Abstract
Strigolactones (SLs) are carotenoid-derived signaling molecules that mediate symbiotic and parasitic communications in the rhizosphere and plant hormones that regulate the growth and development of plants through crosstalk with other hormones. Natural SLs are classified into two groups based on the stereochemistry of the B–C ring junction. Rice and sorghum plants, both gramineous crops, produce orobanchol-type and strigol-type SLs, respectively, while tobacco plants produce both types. In the present study, we demonstrate that such species-specific phenomena in SL production also occur in the transport of exogenous SLs from roots to shoots. In rice plants, strigol-type SLs such as 5-deoxystrigol have been reported to actively inhibit tiller bud outgrowth, whereas root-applied strigol-type SLs could not be detected in shoots harvested 20 hr after treatment, indicating that metabolites of SLs or other signaling compounds downstream of SLs—but not SLs themselves—are the true inhibitors of tiller bud outgrowth.
Keywords: strigolactones, orobanchol-type, strigol-type, transportation
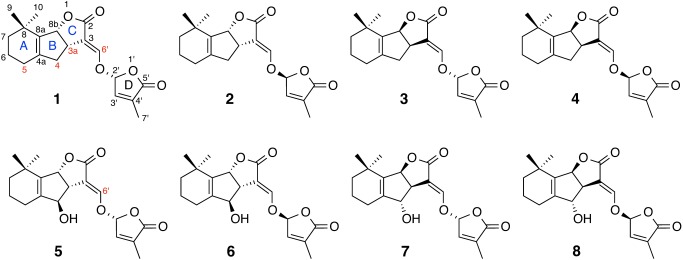
Strigolactones (SLs) are carotenoid-derived signaling molecules that mediate symbiotic and parasitic communications in the rhizosphere and plant hormones that regulate the growth and development of plants through crosstalk with other hormones.1–3) Canonical SLs contain a tricyclic ring system (ABC moiety) that connects to the methylbutenolide D-ring via an enol-ether bridge. These SLs can be classified into two groups based on the stereochemistry of the B–C ring junction.2–4) Orobanchol-type SLs, such as 4-deoxyorobanchol (4DO, 1), orobanchol (5), fabacol, 7-oxoorobanchol, 7-hydroxyorobanchol, and solanacol, have α-oriented C rings; while strigol-type SLs such as strigol, sorgolactone, sorgomol, 5-deoxystrigol (5DS, 3), and strigone, contain β-oriented C rings. Studies on the distribution of SLs in the plant kingdom have revealed that some plant species produce either orobanchol-type or strigol-type SLs, while others, like tobacco, produce both types.4) Since carlactone, the common intermediate for SLs,5) exists as an optically pure form with an (11R) configuration,6) all natural SLs have a (2′R) configuration. Therefore, the cyclization of an oxidized-metabolite of carlactone, presumably 18-hydroxycarlactonoic acid, to either 4DO (1) or 5DS (3) appears to be strictly regulated in some plant species but not in others (Fig. 1). In our previous report, we demonstrated that root-applied SLs are transported to shoots, although not through the xylem.7) In the present study, we examined whether this root-to-shoot transport was dependent on the structure and/or stereochemistry of SLs in rice (orobanchol-type SL producer), sorghum (strigol-type SL producer), and tobacco (producing both types).
Fig. 1. Chemical structures of stereoisomers of 4-deoxyorobanchol (1–4) and orobanchol (5–8). Deuterated positions in d6-deoxyorobanchol and d1-orobanchol are shown in red.
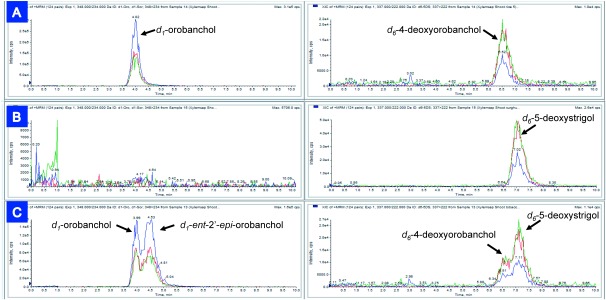
A mixture of four stereoisomers of 3a,4,4,5,5,6′[2H]6-4DO (or 5DS) (1–4) and four stereoisomers of 6′[2H]-orobanchol (5–8) was applied to the roots of rice, sorghum, and tobacco plants; shoots were cut at 2 cm above the shoot/root junction 20 hr after treatment. SLs in the harvested shoot tissues were extracted and analyzed by LC-MS/MS as described previously.7) These stereoisomers were detected with the same or with very similar sensitivities in the LC-MS/MS analyses conducted in the present study. The separation of SL stereoisomers with chiral LC-MS/MS was essentially the same as that reported previously.4) In the case of rice plants, only orobanchol-type SLs, d6-4DO (1) and d1-orobanchol (5) were detected in shoots harvested 20 hr after treatment (Fig. 2A). By contrast, the shoots of sorghum plants were found to contain only d6-5DS (3), while no orobanchol stereoisomers 5–8 were detected (Fig. 2B). Under the experimental conditions, levels of endogenous SLs (d0-SLs) were below the detection limit; thus, the signals observed for d1-orobanchol in Fig. 2A are not isotope peaks of endogenous d0-orobanchol. Four of the eight stereoisomers with (2′R) configurations (1, 3, 5, 7) were detected in the shoots of tobacco plants harvested 20 hr after treatment (Fig. 2C). Therefore, orobanchol-type SLs and strigol-type SLs are stereoselectively produced and transported in rice and sorghum plants, respectively, whereas both types are produced and transported in tobacco plants. These results clearly demonstrate that root-to-shoot transport is a highly structure- and stereospecific process in rice and sorghum plants. This is also true in tobacco plants, which developed a transport system for both types or modified its original system to transport the other type of SLs as well. It should be noted that ent-2′-epi-orobanchol (4-hydroxy-5DS, 7) was not transported in sorghum whose major hydroxy-SL is sorgomol (9-hydroxy-5DS), indicating that the root-to-shoot transport system in sorghum is able to distinguish the positions of the hydroxyl group.
Fig. 2. LC-MS/MS (MRM) chromatograms of extracts of shoots of rice (A), sorghum (B), and tobacco (C) harvested 20 hr after treatment. In the chromatograms, blue, red, and green traces indicate transitions of m/z 348–234, 348–206, and 348–97 for d1-orobanchol and its isomers, and transitions of m/z 337–240, 337–222, and 337–97 for d6-4-deoxyorobanchol and its isomers.
According to the structure–activity relationships of natural SLs and synthetic analogs in shoot-branching inhibition in Arabidopsis and rice, SL stereoisomers with (2′R) configurations are more active.8,9) Furthermore, the putative SL receptor DWARF14 (D14) can interact with DWARF53 (D53), a repressor in the SL signaling pathway,10,11) only in the presence of (2′R) stereoisomers irrespective of the C-ring orientation.8) This is in good agreement with the results obtained in the present study because only naturally occurring SLs, stereoisomers with (2′R) configurations, were found to move from roots to shoots. Since the hydrolysis of (2′R) and (2′S) isomers of 5DS was shown to proceed at a similar rate, the lack of detection of (2′S) isomers was not attributable to a rapid degradation of (2′S) isomers in the test solution.8) Fridlender et al. indicated that both the influx and efflux of SLs in the root are ATP-dependent processes. In addition, the fluorescent SL analog with (2′R) configuration, conforming to the natural SL structure, was better incorporated than its (2′S) isomer and present mostly in the cell cytoplasm, suggesting that SLs are transported symplastically in plants.12) In tiller bud outgrowth inhibition in rice plants, 5DS (3) and ent-2′-epi-orobanchol (7), strigol-type SLs, were as active as 4DO (1) and orobanchol (5).8) However, it is likely that strigol-type SLs hardly move from the roots to shoots in rice plants because strigol-type SLs applied to the roots were not detected in the shoots. These results suggest that metabolites of SLs or other signaling compounds downstream of SLs but not SLs themselves are active inhibitors of axillary bud outgrowth. This hypothesis is in accordance with an observation that GR5 was a potent axillary bud outgrowth inhibitor when applied to the roots but only a weak one when applied directly to axillary buds in Arabidopsis.8,13) Such activity dependence on the application method was not observed with 5DS and its stereoisomers.8) Further study is necessary to understand whether SLs transported from roots to shoots themselves are active inhibitors of tiller bud outgrowth or SL root treatment elicits production of an as yet unknown active inhibitor which moves from the roots to shoots. Furthermore, differences in uptake and metabolism of root-applied SL stereoisomers need to be considered.
Experimental
1. Instruments
LC-MS/MS analysis of proton adduct ions was performed with a triple quadrupole/linear ion trap instrument (LIT) (QTRAP5500; AB SCIEX) with an electrospray source. The LC-MS/MS analytical conditions were the same as those described previously.7)
2. Chemicals
The stereoisomers of 6′[2H]orobanchol14,15) and 3a,4,4,5,5,6′[2H]6-4-deoxyorobanchol16) were synthesized in accordance with the reported methods. The other analytical grade chemicals and HPLC solvents were obtained from Kanto Chemical Co. Ltd. and Wako Pure Chemical Industries Ltd.
3. Plant material
Seeds of rice (Oryza sativa L. cv. Nipponbare) and sorghum (Sorghum bicolor (L.) Moench cv. Hybrid) were purchased from a local market. Tobacco seeds (Nicotiana tabacum L. cv. Michinoku No. 1) were a generous gift from Japan Tobacco Inc.
4. Feeding experiments
Seeds of rice, sorghum, and tobacco were surface-sterilized in 70% EtOH for 2 min. After being thoroughly rinsed with sterile Milli-Q water, the seeds were soaked in water at 25°C for 5 days. Germinated seeds were transferred to a strainer (22×18×7 cm, width×length×height (W×L×H)) lined with a sheet of gauze moistened by placing it in a slightly larger container (22.5×18.5×9 cm, W×L×H) containing 1.5 L of tap water as the culture medium in a growth chamber with a 14/10-hr photoperiod at 120 µmol photons/m2/s at 30/24°C for rice and sorghum and 25/22°C for tobacco plants. Rice (20 plants), sorghum (15 plants), and tobacco (20 plants) grown hydroponically for 16, 16, and 33 days, respectively, were treated with tap water medium (300 mL) containing stereoisomers of d6-4DO (1–4, each at a concentration of 0.15 µg/mL) or d1-orobanchol (5–8, each at a concentration of 0.15 µg/mL). Twenty hr after treatment, shoots were cut at 2 cm above the shoot/root junction to eliminate possible contamination from the media, and the shoot tissues (ca. 20 g FW) were extracted with acetone at 4°C for 24 hr. After removal of the tissues by filtration, the acetone was evaporated in vacuo. Preparation of the samples for LC-MS/MS analyses was conducted as reported previously.17) The experiment was conducted with three replications.
Acknowledgments
We thank Dr. Christopher McErlean (The University of Sydney, Australia) for critical reading of the manuscript and fruitful discussions. This work was supported by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry, by KAKENHI (15K07093, 26850069), and by a grant from JGC-S Scholarship Foundation to XX. Kaori Yoneyama is supported by RPD project (JSPS).
The online version of this article contains supplementary material (analytical conditions), which is available at http://www.jstage.jst.go.jp/browse/jpestics/.
supplementary materials
References
- 1).X. Xie, K. Yoneyama and K. Yoneyama: Annu. Rev. Phytopathol. 48, 93–117 (2010). [DOI] [PubMed] [Google Scholar]
- 2).S. Al-Babili and H. J. Bouwmeester: Annu. Rev. Plant Biol. 66, 161–186 (2015). [DOI] [PubMed] [Google Scholar]
- 3).M. Lopez-Obando, Y. Ligerot, S. Bonhomme, F.-D. Boyer and C. Rameau: Development 142, 3615–3619 (2015). [DOI] [PubMed] [Google Scholar]
- 4).X. Xie, K. Yoneyama, T. Kisugi, K. Uchida, S. Ito, K. Akiyama, H. Hayashi, T. Yokota, T. Nomura and K. Yoneyama: Mol. Plant 6, 153–163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).A. Alder, M. Jamil, M. Marzorati, M. Bruno, M. Vermathen, P. Bigler, S. Ghisla, H. Bouwmeester, P. Beyer and S. Al-Babili: Science 335, 1348–1351 (2012). [DOI] [PubMed] [Google Scholar]
- 6).Y. Seto, A. Sado, K. Asami, A. Hanada, M. Umehara, K. Akiyama and S. Yamaguchi: Proc. Natl. Acad. Sci. U.S.A. 111, 1640–1645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).X. Xie, K. Yoneyama, T. Kisugi, T. Nomura, K. Akiyama, T. Asami and K. Yoneyama: J. Pestic. Sci. 40, 214–216 (2015). [Google Scholar]
- 8).M. Umehara, M. Cao, K. Akiyama, T. Akatsu, Y. Seto, A. Hanada, W. Li, N. Takeda-Kamiya, Y. Morimoto and S. Yamaguchi: Plant Cell Physiol. 56, 1059–1072 (2015). [DOI] [PubMed] [Google Scholar]
- 9).A. Scaffidi, M. T. Waters, Y. K. Sun, B. W. Skelton, K. W. Dixon, E. L. Ghisalberti, G. R. Flematti and S. M. Smith: Plant Physiol. 165, 1221–1232 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).F. Zhou, Q. Lin, L. Zhu, Y. Ren, K. Zhou, N. Shabek, F. Wu, H. Mao, W. Dong, L. Gan, W. Ma, H. Gao, J. Chen, C. Yang, D. Wang, J. Tan, X. Zhang, X. Guo, J. Wang, L. Jiang, X. Liu, W. Chen, J. Chu, C. Yan, K. Ueno, S. Ito, T. Asami, Z. Cheng, J. Wang, C. Lei, H. Zhai, C. Wu, H. Wang, N. Zheng and J. Wan: Nature 504, 406–410 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).L. Jiang, X. Liu, G. Xiong, H. Liu, F. Chen, L. Wang, X. Meng, G. Liu, H. Yu, Y. Yuan, W. Yi, L. Zhao, H. Ma, Y. He, Z. Wu, K. Melcher, Q. Qian, H. E. Xu, Y. Wang and J. Li: Nature 504, 401–405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).M. Fridlender, B. Lace, S. Wininger, A. Dam, P. Kumari, E. Belausov, H. Tsemach, Y. Kapulnik, C. Prandi and H. Koltai: Mol. Plant 8, 1809–1812 (2015). [DOI] [PubMed] [Google Scholar]
- 13).F.-D. Boyer, A. de Saint Germain, J.-P. Pillot, J.-B. Pouvreau, V. X. Chen, S. Ramos, A. Stévenin, P. Simier, P. Delavault, J.-M. Beau and C. Rameau: Plant Physiol. 159, 1524–1544 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).K. Mori, J. Matsui, T. Yokota, H. Sakai, M. Bando and Y. Takeuchi: Tetrahedron Lett. 40, 943–946 (1999). [Google Scholar]
- 15).K. Akiyama, K. Matsuzaki and H. Hayashi: Nature 435, 824–827 (2005). [DOI] [PubMed] [Google Scholar]
- 16).K. Ueno, A. Hanada, S. Yamaguchi and T. Asami: J. Labelled Comp. Radiopharm. 53, 763–766 (2010). [Google Scholar]
- 17).K. Yoneyama, X. Xie, T. Kisugi, T. Nomura and K. Yoneyama: Planta 238, 885–894 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.