Abstract
Five spraying techniques were evaluated for the coverage of bifenthrin on peas under open field conditions. Ultra-low volume sprayer (ULVA) and Ground hydraulic motor with conventional spray gun (GMG) gave number mean diameters (NMDs) from 27 to 68 and from 33 to 73 N/cm2 with volume mean diameter (VMD) from 50 to 120 and from 320 to 508, respectively. Homogeneity factor values were 2, 2, 2.5, 2.8, and 13.5 in ULVA, Domestic modification of the ground hydraulic motor sprayer with one nozzle (GMO), Ground hydraulic motor sprayer with vertical boom (GMV), Motorized knapsack mist blower sprayer (MKM) and GMG. The percentages of the lost spray in the ground were 23, 39, 21, 24, and 36% for ULVA, MKM, GMG, GMO and GMV, respectively. An analytical method was developed using QuEChERS and GC-ECD to determine the initial deposit of bifenthrin in pods and leaves. Initial deposits were from 0.006 to 0.05 mg/kg in pods and from 0.03 to 0.66 mg/kg in leaves. The most efficient technique was single nozzle Twinjet, followed by the motorized knapsack sprayer.
Keywords: pesticide spraying techniques, pea, bifenthrin residues, method development and validation
Introduction
Peas (Pisum sativum), pods and seeds, are eaten fresh or cooked. Peas’ leaves can be used as animal feed.1) Peas plants are infested with many insect pests, e.g., two spotted mite (Tetranychus urticae), thrips, caterpillars and aphids. Most of these pests attack the lower surface of the leaves which provides good shelter for such pests underneath it. This situation becomes more challenging when the plant canopy is fully developed, which prevents pesticide spray droplets from reaching the target. Delivery, uniformity and good coverage of the pesticide solution are instrumental for pesticides’ achieving efficient control of the target pest.2) Also, well-atomized spray solutions allow the drops to be affixed to crop leaves. Spray deposit on the target surface is influenced by many factors, such as sprayer design and settings, nozzle type, and target canopy characteristics.3) Pesticide application efficiency, minimizing the loss of spray solution4) as well as diminishing pesticide residues in the consumed part, can all be achieved by selecting the appropriate application equipment. Many studies have shown that droplet size is a major factor influencing deposition on the target and the drift of the spray solution.5) Also, different spraying machines use different amounts of water and different deposits are expected to occur. The initial deposit affects the control efficiency, required application frequency and eventually pesticide residues in the produce.6)
Egypt is a producer and exporter of peas. Egypt produced 180,631 tones of green peas and 124 tones of dry peas in 2012.7) Peas with pods are totally dedicated for exportation, especially to the European market. Growers mainly use motorized ground spray guns with high-pressure hydraulic nozzles for better penetration of the spray solution into the canopy. they produce large droplet-size sprays which do not provide efficient control of pests inhabiting the leaves’ lower surfaces. Thus, higher pesticide doses and multiple pesticide applications are practiced; consequently, more pesticide waste is delivered into the environment, and the incidence of pest resistance increases. Also, overdosage and multiple pesticide applications increase the potential of exceeding the acceptable residue limits.6) Pesticide residues in food are controlled and regulated by maximum residue limit (MRL) values that depend on the country or the regulating authority.8) according to European Food Safety Authority, 8.6% of peas pods samples violated the MRL value in 2015.9)
In this study, the authors investigated the effect of using different application techniques on the characteristics of bifenthrin deposition on pea pods and leaves, e.g., droplet size and volume, homogeneity factor, vertical distribution of the droplets on the plant leaves, and the expected loss of spray solution. Also, an analytical method was developed to determine the bifenthrin deposit in pods and leaves of pea plant using QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) followed by gas chromatograph connected to a micro-Electron Capture Detector 63Ni (GC-µECD). In addition, recommending the most suitable application machine was of concern.
Materials and Methods
1. Chemicals and reagents
Analytical standard bifenthrin (99.2%) was purchased from Sigma-Aldrich, Germany. Acetic acid was obtained from EL Nasr Chemicals Company, Cairo, Egypt. Acetonitrile 99% pure was purchased from Riedel-de Haën, UK. Sodium chloride and anhydrous sodium sulfate (activated at 250°C overnight prior analysis) were obtained from El Nasr Chemicals Company. Primary secondary amine (PSA) was purchased from Waters Corporation, USA. Bifenthrin formulation (killer, 2.5% emulsifiable concentrate EC) was secured from the local market.
2. Spraying equipment
The following spraying equipment or modifications were tested.
i) Ultra-Low Volume Sprayer “ULVA”
ii) Motorized Knapsack Mistblower Sprayer (MKM) (Cifarelli, Italy)
iii) Ground hydraulic Motor with conventional spray Gun (GMG)
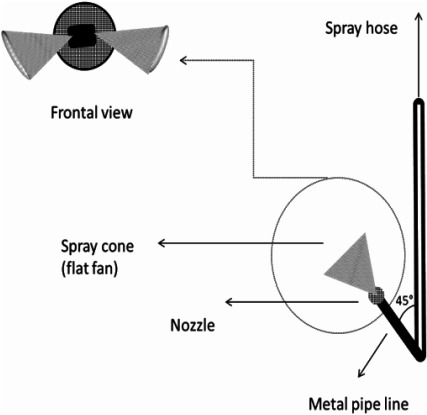
iv) Domestic modification of the Ground hydraulic Motor sprayer with One nozzle (GMO): One nozzle TwinJet (Twin Flat spray Tip stainless steel with a spray angle of 110°, 1.82 L/min 4 bar) was fitted with a metallic pipe with an upward angle of 45° (Fig. 1).
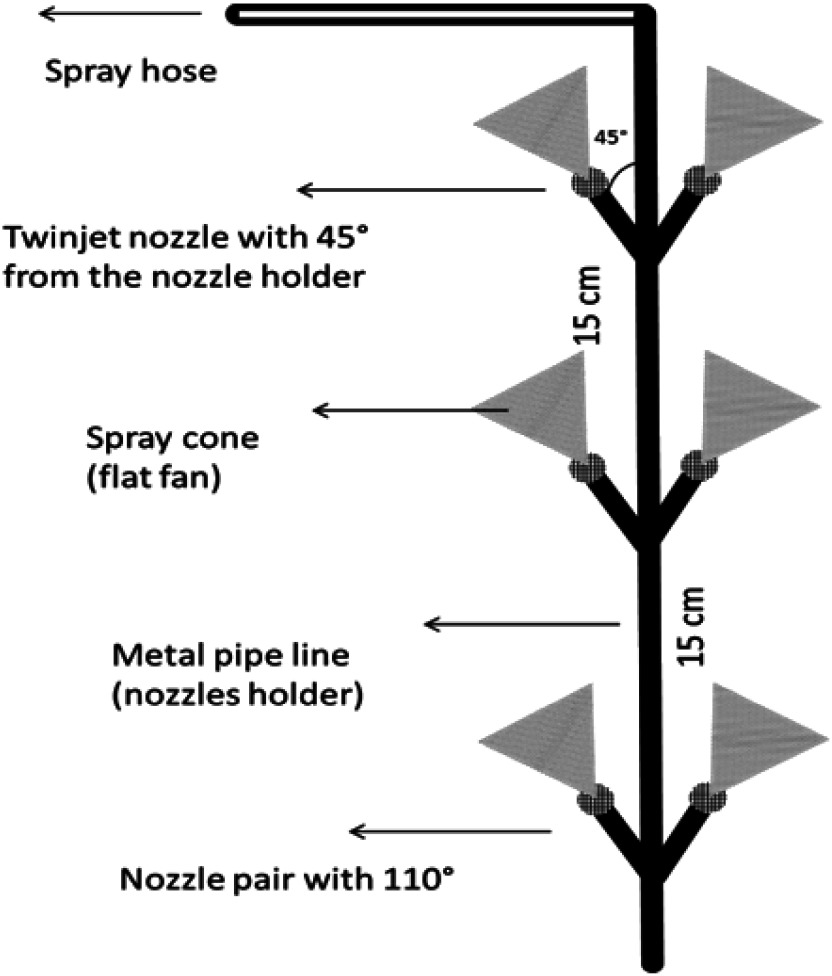
v) Domestic modification of the Ground hydraulic Motor sprayer with 6 nozzles (Vertical boom) (GMV): vertical boom with six TwinJet nozzles that were placed in three pairs and fitted to a stainless steel vertical carrier with an upward angle of 45°. The angle between the two nozzles in each pair was 110°. The distance between each nozzle pair was 15 cm (Fig. 2).
Fig. 1. Illustrative diagram of Ground hydraulic Motor sprayer with one nozzle GMO.
Fig. 2. Illustrative diagram of Ground hydraulic Motor sprayer with 6 nozzles GMV.
ULVA, MKM and GMG are already used at different export farms, and they achieve satisfactory results from the grower’s perspective. GMO and GMV were crafted for the purpose of enhancing application efficiency. TwinJet nozzles were purchased from Spraying Systems Company, USA.
Technical data of the investigated spraying equipment are presented in Table 1.
Table 1. Technical data of the tested ground sprayers applied on peas.
| Parameter | ULVA | MKM | GMG | GMO | GMV |
|---|---|---|---|---|---|
| Type of machine | Rotary (spinning disc) | Pneumatic | Hydraulic | Hydraulic | Hydraulic |
| Number of nozzles | 1 | 1 | 1 | 1 | 6 |
| Nozzle type | Restrictor | Shear nozzle | Spray gun | Tj60-1104 | Tj60-1104 |
| Operational pressure (kg/cm2) | — | — | 10 | 4 | 25 |
| Flow rate (L/min) | 0.18 | 1 | 11.43 | 1.82 | 10.92 |
| Spray volume (L/fed) | 20 | 200 | 600 | 200 | 1200 |
| Power of sprayer | 6 V/7 W motor, 7000 rpm | 4.8 horse power | 6 horse power | 6 horse power | 6 horse power |
| Height of nozzles (cm) | 50 | 50 | 50 | 50 | 50 |
| Type of spraying | Target spraying technique | ||||
| Working speed | 2.4 km/hr | ||||
3. Measurement of spray droplet size and volume
The distribution and homogeneity of bifenthrin deposits on pea plants were investigated using water-sensitive papers (Ciba-Geigy, Switzerland). Water-sensitive paper was stuck to the lower side of leaves on three vertical levels of pea plants. Water-sensitive paper was also placed on the ground between two adjacent plants in order to estimate the spray deposited on the soil. The trial was carried out in triplicate. Measurement of droplet sizes as the volume mean diameter (VMD) and the numbers of spray droplets per square centimeter as the number mean diameter (NMD) were carried out by means of specially scaled monocular lens (Strüben®, Japan).10) The homogeneity factor, symmetric distribution, and spray bulk were calculated.
4. Instrumentation
The Agilent 7890A gas chromatograph was used to determine bifenthrin residues. The system was equipped with two fused silica capillary columns HP-5 and HP-35, with the same parameters, i.e., 30-m length, 0.25-mm i.d., and 0.25-µm film thickness. Each column was connected to a µECD. The oven temperature program was started at 100°C for 1 min, then raised to 170°C at a rate of 25°C/min, held for 1 min, raised at a rate of 3°C/min to 230°C, and held for 1 min before being raised to 300°C, and then being kept for 2 min. The injector temperature was 300°C/min. The flow of the carrier gas, nitrogen, was set at 2 mL/min, while that of the makeup gas nitrogen was set at 50 mL/min. The detector temperature was 320°C. The injection volume was 1 µL (splitless). Chromatographic conditions were optimized to ensure that no interfering peaks were present during the retention window of bifenthrin. A matrix-matched standard approach was utilized in residue determination to compensate for the matrix effect. Matrix-matched standard solutions were freshly prepared in either blank peas’ pods or leaves extracts accordingly.
5. Field trial
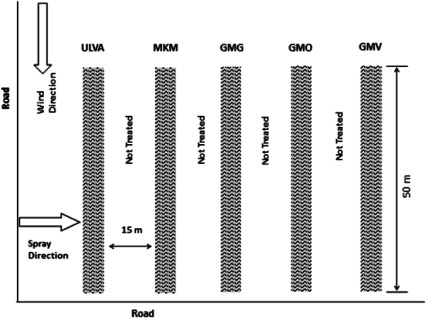
The field trial was carried out at Blue Nile Farm (70 km Cairo, Alexandria Desert Road). Bifenthrin insecticide (Killer, 2.5% EC) was sprayed at the recommended rate of application (2.5 g a.i. /100 L water). Pea plant var. Snow Green peas were cultivated in rows and grown vertically to about 160 cm tall. The experimental area was divided into 50-m2 plots. Each plot was sprayed with a single dose using one of the tested application machines. A complete block random design was followed. To avoid spray drift a 15-m2 barrier was left untreated between the treated plots. The mean meteorological conditions during field testing were suitable for spraying. Temperature was 30°C, relative humidity was 55%, and the average wind velocity was 2–3 m/sec, northwest. A diagrammatic layout of the field experiment is shown in Fig. 3. Sprayed plants were left to dry for 2 hr before samples of leaves and pods were collected for bifenthrin residue analysis. Water-sensitive paper was also collected 2 hr after application. Representative samples of about 0.5 kg of pea pods and 50 pea leaves were collected from each treatment. Each sample was placed in labeled plastic bag. Blank (untreated) samples of pea pods and leaves were collected before application. Samples were kept frozen (−20°C) until analysis. Each sample was homogenized using high-speed blender.
Fig. 3. Diagrammatic layout of the field trial.
6. Sample preparation for bifenthrin residue determination
A modified QuEChERS method was used to extract and clean up the samples as follows: 10 g of a homogenized sample was placed into an extraction tube (50 mL) and 10 mL of 1% acetic acid in acetonitrile was added, and the tube was shaken for 1 min. Next, 5 g of activated anhydrous sodium sulfate and 1 g of sodium chloride were added, and the tube was shaken again by hand for 1 min and then centrifuged at 4000 rpm for 5 min. Then a 1-mL aliquot of extract was transferred into a cleanup tube (2 mL) that contained 50 mg PSA and 150 mg anhydrous sodium sulfate; it was hand shaken for 1 min then centrifuged at 4000 rpm for 5 min. Finally, 0.5 mL of the clean extract was placed into a glass vial and subjected to residue determination by GC.
7. Recovery and precision
A recovery and repeatability study was performed at two levels, i.e., at 0.05 and 0.1 mg/kg in pea pods and at 0.1 and 0.5 mg/kg in leaves, to evaluate the trueness and precision of the method. Ten grams blank pod or leaf samples were put into extraction tubes and then spiked with bifenthrin standard solution to give the required concentration level. Samples were mixed with a clean spatula and left to soak for 30 min before extraction and cleanup using the procedure described above. The experiments were performed in triplicate.
Results and Discussion
1. Analytical method
An analytical method was developed and validated to fulfill requirements for the analysis of bifenthrin residues in pea pods and leaves in terms of sensitivity, specificity, linearity, trueness, and repeatability criteria. The method relied on the QuEChERS technique for sample preparation and GC/µECD for residue determination. µECD offered high sensitivity and specificity for bifenthrin. Using two columns of different polarities, i.e., HP 5% and HP 35%, provided satisfactory confirmation of the results. Validation parameters, according to the European Commission,11) were assessed to ensure the ability of the analytical method to provide accepted analytical results of bifenthrin residues.
2. Linearity
Standard solutions of calibration curves were prepared in blank extract of pods or leaves as required, considering the same matrix concentration as in the samples (1 : 1 sample-to-solvent ratio). Bifenthrin showed good linear ranges over 3 times in magnitude, from 0.01 to 0.5 mg/kg in pods and from 0.02 to 1 mg/kg in leaves with determination coefficients (r2) of 0.999 and 0.997 for pods and leaves, respectively (Table 2). Five concentrations of the standard in matrix solutions (0.01, 0.02, 0.05, 0.1 and 0.5 mg/L) for pod samples and (0.02, 0.05, 0.1, 0.5, 1 mg/L) for leaf samples were used to construct the calibration curves. Each calibration level was injected 3 times, and the average was used in calculations. Slopes of standard calibration curves of pods and leaves were 55429 and 49556, while the intercepts were 104.76 and 1570.4, respectively.
Table 2. Validation parameters of the developed method.
| Parameter | Pods | Leaves | Criteriab) |
|---|---|---|---|
| Linearity (r2) | 0.999 | 0.997 | ND |
| Repeatability (RSD %) | 3.75 | 5.43 | ≤ 20% |
| LOD (µg/kg) | 0.83 | 2.7 | ND |
| LOQ (µg/kg) | 2.5 | 8.2 | ≤ MRL |
| Accuracya) (%) | 109.38 | 108.5 | 70–120% |
r2 determination coefficient; LOD limit of detection was calculated as 3 : 1 S/N ratio; LOQ limit of quantification was calculated as 10 : 1 S/N ratio from calibration measurements. a) Accuracy was calculated as the average recovery from all spike levels. b) European Commission requirements (SANCO Document 12571, 2013), unless not determined ND.
3. Recovery study
The mean recovery of bifenthrin (n=3) in pods was 109.38% while in leaves it reached 108.50%. Method repeatability, as the Relative Standard Deviation (RSD), reached 3.75% in pea pods and 5.43% in leaves.
4. Deposit of bifenthrin after application
The residue of bifenthrin in pods and leaves of pea plants after application with the tested machines was determined using the validated method. Data are summarized in Table 3. Treatment with the vertical boom of 6 nozzles gave the highest residue level in both pods (0.05 mg/kg) and leaves (0.66 mg/kg), as compared to treatment with ULVA, which deposited 0.006 mg/kg on pods and 0.03 mg/kg on leaves. The treatments can be placed in this order: GMV>GMG>GMO>MKM>ULVA. Results of the present study are comparable to results reported by Yarpuz-Bozdogan et al.,12) who found that the initial residues of dicofol on strawberries after treatment with different spraying techniques ranged from 0.005 to 0.14 mg/kg. Data in Table 3 show that there is a clear positive relation of bifenthrin deposits on leaves with the spray volume applied. The same relation is also found in pods, with one exception in the GMG case which can be due to the large droplet size with GMG.
Table 3. Residues of bifenthrin on pods and leaves after spraying by the tested machines.
| Spraying machines | Spray volume L/fed | Pods | Leaves | ||
|---|---|---|---|---|---|
| Deposit (mg/kg) | RSD% | Deposit (mg/kg) | RSD% | ||
| ULVA | 20 | 0.006 | 9.99 | 0.03 | 18.9 |
| MKM | 200 | 0.02 | 10.9 | 0.17 | 4.2 |
| GMG | 600 | 0.03 | 12.4 | 0.56 | 1.5 |
| GMO | 200 | 0.025 | 27.9 | 0.23 | 6.3 |
| GMV | 1200 | 0.05 | 19.8 | 0.66 | 21.2 |
The average RSD was 13.31% in pod and leaf samples, implying accepted repeatability of the results. None of the treatments exceeded the maximum residues limit (MRL) (0.1 mg/kg) of bifenthrin in pods.13) MRL regulations do not apply to leaves.6)
Despite the fact that residues of bifenthrin were below the MRL in all tested treatments, residues may accumulate in peas to violate the MRL due to repeated applications to control, for instance, spider mite.
5. Spraying techniques
Data in Table 1 illustrate that the performance rate of the tested techniques could be arranged in a descending order according to spray volume (l/fed) as follows: GMV, GMG, MKM, GMO, and ULVA, i.e., 1200, 600, 200, 200, and 20 L/fed, respectively.
6. Droplets VMD and NMD
The results reported in literature indicated that the optimum spectrum of droplets required for controlling insects in field crops should be sized between 140 and 200 µm (VMD) with NMDs of 30–50 droplets/cm2 distributed homogeneously on the treated target.14,15) Also, Halawa et al.16) mentioned that the tested spraying equipment for controlling citrus brown mites gave an NMD of 50 droplets/cm2 and VMDs of 111 to 177 µm. Accordingly, in the present study, ULVA and GMG delivered the least coverage on plant vertical levels, as NMDs ranged from 27 to 68 N/cm2 and 33 to 73 N/cm2 and VMDs ranged from 50–120 and 320–508 for ULVA and GMG, respectively (Table 4).
Table 4. NMD and VMD of the spray droplets generated by the tested sprayers at the different levels of pea plants.
| Plant levels | ULVA | MKM | GMG | GMO | GMV | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NMDa) | VMDb) | NMD | VMD | NMD | VMD | NMD | VMD | NMD | VMD | |
| Upper | 68 | 120 | 73 | 156 | 37 | 320 | 84 | 233 | 91 | 240 |
| Middle | 34 | 85 | 58 | 83 | 22 | 500 | 102 | 180 | 147 | 350 |
| Lower | 27 | 50 | 33 | 167 | 55 | 508 | 83 | 117 | 69 | 180 |
| Ground | 35 | 65 | 83 | 167 | 23 | 600 | 68 | 217 | 140 | 350 |
a) in N/cm2. b) in µm.
7. Homogeneity factor (HF)
The homogeneity factor was calculated from Eq. (1) as follows:
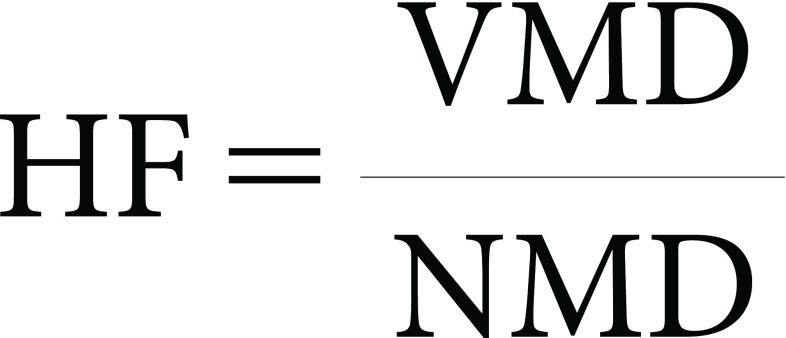 |
(1) |
Homogeneity factor is an indication of the droplet size range. As the HF tends to be 1, spray droplets tend to have the same size, which can only be generated from a uniform droplet generator;17) this is not the case in real spraying machines. The smaller this ratio, the more uniform in size and the narrower in spectrum are the droplets.18) Results in Table 5 reveal that ULVA, GMO, GMV, and MKM showed satisfactory average homogeneity factors of 2, 2, 2.5, and 2.8, respectively. On the other hand, GMG revealed the worst homogeneity factor (13.5 on average) indicating that the droplet size range was less uniform with large droplets of 320–508 µm.
Table 5. Spray coverage on pea’s plants, as produced by the tested sprayers with the recommended rate of bifenthrin.
| Plant levels | ULVA | MKM | GMG | GMO | GMV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SB | HF | VD% | SB | HF | VD% | SB | HF | VD% | SB | HF | VD% | SB | HF | VD% | |
| Upper | 12308 | 1.7 | 60 | 11388 | 2.1 | 32 | 11840 | 8.6 | 18 | 19572 | 2.8 | 31 | 21840 | 2.6 | 16 |
| Middle | 1484 | 2.5 | 7 | 4814 | 1.4 | 14 | 11000 | 22.7 | 17 | 18360 | 1.8 | 29 | 51450 | 2.4 | 38 |
| Lower | 1938 | 1.8 | 10 | 5511 | 5.1 | 15 | 27940 | 9.2 | 43 | 9711 | 1.4 | 16 | 12420 | 2.6 | 9 |
| Ground | 4760 | 23 | 13861 | 39 | 13800 | 21 | 14756 | 24 | 49000 | 36 | |||||
8. Spraying bulk and vertical distribution
The spraying bulk (SB) was calculated from Eq. (2) as follows:
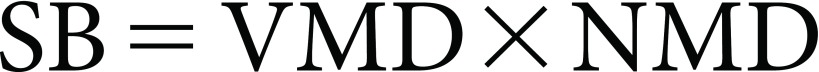 |
(2) |
The percent of vertical distribution (VD) was calculated from Eq. (3) as follows:
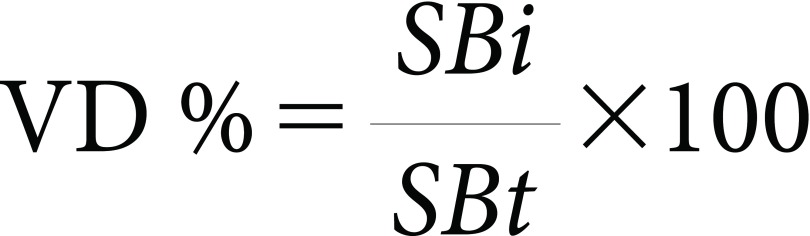 |
(3) |
where SBi is the spray bulk at a certain level, and SBt is the total spray bulk at all levels.
The symmetric distribution in the upper, middle and lower levels were (60, 7, 10%), (32, 14, 15%), (18, 17, 43%), (31, 29, 16%), and (16, 38, 9%) for ULVA, MKM, GMG, GMO, and GMV, respectively. Most of the deposition of the ULVA sprayer (60%) settled in the upper level of the plant, which implies that the lower and middle parts of the plant received insufficient deposits for good protection, while 43% of the deposition of GMG settled at the lower level. This is not the case with GMO, which showed the best vertical distribution among the tested spraying techniques.
The percentages of the spray lost in the ground were 23, 39, 21, 24, and 36% for ULVA, MKM, GMG, GMO, and GMV, respectively. Halawa et al.16) mentioned that the spray lost with the tested spraying machines ranged from 15–40%. Although ULVA showed the best spraying technique from environmental and consumer safety points of view, it has the poorest symmetric distribution of deposits on the vertical levels of the plant.
After careful examination of the results of the bifenthrin residue in pods and leaves, homogeneity factor, symmetric distribution pattern, and spray amount lost on the ground, the authors suggest that the most efficient technique tested was the single nozzle TwinJet GMO, followed by the motorized knapsack sprayer, MKM. However, the authors recommend that the use of vertical boom should be avoided in pea fields because of its undesired impact on the environment and consumers, e.g., high residues in pods and leaves, increased rate of spray solution and loss of pesticide solution on the ground.
Conclusion
The results of the present study revealed that the highest residues of bifenthrin in pea pods and leaves were detected in the samples sprayed by vertical boom machine, while ULVA sprayer showed the lowest residues among all tested spraying machines. Also, the worst HF occurred with the GMG machine, while the best HF was with the GMO. The droplets in the ULVA sprayer treatment were mostly distributed on the upper part of the plants which indicates a lack of symmetric coverage on different levels of the plant. GMO and MKM are suggested for pesticide application in pea fields.
Acknowledgments
The authors wish to thank staff and management of Blue Nile Egypt farm for provision of resources for this work.
References
- 1).G. Borreani, P. G. Peiretti and E. Tabacco: Field Crops Res. 100, 1–9 (2007). [Google Scholar]
- 2).E. Gil, J. Llorens, A. Landers, J. Llop and L. Giralt: Eur. J. Agron. 35, 33–46 (2011). [Google Scholar]
- 3).P. A. Larbi and M. Salyani: Trans. ASABE 55, 41–48 (2012). [Google Scholar]
- 4).G. J. Dorr, A. J. Hewitt, S. W. Adkins, J. Hanan, H. Zhang and B. Noller: Crop Prot. 53, 109–117 (2013). [Google Scholar]
- 5).M. De Schampheleire, D. Nuyttens, K. Baetens, W. Cornelis, D. Gabriels and P. Spanoghe: Precis. Agric. 10, 409–420 (2009). [Google Scholar]
- 6).S. B. Abdel Ghani and O. I. Abdallah: Food Chem. 194, 516–521 (2016). [DOI] [PubMed] [Google Scholar]
- 7).http://faostat.fao.org/site/535/DesktopDefault.aspx?PageID=535#ancor (accessed Aug., 2015)
- 8).S. Hrouzková, M. Andraščíková, S. B. Abdel Ghani and A. Purdešová: Food Anal. Methods 6, 969–977 (2013). [Google Scholar]
- 9).European Food Safety Authority (EFSA): The 2013 European Union report on pesticide residues in food. EFSA J. 13, 66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).M. A. Hindy, R. F. Bakr, N. M. Guniedy, N. S. Ahmed and R. A. Dar: J. Am. Sci. 7, 713–719 (2011). http://www.jofamericanscience.org/journals/am-sci/am0712/092_7682am0712_713_719.pdf (Accessed Oct., 2015) [Google Scholar]
- 11).http://ec.europa.eu/food/plant/pesticides/guidance_documents/docs/qualcontrol_en.pdf (Accessed 2 Aug., 2015)
- 12).N. Yarpuz-Bozdogan, E. Atakan, A. M. Bozdogan, H. Yilmaz, N. Daglioglu, T. Erdem and E. Kafkas: Afr. J. Agric. Res. 6, 660–670 (2011). [Google Scholar]
- 13).http://ec.europa.eu/sanco_pesticides/public/?event=pesticide.residue.selection&language=EN (Accessed 25 Aug., 2015)
- 14).C. M. Himel and A. D. Moore: J. Econ. Entomol. 62, 916–918 (1969). [Google Scholar]
- )15.E. C. Burt, E. P. Lloyed and W. P. Scott, J. R. Mccoy and F. C. Tingles: J. Econ. Entomol. 63, 365–370 (1970). [Google Scholar]
- 16).A. M. Halawa, T. A. Abd-El Rahman, R. A. Dar and N. S. Hiekel: Egypt. Acad. J. Biol. Sci. F. Toxicol. Pest Control 6, 25–33 (2014). [Google Scholar]
- 17).G. A. Matthews: Determination of droplet size. Pest Articles & News Summaries (PANS) 21, 213–225 (1975). [Google Scholar]
- 18).K. Kathirve, T. V. Job and R. Manian: Agricultural Mechanization in Asia, Af. Latin Am 31, 18–21 (2000). [Google Scholar]