Abstract
The target site of tolprocarb has been reported to be polyketide synthase (PKS). Here, we evaluated the activities for Pyricularia oryzae PKS and melanin biosynthesis as well as the control efficacy of rice blast using a series of tolprocarb derivatives. A comparison of the inhibitory activities of PKS and melanin biosynthesis revealed a linear relationship (r2=0.90), confirming PKS as the target site of tolprocarb. A compound beyond this relationship was metabolized by P. oryzae to an inactive compound. The control efficacy of rice blast was explained using the melting point and either the inhibitory activity of PKS or melanin biosynthesis. Structure–activity analysis revealed that both end parts of tolprocarb preferred hydrophobic groups, and the chirality of the substituent in the middle part significantly influenced the activities. These relationships will provide useful information for the development of novel PKS inhibitors.
Keywords: melanin biosynthesis inhibitors, polyketide synthase, tolprocarb, P. oryzae, rice blast, structure-activity relationship
Introduction
The ascomycete Pyricularia oryzae (syn. Magnaporthe oryzae) is the cause of one of the most widespread and destructive diseases of rice plants, known as rice blast disease.1) During the infection process, P. oryzae produces unicellular infection structures, called appressoria, which produce slender infection pegs that pierce the underlying cell wall of the host. The cell wall of an appressorium contains a dense layer of melanin synthesized from 1,8-dihydroxynaphthalene (1,8-DHN).2) The accumulation of the dark-colored melanin is an essential step before the appressoria of P. oryzae can penetrate host plants.3–7) It was also shown that melanin-deficient mutants of P. oryzae were unable to infect intact host plants.8) The melanin biosynthetic pathway has been reported in various fungal species.2,6,8–10) The biosynthetic pathway for melanin in fungi starts from the formation of 1,3,6,8-tetrahydroxynaphthalene by polyketide synthase (PKS). Subsequently, two sequential reduction and dehydration steps lead to the intermediate, 1,8-DHN. Finally, 1,8-DHN is polymerized into melanin.
Melanin biosynthesis inhibitors (MBIs) have long been used to control rice blast disease. They can be divided into two subgroups based on the inhibitory step, reductase or dehydratase.2) Reductase inhibitors target two hydroxynaphthalene reductases involved in melanin biosynthesis and comprise compounds such as tricyclazole, pyroquilon, or fthalide.3–5,11,12) The dehydratase inhibitors target scytalone dehydratase and include compounds such as carpropamid, diclocymet, or fenoxanil.13–15) However, the development of resistance to conventional MBIs that inhibit dehydratase has posed a problem for controlling rice blast and other plant diseases.16) Therefore, fungicides with different modes of action are required to combat the development of resistance to the currently used fungicides.
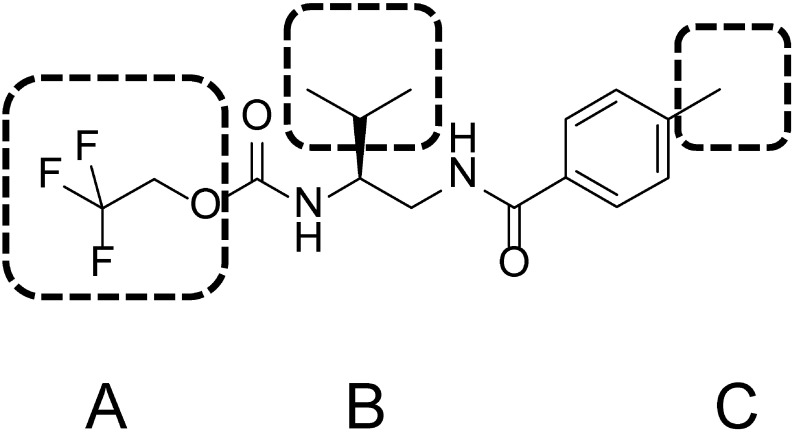
Tolprocarb, 2,2,2-trifluoroethyl N-[(1S)-2-methyl-1-[[(4-methylbenzoyl)amino]methyl] propyl] carbamate, which is being developed by Mitsui Chemicals Agro Inc., has proven to be highly effective for controlling rice blast.17–19) In our previous report, the target site of tolprocarb was found to be PKS, which is different from that of conventional fungicides.20) As a result, tolprocarb was classified into a new mode-of-action category in the Fungicide Resistance Action Committee code list. In the present study, we evaluated a series of tolprocarb derivatives for P. oryzae polyketide synthase inhibitory activity (PKS-A), melanin biosynthesis inhibitory activity (MBI-A), and rice blast control efficacy (RBC-E) to verify their target sites and to investigate the structure–activity relationships (SARs) and activity–activity relationships. For practical reasons, we selected tolprocarb as a reference and divided its derivatives into three substructures to facilitate organization of the SAR exploration: part A, part B, and part C (Fig. 1).
Fig. 1. Structure of tolprocarb and SAR design.
Materials and Methods
1. Chemicals, culture media, and strain of P. oryzae
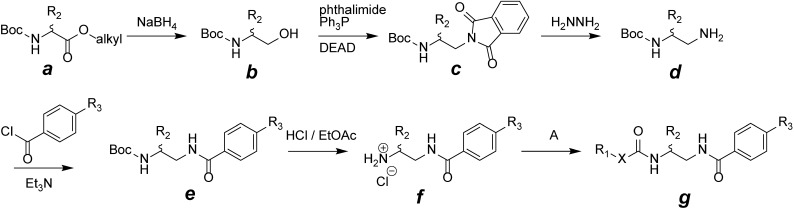
All tolprocarb derivatives evaluated here were synthesized at the Agrochemicals Research Center, Mitsui Chemicals Agro, Inc. (Chiba, Japan), according to the general procedures described previously17,18) and summarized in Fig. 2. Generally, all derivatives were synthesized from their respective amino acids or amino alcohols such as N-tert-butoxycarbonyl (N-Boc)-protected amino acid esters a or amino alcohols b. Tolprocarb derivatives g were synthesized by various reagents or reactions such as chloroformate derivatives, p-nitrophenylcarbonates, O-(p-nitrophenyl) thiocarbonates, isocyanates, isothiocyanates, carbamoyl chlorides, acid chlorides, acid anhydrides, or mixed acid anhydrides or by direct coupling with acid using coupling reagents such as dicyclohexylcarbodiimide or carbonyldiimidazole. Melting points (Tms) were measured with a Mettler FP62 melting point apparatus with a heating rate of 3°C/min and were uncorrected. Proton nuclear magnetic resonance (1H NMR) spectra (400 or 500 MHz) were obtained using JEOL JNM-400 FT-NMR or JEOL JNM-ECA-500 with tetramethylsilane as the internal standard and are listed in the electronic supplementary material (Supplemental Table S1). Malonyl-CoA and oatmeal agar were purchased from Sigma (St. Louis, MO, USA). Potato dextrose agar (PDA) and potato dextrose broth (PDB) were purchased from Kyokuto Seiyaku (Tokyo, Japan) and Difco Laboratories (Detroit, MI, USA), respectively. P. oryzae 40901 maintained at the Agrochemicals Research Center, Mitsui Chemical Agro, Inc., was used in all experiments described here.
Fig. 2. Overall reaction pathway for synthesis of tolprocarb derivatives. Alkyl=ethyl or methyl; Boc=tert-butoxycarbonyl; DEAD=diethyl azodicarboxylate; X=N (urea), O (carbamate), C (amide) or S (thiocarbamate); A=chloroformates, p-nitrophenylcarbonates, O-(p-nitrophenyl)thiocarbonates, isocyanates, isothiocyanates, carbamoyl chlorides, acid chlorides, acid anhydrides or mixed acid anhydrides.
2. Cell-free assay of PKS
A cell-free extract containing P. oryzae PKS was extracted from Aspergillus oryzae heterologously expressing P. oryzae PKS by a previously described method.20,21) The cell-free assay of PKS was performed using the previously described procedure20,21) with some modifications. A reaction mixture (final volume, 200 µL) containing 50 mM potassium phosphate (pH 7.2), 500 µM malonyl-CoA, 450 µg/mL protein, and a tolprocarb derivative was incubated for 2 hr at 25°C. The reaction mixture was then added to 6 M HCl to facilitate the oxidation of 1,3,6,8-tetrahydroxynaphthalene to flaviolin and to terminate the reaction. After filtration using a 0.2-µm membrane, the flaviolin in the reaction mixture was analyzed by high-performance liquid chromatography (HPLC). The IC50 (concentration where the response was reduced by half) values for PKS-A were determined using a four-parameter logistic curve-fitting program (Prism Graph Pad 6.00) with an upper constraint of 100% extension and lower constraint of 0% extension.
3. Melanin biosynthesis inhibitory assay using a potato dextrose agar (PDA) plate
P. oryzae mycelial disks (6 mm in diameter), cut from the edge of precultured mycelia on a PDA, were placed on PDA plates with or without tolprocarb derivatives. After incubation for 7 days at 25°C, minimal inhibitory concentrations (MICs) of mycelial melanization on PDA plates were assayed for tolprocarb derivatives at 500, 100, 5, 1, 0.2, and 0.04 ppm. MICs for MBI-A were determined by visually observing the mycelial color change because melanin synthesized in the mycelia can be observed as dark gray, and the inhibition of melanin biosynthesis, such as the addition of tolprocarb, yields colorless mycelia.20) A distinct growth inhibition was not observed in the melanin biosynthesis inhibitory assay with all compounds studied here.
4. Pot tests to evaluate RBC-E
After soaking in water for 2 d at room temperature, approximately 30 rice seeds (Oryza sativa cv. Tsukimimochi) were sown in a 6-cm-diameter plastic pot filled with soil and grown for 10–14 days before the inoculation test. P. oryzae was grown on oatmeal agar plates to produce conidiospores. A solution of 50 mg of tolprocarb derivatives in 10 mL of acetone was then diluted with water to adjust the specified concentration. The solution was sprayed on two-leaf-stage rice seedlings at 250, 50, 12.5, 3.13, 0.781, and 0.195 ppm. Sample solution volumes were 50 mL/three pots. After air-drying, treated plants were sprayed with a spore suspension of P. oryzae adjusted to 1×105 spores/mL and then kept in a chamber with a 12-hr light/12-hr dark cycle in high humidity at 25°C for 7 days. The lesions on the 10 inoculated seedlings (one unit) were counted, and the disease index was adapted to the following index: 0, no symptoms; 1, 1–5 lesions; 2, 6–10 lesions; 3, 11–20 lesions; and 4, >20 lesions. The disease severity and control efficacy were calculated using the following formula:
 |
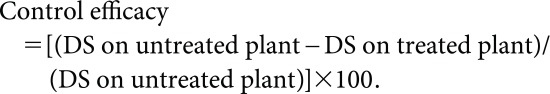 |
The activity levels of tolprocarb derivatives for RBC-E are described by index in Table 1.
Table 1. Index in pot tests.
| RBC-E index | control efficacy |
|---|---|
| 0 | <50% at 250 ppm |
| 1 | ≥50% and <95% at 250 ppm |
| 2 | ≥95% at 250 ppm and <50% at 50 ppm |
| 3 | ≥50% and <80% at 50 ppm |
| 4 | ≥80% and <95% at 50 ppm |
| 5 | ≥95% at 50 ppm and <50% at 12.5 ppm |
| 6 | ≥50% and <80% at 12.5 ppm |
| 7 | ≥80% and <95% at 12.5 ppm |
| 8 | ≥95% at 12.5 ppm and <50% at 3.13 ppm |
| 9 | ≥50% and <80% at 3.13 ppm |
| 10 | ≥80% and <95% at 3.13 ppm |
| 11 | ≥95% at 3.13 ppm and <50% at 0.781 ppm |
| 12 | ≥50% and <80% at 0.781 ppm |
| 13 | ≥80% and <95% at 0.781 ppm |
| 14 | ≥95% at 0.781 ppm and <50% at 0.195 ppm |
5. Metabolite assay
P. oryzae was cultured in PDB with a compound at 21°C with a rotary shaker (110 rpm), and the spore suspension (1×104 spores/mL) was used as the inoculum. A parent compound and its metabolite in culture were analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS). After filtration with a 0.45-µm membrane, the diluted culture was injected into the liquid chromatography column. The liquid chromatography column was connected to the mass spectrometer through a Valco valve that diverted the first 2.4 min of eluent (post-injection) to waste. The parent and metabolite were quantitated in positive-ion multiple-reaction monitoring mode. LC-MS/MS operating conditions are presented in Supplemental Table S2.
6. Computational analysis
Statistical analyses were conducted using genetic algorithms with partial least squares (GAPLS) in Chemish (ver. 5.07)22) to select the best possible variable. The values of empirical parameters affecting the performance of genetic algorithms are defined as follows: number of population is 500, selection rate is 0.3, crossover frequency is 0.05, and mutation rate is 0.01.
Results
1. Relationship between PKS-A and MBI-A
The activities and Tms of tolprocarb derivatives are listed in Tables 2–4. A linear relationship with r2=0.90 between PKS-A(pIC50) and MBI-A(pMIC) was evident when expressed in logarithmic form using all compounds listed in Tables 2–4 except for inactive compounds (Fig. 3). Furthermore, inactive compounds in the PKS assay were shown to be very weak or inactive in the melanin biosynthesis inhibitory assay, except for compound 34. From this significant correlation, we verified that PKS is the target site of tolprocarb in the melanin biosynthetic pathway. The calculated values of AlogP are also presented in Tables 2–4. AlogP showed a positive correlation with PKS-A, particularly for part A(r2=0.77) and part C (r2=0.67). These relationships indicate that the compounds containing more hydrophobic groups in part A or part C are expected to possess higher PKS-A than those listed in Tables 2–4.
Table 2. Activities and physical parameters of tolprocarb derivatives (part A).
| No. | R | PKS-A IC50(μM) | MBI-A MIC (ppm) | RBC-E Index | Tm (°C) | AlogP |
|---|---|---|---|---|---|---|
| 1 | OCH2CF3 | 3.05×10−2 | 0.2 | 8 | 132.2 | 3.51 |
| 2 | O-i-Pr | 9.75×10−2 | 1 | 5 | 132.4 | 3.30 |
| 3 | OCH3 | 1.04×10−1 | 5 | 5 | 112.0 | 2.54 |
| 4 | O-i-Bu | 4.15×10−2 | 1 | 5 | 117.6 | 3.75 |
| 5 | OCH(CH3)CF3 | 2.05×10−2 | 1 | 8 | 155.9 | 3.93 |
| 6 | OCH2Ph | 8.30×10−3 | 0.2 | 5 | 134.0 | 4.32 |
| 7 | CH3 | 1.76 | 500 | 2 | 143.9 | 1.91 |
| 8 | CF3 | 5.61×10−1 | 100 | 2 | 151.4 | 3.03 |
| 9 | CH2CF3 | 7.06×10−1 | 100 | 1 | 193.9 | 2.96 |
| 10 | CH2CH2CF3 | 1.64×10−1 | 5 | 1 | 172.0 | 3.20 |
| 11 | CH2-p-Cl-Ph | 1.82×10−2 | 0.2 | 4 | 190.5 | 4.28 |
| 12 | SCH2CH3 | 4.38×10−2 | 5 | 6 | 128.5 | 3.23 |
| 13 | SCH2CF3 | 6.18×10−2 | 5 | 4 | 122.1 | 3.86 |
| 14 | S-i-Bu | 3.02×10−2 | 1 | 6 | 109.8 | 4.10 |
| 15 | NHCH2CF3 | 5.66×10−1 | 100 | 1 | 207.7 | 2.86 |
| 16 | 4-Morpholinyl | 2.97×101 | >500 | 0 | 171.9 | 1.79 |
Fig. 3. Relationships between (a) PKS-A and MBI-A and (b) PKS-A and AlogP. (a) pIC50 [=log (1/IC50(ppm))] and pMIC [=log (1/MIC(ppm))]. For tolprocarb derivatives listed in Table 2–4, a linear fit coefficient of determination, r2 of 0.90 was obtained. (b) pIC50 [=log (1/IC50(ppm))]. AlogP was calculated using Accelrys Draw (4.1 SP1).
2. Relationship between RBC-E and either PKS-A or MBI-A
GAPLS calculations were conducted to identify the parameters that connect either PKS-A or MBI-A with RBC-E. The possible two-dimensional (2D) descriptors were calculated using MOE (2014.09). Although many 2D descriptors such as the polar surface area and molar refractivity are involved in GAPLS calculations, measured Tms were selected for the parameter connecting RBC-E (index) and either PKS-A (pIC50) or MBI-A (pMIC). The index for RBC-E was explained using the Tm and either pIC50 or pMIC as follows:
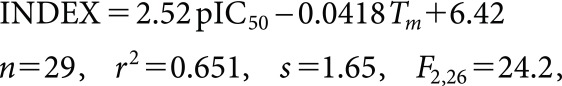 |
(1) | INDEX=2.52 pIC 50 −0.0418 T m + 6.42 n = 29 , r 2 = 0.651 , s= 1.65 , F 2,26 = 24.2 , |
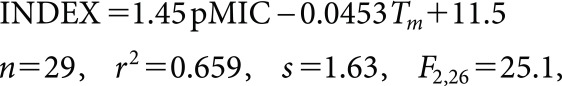 |
(2) | INDEX= 1.45 pMIC−0.0453 T m + 11.5 n = 29 , r 2 = 0.659 , s= 1.63 , F 2,26 = 25.1 , |
where pIC50=log(1/IC50 (ppm)), pMIC=log(1/MIC (ppm)), and Tm is the Celsius melting point. In Eqs. 1 and 2, n is the number of compounds, r is the correlation coefficient, s is the standard deviation, and F is the Fischer statistic.
3. Metabolic action in the P. oryzae mycelial colony
The melanin biosynthesis inhibitory assay involves the metabolic action by P. oryzae, whereas the cell-free PKS assay does not. Therefore, a comparison of PKS-A with MBI-A is expected to reveal the metabolic action in P. oryzae. A compound possessing a methyl ester group at part C (34) is beyond the linear relationship between PKS-A and MBI-A. In general, the methyl ester is metabolized to carboxylic acid. To investigate the metabolic effect by P. oryzae, the stabilities of 34 and its expected inactive metabolite (36) as well as tolprocarb (1) were evaluated with or without P. oryzae in PDB. The molar ratios calculated with the amount of the parent measured at 0 hr as 100% are listed in Table 5. As shown in Table 5, the methyl ester in 34 was completely metabolized to carboxylic acid, which corresponds to 36, but only with P. oryzae. In contrast, 1 and 36 were stable for 140 hr with P. oryzae. Therefore, the extreme deviation of 34 from the linear relationship appears to be accounted for by its fast metabolism.
Table 5. Biotransformation of 1, 34, and 36 by P. oryzae (molar ratio).
| treatment | detection | incubation time (h) | ||
|---|---|---|---|---|
| 0 | 42 | 140 | ||
| 1 | 1 | 100% | 105% | 96% |
| 34 | 34 | 100% | 63% | 0% |
| 36 | 1% | 31% | 104% | |
| 36 | 34 | 0% | 0% | 0% |
| 36 | 100% | 95% | 96% | |
| 1a) | 1 | 100% | NTb) | 98% |
| 34a) | 34 | 100% | NTb) | 97% |
a) P. oryzae mycelia was not added. b) Not tested.
4. Relationship between structure and PKS-A
4.1. Part A
The activities of the compounds modified at part A are listed in Tables 2 and 3. As shown in Table 2 and Fig. 3(b), hydrophobic groups such as the benzyl group (6 and 11) exhibited high PKS-A. The compounds possessing a trifluoroethyl group in part A (1, 26, and 28) showed higher PKS-A than the compounds possessing an isopropyl group (2, 27, and 29). By comparing the two pairs (7 versus 3 and 9 versus 1), we observed that the carbamate bond provided a higher PKS-A than the amide bond. The introduction of the urea bond decreased PKS-A as compared with 15 with 1. The thiocarbamate bond was found to provide the same level of PKS-A as the carbamate bond when the two pairs were compared (14 versus 4 and 13 versus 1).
4.2. Part B
The activities of the compounds modified at part B are listed in Table 3. The hydrogen atoms (17) or dimethyl groups (18) at R1 and R2 reduced PKS-A. The enantiomers possessed very different activities; the S-enantiomer (1) exhibited one of the highest PKS-A, whereas the R-enantiomer (19) exhibited no PKS-A. Bulky substituents such as cyclohexane (24) and the tert-butyl group (22) at R1 maintained a high PKS-A, suggesting that the size of steric tolerance is considerably large around the R1 substituent; however, bulky substituents in part B did not enhance the activities. The hydrophilic methyl ester (25) also maintained a high PKS-A. The substituent possessing a β-branch (1) at R1 showed higher activity than the linear (21) or γ-branched substituents (23).
Table 3. Activities and physical parameters of tolprocarb derivatives (part B).
| No. | A | R1 | R2 | PKS-A IC50(μM) | MBI-A MIC (ppm) | RBC-E Index | Tm (°C) | AlogP |
|---|---|---|---|---|---|---|---|---|
| 1 | CH2CF3 | i-Pr | H | 3.05×10−2 | 0.2 | 8 | 132.2 | 3.51 |
| 2 | i-Pr | i-Pr | H | 9.75×10−2 | 1 | 5 | 132.4 | 3.30 |
| 17 | CH2CF3 | H | H | 2.01×10−1 | 5 | 2 | 169.3 | 2.23 |
| 18 | i-Pr | CH3 | CH3 | 1.39 | 500 | 2 | 110.0 | 2.50 |
| 19 | CH2CF3 | H | i-Pr | >100 | 500 | 2 | 129.0 | 3.51 |
| 20 | i-Pr | H | i-Pr | >100 | >500 | 1 | 159.7 | 3.30 |
| 21 | CH2CF3 | n-Pr | H | 6.93×10−2 | 1 | 3 | 170.8 | 3.51 |
| 22 | CH2CF3 | t-Bu | H | 2.82×10−2 | 0.2 | 12 | 101.0 | 4.02 |
| 23 | CH2CF3 | i-Bu | H | 4.68×10−1 | 100 | 1 | 147.5 | 3.84 |
| 24 | CH2CF3 | c-Hex | H | 4.32×10−2 | 1 | 3 | 155.0 | 4.20 |
| 25 | CH2CF3 | COOCH3 | H | 2.87×10−2 | 1 | 3 | 139.3 | 2.12 |
| 26 | CH2CF3 | CH2SCH3 | H | 3.03×10−1 | 5 | 2 | 153.0 | 2.58 |
| 27 | i-Pr | CH2SCH3 | H | 1.65 | 500 | 2 | 162.2 | 2.36 |
| 28 | CH2CF3 | CH2SOCH3 | H | 1.04×101 | >500 | 0 | 153.7 | 1.44 |
| 29 | i-Pr | CH2SOCH3 | H | >100 | >500 | 0 | 192.7 | 1.22 |
4.3. Part C
The activities of the compounds modified at part C are listed in Table 4. The hydrophobic substituents (methyl, 1; ethyl, 31; chloro, 32; and trifluoromethyl, 33) gave higher PKS-A than the hydrophilic substituents (methyl sulfonyl, 35 and carboxy, 36). In contrast, hydrophilic methyl ester (34) exhibited one of the highest PKS-A values, suggesting that nonhydrophobic interaction also contributes to the PKS-A in part C. The electron-donating group (methyl, 1) showed higher PKS-A than the electron-withdrawing group (trifluoromethyl, 33).
Table 4. Activities and physical parameters of tolprocarb derivatives (part C).
| No. | R | PKS-A IC50(μM) | MBI-A MIC (ppm) | RBC-E Index | Tm (°C) | AlogP |
|---|---|---|---|---|---|---|
| 1 | CH3 | 3.05×10−2 | 0.2 | 8 | 132.2 | 3.51 |
| 30 | H | 1.66×10−1 | 5 | 3 | 124.0 | 3.05 |
| 31 | CH2CH3 | 1.67×10−2 | 0.2 | 10 | 117.9 | 3.91 |
| 32 | Cl | 3.23×10−2 | 1 | 4 | 153.9 | 3.57 |
| 33 | CF3 | 9.82×10−2 | 2.5 | 3 | 154.8 | 3.93 |
| 34 | COOCH3 | 1.11×10−2 | >500 | 2 | 160.8 | 2.78 |
| 35 | SO2CH3 | 4.16×10−1 | 100 | 1 | 166.3 | 2.19 |
| 36 | COOH | >100 | >500 | 0 | 225.0 | 2.75 |
Discussion
P. oryzae causes rice blast, one of the major diseases in rice. During the infection process, the accumulation of melanin is essential for the penetration of P. oryzae into host plants.1–6) MBIs are widely used as practical fungicides for P. oryzae. However, the evolution of resistance to conventional MBIs that inhibit dehydratase has become a problem for the control of rice blast.16) Tolprocarb developed by Mitsui Chemicals Agro, Inc., showed a high controlling effect on rice blast by both nursery-box application and broadcasting on paddy rice.19) In our previous paper,20) we demonstrated that tolprocarb inhibited PKS activity with an IC50=0.03 µM. In the present study, a series of tolprocarb derivatives were evaluated for PKS-A, MBI-A, and RBC-E. A linear relationship with r2=0.90 between PKS-A and MBI-A was evident. This relationship further confirms that the inhibition of PKS by tolprocarb and its derivative leads directly to the inhibition of melanin biosynthesis in P. oryzae.
RBC-E showed a positive correlation with PKS-A (r2=0.51), further validating our conclusion that PKS is the target site of tolprocarb. The index in RBC-E was explained by the Tm and either PKS-A or MBI-A. The Tm has numerous applications due to its relationship with solubility, where the Tm term accounts for the effects of solute crystallinity.23–25) MBIs, including tolprocarb, do not directly interfere with the viability of P. oryzae but rather inhibit penetration of the pathogen into the host plant. Therefore, P. oryzae could penetrate the host where MBIs do not exist on the leaves, and a uniform coating on the leaves might be important for protecting the host from infection by P. oryzae. We speculate that readily crystalized compounds possessing a high Tm may impede the formation of a uniform coat of MBIs on the surface of leaves, thereby resulting in weak efficacy in the pot tests. Further experiments, such as the addition of a spreading agent or auxiliary agent to prevent MBIs from crystallizing, will be necessary to validate our speculation.
One of the tolprocarb derivatives possessing a methyl ester group at part C (34) is beyond the linear relationship between PKS-A and MBI-A, that is, 34 showed a high PKS-A and no MBI-A, and its methyl ester was completely metabolized to carboxylic acid in the P. oryzae mycelia. The corresponding metabolite (36) lost PKS-A and MBI-A. Thus, the loss of MBI-A with 34 appears to be due to the detoxification of methyl ester to carboxylic acid by P. oryzae. By contrast, high levels of the compound possessing the methyl ester group in part B (25) were found in both PKS-A and MBI-A, indicating that the metabolic rate of methyl ester in part B is slow or that its expected metabolite, carboxylic acid in part B, also exhibits high PKS-A and MBI-A. Moreover, the strong correlation between PKS-A and MBI-A suggested that most of the tolprocarb derivatives examined in the present study were not significantly metabolized in P. oryzae mycelia. These comparisons between PKS-A and MBI-A indicate that fungus metabolism is an important factor for designing novel fungicides.
In vivo transport is generally explained by logP.26) The melanin biosynthesis inhibitory assay, which used the whole cell, is expected to be influenced by in vivo transport, whereas the cell-free PKS assay may not be influenced by in vivo transport. Thus, the linear relationship between hydrophobicity (AlogP) and PKS-A observed in the present study may not be derived from the in vivo transport effect but rather from the activity in the target site. Furthermore, the strong correlation between PKS-A and MBI-A indicates that MBI-A was not influenced by in vivo transport. An alternative explanation for the positive correlation between AlogP and PKS-A is the partition of compounds between membrane and solution in the PKS-assay system, because the regression coefficient was nearly one unit (a regression equation of the compounds listed in Table 2: pIC50=1.03 AlogP −2.01), which is expected when partitioning is the controlling factor.27)
In conclusion, the target site of tolprocarb was verified to be PKS in P. oryzae based on the strong correlation between PKS-A and MBI-A. Comparisons of the target site and in vitro and in vivo activities revealed the effects of metabolism and physical parameters, which will be valuable for designing novel fungicides. Furthermore, the structure–activity relationships and activity–activity relationships will provide insight and information regarding the structural requirements to develop novel PKS inhibitors.
Acknowledgements
We acknowledge M. Endo and M. Kawashima at Mitsui Chemicals Agro, Inc. for their assistance in PKS assay and LC-MS/MS analysis.
Electronic supplementary material
The online version of this article contains supplementary materials (Supplemental Tables S1 and S2), which is available at http://www.jstage.jst.go.jp/browse/jpestics/.
supplementary materials
References
- 1).J. Liu, X. Wang, T. Mitchell, Y. Hu, X. Liu, L. Dai and G.-L. Wang: Mol. Plant Pathol. 11, 419–427 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).A. A. Bell and M. H. Wheeler: Annu. Rev. Phytopathol. 24, 411–451 (1986). [Google Scholar]
- 3).T. Okuno, K. Matsuura and I. Furusawa: J. Pestic. Sci. 8, 357–360 (1983). [Google Scholar]
- 4).C. P. Woloshuk and H. D. Sisler: J. Pestic. Sci. 7, 161–166 (1982). [Google Scholar]
- 5).I. Yamaguchi, S. Sekido and T. Misato: J. Pestic. Sci. 7, 523–529 (1982). [Google Scholar]
- 6).Y. Kubo, K. Suzuki, I. Furusawa and M. Yamamoto: Pestic. Biochem. Physiol. 23, 47–55 (1985). [Google Scholar]
- 7).K. Suzuki, Y. Kubo, I. Furusawa, N. Ishida and M. Yamamoto: Can. J. Microbiol. 28, 1210–1213 (1982). [Google Scholar]
- 8).F. G. Chumley and B. Valent: Mol. Plant Microbe Interact. 3, 135–143 (1990). [Google Scholar]
- 9).M. H. Wheeler: Exp. Mycol. 6, 171–179 (1982). [Google Scholar]
- 10).R. D. Stipanovic and A. A. Bell: J. Org. Chem. 41, 2468–2469 (1976). [DOI] [PubMed] [Google Scholar]
- 11).C. P. Woloshuk, P. M. Wolkow and H. D. Sisler: Pestic. Sci. 12, 86–90 (1981). [Google Scholar]
- 12).S. Inoue, K. Maeda, T. Uematsu and T. Kato: J. Pestic. Sci. 9, 731–736 (1984). [Google Scholar]
- 13).Y. Kurahashi, S. Sakawa, T. Kinbara, K. Tanaka and S. Kagabu: J. Pestic. Sci. 22, 108–112 (1997). [Google Scholar]
- 14).A. Manabe, K. Maeda, M. Enomoto, H. Takano, T. Katoh, Y. Yamada and Y. Oguri: J. Pestic. Sci. 27, 257–266 (2002). [Google Scholar]
- 15).A. Nishimura and I. Hino: Agrochem. Jpn. 81, 13–15 (2002). [Google Scholar]
- 16).F. Suzuki, J. Yamaguchi, A. Koba, T. Nakajima and M. Arai: Plant Dis. 94, 329–334 (2010). [DOI] [PubMed] [Google Scholar]
- 17).Y. Chiba, H. Daido, T. Akase, H. Matsuno and J. Kishi (Mitsui Chemicals, Inc.): Jpn. Kokai Tokkyo Koho JP2002-07319 (2002).
- 18).K. Ebihara, K. Morizane, N. Tomura, R. Ezaki, M. Yoshida and Y. Osada (Mitsui Chemicals, Inc.): Jpn. Kokai Tokkyo Koho JP2004-015471 (2004).
- 19).T. Akase, N. Araki, R. Ezaki, N. Tomura, K. Morizane and K. Ebihara: Abstr. 40th Annu. Meeting Pestic. Sci. Soc. Jpn., B303, 2015. (in Japanese).
- 20).T. Hamada, M. Asanagi, T. Satozawa, N. Araki, S. Banba, N. Higashimura, T. Akase and K. Hirase: J. Pestic. Sci. 39, 152–158 (2014). [Google Scholar]
- 21).I. Fujii, Y. Mori, A. Watanabe, Y. Kubo, G. Tsuji and Y. Ebizuka: Biochemistry 39, 8853–8858 (2000). [DOI] [PubMed] [Google Scholar]
- 22).T. Tanada, M. Arakawa, R. Nishimura and K. Funatsu: J. Comput. Aided Chem. 1, 35–46 (2000), (in Japanese). [Google Scholar]
- 23).S. H. Yalkowsky and S. C. Valvani: J. Pharm. Sci. 69, 912–922 (1980). [DOI] [PubMed] [Google Scholar]
- 24).J. C. Dearden: Sci. Total Environ. 109-110, 59–68 (1991). [DOI] [PubMed] [Google Scholar]
- 25).L. E. Matheson and Y. Chen: Int. J. Pharm. 125, 297–307 (1995). [Google Scholar]
- 26).J. C. Dearden: Environ. Health Perspect. 61, 203–228 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).J. K. Seydel: “Quantitative Approaches to Drug Design” ed. by J. C. Dearden, Elsevier, Amsterdam, pp. 163–181, 1983.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Fig. 3. Relationships between (a) PKS-A and MBI-A and (b) PKS-A and AlogP. (a) pIC50 [=log (1/IC50(ppm))] and pMIC [=log (1/MIC(ppm))]. For tolprocarb derivatives listed in Table 2–4, a linear fit coefficient of determination, r2 of 0.90 was obtained. (b) pIC50 [=log (1/IC50(ppm))]. AlogP was calculated using Accelrys Draw (4.1 SP1).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/5b85/6140631/6547e49a2459/jps-42-2-D16-100-figure03.jpg)