Abstract
This study aimed to improve the efficacy of azadirachtin (Azadirachta indica. A. Juss) against two serious pest species of stored products, Sitophilus oryzae (L.) and Tribolium castaneum (Herbst), through nano-emulsion formulations. Pseudoternary phase diagrams were constructed consisting of an emulsion system of an active ingredient (neem oil), surfactant (polysorbate or alkylpolyglucoside), and water. Isotropic regions were formed in the pseudoternary phase diagrams, and four formulations were selected from the isotropic regions and characterized according to particle size, particle aging, zeta potential, stability and thermostability, surface tension, viscosity, and pH. The selected formulations showed particle sizes of 208–507 nm in diameter. The result of contact toxicity demonstrated excellent mortality of S. oryzae and T. castaneum adults, with a mortality range of 85–100% and 74–100%, respectively, at a 1% azadirachtin concentration after only 2 days of exposure. Compared to non-formulated neem oil, the nano-emulsion formulations significantly increased the mortality of the tested species.
Keywords: nano-emulsion formulation, neem oil, nonionic surfactant, Tribolium castaneum, Sitophilus oryzae, azadirachtin
Introduction
Stored products are in serious danger of infestation and weight loss due to insect pests, particularly in developing countries. Insect infestations could lead to warm and moist spots that create conditions suitable for fungal growth, leading to further grain loss.1) Among the 600 species of reported beetle pests, the rice weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae), and the red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae), are the most destructive of stored products in different parts of the world, and they cause both qualitative and quantitative damage to various types of grains.2)
Along with the occurrence of plant diseases and pests, chemical control has been considered as one of the most important strategies in the control and reduction of damage and plant loss in agricultural production worldwide. Biopesticides employed against various insect pests from naturally based products such as powders, extracts, and essential oils have been proven to be non-hazardous, have low toxicity, have low residue, and be eco-friendly to humans and the environment.3,4)
One of the most effective plants against insect pests is neem (Azadirachta indica. A. Juss) (Sapindales: Meliaceae). The main component of neem is a tetranotriterpenoid named azadirachtin that is known for its variety of properties such as antifeedancy, repellency, and insect growth inhibition effects. Although neem and other botanical insecticides have exhibited a wide range of promising properties, such as toxicity and biological activities against insect pests, the problems associated with botanical insecticides, such as volatility, poor water solubility, and oxidation tendency, must be resolved when they are prepared to be used in a pest control system.5) In addition, most studies utilize a relatively high concentration of azadirachtin to achieve satisfactory control.6)
A nano-emulsion formulation system is a recent favorable method to improve botanical insecticide characteristics and effectiveness for commercial use.7) Nano-emulsion formulations have good storage stability under a broad range of temperatures (−10 to 55°C). The production cost of nano-emulsion formulations is lower than that of micro-emulsion formulations of pesticides due to high water solubility and their capacity to solubilize hydrophilic and lipophilic compounds, leading to the use of less inert and active ingredients. In a nano-emulsion formulation system, the biological performance of the pesticides is improved by using adjuvants and surfactants.8) Formulation development through the construction of a pseudoternary phase diagram is based on the miscibility of the surfactant, water and oil, and points selected in the isotropic region. Formulations that are stable and transparent are then physically characterized.9)
In the present study, non-ionic naturally based polysorbate (PolyS) and alkylpolyglucoside (APG) surfactants were used as inert ingredients due to their low toxicity, biodegradability, and non-hazardous impacts on humans and the environment.10) The objectives of the study were as follows: 1) Formulate and characterize neem oil nano-emulsion formulations and increase the stability of the formulation with the help of non-ionic surfactants. The present work focused on the nano-emulsion preparation of neem oil using a low-energy centrifugation method to achieve the benefit of less energy consumption, ease of use, and low-cost production. The formulations were selected from pseudoternary phase diagram plots, and the physicochemical behavior of the emulsions was determined for stability, thermostability, particle size, zeta potential, surface tension, viscosity, and pH. 2) Evaluate the insecticidal potency of the neem oil nano-emulsion formulations against adults of S. oryzae and T. castaneum.
Materials and Methods
1. Chemicals
The non-ionic alkylpolyglucoside surfactant (Agnique® MBL 510H) was provided by Cognis Oleochemicals (M) Sdn Bhd (Selangor, Malaysia), and the non-ionic polysorbate surfactant (Tween80) was supplied by Duchefa Biochemie B.V. (Haarlem, Netherlands). Neem oil (3% azadirachtin (a.i.)) was provided by VM Consolidated (M) Sdn Bhd (Seri Kembangan, Malaysia), and the commercial EC formulation, Neemix® (4.5% azadirachtin (a.i.)), was from Zeenex AgroScience (M) Sdn Bhd (Kuala Lumpur, Malaysia).
2. Construction of pseudoternary phase diagrams
Neem oil (azadirachtin) and a non-ionic surfactant (polysorbate or alkylpolyglucoside) were mixed in ratios (w/w) of 10 : 0, 9 : 1, 8 : 2, 7 : 3, 6 : 4, 5 : 5, 4 : 6, 3 : 7, 2 : 8, 1 : 9, and 0 : 10. The prepared compositions were left for a minute to obtain equilibrium. Afterward, water (5% (w/w)) was added by titrating the mixtures of neem oil and surfactant until a 95% water content was achieved in the emulsion system. An analytical balance (Mettler Toledo Model Dragon 204, Spain) was used to weigh each of the components. All of the components were sealed and homogenized by a vortex mixer (Model VTX-3000L, Japan). Afterward, the components were centrifuged at 3500 rpm at 25°C for 30 min.11,12) The samples were visually investigated to determine their phase transition on the basis of clarity, stability, and transparency. The phase domains of the components were determined to be isotropic (transparent and one-phase) or anisotropic (cloudy or two-phase). The phase diagrams were constructed using a Chemix software, version 3.5, phase diagram plotter (UK).
3. Selection of formulations
Four formulations were selected from the phase diagram plots with a priority of being optically isotropic, clear, one-phase, and physically stable at ambient temperature (25°C).12)
4. Characterization of the selected formulations
4.1. Stability and thermostability test
The chosen formulations were prepared and kept at room temperature (25°C) for 90 days and at 54°C for 14 days, which the Food and Agricultural Organization (FAO)–approved standard evaluation for agrochemical products to show the stability of formulations in a tropical climate.13) The test was repeated for two cycles, and the physical appearance of the formulations was visually investigated.
4.2. Particle-size distribution and zeta potential analysis
A Nanophox particle-size analyzer (Clausthal-Zellerfeld, Germany) outfitted with a photon cross-correlation spectrometer (PCCS) was used to determine the particle size and zeta potential of the selected formulations. The formulations were diluted and dispersed into deionized water at a ratio of 1 : 250 (w/w) for the measurement. The particle size and zeta potential growing rates of the formulations were measured at the 1st, 30th, 60th, and 90th days at a temperature of 25°C. The reading was repeated three times for each formulation.
4.3. Surface tension
The interaction of the platinum ring with the surface of the formulations was utilized according to the du Noüy ring method, using a Krüss K6 tension meter (Krüss, UK), to determine the surface tension. Deionized water was used for calibration, with a surface tension of 72–73 mNm−1. The reading was repeated three times for each formulation.
4.4. Viscosity and pH
The viscosity of formulations 1 day old was determined by using a viscometer (Model: RheolabQC; Anton Paar, Austria) at room temperature (25°C) (5-sec–1 shear rate). Each sample was read after equilibrium of the sample at the end of 2 min. The reading was repeated three times.
The pH of the formulations (1 day old) was evaluated using a pH meter (Model: pH-Meter GLP 21; Crison Instruments, Spain) at room temperature (25°C). The device was calibrated at pH 7.0 and pH 4.0. The reading was repeated three times for each formulation.
5. Insects
Colonies of S. oryzae and T. castaneum were obtained from the entomology laboratory stock culture of Universiti Putra Malaysia (UPM). Rice kernels and wheat germ were stored at −15°C for 2 weeks to annihilate any previous contamination and then used to rear S. oryzae and T. castaneum, respectively, at 27±1°C, 75±1% R.H., and a 12 : 12 hr light : dark cycle. Adults 7–14 days old of S. oryzae and T. castaneum were used for the experiments.2)
6. Contact toxicity test
A filter-paper impregnation method was used to evaluate the contact toxicity of the formulations against adults of S. oryzae and T. castaneum as described by Hameed et al.6). Neem oil and a commercial EC formulation of Neemix® were used as a control. The formulations and controls were diluted into 0.5, 0.6, 0.75, and 1% of the azadirachtin concentration using deionized water as a solvent. One (1) mililiter of each formulation was applied to a filter paper 5 cm in diameter, and after evaporation of the solvent, 20 adults of S. oryzae or T. castaneum (7–14 days old) were placed into each petri dish and replicated five times under laboratory conditions (27±1°C, 75±1% R.H., with a 12 : 12 hr light : dark cycle). Mortality was recorded after 1 and 2 days of application. Adults with no response to firm prodding with a fine camel-hair brush were considered dead.
7. Data analysis
Values were expressed as the mean±standard error (SE). Prior to each analysis, the mortality was tested for normal distribution using Bartlett’s test and transformed using a Box–Cox transformation if necessary.14) Since the same petri dishes were examined for mortality after 1 and 2 day of exposure, the data from mortality were analyzed using a repeated measure Analysis of Variance (ANOVA) with exposure as the repeated variable and formulation with concentration as the main effects using the statistical software SAS 9.2 (USA). Means were separated by using the Tukey HSD test at the 5% level.
Results
1. Ternary phase diagram
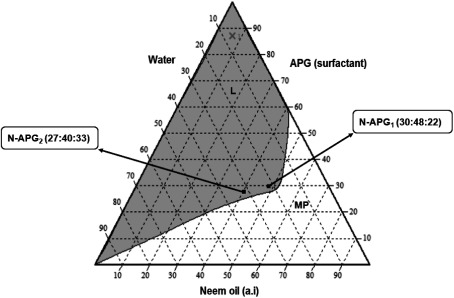
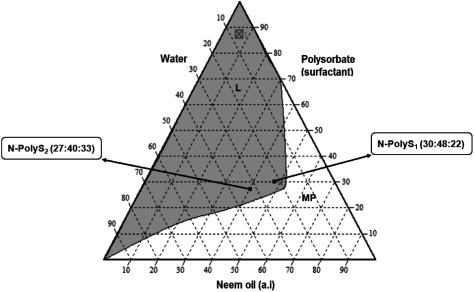
The ternary phase diagrams of the three-component system of water, surfactant, and neem oil are shown in Fig. 1 (neem oil/non-ionic alkylpolyglucoside surfactant/water) and Fig. 2 (neem oil/non-ionic polysorbate surfactant/water). A continuous one-phase region was observed in each phase diagram plot. The grey area (L) in each plot indicates the single-phase and transparent region (isotropic), while the white area (MP) refers to the multiphase and cloudy region (anisotropic).
Fig. 1. Pseudoternary phase diagram of the APG surfactant (Agnique® MBL 510H/neem oil/water) system, showing the selected nano-emulsion formulations with the ratios of N-APG1 (30 : 48 : 22) and N-APG2 (27 : 40 : 33). L=Isotropic. MP=Multiphase.
Fig. 2. Pseudoternary phase diagram of the polysorbate surfactant (Tween80/neem oil/water) system, showing the selected nano-emulsion formulations with the ratios of N-PolyS1 (30 : 48 : 22) and N-PolyS2 (27 : 40 : 33). L=Isotropic. MP=Multiphase.
Two points were selected form the isotropic region of each phase diagram plot according to the criteria of an adequate amount of water, surfactant, and neem oil in order to formulate oil nano-emulsions. The selected points were coded as N-APG1, N-APG2, N-PolyS1, and N-PolyS2, and they were to have equal proportions of neem oil (N), surfactant (APG or PolyS), and water (Table 1).
Table 1. Percentage (w/w) compositions of surfactants, neem oil and water containing in the nano-emulsion formulations.
| Formulation (w/w) | Neem oil (a.i)a) | Agnique® MBL 510Hb) | Tween 80c) | Deionized Waterd) |
|---|---|---|---|---|
| N-APG1 | 48% | 30% | — | 22% |
| N-APG2 | 40% | 27% | — | 33% |
| N-PolyS1 | 48% | — | 30% | 22% |
| N-PolyS2 | 40% | — | 27% | 33% |
a) Active ingredient. b) Non-ionic alkylpolyglucoside surfactant. c) Non-ionic polysorbate surfactant. d) Solvent.
2. Characterization of the nano-emulsion formulations
2.1. Stability and thermostability analysis
The formulations were tested for stability (at 25°C for 90 days) and thermostability (at 54°C for 14 days). All of the formulations were stable at 25°C with no phase separation, creaming and sedimentation and accelerated stability evaluation for 90 days. However, separation was observed for N-APG2 at 54°C after 14 days of exposure.
2.2. Particle-size analysis
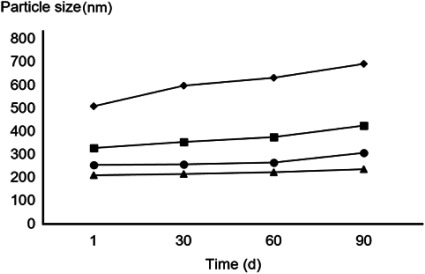
The selected formulations were measured for particle size 1 hr after preparation (Fig. 3). The smallest particle sizes were detected in formulations comprised of a polysorbate surfactant, ranging from 208±1.2 (N-PolyS1) to 253±1.6 (N-PolyS2) nm in diameter, with a significant difference (p<0.05). N-APG1 and N-APG2, composed of an alkylpolyglucoside surfactant, showed the biggest particle sizes, 328±2.3 and 507±2.7 nm, respectively, with a significant difference (p<0.05). The commercial product (Neemix®) is the conventional emulsifiable concentrated formulation of an insecticide that, when mixed with water, forms a spontaneous milky emulsion with dispersed phase droplets in a size range of 1 to 25 µm.
Fig. 3. Effect of time on the mean particle size distribution of nano-emulsions in the system (surfactant/neem oil/water) over a 90 days time period of the nano-emulsion formulations at 25°C. ■: N-APG1=APG surfactant (Agnique® MBL 510H/neem oil/water, 30 : 48 : 22). ◆: N-APG2=APG surfactant (Agnique® MBL 510H/neem oil/water, 27 : 40 : 33). ▲: N-PolyS1=Polysorbate surfactant (Tween80/neem oil/water, 30 : 48 : 22). ●: N-PolyS2=Polysorbate surfactant (Tween80/neem oil/water, 27 : 40 : 33).
The long-term physical stability of the selected formulations was evaluated via analysis of particle aging (Fig. 3). Each of the formulations demonstrated growth in particle size with no significant difference (p<0.05) within 90 days. N-PolyS1 and N-PolyS2, which comprised the polysorbate surfactant, showed the least particle size growth rate. Conversely, N-APG1 and N-APG2, with an alkylpolyglucoside surfactant, showed greater particle aging rates.
2.3. Zeta potential analysis
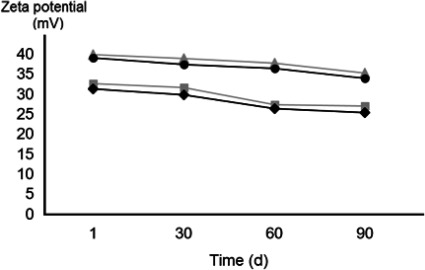
The zeta potential of the prepared formulations ranged from 31.27 to 39.12 mV, indicating the stability of the prepared nano-emulsion formulations, 1 hr after preparation (Fig. 4). The zeta potential of N-PolyS1 and N-PolyS2 with a polysorbate surfactant showed higher stability than N-APG1 and N-APG2 with an alkylpolyglucoside surfactant. The zeta potential of all of the formulations decreased within 90 days, with no significant difference (p<0.05).
Fig. 4. Effect of time on the mean zeta potential of nano-emulsions in the system (surfactant/neem oil/water) over a 90 day time period of the nano-emulsion formulations at 25°C. □: N-APG1=APG surfactant (Agnique® MBL 510H/neem oil/water, 30 : 48 : 22). ◆: N-APG2=APG surfactant (Agnique® MBL 510H/neem oil/water, 27 : 40 : 33). △: N-PolyS1=Polysorbate surfactant (Tween80/neem oil/water, 30 : 48 : 22). ●: N-PolyS2=Polysorbate surfactant (Tween80/neem oil/water, 27 : 40 : 33).
2.4. Surface tension
The surface tensions of all selected formulations were significantly lower than that of the control (water), with a surface tension of 72 mNm−1 at 25°C (p<0.05) (Table 2). N-APG2 showed the highest surface tension, 33.33 mNm−1. On the other hand, N-PolyS2 and N-APG1 both gave readings of 31 mNm−1. The best formulation with the significantly lowest surface tension was observed in N-PolyS1 (30.62 mNm−1).
Table 2. The physicochemical characteristics of the nano-emulsion formulations for stability, thermostability, surface tension, viscosity and pHa).
| Formulationb) | Thermostability c) | Surface Tension (mNm−1)±SE | Viscosity (Pa·s)±SE | pH±SE |
|---|---|---|---|---|
| N-APG1 | √ | 31.74 (±0.43)b | 88.16 (±0.27)a | 3.88 (±0.21)b |
| N-APG2 | × | 33.33 (±0.41)a | 69.03 (±0.61)c | 3.63 (±0.53)b |
| N-PolyS1 | √ | 30.62 (±0.38)c | 81.86 (±0.53)b | 5.11 (±0.39)a |
| N-PolyS2 | √ | 31.05 (±0.52)b | 65.16 (±0.42)d | 4.93 (±0.32)a |
a) Stable at 25°C for 90 days. Data are shown as mean values±standard error (SE) in parentheses. Within the columns, mean values followed by the same letters (a–d) indicate that they are not significantly different at p<0.05; (n=3). b) N-APG1=APG surfactant (Agnique® MBL 510H/neem oil/water, 30 : 48 : 22); N-APG2=APG surfactant (Agnique® MBL 510H/neem oil/water, 27 : 40 : 33); N-PolyS1=Polysorbate surfactant (Tween80/neem oil/water, 30 : 48 : 22); N-PolyS2=Polysorbate surfactant (Tween80/neem oil/water, 27 : 40 : 33). c) Thermostability at 54°C for 14 days. √, stable; ×, not stable.
2.5. Viscosity and pH
The study showed that the formulations became more viscous with increase of neem oil content (Table 2). As the neem oil content increased from 40 to 48% (w/w), an increase in the viscosity of the formulations was observed. N-PolyS2 with 40% neem oil showed the least viscosity, 65.16 (Pa·s).
The nano-emulsion formulations demonstrated a pH range of 3.63–5.11 (Table 2). N-APG1 and N-APG2 were less acidic than their surfactant (Agnique® MBL 510H: 2.51), but the incorporation of neem oil with a pH value of 4.88 explains the positive charge of N-APG1 and N-APG2. N-PolyS1 and N-PolyS2 are more acidic than their surfactant (Tween80: 8.12). On the other hand, in the formulations comprised of a similar surfactant, the pH of the formulations increased as the percentage of surfactant increased. This is in agreement with data reported by Mortti et al.5)
3. Bioassay study
The insecticidal activity of the nano-emulsion formulations of neem oil was studied against S. oryzae and T. castaneum adults. The mortality rates for adults 7–14 days old that were treated with various concentrations of azadirachtin (0.5, 0.6, 0.75, and 1%) after 1 and 2 day of exposure are shown in Figs. 5 and 6.
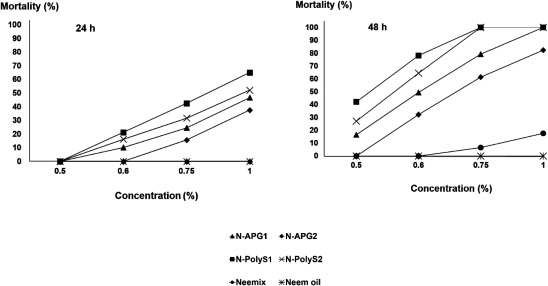
Fig. 5. Contact toxicity of different concentrations of nano-emulsion formulations, Neemix® (EC formulation), and neem oil (crude extract) on the mortality of Sitophilus oryzae adults after 1 and 2 days of exposure. Bioassays were conducted at 27±1°C, 75±1% R.H., and a 12 : 12 hr light : dark cycle. ▲: N-APG1=APG surfactant (Agnique® MBL 510H/neem oil/water, 30 : 48 : 22). ◆: N-APG2=APG surfactant (Agnique® MBL 510H/neem oil/water, 27 : 40 : 33). ■: N-PolyS1=Polysorbate surfactant (Tween80/neem oil/water, 30 : 48 : 22). ×: N-PolyS2=Polysorbate surfactant (Tween80/neem oil/water, 27 : 40 : 33). ●: Neemix® (control). *: neem oil (control).
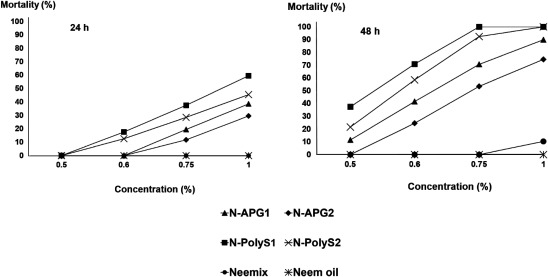
Fig. 6. Contact toxicity of different concentrations of nano-emulsion formulations, Neemix® (EC formulation), and neem oil (crude extract) on the mortality of Tribolium castaneum adults after 1 and 2 days of exposure. Bioassays were conducted at 27±1°C, 75±1% R.H., and a 12 : 12 hr light : dark cycle. ▲: N-APG1=APG surfactant (Agnique® MBL 510H/neem oil/water, 30 : 48 : 22). ◆: N-APG2=APG surfactant (Agnique® MBL 510H/neem oil/water, 27 : 40 : 33). ■: N-PolyS1=Polysorbate surfactant (Tween80/neem oil/water, 30 : 48 : 22). ×: N-PolyS2=Polysorbate surfactant (Tween80/neem oil/water, 27 : 40 : 33). ●: Neemix® (control). *: neem oil (control).
The mortality of S. oryzae adults was significantly affected by the main effects and associated interactions of formulation, concentration, and exposure interval (Table 3). The mortality of S. oryzae 1 day after exposure ranged from 0 to 65%, and all of the formulations showed significant differences within the same concentration (Fig. 5). The mortality rate of S. oryzae abruptly increased after 2 days of exposure, while all of the exposed S. oryzae individuals were dead on the filter papers treated with the highest concentration (1%) of nano-formulations, with the exception of N-APG2 with 85% mortality. However, the same trends resulted in significantly lower mortality of 17.5 with Neemix® and 0% with neem oil (Fig. 5).
Table 3. Repeated measure ANOVA parameters for main effects and associated interactions for mortality of Sitophilus oryzae and Tribolium castaneum adults.
| Source | dfa) | S. oryzae | T. castaneum | ||
|---|---|---|---|---|---|
| F | P | F | P | ||
| Exposure time | 2 | 6.88 | 0.008 | 6.08 | 0.011 |
| Formulation | 5 | 13.01 | 0.000 | 11.12 | 0.001 |
| Concentration | 4 | 15.64 | 0.001 | 14.34 | 0.001 |
| Exposure time×formulation | 10 | 7.04 | 0.000 | 8.07 | 0.000 |
| Exposure time×concentration | 8 | 8.68 | 0.000 | 8.96 | 0.000 |
| Formulation×concentration | 20 | 10.33 | 0.000 | 11.03 | 0.000 |
| Exposure time×formulation×concentration | 40 | 19.43 | 0.000 | 18.17 | 0.000 |
a) Error df=360.
The mortality of T. castaneum was also significantly affected by the main effects and associated interactions of formulation, concentration, and exposure interval (Table 3). The mortality levels of T. castaneum exposed to nano-emulsion formulations of N-PolyS1 and N-PolyS2 reached 100% 2 day after application at a 1% azadirachtin concentration. However, under the same condition, the mortality of T. castaneum adults did not exceed 11% for Neemix® and neem oil (Fig. 6).
Generally, the overall mortality was higher for S. oryzae than for T. castaneum. There was no mortality for any of the nano-emulsion formulations and controls against both insect species at a 0.5% concentration after 1 day of treatment. Remarkably, a 1% concentration of most nano-emulsion formulations showed 100% mortality after 2 days of exposure against S. oryzae and T. castaneum. However, N-APG2 with particle size of 507 nm showed a lower mortality of 85 and 74% against S. oryzae and T. castaneum, respectively. Overall, the toxicity effects were significantly more pronounced for the nano-emulsion formulations compared with the crude extract of neem oil and Neemix® under all conditions (Figs. 5 and 6).
Discussion
Phase behavior provides a crucial suggestion of macroscopic behavior, as it is a key factor in the thermodynamic characterization of the system. The crude extract of neem oil showed poor miscibility when mixed with water, while the addition of surfactants greatly affected the miscibility and distribution of neem oil upon reduction of droplet size and turbidity of the emulsion system. Reddy and Fogler15) stated that the turbidity of the emulsion system is a function of particle size and concentration.
In this study, non-ionic surfactants were used in order to benefit from less irritation, toxicity, and environmental pollution.16) The existence of a wide homogenous isotropic nano-emulsion region in the system prepared with the polysorbate and alkylpolyglucoside surfactants with water (Figs. 1 and 2) suggested the high efficiency of the surfactants. Asib et al.10) and Fernandes et al.17) reported that non-ionic polysorbate and alkylpolyglucoside surfactants were able to produce nano-emulsion formulations with a smaller mean droplet size using low-energy emulsification as compared with other surfactants. These surfactants can generate nano-emulsions by inducing the formation of a looser film.18)
The presence of the surfactants in the emulsion system perhaps reduced the interfacial free energy and provided a mechanical barrier to coalescence, which resulted in the long-term stabilization of the nano-emulsion formulations.19) However, by increasing the temperature, the surfactants were loosely adsorbed on the oil/water interface due to the increased kinetic motion of molecules, leading to collision, coalescence, and destabilizing of the emulsion system.20) It was observed that the physical stability of a nano-emulsion over a long period of time is related to its droplet size; therefore, the N-APG2 with the biggest droplet size, 507 nm, showed instability at 54°C.21)
All of the formulations showed a particle size range of 200–600 nm, which is considered to be a nano-emulsion formulation.7,9) The factors that affect droplet size could depend on the nature and power of the formulation’s structure and the composition of the adsorbed layer between the oil/water phase or the composition of the emulsion system. The formulations with a higher ratio of surfactant (N-PolyS1 and N-APG1) showed a smaller droplet size compared to that of the formulation composed of the same sort of surfactant but with a lesser amount (N-PolyS2 and N-APG2). This is in agreement with the data reported by Kale and Allen (1989),22) according to which the interfacial film is reduced by the accumulation of surfactant to the nano-emulsion system, resulting in higher stability and a smaller formulation droplet size. The quantity of the surfactant used in the study is the typical amount for a commercial formulation. A higher percentage of surfactant (more than 1.5 times the percentage of neem) is required to obtain a particle size of less than 100 nm; that is not commercially viable. The particle aging could be due to the occurrence of creaming, flocculation, coalescence, and Ostwald ripening in the emulsion system.23)
The zeta potential is a parameter for measuring the stability of nano-emulsions and is allied to the surface potential of the droplets. Formulations with zeta potential values above ±30 mV are considered highly stable.24) Van der Waals forces obtained from repulsive forces result in dispersed droplets and a deflocculated system with a great zeta potential value. N-PolyS1 and N-PolyS2 showed the least decrease in zeta potential, which might be due to the type of surfactant. Therefore, the type of surfactant plays a key role on the stability of the colloid. If the zeta potential decreases below a certain level, it leads to aggregation of the colloid because of the attractive forces. In an opposite manner, a high zeta potential leads to a stable system.25)
In the present investigation, the addition of non-ionic surfactants increased the viscosity of the nano-emulsion formulations. This result parallels the report of El Eini et al.,26) which showed that crosslinking chains of the non-ionic surfactants trapped water molecules, causing an increase in the hydrated surfactant’s hydrophilic tail due to water molecules, thus decreasing the viscosity.
Few reports are available on the insecticidal activity of neem oil nano-emulsion formulations with fast and high mortality against stored-product pests. This study showed an increase in the insecticidal effect of azadirachtin against the insect species when formulated as a nano-emulsion. This is in agreement with the report of Anjali et al.,7) which showed improvement of the azadirachtin insecticidal effect against Culex quinquefasciatus, with a mortality of 86.6%. The neem oil nano-emulsion formulations for this work increased the effectiveness of azadirachtin with the help of a surfactant as compared with a simple, non-formulated crude extract of this compound. Similar to this result, Hameed et al.6) reported that a crude extract of neem showed only 30% mortality at a 1% concentration after 72 hr of exposure against T. castaneum.
The mortality data from the contact toxicity test showed that the nano-emulsion formulations with the highest azadirachtin concentration (1%) caused the maximum mortality (100%) of both insects. The difference between the mortality rate of the nano-emulsion formulations and that of the controls could be due to the reduction of the particle size and uniform spreading of the nano-emulsion particles that lead to a higher opportunity for the particles to come into contact with the target insects. This supports the finding by Anjali et al.7) that the accumulation of the insecticide in insects decreased upon increasing the droplet size and, hence, decrease surface area of the droplets with insect. The study also demonstrated that N-APG2, with the biggest particle size, 507 nm, caused lower mortality in both pests (85% for S. oryzae and 74% for T. castaneum) than the other nano-emulsion formulations at a 1% concentration and after 1 day of exposure.
We observed that the nano-emulsion formulations of neem oil not only caused greater mortality of the insects but also increased the speed of azadirachtin action to obtain 100% mortality. The increase in azadirachtin’s speed of action probably is because of the greater bioavailability of the active compound of azadirachtin presented in the nano-emulsion formulations.
Overall, the present study proved that neem oil nano-emulsions are effective in controlling S. oryzae and T. castaneum adults. These nano-emulsions may be used as an alternative for the control of other stored-product insect pests. They have the advantage of promising insecticidal activity and being eco-friendly and less toxic than synthetic pesticides.
In conclusion, the nano-emulsion formulations of neem oil containing polysorbate and alkylpolyglucoside surfactants were successfully created via the low-energy method. All of the formulations provided a nano particle-size, with the smallest size being 208 nm. The N-PolyS1 with the smallest particle size was found to be most effective in controlling S. oryzae and T. castaneum adults. Overall, the study showed that the particle size between the EC formulation of Neemix® and nano-emulsions and within the nano-emulsion formulations contributed to the effectiveness, speed of action, and stability of azadirachtin. Thus, they could be an alternative to conventional insecticides for the control of S. oryzae and T. castaneum adults.
References
- 1) Y. Rajashekar, N. Bakthavatsalam and T. Shivanandappa: Psyche A J. Entomol. 2012, 1–13 (2012). [Google Scholar]
- 2) S. Padin, G. Dal Bello and M. Fabrizio: J. Stored Prod. Res. 38, 69–74 (2002). [DOI] [PubMed] [Google Scholar]
- 3) G. Rosell, C. Quero, J. Coll and A. Guerrero: J. Pestic. Sci. 33, 103–121 (2008). [Google Scholar]
- 4) M. L. Bittner, M. E. Casanueva, C. C. Arbert, M. A. Aguilera, V. J. Hernandez and J. V. Becerra: J. Chil. Chem. Soc. 53, 1444–1448 (2008). [Google Scholar]
- 5) M. D. Mortti, G. Sanna-Passino, S. Demontis and E. Bazzoni: AAPS PharmSciTech 3, 13 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6) A. Hameed, S. Freed, A. Hussain, M. Iqbal, M. Hussain, M. Naeem and A. L. Tipu: Afr. J. Agric. Res. 7, 555–560 (2012). [Google Scholar]
- 7) C. H. Anjali, Y. Sharma, A. Mukherjee and N. Chandrasekaran: Pest Manag. Sci. 68, 158–163 (2012). [DOI] [PubMed] [Google Scholar]
- 8) J. M. Green and G. B. Beestman: Crop Prot. 26, 320–327 (2007). [Google Scholar]
- 9) S. Shafiq, F. Shakeel, S. Talegaonkar, F. Ahmad, R. K. Khar and M. Ali: J. Biomed. Nanotechnol. 3, 28–44 (2007). [Google Scholar]
- 10) N. Asib, D. Omar, R. M. Awang, N. Ashikin and P. Abdullah: JCBPSC 5, 3989–3997 (2015). [Google Scholar]
- 11) J. Flanagan, K. Kortegaard, D. N. Pinder, T. Rades and H. Singh: Food Hydrocoll. 20, 253–260 (2006). [Google Scholar]
- 12) C. J. Lim, M. Basri, D. Omar, M. B. Rahman, A. B. Salleh and R. N. Rahman: Ind. Crops Prod. 36, 607–613 (2012). [Google Scholar]
- 13) F. Chen, Y. Wang, F. Zheng, Y. Wu and W. Liang: Colloids Surf. A Physicochem. Eng. Asp. 175, 257–262 (2000). [Google Scholar]
- 14) J. W. Osborne: Pract. Assess., Res. Eval. 15, 1–9 (2010). [Google Scholar]
- 15) S. R. Reddy and H. S. Fogler: J. Colloid Interface Sci. 79, 101–104 (1981). [Google Scholar]
- 16) A. Azeem, M. Rizwan, F. J. Ahmad, Z. Iqbal, R. K. Khar, M. Aqil and S. Taleganokar: AAPS PharmSciTech 10, 69–76 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17) A. C. Fernandes, V. Uytterhoeven, S. Kuenen, Y. C. Wang, J. R. Slabbaert, J. Swerts and P. Verstreken: J. Cell Biol. 207, 453–462 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18) L. Wang, J. Dong, J. Chen, J. Eastoe and X. Li: J. Colloid Interface Sci. 330, 443–448 (2009). [DOI] [PubMed] [Google Scholar]
- 19) H. Reiss: J. Colloid Interface Sci. 53, 61–70 (1975). [Google Scholar]
- 20) G. Chen and D. Tao: Fuel Process. Technol. 86, 499–508 (2005). [Google Scholar]
- 21) P. Izquierdo, J. Esquena, T. F. Tadros, C. Dederen, M. J. Garcia, N. Azemar and C. Solans: Langmuir 18, 26–30 (2002). [Google Scholar]
- 22) N. J. Kale and A. V. Allen Jr.: Int. J. Pharm. 57, 87–93 (1989). [Google Scholar]
- 23) J. M. Gutierrez, C. Gonzalez, A. Maestro, I. Sole and J. Nolla: Curr. Opin. Colloid Interface Sci. 13, 245–251 (2008). [Google Scholar]
- 24) F. Araujo, R. Kelmann, B. Araujo, R. Finatto, H. Teixeira and L. Koester: Eur. J. Pharm. Sci. 42, 238–245 (2011). [DOI] [PubMed] [Google Scholar]
- 25) E. S. Mahdi, A. M. Noor, M. H. Sakeena, G. Z. Abdullah, M. F. Abdulkari and M. A. Sattar: Int. J. Nanomedicine 6, 2499–2512 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26) D. I. El Eini, B. W. Barry and C. T. Rhodes: J. Colloid Interface Sci. 54, 348–351 (1976). [Google Scholar]