ABSTRACT
Vitamin D deficiency is frequently observed in human cancer patients and a prognostic relevance could be shown for some entities. Additionally, it is known that vitamin D can stimulate the patients’ antitumor immunity. However, valid epidemiological data for head and neck squamous cell carcinoma (HNSCC) patients are sparse and functional studies on a possible connection between vitamin D and the patients’ immune system are missing.
25-OH vitamin D serum levels were analyzed in 231 HNSCC patients and 232 healthy controls and correlated with clinical data and patient survival. Intra- and peritumoral infiltration with T-cell, NK-cell and macrophage populations was analyzed in 102 HNSCC patients by immunohistochemistry. In 11 HNSCC patients, NK-cells were isolated before and after vitamin D substitution and analyzed for their cytotoxic activity directed against a HNSCC cell line.
Vitamin D serum levels were significantly lower in HNSCC patients compared with healthy controls. Low vitamin D levels were associated with lymphatic metastasis and a negative HPV status and were a significant predictor of poor overall survival. HNSCC patients with severe vitamin D deficiency showed significantly altered intra- and peritumoral immune cell infiltrate levels. After vitamin D substitution, the patients’ NK cells showed a significant rise in cytotoxic activity.
Taken together, we could show that Vitamin D deficiency is highly prevalent in HNSCC patients and is a predictor of poor survival. Vitamin D substitution used as an adjuvant in immune therapies such as cetuximab and nivolumab treatment could support antitumorigenic immune responses, thus contributing to the improvement of the patients’ prognosis in the context of a multimodal therapy.
Keywords: head and neck cancer, vitamin D, immunooncology, natural killer cells, T cells
Introduction
Head and neck squamous cell carcinomas (HNSCC) belong to the six most common cancers worldwide and account for more than 5% of human malignancies1,2. In addition to tobacco and alcohol consumption3, an infection of the oral mucosa with high-risk human papillomavirus (HR-HPV) was identified as a relevant risk factor, especially for oropharyngeal cancer4-6. The prognosis of the patients is influenced by several factors including lymphatic metastasis7, general condition8, primary tumor localization9 and UICC stage10. On the molecular level, only few prognostic and predictive biomarkers apart from HPV tumor status have been identified11, with inconsistent and partially contradictory results12. Therefore, further studies are needed to enable a better prediction of outcome and more precise choice of treatment in HNSCC patients.
Vitamin D (cholecalciferol) belongs to the group of human steroid hormones and is involved in the regulation of Ca2+ and HPO42 – homeostasis by controlling intestinal and renal Ca2+ resorption, parathormone secretion and bone homeostasis13,14. Beneficial effects of sufficient vitamin D supply include an increase in bone density15, a reduced risk of falls in older people16 and a lower rate of pathologic bone fracture17. In the 1940s, Peller18 and Apperly19 found first hints of an antitumor effect of vitamin D when investigating the prevalence of cancer in sun exposed US farmers and US Navy soldiers. In these studies, probands with a higher sun exposure showed a higher incidence of skin tumors compared to individuals with low sun exposure, but a lower rate of all other malignancies. In accordance to this several studies of the last years found a correlation between vitamin D serum level and the incidence of tumor diseases, with an increased risk of colorectal cancer, prostate cancer and breast cancer under conditions of vitamin D deficiency20-22. For head and neck cancer, it was shown that the majority of patients have a higher incidence of vitamin D deficiency compared to healthy controls matched for age and sex23 and that a low vitamin D serum level is associated with a higher tumor incidence24. Furthermore, the antitumor function of the immune system also seems to depend on a sufficient vitamin D supply25,26. To our knowledge, a prognostic relevance of vitamin D serum level in HNSCC patients has so far only been shown in one study comprising 88 HNSCC patients with a shorter overall survival in patients with a vitamin D serum level below 10 ng/ml27. In vitro studies revealed an inhibitory effect of vitamin D on HNSCC cell proliferation, apoptosis induction, cell cycle arrest as well as angiogenesis, associated with a higher sensitivity to chemotherapeutic agents28,29. In an in vivo model, Meier et al. induced the growth of a mucosal cheek tumor in hamsters using 7,12-Dimethylbenzanthrazen (DMBA) and showed that vitamin D supplementation of the animals could suppress tumor development as well as tumor growth30.
Building on this, our study analyzed the prevalence of vitamin D deficiency in a collective of 463 patients (231 HNSCC patients and 232 control patients) and correlated vitamin D serum levels with clinical and pathological data as well as the patients’ overall survival. Furthermore, we investigated the underlying immunomodulatory effect of vitamin D by analyzing intra- and peritumoral immune cell infiltration and the antitumoral activity of natural killer cells (NK cells) isolated from HNSCC patients.
Results
Prevalence of vitamin d deficiency in HNSCC and healthy control patients and correlation with clinical data
To evaluate the prevalence of vitamin D deficiency in HNSCC patients, 25-OH vitamin D serum levels were analyzed in 231 HNSCC patients compared with 232 healthy controls matched for age and sex. Detailed clinical and epidemiological data are shown in Table 1. The most common primary tumor localization in the HNSCC group was tonsil cancer (n = 80; 35%), followed by laryngeal cancer (n = 56; 24%) and hypopharyngeal cancer (n = 30; 13%). Most patients were diagnosed in UICC stage IVa. In 35 HNSCC patients (15,2%) high-risk HPV-DNA was detected in the primary tumor tissue; 28 of these patients were diagnosed with oropharyngeal cancer (82,3%). The median 25-OH vitamin D serum level was significantly lower in the HNSCC group (11,1 ng/ml) than in the healthy control group (21,8 ng/ml; p < 0,0001; Figure 1A – C). Only 15 HNSCC patients had a sufficient vitamin D serum level (30 – 100 ng/ml). To assess the influence of general nutrition status on the vitamin D serum level, we analyzed the body mass index (BMI) and serum albumin level in all patients. We found no significant difference in either serum albumin level (VitD low: median 35 g/l; VitD high: median 34 g/l) or BMI (VitD low: median 25,8 kg/m2; VitD high: median 24,4 kg/m2) when comparing HNSCC patients with 25-OH vitamin D serum levels < 15 ng/ml (VitD low; n = 155) and ≥ 15 ng/ml (VitD high; n = 76) indicating that vitamin D deficiency is no indicator of a general malnutrition status. Furthermore, we found that serum calcium (Ca2+) levels were not significantly different between the two groups (VitD low: median 2,4 mmol/l; VitD high: median 2,4 mmol/l) and did not have a significant influence on the survival of HNSCC patients (Supplementary Figure 1).
Table 1.
Epidemiological and clinical data of HNSCC and control patients.
| HNSCC patients |
Control patients |
||
|---|---|---|---|
| no. of patients | 231 | 232 | |
| sex | male | 184 (80%) | 182 (78%) |
| female | 47 (20%) | 50 (22%) | |
| median age (years) | 63,0 | 60,5 | |
| primary tumor | tonsil | 80 (35%) | |
| larynx | 56 (24%) | ||
| hypopharynx | 30 (13%) | ||
| base of tongue | 26 (11%) | ||
| tongue border | 22 (10%) | ||
| floor of the mouth | 16 (7%) | ||
| cheek | 1 (0,4%) | ||
| UICC stage (7th TNM version) |
I | 26 (11%) | |
| II | 31 (13%) | ||
| III | 42 (18%) | ||
| IVa | 102 (44%) | ||
| IVb | 16 (7%) | ||
| IVc | 14 (6%) | ||
Figure 1.
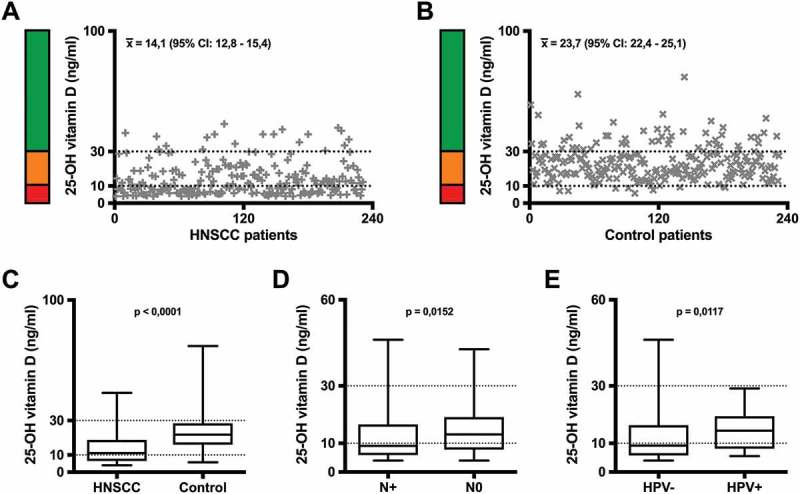
25-OH vitamin-D serum levels in (A) 231 HNSCC patients and (B) 232 healthy control patients matched for age and sex. Each individual value is illustrated on the graph. The range of vitamin D deficiency (< 10 ng/ml) is marked by a red bar and a dotted line, the range of insufficient vitamin D levels (10 – 30 ng/ml) by an orange bar and a dotted line, the range of sufficient vitamin D serum levels (> 30 ng/ml) by a green bar. (C) Comparison between 25-OH vitamin D serum levels of HNSCC and control patients. (D) Correlation of 25-OH vitamin D serum level with the lymph node status of HNSCC patients and (E) with the HPV-status of HNSCC patients. In the box and whisker blots in (C) – (E), each box represents the range from the first quartile to the third quartile. The median is indicated by a line inside the box. The whiskers outside the boxes represent the ranges from the minimum to the maximum value of each group.
x̅: arithmetic mean; 95% CI: 95% confidence interval of the mean; N+: positive lymph node status; N-: negative lymph node status; HPV+: positive HPV status; HPV-: negative HPV status
When correlating the patients’ vitamin D serum level with their clinical and pathological data, we found a significant correlation of low vitamin D serum levels with a positive lymph node status (Figure 1D) and of higher vitamin D serum levels in HPV positive patients compared with HPV negative patients (Figure 1E).
Prognostic relevance of vitamin d serum level in HNSCC patients
To evaluate the prognostic relevance of vitamin D supply in HNSCC patients, we correlated 25-OH vitamin D serum levels with the patients’ overall survival (OS). We found a significant shorter OS in HNSCC patients with low vitamin D (VitD low; n = 155) compared with the vitamin D high HNSCC group (VitD high; n = 76). During the follow-up time, 66 patients of the VitD low group (66/155; 42,6%) and 23 patients of the VitD high group died (23/76; 30,3%) from their tumor disease. The respective Kaplan-Meier curves are shown in Figure 2A.
Figure 2.
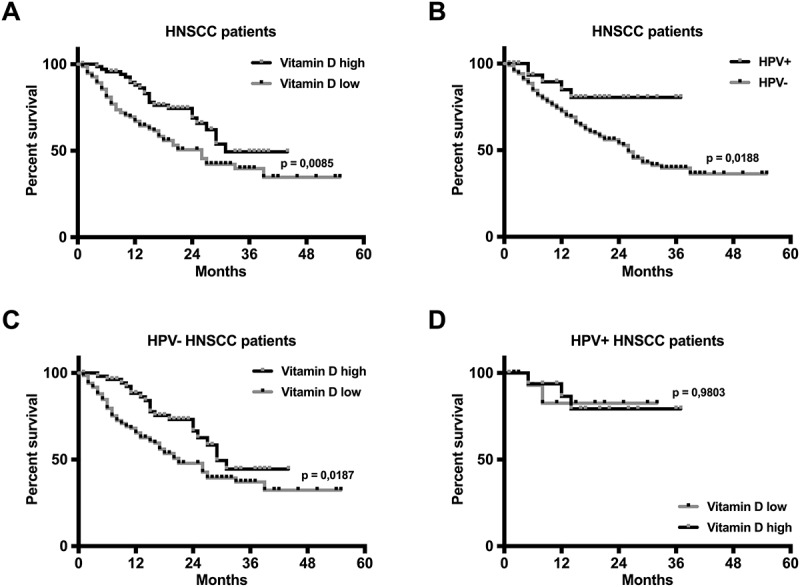
Correlation of 25-OH vitamin D serum level and HPV status in HNSCC patients with overall survival. A Kaplan-Meier analysis is shown in (A) for a vitamin D high group (black line; 25-OH vitamin D ≥ 15 ng/ml; n = 76) and a vitamin D low group (grey line; 25-OH vitamin D < 15 ng/ml; n = 155). It revealed a significantly worse prognosis for vitamin D deficient patients (p = 0,0085; log-rank test). HPV positive tumor status (n = 35) correlated with improved overall survival in the analyzed HNSCC patients (B). Low vitamin D serum levels were a predictor of poor overall survival (OS) in HPV negative patients (C), while vitamin D supply had no influence on the OS in HPV positive HNSCC patients (D). Black or grey dots represent censored data.
HPV+: positive HPV status; HPV-: negative HPV status
HPV positive tumor status was a significant predictor of better overall survival in HNSCC patients, in concordance with previous reports4,(Figure 2B). In HPV positive patients, vitamin D supply was not found to bear prognostic relevance with regards to OS, with the caveat that this group only included 35 patients (HPV+ VitD low, n = 18; HPV+ VitD high, n = 17). In contrast, low vitamin D serum level correlated with significantly shorter OS in HPV negative patients (Figure 2C and D).
Influence of vitamin d on intra- and peritumoral immune cell infiltration
To assess the influence of vitamin D serum level on the antitumor activity of the immune system, we performed immunohistochemical stainings of the primary tumor tissue, detecting T cells (using CD3 as a marker), helper T cells (CD4), cytotoxic T cells (CD8), natural killer cells (CD56), macrophages (CD68), M1 macrophages (CD11c) and M2 macrophages (CD163) in order to analyze the intra- and peritumoral immune cell infiltration in a representative subset of VitD high patients (n = 43) and VitD low patients (n = 59). A modified immunoreactive score (mIRS) was used to evaluate the staining results, as described in materials and methods.
Overall, a significantly higher intratumoral and/or stromal infiltration of CD3+ T cells, helper T cells, cytotoxic T cells, NK cells, CD68+ macrophages, and M1 macrophages was observed in the VitD high patients compared with the VitD low patients (Figure 3A – E; Supplementary Figure 2). For M2 macrophages, staining results showed a tendency towards a significantly lower peritumoral infiltration in the VitD high group (Figure 3F). Representative results of immunohistochemical stainings are shown in Figure 3G & H.
Figure 3.
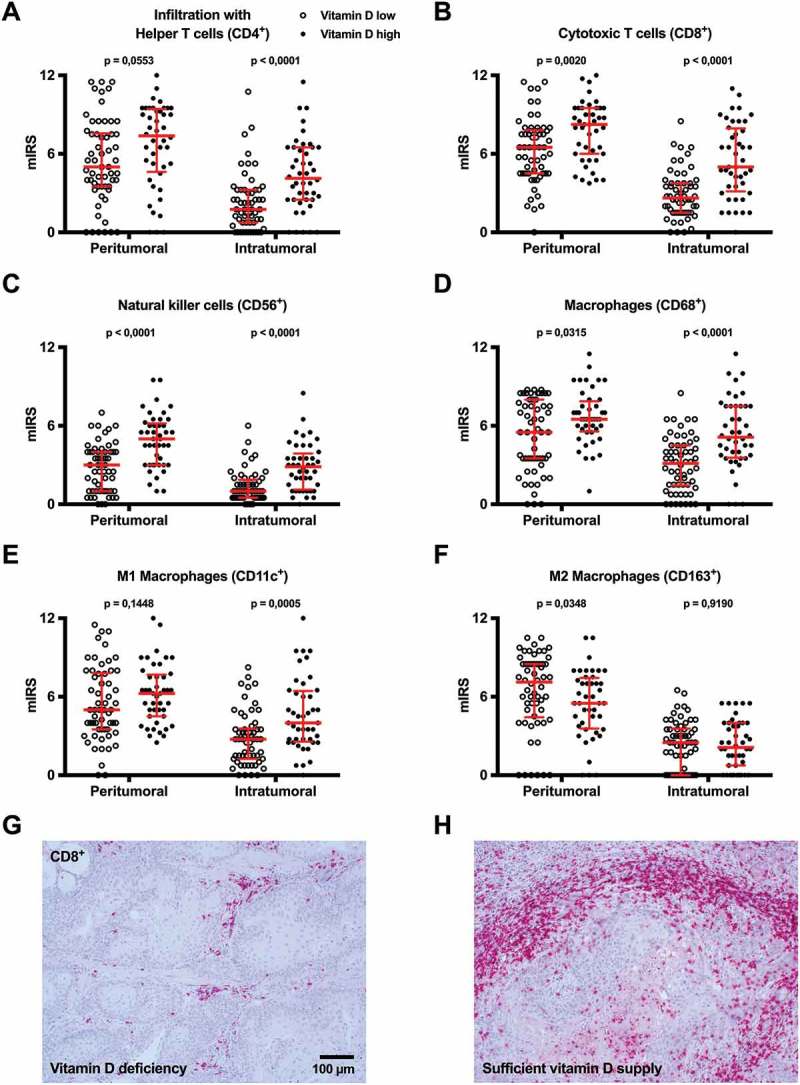
Immunohistochemical analysis of peri- and intratumoral immune cell infiltration in HNSCCs. The immune cell infiltration in the peritumoral stroma and the tumor was analyzed by immunohistochemistry in HNSCC patients with vitamin D deficiency (Vitamin D low; n = 59) and sufficient vitamin D levels (Vitamin D high; n = 43). (A) helper T cells, (B) cytotoxic T cells, (C) NK cells, (D) CD68+ macrophages, (E) M1 macrophages and (F) M2 macrophages were quantified using the mIRS and each individual score is illustrated as dot on the graph. In the plots in (A) – (F) the median is indicated by a red line and the whiskers represent the range from the first quartile to the third quartile. (G & H) Representative images of CD8 immunhistochemical staining in a patient with vitamin D deficiency (G) and sufficient vitamin D supply (H); both are squamous cell carcinomas of the floor of the mouth.
mIRS: modified immunoreactive score
In regards to the prognostic relevance of immune cell infiltration, intratumoral infiltration with cytotoxic T cells (CD8+) and NK cells (CD56+) as well as peritumoral infiltration with CD3+ T cells and T helper cells (CD4+) were predictors of a significantly longer overall survival, whereas high levels of intratumoral infiltration with M2 macrophages (CD163+) were associated with a significantly shorter overall survival (Figure 4; Supplementary Figure 3).
Figure 4.
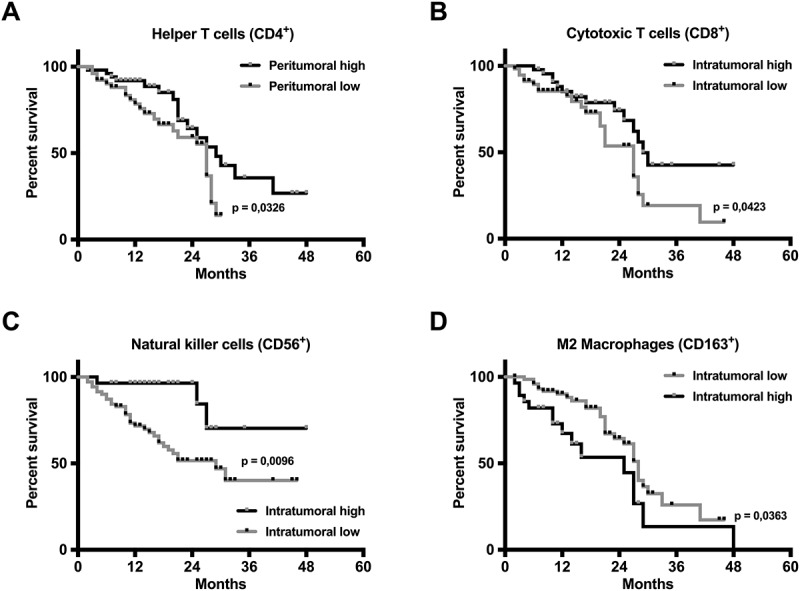
Prognostic relevance of peri- and intratumoral immune cell infiltration in HNSCC patients. The peritumoral infiltration with helper T cells (A) as well as the intratumoral infiltration with (B) cytotoxic T cells, (C) NK cells and (D) M2 macrophages showed a significant correlation with patient overall survival (OS). A higher infiltration of CD4+, CD8+, and CD56+ cells correlated with an improved OS, whereas a higher intratumoral infiltration with CD163+ cells correlated with a poorer OS (log-rank test). In the Kaplan-Meier curves, black or grey dots indicate censored survival data.
Influence of vitamin d on antitumor NK cell cytotoxicity in HNSCC patients
Based on our observation that high vitamin D serum levels promotes peri- and intratumoral infiltration with NK cells in HNSCC patients (Figure 3C) and therefore may lead to a significant improvement of the patients’ prognosis (Figure 4C), we next analyzed if vitamin D also affects the cytotoxic activity of NK cells directed against HNSCC cells. Therefore, we isolated NK cells from peripheral blood samples of 11 representative HNSCC patients showing a vitamin D deficiency (25-OH vitamin D < 10 ng/ml) and incubated these cells with cultured hypopharyngeal cancer cells (FaDu cell line) with or without addition of anti-EGFR antibody cetuximab – a therapeutic antibody approved for the treatment of locally advanced and metastasized HNSCC in combination with radiation. After this first analysis of antitumor NK cell activity, all patients were substituted with vitamin D for 3 months, after which cytotoxicity was assessed again.
After 3 months of vitamin D substitution, a group of 9 out of the 11 patients defined as vitamin D responders showed a rise in vitamin D serum level above 10 ng/ml. In contrast, 2 patients showed no relevant change (Δ vitamin D < 10 ng/ml) and were defined as vitamin D non-responders. All vitamin D responders showed an improvement in cytotoxic NK cell activity directed against FaDu cells dependent and independent of cetuximab (Figure 5).
Figure 5.
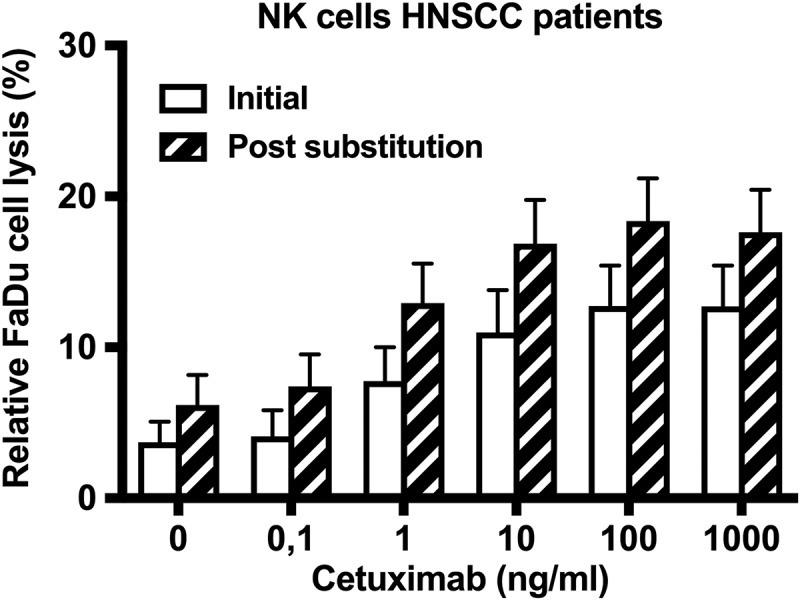
Effect of vitamin D supplementation on antibody-dependent and antibody-independent antitumor NK cell activity. The antitumoral activity of NK cells isolated from HNSCC patients, directed against FaDu cells is shown before and after substitution as relative FaDu cell lysis. Each bar represents the mean value of 9 HNSCC patients with a significant rise in vitamin D serum level (Δ > 10 ng/ml; vitamin D responder). The error bars indicate the standard error of the mean.
Discussion
Our study demonstrated that vitamin D deficiency is frequent in HNSCC patients and is further on also associated with lymphatic metastasis, a significantly shorter overall survival and an altered profile of peri- and intratumoral immune cell infiltration. Moreover, the substitution of vitamin D increased the antitumor cytotoxic activity of the patients’ NK cells and thus has the potential to improve their prognosis by promoting the antitumor immune response.
Our epidemiological data on vitamin D serum levels in HNSCC patients are consistent with the data from previous studies performed on lower numbers of patients. For example, Orell-Kotikangas et al. found vitamin D serum levels ≤ 20 ng/ml in 42 of 65 HNSCC patients (65%)23. An influence of vitamin D on the immune system of HNSCC patients has been described by other groups, as well. However, no study addressed the function of NK cells. Walsh et al. analyzed the infiltration with CD8+ and CD68+ cells in the peritumoral tissue of 16 HNSCC patients before and after vitamin D substitution and found a stimulation of immune cell infiltration by vitamin D31. Walker et al. substituted 35 HNSCC patients with 1,25(OH)2 vitamin D and detected increased levels of intratumoral proinflammatory cytokines such as IL-6, IL-10 and IFN-γ compared with 18 non-substituted control patients25. In a study by Kulbersh et al., vitamin D substitution in 11 HNSCC patients reduced the number of immunosuppressive CD34+ cells and immature dendritic cells in the peritumoral tissue compared with 12 non-substituted HNSCC patients32. In concordance with our results, these studies suggest that vitamin D can stimulate the antitumoral activity of the immune system in HNSCC patients and therefore can play a role as immunotherapeutic agent in head and neck oncology.
A prognostic relevance of vitamin D serum level for head and neck cancer as demonstrated in our study was shown to our knowledge by only one other study with a relatively lower number of 88 patients27. For other tumor entities, a higher evidence for a beneficial effect of vitamin D on patient survival already exists. In a large meta-analysis comprising 44,165 cancer patients with different tumor entities, higher 25-OH vitamin D serum levels were shown to be associated with better overall survival and progression-free survival33. A further accumulation of data will clarify to which extent vitamin D serum levels influence the survival of HNSCC patients.
Several studies have shown that HNSCC patients frequently harbor signs of immunosuppression, making vitamin D substitution a promising approach for immunostimulatory activity34. Meneses et al. examined tumor free cervical lymph nodes in HNSCC patients and compared them with cervical lymph nodes of tumor free control patients, which revealed a reduced size of lymph nodes and a lower number of functional T cells in the HNSCC group35. Moreover, T cells isolated from HNSCC patients were reported to show reduced CD3 receptor responsiveness36 and studies on peripheral blood samples taken from HNSCC patients demonstrated an impaired maturation of dendritic cells, which are key coordinators of the antitumor immune response37,38. Additionally, several studies have found an increased number of immunosuppressive CD34+cells in the peripheral blood of HNSCC samples, resulting in a substantial impairment of T cell function39-41. Gasparoto et al. investigated the immunosuppressive activity of CD4+/CD25+ T cells in the blood and tissue samples from 12 HNSCC patients and 10 healthy control patients and found an increased number of immunosuppressive regulatory T cells in both blood and peritumoral tissue of the HNSCC patients compared to healthy controls42. Our observation that higher vitamin D serum levels correlate with a higher number of T cells, NK cells and immunostimulatory M1 macrophages as well as a lower number of immunosuppressive M2 macrophages in the tumor and peritumoral tissue suggests that an adequate vitamin D status improves prognosis by promoting the antitumor immune response. Taken together, these results are in in line with our observation that lower vitamin D serum levels are associated with a higher number of immunosuppressive M2 macrophages in the tumor and peritumoral tissue. The adverse prognostic effect of these numerous mechanisms of tumor-induced immunosuppression in HNSCC patients was already proven, as well43,44.
Our correlation of vitamin D serum level and clinical patient data revealed a significant association of higher vitamin D serum levels with a negative lymph node status, pointing towards a possible inhibitory effect of vitamin D on tumor cell metastasis. Accordingly, first in vitro studies showed an inhibition of HNSCC cell proliferation by vitamin D28. Apart from cancer cell metastasis, the results mentioned before also raise the question if the beneficial prognostic effect of vitamin D in HNSCC patients could possibly be mediated not only by the immune system but also by a direct effect of vitamin D on cancer cell biology. These are important questions that need to be addressed by future studies.
In the interest of transparency, it must be stated that the distribution of tumor subsites was comparable but not completely equal between the VitD low and the VitD high groups, with a higher percentage of floor of the mouth cancer (VitD low: 8%; VitD high: 4%) and hypopharyngeal cancer (VitD low: 14%; VitD high: 11%) as well as a lower number of tongue cancer (VitD low: 7%; VitD high: 16%) in the VitD low group. In both groups, tonsil cancer was the most common tumor subsite followed by laryngeal cancer (Supplementary Figure 4).
Overall, our study demonstrated that vitamin D deficiency is highly frequent in HNSCC patients and can act as a predictor of worse survival. While this fully applied to HPV negative patients, the role of vitamin D supply in patients with a HPV positive tumor status should be analyzed in further studies comprising larger cohorts. Based on our data, vitamin D substitution represents a promising approach for an immunostimulatory therapy in HNSCC patients that can contribute to the improvement of the patients’ prognosis in the context of a multimodal therapy concept. In times of successful treatment of locally advanced and even metastasized cancer patients with checkpoint inhibitors as core elements of immunotherapy34,45, vitamin D represents a feasible, cost-effective and well-tolerated option for an immune supportive treatment of HNSCC patients.
Patients and methods
Patients and tissue samples
A total of 463 patients (231 HNSCC patients, 232 non-HNSCC control patients) were included in our study. Blood samples were taken from all patients before treatment for the analysis of 25-OH vitamin D, albumin and calcium serum levels. In addition, formalin-fixed, paraffin embedded (FFPE) tumor tissue samples from 103 HNSCC patients were used for immunohistochemical detection of peri- and intratumoral immune cell infiltration. 11 of these patients were included in the analysis of antitumor NK cell function before and after vitamin D supplementation. For all included HNSCC cases, the histological diagnosis was squamous cell carcinoma (SCC). The primary tumor localizations for the HNSCC patients were tonsil (n = 80), larynx (n = 56), hypopharynx (n = 30), tongue base (n = 26), tongue border (n = 22), floor of the mouth (n = 16) and cheek (n = 1). The median follow-up time, as calculated with the Kaplan Meier estimate of potential follow-up, was 21 months for the HNSCC patients. The detailed distribution of clinical diagnoses and UICC stage for the HNSCC group as well as age and sex for both groups is shown in Table 1. The patients were diagnosed and treated at three different hospitals in the Saar-Lor-Lux region (Saarland University Medical Center, Homburg, Germany; Zitha Hospital, Luxemburg-City, Luxemburg; Caritas Hospital, Saarbrücken, Germany). The Saarland Medical Association ethics review committee approved the scientific use of the patients’ tissue, blood and clinical data (index numbers 218/10 and 227/12). All experiments were performed according to the relevant guidelines and regulations. Written informed consent was obtained from all patients.
Immunohistochemistry
FFPE tissue samples of the patients’ primary tumor were used for immunohistochemical staining. After omitting the first three slides of 10 μm thickness each, 3 μm slides were prepared using the Leica RM 2235 rotation microtome (Leica Microsystems, Wetzlar), transferred onto Superfrost Ultra PLUS microscope slides (Menzel-Gläser, Braunschweig, Germany) and dried in an incubator overnight at 37°C. HE staining was performed for each tissue sample according to a standard protocol for morphological control. For the immunohistochemical detection of the markers CD3, CD4, CD8, CD11c, CD56, CD68 and CD163, heat-induced epitope unmasking was performed upon deparaffinization in a rice cooker using Tris-EDTA retrieval buffer (10 mM TRIS, 1 mM EDTA, pH 9). Afterwards, unspecific protein binding was blocked by an incubation in PBS (pH 7,2) with 3% bovine serum albumine (BSA; Sigma Aldrich, St. Louis, USA) for 30 min at room temperature (RT). Slides were then incubated with the respective primary antibody for 1 h at RT. The final concentrations of the primary antibodies were 1:300 for CD3 (Thermo Fisher, Waltham, MA, USA; clone SP7, RM-9107), 1:40 for CD4 (Abcam, Cambrigde, UK; clone BC/1F6, ab846), 1:800 for CD8 (Abcam, ab4055), 1:100 for CD11c (Abcam, clone EP1347Y, ab52632), 1:50 for CD56 (Dako, clone 123C3, M7304), 1:200 for CD68 (Abcam, clone KP1, ab955) and 1:16000 for CD163 (Abcam, ab87099); each concentration in PBS/1% BSA. Every staining series included negative controls performed by omitting the primary antibody and appropriate tissue samples as positive controls. Visualization was performed with streptavidin-labeled alkaline phosphatase and chromogen red using the Dako REAL Detection System Alkaline Phosphatase/RED (Dako, Glostrup, Denmark) following the manufacturer’s instructions. Finally, the slides were counterstained with hematoxylin (Sigma Aldrich) and permanently mounted with Entellan (Merck, Darmstadt, Germany).
Semiquantitative analysis of immunohistochemical staining results was performed using an immunohistochemical score modified according to Remmele and Stegner46. The absolute number of immune cells in the tumor and peritumoral tissue was valued from 1 to 4 (1 – no immune cell infiltration; 2 – < 25%; 3 – 25 – 50%; 4 – > 50% of tissue infiltrated with immune cells) and the relative number of positively stained leucocytes was valued from 1 to 3 (1 – < 30%; 2 – 30 – 60%; 3 – > 60%). Finally, both values were multiplied, resulting in a modified IRS (mIRS) ranging from 1 to 12. Every analysis was performed independently by three different examiners including one pathologist blinded for the clinical diagnosis and the vitamin D level as well as the other examiner’s score.
HPV status
For the determination of HPV tumor status, we combined HPV-DNA-PCR and immunohistochemical p16-Ki67 dual staining. HPV-DNA-PCR was performed using fresh-frozen tumor tissue, whereas the p16-Ki67 dual staining was performed on FFPE tumor tissue samples.
For the detection of HPV-DNA, DNA was extracted from fresh-frozen tumor tissue using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Then, HPV-DNA-PCR was performed with the LightCycler 2.0 (Roche Diagnostics, Mannheim, Germany) using GP5+/6+ primers as described previously47. PCR amplification products were detected with SYBR Green as well as gel electrophoresis. Following an initial denaturation at 95°C for 15 min, 45 PCR cycles were performed with a denaturation at 95°C for 10 s, an annealing at 45°C for 5 s and an elongation at 72°C for 18 s. After amplification of the PCR products, a melting curve analysis was conducted with temperatures between 45°C and 95°C with a rise in temperature of 0.2°C/s. Every PCR analysis included a HPV16 positive control (Tm 79°C) and a HPV18 positive control (Tm 82°C). Additionally, the Glyceraldehyde-3-phosphat-dehydrogenase (GAPDH) gene was amplified and used as internal positive control48.
For immunohistochemical detection of p16 and Ki67 expression, we used the CINtec PLUS kit (Roche Diagnostics). Heat-induced epitope unmasking was performed upon deparaffinization in a rice cooker for 20 minutes using the supplied retrieval buffer. Incubation with the respective antibodies and the detection of staining signals was performed as described by the manufacturer of the CINtec PLUS kit for immunocytochemistry. Every staining series included the supplied negative and positive controls.
Analysis of antitumoral NK cell function
To analyze the influence of 25-OH vitamin D serum level on NK cell-mediated antitumoral cytotoxicity, the epidermal growth factor receptor (EGFR) positive hypopharyngeal cancer cell line FaDu (ATCC HTB-43) was used as target in a NK cell cytotoxicity assay. For this, FaDu cells were harvested, washed, diluted in “cytotoxicity medium” (see below) to a concentration of 4000 cells/75 µl, incubated with different concentrations of the chimeric anti-EGFR-antibody cetuximab (Merck) for 20 min at 37°C and afterwards seeded in a 96 well plate (Sarstedt, Nümbrecht, Germany) with 4000 cells per well. Cetuximab was used in the following concentrations to tag the target cells: 0 – 0,1 – 1 – 10 – 100 – 1000 ng/ml. Peripheral blood mononuclear cells were isolated from 11 representative HNSCC patients before and after a supplementation with vitamin D by density gradient centrifugation (Pancoll, PAN-Biotec, Aidenbach, Germany). The isolation of NK cells was performed by magnetic depletion of all non-NK cells using the NK cell isolation kit human (Miltenyi Biotec, Bergisch-Gladbach, Germany) following the manufacturer’s instructions. Thereafter, NK (effector) cells were incubated with the unlabeled or cetuximab-labeled FaDu (target) cells at 37°C in 5% (v/v) CO2 at effector/target ratios of 5:1 and 2,5:1 respectively. At this step, all cells were cultured in indicator free X-Vivo-15 medium (Lonza, Verviers, Belgium) added with 2% human serum albumin (CLS Behring, Marburg, Germany) so-called “cytotoxicity medium”. The suspension volume was 75 µl per well. Then, the antitumor cytotoxic activity of the NK cells was analyzed by measuring the release of lactate dehydrogenase (LDH) from the target cells. Spontaneous lysis of target cells was analyzed in wells without NK cells. The maximum lysis was determined by adding a detergent 15 min before the end of incubation time. After 4 hours, the 96 well plates were centrifuged (200 g, 6 min, RT) and 50 µl of the supernatant was transferred into another 96 well plate (Sarstedt) and incubated with 50 µl LDH substrate (Cytotoxicity Detection KitPlus [LDH], Roche Diagnostics) at RT. After 30 min, reaction was stopped by adding 25 µl stop solution and the extinction at 490 nm was determined photometrically (Wallac Victor 2 Microplate Reader, PerkinElmer LAS, Rodgau, Germany). Percental lysis rates as an indicator for antitumoral cytotoxic NK cell activity were calculated according to the following formula:
Lysis rate = (test lysis – spontaneous lysis)/(maximum lysis – spontaneous lysis) x 100)
The clinical diagnoses of the 11 patients that were included in the analysis of antitumoral NK cell function comprised cancer of the tongue base (n = 3; UICC stages II, III and IVa; all HPV negative), tonsil cancer (n = 3; two HPV-positive, UICC stage II; one HPV-negative, UICC stage IVa), hyopharyngeal cancer (n = 3; UICC stages IVa (2x) and II (1x); all HPV negative) and laryngeal cancer (n = 2; UICC stages IVa and IVc; all HPV negative). The patients were treated with surgery followed by adjuvant radiotherapy (n = 4), surgery followed by adjuvant radiochemotherapy (n = 4) or radiochemotherapy alone (n = 3).
Analysis of vitamin D, albumin and calcium serum levels
The analysis of 25-OH vitamin D serum level in HNSCC and control patients was performed with the CLIA technology (chemiluminescence immunoassay) using the LIAISON 25 OH Vitamin D TOTAL Assay (DiaSorin Inc., Stillwater, MN, USA).
Serum albumin level in HNSCC patients was analyzed photometrically (Bromocresol green) using the cobas Albumin Gen.2 assay (Roche Diagnostics). Serum calcium level in HNSCC patients was analyzed photometrically (5-nitro-5ʹ-methyl-BAPTA and EDTA) using the cobas Calcium Gen.2 assay (Roche Diagnostics).
Vitamin d supplementation
Vitamin D supplementation of HNSCC patients started after the inital NK cell cytotoxicity measurement. Depending on the possibility of oral food intake, either enteral Vigantoletten 1.000 IE 1x/d oral/via feeding tube (Merck) or Dekristol 20.000 IE 1x/week oral (Mibe, Grünwald, Germany) or parenteral (D3-Vicotrat 100.000 IE 1x/month intramuscular (Heyl, Berlin, Germany) were administered over three months. Vitamin D serum level was measured at the time of each NK cell cytotoxicity assay before and after supplementation.
Statistical analyses
Statistical analysis of the overall survival was performed with the Kaplan-Meier and Mantel-Cox test (log-rank test) using GraphPad Prism 7.0d (GraphPad Software, La Jolla, CA, USA).
The high versus low immune infiltration (Figure 4; Supplementary Figure 3) and calcium serum level cohorts (Supplementary Figure 1) were defined as patients with values greater than or equal to the mean of the corresponding group (high) and less than the mean (low), respectively. Analyses of the 25-OH vitamin D, albumin and calcium serum levels as well as of the immune reactive scores were performed using the D’Agostino & Pearson normality test and a two-tailed, unpaired Student’s t-test or a two-tailed Mann-Whitney test. P-values < 0,05 were considered statistically significant (α = 0,05).
Funding Statement
This work was supported by a HOMFOR (Homburger Forschungsförderungprogramm) grant to M.L.
Acknowledgments
The excellent technical assistance of Claudia Schormann, Ulrike Bechtel and Monika Hoffmann is gratefully acknowledged. We also would like to sincerely thank Anna Meier for her valuable contribution during the preparation of the manuscript.
Disclosure of Potential Conflicts of Interest
The authors report no conflict of interest.
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Barnes L, Eveson JW, Reichart P, Sidransky D.. Pathology and genetics of head and neck tumors. World Health Organization Classification of Tumors. Lyon, France: IARC Press; 2005. [Google Scholar]
- 2.Cooper JS, Porter K, Mallin K, Hoffman HT, Weber RS, Ang KK, Gay EG, Langer CJ. National cancer database report on cancer of the head and neck: 10-year update. Head Neck. 2009;31(6):748–758. doi: 10.1002/hed.v31:6. [DOI] [PubMed] [Google Scholar]
- 3.Pavlidis N, Pentheroudakis G, Plataniotis G. Cervical lymph node metastases of squamous cell carcinoma from an unknown primary site: a favourable prognosis subset of patients with CUP. Clinical Transl Oncol. 2009;11(6):340–348. doi: 10.1007/s12094-009-0367-1. [DOI] [PubMed] [Google Scholar]
- 4.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’ Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Vigilone M, Symer DE et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 7.Zätterström UK, Wennerberg J, Ewers SB, Willén R, Attewell R. Prognostic factors in head and neck cancer: histologic grading, DNA ploidy, and nodal status. Head Neck. 1991;13(6):477–487. doi: 10.1002/hed.2880130603. [DOI] [PubMed] [Google Scholar]
- 8.De Cássia Braga Ribeiro K, Kowalski LP, Latorre Mdo R. Perioperative complications, comorbidities, and survival in oral or oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2003;129(2):219–228. doi: 10.1001/archotol.129.2.219. [DOI] [PubMed] [Google Scholar]
- 9.Snow GB, Boom RP, Delemarre JF, Bangert JA. Squamous carcinoma of the oropharynx. Clin Otolaryngol Allied Sci. 1977;2(2):93–103. doi: 10.1111/j.1365-2273.1977.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 10.Polanska H, Raudenska M, Gumulec J, Sztalmachova M, Adam V, Kizek R, Masarik M. Clinical significance of head and neck squamous cell cancer biomarkers. Oral Oncol. 2014;50(3):168–177. doi: 10.1016/j.oraloncology.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rainsbury JW, Ahmed W, Williams HK, Roberts S, Paleri V, Mehanna H. Prognostic biomarkers of survival in oropharyngeal squamous cell carcinoma: systematic review and meta-analysis. Head Neck. 2013;35(7):1048–1055. doi: 10.1002/hed.22950. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF. Vitamin D deficiency. New Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 16.Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel DP. A higher dose of vitamin d reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc. 2007;55(2):234–239. doi: 10.1111/j.1532-5415.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 17.Sato Y, Iwamoto J, Kanoko T, Satoh K. Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in hospitalized, elderly women with Alzheimer’s disease: a randomized controlled trial. J Bone Miner Res. 2005;20(8):1327–1333. doi: 10.1359/JBMR.050402. [DOI] [PubMed] [Google Scholar]
- 18.Peller S, Stephenson CS. Skin irritation and cancer in the United States navy. AM J M Sc. 1937;194:326–333. doi: 10.1097/00000441-193709000-00004. [DOI] [Google Scholar]
- 19.Apperly FL. The relation of solar radiation to cancer mortality in North America. Cancer Res. 1941;1:191–195. [DOI] [PubMed] [Google Scholar]
- 20.Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, Giovannucci E. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1502–1508. [PubMed] [Google Scholar]
- 21.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 22.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96(2):252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orell-Kotikangas H, Schwab U, Österlund P, Saarilahti K, Mäkitie O, Mäkitie AA. High prevalence of vitamin D insufficiency in patients with head and neck cancer at diagnosis. Head Neck. 2012;34(10):1450–1455. doi: 10.1002/hed.21954. [DOI] [PubMed] [Google Scholar]
- 24.Afzal S, Bojesen SE, Nordestgaard BG. Low plasma 25-hydroxyvitamin D and risk of tobacco-related cancer. Clin Chem. 2013;59(5):771–780. doi: 10.1373/clinchem.2012.201939. [DOI] [PubMed] [Google Scholar]
- 25.Walker DD, Reeves TD, De Costa AM, Schuyler C, Young MR. Immunological modulation by 1α,25-dihyroxyvitamin D3 in patients with squamous cell carcinoma of the head and neck. Cytokine. 2012;58(3):448–454. doi: 10.1016/j.cyto.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young MRI, Day TA. Immune regulatory activity of vitamin D3 in head and neck cancer. Cancers. 2013;5(3):1072–1085. doi: 10.3390/cancers5031072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gugatschka M, Kiesler K, Obermayer-Pietsch B, Groselj-Strele A, Griesbacher A, Friedrich G. Vitamin D status is associated with disease-free survival and overall survival time in patients with squamous cell carcinoma of the upper aerodigestive tract. Eur Arch Otorhinolaryngol. 2011;268(8):1201–1204. doi: 10.1007/s00405-010-1481-y. [DOI] [PubMed] [Google Scholar]
- 28.Chiang KC, Yeh CN, Hsu JT, Chen LW, Kuo SF, Sun CC, Huang CC, Pang JH, Flanagan JN, Takano M, et al. MART-10, a novel vitamin D analog, inhibits head and neck squamous cell carcinoma cells growth through cell cycle arrest at G0/G1 with upregulation of p21 and p27 and downregulation of telomerase. J Steroid Biochem Mol Biol. 2013;138:427–434. doi: 10.1016/j.jsbmb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Satake K, Takagi E, Ishii A, Kato Y, Imagawa Y, Kimura Y, Tsukuda M. Anti-tumor effect of vitamin A and D on head and neck squamous cell carcinoma. Auris Nasus Larynx. 2003;30(4):403–412. doi: 10.1016/S0385-8146(03)00091-9. [DOI] [PubMed] [Google Scholar]
- 30.Meier JD, Enepekides DJ, Poirier B, Bradley CA, Albala JS, Farwell DG. Treatment with 1-alpha,25-dihydroxyvitamin D3 (vitamin D3) to inhibit carcinogenesis in the hamster buccal pouch model. Arch Otolaryngol Head Neck Surg. 2007;133(11):1149–1152. doi: 10.1001/archotol.133.11.1149. [DOI] [PubMed] [Google Scholar]
- 31.Walsh JE, Clark AM, Day TA, Gillespie MB, Young MR. Use of alpha,25-dihydroxyvitamin D3 treatment to stimulate immune infiltration into head and neck squamous cell carcinoma. Hum Immunol. 2010;71(7):659–665. doi: 10.1016/j.humimm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulbersh JS, Day TA, Gillespie MB, Young MR. 1α,25-Dihydroxyvitamin D3 to skew intratumoral levels of immune inhibitory CD34+ progenitor cells into dendritic cells. Otolaryngol Head Neck Surg. 2009;140(2):235–240. doi: 10.1016/j.otohns.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaughan-Shaw PG, O’Sullivan F, Farrington SM, Theodoratou E, Campbell H, Dunlop MG, Zgaga L. The impact of vitamin D pathway genetic variation and circulating 15-hydroxyvitamin D on cancer outcome: systematic review and meta-analysis. Br J Cancer. 2017;116(8):1092–1110. doi: 10.1038/bjc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young MR. Trials and tribulations of immunotherapy as a treatment option for patients with squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2004;53(5):375–382. doi: 10.1007/s00262-003-0456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meneses A, Verastegui E, Barrera JL, De La Garza J, Hadden JW. Lymph node histology in head and neck cancer: impact of immunotherapy with IRX-2. Int Immunol. 2003;3(8):1083–1091. [DOI] [PubMed] [Google Scholar]
- 36.Shibuya TY, Wei WZ, Zormeier M, Ensley J, Sakr W, Mathog RH, Meleca RJ, Yoo GH, June CH, Levine BL, et al. Anti-CD3/anti-CD28 bead stimulation overcomes CD3 unresponsiveness in patients with head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126(4):473–479. doi: 10.1001/archotol.126.4.473. [DOI] [PubMed] [Google Scholar]
- 37.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6(5):1755–1766. [PubMed] [Google Scholar]
- 38.Egan JE, Quadrini KJ, Santiago-Schwarz F, Hadden JW, Brandwein HJ, Signorelli KL. IRX-2, a novel in vivo immunotherapeutic, induces maturation and activation of human dendritic cells in vitro. J Immunother. 2007;30(6):624–633. doi: 10.1097/CJI.0b013e3180691593. [DOI] [PubMed] [Google Scholar]
- 39.Lathers DMR, Achille N, Kolesiak K, Hulett K, Sparano A, Petruzzelli GJ, Young MR. Increased levels of immune inhibitory CD34+ progenitor cells in the peripheral blood of patients with node positive head and neck squamous cell carcinomas and the ability of these CD34+ cells to differentiate into immune stimulatory dendritic cells. Otolaryngol Head Neck Surg. 2001;125(3):205–212. doi: 10.1067/mhn.2001.117871. [DOI] [PubMed] [Google Scholar]
- 40.Pandit R, Lathers DM, Bel NM, Garrity T, Young MR. CD34+ immune suppressive cells in the peripheral blood of patients with head and neck cancer. Ann Otol Rhinol Laryngol. 2000;109(8 Pt 1):749–754. doi: 10.1177/000348940010900809. [DOI] [PubMed] [Google Scholar]
- 41.Garrity T, Pandit R, Wright MA, Benefield J, Keni S, Young MR. Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into dendritic cells. Int J Cancer. 1997;73(5):663–669. doi: 10.1002/(ISSN)1097-0215. [DOI] [PubMed] [Google Scholar]
- 42.Gasparato TH, De Souza Malaspina TS, Benevides L, De Melo EJ Jr, Costa MR, Damante JH, Ikoma MR, Garlet GP, Cavassani KA, Da Silva JS, et al. Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol Immunother. 2010;59(6):819–828. doi: 10.1007/s00262-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heimdal JH, Aastad HJ, Klementsen B, Olofsson J. Peripheral blood mononuclear cell (PMBC) responsiveness in patients with head and neck cancer in relation to tumour stage and prognosis. Acta Otolaryngol. 1999;119(2):281–284. doi: 10.1080/00016489950181828. [DOI] [PubMed] [Google Scholar]
- 44.Heimdal JH, Aarsatd HJ, Olofsson J. Peripheral blood T-lymphocyte and monocyte function and survival in patients with head and neck carcinoma. Laryngoscope. 2000;110(3 Pt 1):402–407. doi: 10.1097/00005537-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Economopolou P, Kotsantis I, Psyrri A. Checkpoint inhibitors in head and neck cancer: rationale, clinical activity, and potential biomarkers. Curr Treat Options Oncol. 2016;17(8):40. doi: 10.1007/s11864-016-0419-z. [DOI] [PubMed] [Google Scholar]
- 46.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8(3):138–140. [PubMed] [Google Scholar]
- 47.De Roda Husman AM, Walboomers JM, Van Den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3ʹ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76(Pt 4):1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 48.Ruprecht K, Ferreira H, Flockerzi A, Wahl S, Sauter M, Mayer J, Mueller-Lantzsch N. Human endogenous retrovirus family HERV-K(HML-2) RNA transcripts are selectively packaged into retroviral particles produces by the human germ cell tumor cell line Tera-1 and originate mainly from a provirus on chromosome 22q11.21. J Virol. 2008;82(20):10008–10016. doi: 10.1128/JVI.01016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


