ABSTRACT
In preclinical models, IL-1β inhibition could enhance the efficacy of fluorouracil (5-FU). In this phase 2 study, we assessed the activity and safety of 5-FU plus bevacizumab and anakinra (an IL-1β and α inhibitor) in patients with metastatic colorectal (mCRC) refractory to chemotherapy and anti-angiogenic therapy.
Eligible patients had unresectable mCRC; were refractory or intolerant to fluoropyrimidine, irinotecan, oxaliplatin, anti-VEGF therapy, and anti-EGFR therapy (for tumors with wild-type KRAS). Patients were treated with a simplified acid folinic plus 5-FU regimen and bevacizumab (5 mg/kg) both administered by intravenous infusion for 30 min every 2 weeks. Anakinra (100 mg) was injected subcutaneously once daily. The primary endpoint was the 2-month response rate determined upon CHOI criteria.
Thirty two patients with metastatic colorectal cancer were enrolled. Five patients demonstrated response (Choi criteria) and 22 patients had stable disease as the best 2-month overall response. Median progression-free and overall survival were 5.4 (95% CI, 3.6–6.6) and 14.5 months (95% CI, 9–20.6) respectively. Twenty patients experienced grade 3 toxicity. No grade 4 or 5 toxicity related to therapy occurred. The most common grade 3 adverse events were neutropenia in 8 (25%) patients, digestive side effects in 7 (21.9%) patients and hypertension in 6 (18.75%) patients. No treatment-related deaths or serious adverse events were reported.5-FU plus bevacizumab and anakinra has promising activity and a manageable safety profile, suggesting that this combination might become a potential treatment option for patients with refractory mCRC.
KEYWORDS: IL1, chemoimmunotherapy, clinical trial, colorectal, MDSC, clicial trial optimization, new targets, therapeutic trials
Introduction
Colorectal cancer (CRC) is the second most commonly diagnosed cancer in Europe and a leading cause of death both in Europe and worldwide.1,2 Chemotherapy remains the cornerstone treatment for metastatic colorectal cancer (mCRC). When all metastatic sites cannot be surgically removed, treatment remains palliative and requires different chemotherapeutic protocols. However, the use of palliative systemic chemotherapy dramatically enhances response rates, progression-free survival (PFS) and overall survival (OS). Treatment is currently based on the use of three cytotoxic chemotherapy drugs – fluoropyrimidine, oxaliplatin and irinotecan – combined with targeted therapies (anti-EGFR (panitumumab and cetuximab) or anti-VEGF (bevacizumab or aflibercept) monoclonal antibodies). Regorafenib and TAS-102 have recently been added to the therapeutic arsenal as third line of therapy.3
Chronic inflammation is recognized as a promoting factor in carcinogenesis and tumor progression.4 Interleukin-1β (IL-1β) plays a role in chronic inflammation, and tumor growth is encouraged by an overly active IL-1β system. These factors weigh in favor of IL-1β inhibition for cancer prevention or therapy.5,6 Recent clinical data has shown that IL-1 β inhibition using canakinumab could prevent lung cancer growth,7 and another recent trial has underlined that MAbP1, a monoclonal antibody which targets IL1α, improves quality of life and survival in mCRC.8 Recently we observed that fluorouracil (5-FU) could activate the NOD-like receptor family, the pyrin domain containing 3-protein (NLRP3)-dependent caspase-1 activation complex (termed the inflammasome) in myeloid-derived suppressor cells (MDSCs), leading to IL-1β production, which curtails anticancer immunity. IL-1β was also shown to induce the generation and expansion of TH17 cells, which could promote tumor growth by favoring proangiogenesis and immunosuppression in a IL-17A dependent manner.9 Based on this rational we suggested that inhibition of IL-1β could reverse resistance to 5-FU plus bevacizumab therapy in mCRC.
Anakinra is a recombinant, non-glycosylated version of human IL-1RA (RA for receptor antagonist) and inhibits both IL-1α and IL-β. This first-in-man study was designed to test the safety, tolerability, and efficacy of anakinra in combination with 5-FU and bevacizumab in mCRC patients refractory to previous treatment with 5-FU and bevacizumab alone or in combination with oxaliplatin or irinotecan.
Methods
Patients
This open-label phase II study investigated the effects of combined 5-FU, bevacizumab and anakinra in patients with pathologically confirmed mCRC disease refractory to standard treatment (Fluoropyrimidine, oxaliplatin irinotecan, anti-angiogenic and anti-EGFR therapies). Notably, we only included patients with progressive disease on Fluoropyrimidine plus bevacizumab based chemotherapy. Patients were screened at Georges Francois Leclerc Cancer Center (Dijon, France) and the Besancon University Hospital (Besancon, France). The main criteria for eligibility were: minimum age 18 years, Eastern Cooperative Oncology Group (ECOG) score of 0, 1, or 2; and adequate hematological profile, renal function and hepatic function. The study was approved by the Burgundy Committee for the Protection of Persons. All patient gave writing inform consent. The trial was registered on ClinicalTrials.gov (Identifier: NCT02090101). The study was stopped after the recruitment of the first 9 patients in order for an external Review Board to evaluate the toxicity and determine the potential treatment benefit versus risk in this patient group. After evaluation, we continued inclusion to a total of 32 patients.
Treatment plan and evaluation
After inclusion, patients were given 2-week cycles of bevacizumab + LV5FU2 (folinic acid + 5-FU) + anakinra. On day 1, patients received bevacizumab 5 mg/kg intravenous infusion over 90 (±15) min followed by 2-hour intravenous infusion of folinic acid infusion 400 mg/m2, followed by 5-FU bolus 400 mg/m2 and then 5-FU continuous infusion 1200 mg/m2 over 46 hours. Anakinra (100mg) was injected subcutaneously once per day, every day. Treatment was repeated until disease progression or unacceptable toxicity (≥grade 4) were observed. A chest-abdomen-pelvis CT scan was performed every 8 weeks to assay tumor progression. Patients had to have received at least four cycles of therapy to be evaluable. Tumor response was evaluated using CHOI criteria10 and RECIST 1.111 (response evaluation criteria in solid tumors) criteria by computerized tomography (CT)-scan. Patients were followed until tumor progression.
Outcomes
The primary endpoint was the assessment of the response rate after 2 months of therapy using CHOI criteria. CHOI criteria were selected as primary end point because we suspect than chemotherapy combination has mainly antiangiogenic effect. Secondary endpoints were response according to RECIST criteria, tumor control and overall survival rates at 2, 4 and 6 months. The incidence of all AE or SAE, irrespective of the link with treatment, were also analyzed.
Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTC AE) v 4.0.
Sample size and statistical analysis
Without therapy, an estimated 5% of patients can expect stable disease or response at two months in this context. Consequently, our study needed at least 30 patients in order to show a difference between the standard proportion of 5% (null hypothesis) and the expected proportion of 25% (alternative hypothesis) with α = 0.10 and a statistical power of 90% while using one-sided alpha risk. Calculation of the required number of patients was performed using Simon’s two-step design. Overall, 32 patients had to be included taking into account the 10% of patients lost to follow-up or prematurely withdrawn from the study. Using Simon’s two-step design, an intermediate analysis was performed on the first 9 evaluable patients. If one or more patients were stable or responded to therapy, the study could go on. The final analysis was done on the first 30 evaluable patients and concluded treatment efficacy if 4 or more patients had therapy response or stable disease.
Analyses were performed on the modified intention-to-treat population (i.e. patients with at least 4 cycles of therapy). Descriptive analysis was performed using median with range for quantitative variables and percentages for qualitative ones. The median follow-up was determined using the reverse Kaplan Meier method. Survival curves were plotted using the Kaplan Meier. Analyses were performed using SAS 9.4.
Biological analysis
Serum levels for IFN-γ and IL-17A were measured using ELISA (Biolegend) before therapy and after 15 and 30 days. IL-1β (R&D Systems) serum levels were measured before therapy and after 1 day of 5-FU injection. Blood granulocyte, lymphocyte numbers were estimated using complete blood could. Number of monocytic Myeloid-derived suppressor cell (mMDSC) (CD11b+CD14+HLA-DR−/loCD15−CD33+) was determined upon Flow cytometry. mMDSC expression of bioactive caspase-1 was determined by cytometry using FLICA-1 staining. Slices of tissue 4µm thick were cut from formalin-fixed paraffin-embedded specimens. All IHC procedures were performed using a BenchMark device (Ventana Medical Systems) and diaminobenzidine (DAB) for immunohistochemical staining. Once stained and permanently mounted, slides were magnified (x20) and scanned with a Nanozoomer HT2.0 (Hammamatsu). For Il-17a analysis, two physicians analyzed the corresponding files independently. The scores were compared, and a third pathologist reviewed the slides when there were inconsistencies in the initial evaluations. IL17a staining was performed using polyclonal goat antibody AF-317 (R&D).Cytotoxic T-cell infiltrates were evaluated using CD8 labelling with the C8/144B clone (Dako) and analyzed with QuPath software.
Results
Patient population
Between October 6th, 2014 and May 15th, 2017, 42 patients were screened: 8 were not retained on the basis of protocol inclusion or exclusion criteria and 2 withdrew consent during the screening period. Overall, 32 patients were enrolled in 2 centers and received at least one dose of therapy. Median follow-up was 8.7 months (CI 1.8–32.9). Median age was 65.6 years (range 41.3–82.0). We included 20 RAS mutated patients, 11 WT patients and 1 BRAF mutated patient. No patients with MSI tumors were included in this clinical trial. Six patients had right-sided colon cancer. Seventeen patients had previously received two lines of therapy and 15 patients 3 lines of therapy or more. All patients previous received antiangiogenic therapies and experiment progression under this treatment. The baseline characteristics of patients with mCRC are listed in Table 1.
Table 1.
Summary of baseline patient characteristics.
| Number (%) | |
|---|---|
| Age at Diagnosis, in years | |
| Median | 65.6 |
| Range | 41–82 |
| Sex-No. (%) | |
| Male | 21 (65.5) |
| Female | 11 (34.5) |
| ECOG-No. (%) | |
| 0 | 20 (62.25) |
| 1 | 12 (37.75) |
| Primary site-No. (%) | |
| Colon Right side | 6 (18.75) |
| Colon Left side | 17 (53.1) |
| Rectum | 9 (28.15) |
| Mutational Status-No. (%) | |
| Wild Type | 11 (34.5) |
| RAS mutated | 20 (62.25) |
| Braf Mutated | 1 (3.25) |
| Number of Prior metastatic treatments-No. (%) | |
| 2 | 17 (53.1) |
| 3 or more | 15 (46.9) |
| Prior systemic anticancer agents-No. (%) | |
| Fluropyrimidine | 32 (100) |
| Oxaliplatin | 32 (100) |
| Irinotecan | 32(100) |
| Bevacizumab | 32(100) |
| Aflibercept | 15 (46.9) |
| Regorafenib | 5 (15.5) |
| Anti EGFR | 8 (25.0) |
| Refractory to fluoropyrimidine plus antiangiogenic regimen-No. (%) | |
| As part of last therapy | 27 (84.5) |
| As part of any therapy | 32 (100) |
ECOG: Eastern Cooperative Oncology Group performance status
Safety
The adverse events (AEs) reported during the study period were consistent with those typically reported with a regimen of bevacizumab and 5-FU (Table 2). Therapy-related grade 3 toxicity occurred in 25 patients. No therapy-related grade 4 or 5 toxicity was reported, but one patient died during the protocol. This patient presented a fatal digestive perforation as the result of occlusion due to tumor obstruction. The most frequent grade 3 AEs were neutropenia, diarrhea, high blood pressure and hepatic cholestasis. Only 5 serious AE occurred. AEs resulting from anakinra injections were grade 1 and 2 pains and erythema on the injection site. Overall, 8 AEs led to dose reduction, 7 AEs led to dose interruption, and no patients discontinued treatment because of an AE.
Table 2.
Frequency of adverse event and laboratory abnormality.
| Any Grade | Grade ≥ 3 | |
|---|---|---|
| Any event-No. (%) | 32 (100) | 25 (78) |
| Any serious event-No. (%) | 5 (15.5) | 1 (3.25) |
| General event-No. (%) | ||
| Asthenia | 20 (62.5) | 1(3.25) |
| Weight loss | 7 (22) | 0 |
| Fever | 3 (9.4) | 0 |
| Digestive-No. (%) | ||
| Nausea | 9 (28) | 1 (3.25) |
| Vomiting | 3 (9.4) | 0 |
| Diarrhea | 14 (43.75) | 5 (15.5) |
| Abdominal pain | 8 (25) | 0 |
| Related to antiangiogenic activity-No. (%) | ||
| High blood pressure | 7 (22) | 6 (18.75) |
| Bleeding | 7 (22) | 0 |
| Hematologic-No. (%) | ||
| Neutropenia | 13 (40.5) | 8 (25) |
| Leukopenia | 6 (18.75) | 0 |
| Anemia | 3 (9.4) | 0 |
| Thrombopenia | 6 (18.75) | 0 |
| Cutaneous-No. (%) Hand-foot syndrome |
3 (9.4) | 0 |
| Liver abnormality-No. (%) | ||
| Increase in asparate aminotransferase level | 10 (31.25) | 0 |
| Increase in alanine aminotransferase level | 9 (28) | 1 (3.25) |
| Increase in Bilirubin | 1 (3.25) | 1 (3.25) |
| Increase in Alkaline phosphatase | 6 (18.75) | 1 (3.25) |
| Event related to anakinra-No. (%) | ||
| Skin reaction at injection site | 11(34.5) | 0 |
| Infection at injection site | 0 | 0 |
All Adverse Events were graded according to National Cancer Institute Common Terminology Criteria for adverse Events, version 4.03
Efficacy
An intermediary analysis was performed after the first 9 inclusions. Six patients had stable disease and one partial response (PR) upon analysis (CHOI criteria). This result allowed us to continue the study and complete the inclusions. Of the 32 patients enrolled, 30 were evaluable. Upon analysis with CHOI criteria, five (15.5%) achieved PR as a best response and 22 (68.5%) patients had stable disease. No patients achieved response with RECIST criteria. However, stable disease was observed in 27 (84%) patients at two months and in 12 (37.5%) of patients, stable disease was maintained 6 months or more. Overall, the target lesion shrunk ≥ 10% according to RECIST in 6 patients (18%). At the end of the study, 26 patients had progressive disease and 14 had died. Median PFS was 5.4 months (95% CI, 3.6–6.6 months), and overall survival was 8.7 months (95% CI, 9–20.6). Eighteen patients in the study were treated for 6 months or more (Figure 1).
Figure 1.
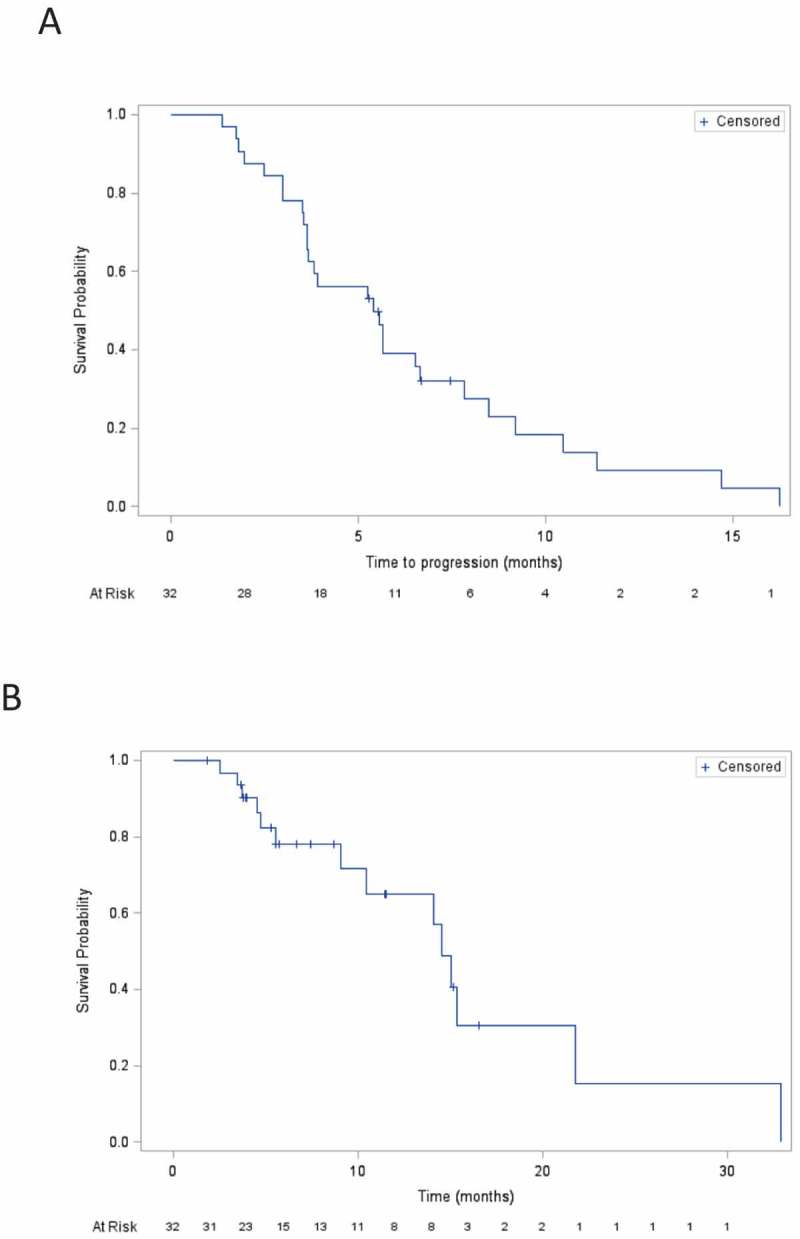
Kaplan-Meier curve for the overall survival (A) and progression free survival (B).
Biological effect of the therapy
No blood parameter taken as a continuous variable at baseline is associated with PFS. However high number of lymphocyte at baseline is associated with better OS (HR = 0.85; p = 0.01) while high level of granulocyte and mMDSC at baseline were associated with poor OS (HR = 1.11; p = 0.04 and HR = 1.05 p = 0.01 respectively). In mice, 5-FU induces caspase-1 activation and IL-1β release and induces the generation Th17 cells, which is inhibited by anakinra.9 We observed a significant activation of caspase-1 in monocytic Myeloid derived suppressor cells (mMDSC) and a significant increase of serum IL-1β
24h after the first injection of 5-FU (Figure 2A and B). After 2 cycles of therapy, blood tests showed a significant decrease in mMDSC and granulocytes and a significant increase in lymphocytes (Figure 2C). At this time point, we observed a significant increase in IFN-γ and a significant decrease in IL-17A cytokine in the serum (2D, E). High IFN-γ serum level at day 15 is associated with better outcome HR = 0.98, p = 0.02). Ten patients underwent a tumor biopsy 2 months after the start of therapy. Here, we observed an increase in CD8 infiltration after 2 months of therapy (Supplementary figure 1) and a correlation between CD8 infiltration at 2 months and PFS (r2 = 0.71) (Figure 2F). IL-17A staining was also evaluated by semi-quantitative evaluation by pathologists. Only 3 patients had detectable IL-17 labelling in their first biopsy. For these 3 patients IL17A staining was decreased or disappeared in the second biopsy.
Figure 2.
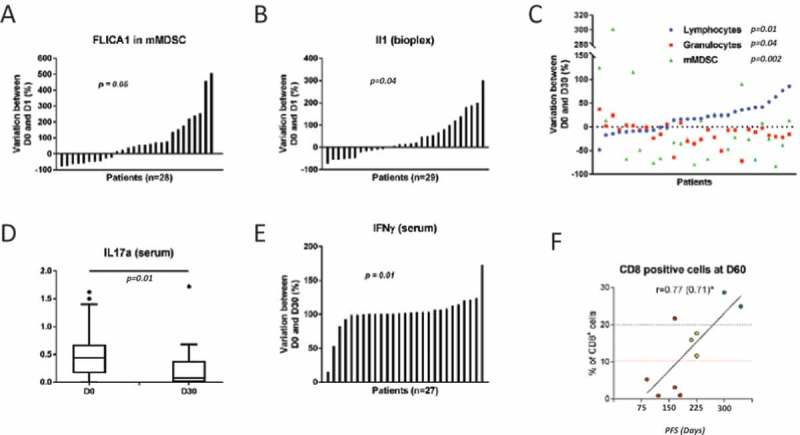
Biological effect of the therapy.
A. Caspase 1 activation determined by flow cytometry before first injection of fluororuracil and after 24 h of infusion. B. IL-1β presence in serum samples determined by ELISA assay before first injection of fluororuracil and after 24 h of infusion. C. Evolution in lymphocyte, granulocyte and mMDSC count for each patient determined upon complete blood count or cytometry (before and after 2 cycles of therapy). Evolution in IL-17A (D) and IFN-g (E) for each patient determined upon ELISA (before and after 2 cycles of therapy). F. Correlation between PFS and CD8 immune infiltration after 2 months of therapy.
Discussion
To our knowledge, this is the first trial to test a combination of anakinra with 5-FU plus bevacizumab in heavily treated mCRC patients. We have reported the safety of the combination therapy as well as the low number of adverse events related to anakinra with exception of skin reaction at the injection site. Toxicity related to 5-FU plus bevacizumab therapy was typical and manageable. The addition of anakinra did not seem to enhance the toxicity of the 5-FU and bevacizumab combination.
IL-1α and β are major pro-inflammatory cytokines. In the context of CRC, chronic intestinal inflammation, for example inflammatory bowel disease, is associated with colorectal carcinogenesis. Polymorphisms in IL1B and IL1RA genes, which induce activation of IL-1 pathway, are associated with a higher risk of tumor recurrence.12 For malignant disease, inflammation signals are essential for tumoral processes such as angiogenesis, remodeling of the tumor stroma, tumor invasion, metastatic spread, and cachexia. IL-1β is predominantly expressed on myeloid lineages within the tumor microenvironment. Conversely, IL-1α is typically expressed by malignant cells.13 The pro-oncogenic properties of IL-1α are its capacity to induce activation of NFKB and STAT3 pathways and to promote tumor aggressiveness.14,15 Neutralization of IL-1α was proposed to have the potential to lessen tumor growth and to reverse or improve debilitating morbidities associated with the disease. MABp1 monoclonal antibody neutralises IL-1α when compared to placebo, and MABp1 has been shown to improve symptoms and quality of life. In an exploratory analysis in a subgroup of patients, the median survival was 6.1 months (95% CI 4.4–7.2) in the MABp1 group compared with only 2.4 months (95% CI 1.9–3.2) in the placebo group, which reinforces the idea that targeting IL-1 pathways is relevant for the treatment of mCRC.8
So far, no clinical study has tested the therapeutic effect of simultaneous IL1α and IL-1β inhibition in mCRC. While both cytokines interact with the same receptor, is it logical to think that they are complementary, and using anakinra (a recombinant IL1 soluble receptor) is therefore appropriate. Importantly, IL-1β related inflammation is not only dependent on tumor cells because 5-FU promotes the release of IL-1β by mMDSC.9 In addition, studies in mice have shown that Il-1β favor tumor growth via Th17 generation and production of IL-17A. IL-17A and Th17 were previously described as factors for poor prognosis in human with CRC.16 Ancillary biological studies in our trial corroborate our preclinical rational. Indeed, Caspase-1 activation and IL-1β release has been observed 24 hours after the first 5-FU injection. We previously observed that 5-FU regimen or FOLFOX regimen9,17 prompts the accumulation of Th17 in patients’ blood and reduces the number of mMDSC. Immunomonitoring underlines a decrease in the number of mMDSC and the inversion of granulocyte/lymphocyte ratio after therapy. Both mMDSC accumulation and granulocyte/lymphocyte ratio have been associated with poor outcome in mCRC.17 For cytokines, therapy decreases the level of harmful IL-17A and increases levels of anti-tumoral IFN-γ. We also observed the accumulation of CD8 in the tumor which suggests a positive in situ immune response with T-cell recruitment. The mechanism of IFN-γ increase is not currently explore. We could suspect that NLRP3 activation induce by 5-Fluorouracil would increase in IL 18 or a decrease in IL 18 Binding Protein in addition to IL-1β activation18
After failure of irinotecan and oxaliplatin based chemotherapy, few therapeutic options are available for mCRC. Regorafenib and TAS-102 demonstrated superiority to best supportive care in phase III trial. For the regorafenib study, median progression free survival was 2.8 months (95% CI 1.4–3.7) versus 1.8 months (95% CI 1.3–1.7), (hazard ratio 0.49; p < 0 · 0001). The median overall survival was 6.4 months (95% CI 3.6–11.8) versus 5.0 months (95% CI 2.8–10.4) (hazard ratio 0.77; p = 0.0052).19 For the more recent TAS-102, a similar study underlines a median progression-free survival of 2.0 months (95% CI, 1.9 to 2.1) in the TAS-102 group and 1.7 months (95% CI, 1.7 to 1.8) in the placebo group (hazard ratio 0.48; p < 0.001). The median overall survival improved from 5.3 months (95% CI, 4.6–6) to 7.1 months (95% CI, 6.5–7.8) (hazard ratio 0.68; p < 0.001).20
Our study revealed an impressive survival rate, with a median PFS of 5.4 months (95% CI, 3.6–6.6 months), and overall survival of 8.7 months (range, 1.8–32.9 months). Such data compare favorably to previous phase III clinical trials of third line therapies. However our IRAFU trial was designed as a non-comparative phase 2 trial, and all statistics are therefore exploratory in nature. In addition, we could not exclude the possibility of a selection bias for patients with a better performance status or less heavy treatment were included in contrast to phase III trials. Nonetheless, baseline characteristics from IRAFU and previous phase III trials are very similar.
In conclusion, the IRAFU study reached its objectives which were to demonstrate very good tolerance in mCRC, and to show efficacy with long lasting tumor stabilization. Such data suggest that it would be of interest to test this combination in comparison to TAS-102 or regorafenib in phase III. Biological testing showed that our cases had reduced IL-17A levels, increased IFN-γ release and higher infiltration of CD8, which leads us to the conclusion that the combination of therapies used to treat our patients may led to enhanced checkpoint efficacy.
Funding Statement
This work was supported by the Soby; cancéropole grand est.
Supplementary materials
Supplemental data for this article can be accessed here.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F.. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F.. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer J Int Du Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M.. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagemann T, Balkwill F, Lawrence T. Inflammation and cancer: a double-edged sword. Cancer Cell. 2007;12:300–301. doi: 10.1016/j.ccr.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, Ridker P, Lorenzatti A, Krum H, Varigos J, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 8.Hickish T, Andre T, Wyrwicz L, Saunders M, Sarosiek T, Kocsis J, Nemecek R, Rogowski W, Lesniewski-Kmak K, Petruzelka L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017;18:192–201. doi: 10.1016/S1470-2045(17)30006-2. [DOI] [PubMed] [Google Scholar]
- 9.Bruchard M, Mignot G, Derangère V, Chalmin F, Chevriaux A, Végran F, Boireau W, Simon B, Ryffel B, Connat JL, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 10.Choi H. et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG.. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Lurje G, Hendifar AE, Schultheis AM, Pohl A, Husain H, Yang D, Manegold PC, Ning Y, Zhang W, Lenz HJ.. Polymorphisms in interleukin 1 beta and interleukin 1 receptor antagonist associated with tumor recurrence in stage II colon cancer. Pharmacogenet Genomics. 2009;19:95–102. doi: 10.1097/FPC.0b013e32831a9ad1. [DOI] [PubMed] [Google Scholar]
- 13.Apte R. N, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E.. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 14.Dmitrieva OS, Shilovskiy IP, Khaitov MR, Grivennikov SI. Interleukins 1 and 6 as main mediators of inflammation and cancer. Biochemistry (Mosc). 2016;81:80–90. doi: 10.1134/S0006297916020024. [DOI] [PubMed] [Google Scholar]
- 15.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN.. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F, Galon J.. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 17.Limagne E, Euvrard R, Thibaudin M, Rébé C, Derangére V, Chevriaux A, Boidot R, Végran F, Bonnefoy N, Vincent J, et al. Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res. 2016;76:5241–5252. doi: 10.1158/0008-5472.CAN-15-3164. [DOI] [PubMed] [Google Scholar]
- 18.Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol Rev. 2018;281:138–153. doi: 10.1111/imr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grothey A, Cutsem EV, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 20.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


