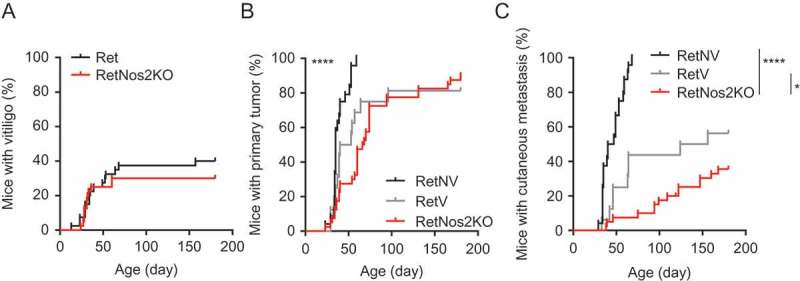
Vitiligo confers an intermediate protection against melanoma development compared to NOS2 deficiency.
(A) Time courses of vitiligo onset from Ret (n = 40) and RetNos2KO (n = 40) mice. (B-C) 6-months follow-up of melanoma development from Ret mice without vitiligo (RetNV, n = 24), Ret mice with vitiligo (RetV, n = 16) and RetNos2KO (n = 40) mice. Time courses of primary tumor (B) and cutaneous metastasis (C) onset. Mice were examined every two weeks. *P < 0.05 and ****P < 0.0001 (Wilcoxon test).
Vitiligo development is associated with a decrease of PMN-MDSCs and γδ T cells in primary tumors
To decipher whether the tumor protection associated to vitiligo relies on a specific immune microenvironment at the primary tumor site, we analyzed ex vivo the cytokine profile of primary tumors derived from 6 month old RetV and RetNV mice. The protein levels of IL-12p70, IFN- γ and tumor necrosis factor-α (TNF- α) were quite similar in both groups (Figure 2A). Primary tumors from RetV mice contained lesser amounts of IL-10 and keratinocyte-derived cytokine (KC), a murine IL-8 homologue involved in PMN cell recruitment, than RetNV mice (Figure 2A). Interestingly, they also displayed less IL-1β and IL-6 (Figure 2A). Although circulating IL-1β was almost undetectable in both groups, the concentration of IL-6 was lower in the blood of RetV mice than in RetNV mice (Figure 2B), consistent with our data from aqueous humors (primary tumor site). This result reinforces that, in presence of vitiligo onset, inflammation is significantly reduced in the course of the melanoma development.
Figure 2.
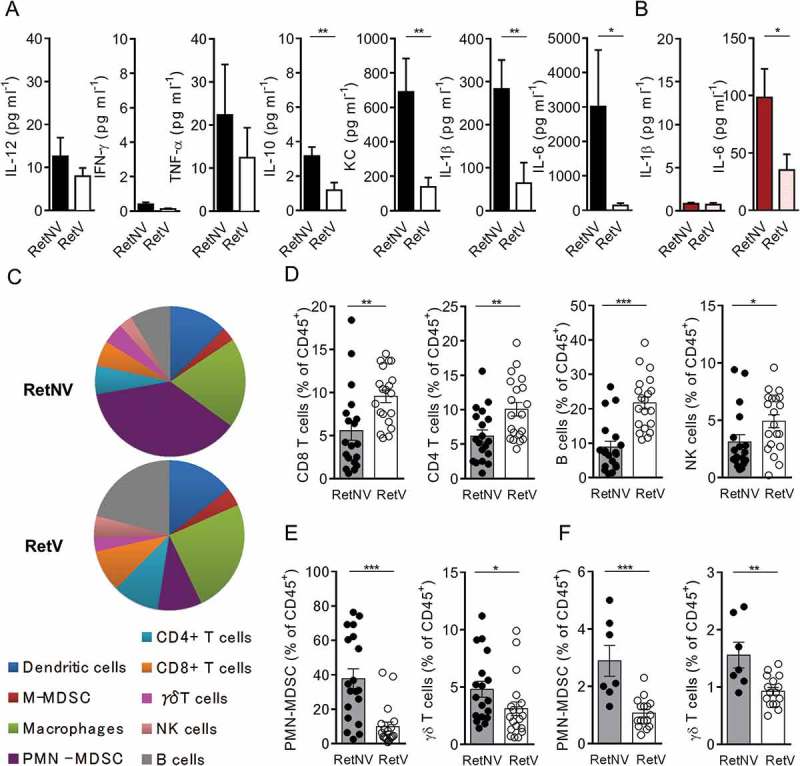
Vitiligo-associated decrease of PMN-MDSCs and γδ T cell infiltration within primary tumors and spleen.
(A) Protein levels of indicated cytokines in aqueous humors from RetNV (n = 14) and RetV (n = 11) mice, determined by multiplex ELISA. (B) IL-1β and IL-6 quantifications in sera from RetNV (n = 8) and RetV (n = 8) mice determined by ELISA. (C-F) Dendritic cells (CD11c+), macrophages (CD11b+CD11c−Ly6C−/lowLy6G−), monocytic MDSCs (M-MDSCs: CD11b+CD11c−Ly6ChighLy6G−), PMN-MDSCs (CD11b+CD11c−Ly6ClowLy6G+), CD4+ T cells (CD3+, CD4+), CD8+ T cells (CD3+, CD8+), γδ T cells (CD3+, δ TCR+), NK cells (CD3−, NK1.1+), B cells (CD3−, CD19+) were analyzed by flow cytometry. Parts of whole graphs describing immune cells found in primary tumors (C). Percentages of CD8+ T cells, CD4+ T cells, NK cells and B lymphocytes (D) PMN-MDSCs and γδ T cells among CD45+ cells within primary tumors (E) from RetNV (n = 18) and RetV (n = 19) or within spleen (F) from RetNV (n = 7) and RetV (n = 16). Each point represents individual mouse. Bars are mean ± SEM. *P < 0.05, **P < 0.01 ***P < 0.001 (Mann-Whitney test).
In parallel, we quantified the immune cells within primary tumors of 6 month old mice (Figure 2C-F). Primary tumors of RetV mice are more infiltrated by anti-tumor effector cells, such as B cells, NK cells and tumor infiltrating lymphocytes (TILs), than those from RetNV mice (Figure 2D). Remarkably they also exhibited significantly less PMN-MDSCs (CD11b+CD11c−Ly6ClowLy6G+, mean: 36.8% vs 9.8%) and γδ T cells (CD3+, pan γδ TCR, mean: 5.1% vs 3%) (Figure 2E), consistent with the low levels of KC and with the key roles of these immune subsets in the Ret melanoma progression.4,13 Similarly PMN-MDSCs and γδ T cells were less frequent in the spleen of RetV mice than of RetNV mice (Figure 2F). Collectively, our results suggest that the reduced proportion of γδ T cells at the primary tumor site and the subsequent reduced proportion of PMN-MDSCs contribute to delay the tumor development in Ret mice with melanoma-associated vitiligo.
Vitiligo development is associated with a decrease of γδ T cells expressing NOS2
Our recent data evidenced that NOS2 expressing γδ T cells lead to a strong recruitment of PMN-MDSCs.13 Because RetV and RetNos2KO mice display the same delay in primary tumor onset, we hypothesized that γδ T cells in RetV mice express a lower level of NOS2 than RetNV mice. In this aim, we isolated CD45+ cells from primary tumors and tumor draining lymph nodes (LN), and stained them for NOS2 in green and TCRδ chain in red. The Figure 3A illustrates a representative staining from LN derived cells. After undergoing the cytospin process, γδ T cells producing or not NOS2 were quantified. Interestingly, we found that the NOS2 expressing γδ T cells among all γδ T cells are significantly less frequent in LN (Figure 3B) as well as within the primary tumor (Figure 3C) of RetV mice compared to RetNV mice. Our results indicate that vitiligo occurrence is associated with a decrease of γδ T cells producing NOS2.
Figure 3.
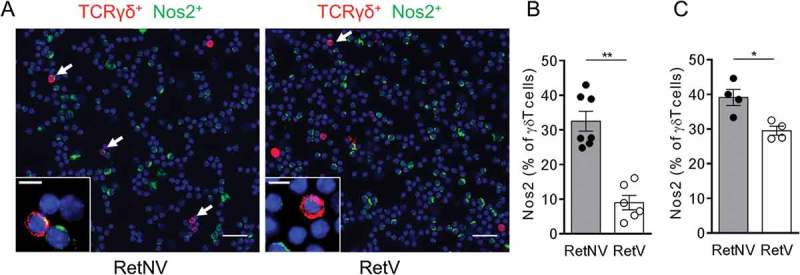
Vitiligo-associated decrease of the proportion of NOS2 positive γδ T cells in primary melanoma and TdLNs.
(A) Representative microscopy images showing γδ T cells positive for NOS2 from cells derived from TdLNs of RetNV or RetV mice and stained with antibodies to TCR γδ (red), NOS2 (green) and counterstained with DAPI (blue). Arrows indicate NOS2 expressing γδ T cells. Bars 10 µM. 40 X objective. (B, C) Quantification of NOS2 positive γδ T cells in TdLN (B) from RetNV (n = 7) and RetV (n = 6) and in primary melanoma (C) from RetNV and RetV (n = 4 each). It was performed from 500 to 1500 γδ T cells. Each point represents individual mouse. Bars are mean ± SEM. *P < 0.05, **P < 0.01 (Mann-Whitney test).
In vivo neutralization of IL-1β and IL-6 slows down metastasis formation, induces vitiligo incidence and lessens recruitment of γδ T cells and PMN-MDSCs
As weak amounts of IL-1β and IL-6 were detected in primary tumor extracts from RetV mice (Figure 2A), we assumed that these cytokines play a key role in inducing NOS2 in γδ T cells that could subsequently accelerate tumor onset. To address this point, Ret mice were treated either with antibodies neutralizing IL-1β and IL-6 or with the IL-1 receptor antagonist anakinra during three months (Figure 4). These treatments did not affect primary tumor incidence (Figure 4A), but significantly delayed metastasis formation (Figure 4B). IL-1β and IL-6 neutralization tends to control metastasis dissemination more efficiently than pharmaceutical inhibition of the IL-1 receptor (Figure 4B). The frequency of vitiligo was similar in Ret mice either untreated (38.6%, Figure 1A) or treated with anakinra (35.6%, Figure 4C) at the end of the follow-up period. Among the twelve Ret mice used to neutralize IL-1β and IL-6, four mice (33.3%) exhibited vitiligo before treatment. Interestingly, four RetNV mice became depigmented during treatment. The comparison of the occurrence of vitiligo between treated mice (66.7%, Figure 4C) and untreated mice (Figure 1A) suggests that cytokine neutralization induced the development of vitiligo. Nor IL-1β or IL-6 amounts were found in aqueous humors of 3 month treated mice confirming the neutralization efficiency (Figure 4D). As shown by cytometry, the neutralization of IL-1β and IL-6 significantly reduced the proportion of PMN-MDSCs and γδ T cells within primary tumors (Figure 4E, F) and spleen (Figure 4G, H), consistent with the effect on the metastatic control. Taken together, these data showed that IL-1β and IL-6 promote metastasis formation via an effect on PMN-MDSCs and γδ T cells in our melanoma model.
Figure 4.
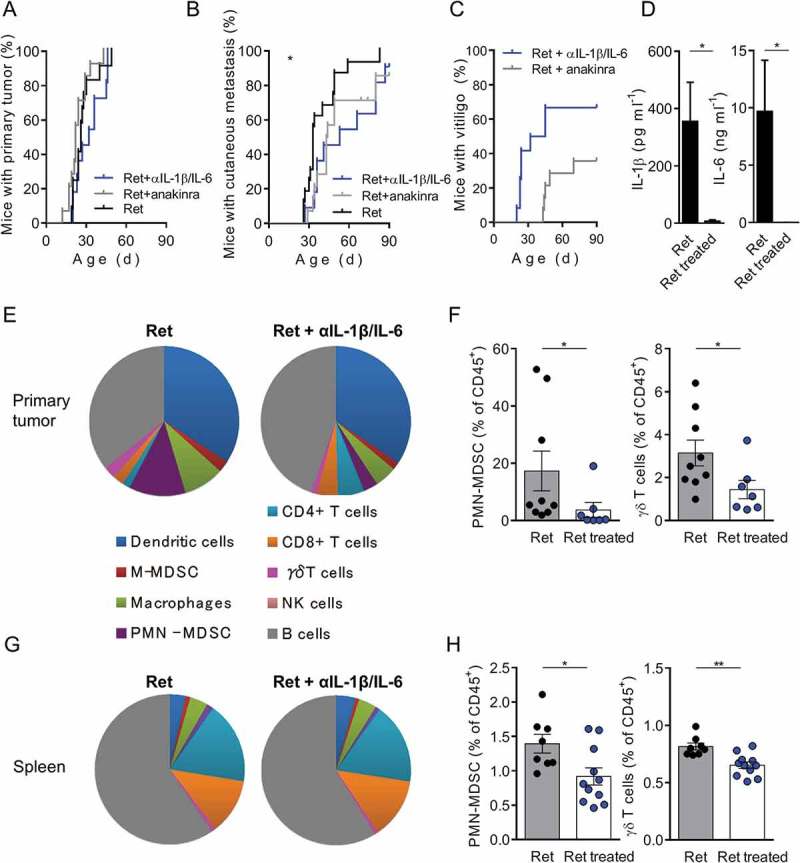
In vivo neutralization of IL-1β and IL-6 slows down metastasis formation and reduced PMN-MDSCs and γδ T cell infiltration within primary tumors and spleen.
(A, B) 3-month follow-up of melanoma development from Ret mice untreated (n = 12) or treated either with antibodies neutralizing IL-1β and IL-6 (n = 12) or with IL-1 receptor antagonist (anakinra) (n = 14). Time courses of primary tumor (A), cutaneous metastasis (B) and vitiligo (C) onsets. (D) Protein levels of IL-1β and IL-6 in primary tumor extracts, from Ret (n = 4) and Ret treated (n = 4) mice, determined by multiplex ELISA. (E-H) Analyses of immune cell proportions within primary tumors (E) and spleen (G) represented as parts of whole. Percentages of PMN-MDSCs and γδ T cells among CD45+ cells within primary tumors (F) or spleen (H) from Ret (n = 9 and 8) and Ret treated (n = 7 and 11 respectively) mice. Each point represents individual mouse. Bars are mean ± SEM. *P < 0.05, **P < 0.01. Wilcoxon test (A, B), Mann-Whitney test (D, F, H).
In vivo neutralization of IL-1β and IL-6 reduces NOS2 expression in pro-tumorogenic γδ T cells
We next evaluated if IL-1β and IL-6 contribute to NOS2 expression in γδ T cells. Three month old RetNV mice were treated with anti-IL-1β and anti-IL-6 neutralizing antibodies. After fifteen days of treatment, the proportion of NOS2 expressing γδ T cells, assessed by microscopy, was significantly reduced (Figure 5A and B). These cells represented less than 10% of γδ T cells in treated RetNV mice (Figure 5B), a proportion quite similar to that observed in untreated RetV mice (Figure 3B). Interestingly, we also found lower amounts of IL-17 in primary tumors extracts from treated RetNV mice (Figure 5C), suggesting that such a short-term treatment already affects the proportion of IL-17 producing γδ T cells at the tumor site. Collectively, our results showed that high concentrations of IL-1β and IL-6 within the tumor microenvironment strongly supported NOS2 expression in pro-tumorigenic γδ T cells in melanoma and that their low NOS2 expression may, at least in part, explain the reduced tumor aggressiveness in Ret mice with melanoma-associated vitiligo.
Figure 5.
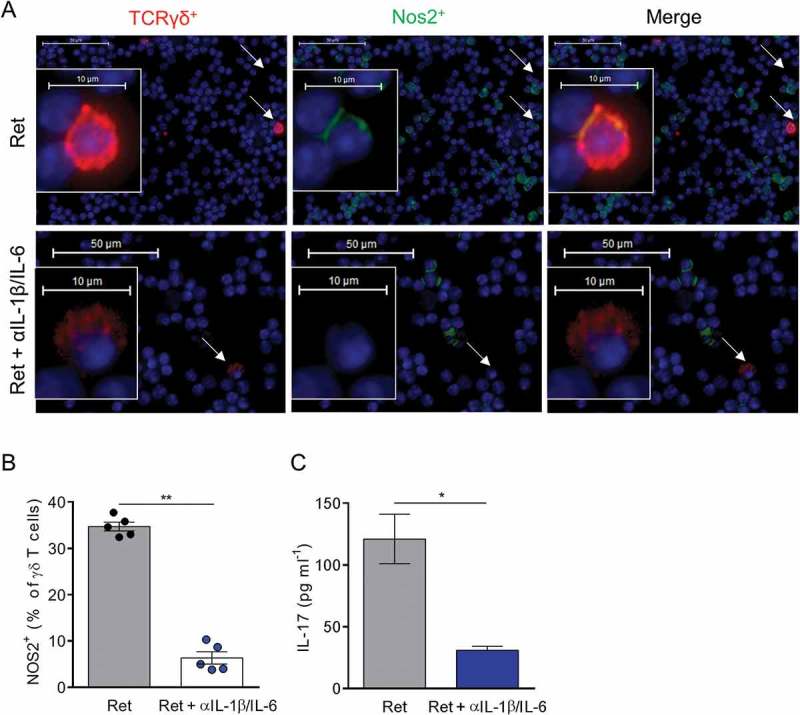
In vivo neutralization of IL-1β and IL-6 suppresses NOS2 expression in γδ T cells.
(A-C) 3 month old Ret mice were treated or not during 2 weeks with antibodies neutralizing IL-1β and IL-6. (A, B) NOS2 expression was analyzed by microscopy in TdLNs from 5 mice in each group. Representative images showing γδ T cells positive for NOS2 derived either from untreated or treated Ret mice and stained with antibodies to TCR γδ (red), NOS2 (green) and counterstained with DAPI (blue). Bars 10 µM. 40 X objective. Arrows indicate γδ T cells. (B) NOS2 positive γδ T cells were quantified from 500 to 1500 γδ T cells. (C) Protein levels of IL-17 in primary tumor extracts, from untreated (n = 5) and treated (n = 5) Ret mice, were determined by ELISA. Each point represents individual mouse. Bars are mean ± SEM. *P < 0.05, **P < 0.01. (Mann-Whitney test).
Discussion
Here, we demonstrated that IL-1β and IL-6 inflammatory cytokines induce NOS2 expression by γδ T cells in a spontaneous model of melanoma. We previously reported that NOS2 drives optimal IL-2 and IL-17 production, proliferation and glycolysis of γδ T cells promoting their peripheral expansion at steady state and their pro-tumoral properties in the context of melanoma,13,22 nevertheless the molecular mechanisms underlying autocrine NOS2 expression by γδ T cells were not investigated. Murine interferons (IFN-γ and type I IFNs) and microbial products (e.g., LPS/TLR ligand activated NF-kB) up-regulate NOS2 in myeloid cells, particularly in macrophages.23 IL-1β and/or IL-6 induce NOS2 expression in several immune populations including macrophages, plasma cells and Th17 cells.24–27 Recently, the SETD1B histone methyltransferase was shown to activate NOS2 in tumor-induced MDSCs.28 Indeed, the regulation of NOS2 is different depending on immune cell types. Here, firstly we observed that the primary tumor in Ret mice with the more aggressive melanoma is particularly enriched in IL-1β and IL-6 and highly infiltrated within NOS2 expressing γδ T cells and secondly that IL-1β and IL-6 neutralization reduced the capacity of γδ T cells to express NOS2 and their proportion at the tumor site. Collectively, our data support a key role of these inflammatory cytokines in regulating NOS2 expression by γδ T cells in vivo. Moreover we have previously observed that IL-1β and IL-6 were almost absent from primary tumor extracts of RetNos2KO mice13 suggesting a possible feedback loop between NOS2 and these cytokines. Such an hypothesis is supported by the induction of IL-6 transcription by nitric oxide via activating guanosine 3ʹ,5ʹ-cyclic monophosphate-dependent protein kinase in osteoblasts and skeletal myotubes.29,30
Melanoma patients may develop spontaneously a vitiligo that correlates with significant improved 5-year survival.17,18,31 When occurring in response to melanoma treatment (i.e. anti-PD1 and anti-CTL-A4 based immunotherapy), the occurrence of vitiligo may also have prognostic significance.20 An autoimmune etiology has been proposed to explain the development of vitiligo essentially based on the presence of autoantibodies and autoreactive T lymphocytes against melanocyte antigens.32 Nevertheless, an effort has to be done to identify new immune-related actors involved in the balance between autoimmunity and melanoma to provide new insight into the development of more efficient melanoma treatment. Using the Ret mouse model, we have previously reported that mice developing a melanoma-associated vitiligo displayed frequent peripheral melanoma-specific CD8+ T-cells that protected them toward a challenge with syngenic melanoma cells.15 In line with this, CD8+ T cells specific for melanocyte-differentiation antigens are detected in the human related pathology.21 Despite our current observation that tumor infiltrating CD8+ T cells are more frequent in RetV mice compared to RetNV mice, Ret mice either deficient for T cells or depleted for CD8+ T cells from birth developed a melanoma-associated vitiligo,33,34 supporting that CD8+ T cells would be the consequence of the tumor immune surveillance rather than the cause of the vitiligo development. We also evidenced accumulation of inflammatory monocytes/dendritic cells inhibiting tumor cell proliferation in the skin of Ret mice with active vitiligo and demonstrated their key contribution in the tumor control resulting in skin depigmentation.34,35 Here, we analyzed for the first time the cytokine profile and the composition of immune cells infiltrating the primary tumor in Ret mice with or without vitiligo. In addition to CD8+ T cells already discussed, CD4+ T, B and NK cells infiltrate more significantly tumors of RetV mice compared to those of RetNV mice. We have shown that T, B and NK cells are not crucial for the development of vitiligo in Ret mice and that IL-10 neutralization results in increased occurrence of vitiligo34 consistent with the reduced IL-10 expression in RetV mice compared to RetNV mice we observed here. Strikingly, NOS2 expressing γδ T cells and PMN-MDSCs are less frequent in mice developing a spontaneous melanoma associated vitiligo, in agreement with the reduced local concentration of IL-1β, IL-6 and KC. In line with these data, we recently found that NOS2 promoted IL-17 production by tumor infiltrating γδ T cells leading to the recruitment of PMN-MDSCs.13 In addition, the neutralization of IL-1β and IL-6 reduced NOS2 expression in γδ T cells. Collectively, our data suggest that the delay in tumor cell spreading in melanoma associated vitiligo is due, at least in part, to a reduced presence of IL-1β and IL-6 at the primary tumor site, decreasing the proportion of NOS2 expressing γδ T cells and subsequently the recruitment of PMN-MDSCs.
Chronic inflammation in the tumor microenvironment is an hallmark of cancer that promotes tumorigenesis and tumor cell dissemination.36,37 Counteracting cancer induced chronic inflammation may be an attractive therapeutic strategy,38 as exemplified with the reduced B16 melanoma cell tumorogenicity in IL-1β knockout mice39 or the lower incidence of lung cancer incidence and mortality after IL-1β inhibition.40 Here, we show not only a delay in tumor cell dissemination, but also a significant increase of the occurrence of vitiligo in Ret mice in response to IL-1β and IL-6 neutralization, suggesting a key role of inflammatory cytokines in the balance between cancer and autoimmunity.
In conclusion, our data show for the first time that the levels of IL-1β and IL-6 at the tumor site control the recruitment of γδ T cells producing NOS2 and the balance between melanoma and vitiligo associated melanoma.
Materials and methods
Mice
MT/ret± mice (called Ret later) are transgenic for the human Ret oncogene.14 Ret mice were crossed with mice deficient for Nos2 to obtain RetNos2KO mice. The incidences of primary tumors and cutaneous lesions were assessed from day 21 and then twice a month. 3 to 6-month-old mice were used for experiments. Mice were sacrificed at indicated times or when considered moribund (prostrated, bristly, skinny). All mice are on the C57BL/6J background and were maintained in our animal facility under specific pathogen-free conditions.
Protein quantifications at the primary tumor site and in sera
Aqueous humors of 6 month old Ret mice were collected from primary tumors mechanically dissociated in 150 µl of phosphate-buffered saline (PBS). They were frozen until use. IL-17 was quantified by ELISA using R&D Systems. The concentrations in IFN-γ, IL-12, IL-10, TNF-α, KC, IL-1β, and IL-6 were determined using a kit from Meso Scale Discovery according to supplier instruction. IL-1β and IL-6 were also quantified from sera obtained following mandibular blood collection.
Single cell suspension procedures
Spleen were mechanically dissociated, homogenized, and passed through a 100 µM cell strainer in PBS with 5% FCS and 0.5% EDTA. Primary tumors were mechanically dissociated and digested with 1 mg ml−1 collagenase D and 0.1 mg ml−1 DNase I for 20 min 37°C.
Cell staining and flow cytometry
Surface staining was performed by incubating cells on ice, for 20 min, with saturating concentrations of labeled Abs in PBS, 5% FCS and 0.5% EDTA. Mouse cell-staining reactions were preceded by a 15-min incubation with purified anti-CD16/32 antibodies (Abs) (FcγRII/III block; 2.4G2) obtained from hybridoma supernatants. Following anti-mouse Abs were used: FITC – conjugated anti-Ly6G (1A8), anti-B220 (RA3-632), PE – conjugated anti- δ TCR (GL3) and anti-NK1.1 (PK136), APC- conjugated anti-CD45.2 (104), anti-CD11b (M1/70), PerCP-Cy5.5 – conjugated anti-CD3 (145-2C11), anti-βTCR (H57-597), and anti-CD45.2 (104), Pacific Blue- conjugated anti-CD4 (RM4-5), V450-conjugated anti-Ly6C (AL-21), PE-Cy7-conjugated anti-CD11c (HL3), APC-H7-conjugated anti-CD8 (53–6.7). Abs were purchased from BD Biosciences except anti-CD11b, anti-B220 and anti-δ TCR from eBioscience. Data files were acquired on LSRII and analyzed using Diva software (BD Biosciences).
Microscopy
Hematopoietic cells were magnetically sorted from primary tumors with anti-CD45+ MicroBeads (Miltenyi Biotec). Then, CD45+ cells either from primary tumors or tumor draining LN were centrifuged onto a microscope slide using Cellspin 1 (Tharmac). Non-specific reactivity was performed with incubation of PBS, 1% BSA for 20 min at room temperature (RT). Biotin hamster anti-mouse γδ TCR (GL3, BD Biosciences) and rabbit anti-mouse NOS2 (Calbiochem) used as primary Abs were incubated 1 hour at RT. Then, slides were washed in PBS, 1% BSA before being incubated for 30 min at RT with Alexa fluor 488-conjugated goat anti-rabbit (Jackson immuno research) and PE-conjugated streptavidine (BD Biosciences) used as secondary Abs. Finally nuclei were labeled with DAPI and slides were mounted in Vectashield mounting medium (Vector Labs). Images were acquired using an automated high-resolution scanning system (Lamina™, PerkinElmer) with 40X objective. Acquisitions were performed with inForm for Lamina software (PerkinElmer) and images were analyzed with ImageJ and Panoramic Viewer (3DHISTECH).
In vivo treatments
For the neutralization of IL-1β and IL-6 over a three month period, Ret mice received three times a week an i.p. injection of 50 µg of anti-IL-1β (Clone B122, BioXcell) and anti-IL-6 (Clone MP5-20F3, BioXcell) from week 1 to week 3, and then of 100 µg of the same neutralizing antibodies from week 4 to week 12. Alternatively, 200 µg of IL-1 receptor antagonist (Anakinra kindly provided by Pr. L. Mouthon, Cochin Hospital) was injected i.p. three times a week for three months. Diagnosis was performed once a week to detect the occurrence of primary tumor and cutaneous metastases. In the short-term treatment, 3 month old Ret mice were treated three times a week for 2 weeks with 100 µg of anti-IL-1β and anti-IL-6 antibodies.
Statistics
Comparison between incidence curves was performed using Wilcoxon test. Data are expressed as mean ± SEM. The significance of differences between two series of results was assessed using the Mann-Whitney test. (*, P < 0.05; **, P < 0.01; ***, P < 0.001). All statistical analyses were performed using Prism 6 software (GraphPad).
Study approval
Experiments were carried out in accordance with the guidelines of the French Veterinary Department and were approved by the Paris-Descartes Ethical Committee for Animal Experimentation (15–035, APAFIS #3646).
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was reported by the authors.
Funding Statement
This work was supported by the SILAB Jean Paufique Fundation;SFD;La ligue contre le cancer;
Acknowledgments
This work was supported by the SILAB Jean Paufique foundation. L. Douguet was supported by Paris VII University and the “Ligue Nationale contre le Cancer”. A. Prévost-Blondel’s team is supported by the Comité “Ile de France” de la Ligue contre le Cancer and the Société Française de Dermatologie. We thank Pr L. Mouthon (Cochin Institute) for anakinra. We acknowledge Cybio, Histim and animal facilities of the Cochin Institute.
Author’s contributions
L.D., L.B., R.L., and L.L. did experiments; L.D., and A.P.B. designed the study with the help of I.C., and L.D., and A.P.B. wrote the manuscript; and all authors critically reviewed the manuscript.
References
- 1.Eyles J, Puaux AL, Wang X, Toh B, Prakash C, Hong M, Tan TG, Zheng L, Ong LC, Jin Y, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest. 2010;120:2030–2039. doi: 10.1172/JCI42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar V, Patel S, Tcyganov E, Gabrilovich DI.. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res. 2015;128:95–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011;9:e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafont V, Sanchez F, Laprevotte E, Michaud HA, Gros L, Eliaou JF, Bonnefoy N. Plasticity of gammadelta T cells: impact on the anti-tumor response. Front Immunol. 2014;5:622. doi: 10.3389/fimmu.2014.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chitadze G, Oberg HH, Wesch D, Kabelitz D. The ambiguous role of gammadelta T lymphocytes in antitumor immunity. Trends Immunol. 2017;38:668–678. doi: 10.1016/j.it.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Silva-Santos B, Serre K, Norell H. gammadelta T cells in cancer. Nat Rev Immunol. 2015;15:683–691. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 8.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, Barilla R, Torres-Hernandez A, Hundeyin M, Mani VRK, et al. gammadelta T cells support pancreatic oncogenesis by restraining alphabeta T cell activation. Cell. 2016;166:1485–99 e15. doi: 10.1016/j.cell.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M, Hawinkels LJ, Jonkers J, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, Shi L, Wu D, Dong C, Liu H. IL-17A produced by gammadelta T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014;74:1969–1982. doi: 10.1158/0008-5472.CAN-13-2534. [DOI] [PubMed] [Google Scholar]
- 12.Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W, et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douguet L, Bod L, Lengagne R, Labarthe L, Kato M, Avril MF, Prevost-Blondel A. Nitric oxide synthase 2 is involved in the pro-tumorigenic potential of gammadelta17 T cells in melanoma. Oncoimmunology. 2016;5:e1208878. doi: 10.1080/2162402X.2016.1208878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato M, Takahashi M, Akhand AA, Liu W, Dai Y, Shimizu S, Iwamoto T, Suzuki H, Nakashima I. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17:1885–1888. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- 15.Lengagne R, Le Gal FA, Garcette M, Fiette L, Ave P, Kato M, Briand JP, Massot C, Nakashima I, Renia L, et al. Spontaneous vitiligo in an animal model for human melanoma: role of tumor-specific CD8+ T cells. Cancer Res. 2004;64:1496–1501. doi: 10.1158/0008-5472.CAN-03-2828. [DOI] [PubMed] [Google Scholar]
- 16.Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, Luiten RM. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 17.Bystryn JC, Rigel D, Friedman RJ, Kopf A. Prognostic significance of hypopigmentation in malignant melanoma. Arch Dermatol. 1987;123:1053–1055. doi: 10.1001/archderm.1987.01660320095019. [DOI] [PubMed] [Google Scholar]
- 18.Nordlund JJ, Kirkwood JM, Forget BM, Milton G, Albert DM, Lerner AB. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689–696. doi: 10.1016/S0190-9622(83)70182-9. [DOI] [PubMed] [Google Scholar]
- 19.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, Panagiotou P, Polyzos A, Papadopoulos O, Stratigos A, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 21.Le Gal FA, Avril MF, Bosq J, Lefebvre P, Deschemin JC, Andrieu M, Dore MX, Guillet JG. Direct evidence to support the role of antigen-specific CD8(+) T cells in melanoma-associated vitiligo. J Invest Dermatol. 2001;117:1464–1470. doi: 10.1046/j.0022-202x.2001.01605.x. [DOI] [PubMed] [Google Scholar]
- 22.Douguet L, Cherfils-Vicini J, Bod L, Lengagne R, Gilson E, Prevost-Blondel A. Nitric oxide synthase 2 improves proliferation and glycolysis of peripheral gammadelta T cells. PLoS One. 2016;11:e0165639. doi: 10.1371/journal.pone.0165639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinert H, Schwarz PM, Forstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol Chem. 2003;384:1343–1364. doi: 10.1515/BC.2003.152. [DOI] [PubMed] [Google Scholar]
- 24.Obermajer N, Wong JL, Edwards RP, Chen K, Scott M, Khader S, Kolls JK, Odunsi K, Billiar TR, Kalinski P. Induction and stability of human Th17 cells require endogenous NOS2 and cGMP-dependent NO signaling. J Exp Med. 2013;210:1433–1445. doi: 10.1084/jem.20121277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saini AS, Shenoy GN, Rath S, Bal V, George A. Inducible nitric oxide synthase is a major intermediate in signaling pathways for the survival of plasma cells. Nat Immunol. 2014;15:275–282. doi: 10.1038/ni.2806. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Gonon AT, Sjoquist PO, Lundberg JO, Pernow J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc Natl Acad Sci U S A. 2013;110:15049–15054. doi: 10.1073/pnas.1307058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lima-Junior DS, Costa DL, Carregaro V, Cunha LD, Silva AL, Mineo TW, Gutierrez FR, Bellio M, Bortoluci KR, Flavell RA, et al. Inflammasome-derived IL-1beta production induces nitric oxide-mediated resistance to Leishmania. Nat Med. 2013;19:909–915. doi: 10.1038/nm.3221. [DOI] [PubMed] [Google Scholar]
- 28.Redd PS, Ibrahim ML, Klement JD, Sharman SK, Paschall AV, Yang D, Nayak-Kapoor A, Liu K. SETD1B activates iNOS expression in myeloid-derived suppressor cells. Cancer Res. 2017;77:2834–2843. doi: 10.1158/0008-5472.CAN-16-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broderick KE, Zhang T, Rangaswami H, Zeng Y, Zhao X, Boss GR, Pilz RB. Guanosine 3ʹ,5ʹ-cyclic monophosphate (cGMP)/cGMP-dependent protein kinase induce interleukin-6 transcription in osteoblasts. Mol Endocrinol. 2007;21:1148–1162. doi: 10.1210/me.2005-0389. [DOI] [PubMed] [Google Scholar]
- 30.Makris AC, Sotzios Y, Zhou Z, Makropoulou M, Papapetropoulos N, Zacharatos P, Pyriochou A, Roussos C, Papapetropoulos A, Vassilakopoulos T. Nitric oxide stimulates interleukin-6 production in skeletal myotubes. J Interferon Cytokine Res. 2010;30:321–327. doi: 10.1089/jir.2009.0022. [DOI] [PubMed] [Google Scholar]
- 31.Quaglino P, Marenco F, Osella-Abate S, Cappello N, Ortoncelli M, Salomone B, Fierro MT, Savoia P, Bernengo MG. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21:409–414. doi: 10.1093/annonc/mdp325. [DOI] [PubMed] [Google Scholar]
- 32.Le Poole IC, Wankowicz-Kalinska A, van den Wijngaard RM, Nickoloff BJ, Das PK. Autoimmune aspects of depigmentation in vitiligo. J Invest Dermatol Symp Proc. 2004;9:68–72. doi: 10.1111/j.1087-0024.2004.00825.x. [DOI] [PubMed] [Google Scholar]
- 33.Lengagne R, Pommier A, Caron J, Douguet L, Garcette M, Kato M, Avril MF, Abastado JP, Bercovici N, Lucas B, et al. T cells contribute to tumor progression by favoring pro-tumoral properties of intra-tumoral myeloid cells in a mouse model for spontaneous melanoma. PLoS One. 2011;6:e20235. doi: 10.1371/journal.pone.0020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pommier A, Audemard A, Durand A, Lengagne R, Delpoux A, Martin B, Douguet L, Le Campion A, Kato M, Avril MF, et al. Inflammatory monocytes are potent antitumor effectors controlled by regulatory CD4+ T cells. Proc Natl Acad Sci U S A. 2013;110:13085–13090. doi: 10.1073/pnas.1300314110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pommier A, Lucas B, Prevost-Blondel A. Crucial role of inflammatory monocytes in antitumor immunity. Oncoimmunology. 2013;2:e26384. doi: 10.4161/onci.26384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]


