Abstract
Strigolactones (SLs) are a series of sesquiterpene lactones that serve as plant hormones to regulate plant growth and development, such as shoot branching, lateral root formation, and root hair elongation. Recently, SLs have been reported to accelerate the leaf senescence, which is also regulated by sugar signals. In this study, we utilized segments of a bamboo leaf to observe leaf senescence and confirmed that SL accelerates leaf senescence and triggers cell death under a dark condition rather than under a light condition. Further studies showed that the co-treatment of sugars suppressed SL-induced leaf senescence and cell death under dark conditions, suggesting a crosstalk between SL and the sugar signal in regulating leaf senescence.
Keywords: strigolactone, leaf senescence, sugar, bamboo, leaf segment
Introduction
Strigolactones (SLs), a family of sesquiterpene lactones, were first identified to be stimulants that induce the seed germination of parasitic plants.1) Subsequent studies demonstrated versatile roles of SLs, which promote the hyphal branching of arbuscular mycorrhizal (AM) fungi and regulate plant stature as plant hormones by suppressing shoot branching, such as in rice and Arabidopsis.2,3) SLs have also been confirmed to enhance lateral root formation and root hair elongation, stimulate cambial activity, and counteract seed dormancy. Recently, Yamada et al.4) reported that GR24, a synthetic SL analog, accelerated darkness-induced leaf senescence in SL-deficient rice mutants (dwarf27/d27, d17, and d10) but not in SL-insensitive mutants (d14 and d13), which verified the former observations that SL-deficient and SL-insensitive mutants display a phenotype with delayed leaf senescence.5–9) Further studies showed that leaf senescence is much more sensitive to GR24 in a phosphate-deficient condition, indicating that SLs regulate leaf senescence in response to available levels of phosphate.4) Similar results were obtained by Ueda & Kusaba9) in Arabidopsis, where SL-deficient mutants (more axillary growth3/max3, max4, and max1) and SL-insensitive mutants (Atd14 and max2) displayed delayed leaf senescence in the dark when compared to the wild type. Treatment with GR24 accelerated the leaf senescence in SL-deficient mutants but not in SL-insensitive mutants. Ueda & Kusaba9) further demonstrated that SL accelerates leaf senescence in an ethylene-dependent manner and an ethylene-independent manner, suggesting that other unknown factors play roles in SL-induced leaf senescence.
Here, we established a test system using a bamboo leaf segment to investigate the roles of sugar in SL-induced leaf senescence. Our results showed that GR24 induced the senescence of bamboo leaf segments and cell death with dark treatment rather than with light treatment. GR24-induced leaf senescence is attributed to apoptotic-like cell death, which can be alleviated by co-treatment with sugars, indicating the crosstalk between SL and sugars in leaf senescence.
Materials and Methods
1. Plant materials
The bamboo leaf segments were produced from the third expanding leaves on the mature stalks of Bambusa oldhamii (B. oldhamii) cultivated in the biotron of Zhejiang Agriculture and Forest University. The leaves were cut into 1 cm×1.5 cm segments and washed with distilled water.
2. Plant growth conditions and treatments
The leaf segments were immersed and cultivated in distilled water containing GR24 or not, or both GR24 and sugars (Glc, Fru, or Suc) or mannitol as a control at 25°C under a constant light (120 µmol/m2/s) or dark condition for 14 days.
3. Measurement of chlorophyll content and the photochemical efficiency of PSII
The chlorophylls of leaf segments with or without treatment were extracted with 95% ethanol for 24 hr in the dark. The absorbance of extractions was recorded with a microplate reader (Infinite 200, TECAN Inc., Maennedorf, Austria) at 664.2 nm and 648.6 nm. The chlorophyll (Chl) content was calculated according to the equation Ca+b=5.24×A664.2+22.24×A648.6.10) Measurements of the photochemical efficiency of PSII were carried out with Imaging-PAM (Walz GmbH, Effeltrich, Germany). Briefly, the fluorescence of leaf segments was detected after dark adaption for 10 min (F0). Then another detection was performed with a saturation pulse (2800 µmol/m2/s) (Fm). The photochemical efficiency of PSII (represented by Fv/Fm) was calculated with the equation Fv/Fm=(Fm-F0)/Fm.
4. DNA extraction and electrophoresis
Genome DNA was isolated using the CTAB method and detected with electrophoresis (1% agar).
5. Trypan blue staining
Leaf segments were stained by trypan blue as described by Keogh et al.11) Briefly, leaf segments immersed in trypan blue solution were boiled for 1 min and left overnight. Then the staining solution was replaced with a chloral hydrate solution for distaining.
6. Real-time RT-PCR analysis
Total RNA from B. oldhamii was isolated by using RNAiso Plus (Takara, Shiga, Japan), then reverse transcriptase M-MLV (Takara) was used to synthesize first-strand cDNA, according to the manufacturers’ protocols. The CFX96TM Real-Time PCR Detection System (Bio-Rad) and the SYBR Premix Ex Taq II Mix (Takara) were used for PCR amplification. The program was 95°C for 3 min, followed by 40 cycles of amplification (95°C for 15 sec, 60°C for 30 sec). Reactions were performed in 20 µL mixtures consisting of 10 µL of 2× SYBR Premix Ex Taq II Mix, 0.5 µL of a forward or reverse primer, 1 µL of cDNA template (50 ng/µL), and 8 µL of double-distilled H2O. The data were analyzed using the 2-ΔΔCt method. The transcript levels of Bo_eIF-5A were normalized to those of Actin (5′-GAG CGA GAA ATT GTC AGG GA-3′ and 5′-GAT GGC TGG AAG AGG ACC T-3′) using specific primers for Bo_eIF-5A (5′-TGC TAA ATG TCA CTT CGT GGC-3′ and 5′-CAG TGG GGA GCC TCA AAT CA-3′).
Results
1. GR24 accelerated the senescence of bamboo leaf segments in the dark condition
Leaf senescence has been reported to be involved in several plant hormones, such as abscisic acid, ethylene, jasmonic acid, cytokinins, and brassinosteroids.12) Recent studies of Yamada et al. and Ueda & Kusaba demonstrated the roles of SL in leaf senescence.4,9) Intriguingly, the SL-induced leaf senescence reported by Yamada et al. and Ueda & Kusaba was observed with dark treatment rather than light treatment, indicating an unknown mechanism of SL-induced senescence. The mode of action of SL-induced leaf senescence is still largely unknown.
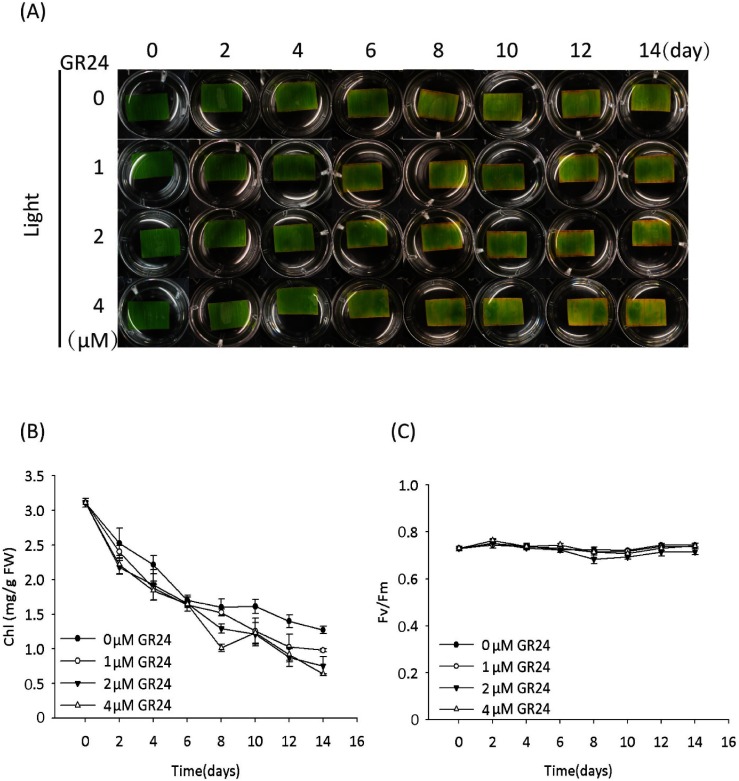
To give further insight into the mechanism of SL-induced leaf senescence, we prepared bamboo leaf segments and tested their senescence in response to the treatment with racemic GR24 (GR24 will be used hereafter for convenience). In the light condition, the leaf segments started to turn yellow on day 2 (Fig. 1a). In addition to leaf yellowing, the decrease in chlorophyll (Chl) content but not in the photosynthetic efficiency of the PSII system (Fm/Fv)13–15) was confirmed (Fig. 1b, c), suggesting that the leaf segments undergo senescence under light together with the degradation of Chl but retain a functional PSII system. These results correlate with the results observed in detached leaves of tobacco and barley,16,17) and suggest the possibility that photosynthesis in the detachment of leaves led to the accumulation of sugars, which are hypothesized to trigger the senescence.10) Treatment with GR24 had no obvious impact on the light-cultured leaf senescence process (the starting day of senescence) or photosynthetic efficiency (Fig. 1), which is in accordance with the results reported by Yamada et al.4) and Ueda & Kusaba9) in rice and Arabidopsis, respectively.
Fig. 1. GR24 displayed no effect on the senescence of detached bamboo leaf segments cultured under a light condition. (A) Time course of representative leaf segments with or without GR24 treatment. (B) Time course of the chlorophyll (Chl) content of leaf segments with or without GR24 treatment. Error bars indicate±SD, n=3. (C) Time course of photosynthesis efficiency (represented by Fv/Fm) of leaf segments with or without GR24 treatment. Error bars indicate±SD, n=3. The GR24 mentioned here stands for racemic GR24.
In the dark condition, the leaf segments with the mock treatment (0 uM GR24) remained green on day 14 (Fig. 2a), though they turned yellow on day 20 (data not shown). However, the leaf segments with GR24 treatment started to turn yellow on day 10, indicating that SL accelerates the senescence of leaf segments, although no obvious GR24 concentration dependence was observed (Fig. 2a). In addition to the yellowing of leaf segments, GR24 rather than the mock treatment decreased the Chl content (Fig. 2b). GR24 also decreased the photosynthetic efficiency (Fig. 2c), suggesting its direct or indirect impact on the PSII system under the dark condition rather than the light condition (Fig. 1c). These results demonstrated that SL induces leaf senescence in the dark, and this process is in accordance with the decrease in both Chl content and photosynthetic efficiency, although the mechanism remains unknown.
Fig. 2. GR24 accelerated the senescence of detached bamboo leaf segments cultured under a dark condition. (A) Time course of representative leaf segments with or without treatment with 2 µM GR24. (B) Time course of the chlorophyll (Chl) content of leaf segments with or without treatment with 2 µM GR24. Error bars indicate±SD, n=3. (C) Time course of photosynthesis efficiency (represented by Fv/Fm) of leaf segments with or without treatment with 2 µM GR24. Error bars indicate±SD, n=3.
2. Sugar treatment antagonized the GR24-induced senescence of leaf segments
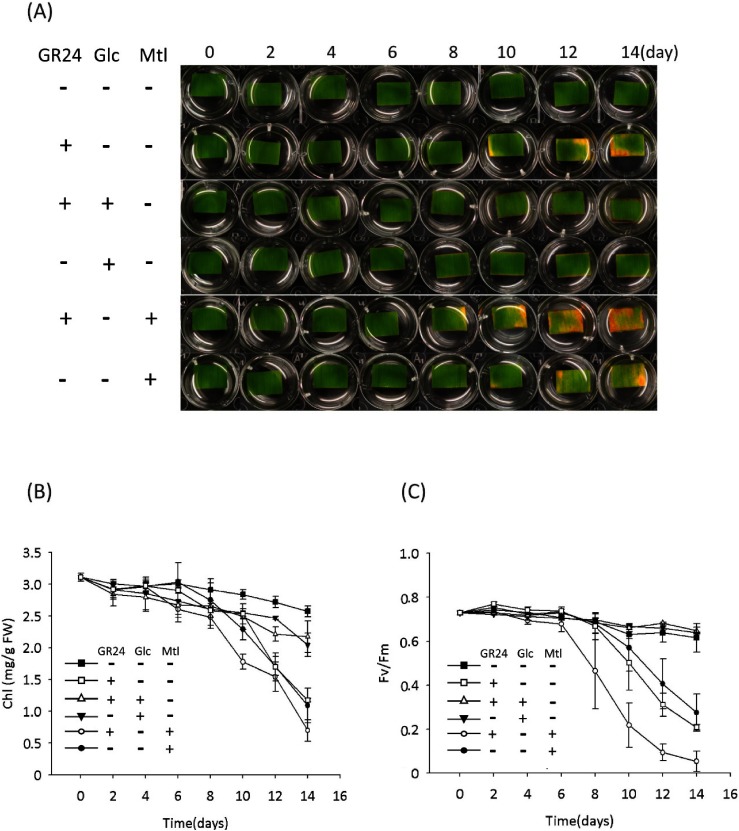
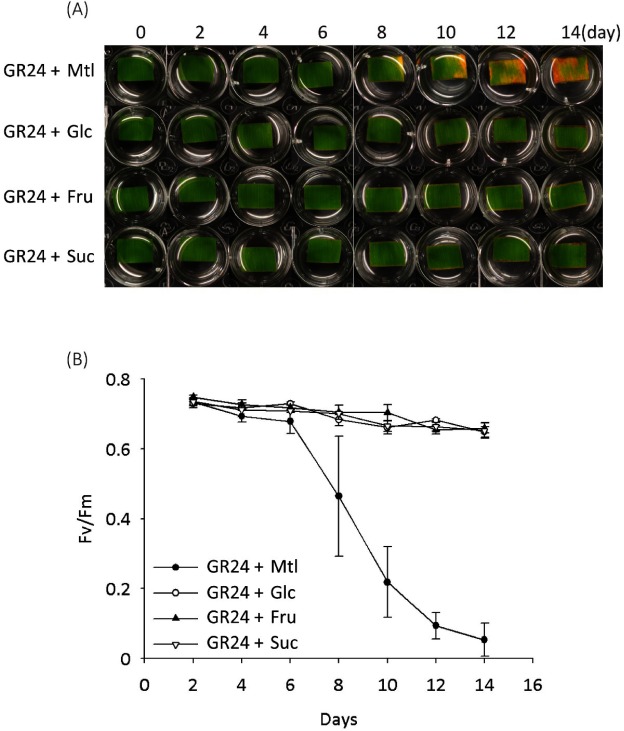
In addition to plant hormones, the sugars that serve as signal molecules and energy resources are involved in regulating leaf senescence, though there is a debate over whether sugar starvation or over-accumulation triggers the leaf senescence.18) Our recent report showed that SL plays a synergistic role in sugar-induced suppression on seedling establishment, and hexose levels (glucose and fructose) were confirmed to be lower in SL mutants (max1 and max2) than that in the wild type,19) suggesting a crosstalk between the SL signal and the sugar signal in regulating seedling establishment. In view of these findings, we tested the SL-induced leaf senescence in response to treatment with sugars in the dark. The results showed that senescence induced by GR24 was suppressed by adding 0.8% glucose (Glc) rather than mannitol (Mtl) as an osmotic control (Fig. 3a). Similarly, Glc rather than Mtl suppressed the GR24-induced decreases in Chl content and photosynthetic efficiency (Fig. 3b, c). Further treatments with fructose (Fru) and sucrose (Suc) also suppressed the GR24-induced senescence of leaf segments (Fig. 4). These results indicated that sugars, but not sugar alcohol, antagonize SL-induced senescence in the dark.
Fig. 3. Glucose (Glc) antagonized the GR24-induced senescence of detached bamboo leaf segments cultured under a dark condition. (A) Time course of representative leaf segments with treatment of GR24 (2 µM) or co-treatment of GR24 (2 µM) with 0.8% Glc or 0.8% mannitol (Mtl) as an osmotic control. (B) Time course of the chlorophyll (Chl) content of leaf segments with treatment of GR24 (2 µM) or co-treatment of GR24 (2 µM) with 0.8% Glc or 0.8% mannitol (Mtl) as an osmotic control. Error bars indicate±SD, n=3. (C) Time course of photosynthesis efficiency (represented by Fv/Fm) of leaf segments with treatment of GR24 (2 µM) or co-treatment of GR24 (2 µM) with 0.8% Glc or 0.8% mannitol (Mtl) as an osmotic control. Error bars indicate±SD, n=3.
Fig. 4. Sugars antagonized the GR24-induced senescence of detached bamboo leaf segments cultured under a dark condition. (A) Time course of representative leaf segments with treatment of GR24 (2 µM) or co-treatment of GR24 (2 µM) with sugar (0.8% glucose, 0.8% fructose, or 1.6% sucrose) or 0.8% mannitol (Mtl) as an osmotic control. (B) Time course of the chlorophyll (Chl) content of leaf segments with treatment of GR24 (2 µM) or co-treatment of GR24 (2 µM) with 0.8% Glc or 0.8% mannitol (Mtl) as an osmotic control. Error bars indicate±SD, n=3.
3. GR24 accelerates leaf senescence and triggers cell death under a dark condition
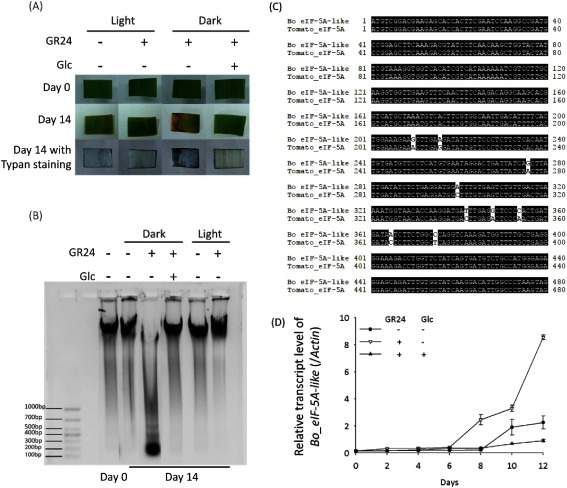
Leaf senescence contains several phases, including apoptotic-like cell death. The results from trypan blue staining showed that the leaf segments treated with GR24 under a dark condition displayed a blue color, which indicates cell death (Fig. 5a). This GR24-induced cell death can be suppressed by co-treatment with 0.8% Glc. Cell death is accompanied by the degradation of genomic DNA.20) We detected the genomic DNA isolated from the dark-cultured leaf segments with GR24 treatment, or co-treatment with GR24 and Glc, and found that treatment with GR24 triggered degradation of the genomic DNA of leaf segments under the dark condition rather than the light condition (Fig. 5b). Co-treatment with 0.8% Glc or culture under a light condition stabilized the genomic DNA in the presence of GR24. Upon the transcriptomic analysis of B. oldhamii (unpublished), we identified a gene displaying high similarity (> 98%) to the senescence-induced cDNA in tomatoes that encodes eucaryotic initiation factor 5A (eIF-5A), which is involved in the translation of mRNAs required for cell division and cell death21,22) (Fig. 5c), and thus we designated it as Bo_eIF-5A-like. A relative quantitative PCR was performed to check the expression pattern of Bo_eIF-5A-like. As shown in Fig. 5d, the expression level of Bo_eIF-5A-like was dramatically triggered with the treatment of GR24 in the dark from day 8, and it can be suppressed with a co-treatment of 0.8% Glc. These results confirmed that SL promotes the cell death of leaf segments in the dark condition, and upregulation on Bo_eIF-5A-like is one reason for its impact on leaf senescence, despite the lack of detailed information.
Fig. 5. Treatment with GR24 triggered cell death, which is alleviated by co-treatment with Glc. (A) Trypan blue staining was performed to detect the cell death of leaf segments with treatment of GR24 (2 µM) or co-treatment of GR24 (2 µM) and Glc (0.8%) under a light or dark condition on day 14. (B) Electrophoresis was performed to check the degradation of genomic DNA. (C) Homology analysis of cDNAs from B. oldhamii and a tomato (Solanum lycopersicum). Bo_eIF-5A-like displays over 98% similarity to tomato_eIF-5A. (D) Real-time RT-PCR analysis was performed to monitor transcript levels of the Bo_eIF-5A-like of leaf segments with a treatment of GR24 (2 µM) or co-treatment of GR24 (2 µM) and Glc (0.8%) under a dark condition. Error bars indicate±SD, n=3.
Discussion
Leaf senescence, the final phase of leaf development, is an important process for nutrient relocation in plants, which adapts plants to the ambient environment.23,24) SL, as a signal molecule in regulating plant growth and development, has been reported to play a role in leaf senescence, despite the lack of details about its mode of action in this process. Our observation of bamboo leaf segment response to GR24 confirmed that SL accelerates senescence and induces cell death in the dark condition rather in the light condition (Figs. 1–3, 5). Furthermore, the SL-accelerated senescence and SL-induced cell death in leaf segments can be blocked by treatment with sugars such as Glc (Figs. 3 and 5). We also observed that the sucrose level in leaf segments dramatically decreased in a dark-treatment-time-manner (data not shown). These results indicate a potential crosstalk between SL and sugars in regulating leaf senescence, and the low sugar condition in the dark condition is a key point for further studies on this issue.
In our assay system, we also noticed that the leaf segments cultured in the light condition turned yellow earlier (on day 2) than the ones cultured in the dark condition (after 14 days) (Fig. 1). In view of the fact that the light condition we used was moderate (120 µmol/m2/s) and that there were no obvious changes in photosynthetic efficiency despite the decrease in chlorophyll content, we assume that the senescence could be induced by the accumulation of higher sugar levels, and this is supported by former studies on the senescence of detached leaves.16,17) GR24 displayed no effects on the process of senescence in the light condition (Fig. 1). This phenomenon is assumed to be involved in the sugar level, as mentioned above.
In summary, the test system using bamboo leaf segments provides a tool for investigating the mechanism of SL-induced leaf senescence. Our findings of crosstalk between SL and sugar in regulating leaf senescence provide a novel understanding of SL-related leaf senescence, although further studies are needed to elucidate its mode of action.
References
- 1).C. E. Cook, L. P. Whichard, B. Turner, M. E. Wall and G. H. Egley: Science 154, 1189–1190 (1966). [DOI] [PubMed] [Google Scholar]
- 2).M. T. Waters, C. Gutjahr, T. Bennett and D. C. Nelson: Annu. Rev. Plant Biol. 68, 291–322 (2017). [DOI] [PubMed] [Google Scholar]
- 3).R. Yao, J. Li and D. Xie: Sci. China Life Sci. 61, 277–284 (2018). [DOI] [PubMed] [Google Scholar]
- 4).Y. Yamada, S. Furusawa, S. Nagasaka, K. Shimomura, S. Yamaguchi and M. Umehara: Planta 240, 399–408 (2014). [DOI] [PubMed] [Google Scholar]
- 5).H. R. Woo, K. M. Chung, J.-H. Park, S. A. Oh, T. Ahn, S. H. Hong, S. K. Jang and H. G. Nam: Plant Cell 13, 1779–1790 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).K. C. Snowden, A. J. Simkin, B. J. Janssen, K. R. Templeton, H. M. Loucas, J. L. Simons, S. Karunairetnam, A. P. Gleave, D. G. Clark and H. J. Klee: Plant Cell 17, 746–759 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).H. Yan, H. Saika, M. Maekawa, I. Takamure, N. Tsutsumi, J. Kyozuka and M. Nakazono: Genes Genet. Syst. 82, 361–366 (2007). [DOI] [PubMed] [Google Scholar]
- 8).C. Hamiaux, R. S. M. Drummond, B. J. Janssen, S. E. Ledger, J. M. Cooney, R. D. Newcomb and K. C. Snowden: Curr. Biol. 22, 2032–2036 (2012). [DOI] [PubMed] [Google Scholar]
- 9).H. Ueda and M. Kusaba: Plant Physiol. 169, 138–147 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).H. K. Lichtenthaler: Methods Enzymol. 148, 350–382 (1987). [Google Scholar]
- 11).R. C. Keogh, B. J. Deverall and S. McLeod: Trans. Br. Mycol. Soc. 74, 329–333 (1980). [Google Scholar]
- 12).E. Fedina, A. Yarin, F. Mukhitova, A. Blufard and I. Chechetkin: Steroids 117, 25–28 (2017). [DOI] [PubMed] [Google Scholar]
- 13).N. Pourtau, R. Jennings, E. Pelzer, J. Pallas and A. Wingler: Planta 224, 556–568 (2006). [DOI] [PubMed] [Google Scholar]
- 14).Y. Sun, Z. Han, J. Tang, Z. Hu, C. Chai, B. Zhou and J. Chai: Cell Res. 23, 1326–1329 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).M. Viljevac, K. Dugalic, I. Mihaljevic, D. Simic, R. Sudar, Z. Jurkovic and H. Lepedus: Acta Bot. Croat. 72, 221–235 (2013). [Google Scholar]
- 16).A. Krapp, W. P. Quick and M. Stitt: Planta 186, 58–69 (1991). [DOI] [PubMed] [Google Scholar]
- 17).D. Parrott, L. Yang, L. Shama and A. M. Fischer: Planta 222, 989–1000 (2005). [DOI] [PubMed] [Google Scholar]
- 18).W. G. van Doorn: J. Exp. Bot. 59, 1963–1972 (2008). [DOI] [PubMed] [Google Scholar]
- 19).G. D. Li, L. N. Pan, K. Jiang, I. Takahashi, H. Nakamura, Y. W. Xu, T. Asami and R. F. Shen: Plant Biotechnol. 33, 87–97 (2016). [Google Scholar]
- 20).W. Sakamoto and T. Takami: J. Exp. Bot. 65, 3835–3843 (2014). [DOI] [PubMed] [Google Scholar]
- 21).T. W. Wang, L. Lu, D. Wang and J. E. Thompson: J. Biol. Chem. 276, 17541–17549 (2001). [DOI] [PubMed] [Google Scholar]
- 22).J. E. Thompson, M. T. Hopkins, C. Taylor and T.-W. Wang: Trends Plant Sci. 9, 174–179 (2004). [DOI] [PubMed] [Google Scholar]
- 23).P. O. Lim, H. J. Kim and H. Gil Nam: Annu. Rev. Plant Biol. 58, 115–136 (2007). [DOI] [PubMed] [Google Scholar]
- 24).D. Liebsch and O. Keech: New Phytol. 212, 563–570 (2016). [DOI] [PubMed] [Google Scholar]