Key Teaching Points.
-
•
Frequent multifocal fascicular-Purkinje-related premature ventricular complexes with or without dilated cardiomyopathy should raise the suspicion of SCN5A channelopathy. This can imply an increased risk of sudden cardiac arrest.
-
•
Amiodarone-associated excessive QT prolongation and torsades de pointes should prompt genotyping of the patient.
-
•
Combined clinical, genetic, and cellular analyses enable individualized patient care and facilitate personalized arrhythmia therapy.
Introduction
A new SCN5A-related cardiac syndrome, called “multifocal ectopic Purkinje-related premature contractions” (MEPPC), was first reported in 2012.1 In this autosomal-dominant condition, mutant sodium channels cause hyperexcitability of the fascicular-Purkinje system. Here we describe the case of a patient with MEPPC, nonsustained ventricular tachycardia (VT), and dilated cardiomyopathy, who developed drug-induced torsades de pointes (TdP) upon treatment with amiodarone.
Case report
A 24-year-old woman of Polish descent presented at the emergency department with exertional syncope that had occurred during housekeeping. Her unconsciousness struck without prior palpitations, chest discomfort, or light-headedness. She regained full consciousness after several seconds, without confusion or amnesia. She had no tongue bite or incontinence. In the days before, no diarrhea, vomiting, or febrile events had occurred. Her medical history was unremarkable. She did not take medication and denied the use of recreational drugs.
Exertional syncope without prodromi in young adults should raise the suspicion of ventricular arrhythmias with sympathetic susceptibility, such as in long QT syndrome, catecholaminergic polymorphic VT, arrhythmogenic (right ventricular) cardiomyopathy, and Gallavardin’s VT. Structural heart diseases that may be considered are obstructive hypertrophic cardiomyopathy, severe aortic valve stenosis, premature coronary artery stenosis or spasm, and coronary artery anomalies. Epileptic seizures can manifest without generalized convulsions, but often cause aura, tongue injury, and involuntary loss of bladder/bowel control. It is important to obtain a detailed family history on the occurrence of unexplained sudden (infant) death or cardiac arrest and other cardiac abnormalities, as this may allude to the presence of inherited arrhythmia syndromes.
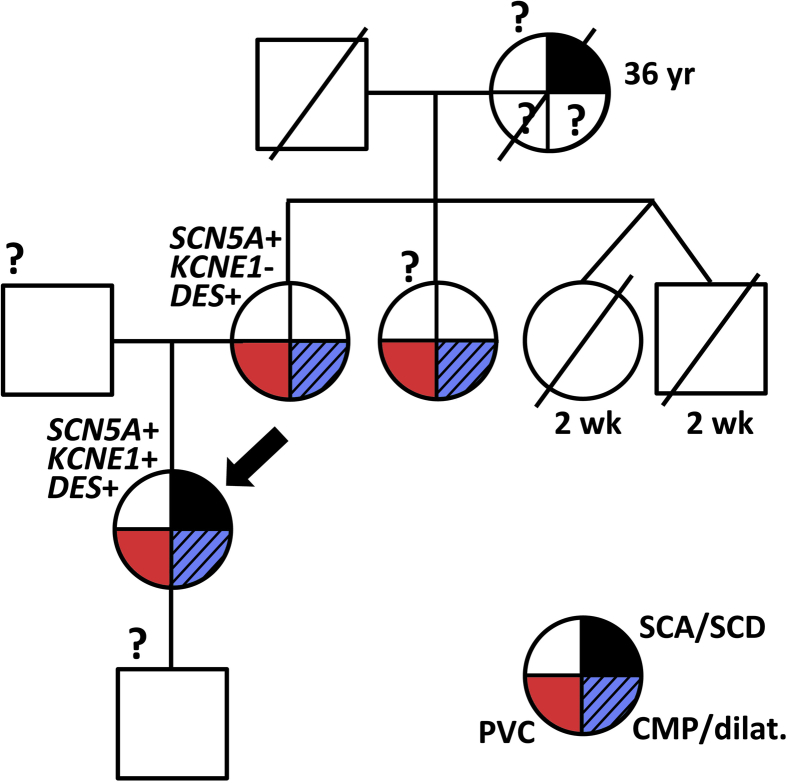
The patient was a slender woman with a normal blood pressure. She smoked and was not active in sports. There was no hypercholesterolemia or diabetes mellitus. Over a period of months, she had experienced progressive fatigue with slight exertional dyspnea and sporadic light-headedness. Her mother and a maternal aunt were known to have frequent premature ventricular complexes (PVCs) of left fascicular origin, a normal QTc, mild ventricular dilatation (mother), and impaired left ventricular (LV) function (maternal aunt) since adolescence. The mother’s twin sister and brother died postnatally after full-term gestation. The patient’s maternal grandmother died suddenly at age 33 (Figure 1).
Figure 1.
Partial pedigree. Circles are females; squares, males. Cardiac phenotypes are depicted as the red (premature ventricular complex [PVC]), dashed blue (cardiomyopathy/dilatation [CMP/dilat.]), or black (sudden cardiac arrest/death [SCA/SCD]) quadrants. Genotypes for the patient (arrow) and her mother are indicated. ? indicates missing information.
The family history of sudden death and ventricular arrhythmia may indicate an inherited arrhythmia syndrome or structural cardiomyopathy. The suspicion of premature coronary artery disease with ischemia is low, as her cardiovascular risk profile appears small. Coronary artery anomalies usually do not show familial clustering. Coronary spasm cannot yet be excluded.
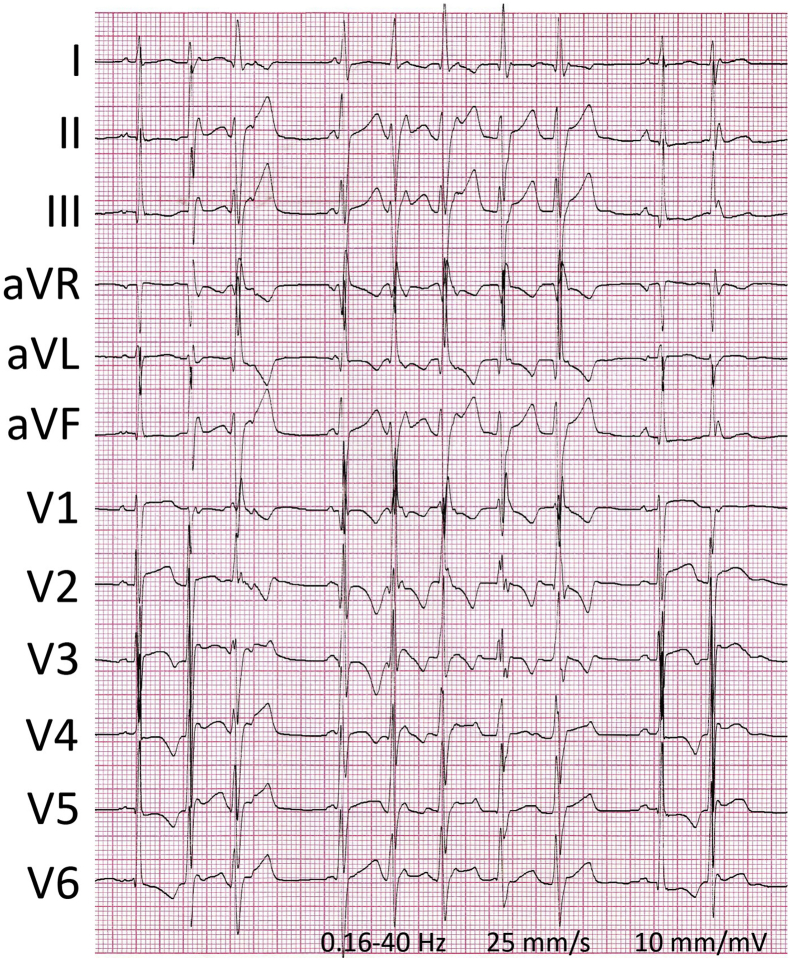
Physical examination revealed a blood pressure of 120/70 mm Hg with an irregular heart rate of 114 beats per minute and a body temperature of 36.0°C (96.8°F). Breathing rate and oxygen saturation were normal. Body height was 180 cm and weight 68 kg. The ictus cordis was displaced laterally with a grade 2/6 blowing holosystolic murmur at the apex and a third heart sound. Her jugular venous pressure was not elevated. Pulmonary and abdominal investigations were normal. The extremities were warm with intact pulses and without ankle edema. Besides elevated NT-proBNP levels (266 pmol/L), extensive laboratory testing revealed no abnormalities. The patient’s 12-lead electrocardiogram showed sinus rhythm with frequent multiform PVCs and nonsustained VTs, with swift intrinsicoid deflections (Figure 2) and left or right bundle branch block–like morphologies. Ventricular ectopy increased upon emotional distress and excitement, and reached a daily burden of 25,000 (∼17%). PQ and QT intervals were normal. The QRS amplitudes and inferolateral T-wave abnormalities fulfilled the criteria of LV hypertrophy: S V1 + R V5/V6 ≥ 35 mm (Sokolow-Lyon), and R V5/V6 ≥ 30 mm with typical ST-T wave changes (Romhilt-Estes).
Figure 2.
A 12-lead electrocardiogram on admission showing ventricular ectopy and nonsustained ventricular tachycardia.
The patient’s physical and electrocardiographic findings suggest a sympathetically driven primary electrical disorder or a proarrhythmic structural cardiomyopathy with signs of LV hypertrophy and reduced systolic function. Catecholaminergic polymorphic VT may be considered, but this is typically associated with preserved cardiac contractility. The morphology of the dominant PVCs is reminiscent of left fascicular and/or Purkinje-related foci, which is not typical for arrhythmogenic (right ventricular) cardiomyopathy or Gallavardin’s VT. A normal QT duration does not rule out long QT syndrome, but this is an unlikely cause of the abundant ventricular ectopy observed. For subsequent diagnostic evaluation an echocardiogram would be appropriate.
On echocardiography, the LV was dilated (end-diastolic diameter 64 mm, end-systolic diameter 56 mm) and its ejection fraction (LVEF) reduced (23%). A secondary grade B mitral regurgitation was found. No signs of active myocarditis, significant fibrosis, storage, or infiltrative diseases were observed with cardiac magnetic resonance imaging and in right ventricular septal endomyocardial biopsies. Coronary angiography was normal without aberrant coronary artery originations. Additional laboratory testing, including viral serology, autoimmune markers, thyroid function, iron status, corticosteroids, soluble IL-2 receptor, and urinary metanefrine concentrations, remained unremarkable. During invasive electrophysiological study, intracardiac conduction times were normal, and no VT could be induced by programmed electrical stimulation. Spontaneous PVCs were often preceded by high-frequency potentials in the ventricle, suggesting ectopic sites in the fascicular-Purkinje system. No ablation was attempted owing to the multifocality of the PVCs.
The combination of multifocal fascicular-Purkinje-related ectopy, nonsustained VT, and dilated cardiomyopathy without identifiable structural substrate suggests irregulopathy as the cause of heart failure. Along with the autosomal dominant pattern of arrhythmic disease in the family, these features imply a genetic origin, warranting molecular-genetic analysis.
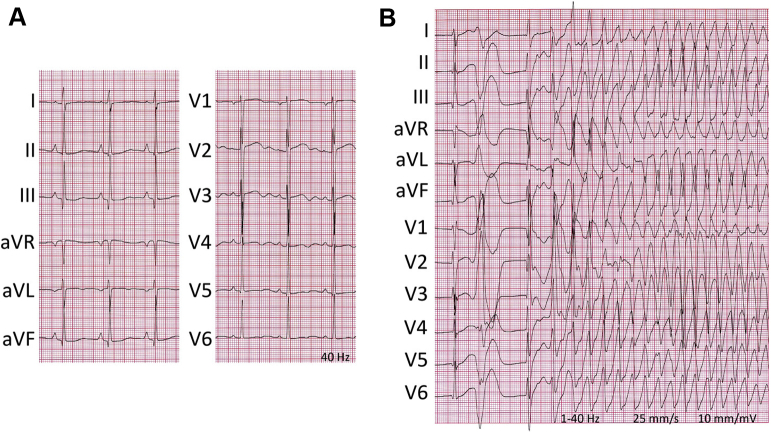
With informed consent of the patient, genomic DNA was extracted and screened for gene mutations associated with inherited arrhythmia and/or cardiomyopathy. Meanwhile, several antiarrhythmic drugs proved inefficacious: metoprolol, carvedilol, labetalol, and lidocaine. Amiodarone administration successfully suppressed all ectopic activity, albeit at the expense of substantial QT prolongation (∼730 ms; Figure 3A). Alas, recurrent episodes of TdP [(N-desethyl)amiodarone plasma level (0.4) 1.0 mg/L; Figure 3B] emerged after 9 days, requiring immediate discontinuation of the drug; this normalized the QT interval within a week. Ventricular ectopy recurred and an implantable cardioverter-defibrillator was inserted. Symptomatic hypotension and quinapril-induced angioedema hindered maintenance therapy with renin-angiotensin-aldosterone system blockers.
Figure 3.
Electrocardiograms at 48 hours (A) and 9 days (B) after initiation of amiodarone showing repolarization prolongation and pause-dependent onset of torsades de pointes.
Amiodarone has class I, II, III, and IV antiarrhythmic properties according to the Vaughan-Williams classification and can be beneficially used in heart failure patients. The incidence of amiodarone-induced proarrhythmia is low (0.7%), despite concomitant QT prolongation.2 The patient’s TdP likely resulted from ventricular ectopics impacting on a substrate of amiodarone-exaggerated dispersion of repolarization in the setting of reduced repolarization reserve.
DNA analysis revealed allelic variation in the SCN5A gene [c.2482C>T, p.(Leu828Phe)], KCNE1 gene [c.253G>A, p.(Asp85Asn)], and DES gene [c.638C>T, p.(Ala213Val)]. In 36 other cardiomyopathy- and arrhythmia-associated genes variations were excluded. The patient’s mother harbored the SCN5A and DES variant.
Although at this point the effects of the novel SCN5A variant on the sodium current (INa) are unclear, these features are reminiscent of the SCN5A channelopathy MEPPC.1 In the first report of MEPPC the SCN5A mutation p.(Arg222Gln) was described, which caused hyperexcitability of Purkinje fibers and was associated with cardiac dilatation. In our case, dilated cardiomyopathy could have been exaggerated by the rare polymorphism in DES. According to the MOGES classification the patient would be classified as MD-PVCOHGADEG-SCN5A p.(Leu828Phe)+DES p.(Ala213Val)S(C-II).3 Heart failure–induced electrical remodeling in combination with reduced potassium current by likely pathogenic KCNE1-p.(Asp85Asn) led to a diminished repolarization reserve.4 Amiodarone-induced proarrhythmia coincided with the time-dependent inhibition of the slowly activating delayed rectifier potassium current (IKs).5
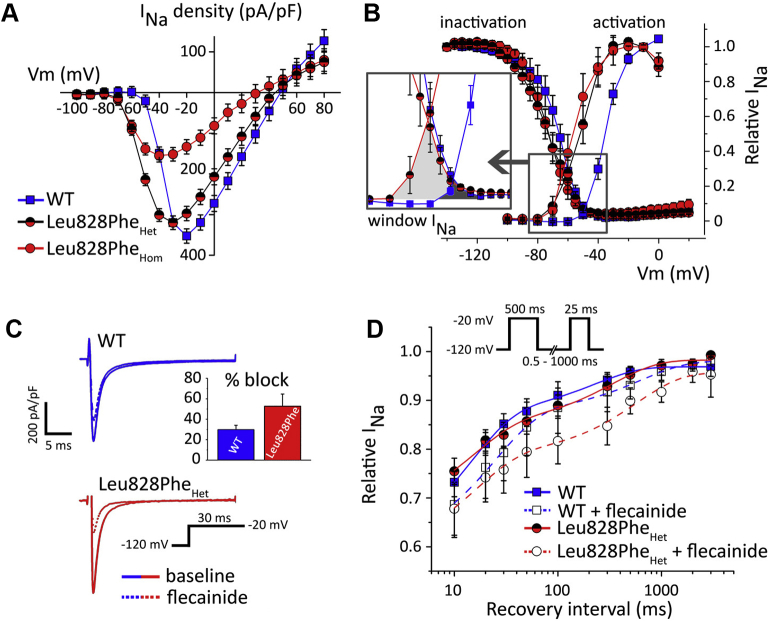
The class 3 missense mutation SCN5A-p.(Leu828Phe) affected the highly conserved leucine residue at domain II (S4–S5) of the cardiac sodium channel, juxtaposed to the voltage-sensing domain, and was absent in the ExAC, gnomAD, and ClinVar databases. We characterized SCN5A-p.(Leu828Phe) in transiently transfected Chinese hamster ovary cells (Figure 4). Heterozygous mutant INa showed similar amplitudes as wild-type INa, but with a significant leftward shift of the voltage dependence of activation. Because steady-state voltage-dependent inactivation remained unaltered, window INa increased, creating a broad voltage range of open-channel probability (between -80 and -50 mV), and a wide substrate for open and inactivated-state block by flecainide (10 μmol/L), reflected by increased tonic and blunted use-dependent SCN5A-p.(Leu828Phe) INa inhibition.
Figure 4.
Functional characteristics of SCN5A-p.(Leu828Phe). A: Current–voltage relationship normalized for cell capacitance in wild-type (WT), heterozygous (Het), and homozygous (Hom) mutant expression; beta subunits were not co-expressed. B: Voltage-dependent steady-state (in)activation and enlarged window INa. C: Tonic Leu828PheHet block at first test pulse (-20 mV). D: Reduced Leu828PheHet current recovery from flecainide block.
The functional consequences of SCN5A-p.(Leu828Phe) resemble those of the p.(Arg222Gln) variant.1 Flecainide’s preferential block of p.(Leu828Phe)-mutant INa at short recovery intervals, and at clinically relevant concentrations, suggests beneficial treatment of the patient with a class I antiarrhythmic agent. Screening for adverse class I side effects is advisable.
Short-acting sodium channel inhibition with intravenous ajmaline (1 mg/kg in 10 minutes) almost completely suppressed PVCs without Brugada type 1 electrocardiogram abnormalities, QT prolongation, or other proarrhythmic characteristics. Oral maintenance therapy with flecainide (100 mg, twice daily) was successfully started thereafter. Vigilant monitoring of the patient revealed very low PVC counts and complete LV function recovery within months. Now, 9 years later, the patient is doing well and has not received implantable cardioverter-defibrillator therapy. LVEF is 53%.
Discussion
Our translational approach with combined clinical, genetic, and cellular investigations identified the Achilles’ heel of the disease of this patient, and it led to personalized therapy. Two important lessons were learned: (1) frequent multifocal fascicular-Purkinje-related PVCs with or without dilated cardiomyopathy should raise the suspicion of SCN5A channelopathy; and (2) amiodarone-associated excessive QT prolongation and TdP should prompt genotyping of the patient.6
Sporadic PVCs are common in the general population, affecting about 39% of people (in 4% >100/day).7 They carry a favorable prognosis if occurring in the structurally normal heart. The risk of developing cardiomyopathy depends on the PVC burden (≥16%–24%)8, 9 and dyssynchronous myocardial activation, as is the case with wide PVC-QRS durations and/or epicardial foci,10 irrespective of a LV or right-ventricular origin. Multifocal PVCs originating in the conduction system often have narrow QRS widths and may be associated with familial sudden death (this study and ref [1]).
MEPPC syndrome was first described in 2012 in 3 unrelated families segregating SCN5A-p.(Arg222Gln).1 Patients can present with irregular palpitations with light-headedness, syncopal attacks, or sudden death (21% of affected family members, at mean age of 32 years).1 Symptoms of congestive cardiomyopathy can be present. The age at diagnosis varies from 24 weeks of gestation to 62 years (mean age 20 years) without gender predisposition.1 Multifocal PVCs occur in isolation, as doublets, or as nonsustained VT. Contractile dysfunction can accompany this syndrome.1, 11, 12 Differential diagnoses to be considered are ischemic cardiomyopathy, viral myocarditis, digitalis intoxication, genetic cardiomyopathies such as laminopathy, primary arrhythmia syndromes like catecholaminergic polymorphic VT, and short-coupled PVCs and TdP.13
An erratic pattern of abundant junctional ectopics and PVCs with left and right bundle branch block–like morphologies but with sharp intrinsicoid deflections is characteristic and suggests an origin in the conduction system. Atrioventricular node physiology and ventricular repolarization are unaffected. Although the echocardiographic features of LV dilatation and contractile dysfunction are nonspecific, their co-occurrence with multilevel hyperexcitability should raise the suspicion of MEPPC syndrome. Typically, no signs of myocardial inflammation or fibrosis are found on cardiac magnetic resonance imaging.
DNA sequencing of the SCN5A gene hitherto identified 2 mutations associated with MEPPC: p.(Arg222Gln)1 and p.(Arg225Pro).12 These mutations reside in homologous positions of the voltage-sensor region (S4 segment, domain I) of the cardiac sodium channel. Our novel mutation p.(Leu828Phe) lies close to the S4 segment of SCN5A domain II. Common to all 3 SCN5A mutations is the leftward shift of voltage-dependent activation with resultant triggering of inward INa at more negative resting membrane potentials. This can facilitate PVCs in fascicular and Purkinje fibers in which INa channels are more abundantly expressed compared to ventricular myocytes, and as is predicted by in silico analysis.1
Sodium-channel blockade is the preferred therapy of this SCN5A disease to reduce the PVC burden. Besides flecainide, quinidine or amiodarone can be considered, but the latter can turn adverse in cases with an inherently reduced repolarization reserve. In our patient, the KCNE1-p.(Asp85Asn) with known effect on repolarizing potassium currents IKs and IKr4 likely facilitated drug-induced repolarization prolongation and TdP.14 Proarrhythmic side effects of flecainide15 will be avoided by careful characterization (including class I provocation testing) and on-treatment monitoring of the patient. Long-term outcome is beneficial if antiarrhythmic treatment suppresses the PVCs that cause the irregulopathy, thus enabling the recovery of LV function. Indeed, the recovery of dilated cardiomyopathy and reduced LVEF in our patient indicates an important pathogenic role of SCN5A-related irregulopathy. Besides, a disease-modifying effect of the DES variant in accompanying the SCN5A mutation cannot be excluded.16
Acknowledgments
This work was supported by The Netherlands CardioVascular Research Initiative, CVON PREDICT (P.G.A.V.).
References
- 1.Laurent G., Saal S., Amarouch M.Y. Multifocal ectopic Purkinje-related premature contractions: a new SCN5A-related cardiac channelopathy. J Am Coll Cardiol. 2012;60:144–156. doi: 10.1016/j.jacc.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 2.Hohnloser S.H., Klingenheben T., Singh B.N. Amiodarone-associated proarrhythmic effects. A review with special reference to torsade de pointes tachycardia. Ann Intern Med. 1994;121:529–535. doi: 10.7326/0003-4819-121-7-199410010-00009. [DOI] [PubMed] [Google Scholar]
- 3.Arbustini E., Narula N., Tavazzi L., Serio A., Grasso M., Favalli V., Bellazzi R., Tajik J.A., Bonow R.O., Fuster V., Narula J. The MOGE(S) classification of cardiomyopathy for clinicians. J Am Coll Cardiol. 2014;64:304–318. doi: 10.1016/j.jacc.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Nishio Y., Makiyama T., Itoh H. D85N, a KCNE1 polymorphism, is a disease-causing gene variant in long QT syndrome. J Am Coll Cardiol. 2009;54:812–819. doi: 10.1016/j.jacc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Kamiya K., Nishiyama A., Yasui K., Hojo M., Sanguinetti M.C., Kodama I. Short- and long-term effects of amiodarone on the two components of cardiac delayed rectifier K+ current. Circulation. 2001;103:1317–1324. doi: 10.1161/01.cir.103.9.1317. [DOI] [PubMed] [Google Scholar]
- 6.Behr E.R., Roden D. Drug-induced arrhythmia: pharmacogenomic prescribing? Eur Heart J. 2013;34:89–95. doi: 10.1093/eurheartj/ehs351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostis J.B., McCrone K., Moreyra A.E., Gotzoyannis S., Aglitz N.M., Natarajan N., Kuo P.T. Premature ventricular complexes in the absence of identifiable heart disease. Circulation. 1981;63:1351–1356. doi: 10.1161/01.cir.63.6.1351. [DOI] [PubMed] [Google Scholar]
- 8.Baman T.S., Lange D.C., Ilg K.J. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Hasdemir C., Ulucan C., Yavuzgil O., Yuksel A., Kartal Y., Simsek E., Musayev O., Kayikcioglu M., Payzin S., Kultursay H., Aydin M., Can L.H. Tachycardia-induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: the incidence, clinical and electrophysiologic characteristics, and the predictors. J Cardiovasc Electrophysiol. 2011;22:663–668. doi: 10.1111/j.1540-8167.2010.01986.x. [DOI] [PubMed] [Google Scholar]
- 10.Yokokawa M., Kim H.M., Good E. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm. 2012;9:1460–1464. doi: 10.1016/j.hrthm.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 11.McNair W.P., Ku L., Taylor M.R., Fain P.R., Dao D., Wolfel E., Mestroni L. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation. 2004;110:2163–2167. doi: 10.1161/01.CIR.0000144458.58660.BB. [DOI] [PubMed] [Google Scholar]
- 12.Beckermann T.M., McLeod K., Murday V., Potet F., George A.L., Jr. Novel SCN5A mutation in amiodarone-responsive multifocal ventricular ectopy-associated cardiomyopathy. Heart Rhythm. 2014;11:1446–1453. doi: 10.1016/j.hrthm.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leenhardt A., Glaser E., Burguera M., Nürnberg M., Maison-Blanche P., Coumel P. Short-coupled variant of Torsade de Pointes. A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation. 1994;89:206–215. doi: 10.1161/01.cir.89.1.206. [DOI] [PubMed] [Google Scholar]
- 14.Kääb S., Crawford D.C., Sinner M.F. A large candidate gene survey identifies the KCNE1 D85N polymorphism as a possible modulator of drug-induced torsades de pointes. Circ Cardiovasc Genet. 2012;5:91–99. doi: 10.1161/CIRCGENETICS.111.960930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Echt D.S., Liebson P.R., Mitchell L.B. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 16.Goudeau B., Rodrigues-Lima F., Fischer D. Variable pathogenic potentials of mutations located in the desmin alpha-helical domain. Hum Mutat. 2006;27:906–913. doi: 10.1002/humu.20351. [DOI] [PubMed] [Google Scholar]