Abstract
Background and objectives
Angiogenesis is the main cause of lung adenocarcinoma (LAC) poor prognosis. This study aimed to investigate the effect of sex-determining region Y-box protein 5 (SOX5) expression on angiogenesis of LAC and explore its possible mechanism.
Patients and methods
The effect on angiogenesis was tested by tube formation assays using human umbilical vein endothelial cells cocultured with A549 cells. Lentivirus shRNA of SOX5 and lentivirus of SOX5 overexpression system were used to establish LAC cell lines, which expressed SOX5 of different levels. SOX5 downstream signaling targets were analyzed by real-time qPCR and Western blot. We collected 90 LAC cases and the tissues were examined by immunohistochemistry for SOX5 and vascular endothelial growth factor (VEGF).
Results
We found that SOX5 overexpression in A549 cells significantly promoted tube formation capacity of the cocultured human umbilical vein endothelial cells. SOX5 increased VEGF expression and signal transducer activator of transcription 3 phosphorylation; however, SOX5 had no effect on extracellular signal-regulated kinase and protein kinase B pathway. Furthermore, the expression of SOX5 and VEGF had a significantly positive correlation (r=0.399, P=0.001) according to the tissue microarray data.
Conclusion
These findings suggest that SOX5 induces angiogenesis by activating signal transducer activator of transcription 3/VEGF signaling and confer its candidacy as a potential therapeutic target in LAC.
Keywords: SOX5, VEGF, STAT3, angiogenesis, lung adenocarcinoma, tissue microarray, tube formation
Introduction
Lung cancer is the leading cause of cancer-related death worldwide, and its incidence is still increasing every year. The most common pathological type of lung cancer is lung adenocarcinoma (LAC).1,2 Although the comprehensive treatment of lung cancer has progressed in recent years, most LAC patients eventually die due to recurrence and drug resistance.3,4 Angiogenesis is essential in the process of cancer cells proliferation and metastasis, which is the main cause of poor prognosis. In most types of cancers, including LAC, tumor angiogenesis pathways have been identified as important therapeutic targets. However, the exact mechanisms underlying LAC angiogenesis still remain to be further explored.5,6
Sex-determining region Y-box (SOX) genes are reported to regulate cell growth, metastasis, drug resistance, epithelial–mesenchymal transition, and angiogenesis.7 Accumulating studies have indicated that SOX genes play a crucial role in angiogenesis. For example, SOX18 was reported as a transcription factor involved in the induction of angiogenesis during wound healing.8 Kim et al showed that SOX7 and SOX17 are indispensable factors in the developmental angiogenesis acting as positive feedback regulators of vascular endothelial growth factor (VEGF) signaling.9 Yang et al also demonstrated that SOX17 plays an important role in tumor angiogenesis and tumor progression.10 Existing experiments showed that the expression of SOX5 is associated with the development of different types of cancer, including prostate cancer, breast cancer, glioma, hepatocellular carcinoma, and nasopharyngeal carcinoma.11–16 Our previous study showed that SOX5 is highly expressed in LAC and is closely associated with poor prognosis. We also found that SOX5 promotes LAC cell proliferation and metastasis, but the mechanism was still unclear.17
Angiogenesis plays an important role in tumor metastasis.18 In this study, in order to clarify whether SOX5 correlated with angiogenesis, tube formation assay was performed. Then underlying mechanism of SOX5 and its relationship with VEGF were investigated using A549 cells and LAC tissues from patients. Based on the previous study, we further explored the underlying mechanisms of LAC metastasis induced by SOX5 overexpression. Herein, we found out a novel SOX5/signal transducer activator of transcription 3 (STAT3)/VEGF pathway, which contributed to LAC angiogenesis. As far as we know, the relationship of SOX5 and VEGF is first reported in this study.
Patients and methods
Patients’ information and tissue specimens
This study was a retrospective study. The patients’ samples with LAC were collected from the National Human Genetic Resources Sharing Service Platform (No. 2005DKA21300). A total of 90 cases of LAC were included in this study, all of which had undergone section surgery between 2004 and 2009. All the participants received a written consent form for the preservation of the tissue samples, and the use of the tissue samples was approved by the ethics committee of the First Affiliated Hospital of Zhejiang University. Tissues were analyzed by tissue microarray.
Cells and reagents
The human LAC cell line A549 was purchased from the Cell bank of the Chinese Academy of Sciences (Shanghai, China) with STR verification. The A549 cells were cultured in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific). All the cells were maintained at 37°C in a humidified incubator with 5% CO2. The human umbilical vein endothelial cells (HUVECs; passage number: P3–P4) were gifted by Professor Wang Huiming, a professor in the first affiliated hospital of Zhejiang University. The usage of HUVECs was approved by the ethics committee of the first affiliated hospital of Zhejiang University. HUVEC cells were cultured in endothelial cell medium supplemented with 5% FBS and 1% endothelial cell growth supplement (all from ScienCell Research Laboratories, Carlsbad, CA, USA).
Immunohistochemical staining
The immunohistochemical (IHC) analysis was performed to investigate SOX5 and VEGF expression in LAC. After blocking the endogenous peroxidase activity with 3% H2O2 and target retrieval solution (S1699; Agilent Techologies Inc., Santa Clara, CA, USA) according to the manufacturer’s instructions, the sections were incubated with 10% normal goat serum (S-1000; Vector Laboratories, Burlingame, CA, USA) in PBS for 30 minutes. Next, the sections were incubated with the primary antibody including SOX5 (ab26041, 1:100; Abcam, Cambridge, UK) and VEGF (1:1; Maixin Technology Co., Ltd., Fuzhou, China) at 4°C overnight. After three times of rinses with PBS for 5 minutes each, the slides were incubated for 30 minutes with biotinylated goat antirabbit secondary antibody (BA-1000, 1:200; Vector Laboratories). After another triple PBS rinse for 5 minutes each, the slides were incubated for 30 minutes with Vexta stain Elite ABC reagent (PK-6101, Vector Laboratories). Finally, the sections were incubated in peroxidase substrate solution (SK-4100; Vector Laboratories) until the desired stain intensity was attained. The sections were rinsed in tap water, counterstained, and coverslipped. The sections were observed and scanned with a 3D Histech Pannoramic MIDI microscope (3DHISTECH, Budapest, Hungary). The results of IHC staining were determined by two experienced pathologists. There is no significant difference between the two reports. The two results were then verified by a senior pathologist to avoid the error between observers, and one of the results was selected as the trustable one. The total histological scores, which were the products of the intensity (scored 0–3) and percentage scores (scored 0–5), were utilized to determine the results.
RNA isolation and quantitative RT-PCR
Total RNA was extracted from the tissues or cells using the TRIzol reagent (Thermo Fisher Scientific) and reverse transcription was performed using the HiFi Script gDNA Removal cDNA Synthesis Kit (KangWeiShiJi, Beijing, China) according to the manufacturer’s instructions. Each cDNA sample was analyzed in triplicate on an Applied Biosystems, the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer’s protocol. The primers are shown in Table 1. The results were analyzed by SDS 2.2.2 software (Applied Biosystems) and then calculated as threshold cycle (Ct) values. The relative mRNA levels of the targeted genes were adjusted according to GAPDH and determined as 2−ΔΔCt.
Table 1.
Primer sequences
| Genes | Primer sequences | Product length (bp) |
|---|---|---|
| SOX5 | Forward: 5′-ATAAAGCGTCCAATGAATGCCT-3′ | 114 |
| Reverse: 5′-GCGAGATCCCAATATCTTGCTG-3′ | ||
| VEGF | Forward: 5′-AGGGCAGAATCATCACGAAGT-3′ | 143 |
| Reverse: 5′-AGGGTCTCGATTGGATGGCA-3′ | ||
| GAPDH | Forward: 5′-ACAACTTTGGTATCGTGGAAGG-3′ | 101 |
| Reverse: 5′-GCCATCACGCCACAGTTTC-3′ |
Note: The sequences of primers were demonstrated.
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SOX5, sex-determining region Y-box 5; VEGF, vascular endothelial growth factor.
Western blot analysis
The cells were harvested and lysed as previously described.17 Protein lysates were quantified using the BCA kit (Thermo Fisher Scientific). Protein extracts were mixed with Laemmli loading buffer and incubated at 99°C for 10 minutes. Equal amounts of proteins were separated by 10% SDS-PAGE gels. After blotting onto polyvinylidene fluoride membranes, 5% skim milk powder in TBS-T (TBS plus 0.5% Tween-20) was used for blocking, and the membranes were probed with primary antibodies at 4°C overnight. The primary antibodies included SOX5 (AF5286, 1:1,000; R&D systems, Minneapolis, MN, USA), VEGF (1:1,000; Huabio, Hangzhou, China), and β-Actin (1:3,000, Sigma-Aldrich, Merck KGaA, St Louis, MO, USA). The membrane was then exposed to a peroxidase-conjugated secondary antibody (1:5,000; MultiSciences (Lianke) Biotech Co, Ltd, Hangzhou, China). The signals were visualized by an enhanced chemiluminescence kit (Millipore, Bedford, MA, USA) according to the manufacturer’s instructions. β-actin was used as a loading control. The bands were detected by using ECL reagent (Thermo Fisher Scientific).
Lentivirus packaging and infection
Lentiviral short hairpin RNAs (shRNAs) in hU6-MCS- Ubiquitin-EGFP-IRES-puromycin (Genechem, Shanghai, China) were used to express shRNA. The RNAi sequences targeting the SOX5 gene were 5′-ACATATCAAAGAAGAGATA-3′ and 5′-ATGCAATGATGGATTTCAA-3′. The negative control sequence was 5′-TTCTCCGAACGTGTCACGT-3′. The SOX5 overexpression lentiviral vector, pGC-FU-SOX5-3FLAG-SV40-EGFP-IRES-puromycin, and the empty lentiviral vector for control were purchased from Genechem (Shanghai, China). Lentivirus packaging and infection methods followed the assay as previously published.17 The knockdown or overexpression efficiency was evaluated by fluorescence microscopy, RT-qPCR, and Western blot analysis.
Tube formation assay
Matrigel Basement Membrane Matrix (BD Biosciences, San Jose, CA, USA) was diluted with EBM-2 medium and was coated in 24-well plates at 37°C for 1 hour. Then, 5×104 HUVECs were seeded and cocultured with an equivalent number of A549 cells in the EBM-2 medium on Matrigel. Cocultured A549 cells were seeded in transwells and were incubated in the same well with HUVECs. The tube formation ability of the HUVECs was measured at 16 hours with or without A549 cells. After the incubation, the numbers of tubes and nodes of the tubular structures were quantified.
Statistical analyses
The statistical analyses were performed with GraphPad Prism 5 software. The results of the experiments are depicted as the mean ± SD. The significance of the data was analyzed by Student’s t-test. P-value <0.05 was considered statistically significant.
Results
SOX5 overexpressed A549 cells facilitate endothelial angiogenesis
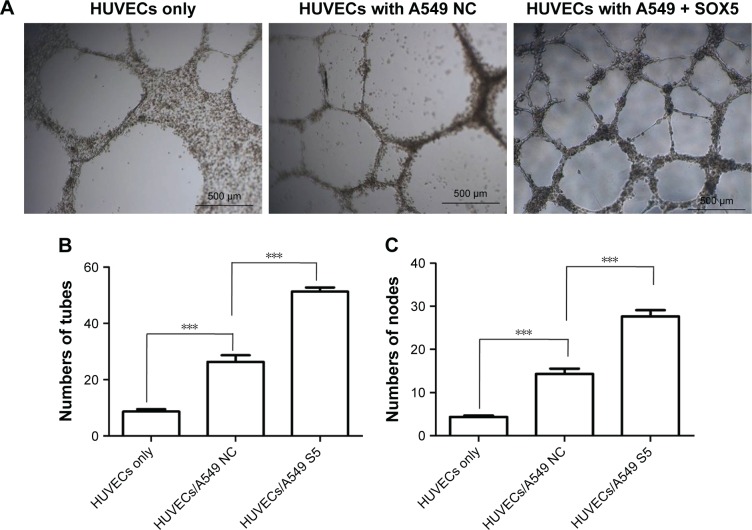
SOX5 significantly promotes LAC cells metastasis in a previous study.17 The formation of new blood vessels is a key step in the growth and metastasis of solid tumors and is also a necessary prerequisite for tumor invasion and metastasis.19 Therefore, in this study, we investigated whether SOX5 contributed to angiogenesis in LAC. The tube formation assay was used to evaluate the role of SOX5 in angiogenesis. When HUVECs were seeded on Matrigel, they gradually extended capillary-like tubular structures and the tubes connected together to create a mesh-like structure. The tube formation ability of the HUVECs was measured at 16 hours with or without A549 cells in the transwell coculture system. After the incubation, the number of tubes and nodes of the tubular structures were quantified. It showed that the morphology of HUVECs alone revealed a fusiform cell shape and formed a tight cluster. In contrast, after coculturing with A549 cells, the HUVECs elongated and spread like a mesh (Figure 1A). To further investigate whether SOX5 enhanced endothelial angiogenesis, SOX5 overexpressed A549 cells were cocultured with HUVECs. The capillary-like tubular structures on Matrigel were increased significantly in HUVEC/A549 SOX5 over-expressed group at 24 hours compared with that of HUVEC/A549 negative control group (Figure 1A). Number of tubes and nodes in each group were showed in bars. Obviously, number of tubes and nodes were increased when HUVECs cocultured with A549 cells (***P<0.001). SOX5 overexpressed A549 cocultured group significantly enhanced the formation ability of tubes and nodes (***P<0.001) (Figure 1B and C). These data revealed that SOX5 promoted angiogenesis in LAC.
Figure 1.
SOX5 expression in A549 cells changes the phenotypes of HUVECs.
Notes: Tube formation assays for HUVECs in a coculture system. (A) Phase contrast micrographs of capillary-like tubular structures on Matrigel in HUVECs-only group, HUVECs with A549 NC group, and HUVECs with SOX5 overexpressed A549 group. HUVECs and A549 cells were cocultured in 0.8 µm transwell plates for 16 hours. Magnification ×200. (B) The number of tubes in each group was calculated in three replicated plates. ***P<0.001. (C) The number of nodes in each group was calculated in three replicated plates. ***P<0.001.
Abbreviations: HUVECs, human umbilical vein endothelial cells; NC, negative control; SOX5, sex-determining region Y-box 5; +SOX5 or S5, transfected with SOX5 lentivirus A549 cells.
SOX5 enhances VEGF expression in A549 cells
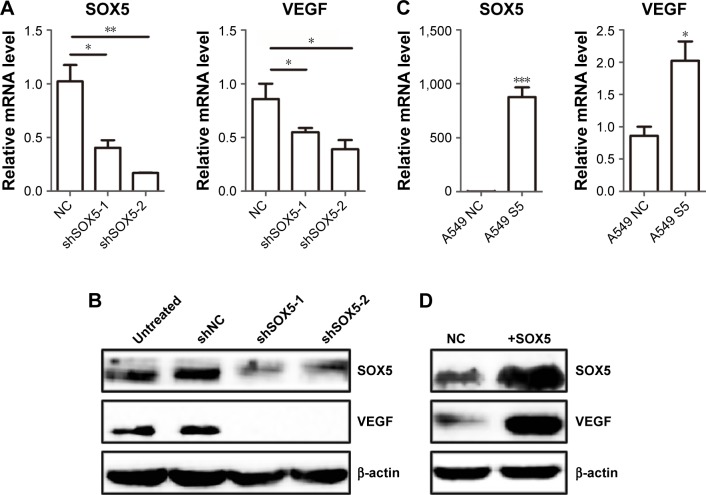
To investigate the relationship between SOX5 and VEGF in LAC, the mRNA and protein expression level of VEGF were measured by RT-qPCR and Western blot analysis in SOX5-silenced cells, overexpression cells, and corresponding control cells. First, the expression levels of VEGF were detected in A549 cells transfected with SOX5 lentivirus packaged shSOX5 or shNC. As shown in Figure 2A, VEGF mRNA level decreased in the SOX5-knockdown A549 cells (shSOX5 cells vs shNC cells, P<0.05). We found that the knockdown of SOX5 significantly decreased the protein expression level of VEGF in the shSOX5-1 and shSOX5-2 cells compared with the shNC cells (Figure 2B). In addition, after we constructed SOX5 overexpressed A549 cells, RT-qPCR and Western blot analysis were used to detect the expression level of SOX5 and VEGF (Figure 2C and D). It was also shown that SOX5 overexpression was followed by increased VEGF transcriptional mRNA level and protein expression level (Figure 2C and D). Taken together, SOX5 and VEGF expressions were positively correlated in LAC cells.
Figure 2.
SOX5 regulates VEGF expression in A549 cells.
Notes: RT-qPCR and Western blot analysis of SOX5 reduced cells and overexpressed cells. (A) Relative mRNA level of SOX5 and VEGF when SOX5 was knocked down in A549 cells by shRNAs. *P<0.05, **P<0.01. (B) Western blot analysis of SOX5 and VEGF in untreated group (untreated), negative control group (shNC), and SOX5-silencing group (shSOX5-1 and shSOX5-2). (C) Relative mRNA level of SOX5 and VEGF when SOX5 overexpressed in A549 cells. *P<0.05, ***P<0.001. (D) SOX5 and VEGF expression levels were examined by Western blot analysis in A549 control group (NC) and SOX5 overexpressed group (+SOX5).
Abbreviations: SOX5, sex-determining region Y-box 5; +SOX5, transfected with SOX5 lentivirus A549 cells; VEGF, vascular endothelial growth factor.
SOX5 induces STAT3 activation in A549 cells
A previous study showed that VEGF expression is always regulated by the transcription factor STAT3 and the extracellular signal-regulated kinase (ERK) or protein kinase B (AKT) signaling pathway.20 It is reported that the Janus Kinase 2/STAT3/VEGF signaling pathway mediates tumor angiogenesis in NSCLC.21 Thus, to further clarify the mechanism of SOX5-induced angiogenesis in A549 cells, the expression levels of ERK, STAT3, AKT, p-ERK, p-STAT3, and p-AKT were also detected by Western blot analysis. The results showed that SOX5 silencing did not affect the ERK or AKT signaling, whereas it significantly downregulated the level of p-STAT3-Y705 (Figure 3A). Besides, the change of p-STAT3 expression level was also examined in SOX5 overexpressed A549 cells and control cells. We showed that there was high activated p-STAT3 when SOX5 overexpressed in A549 cells (Figure 3B). Taken together, these results indicated that SOX5 induced STAT3 activation and increased VEGF expression, which might lead to angiogenesis in LAC.
Figure 3.
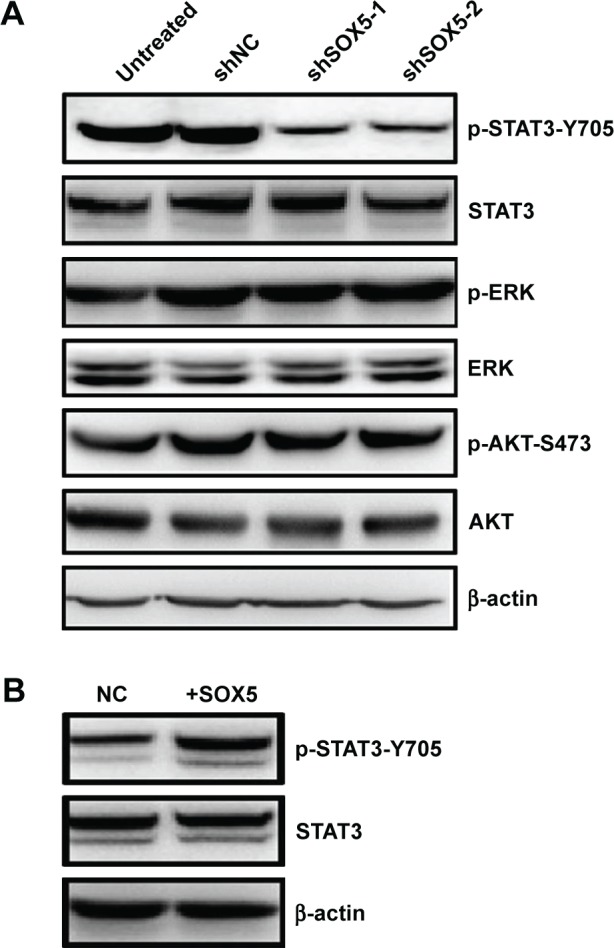
SOX5 activates STAT3 in A549 cells.
Notes: Western blot analysis in SOX5 reduced cells and overexpressed cells. (A) STAT3, ERK, AKT, and their phosphorylated sites were detected by Western blot in control cells (Untreated and shNC) and SOX5-silencing cells (shSOX5-1 and shSOX5-2). (B) p-STAT3 and STAT3 protein level were detected by Western blot in control cells (NC) and SOX5 overexpressed cells.
Abbreviations: AKT, protein kinase B; ERK, extracellular signal-regulated kinase; p-AKT-S473, phosphorylated AKT on serine 473 site; p-ERK, phosphorylated ERK; p-STAT3-Y705, phosphorylated STAT3 on tyrosine 705 site; +SOX5, transfected with SOX5 lentivirus A549 cells; STAT3, signal transducers and activators of transcription 3.
The expression of SOX5 is positively correlated with VEGF expression in lung adenocarcinoma patients
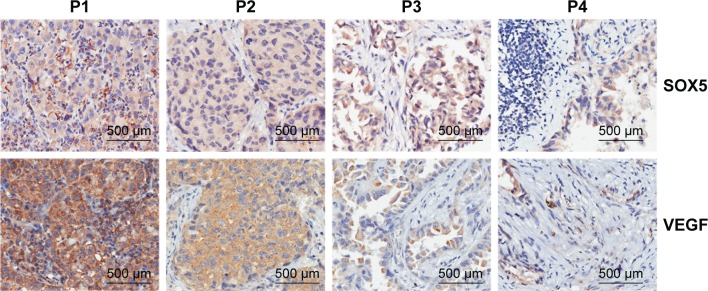
In a previous study, we found SOX5 was upregulated in LAC tumor tissues.17 To further investigate the relationship between SOX5 and VEGF, 90 pairs of LAC tissues were examined by IHC. After three-dimensional microscope scanning, the staining intensity and the positive rate of SOX5 and VEGF were measured. Analysis revealed that SOX5 and VEGF were frequently expressed in LACs, with only six cases (6.67%) negative for SOX5 and all positive cases for VEGF. We also found that 77 of 90 (85.55%) LACs had high SOX5 expression and 78 of 90 (86.67%) LACs had high VEGF expression (scored 4 and 5). Patients’ information and staining scores of SOX5 and VEGF were shown in Table 2 (two cases’ data loss). The representative images for IHC staining of SOX5 and VEGF were shown in Figure 4. The intensity of VEGF staining was positively correlated with the intensity of SOX5 staining (Figure 4). Spearman correlation analysis was done by SPSS software. The analysis revealed that the expression of SOX5 was positively correlated with the expression of VEGF (r=0.399, P=0.001). The statistical results were shown in Table 3. The number of different groups according to staining intensity was shown in Table 4 (two cases’ data loss). These data indicate that SOX5 has close relationship with VEGF in LAC patients, which is consistent with the result of in vitro experiments.
Table 2.
Description of the population studied by immunohistochemistry
| Variables | n=90 |
|---|---|
| Age (years) | |
| ≤60 | 41 (45.55%) |
| >60 | 49 (54.44%) |
| Sex | |
| Male | 49 (54.44%) |
| Female | 41 (45.55%) |
| Subtypes | |
| Lung adenocarcinoma | 71 (78.89%) |
| Bronchioloalveolar carcinoma | 13 (14.44%) |
| Mucinous carcinoma | 5 (5.56%) |
| Papillary carcinoma | 1 (1.11%) |
| Tumor size (cm) | |
| ≤5 | 74 (82.22%) |
| >5 | 16 (17.78%) |
| Histologic grade | |
| I | 3 (3.33%) |
| II | 66 (73.33%) |
| III | 21 (23.33%) |
| Tumor stage (T) | |
| I | 17 (18.89%) |
| II | 50 (55.55%) |
| III | 17 (18.89%) |
| IV | 6 (6.67%) |
| Lymph node metastases (N) | |
| N0 | 39 (43.33%) |
| N1 | 17 (18.89%) |
| N2 | 15 (16.67%) |
| N3 | 4 (4.44%) |
| Nx | 12 (4.58%) |
| Unknown | 3 (3.33%) |
| Distance metastases (M) | |
| M0 | 88 (97.78%) |
| M1 | 1 (1.11%) |
| Unknown | 1 (1.11%) |
| SOX5-positive incidence | |
| 0 | 6 (6.67%) |
| 1 (1%–20%) | 0 (0%) |
| 2 (21%–40%) | 3 (3.33%) |
| 3 (41%–60%) | 2 (2.22%) |
| 4 (61%–80%) | 20 (22.22%) |
| 5 (81%–100%) | 57 (63.33%) |
| Unknown | 2 (2.22%) |
| SOX5 staining intensity | |
| 0–1 (including 0) | 22 (24.44%) |
| 1–2 (including 1) | 48 (53.33%) |
| 2–3 (including 2) | 15 (16.67%) |
| 3 | 3 (3.33%) |
| Unknown | 2 (2.22%) |
| VEGF-positive incidence | |
| 0 | 0 (0%) |
| 1 (1%–20%) | 4 (4.44%) |
| 2 (21%–40%) | 3 (3.33%) |
| 3 (41%–60%) | 3 (3.33%) |
| 4 (61%–80%) | 9 (10%) |
| 5 (81%–100%) | 69 (76.67%) |
| Unknown | 2 (2.22%) |
| VEGF staining intensity | |
| 0–1 (including 0) | 26 (28.89%) |
| 1–2 (including 1) | 49 (54.44%) |
| 2–3 (including 2) | 3 (3.33%) |
| 3 | 10 (11.11%) |
| Unknown | 2 (2.22%) |
Abbreviations: SOX5, sex-determining region Y-box 5; VEGF, vascular endothelial growth factor.
Figure 4.
SOX5 and VEGF are positively correlated in lung adenocarcinoma patients’ tumor tissues.
Notes: Immunohistochemistry of SOX5 and VEGF in lung adenocarcinoma patients’ tumor tissues. Magnification ×200.
Abbreviations: P, patient; SOX5, sex-determining region Y-box 5; VEGF, vascular endothelial growth factor.
Table 3.
Correlation analysis of VEGF and SOX5 (Spearman’s correlation analysis)
| Spearman’s rho | SOX5 | VEGF | |
|---|---|---|---|
| SOX5 | Correlation coefficient | 1.000 | 0.399 |
| Signification (2-tailed) | 0.001 | ||
| N | 88 | 88 |
Notes: P=0.001. The P-values that were bold mean statistically positive correlation between VEGF and SOX5 expression in lung adenocarcinoma.
Abbreviations: SOX5, sex-determining region Y-box 5; VEGF, vascular endothelial growth factor.
Table 4.
The number of different groups (according to SOX5 staining intensity and VEGF staining intensity)
| Variables | SOX5
|
|||
|---|---|---|---|---|
| 0–1 (including 0) | 1–2 (including 1) | 2–3 (including 2 and 3) | Total | |
| VEGF | ||||
| 0–1 (including 0) | 8 | 16 | 2 | 26 |
| 1–2 (including 1) | 11 | 28 | 10 | 49 |
| 2–3 (including 2 and 3) | 3 | 4 | 6 | 13 |
| Total | 22 | 48 | 18 | |
Abbreviations: SOX5, sex-determining region Y-box 5; VEGF, vascular endothelial growth factor.
Discussion
As an essential process in lung cancer development and progression, angiogenesis provides not only oxygen and nutrients for tumor growth but also more opportunities for tumor cells to migrate and spread.22 Many studies have revealed that angiogenesis is an important indicator of poor prognosis in lung cancer.23,24 However, the molecular mechanisms involved in LAC angiogenesis are not well understood yet. In previous study, we found that SOX5 overexpressed in LAC, which promoted cancer metastasis and correlated with overall survival time in LAC patients.17 Therefore, we investigated whether SOX5 was involved in the regulation of LAC angiogenesis. In this study, we demonstrated that SOX5 facilitates tube formation ability of endothelial cells and enhances the angiogenesis of LAC cells. Combined with our previous findings that SOX5 expression was high in LAC cell lines and tissues, we assumed that SOX5 upregulation may be a crucial cause of angiogenesis and metastasis of LAC.
VEGF is probably the most commonly involved proangiogenic factor. Because VEGF plays crucial roles in promoting tumor angiogenesis, inhibiting VEGF signaling has been an effective method to block tumor angiogenesis and metastasis.25,26 VEGF is a powerful regulation factor inducing tumor angiogenesis, which is closely related to the occurrence, development, and metastasis of lung cancer.23 SOX family genes are important for a wide range of fate determination processes in vascular development, but their contribution to tumor angiogenesis remains to be determined.9,27–30 Previous studies showed that SOX9 regulates the expression of VEGF.31 SOX5 and SOX9 belong to the SOX family, and several studies showed that SOX5 had similar biological functions to SOX9.32 Therefore, the relationship between VEGF and SOX5 was further investigated in this study. Clinical trials in several cancer types have indicated that therapeutics that target the VEGF pathway, such as bevacizumab, potently suppress tumor growth and metastasis.33 In this study, through in vitro SOX5 knockdown and overexpression analysis and tube formation assays, we showed that SOX5 can facilitate angiogenesis by regulating VEGF expression. Furthermore, by analyzing 90 clinical LAC samples, we found that VEGF expression was positively correlated with SOX5 expression. Taken together, our data suggest that SOX5 plays an important role in VEGF-mediated angiogenesis in LAC. A previous study showed that VEGF expression is always regulated by the STAT3, ERK, or AKT signaling pathway.34 STAT3 plays important roles in the progression of various types of cancers, including proliferation, invasion, angiogenesis, immune surveillance evasion, etc.35,36 It is activated by the phosphorylation of tyrosine 705 site via signaling from upstream regulators.37,38 Studies showed that STAT3 activation in lung cancer increases the expression of VEGF and stimulates angiogenesis.39–41 Therefore, we further investigated whether SOX5 regulated STAT3, ERK, or AKT signaling leading to VEGF stimulation. In this study, we provided evidence for the first time that STAT3/VEGF activation of A549 cells can be attributed to SOX5 overexpression.
Conclusion
Our in vitro and in vivo analyses provided preliminary evidence that SOX5 is indeed an inducer of angiogenesis in LAC. We indicated that SOX5 overexpression had stronger impact on HUVECs tube formation ability. We also demonstrated that SOX5 can promote LAC angiogenesis via the transcriptional activation of VEGF. Our results showed that knockdown of the expression of SOX5 led to a decrease in VEGF at both mRNA and protein levels in vitro. We found that SOX5 silencing led to less phosphorylated STAT3 and less VEGF expression. Overexpression of SOX5 led to the activation of STAT3 and promotion of VEGF expression. Then we further analyzed the correlation between VEGF and SOX5 in 90 LAC patients. Interestingly, the expression of SOX5 and VEGF in LAC was positively correlated (r=0.399, P=0.001). These results suggested that the high level of SOX5 promotes tumor angiogenesis by STAT3/VEGF signaling and that silencing SOX5 may be an important approach to controlling LAC angiogenesis.
Acknowledgments
This study was supported by grants from the National Nature Science Foundation of China (Nos.81603340, 81802887, 81570343, 81670350) and the Natural Sciences Fund of Zhejiang Province (LY15H160029).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 4.Chen K, Zhou F, Shen W, et al. Novel mutations on EGFR Leu792 potentially correlate to acquired resistance to osimertinib in advanced NSCLC. J Thorac Oncol. 2017;12(6):e65–e68. doi: 10.1016/j.jtho.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Hall RD, Le TM, Haggstrom DE, Gentzler RD. Angiogenesis inhibition as a therapeutic strategy in non-small cell lung cancer (NSCLC) Transl Lung Cancer Res. 2015;4(5):515–523. doi: 10.3978/j.issn.2218-6751.2015.06.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: prognostic and therapeutic implications. J Clin Oncol. 2005;23(14):3243–3256. doi: 10.1200/JCO.2005.18.853. [DOI] [PubMed] [Google Scholar]
- 7.Xu YR, Yang WX. SOX-mediated molecular crosstalk during the progression of tumorigenesis. Semin Cell Dev Biol. 2017;63:23–34. doi: 10.1016/j.semcdb.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Darby IA, Bisucci T, Raghoenath S, Olsson J, Muscat GEO, Koopman P. Sox18 is transiently expressed during angiogenesis in granulation tissue of skin wounds with an identical expression pattern to Flk-1 mRNA. Lab Invest. 2001;81(7):937–943. doi: 10.1038/labinvest.3780304. [DOI] [PubMed] [Google Scholar]
- 9.Kim K, Kim I-K, Yang JM, et al. SoxF transcription factors are positive feedback regulators of VEGF signaling novelty and significance. Circ Res. 2016;119(7):839–852. doi: 10.1161/CIRCRESAHA.116.308483. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Lee S, Lee S, et al. Sox17 promotes tumor angiogenesis and destabilizes tumor vessels in mice. J Clin Invest. 2013;123(1):418–431. doi: 10.1172/JCI64547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Han S, Wang X, Peng R, Li X. SOX5 promotes epithelial–mesenchymal transition and cell invasion via regulation of Twist1 in hepatocellular carcinoma. Med Oncol. 2015;32(2):461. doi: 10.1007/s12032-014-0461-2. [DOI] [PubMed] [Google Scholar]
- 12.Renjie W, Haiqian L. MiR-132, miR-15a and miR-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox5. Cancer Lett. 2015;356(2):568–578. doi: 10.1016/j.canlet.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Pei X-H, Lv X-Q, Li H-X, Xq L, Hx L. Sox5 induces epithelial to mesenchymal transition by transactivation of Twist1. Biochem Biophys Res Commun. 2014;446(1):322–327. doi: 10.1016/j.bbrc.2014.02.109. [DOI] [PubMed] [Google Scholar]
- 14.Ma S, Chan YP, Woolcock B, et al. DNA fingerprinting tags novel altered chromosomal regions and identifies the involvement of SOX5 in the progression of prostate cancer. Int J Cancer. 2009;124(10):2323–2332. doi: 10.1002/ijc.24243. [DOI] [PubMed] [Google Scholar]
- 15.Huang D-Y, Lin Y-T, Jan P-S, et al. Transcription factor SOX-5 enhances nasopharyngeal carcinoma progression by down-regulating SPARC gene expression. J Pathol. 2008;214(4):445–455. doi: 10.1002/path.2299. [DOI] [PubMed] [Google Scholar]
- 16.Ueda R, Yoshida K, Kawase T, Kawakami Y, Toda M. Preferential expression and frequent IgG responses of a tumor antigen, SOX5, in glioma patients. Int J Cancer. 2007;120(8):1704–1711. doi: 10.1002/ijc.22472. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Fu Y, Xu H, et al. SOX5 predicts poor prognosis in lung adenocarcinoma and promotes tumor metastasis through epithelial-mesenchymal transition. Oncotarget. 2018;9(13):10891–10904. doi: 10.18632/oncotarget.22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Adjei AA. Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. Oncologist. 2015;20(6):660–673. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang T, Liu J, Xiao X-Q. Cantharidin inhibits angiogenesis by suppressing VEGF-induced JAK1/STAT3, ERK and AKT signaling pathways. Arch Pharm Res. 2015;38(2):282–289. doi: 10.1007/s12272-014-0383-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhao M, Gao F-H, Wang J-Y, et al. JAK2/STAT3 signaling pathway activation mediates tumor angiogenesis by upregulation of VEGF and bFGF in non-small-cell lung cancer. Lung Cancer. 2011;73(3):366–374. doi: 10.1016/j.lungcan.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35(1):75–91. doi: 10.1007/s10555-016-9618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bremnes RM, Camps C, Sirera R. Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer. 2006;51(2):143–158. doi: 10.1016/j.lungcan.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Crino L, Metro G. Therapeutic options targeting angiogenesis in nonsmall cell lung cancer. Eur Respir Rev. 2014;23(131):79–91. doi: 10.1183/09059180.00008913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Chen J, Guo Y, Wang B, Chu H. Strategies targeting angiogenesis in advanced non-small cell lung cancer. Oncotarget. 2017;8(32):53854–53872. doi: 10.18632/oncotarget.17957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goi T, Fujioka M, Satoh Y, et al. Angiogenesis and tumor proliferation/metastasis of human colorectal cancer cell line SW620 transfected with endocrine glands-derived-vascular endothelial growth factor, as a new angiogenic factor. Cancer Res. 2004;64(6):1906–1910. doi: 10.1158/0008-5472.can-3696-2. [DOI] [PubMed] [Google Scholar]
- 27.Wat JJ, Wat MJ. Sox7 in vascular development: review, insights and potential mechanisms. Int J Dev Biol. 2014;58(1):1–8. doi: 10.1387/ijdb.130323mw. [DOI] [PubMed] [Google Scholar]
- 28.Cermenati S, Moleri S, Cimbro S, et al. Sox18 and Sox7 play redundant roles in vascular development. Blood. 2008;111(5):2657–2666. doi: 10.1182/blood-2007-07-100412. [DOI] [PubMed] [Google Scholar]
- 29.Matsui T, Kanai-Azuma M, Hara K, et al. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci. 2006;119(17):3513–3526. doi: 10.1242/jcs.03081. [DOI] [PubMed] [Google Scholar]
- 30.Downes M, Koopman P. SOX18 and the transcriptional regulation of blood vessel development. Trends Cardiovasc Med. 2001;11(8):318–324. doi: 10.1016/s1050-1738(01)00131-1. [DOI] [PubMed] [Google Scholar]
- 31.Perera PM, Wypasek E, Madhavan S, et al. Mechanical signals control SOX-9, VEGF, and c-Myc expression and cell proliferation during inflammation via integrin-linked kinase, B-Raf, and ERK1/2-dependent signaling in articular chondrocytes. Arthritis Res Ther. 2010;12(3):R106. doi: 10.1186/ar3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16(21):2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambrechts D, Lenz H-J, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31(9):1219–1230. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 34.Wang T, Liu J, Xiao X-Q. Cantharidin inhibits angiogenesis by suppressing VEGF-induced JAK1/STAT3, ERK and AKT signaling pathways. Arch Pharm Res. 2015;38(2):282–289. doi: 10.1007/s12272-014-0383-8. [DOI] [PubMed] [Google Scholar]
- 35.Darnell JE., Jr STATs and gene regulation. Science. 1997;277(5332):1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 36.Bromberg J, Darnell JE. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19(21):2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 37.Klemm JD, Schreiber SL, Crabtree GR. Dimerization as a regulatory mechanism in signal transduction. Annu Rev Immunol. 1998;16(1):569–592. doi: 10.1146/annurev.immunol.16.1.569. [DOI] [PubMed] [Google Scholar]
- 38.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19(49):5662–5679. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 39.Zhao M, Gao F-H, Wang J-Y, et al. JAK2/STAT3 signaling pathway activation mediates tumor angiogenesis by upregulation of VEGF and bFGF in non-small-cell lung cancer. Lung Cancer. 2011;73(3):366–374. doi: 10.1016/j.lungcan.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Xue D, Yang Y, Liu Y, et al. MicroRNA-206 attenuates the growth and angiogenesis in non-small cell lung cancer cells by blocking the 14-3-3ζ/STAT3/HIF-1α/VEGF signaling. Oncotarget. 2016;7(48):79805–79813. doi: 10.18632/oncotarget.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan B, Shen J, Cao J, et al. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep. 2015;5(1):16053. doi: 10.1038/srep16053. [DOI] [PMC free article] [PubMed] [Google Scholar]