Abstract
A crude rice extract caused a higher probing response than did the control in the green rice leafhopper, Nephotettix nigropictus. Bioassay-guided separation led to the isolation of four active compounds, isoscoparin 2″-O-glucoside, isoscoparin 2″-O-(6‴-(E)-feruloyl)glucoside, isoscoparin 2″-O-(6‴-(E)-p-coumaroyl)glucoside, and isovitexin 2″-O-(6‴-(E)-feruloyl)glucoside from ODS 40% methanol in water faction. Each of the compounds, or any combination without one of the four compounds, caused weaker probing responses than the crude rice extract. The activity was recovered only when all the compounds were combined.
Keywords: Nephotettix nigropictus, rice plant, probing stimulants, flavonoid
Introduction
The green rice leafhopper (GRLH), Nephotettix nigropictus, is a serious rice sucking pest and is widely distributed in South and Southeast Asia, Japan, and Taiwan. It not only causes direct damage to the crop but also can transmit the rice dwarf virus (RDV), rice tungro virus, and rice transitory yellowing virus.1) It is also capable of transovarially transmitting the RDV to its progeny.2) The GRLH is also a vector of rice yellow dwarf mycoplasma-like organisms.3)
The feeding behavior of sucking rice pests is well known to be classified into two phases: a probing phase and a sucking phase, and these are controlled by physical and chemical factors in plants.4,5) For chemicals relating to their sucking behavior, sucrose and amino acids (L-aspartic acid, L-glutamic acid, L-alanine, L-asparagine, and L-valine) were reported as sucking stimulants for the brown planthopper (BPH), Nilaparvata lugens.6,7) Salicylic acid at an effective concentration suppressed the sucking response when used as a probing and oviposition stimulant.8) Oxalic, maleic, and itaconic acids, as well as aromatic acids (benzoic and salicylic acids), were identified as strong sucking-inhibitory substances for the BPH.9) However, not many studies on sucking behavior have been conducted, and most are limited to the BPH, not the GRLH.
On the other hand, fewer chemicals have been elucidated for probing behavior than for sucking behavior in the rice plant. For instance, eight C-glycosylflavones were partially isolated from the rice plant as probing stimulants for the BPH.10,11) Probing stimulants for the smaller brown planthopper (SBPH), Laodelphax striatellus,12) and the white-backed planthopper (WBPH), Sogatella furcifera,13) also were only partially reported. However, leafhoppers, including Nephotettix virescens, Nephotettix cincticeps and Nephotettix nigropictus, have scarcely been researched with regard to probing stimulants. Since probing and sucking stimulants play a tremendous role in feeding behavior for sucking rice pests and probing behavior is performed prior to sucking behavior,4) it may be useful to develop a new method to control sucking rice pests by manipulating the probing stimulants. Therefore, in this study, we isolated and identified probing stimulants for the GRLH in rice plants.
Materials and Methods
1. Insects
Stock colonies of the GRLH were well bred on rice seedlings at 25–28°C, with a relative humidity of 50–60%, and with 16 : 8 hr (L : D) illumination in a controlled room.
2. Plants
The rice plants (cv. Toyonishiki) were cultivated without insecticides by the Faculty of Agriculture, Kochi University, and harvested before a spike appeared.
3. Assay apparatus
Three adult female insects (GRLH) starved for 1 hr were introduced into the bioassay apparatus.12) Each test treatment containing 1.0 g of a fresh rice plant equivalent (1.0 g eq.) extract solved with 2% sucrose solution (0.5 mL) was fed to the insect. The pH value of each test solution was adjusted to 7 by adding a HCl solution or a KOH solution.14) The control treatment consisted of a 2% sucrose solution.
4. Biological assay
The assay apparatus and sample solutions were kept in a controlled room for 24 hr. After the parafilm membranes were stained with a 1% red fuchsin basic solution, the stylet sheaths on the parafilm membranes were observed under a microscope. Probing sheaths were classified as non-branched, two-branched, three-branched, and more than four-branched. They were assigned coefficients of 0, 1, 2 and 3 points, respectively and the intensity of probing activities was obtained as the total number of points. Five assay apparatuses were tested as one set and each set was replicated three times.
5. Statistical analysis
All of the bioassay data were expressed as mean±SE (n=3) and analyzed using Student’s t-test and Fisher’s LSD (least-significant difference) method at p=0.01 or p=0.05.
6. Extraction
The fresh stems and leaves of rice plants (cv. Toyonishiki, 7.96 kg) were cut into pieces (about 5 cm long) and extracted three times with 90% methanol in water (26.0 L). After the extract was evaporated under reduced pressure, the residue (319.27 g) was defatted with hexane three times to obtain dried “crude rice extract” (281.80 g).
7. ODS separation of the crude rice extract
The crude rice extract (1.0 kg eq.) was loaded on a medium-pressure ODS (Chromatorex DM 1020T, 100-200 mesh; Fuji Silysia Chemical) column (500 mm×50 mm id.) and eluted with an increasing concentration of methanol (5.5 L) to obtain ODS water (34.0 g), ODS 20% methanol in water (0.98 g), ODS 40% methanol in water (1.24 g), and ODS 100% methanol (1.19 g) fractions. Compounds 1 (8.0 mg) and 2 (17.5 mg) and peak 3 were isolated from the ODS 40% methanol in water fraction by using preparative HPLC {column: SHISEIDO CAPCELL PAK C18, UG80, 5 µm, 10 mm i.d.×250 mm, solvent: 35% methanol in water (1% acetic acid); flow rate: 3.0 mL/min; wavelength: 254 nm; column temperature: 30°C}. Peak 3 was further separated into two compounds; 3-1 (11.0 mg) and 3-2 (8.5 mg), by using a different preparative HPLC {column: COSMOSIL 5 C18-PAQ, 5 µm 10 mm i.d.×250 mm; flow rate: 2.0 mL/min; wavelength: 254 nm; solvent: 20% acetonitrile in water (1% acetic acid)}.
8. Instruments
LC-MS spectra were obtained using a Shimadzu LCMS-2020 liquid chromatography mass spectrometer {column: SHISEIDO CAPCELL PAK C18 UG80, 5 µm 1.0 mm i.d.×50 mm; flow rate: 0.2 mL/min; wavelength: 254 nm; column temperature: 30°C; solvent: 35% methanol in water (0.1% formic acid)} in ESI-positive and negative modes. The operation was performed under the following conditions: probing voltage, +4.50 kV for ESI-positive mode, −3.50 kV for ESI-negative mode; DL temperature, 250°C; block heater temperature, 200°C; nebulizing gas flow, 1.50 L/min; drying gas flow, 15.00 L/min. The NMR spectra were obtained using a JEOL JNM-ECX500 spectrometer.
Results and Discussion
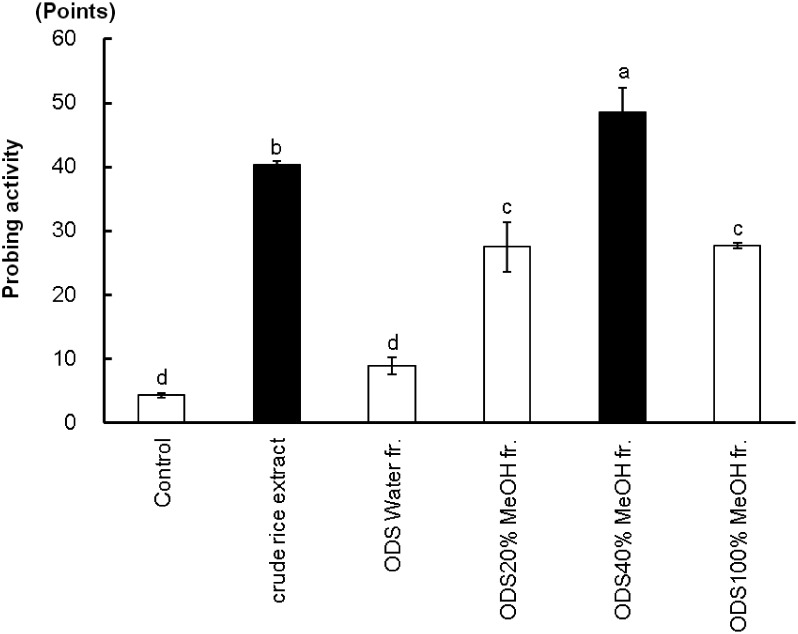
The GRLH made significantly more branched stylet sheaths in the crude rice extract than in the control. Thus, the crude rice extract was further loaded on an medium-pressure ODS column and eluted with water, 20% methanol in water, 40% methanol in water and 100% methanol. The ODS 40% methanol in water fraction yielded the highest probing response compared to the other fractions. As shown in Fig. 1, the ODS 40% methanol in water fraction caused higher activity than did the crude rice extract.
Fig. 1. Probing responses (mean±SE, n=3) of GRLH to control solution, crude rice extract and four ODS fractions. Bars with the same letters are not significantly different (p>0.05) by t-test and Fisher-LSD (Least-significant difference). Fraction was abbreviated to fr.
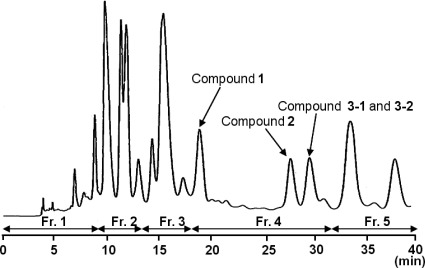
Therefore, the ODS 40% methanol in water fraction (200.0 g eq.) was further separated into five fractions, Fr. 1 (0.0569 g, tR=0.00–9.50 min), Fr. 2 (0.0583 g, tR=9.51–14.40 min), Fr. 3 (0.0250 g, tR=14.41–18.80 min), Fr. 4 (0.0704 g, tR=18.81–32.50 min) and Fr. 5 (0.0403 g, tR=32.51–41.00 min) by a reverse phase HPLC, as shown in Fig. 2.
Fig. 2. HPLC profile of the ODS 40% methanol in water fraction.
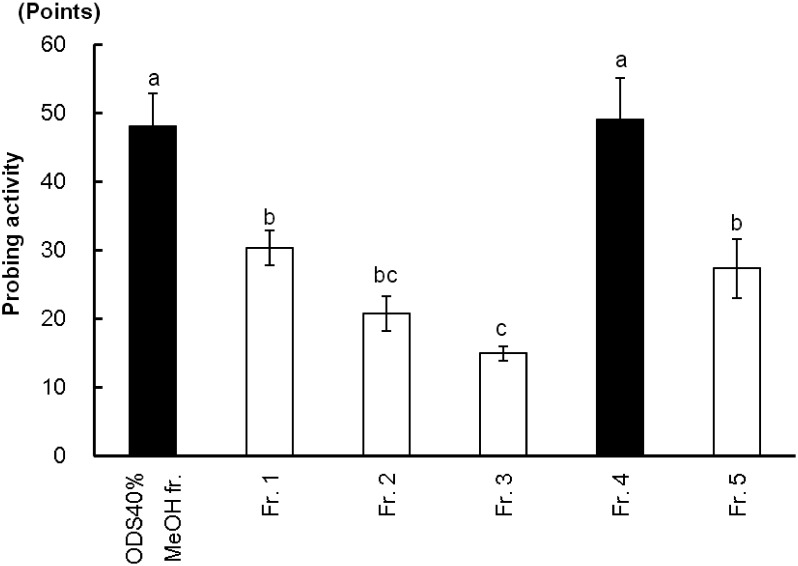
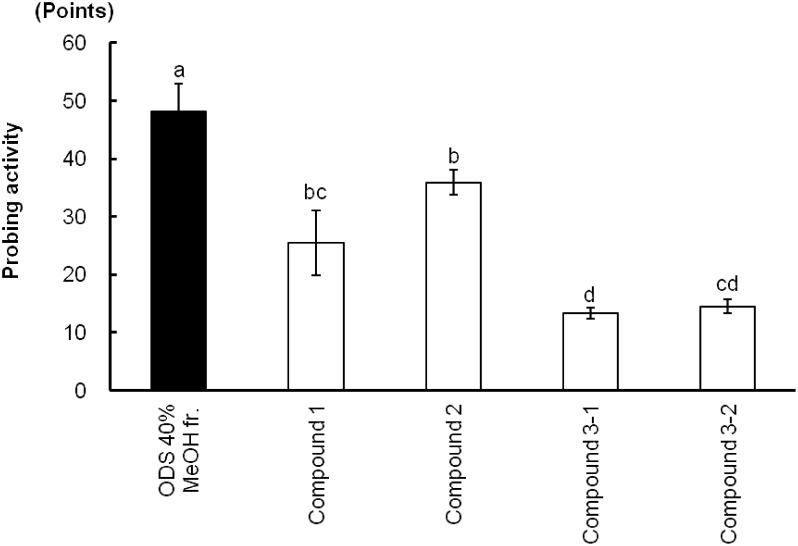
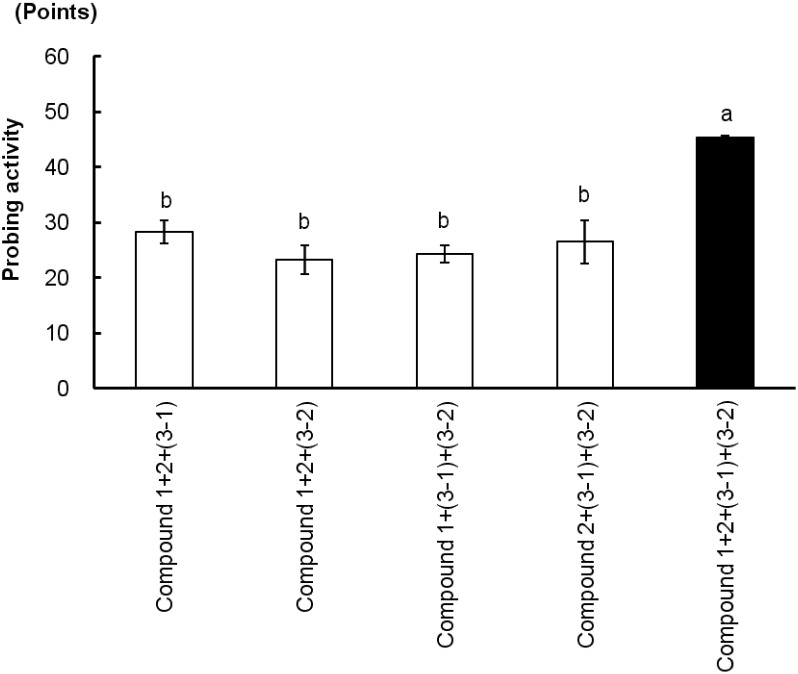
As shown in Fig. 3, Fr. 4 showed the highest probing activity for the GRLH and no significant difference from ODS 40% methanol in water fraction, while Fr. 2, Fr. 3 and Fr. 5 showed a significant difference from Fr. 4 (p<0.05). Consequently, Fr. 4 was further separated into four main compounds, 1, 2, 3-1, and 3-2, by HPLC, as shown in Fig. 2. The bioassay results (Fig. 4) revealed that each of the compounds 1, 2, 3-1, and 3-2 yielded a lower response than the ODS 40% methanol in water fraction for the GRLH. When the mixtures without one of the four peaks were fed to insects, they also caused lower probing responses than did the mixture of all of the peaks, as shown in Fig. 5. However, when all four compounds were combined, activity similar to that with the ODS 40% methanol in water fraction was observed. That means that each of the four compounds was necessary for the GRLH to probe and search the sucking sites. Consequently, a four-compound group was considered as the main probing stimulant toward this insect.
Fig. 3. Probing responses (mean±SE, n=3) of GRLH to ODS 40% methanol in water and five fractions separated from ODS 40% methanol in water fraction. Bars with the same letters are not significantly different (p>0.05) by Fisher-LSD (Least-significant difference).
Fig. 4. Probing responses (mean±SE, n=3) of GRLH to 40% methanol in water fraction and four compounds. Bars with the same letters are not significantly different (p>0.05) by Fisher-LSD (Least-significant difference).
Fig. 5. Probing responses (mean±SE, n=3) of GRLH to several combined peaks. Bars with the same letters are not significantly different (p>0.01) by Fisher-LSD (Least-significant difference).
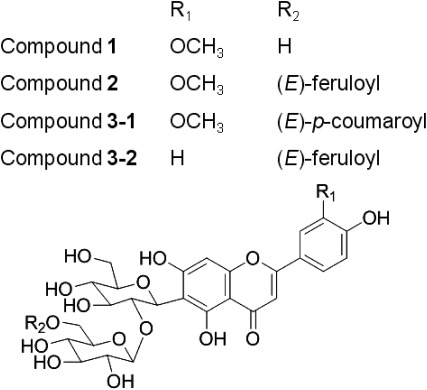
The actual concentration of compounds 1, 2, 3-1, and 3-2 was measured as 10.6 µg, 19.0 µg, 12.6 µg, and 20.6 µg, respectively, in 1.0 g eq. crude rice extract. The structure of compounds 1, 2, 3-1, and 3-2 was determined as isoscoparin 2″-O-glucoside, isoscoparin 2″-O-(6‴-(E)-feruloyl)glucoside, isoscoparin 2″-O-(6‴-(E)-p-coumaroyl)glucoside, and isovitexin 2″-O-(6‴-(E)-feruloyl)glucoside, respectively, as shown in Fig. 6, by comparing the LC-MS and NMR data with the reported data.15,16,18,19)
Fig. 6. Structures of compound 1, 2, 3-1 and 3-2.
In this study, it is demonstrated that these four compounds play a significant role in GRLH probing behavior. This is because each of these four compounds individually showed weak activities, but the combination of all of them showed high activity toward the GRLH. This is the first time the combination of several elucidated compounds has been reported to be highly effective as a probing stimulant for the GRLH in rice plants.
Of these four active flavonoid glycosides, isoscoparin 2″-O-(6‴-(E)-feruloyl)glucoside, isoscoparin 2″-O-(6‴-(E)-p-coumaroyl)glucoside, and isovitexin 2″-O-(6‴-(E)-feruloyl)glucoside have been initially determined from the rice plant by Besson et al.18) and Markham et al.19) Therefore, they are considered as specific components in the rice plant. On the other hand, isoscoparin 2″-O-glucoside was isolated from Alliaria petiolata,15) Passiflora incarnata,16) Silene pratensis,17) and Oryza sativa18) and is widespread as a common flavonoid glycoside in many plants. It is also known to be associated with free radical scavenging activities and antibacterial activities.15) Isoscoparin 2″-O-glucoside, isoscoparin 2″-O-(6‴-(E)-feruloyl)glucoside, and isoscoparin 2″-O-(6‴-(E)-p-coumaroyl)glucoside were reported to stimulate probing in the BPH.18)
As an oligophagous insect, the GPLH requires one common and three specific flavonoid C-glycosides as probing stimulants. The SBPH, another oligophagous insect, also needed six components, including two common flavonoid O-glycosides (tricin 5-O- and 7-O-glucoside)12) and four specific flavonoid C-glycosides (unpublished). The other oligophagous insect, the WBPH, also required one common tricin 5-O-glucoside13) and three specific flavonoid C-glycosides (unpublished). For the monophagous BPH, eight probing stimulants were mainly reported from the rice plant.18) This shows that in order for it to specifically select its host, the BPH requires many specific compounds as probing stimulants. The oligophagous rice pests WBPH and SBPH did not need many specific flavonoid glycosides as probing stimulants in order to find their host plants, because they have dozens or several dozens of host plants. The polyphagous insect N. cincticeps probably needs only a few common flavonoid glycosides as probing stimulants. It is considered that there are some relationships between the quality and quantity of probing stimulants for sucking rice pests and their host ranges. This hypothesis may support the idea that the probing stimulants might play a significant role in host recognition. Since the probing behavior is performed prior to the sucking behavior,4) manipulating the probing stimulants could become a new method to control the GRPH. To elucidate these speculations, further research must be carried out on other monophagous and polyphagous leafhoppers such as N. virescens and N. cincticeps, respectively. More bioassays as well as identification of the rest of the active components for the BPH, SBPH, and WBPH must also be studied in detail in the future.
References
- 1).S. K. De Datta: “Principles and practices of rice production,” John Wiley & Sons, Singapore, pp. 420–449, 1981.
- 2).S. Nasu: Bull. Kyushu Agric. Exp. Sta. 8, 153–349 (1963) (in Japanese with English summary). [Google Scholar]
- 3).S. Nara, M. Sugiura, S. Wakimoto and T. Iida: Ann. Phytopathological Soc. Jpn. 33, 343–344 (1967) (in Japanese). [Google Scholar]
- 4).K. Sōgawa: Annu. Rev. Entomol. 27, 49–73 (1982). [Google Scholar]
- 5).K. Sōgawa: Appl. Entomol. Zool. (Jpn.) 9, 204–214 (1974). [Google Scholar]
- 6).T. Sakai and K. Sōgawa: Appl. Entomol. Zool. (Jpn.) 11, 82–88 (1976). [Google Scholar]
- 7).K. Sōgawa: Jpn. J. Appl. Entomol. Zool. 16, 1–7 (1972) (in Japanese with English summary). [Google Scholar]
- 8).S. Sekido and K. Sōgawa: Appl. Entomol. Zool. (Jpn.) 11, 75–81 (1976). [Google Scholar]
- 9).K. Sōgawa: Appl. Entomol. Zool. (Jpn.) 11, 160–164 (1976). [Google Scholar]
- 10).T. Yoshihara, K. Sogawa, M.-D. Pathak, B.-O. Juliano and S. Sakamura: Entomol. Exp. Appl. 27, 149–155 (1980). [Google Scholar]
- 11).M. Kim, H.-S. Koh and H. Fukami: J. Chem. Ecol. 11, 441–452 (1985). [DOI] [PubMed] [Google Scholar]
- 12).F. Adjei-Afriyie, C.-S. Kim, M. Takemura, M. Ishikawa, S.-I. Tebayashi and M. Horiike: Z. Naturforsch. C 55, 1038–1043 (2000). [DOI] [PubMed] [Google Scholar]
- 13).F. Adjei-Afriyie, C.-S. Kim, M. Takemura, M. Ishikawa and M. Horiike: Biosci. Biotechnol. Biochem. 64, 443–446 (2000). [DOI] [PubMed] [Google Scholar]
- 14).M. Kim, H.-S. Koh, T. Ichikawa, H. Fukami and S. Ishii: Appl. Entomol. Zool. (Jpn.) 10, 116–122 (1975). [Google Scholar]
- 15).Y. Kumarasamy, M. Byres, P.-J. Cox, A. Delazar, M. Jaspars, L. Nahar, M. Shoeb and S.-D. Sarker: Chem. Nat. Compd. 40, 122–128 (2004). [Google Scholar]
- 16).K. Rahman, L. Krenn, B. Kopp, M. Schubert-Zsilavecz, K.-K. Mayer and W. Kubelka: Phytochemistry 45, 1093–1094 (1997). [Google Scholar]
- 17).J. B. Harborne and H. Baxter (eds.): “The Handbook of Natural Flavonoids,” Vol. 1, p. 549, 1999.
- 18).E. Besson, G. Dellamonica, J. Chopin, K.-R. Markham, M. Kim, H.-S. Koh and H. Fukami: Phytochemistry 24, 1061–1064 (1985). [Google Scholar]
- 19).K.-R. Markham, G.-J. Tanner, M. Cassi-Lit, M. I. Whitecross, M. Nayudu and K. A. Mitchell: Phytochemistry 49, 1913–1919 (1998). [Google Scholar]