Abstract
Chronic kidney disease (CKD) progression results in musculoskeletal dysfunction that is associated with a higher likelihood of hospitalization and is predictive of hospitalizations and mortality. Despite this, there is a lack of effective interventions to treat the musculoskeletal dysfunction. We studied treadmill running as an intervention to improve musculoskeletal health in a translational rat model that has slowly progressive CKD. CKD rats were subjected to treadmill exercise or no treadmill exercise for 10 weeks (n = 8 each group). Animals ran for 60 min, 5 times per week starting at a speed of 8 m/min and ending at 18 m/min (1 m/min increase/week). Treadmill training had no effect on muscle strength (assessed as maximally stimulated torque), half-relaxation time (time from peak torque to 50%) or muscle cross-sectional area. Overall, there were no biochemical improvements related to CKD progression. Skeletal muscle catabolism was higher than non-exercised animals without a concomitant change in muscle synthesis markers or regeneration transcription factors. These results suggest that aerobic exercise, achieved via treadmill running was not protective in CKD animals and actually produced potentially harmful effects (increased catabolism). Given the high prevalence and dramatic musculoskeletal mobility impairment in patients with CKD, there is a clear need to understand how to effectively prescribe exercise in order to benefit the musculoskeletal system.
Keywords: Chronic kidney disease, Treadmill training, Skeletal muscle, Sarcopenia
Highlights
-
•
Animals with progressive chronic kidney disease had no skeletal muscle benefit from 10 weeks of treadmill running.
-
•
There were no biochemical improvements related to CKD progression in animals that underwent 10 weeks of treadmill running.
-
•
Skeletal muscle catabolism was higher in treadmill exercised animals without a concomitant increase in synthesis.
1. Introduction
Chronic kidney disease (CKD) is common in the United States, affecting > 30 million adults [1]. Chronic kidney disease progression is associated with muscle atrophy, reduced strength and impaired mobility; known as sarcopenia or frailty [2], [3], [4]. The progressive loss of muscle size and function leads to impaired physical function that is associated with a higher likelihood of being hospitalized [5] and is predictive of mortality in patients with CKD and those on dialysis [6], [7]. Despite this, there is a lack of effective interventions to treat the musculoskeletal dysfunction.
Exercise is often viewed as a primary intervention to aide in musculoskeletal health. In persons with chronic disease conditions, exercise participation leads to a number of beneficial effects [8]. In CKD, a systematic review of clinical exercise studies for patients with stage 2–5 CKD, demonstrated beneficial effects on aerobic capacity and blood pressure, but no difference in muscle size, strength, or walking capacity [9]. In general, the extent to which exercise is beneficial for patients with CKD is inconclusive. Similarly, animal models of forced exercise have shown inconclusive evidence on the beneficial effects of exercise [10], [11], [12], [13], [14]. These animal studies mimic what is seen clinically with no effect on disease progression [10], [13] or muscle protein synthesis [10], [14], and mixed effects on antioxidants [13], [15]. This lack of effect may be due to a number of factors surrounding the exercise prescription, mode, and disease model. We therefore intended to study the effects of aerobic exercise in a translational rat model with slowly progressive CKD with demonstrated muscle dysfunction [16], [17]; to identify the effects of 10 weeks of treadmill aerobic exercise on skeletal muscle properties.
2. Methods
2.1. Experimental Design
We used a rat model of CKD (Cy/+ rats) that develops progressive azotemia with terminal uremia by 40 weeks [18]. Animals were fed a casein diet (Purina AIN-76A, Purina Animal Nutrition, Shreveport, LA, USA; 0.53% Ca and 0.56% P) beginning at 24 weeks (approximately 50% normal kidney function) in order to promote consistent disease development. At 25 weeks, CKD rats were subjected to treadmill exercise or no treadmill exercise for 10 weeks (n = 8 each group). Animals ran for 60 min, 5 × /week starting at a speed of 8 m/min and ending at 18 m/min (1 m/min increase/week). Animals were acclimated for one week prior to initiation of training bout (from weeks 24–25). Animals received a mild electrical stimulus when failing to maintain prescribed speed and an individual treadmill session was terminated if electrical stimulation was given more than 3 times in one minute, or if the animal sat on the coils for greater than 10 s. The Indiana University School of Medicine Institutional Animal Care and Use Committee reviewed and approved all procedures prior to initiating the study.
2.2. Electrically stimulated torque
Prior to euthanasia at 35 weeks, muscle strength/torque was assessed (1305 A Whole Rat Test System, Aurora Scientific Inc., Aurora, ON, Canada). Animals were placed in a supine position and their right foot secured to a foot pedal attached to a servomotor [17]. Electrodes were placed near the common peroneal nerve to stimulate a dorsiflexion twitch response and input current was adjusted to achieve maximum twitch response followed by a 20% increase to ensure supramaximal stimulation of muscle fibers (~15 mA for dorsiflexion). Maximum isometric torque (N·m) was recorded for various stimulation frequencies ranging between 10 Hz and 200 Hz, with a pulse width of 0.2 ms and pulse duration of 200 ms. Following testing, time to maximum torque and half relaxation time were calculated from the peak isometric torque data.
2.3. Tissue procurement
Blood was collected prior to euthanasia, and the extensor digitorum longus (EDL) from the non-stimulated limb was collected. The middle third of EDL was isolated, placed in OCT media and frozen in liquid nitrogen chilled isopentane; then remaining tissue was snap frozen in liquid nitrogen. The remaining EDL was stored for protein and RNA analysis at −80 °C.
2.4. Assays
Blood plasma was analyzed for blood urea nitrogen (BUN), calcium and phosphorus using colorimetric assays (BioAssay Systems, Hayward, CA, USA) and intact PTH by ELISA (Alpco, Salem, NH, USA).
2.5. Muscle cross-sectional area
Immunostaining was performed on frozen EDL cryosections. Sections were incubated overnight with a polyclonal anti-laminin antibody (Sigma, St. Louis, MO) in order to label muscle fiber perimeters for measurement of fiber cross-sectional area. Skeletal muscle cross-sections were imaged at 10x magnification using a Spot RT Color Camera System mounted on an inverted Nikon Diaphot 200 microscope (Nikon Instruments Inc, Melville, NY), 3 images per Section, 3 sections per animals were analyzed. Prior to analysis, each image was inspected for sectioning artifacts, blood vessels, or poor image quality, and any sections that were not acceptable were omitted from the analysis. Muscle fiber cross-sectional area was measured with an automated, custom-written macro in ImageJ (NIH, Bethesda, MD), provided by Drs. Rick Lieber and Samuel Ward [19].
2.6. RNA isolation and real time PCR
Total RNA was isolated using miRNeasy Mini Kit (Qiagen) as previously described [16]. Gene expression was determined by real time PCR using TaqMan mRNA assays (Applied Biosystems, Foster City, CA). Target-specific PCR primers (Pax-7, MyoD, Myostatin, Myogenin, Atrogin 1, IGF-1) were obtained from Applied Biosystems. The cycle number at which the amplification plot crosses the threshold was calculated (CT), and the ΔΔCT method was used to analyze the relative changes in gene expression and normalized by β-actin.
2.7. Western blot
Western blotting was performed as previously described [16], [20]. Briefly, the EDL muscle was homogenized and the total protein lysates analyzed for expression of markers for protein degradation using antibody against Ubiquitin and p70 (total and phosphorylated), respectively (1:500, Santa Cruz Biotechnology, Santa Cruz, CA). The blots were incubated overnight at 4 oC followed by incubating with peroxidase conjugated secondary antibody (1:5000 dilution) and immunodetection assessed with the Enhanced Chemiluminescence Prime Western Blot Detection Reagent (Amersham, Piscataway, NJ). Band intensity was analyzed by ChemiDoc MP Imaging System (Imaging Lab 4.0, Bio-Rad, Richmond, CA) and normalized to total protein expression using Ponceau S (Santa Cruz Biotechnology, Santa Cruz, CA) or phosphorylated to total p70 were utilized as the normalization for p70 expression.
2.8. Statistics
Two-tailed independent sample t-tests were used to compare means between CKD and CKD + exercise. Data are presented as mean ± SD.
3. Results
At 35 weeks of age (approximately 15% of normal kidney function), there was no difference between groups for muscle strength (via maximally stimulated torque; Fig. 1A) or half-relaxation time (time from peak torque to 50% torque reduction; Fig. 1B). There was also no difference in muscle fiber cross-sectional area between CKD rats (2821 µm ± 405) and CKD + exercise (2413 µm ± 449). Biochemical measures, indicative of disease severity were not significantly different between groups (Table 1) with the exception of elevations in Ca++ with exercise. Similarly, there was no difference in body mass.
Fig. 1.
The effect of treadmill training on skeletal muscle function in CKD.
Table 1.
End-point biochemical properties.
| Group | Body mass (g) | BUN (mg/dl) | Calcium (mg/dl) | Phosphorus (mg/dl) | PTH (pg/dl) |
|---|---|---|---|---|---|
| CKD | 534 ± 43 | 58 ± 9 | 7.05 ± 1.70 | 12.3 ± 3.1 | 1375 ± 504 |
| CKD+Ex | 524 ± 7 | 55 ± 4 | 9.69 ± 2.26* | 10.7 ± 1.2 | 1333 ± 284 |
Mean ± SD.
p < 0.05 for difference from CKD.
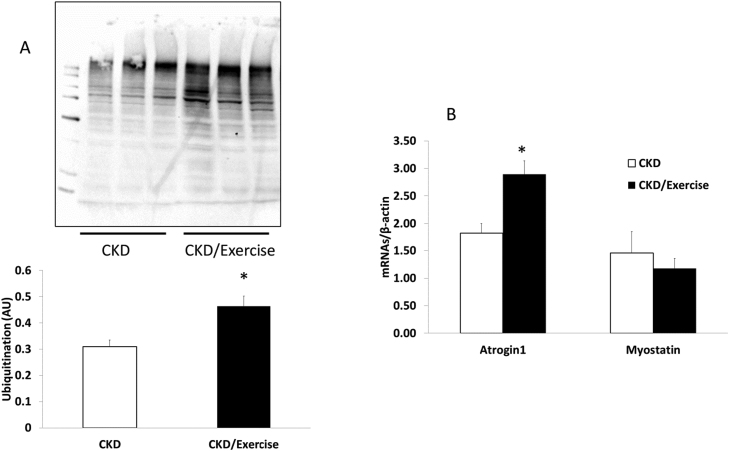
In an attempt to better understand the lack of efficacy of treadmill running, we examined muscle protein turnover. Treadmill exercise did not alter the gene expression of IGF-1, an upstream, global marker for protein synthesis (Fig. 2A) or its downstream target p70 (Fig. 2B). However, treadmill exercise increased muscle catabolism, with levels of ubiquitin protein expression 50% higher compared to non-exercised animals (Fig. 3A). The propensity towards catabolism was also corroborated by higher gene expression levels of the ubiquitin pathway gene atrogin-1 (Fig. 3B, p < 0.05).
Fig. 2.
The effect of treadmill training on skeletal muscle function in CKD.
Fig. 3.
Proteolytic pathways in muscle are activated by treadmill training in CKD.
To determine if skeletal muscle regeneration was altered, we measured RNA transcription factors associated with muscle stem cell regeneration. Treadmill exercise resulted in 44% lower expression of the muscle stem cell quiescence marker PAX7 (Fig. 4, p < 0.05), but there was no difference in expression of the regenerative factors myoD or myogenin (Fig. 4).
Fig. 4.
Skeletal muscle regeneration is altered with treadmill training in CKD.
4. Discussion
Exercise has demonstrated beneficial effects in a number of chronic disease conditions [21], but in CKD the data are conflicting and most often reveal muted responses [9]. In this study, we hypothesized that aerobic exercise (i.e. treadmill exercise) would improve skeletal muscle properties. Overall, we did not find a positive effect from aerobic exercise in improving muscle performance (i.e. maximally stimulated torque) or cross-sectional area. In fact, we found increased skeletal muscle catabolism based on increased atrogin-1 and ubiquitination, without concomitant protein synthesis. There was also reduction in PAX-7 expression, a marker of muscle satellite stem cell quiescence. Reduced quiescence indicates muscle stem cell activation that could be deemed inappropriate or ineffective as there was no further downstream alterations in myogenin or myoD. These results suggest that our exercise protocol was not effective in rats with CKD due to increased catabolism without concomitant and/or impaired satellite cell differentiation and downstream regeneration in CKD.
The lack of exercise efficacy demonstrated in this slowly progressive CKD model is similar to results from other CKD animal models. Yoshida et al. (2017) aerobically exercised rats at 15 m/min for 1 h/day, 5 days/wk for 7 weeks, waiting 5 weeks after 5/6 nephrectomy. Although they additionally compared low protein diet vs a branched-chain amino acids diet, the study found that treadmill exercise in CKD rats did not have a positive effect irrespective of the diet upon skeletal muscle cross-sectional area. Further, they found no effect on skeletal muscle protein synthesis or degradation. De Souza et al. performed a one step 5/6 nephrectomy in rats, followed by a forced exercise intervention of treadmill training at 17 m/min for 50 min/day for 8 weeks. They found beneficial anti-oxidant effects (increased superoxide production and reduced oxidative damage) but there were no assessments of muscle cross-sectional area or function. Moningka et al., 2011, examined age-related kidney disease using Fisher 344 male rats; these rats performed treadmill exercise at 15 m/min, 15° incline for 60 min/day for 10–12 weeks. They found that exercise did not mitigate age-related kidney changes, nor alter protective antioxidant enzymes and oxidative stress. In the present study, we not only assessed morphologic and gene changes, but also function as a surrogate for clinically meaningful improvements in physical performance.
The limited response to treadmill training in our study and other studies in rat models may be due to a number of factors including dysfunction of stem cell differentiation as shown in our study, and increased catabolic state as shown in the present study and by Wang et al., 2009. Uremic toxins may have direct effects on metabolic pathways, and impaired cardiovascular function may alter blood flow with intensive exercise. Another possibility is the behavioral stress related to this type of forced exercise. Treadmill training in animals is similar to humans, occurring over a motorized belt. However, the animals are performing exercise during the day, opposite of their normal awake cycle. Further, the studies require increased human handling, exercise in a non-familiar small compartment, and the animals experience an electrical stimulus when unable to maintain the prescribed speed. These additional factors may result in detrimental behavioral stress [22]. One limitation to our study is that we did not have a normal control group, thus we cannot fully determine if the observed response is unique to CKD or to the exercise regimen itself.
Given the similarity of the rodent models examined to date and the lack of consistent positive effects, there is mounting support that aerobic exercise is ineffective in CKD. However, an alternative hypothesis is that CKD, with its systemic comorbidities and baseline mobility impairment [23], requires a different approach to exercise. In humans, exercise in chronic disease rehabilitation programs is initiated slowly and prescribed under supervision of trained professionals. A properly prescribed and dosed exercise in rats might therefore be effective if there is first an assessment of maximal aerobic capacity (using VO2max), and then an appropriate scaling of exercise intensity. These working intensities determine the treadmill training speeds. This lack of prescription specificity and graded increase may also partially explain the results from the present, and other studies, in rodents. Supporting this, studies using voluntary wheel running in rats with CKD have demonstrated beneficial effects anti-inflammatory signaling and cardiac function [24], [25], [26], [27] although musculoskeletal outcomes were not assessed. Further studies comparing wheel versus treadmill training would demonstrate preferential interventions of increased physical activity versus intensive exercise. Given the high prevalence and dramatic musculoskeletal mobility impairment in patients with CKD, there is a clear need to understand how to effectively prescribe exercise in order to benefit the musculoskeletal system.
There was no significant difference between the groups when assessing in vivo dorsiflexion maximum isometric torque (A) or half relaxation time (B). Data are shown for CKD (white bars) rats and CKD + exercise (black bars) at 35 weeks of age. Values are mean ± SD.
IGF-1 is an upstream, global marker for protein synthesis. (A) There was no difference in IGF-1 expression in the EDL of 35 week old CKD rats and CKD + exercise, as determine by qRT-PCR. Protein synthesis was further assessed for IGF-I downstream target p70; (B) the ratio between total and phosphorylated p70 are shown. There was no difference between the two groups CKD (white bars) and CKD+exercise (black bars). Data are shown as mean ± SD.
(A) Protein expression for total ubiquitination by western blot in skeletal muscle was greater in the CKD animals that were exercised. (B) Atrogin-1 expression RNA expression was also higher in the EDL of 35 week old rats that were exercised compared to CKD without exercise. Data are shown as mean ± SD. *P < 0.05, CKD (white bars) vs. CKD + exercise (black bars).
The expression of myogenic factors was determined by qRT-PCR in the EDL muscle from 35 weeks old chronic kidney disease (CKD); white bars and CKD+exercise (black bars) rats. In the CKD+exercise group there is increased satellite (stem cell) activation due to lower expression of Pax-7, but no difference in downstream myogenic markers MyoD and myogenin expression. These data suggest blockade of normal regenerative response to exercise. Data are shown as mean ± SD. *P < 0.05, CKD vs. CKD + exercise.
Funding source
K08 DK110429-01 NIH/NIDDK Mechanisms of Interventions to Ameliorate Sarcopenia in Chronic kidney disease, Granting Agency: NIDDK (author: KGA). This study was funded by a pilot grant from the National Skeletal Muscle Research Center at the University of California-San Diego, as a subcontract of NIH 5R24HD050837-10 NIH (PI: Lieber)
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.09.001.
Contributor Information
J.M. Organ, Email: jorgan@iupui.edu.
M.R. Allen, Email: matallen@iupui.edu.
A. Myers-White, Email: amyerswh@umail.iu.edu.
W. Elkhatib, Email: welkhati@iupui.edu.
K.D. O'Neill, Email: kaddingt@iu.edu.
N.X. Chen, Email: xuechen@iu.edu.
S.M. Moe, Email: smoe@iu.edu.
K.G. Avin, Email: keigavin@iu.edu.
Appendix A. Transparency document
Supplementary material
References
- 1.Centers for Disease Control and Prevention . US Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2017. National Chronic Kidney Disease Fact Sheet. 2017. [Google Scholar]
- 2.Moon S.J., Kim T.H., Yoon S.Y., Chung J.H., Hwang H.J. Relationship between stage of chronic kidney disease and sarcopenia in korean aged 40 Years and older using the Korea National Health and Nutrition Examination Surveys (KNHANES IV-2, 3, and V-1, 2), 2008–2011. PLoS One. 2015;10:e0130740. doi: 10.1371/journal.pone.0130740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley R.N., Wang C., Ishani A., Collins A.J., Murray A.M. Kidney function and sarcopenia in the United States general population: nhanes III. Am. J. Nephrol. 2007;27:279–286. doi: 10.1159/000101827. [DOI] [PubMed] [Google Scholar]
- 4.Moorthi R.N., Avin K.G. Clinical relevance of sarcopenia in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2017;26:219–228. doi: 10.1097/MNH.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeOreo P.B. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am. J. Kidney Dis. 1997;30:204–212. doi: 10.1016/s0272-6386(97)90053-6. [DOI] [PubMed] [Google Scholar]
- 6.Roshanravan B., Robinson-Cohen C., Patel K.V., Ayers E., Littman A.J., de Boer I.H., Ikizler T.A., Himmelfarb J., Katzel L.I., Kestenbaum B., Seliger S. Association between physical performance and all-cause mortality in CKD. J. Am. Soc. Nephrol. 2013;24:822–830. doi: 10.1681/ASN.2012070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao Y.R., Dalrymple L., Chertow G.M., Kaysen G.A., Johansen K.L. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch. Intern. Med. 2012;172:1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mora J.C., Valencia W.M. Exercise and older adults. Clin. Geriatr. Med. 2018;34:145–162. doi: 10.1016/j.cger.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Heiwe S., Jacobson S.H. Exercise training in adults with CKD: a systematic review and meta-analysis. Am. J. Kidney Dis. 2014;64:383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T., Kakizawa S., Totsuka Y., Sugimoto M., Miura S., Kumagai H. Effect of endurance training and branched-chain amino acids on the signaling for muscle protein synthesis in CKD model rats fed a low-protein diet. Am. J. Physiol. Ren. Physiol. 2017;313:F805–F814. doi: 10.1152/ajprenal.00592.2015. [DOI] [PubMed] [Google Scholar]
- 11.Tucker P.S., Briskey D.R., Scanlan A.T., Coombes J.S., Dalbo V.J. High intensity interval training favourably affects antioxidant and inflammation mRNA expression in early-stage chronic kidney disease. Free Radic. Biol. Med. 2015;89:466–472. doi: 10.1016/j.freeradbiomed.2015.07.162. [DOI] [PubMed] [Google Scholar]
- 12.de Souza P.S., da Rocha L.G., Tromm C.B., Scheffer D.L., Victor E.G., da Silveira P.C., de Souza C.T., Silva L.A., Pinho R.A. Therapeutic action of physical exercise on markers of oxidative stress induced by chronic kidney disease. Life Sci. 2012;91:132–136. doi: 10.1016/j.lfs.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Moningka N.C., Sindler A.L., Muller-Delp J.M., Baylis C. Twelve weeks of treadmill exercise does not alter age-dependent chronic kidney disease in the Fisher 344 male rat. J. Physiol. 2011;589:6129–6138. doi: 10.1113/jphysiol.2011.214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X.H., Du J., Klein J.D., Bailey J.L., Mitch W.E. Exercise ameliorates chronic kidney disease-induced defects in muscle protein metabolism and progenitor cell function. Kidney Int. 2009;76:751–759. doi: 10.1038/ki.2009.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coelho B.L., Rocha L.G., Scarabelot K.S., Scheffer D.L., Ronsani M.M., Silveira P.C., Silva L.A., Souza C.T., Pinho R.A. Physical exercise prevents the exacerbation of oxidative stress parameters in chronic kidney disease. J. Ren. Nutr. 2010;20:169–175. doi: 10.1053/j.jrn.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Avin K.G., Chen N.X., Organ J.M., Zarse C., O'Neill K., Conway R.G., Konrad R.J., Bacallao R.L., Allen M.R., Moe S.M. Skeletal muscle regeneration and oxidative stress are altered in chronic kidney disease. PLoS One. 2016;11:e0159411. doi: 10.1371/journal.pone.0159411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Organ J.M., Srisuwananukorn A., Price P., Joll J.E., Biro K.C., Rupert J.E., Chen N.X., Avin K.G., Moe S.M., Allen M.R. Reduced skeletal muscle function is associated with decreased fiber cross-sectional area in the Cy/+ rat model of progressive kidney disease. Nephrol. Dial. Transplant. 2016;31:223–230. doi: 10.1093/ndt/gfv352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moe S.M., Chen N.X., Seifert M.F., Sinders R.M., Duan D., Chen X., Liang Y., Radcliff J.S., White K.E., Gattone V.H., 2nd A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009;75:176–184. doi: 10.1038/ki.2008.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minamoto V.B., Suzuki K.P., Bremner S.N., Lieber R.L., Ward S.R. Dramatic changes in muscle contractile and structural properties after 2 botulinum toxin injections. Muscle Nerve. 2015;52:649–657. doi: 10.1002/mus.24576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moe S.M., Duan D., Doehle B.P., O'Neill K.D., Chen N.X. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int. 2003;63:1003–1011. doi: 10.1046/j.1523-1755.2003.00820.x. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen B.K., Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports. 2015;25(Suppl 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 22.Svensson M., Rosvall P., Boza-Serrano A., Andersson E., Lexell J., Deierborg T. Forced treadmill exercise can induce stress and increase neuronal damage in a mouse model of global cerebral ischemia. Neurobiol. Stress. 2016;5:8–18. doi: 10.1016/j.ynstr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plantinga L.C., Johansen K., Crews D.C., Shahinian V.B., Robinson B.M., Saran R., Burrows N.R., Williams D.E., Powe N.R., Team C.C.S. Association of CKD with disability in the United States. Am. J. Kidney Dis. 2011;57:212–227. doi: 10.1053/j.ajkd.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martens C.R., Kuczmarski J.M., Kim J., Guers J.J., Harris M.B., Lennon-Edwards S., Edwards D.G. Voluntary wheel running augments aortic l-arginine transport and endothelial function in rats with chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2014;307:F418–F426. doi: 10.1152/ajprenal.00014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams G.R., Zhan C.D., Haddad F., Vaziri N.D. Voluntary exercise during chronic renal failure in rats. Med. Sci. Sports Exerc. 2005;37:557–562. doi: 10.1249/01.mss.0000159006.87769.67. [DOI] [PubMed] [Google Scholar]
- 26.Kuczmarski J.M., Martens C.R., Kim J., Lennon-Edwards S.L., Edwards D.G. Cardiac function is preserved following 4 weeks of voluntary wheel running in a rodent model of chronic kidney disease. J. Appl. Physiol. 1985;117(2014):482–491. doi: 10.1152/japplphysiol.00344.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai Y., Sigala W., Adams G.R., Vaziri N.D. Effect of exercise on cardiac tissue oxidative and inflammatory mediators in chronic kidney disease. Am. J. Nephrol. 2009;29:213–221. doi: 10.1159/000156715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material