Abstract
Pyraziflumid was discovered as a novel SDHI fungicide chemically characterized by the 3-(trifluoromethyl)pyrazine-2-carboxamide group. This chemical series showed particularly high fungicidal activities against a broad spectrum of plant diseases in the case of N-(biphenyl-2-yl) as well as N-(1,1,3-trimethylindan-4-yl)carboxamides. Various N-(biphenyl-2-yl)pyrazine-2-carboxamides were synthesized, and their structure–activity relationships were studied. The optimization of the fungicidal performance of the series finally led to the identification of pyraziflumid, which could control a wide range of plant diseases. In this report, details of the structure–activity relationships from the lead compound to pyraziflumid are described.
Keywords: pyraziflumid, NNF-0721, 3-(trifluoromethyl)pyrazine-2-carboxamide, SDHI, fungicide, structure-activity relationships
Introduction
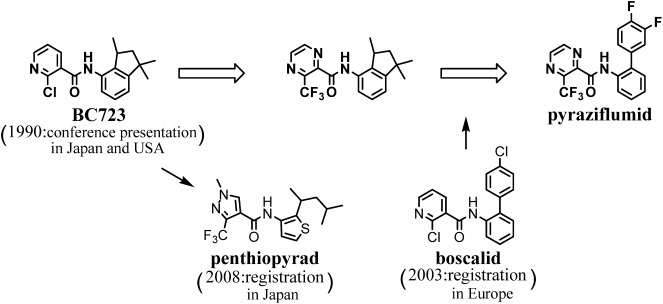
Carboxin-related carboxamides have been prepared, and their selective control over plant diseases caused by Basidiomycetes has been demonstrated.1–6) In 1990, we discovered that BC723, 2-chloro-N-(1,1,3-trimethylindan-4-yl)pyridine-3-carbxamide, had potent fungicidal activity against gray mold caused by Ascomycetes, Botrytis cinerea, as well as wheat brown rust (Puccinia recondita) and rice sheath blight (Rhizoctonia solani) caused by Basidiomycetes.7,8) BC723 was clarified to inhibit the mitochondrial succinate dehydrogenase complex (SDC; mitochondrial complex II) of B. cinerea as well as that of R. solani.9,10) The structure–activity relationships of BC723 analogues on the fungicidal activity against gray mold and rice sheath blight were studied and demonstrated in our previous papers.11–13) Since then, penthiopyrad, N-[2-(1,3-dimethylbutyl)-3-thienyl]-1-methyl-3-(trifluoromethyl)pyrazole-4-carboxamide, has been discovered based on BC723 as the lead compound,14) and some other succinate dehydrogenase inhibitor (SDHI) fungicides having such a broad spectrum have been discovered and launched.15–19) Almost all of these fungicides possess a pyridine or pyrazole group as the carboxylic acid moiety. However, it was suggested in our previous paper13) regarding the structure–activity relationships of N-(1,1,3-trimethylindan-4-yl)carboxamides that the 3-substituted pyrazine-2-carboxamides may also have activities as high as those of the 2-subustituted pyridine-3-carboxamides. Taking the information above and our new chemistry into consideration, we started to reexamine the carboxylic acid moiety in order to discover another novel SDHI fungicide that has a broader spectrum and more potent inhibiting activity against SDC than BC723. As a result of validating the pyrazine compounds, we have found that 3-(trifluoromethyl)pyrazine-2-carboxamides in particular tended to show high fungicidal activities against various plant diseases.20,21) The optimization of the fungicidal performance of the chemical series finally led to the identification of pyraziflumid, N-(3′,4′-difluorobiphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamide as shown in Fig. 1.22) Pyraziflumid has excellent activities against various diseases in the field of vegetables, fruit trees, turf, and rice as well as gray mold, sclerotinia rot, and powdery mildew. It has been practically evaluated in Japan Plant Protection Association (JPPA) official trials with the code number of NNF-0721, and application for registration in Japan has been made. Here we describe the discovery, chemistry, and the structure–activity relationships of pyrazine-2-carboxamides through the evaluation of control activity against cucumber gray mold, wheat brown rust, and barley powdery mildew.
Fig. 1. Discovery of pyraziflumid.
Materials and Methods
1. Preparation of compounds
Chemical structures were confirmed by 1H-NMR spectroscopy using a Bruker ARX-400 NMR spectrometer with tetramethylsilane as an internal standard. Melting points were measured with a Mettler FP80 melting point apparatus and left uncorrected.
1.1. Synthesis
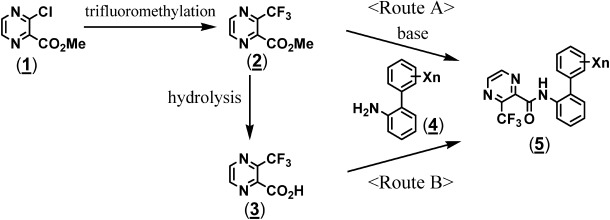
Compounds were prepared according to either one of two routes, as shown in Fig. 2. Some N-(substituted biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamides (5) were obtained by reacting methyl 3-(trifluoromethyl)pyrazine-2-carboxylate (2) with a corresponding biphenyl amine (4) in the presence of a base (Route A).20,23) The others were obtained by condensing 3-(trifluoromethyl)pyrazine-2-carboxylic acid (3) synthesized by the hydrolysis of methyl ester (2) and a corresponding biphenyl amine (4) in the presence of a base (Route B).23–25) Methyl 3-(trifluoromethyl)pyrazine-2-carboxylate (2) was obtained by the trifluoromethylation of methyl 3-chloropyrazine-2-carboxylate (1) synthesized by known methods.13,26) As the respective examples, the procedures used to synthesize pyraziflumid (20) and compound 14 are described below.
Fig. 2. Synthetic pathways of 3-(trifluoromethyl)pyrazine-2-carboxamides.
1.2. Methyl 3-(trifluoromethyl)pyrazine-2-carboxylate (2)
To a slurry mixture of methyl 3-chloropyrazine-2-carboxylate (1) (32.5 g, 188 mmol) and copper (I) iodide (35.8 g, 188 mmol) in dimethylformamide (DMF, 60 mL) and toluene (60 mL), methyl 2-(fluorosulfonyl)difluoroacetate (54.2 g, 282 mmol) was added at 100°C. The resulting mixture was heated at 115°C for 2 hr. under an argon atmosphere. After cooling, the reaction mixture was filtered with Celite, followed by dilution of the filtrate with ethyl acetate. The organic layer was washed with water and brine, dried over sodium sulfate, and concentrated in vacuo. The residue was chromatographed (SiO2, hexane-ethyl acetate=4 : 1) to give a colorless oil (2) (24.0 g, 62% yield). 1H-NMR (CDCl3) δ ppm: 8.85 (d, 1H), 8.83 (d, 1H), 4.05 (s, 3H).
1.3. 3-(Trifluoromethyl)pyrazine-2-carboxylic acid (3)
To a solution of compound (2) (9.80 g, 47.5 mmol) in ethanol (30 mL), aqueous potassium hydroxide solution (3.20 g, 48.0 mmol of KOH in 40 mL of H2O) was added under water cooling and stirred at room temperature for 1.5 hr. The reaction mixture was concentrated in vacuo and the residue was washed with diethyl ether. The aqueous layer was acidified with c-HCl, followed by extracting with ethyl acetate and washing with water and brine in order. The solution was dried over magnesium sulfate anhydride and concentrated in vacuo to give a white crystal (3) (7.00 g, 63% yield). mp 146.5–147°C. 1H-NMR (CDCl3) δ ppm: 8.96 (d, 1H), 8.88 (d, 1H), 3.2–3.9 (br, 1H).
1.4. N-(Substituted biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamides (5)
1.4.1. N-(3′,4′-Difluorobiphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamide (pyraziflumid) by Route A
To a mixture of 3′,4′-difluorobiphenyl-2-amine (4) (40.0 g, 195 mmol), N,N-dimethylacetamide (40 mL), and 28% sodium methoxide in methanol (118 mL, 580 mmol), methyl 3-(trifluoromethyl)pyrazine-2-carboxylate (2) (50.1 g, 243 mmol) was added and stirred at room temperature for 9 hr. The reaction mixture was poured into ice water (250 g) and c-HCl (50 mL) and extracted with ethyl acetate. The extract was washed with water and brine and concentrated in vacuo. The residue was recrystallized from heptane and ethyl acetate to yield a pale yellow crystal (pyraziflumid) (60.4 g, 86% yield). mp 119–120°C. 1H-NMR (CDCl3) δ ppm: 9.56 (s, 1H), 8.83 (d,1H), 8.64 (s, 1H), 8.54 (d, 1H), 7.47 (dt, 1H), 7.3 (m, 4H), 7.19 (m, 1H). Water solubility: 2.32×10−3g / L (20°C). Partition coefficient log Po/w : 3.51 (25°C).
1.4.2. 3-(Trifluoromethyl)-N-[4′-(trifluoromethyl)biphenyl-2-yl]pyrazine-2-carboxamide (compound 14) by Route B
To a slurry mixture of 3-(trifluoromethyl)pyrazine-2-carboxylic acid (3) (192 mg, 1.00 mmol), 4′-(trifluoromethyl)biphenyl-2-amine (4) (238 mg, 1.00 mmol) and 2-chloro-1-methylpyridinium iodide (255 mg, 1.00 mmol) in tetrahydrofuran (10 mL), triethylamine (303 mg, 3.00 mmol) was added and stirred at room temperature for 9 hr. The reaction mixture was diluted with ethyl acetate, followed by washing with water and brine. The organic layer was dried over magnesium sulfate anhydride and concentrated in vacuo. The residue was purified by silica gel chromatography (eluent; hexane–ethyl acetate=2 : 1) to give a white solid (14) (293 mg, 62% yield). mp 147–148°C. 1H-NMR (CDCl3) δ ppm: 9.5 (s, 1H), 8.81 (s, 1H), 8.58 (d, 1H), 8.54 (d, 1H), 7.76 (d, 2H), 7.59 (d, 2H), 7.48 (t, 1H), 7.3 (m, 2H).
1.4.3. Other derivatives
The other derivatives were synthesized in a similar way by the reaction of the pyrazine carboxylic acids or esters and corresponding biphenyl-2-amines, and commercially available reagents and solvents were used.
2. Biological assay
2.1. Application method
Each compound was prepared as 100 g a.i./L of a tentative emulsifiable concentrate (EC) formulation and diluted to certain concentrations (200 to 0.5 mg a.i./L) with water containing a wetting agent (Mai-Rinoh®, 0.1 mL/L). A cucumber plant (Cucumis sativas cv. Suyoh) at 1.5-leaf stage, wheat seedlings (Triticum aestivum cv. Nohrin 61) at 2-leaf stage, and barley seedlings (Hordeum vulgare cv. Kantoh 6) at 2-leaf stage were sprayed with an equivalent of 2000 L per hectare of test solution. After air-drying, treated plants were inoculated with target fungi. Each treatment consisted of two replications.
2.2. Inoculation methods
2.2.1. Cucumber gray mold
Cotyledons were cut from treated cucumber plants and placed in plastic cases. A spore suspension of B.cinerea (1×106 spores/mL) was held on a paper disk (6 mm diameter; Toyo Roshi Kaisha, Ltd.) with potato sucrose medium and the disk was put on the center of cotyledon. The plastic cases were maintained under wet conditions and kept at 15°C for 5 days.
2.2.2. Wheat brown rust
Treated wheat plants were inoculated with a spore suspension of Puccinia recondita (1×106 spores/mL) and kept in a chamber (Koito Electric Industries, Ltd.) at 20°C, 100% relative humidity, under dark conditions for 24 hr, and then inoculated plants were maintained in a greenhouse for 7 days.
2.2.3. Barley powdery mildew
Treated barley plants were dusted with spores of Blumeria graminis f. sp. hordei directly from diseased plants. Inoculated plants were maintained in a greenhouse for 7 days.
2.3. Assessment methods
The control activity was evaluated by the infected area on the leaves. The percentage of infected area was measured visually and the protected value (%) was calculated using the following formula:
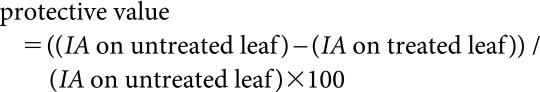 |
where IA means infected area.
The fungicidal activity of test compounds was shown as EC80 values that were determined from the protective value. EC80 means the concentration of test compounds that shows an 80% efficacy of protective value.
Results and Discussion
1. Biological activities of pyridine-3- and pyrazine-2-carboxamides
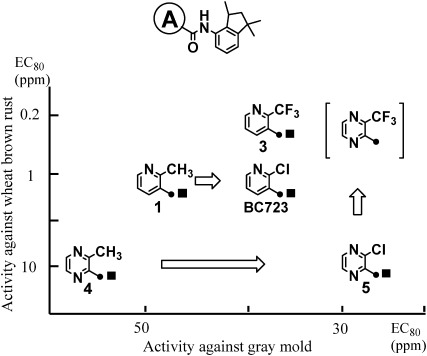
According to our previous paper regarding the structure–activity relationships of N-(1,1,3-trimethylindan-4-yl)carboxamides,13) the 3-subustituted pyrazine-2-carboxamides could give as high activities as the 2-subustituted pyridine-3-carboxamides against both diseases caused by Basidiomycetes and Ascomycetes. The relationships between the compounds, N-(1,1,3-trimethylindan-4-yl)pyridine-3- and pyrazine-2-carbxamides, and their fungicidal activities against gray mold and wheat brown rust are summarized in Fig. 3. In the case of pyridine-3-carboxamides, the 2-methylpyridine-3-carboxamide derivative (1) showed moderate activity against both diseases. BC723, in which the substituent at the 2-position was changed from the methyl group to a chlorine atom, maintained its activity against brown rust and improved its activity against gray mold. Furthermore, the 2-(trifluoromethyl)pyridine-3-carboxamide derivative (3) showed higher activity against brown rust than did the 2-chloro-substituted derivative (BC723), maintaining its activity against gray mold. In the case of pyrazine-2-carboxamides, the 3-methylpyrazine-2-carboxamide derivative (4) showed moderate activity against both diseases. Compound 5, in which the substituent at the 3-position was changed from a methyl group to a chlorine atom, also maintained its activity against brown rust and greatly increased its activity against gray mold. If substituent effects of the pyrazine-2-carboxamides showed a similar tendency to those of the pyridine derivatives, the 3-(trifluoromethyl)pyrazine derivative was expected to give a higher performance than BC723 against both diseases. However, 3-(trifluoromethyl)pyrazine-2-carboxylic acid was previously unknown and was synthesized at our research center as a novel compound.26) We utilized it to synthesize the 3-(trifluoromethyl)pyrazine-2-carboxamides and examined their various fungicidal activities.20,21)
Fig. 3. Fungicidal activities of N-(1,1,3-trimethylindan-4-yl)pyridine and pyrazinecarboxamides.13).
N-(1,1,3-Trimethylindan-4-yl)pyridine-3- or pyrazine-2-carboxamides and their fungicidal activities against barley powdery mildew in addition to cucumber gray mold and wheat brown rust are summarized in Table 1. In the case of pyridine derivatives, 2-(trifluoromethyl)pyridine-3-carboxamide (3) showed almost the same activity against gray mold and only a little higher activity against wheat brown rust in comparison with the chloro-substituted derivative (2, BC723). The activity against barley powdery mildew of the trifluoromethyl-substituted derivative (3) was inferior to that of the chloro-substituted derivative (2). In the case of pyrazine derivatives, 3-(trifluoromethyl)pyrazine-2-carboxamide (6) showed almost the same activity against gray mold as the chloro-substituted derivative (5), as in the case of pyridine derivatives. However, the activity of the (trifluoromethyl)pyrazine derivative (6) against wheat brown rust and barley powdery mildew was more than 10 times and 20–200 times higher than that of the chloro-substituted derivative (5), respectively. Thus, 3-(trifluoromethyl)pyrazine-2-carboxamide tended to show higher activity than any the other carboxamides against a broad spectrum of plant diseases. Such drastic enhancement of activity seemed to be caused by the substituent effect of the trifluoromethyl group peculiar to 3-substituted pyrazine-2-carboxamides, because such a phenomenon was not seen in the case of 2-substituted pyridine-3-carboxamides.
Table 1. N-(1,2,3-Trimethylindan-4-yl)pyridine-3- or pyrazine-2-carboxamides and their biological activities.
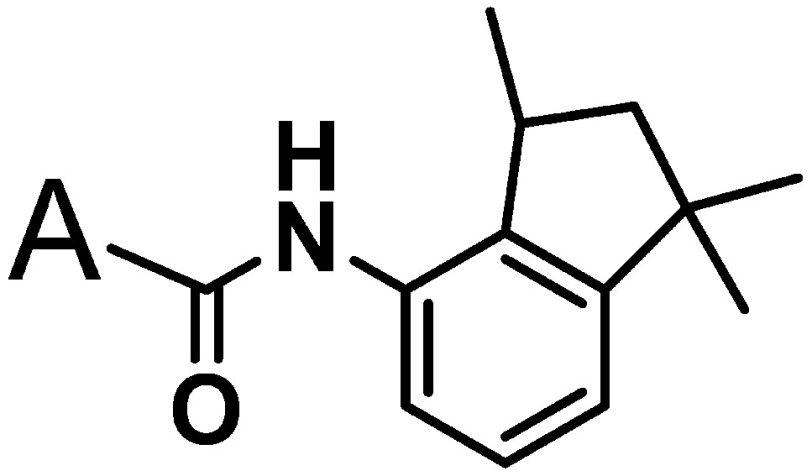 | |||||
|---|---|---|---|---|---|
| Compound No. | A | mp (°C) | Fungicidal activity EC80 (ppm) | ||
| Gray mold | Brown rust | Powdery mildew | |||
| 1 | 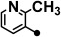 |
123.5–124.5 | 20–100 | 1–10 | 200 |
| 2BC723 | 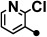 |
133–134 | 10–40 | 1–10 | 200 |
| 3 | 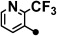 |
174–175 | 10–40 | <1 | >200 |
| 4 | 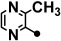 |
78.2–78.7 | 50–200 | 5–20 | >200 |
| 5 | 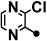 |
124–125 | 10–40 | 5–20 | 200 |
| 6 | 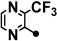 |
169.1–170.1 | 10–40 | <1 | 1–10 |
It has been reported that some N-(biphenyl-2-yl)carboxamides may exhibit biological activities similar to those of N-(1,1,3-trimethylindan-4-yl)carboxamides.14) Therefore, the generality of the particular substituent effect of (trifluoromethyl)pyrazine group on N-(biphenyl-2-yl)carboxamides was examined, and the results are shown in Table 2. 3-(Trifluoromethyl)pyrazine-2-carboxamide (8) showed only a little higher activity against gray mold than did the chloro-substituted derivative (7), whereas it exhibited 10–100 times higher activity against both barley powdery mildew and wheat brown rust than did the chloro-substituted derivative (7). Thus, the particular substituent effect of the (trifluoromethyl)pyrazine group was also observed in the case of N-(biphenyl-2-yl)carboxamides. Since N-(biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamide exhibited well-balanced high activity against all three of these diseases, we started to optimize the substituents of biphenyl moiety in the following research.
Table 2. N-(Biphenyl-2-yl)-3-substituted pyrazine-2-carboxamides and their biological activities.
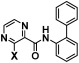 | |||||
|---|---|---|---|---|---|
| Compound No. | X | mp (°C) or nD25 | Fungicidal activity EC80 (ppm) | ||
| Gray mold | Brown rust | Powdery mildew | |||
| 7 | Cl | 1.6495 | 10–40 | 50–200 | >200 |
| 8 | CF3 | 61–66 | 5–20 | 5–20 | 5–20 |
2. Structure–activity relationships
2.1. N-(4′-Substituted biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamides
The fungicidal activities of N-(biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamides substituted by alkyl groups, a trifluoromethyl group or halogen atoms at the 4′-position of biphenyl moiety are shown in Table 3. Methyl or t-butyl derivatives (9, 10) showed almost the same level of activity as the unsubstituted compound (8) against gray mold but lower activity against brown rust and powdery mildew. Halogen atoms or trifluoromethyl derivatives (11–14) showed almost the same level of activity as the unsubstituted compound (8) against brown rust, whereas they exhibited higher activity against gray mold and powdery mildew. This suggested that electron withdrawing groups at the 4′-position on the biphenyl group might be favorable for fungicidal activities. Therefore, the substituent effects at various positions were examined in the following research.
Table 3. N-(4′-Substituted biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamides and their biological activities.
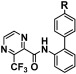 | |||||
|---|---|---|---|---|---|
| Compound No. | R | mp (°C) | Fungicidal activity EC80 (ppm) | ||
| Gray mold | Brown rust | Powdery mildew | |||
| 8 | H | 61–66 | 5–20 | 5–20 | 5–20 |
| 9 | Me | 110–111 | 5–20 | 200 | 20–100 |
| 10 | t-Bu | 127.8–129.1 | 10–40 | 50–200 | 10–40 |
| 11 | F | 133–134 | 2–10 | 20–100 | 2–10 |
| 12 | Cl | 145–146 | 2–10 | 2–10 | 5–20 |
| 13 | Br | 156–157 | 2–10 | 20–100 | 0.5–2 |
| 14 | CF3 | 147–148 | 5–20 | 5–20 | 2–10 |
2.2. N-(Substituted biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamides
N-(Biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamides substituted by a fluorine atom, chlorine atom, or trifluoromethyl group at the 2′-, 3′-, or 4′-position and their fungicidal activities are shown in Table 4. With regard to the activity against gray mold, the derivative substituted by a fluorine atom at the 4′-position (11) exhibited the highest activity, followed by the 3′-substituted derivative (15) and the 2′-substituted derivative (16), which showed the lowest activity. Similarly, the derivatives substituted by a chlorine atom or a trifluoromethyl group at the 4′-position (12, 14) showed the highest activity among their respective regioisomers. With respect to the activity against brown rust, the derivative substituted by a fluorine atom at the 3′-position (15) showed the highest activity, followed by the 4′- and 2′-substituted derivatives (11, 16). Similarly, the derivatives substituted by a chlorine atom or a trifluoromethyl group at the 3′-position (17, 19) gave the highest activity among their respective regioisomers. These evaluation results suggested that the control activity against gray mold might be governed by the specific effect of electron withdrawing groups at the 4′-position, whereas the activity against brown rust was supposed to be specifically influenced by the 3′-position substituents. Compounds 16 and 18, substituted by a fluorine or chlorine atom at the 2′-position, showed the lowest activity against both gray mold and brown rust among their respective regioisomers. This suggested that the substituents at the 2′-position might be unfavorable for activity against gray mold and brown rust.
Table 4. N-(Monosubstituted biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamides and their biological activities.
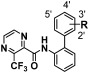 | |||||
|---|---|---|---|---|---|
| Compound No. | R | mp (°C) | Fungicidal activity EC80 (ppm) | ||
| Gray mold | Brown rust | Powdery mildew | |||
| 11 | 4′-F | 133–134 | 2–10 | 20–100 | 2–10 |
| 15 | 3′-F | 69–70 | 10–40 | 2–10 | 2–10 |
| 16 | 2′-F | 96.6–98.7 | 20–100 | 20–100 | 2–10 |
| 12 | 4′-Cl | 145–146 | 2–10 | 2–10 | 5–20 |
| 17 | 3′-Cl | 110.5–111.5 | 5–20 | 0.5–2 | 2–10 |
| 18 | 2′-Cl | 126.7–128.1 | 50–200 | 20–100 | 2–10 |
| 14 | 4′-CF3 | 147–148 | 5–20 | 5–20 | 0.5–2 |
| 19 | 3′-CF3 | 120–121.5 | 50–200 | 1–5 | 2–10 |
With respect to the activity against powdery mildew, the derivatives substituted by a fluorine atom (11, 15, 16) showed almost the same activity as one another. The derivative substituted by a chlorine atom at the 4′-position (12) showed lower activity than its regioisomers (17, 18), whereas the derivative substituted by a trifluoromethyl group at the 4′-position (14) showed higher activity than its regioisomer (19). Thus, no such regiospecific effect of substituents as stated above was observed on the activity against powdery mildew.
2.3. N-(Disubstituted biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamides
As we have clarified the structure–activity relationships of the derivatives having a single substituent on biphenyl moiety, we next examined the fungicidal activity of the derivatives with two substituents on it. N-(Biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamides substituted by a fluorine atom, chlorine atom, or trifluoromethyl group at two positions on the biphenyl group and their fungicidal activities are shown in Table 5. Compounds 20–23, having a fluorine atom, chlorine atom or trifluoromethyl group at the 3′- and 4′-positions, exhibited the same high activity against gray mold as did corresponding monosubstituted compounds 11, 12, and 14, having a fluorine atom, chlorine atom or trifluoromethyl group at the 4′-position, respectively. Compounds 20–23 also showed almost the same level of high activity against brown rust as did corresponding monosubstituted compounds 15 and 17, having a fluorine or chlorine atom at the 3′-position, respectively. These evaluation results of the 3′,4′-disubstituted derivatives suggested that the regiospecific effects of substituents at the 4′- and 3′-position might work independently and contribute to the activity against gray mold and brown rust additively. However, compounds 20 and 23 showed slightly higher activity against brown rust than did the 3′-fluorine derivative (15). In this case, a synergistic effect might be generated by a fluorine atom at the 3′-position and by a trifluoromethyl group or a fluorine atom at the 4′-position in addition to the regiospecific effect at the 3′-positon on the activity against brown rust.
Table 5. N-(Disubstituted biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamides and their biological activities.
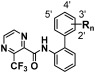 | |||||
|---|---|---|---|---|---|
| Compound No. | Rn | mp (°C) | Fungicidal activity EC80 (ppm) | ||
| Gray mold | Brown rust | Powdery mildew | |||
| 20 (pyraziflumid) | 3′,4′-F2 | 119–120 | 2–10 | 0.5–2 | 0.5–2 |
| 21 | 3′,4′-Cl2 | 132–132.6 | 2–10 | 0.5–2 | 2–10 |
| 22 | 3′-F,4′-Cl | 128–129 | 2–10 | 2–10 | 0.5–2 |
| 23 | 3′-F,4′-CF3 | 142.5–143.5 | 5–20 | 0.5–2 | 0.5–2 |
| 24 | 3′,5′-F2 | 119–120 | 5–20 | 2–10 | 0.5–2 |
| 25 | 3′,5′-Cl2 | 117–118 | 50–200 | 2–10 | 2–10 |
| 26 | 3′,5′-(CF3)2 | 147–148 | >200 | 5–20 | >200 |
| 27 | 2′,4′-F2 | 117–118 | 2–10 | 5–20 | 0.5–2 |
| 28 | 2′,4′-Cl2 | 135.5–136.5 | 5–20 | 5–20 | 2–10 |
| 29 | 2′-F,4′-Cl | 127–128 | 2–10 | 10–50 | 0.5–2 |
| 30 | 2′,5′-F2 | 88–89 | 10–40 | 2–10 | 2–10 |
Compound 24, substituted by two fluorine atoms at the 3′- and 5′-positions, showed the same level of activity against gray mold and brown rust as mono-fluorine derivative substituted at the 3′-position (15). Compounds 25 and 26, substituted by two chlorine atoms or trifluoromethyl groups at the 3′- and 5′-positions, showed a little lower activity against gray mold and brown rust than mono-chlorine or mono-trifluoromethyl derivative substituted at the 3′-position (17, 19). These data supported that the 3′,5′-disubstituted derivatives gave no additive contribution of regiospecific substituent effects at the 3′- and 5′-positions toward the activity against brown rust.
Compounds 27–29, substituted by fluorine or chlorine atoms at the 2′- and 4′-positions, exhibited 10-fold higher activity against gray mold and only a little higher activity against brown rust than did the respective 2′-substituted derivatives (16, 18). These drastic enhancements of fungicidal activity against gray mold might be caused by a specific substituent effect at the 4′-position. Compound 30, substituted by fluorine atoms at the 2′- and 5′-positions, exhibited 10-fold higher activity against brown rust, and almost the same activity against gray mold in comparison with the 2′-substituted derivative (16). These evaluation results also support the hypothesis that the regiospecific substituent effects at the 4′- or 3′-position might be independent and contribute additively to the activity against gray mold and brown rust.
With respect to activity against powdery mildew, compounds 20, 22, and 23, substituted by a fluorine atom either at the 3′- or 4′-position, exhibited relatively high activity among the 3′,4′-disubstituted derivatives. Compound 24, substituted by fluorine atoms at the 3′- and 5′-positions, showed the highest activity among the 3′,5′-disubstituted compounds. Compounds 27 and 29, substituted by a fluorine atom either at the 2′- or 4′-position, also exhibited higher activity than the 2′,4′-dichloro derivative (28). These evaluation results suggested that the disubstituted derivatives including a fluorine atom at any position could show high activity against powdery mildew.
Among the compounds tested, compound 20, substituted by fluorine atoms at the 3′- and 4′-positions, showed the most potent activity against all three of the diseases. Through further examinations and various field trials, compound 20 was finally selected as a development compound (pyraziflumid) that exhibits superior performance as a new fungicide candidate.
Conclusion
Pyraziflumid was discovered as a novel SDHI fungicide chemically characterized by 3-(trifluoromethyl)pyrazine-2-carboxamide, whose chemical series tended to show particularly high activities against various diseases in the case of N-(biphenyl-2-yl) as well as N-(1,1,3-trimethylindan-4-yl)carboxamides. The structure–activity relationships of N-(biphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamides suggested that the control activity against gray mold might be specifically governed by the substituent effect at the 4′-position, whereas activity against brown rust could be influenced by the 3′-position substituents. The regiospecific substituent effects at the 4′- or 3′-position seemed to be independent and contribute additively to the activity against gray mold and brown rust. With respect to control activity against powdery mildew, a regiospecific effect of substituents was not observed, but disubstituted derivatives including a fluorine atom at any position exhibited high activity. These results on structure–activity relationships through the evaluation of control activity against three diseases demonstrate that N-(3′,4′-difluorobiphenyl-2-yl)-3-(trifluoromethyl)pyrazine-2-carboxamide, pyraziflumid, is the most well-balanced compound as a fungicide from the viewpoint of unique regiospecific substituent effects.
Acknowledgments
The authors are deeply indebted to our colleagues at the Reseach Center, Nihon Nohyaku Co., Ltd., for their expert technical support in determining antifungal activity. We also wish to express our thanks to Dr. T. Konno and Dr. K. Tsubata, Nihon Nohyaku Co., Ltd., for their invaluable suggestions.
References
- 1) B. von Schmeling and M. Kulka: Science 152, 659–660 (1966). [DOI] [PubMed] [Google Scholar]
- 2) A. J. P. Frost, K. U. Junk and J. L. Belford: Med. Rijksfac. Landbouwwet. Gent 1, 111 (1973). [Google Scholar]
- 3) S. Kawada, A. Sakamoto and I. Shimazaki: J. Pestic. Sci. 10, 315–324 (1985). [Google Scholar]
- 4) F. Araki: Jpn. Pestic. Inform 47, 23–25 (1985). [Google Scholar]
- 5) G. A. White and G. D. Thorn: Pestic. Biochem. Physiol. 5, 380–395 (1975). [Google Scholar]
- 6) G. A. White, J. N. Phillips, J. L. Huppatz, B. Witrzens and S. J. Grant: Pestic. Biochem. Physiol. 25, 163–168 (1986). [Google Scholar]
- 7) M. Oda, N. Sasaki, T. Sakaki, N. Nonaka and K. Yamagishi: Abstr. 15th Pestic. Sci. Soc. Japan, A210 (1990), (in Japanese).
- 8) M. Oda, T. Sakaki, N. Sasaki, N. Nonaka and K. Yamagishi: Abstr. 15th Pestic. Sci. Soc. Japan, A211 (1990), (in Japanese).
- 9) N. Nonaka, S. Satoh, H. Tomita and M. Oda: Ann. Phytopathological Soc. Jpn. 56, 407 (1990), Abstr. [Google Scholar]
- 10) K. Yamagishi, N. Nonaka, H. Tomita, N. Sasaki and M. Oda: Ann. Phytopathological Soc. Jpn. 56, 407 (1990), Abstr. (in Japanese). [Google Scholar]
- 11) M. Oda, N. Sasaki, T. Sakaki, N. Nonaka, K. Yamagishi and H. Tomita: J. Pestic. Sci. 17, 91–98 (1992). [Google Scholar]
- 12) M. Oda, T. Sakaki, N. Sasaki, N. Nonaka, K. Yamagishi and H. Tomita: J. Pestic. Sci. 18, 49–57 (1993). [Google Scholar]
- 13) M. Oda, T. Sakaki, N. Sasaki, N. Nonaka, K. Yamagishi and H. Tomita: J. Pestic. Sci. 18, 245–251 (1993). [Google Scholar]
- 14) Y. Yoshikawa, H. Katsuta, J. Kishi and Y. Yanase: J. Pestic. Sci. 36, 347–356 (2011). [Google Scholar]
- 15) G. Stammler, H. D. Brix, A. Glaettli, M. Semar and U. Schoefl: Proceedings 16th International Plant Protection Congress Glasgow, pp. 40–45, 2007. (in UK).
- 16) R. Dunkel, H. Rieck, Heiko, H.-L. Elbe, U. Wachendorff-Neumann, K.-H. Kuck (Bayer Cropscience AG): PCT Int. Appl. WO2003/070705 (2003).
- 17) J. Eheenfreuvd, H. Tobler, H. Walter (Syngenta Participations AG): PCT Int. Appl. WO2003/074491 (2003).
- 18) J. Eheenfreuvd, H. Tobler, H. Walter (Syngenta Participations AG): PCT Int. Appl. WO2004/035589 (2004).
- 19) G. Markus, D. Jochen, G. Thomas, B. Carsten, G. Wassilios, H. Udo, M. Bernd, S. Frank, S. Anja, L. Jan Klaas, R. Joachim, S. Peter, S. Siegfried, S. Reinhard (BASF AG): PCT Int. Appl. WO2006/087343 (2006).
- 20) M. Oda, T. Furuya, M. Hasebe and N. Kuroki (Nihon Nohyaku Co., Ltd.): PCT Int. Appl. WO2007/072999 (2007).
- 21) M. Oda, T. Furuya, M. Hasebe, N. Kuroki and K. Kikutake (Nihon Nohyaku Co., Ltd.): PCT Int. Appl. WO2007/125749 (2007).
- 22) M. Oda, T. Furuya, Y. Morishita, Y. Matsuzaki, M. Hasebe and N. Kuroki: Abstr. 41th Pestic. Sci. Soc. Japan, C307 (2016), (in Japanese).
- 23) K. Ohshima, Y. Matsuzaki and M. Oda: (Nihon Nohyaku Co., Ltd.): Jpn. Kokai Tokkyo Koho JP 242244 (2009), (in Japanese).
- 24) M. Oda, Y. Matsuzaki and Y. Morishita (Nihon Nohyaku Co., Ltd.): PCT Int. Appl. WO2010/055884 (2010).
- 25) M. Oda (Nihon Nohyaku Co., Ltd.): PCT Int. Appl. WO2010/122793 (2010).
- 26) T. Furuya, K. Machiya, A. Suwa and S. Fujioka (Nihon Nohyaku Co., Ltd.): PCT Int. Appl. WO2005/115994 (2005).