Abstract
BACKGROUND
Chordomas are rare but challenging neoplasms involving the skull base. A preoperative grading system will be useful to identify both areas for treatment and risk factors, and correlate to the degree of resection, complications, and recurrence.
OBJECTIVE
To propose a new grading system for cranial chordomas designed by the senior author. Its purpose is to enable comparison of different tumors with a similar pathology to clivus chordoma, and statistically correlate with postoperative outcomes.
METHODS
The numerical grading system included tumor size, site of the tumor, vascular encasement, intradural extension, brainstem invasion, and recurrence of the tumor either after surgery or radiotherapy with a range of 2 to 25 points; it was used in 42 patients with cranial chordoma. The grading system was correlated with number of operations for resection, degree of resection, number and type of complications, recurrence, and survival.
RESULTS
We found 3 groups: low-risk 0 to 7 points, intermediate-risk 8 to 12 points, and high-risk ≥13 points in the grading system. The 3 groups were correlated with the following: extent of resection (partial, subtotal, or complete; P < .002); number of operative stages to achieve removal (P < .014); tumor recurrence (P = .03); postoperative Karnofsky Performance Status (P < .001); and with successful outcome (P = .005). The grading system itself correlated with the outcome (P = .005).
CONCLUSION
The proposed chordoma grading system can help surgeons to predict the difficulty of the case and know which areas of the skull base will need attention to plan further therapy.
Keywords: Classification, Cranial, Chordoma, Grading System, Outcome, Prognostic, Treatment, Tumor
ABBREVIATIONS
- ICA
internal carotid artery
- IRB
Institutional Review Board
- MRI
magnetic resonance imaging
- OS
overall survival
- RFS
recurrence-free survival
- SGSCC
Sekhar Grading System for Cranial Chordomas
- TED
tumor equivalent diameter
Skull base chordomas are rare slow-growing tumors.1 However, unless incidentally diagnosed, they are usually large, involve critical neurovascular structures, and are difficult to eradicate by surgery.2,3 Meta-analysis of previously published papers shows that complete resection correlates with recurrence-free survival (RFS).4-6 Adjuvant radiotherapy requires that focused radiation (carbon-ion, proton, or alternate stereotactic radiation) be delivered at high doses to achieve therapeutic levels for this radio-resistant tumor. While many groups, including ours, favor the use of particle-beam radiation (proton or carbon-ion based),7-9 a recent meta-analysis failed to show a difference in either RFS or overall survival (OS) across various radiation modalities.10 There are ongoing efforts to use tumor genetics and proteomics to develop targeted chemotherapies but none are in clinical use beyond experimental studies.11-15
Previous studies indicate that size, location, and degree of surgical resection impact OS and RFS.2,3,5,16 However, there are currently no prognostic scoring systems for cranial chordomas that address these parameters. Based on the prior experience of the senior author (LNS), we developed a scoring system (Sekhar Grading System for Cranial Chordomas [SGSCC]) that incorporates prognostically and surgically important features. Using the SGSCC, we classified skull base chordomas into low, intermediate, and high-risk groups.
METHODS
This a retrospective cohort analysis of 42 consecutive patients operated upon between 2005 and March 2015 by the authors (LNS, RCR, MFJ) at Harborview Medical Center and University of Washington Medical Center, in Seattle, Washington with pathologically confirmed cranial chordoma. The University of Washington Institutional Review Board (IRB) granted approval for this retrospective study. The University IRB Committee judged that patient consent was unnecessary because of the retrospective nature of the study.
We extracted details of clinical history, prior treatment, surgical approach, and patient outcomes from medical records. Clinical information for the study was reviewed and scored by one of the authors not directly involved in patient care (HBS), and extent of resection and recurrence was obtained by the radiological report dictated by a board-certified radiologist. Most tumors have an oval shape, thus tumor equivalent diameter (TED) was used to represent tumor size and was calculated using the formula17 Dmean = (D1 × D2 × D3)1/3. All patients were followed up radiographically and clinically. For those patients who had not been evaluated in the clinic within the last 4 mo, the follow-up data were updated by a phone call (by HBS).
A grading system for skull base chordomas was developed by the senior author (LNS) based on his experience in treating these tumors, accounting for tumor size, site, vascular encasement by the tumor, intradural extension, brainstem involvement, and prior treatments (Table 1). Some of the listed grading criteria overlap occasionally but are different; size (criterion 1) refers to a physical measurable dimension, and the criteria 2, 3, and 4 are anatomic but with completely different implications for the surgeon. Preoperative imaging was used to establish the encasement of the vessels, and intraoperative and histological findings to evaluate the tumor invasion. The scoring system is summarized below.
TABLE 1.
Criteria Needed to Calculate the Chordoma Grading System
| Sekhar chordoma grading: | |
|---|---|
| Tumor size (scored 1-4 based on calculated TED) | |
| 1 (>0-1.9 cm) | |
| 2 (2-3.9 cm) | |
| 3 (4-5.9 cm) | |
| 4 (>6 cm) | |
| Site (scored 1-9 based on anatomic regions involved) | |
| Clivus: upper, mid, lower | |
| Cavernous sinus: left, right | |
| Petrous bone: left, right | |
| Cervical C1/2/3: left, right | |
| Vascular involvement (scored 0-5 for each artery with >50% encasement) | |
| ICA: left, right | |
| Vertebral: left, right | |
| Basilar artery | |
| Intradural invasion (scored 0-2) | |
| None | |
| Small without brainstem displacement | |
| Large with brainstem displacement | |
| Tumor regrowth after prior treatment (scored 0-5) | |
| After surgery (2 points) | |
| After radiation (3 points) |
TED, tumor equivalent diameter; Dmean = (D1 × D2 × D3)1/3
Sekhar Grading System for Cranial Chordomas (Table 1)
Tumor size: scored 1 to 4 based on average diameter: >0 to 1.9 cm, 1 point; 2 to 3.9 cm, 2 points; 4 to 5.9 cm, 3 points; >6 cm, 4 points.
Tumor site: scored 1 to 9 based on anatomic regions involved, 1 point for each region: upper, mid, lower clivus; left and right cavernous sinus; left and right petrous bone; cervical areas C1-C3 left and right. For the purpose of this classification, we defined the limit between upper, middle, and lower clivus as follows. The upper clivus is above petrous apices and above the crossing points of the trigeminal nerves from the posterior of the middle fossa. The mid clivus extends from the trigeminal nerve down to the exit foramina (pars nervosa) of the jugular foramen. The lower clivus is the area below the ninth, tenth, and eleventh nerves and includes the jugular tubercle, occipital condyles, the foramen magnum, and the hypoglossal canals.18 The clivus is shaped like a truncated triangle.
Vascular encasement: scored 0 to 5 for each artery with >50% encasement, 1 point for each vessel: left and right internal carotid artery (ICA), left and right vertebral arteries, and basilar artery. We did not consider vessel displacement as vessel encasement.
Intradural invasion: scored 0 to 2. Degree of extension: small with no brainstem displacement, 1 point; large with brainstem displacement, 2 points. Thus, intradural invasion, in contrast to interdural extension, is when preoperative magnetic resonance imaging (MRI) showed intradural extension of the tumor.
Prior treatment: scored 0 to 5: prior surgery 2 points, prior radiotherapy 3 points. This allows us to include patients treated elsewhere in our study. Therefore, it is strengthening the usefulness of the grading system.
Each patient was graded using information available preoperatively. Grades were correlated with the following: completeness of resection, complications (death, permanent deficit, cerebrospinal fluid leak, cranial nerve palsy, stroke, and reoperation for complication), OS, RFS, Karnofsky Performance Status (KPS), and overall success (complete resection, KPS > 70, without complication or recurrence).
Statistical analysis was performed by a statistician (JKB) on IBM SPSS version 19 (IBM Corp, Armonk, New York) and StatXact version 4 (CYTEL Software Corp, Cambridge, Massachusetts). Differences among the chordoma groups were tested for statistical significance using Spearman correlation coefficients (for KPS scores and other continuous measures), Cochran–Armitage exact tests for trend (for complications and other dichotomous measures), and Cox proportional hazards regression (overall and progression-free survival).
RESULTS
Main Results
We retrospectively studied 42 consecutive patients who met our inclusion criterion of having at least 12 mo of follow-up. Mean patient age was 40.9 yr and ranged from 5 to 69 yr. Sixty-two percent of patients were male and 38% female. Patients had an average preoperative KPS of 87, with 15 patients with a KPS of 80 or lower (Table 2). TED was between 0 and 1.9 cm in 17%, between 2.0 and 3.9 in 38%, between 4.0 and 5.9 in 29%, and greater than 6.0 cm in 17% of cases, mean TED was 3.29 cm. Surgical resection utilized open microsurgical technique with skull base exposures in the majority of cases (88%). Seven percent underwent totally endoscopic resection while staged endoscopic and microsurgical resections were performed in 5% of cases. Staged surgical procedures using different skull base exposures were common, occurring in 45% of cases (average number of stages 1.6, range 1-3). The extreme lateral approach was used most commonly, employed in 24% of cases. Extended subfrontal (21%) and frontotemporal-orbitozygomatic (16%) approaches were also commonly utilized. LeFort osteotomies, endoscopic endonasal, posterior transpetrosal, and subtemporal-infratemporal were the other approaches used in this series. Demographic and preoperative data are shown in Table 2.
TABLE 2.
Demographic and Preoperative Data
| Subjects | 42 |
|---|---|
| Age mean (SD) | 41 (17) |
| 0-29 | 13 (31%) |
| 30-49 | 15 (36%) |
| 50+ | 14 (33%) |
| Sex | |
| Male | 16 (38%) |
| Female | 26 (62%) |
| Year of surgery | |
| 2005-09 | 15 (36%) |
| 2010-12 | 12 (29%) |
| 2013+ | 15 (36%) |
| Preoperative KPS | |
| Mean (SD) | 87 (10) |
| ≤80 | 15 (36%) |
| 90 | 20 (48%) |
| 100 | 7 (17%) |
| Stages | 1.6 (0.7) |
| 1 | 23 (55%) |
| 2 | 15 (36%) |
| 3 | 4 (10%) |
| TED | |
| Mean | 3.29 cm |
| Range | 1.16-6.66 cm |
| Approach | |
| Open | 37 (88%) |
| Extreme lateral | 16 (24%) |
| Extended subfrontal | 14 (21%) |
| Frontotemporal OZ | 11 (16%) |
| Lefort 1/2 | 7 (10%) |
| Transpetrosal | 5 (7%) |
| Subtemporal-infratemporal | 4 (6%) |
| Other | 4 (6%) |
| Endoscopic endonasal | 3 (7%) |
| Combined | 2 (5%) |
KPS, Karnofsky Performance Scale; TED, tumor equivalent diameter; Dmean = (D1 × D2 × D3)1/3
Tumor involved the upper clivus in 43%, the middle clivus in 88%, and the lower clivus in 69% of cases. Cavernous sinus involvement was present in 45% of patients (19% on the right, 7% on the left, and 19% bilaterally). Tumors frequently invaded the petrous bone, occurring in 88% of cases (19% on the right, 17% on the left, and 52% bilaterally). Tumor extended inferiorly into the occipital condyles, craniocervical junction, and upper cervical spine in 43% of cases (10% on the right, 19% on the left, and 14% bilaterally). Tumors, on average, extended into 4.6 different anatomic regions. Tumor encased >50% of the vertebral arteries in 33% of cases (14% on the right, 7% on the left, and 12% bilaterally). The basilar artery was involved in 28% of cases. Tumor encased the ICAs in 38% of patients (19% on the right, 5% on the left, and 14% bilaterally). Tumor invasion through the dura was present in 73% of cases, while the brainstem was displaced in 52% of cases, intradural invasion without brainstem displacement was found in 21% of cases (Table 3). Patients presented after having previously been treated for their tumors in 40% of cases: 24% had prior surgical resections and 17% had both previous surgery and postoperative radiation therapy.
TABLE 3.
Summary of Anatomic Regions With Tumor Involvement and Invasion
| Clivus regions with tumor | |
| Upper | 18 (43%) |
| Mid | 37 (88%) |
| Lower | 29 (69%) |
| Cavernous sinus invasion | |
| Left | 3 (7%) |
| Right | 8 (19%) |
| Bilateral | 19 (45%) |
| Petrous bone invasion | |
| Left | 7 (17%) |
| Right | 8 (19%) |
| Bilateral | 22 (52%) |
| Cervical C1/2/3 invasion | |
| Left | 8 (19%) |
| Right | 4 (10%) |
| Bilateral | 6 (14%) |
| ICA involvement | |
| Left | 2 (5%) |
| Right | 8 (19%) |
| Bilateral | 6 (14%) |
| Vertebral artery involvement | |
| Left | 3 (7%) |
| Right | 6 (14%) |
| Bilateral | 5 (12%) |
| Basilar artery involvement | 12 (28%) |
| Intradural invasion | |
| None | 11 (26%) |
| No brainstem displacement | 9 (21%) |
| With brainstem displacement | 22 (52%) |
There were no surgical or perioperative mortalities. Our goal was to remove the tumors completely, but after 3 stages or based on the patients’ wishes, a small remnant was accepted. The assessment of degree of resection was independent of the intraoperative impression by the surgeon, it was confirmed through a follow-up MRI. Complete resection was achieved in 36% of cases and subtotal resection in 64% of the cases. The tumor residual was more frequent in the lower clivus cranial-cervical junction (6 cases), and cavernous sinus region (5 cases); Table 4 summarizes the residual tumor locations. Perioperative complications occurred in 31% of patients. Cerebrospinal fluid leak was the most commonly seen complication, occurring in 14% of cases. Seven percent of patients had a new permanent deficit and 5% had new cranial nerve palsy, and 5% had new ischemic lesions. In total, 19% of patients underwent an additional surgical intervention to treat a postoperative complication. Tumor recurrence at 12 mo occurred in 19% of cases. Patients had an average KPS of 88 at 12 mo: 27% had a KPS of 100 and only 7% of the patients had a KPS < 70. Average time to recurrence was estimated at 102.5 mo. Mean OS time was estimated at 120.5 mo. Outcomes are shown in Table 5. A complete discussion of radiotherapy for chordomas is beyond the scope of this paper; however, proton beam therapy was the most frequent type used as an adjuvant therapy in 28 of our patients. The type of radiation and the amount received by each patient is summarized in Table 6. Proton beam therapy is our preferred option of adjuvant therapy, and has been shown to play an important role in progression-free survival for chordomas.8
TABLE 4.
Summary of Residual Tumor Locations
| Lower clivus cranial-cervical junction | 6 |
| Cavernous sinus region | 5 |
| Petrous bone | 4 |
| Retropharyngeal and soft issues | 3 |
| Cervical vertebrae | 2 |
| Condyles | 2 |
| Sphenoid bone and orbita | 1 |
| Cerebellopontine angle | 1 |
TABLE 5.
Clinical Outcomes of the Cohort
| Resection | |
| Complete | 15 (36%) |
| Subtotal | 27 (64%) |
| Complication | 13 (31%) |
| Death | 0 (0%) |
| Permanent deficit | 3 (7%) |
| CSF leak | 6 (14%) |
| CN palsy | 2 (5%) |
| Stroke | 2 (5%) |
| Reop. for comp. | 8 (19%) |
| OS | |
| 12 mo mortality | 0 (0%) |
| Overall mortality | 4 (10%) |
| Mean survival (mo) | 120.5 |
| Mean follow-up (mo) | 60 (42) |
| Recurrence | |
| 12 mo recurrence | 8 (19%) |
| Overall recurrence | 8 (19%) |
| Mean recurrence free survival (mo) | 102.5 |
| Mean follow-up (mo) | 50 (39) |
| KPS (12 mo) | 88 (13) |
| Improved | 14 (33%) |
| Unchanged | 22 (52%) |
| Worsened | 6 (14%) |
| Overall successa | 13 (31%) |
CSF, cerebrospinal fluid; CN, cranial nerve; KPS, Karnofsky Performance Scale
acomplete resection, no complication, no recurrence, and KPS > 70.
TABLE 6.
Type and Amount of Radiotherapy Received by the Patients
| Type of radiation therapy | Number of patients | Gray dosimetry range |
|---|---|---|
| Proton beam therapy | 28 | 50-76 Gy |
| Fractioned stereotactic radiotherapy | 12 | 55-68 Gy |
| Gama Knife radiosurgery | 1 | 31 Gy |
| Cyberknife | 1 | 50 Gy |
Key Results
We applied the scoring system, described above, to this cohort. The total score distribution is shown in Table 7. This table summarizes the statistical significance of all bivariate relationships between clinical variables of interest and the chordoma classification scale, the clinical variables studied were as follows: number and severity of complications, survival, recurrence, change in the KPS score at 12-mo follow-up, and composite outcome. The grading system, when correlated with the composite outcome, showed a P-value of .005, composite outcome was defined by total resection, no mortality, no major new neurological deficit, and no recurrence, and KPS > 70. All significance values are from t-tests, Mann–Whitney tests, Spearman correlation tests, and Cox regression models as appropriate. However, we were concerned about the possibility of incurring in type 1 error (false positive) because of the size of the cohort. Hence, we performed a Holm–Bonferroni adjustment for multiple comparisons (m = 126), and it resulted that none of these P-values remained statistically significant. Moreover, a regression analysis for each individual criterion used to create the grading system was not possible since giving numbers to the categorical data would not turn them into quantitative variables, they are still categorical variables, just ones that have been assigned numbers. Therefore, we could not perform regression on the types of variables used for the grading system because they do not meet to quantitative variables condition. However, despite the small cohort used for this study, the significance of the composite outcome suggests a validation of the grading system.
TABLE 7.
Correlation of Clinical Variables With the Chordoma Grading System (P-values only)
| N (%) | Mean chordoma score | Sig. | |
|---|---|---|---|
| Number of complications | |||
| Mean (SD) | 0.6 (0.9) | .858 Spearman correlation | |
| 0 | 26 (62%) | 10.5 | |
| 1 | 6 (14%) | 11.7 | |
| 2+ | 10 (24%) | 10.4 | |
| Severe complicationa | |||
| No | 29 (69%) | 10.7 | .910 t-test |
| Yes | 13 (31%) | 10.5 | |
| Time to death | |||
| No | 38 (90%) | 10.3 | .531 Cox regression |
| Yes | 4 (10%) | 13.5 | |
| Cumulative survival | 78% | ||
| Mean survival (mo) | 121 | ||
| Mean follow-up (mo) | 60 (42) | ||
| Time to recurrence | |||
| No | 34 (81%) | 9.8 | .029 Cox regression |
| Yes | 8 (19%) | 14.3 | |
| Cumulative survival | 59% | ||
| Mean survival (mo) | 103 | ||
| Mean follow-up (mo) | 50 (39) | ||
| Change in KPS at 12 mo | |||
| Mean (SD) | 1 (11) | .534 Spearman correlation | |
| Worse | 6 (14%) | 10.3 | |
| Same | 22 (52%) | 11.3 | |
| Better | 14 (33%) | 9.7 | |
| Composite outcomeb | .005 Mann–Whitney | ||
*All significance values are from t-tests, Mann–Whitney, Spearman correlations, and Cox models.
aCerebrospinal fluid leak or major permanent deficit or cranial nerve palsy or reoperation for complication.
bTotal resection and no death/stroke/maj. deficit and no recurrence by 12 mo and KPS 12 > 70.
Total scores showed an approximately normal distribution across their range of 2 to 20 (Figure 1), this normal distribution of the scores observed identified the 3 groups described below. There was not a natural break in the curve; however, the curve suggested that the patients should be broken down in 3 groups. Patients were grouped based on their total score using the SGSCC. Group 1 included those with a score ≤7 (n = 8, 19%), group 2 included patients with scores of 8 to 12 (n = 23, 55%), and group 3 consisted of all patients with a score of ≥13 (n = 11, 26%). There was a significant decrease in the rate of complete resection across the 3 groups, declining from 78% in group 1, to 26% in group 2, and to 21% in group 3 (P = .002). KPS scores at 12 mo also decreased significantly across the 3 groups, from an average score of 98 in group 1 to 88 in group 2 and down to 81 in group 3 (P = .001). The rate of patients who achieved complete resection, without any surgical complication or any recurrence at 12 mo, who maintained a KPS > 70 was termed “Overall Success.” It declined across the 3 groups as well (group 1 67%, group 2 26%, group 3 14%, P = .005; Figure 2). The 12-mo RFS decreased significantly across the 3 groups, from 100% in group 1, to 89% in group 2, and to 57% in group 3 (P = .003). The complication rate increased across the 3 groups as well; however, this was not statistically significant (P = .91). Correlation between the extent of resection and recurrence showed a trend, but not statistical significance (P = .1). This may be due to the small number of patients in this series.
FIGURE 1.
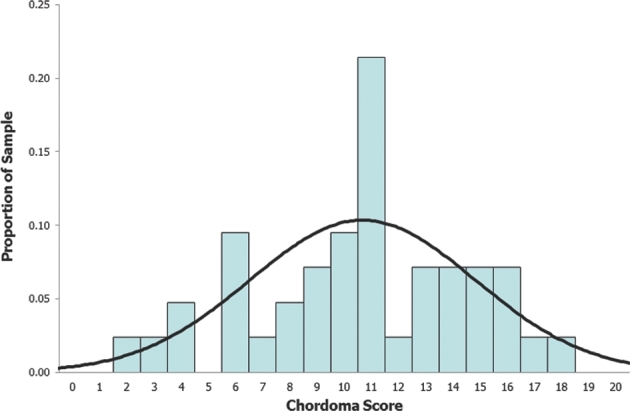
Normal distribution of the scores.
FIGURE 2.
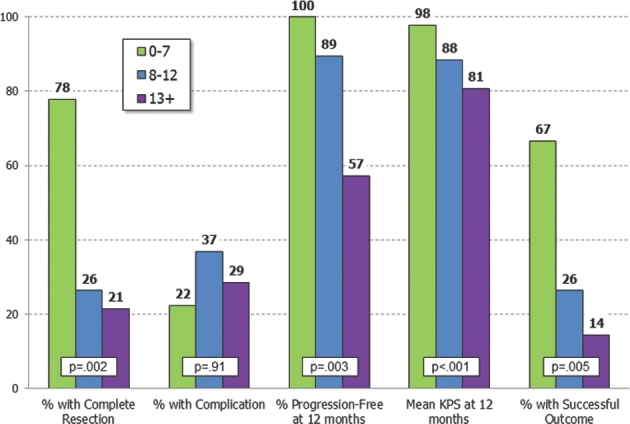
Outcomes using the SGSCC.
Kaplan–Meier survival curves are shown for OS and RFS in Figures 3 and 4, respectively. There were only 4 deaths observed during the follow-up time period, and we did not find a significant difference in a Cox proportional hazards model using the SGSCC (P = .68). RFS showed a statistical trend to decrease across the SGSCC groups (P = .10).
FIGURE 3.
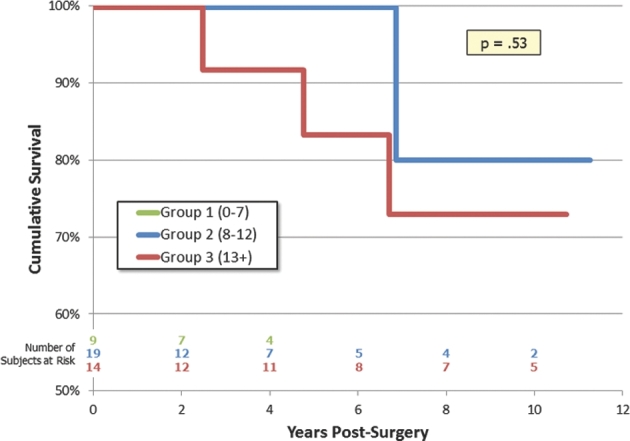
Kaplan–Meier survival curve.
FIGURE 4.
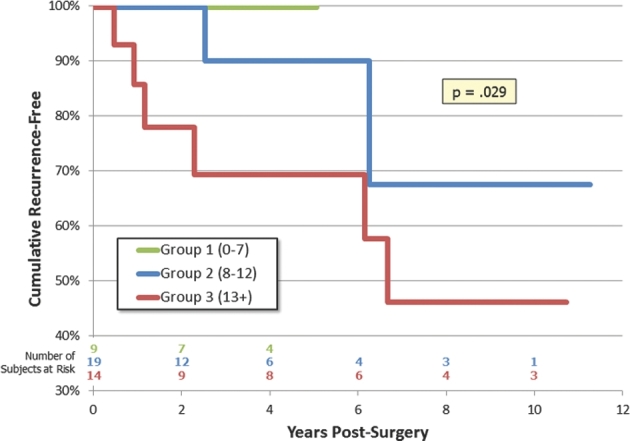
Kaplan–Meier survival curve for RFS.
Illustrative Cases
Case 1
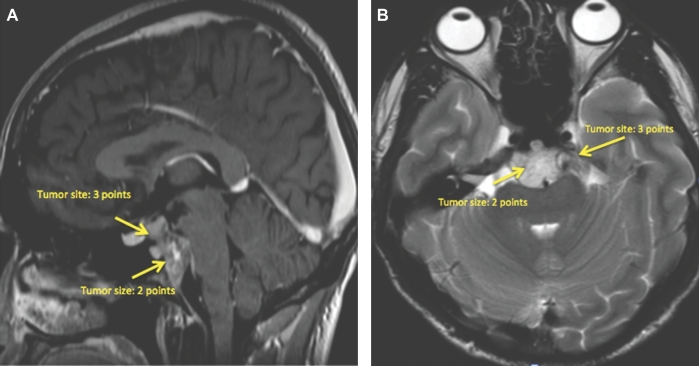
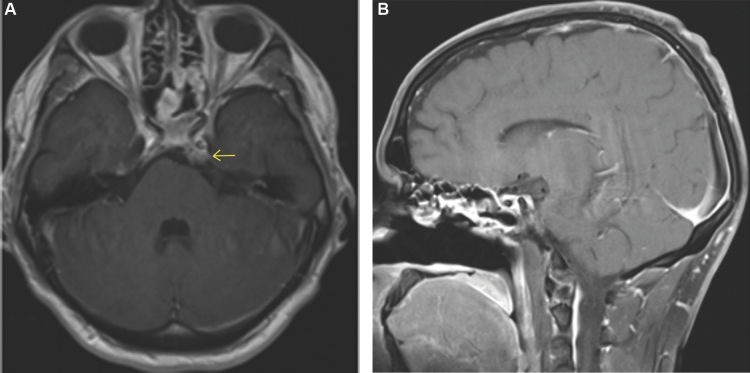
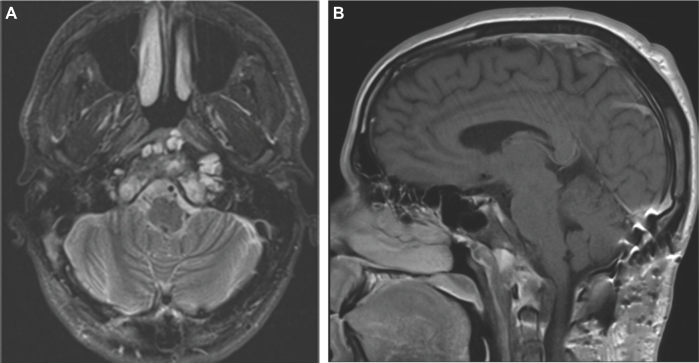
This is a 26-yr-old patient with an incidental brain tumor finding on MRI obtained during work-up following an assault. Subsequently, he developed double vision and represented to the emergency room. An MRI showed tumor growth (Figures 5A and 5B). This tumor scored 6 points using the SGSCC and was classified as low risk: 2 points for the size (2.3 cm maximal diameter), 3 points for the site presence in the upper, middle, and right petrous bone, and 1 point for the small intradural extension (<1 cm). He underwent an endonasal endoscopic subtotal resection with nasoseptal flap, a small tumor remnant was left at the right petroclival region (Figures 6A and 6B). Postoperatively, he received 66.6 Gy through proton beam radiation therapy. Currently, the patient has a KPS of 100, the abducens paresis has resolved. There is still an unchanged tumor remnant in his last MRI (Figures 7A and 7B).
FIGURE 5.
A and B, Preoperative MRI of a chordoma that scored 6 points in the grading system, and that was operated through a transnasal endoscopic approach.
FIGURE 6.
A and B, Postoperative MRI of the chordoma operated through a transnasal endoscopic approach, showing a small intradural tumor remnant.
FIGURE 7.
A and B, Twelve-month follow-up MRI imaging of the chordoma operated through a transnasal endoscopic approach, shows no tumor regrowth.
Case 2
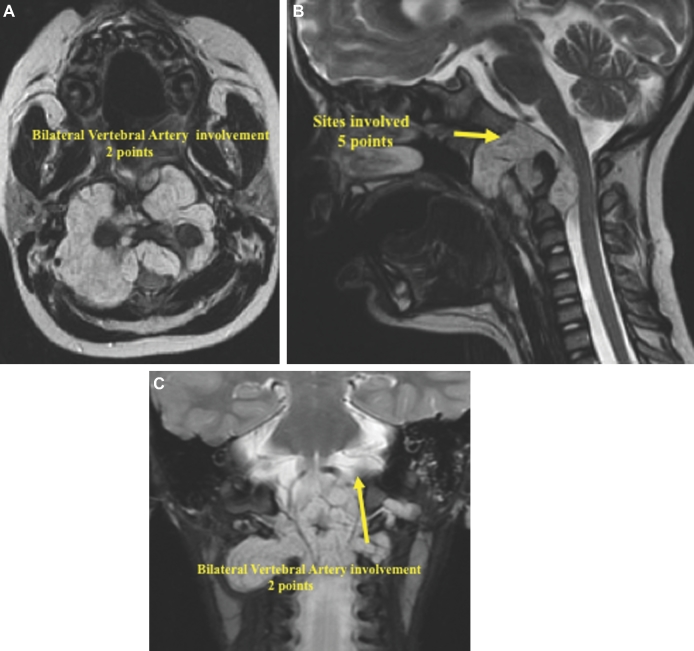
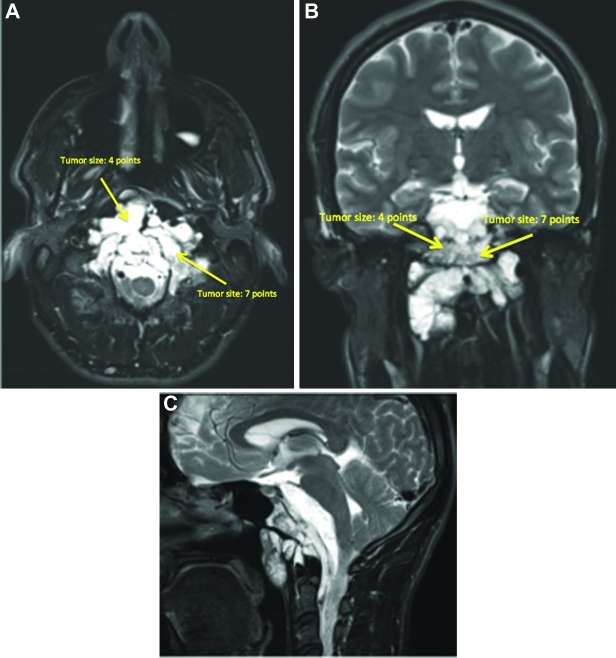
This is a 5-yr-old girl with an extensive lower clival and upper cervical chordoma, who clinically presented with a severe neck and shoulder pain and the inability to keep her head up straight indicating cranial–cervical junction instability, as well as inability to eat. Furthermore, she was dragging both the feet, particularly the right side. The overall SGSCC was 11: 3 points for tumor size, 5 points for sites involved (lower clivus 1 point, petrous bone bilaterally 2 points, and cervical region bilaterally 2 points), 2 points for bilateral vertebral artery encasement, and 1 point for intradural invasion (Figures 8A-8C). She underwent a 3-staged surgical procedure. The first operation was a right retrosigmoid craniotomy and an extreme lateral approach with resection of C1 lateral mass and odontoid process, followed by microsurgical tumor resection. Two weeks later, a posterior occiput to C3-C4 fusion was performed. After 2 more weeks, the third stage surgical procedure was done consisting of a left-sided extreme lateral transcondylar approach with retrosigmoid craniotomy for the microsurgical removal of the tumor remnant. A bone graft between C2 and the occipital condyle was placed at this stage. A small tumor remnant was found at C2 (Figures 9A-9C). Subsequently, the patient received proton beam radiation. Clinically, she recovered both the lower cranial nerves and the motor functions. She remains free of tumor regrowth at 7 yr after the surgery, and is a normal 12-yr-old girl.
FIGURE 8.
A-C, The preoperative MRI imaging showed a large tumor. The overall SGSCC was 11: 3 points for tumor size, 5 points for sites involved (lower clivus 1 point, petrous bone bilaterally 2 points, cervical region bilaterally 2 points), 2 points for bilateral vertebral artery encasement, and 1 point for intradural invasion.
FIGURE 9.
A-C, Postoperative MRI imaging 48-mo follow-up.
Case 3
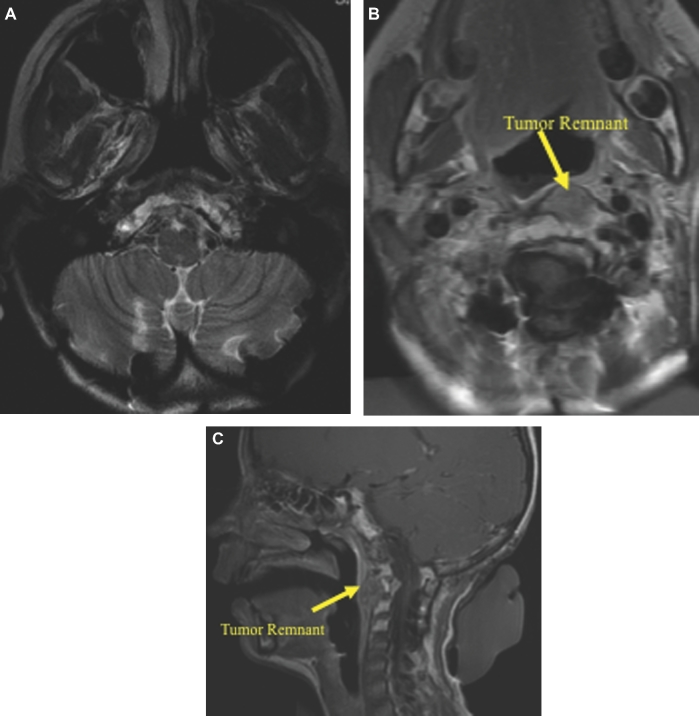
This 28-yr-old male experienced headaches diplopia, left-sided tongue numbness, and shoulder pain. MRI revealed a large clivus chordoma, with extensive intradural invasion through all segments of the clivus (Figures 10A-10C). The overall SGSCC was 21 points and is classified as high risk. The tumor was removed in 3 stages. First using a right transpetrosal approach to resect the intradural tumor. The second stage consisted of right-sided extreme lateral transcondylar approach and resection of extradural clival tumor in the lower clival and retropharyngeal area with an occiput to C3 fusion. The third stage consisted of an extended subfrontal approach with olfactory nerve preservation and gross total resection of the extradural tumor (Figures 11A and 11B). Adjuvant proton beam radiotherapy treatment was given (67 Gy), and he has a stable and small tumor residual and no regrowth at 24-mo follow-up. Postoperatively, the patient did very well, and he currently has a KPS of 90 with a mild abducens paresis.
FIGURE 10.
A-C, Preoperative MRI imaging of a large clivus chordoma that scored 21 points in our grading system.
FIGURE 11.
A and B, Postoperative MRI imaging after the third stage tumor resection for this case.
DISCUSSION
Complete surgical resection of skull base chordomas provides the best possible patient outcomes. However, there are large differences between a small extradural-intraosseous tumor confined to the mid clivus and a large tumor encasing arteries in the circle of Willis, invading the brainstem, and extending into multiple different anatomic regions of the skull base. Moreover, there remain important clinical questions surrounding the management of patients with skull base chordomas, for example, where endoscopic surgery vs open microsurgery is best applied,5,19-22 when and using what modality is postoperative radiation best delivered.5 An effective clinical grading system would fill 3 important purposes in chordoma patients. It would provide individualized prognostic information with greater precision and accuracy, allowing both patients and surgeons to appropriately set clinical priorities and plans. It would also enhance the research potential for investigations into this disease, providing a better mechanism to form comparisons of patients across different case series in a relatively rare disease where research is often limited by sample size. And finally, by focusing on different areas of involvement, and types of involvement, the attention of the surgeon and the radiotherapist can be focused on those regions. Previously published grading systems have focused on anatomic features that relate to surgical planning only. These include the grading system presented by Al Mefty et al23 which divided tumors into 3 groups: (1) those confined to 1 anatomic compartment of the skull base, (2) those extending into multiple compartments, but accessible using a single surgical approach, and (3) those in multiple compartments, which require staged approaches to access. More recently, Gui et al21 published an endoscopic classification system—similarly focused on surgical planning—that classified tumors as being situated in the midline or in the paramedian regions and as occupying the anterior skull base, the upper, middle, or lower clivus. While both of these classification systems offer important information for surgeons in planning their surgical procedure, neither made attempts to correlate their classification to either surgical or patient outcomes. Moreover, neither facilitate comparisons across different surgical techniques or series.
Generalizability
The senior author developed the SGSCC with the goal of providing a tool that could easily be applied in a clinical setting and that would also provide a mechanism to group patients into prognostic significant categories. The scoring system we devised was based both on prior clinical experience and known risk factors from published data. Previous studies have found tumor location, size, and prior treatment, where all have been significantly associated increased risk of tumor recurrence.3,6,10 Anecdotal experience shows that encasement of major intracranial blood vessels, dural transgression, and brainstem invasion all increase surgical risks and decrease the likelihood of safely achieving a complete resection in patients with skull base chordomas.1,5 Assigning a simple point-based scoring system to these 6 easily identified preoperative features yielded a normally distributed total score that enabled us to successfully group patients into low-risk (group 1), intermediate-risk (group 2), and high-risk (group 3) cohorts. These groups demonstrated significant difference in rate of complete resection, postoperative performance status, and overall success rate. An increasing trend was seen in the rate of tumor recurrence and, similarly, a decreasing trend was seen in RFS across groups. Overall complication rate, however, was not found to have a statistically significant correlation across groups. Examining the contribution of each subscore to our outcome measures in a multivariate regression analysis did not isolate any subscore component that was not contributing or driving our results, for this reason it is not reported in this manuscript. The SGSCC provided a useful mechanism, consistent with previously published data, by which to categorize skull base chordomas into prognostic significant groups.
Limitations
The present study has single-institution limitations. Skull base chordomas are a relatively rare disease; this series spanning the past decade has a relatively small sample size. We acknowledge that there is an inherent bias in the approach selection by the surgeons involved in the study. The sample size may have underpowered this analysis, especially in relation to the failure to find statistical significance in the increase in complication rates across the groups, and fails to identify the statistical weight of each of the criteria factors. Importantly, selection bias is likely present in this series of 42 patients, 40% of whom presented to us after having undergone previous treatments. This is an important consideration, and validation of the SGSCC in a larger independent group of patients with skull base chordomas is a critical next step, both to address questions of reproducibility/validity and to increase the statistical power to analyze this grading system.
CONCLUSION
The GSCC can successfully categorize patients with skull base chordomas into prognostically important groups. This provides a useful tool that will improve patient counseling and help to set therapeutic priorities. It may also facilitate research efforts by enabling better cross comparisons of different case series within this heterogeneous disease. We acknowledge that the surgical expertise of our department might have skewed the results. The outcomes presented should be viewed as goalposts for the surgical treatment of chordomas. In addition, further validation of the grading scale in an independent patient population and a larger cohort is needed. Furthermore, future developments of the genetic knowledge of tumors, in particular of chordomas, will result in reappraisal of this scoring system.
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Neurosurgery Speaks! Audio abstracts available for this article at www.neurosurgery-online.com.
COMMENTS
The authors have reviewed their experience with cranial chordomas and have retrospectively created a grading system that may be useful for surgeons taking on these challenging lesions. The grading system includes the expected measures of size, location, vascular involvement, and brainstem involvement. Not surprisingly, these measures overlap to a great extent and predict outcome in their grading system. Although the dividing lines between risk groups seems necessarily arbitrary, the data, the experience, and the case discussions will likely be of value to practicing neurosurgeons.
Richard W. Byrne
Chicago, Illinois
The authors present their extensive experience with the treatment of cranial chordomas in an effort to correlate preoperative imaging characteristics with postoperative outcomes. They propose a grading system that allows for classification of lesions into low, intermediate, and high risk and show good correlation with outcomes. Although the literature is replete with grading systems that offer little validity or usefulness in clinical everyday practice, the one proposed here is relatively intuitive, comprehensive, and seemingly predictive. Similar to our own experience, size, degree of involvement of the neuromuscular skull base, as well as degree of intradural invasion all are factors that they find to be suggestive of both less of a chance of complete resection as well as worse outcome. More work on its application of chroma series of others is necessary now to try to assess whether the findings presented here are generalizable and, even more importantly, whether this grading system would allow one to make clinical decisions prior to surgical resection that impact outcome.
Philip Theodosopoulos
San Francisco, California
The good physician treats the disease; the great physician treats the patient who has the disease. – William Osler
Chordomas have remained essentially unconquered. To say the least, these tumors are enigmatic in their behavior. Prediction of outcome is impossible; more often than not it is slow progressive disability leading to crippling consequences and death. The gratification of clinical recovery following surgery is short-lived as tumor recurrence and its further extension into deeper crevices mars the unfinished celebration. Despite being essentially ‘non-cancerous’ in histological behavior, the growth of tumor is relentlessly progressive and invasive. The effectiveness of radiation treatment is not proven. Surgery alone forms the basis of treatment for these tumors.1,2 The radicality of resection and its role in controlling tumor recurrence, growth, and progression is uncertain. The terms ‘radical and total’ surgical resection must be redefined.
Due to uncertainty of outcome that characterizes these tumors, surgical endeavors have to be controlled and safe despite being aggressive.3 The term ‘radical surgery’ sounds rather enticing to all surgeons. Such surgery provides an opportunity to the surgeon to test personal anatomic and surgical skills, explore further treatment options, and finally have the most pleasant satisfaction of seeing an image that is free of tumor. However, one may ask: Is the aim of surgery in chordomas ‘curative’ or is it palliative? Can a surgeon change the natural course of chordoma by radical surgery? Can he increase the longevity of life? Is the surgical procedure that threatens existing neurological function justified when operation on a chordoma is in question?
With more than 35 years of treating complex brain and spine tumors, my personal opinion is that the aim of surgery should be to maximize tumor resection, relieve the patient of his or her preoperative symptoms, and attempt to improve function. Wherever any functional compromise is expected during the attempt at radical surgery, the surgeon has to necessarily back away. I believe any complication or neurologic deficit is related to inadequate understanding and evaluation, or less than perfect execution of operation. The so-called surgical cure is more appropriately ‘surgical care’; the dream of total removal of the chordoma, in my view, is illusory. Even if the tumor were removed, the adjoining normal cell can throw a ‘malignant’ tantrum, and then the process starts all over again.4 Moreover, for any tumor removal, the surgeon must realize the ‘infinite potential to harm’. The surgical philosophy for chordomas, like most other tumors, is to remove the tumor radically and then pray and wait for it not to recur. The success of surgery will be to have maximum space creation, maximum bulk removal, and safe outcome. The complex terrain of chordomas makes likelihood of complications greater. It is clear to me that the rate of recurrence of any tumor, chordomas in particular, is independent of the extent of tumor resection. Each tumor is unique. It is not the treatment but the cellular behavior that decides the outcome. The more extensive the presence of the tumor, the more difficult is the resection and higher the likelihood of recurrence. More circumscribed tumors are easier to remove, and the long-term outcome is relatively better. The answer to treatment of chordomas may be safe resection to obtain symptom-free time for the patient, an act that can be repeated when mandatory. One can say that chordomas have refused to unveil their secret codes to persistently aggressive oncolytic treatment strategy.
In other words, as neurosurgeons, can we dare to ask of ourselves: Yes we can, but should we? With all our advanced technology and our growing techniques, can we learn to practice a ‘Medicine of limits’?
The art and skill of using skull base surgical approaches to maximize tumor resection has been initiated and propagated by the senior authors of this article. The classification of chordomas as proposed by them on the basis of their large experience on the subject of chordomas has the merit of evaluating the status of the tumor and designing a suitable surgical strategy.
Atul Goelk
Maharashtra, India
References
- 1. Goel A. Chordoma and chondrosarcoma: Internal carotid artery relationships. ActaNeurochir (Wien) 1995;133:30-35. [DOI] [PubMed] [Google Scholar]
- 2. Goel A. Middle fossa sub-Gasserian ganglion approach for clivalchordomas. Acta Neurochir (Wien) 1995;136:212-216. [DOI] [PubMed] [Google Scholar]
- 3. Goel A. Surgical approach for Chordomas Commentary on The Endoscope-assisted ventral approach compared with open microscope – assisted surgery for clival chordomas by Komotar et al World Neurosurgery 2011;76(3-4):318-327. [DOI] [PubMed] [Google Scholar]
- 4. Goel A, Kothari M. Meningiomas: Are they curable? J Craniovertebr Junction and Spine. 2016;7(3):133-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neurosurgery Speaks (Audio Abstracts)
Listen to audio translations of this paper's abstract into select languages by choosing from one of the selections below.
REFERENCES
- 1. George B, Bresson D, Herman P, Froelich S. Chordomas: a review. Neurosurg Clin N Am. 2015;26(3):437-452. [DOI] [PubMed] [Google Scholar]
- 2. Sekhar LN, Pranatartiharan R, Chanda A, Wright DC. Chordomas and chondrosarcomas of the skull base: results and complications of surgical management. Neurosurg Focus. 2001;10(3):E2. [DOI] [PubMed] [Google Scholar]
- 3. Tzortzidis F, Elahi F, Wright D, Natarajan SK, Sekhar LN. Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chordomas. Neurosurgery. 2006;59(2):230-237; discussion 230-237. [DOI] [PubMed] [Google Scholar]
- 4. Di Maio S, Rostomily R, Sekhar LN. Current surgical outcomes for cranial base chordomas: cohort study of 95 patients. Neurosurgery. 2012;70(6):1355-1360; discussion 1360. [DOI] [PubMed] [Google Scholar]
- 5. Di Maio S, Temkin N, Ramanathan D, Sekhar LN. Current comprehensive management of cranial base chordomas: 10-year meta-analysis of observational studies. J Neurosurg. 2011;115(6):1094-1105. [DOI] [PubMed] [Google Scholar]
- 6. Choy W, Terterov S, Kaprealian TB et al. Predictors of recurrence following resection of intracranial chordomas. J Clin Neurosci. 2015;22(11):1792-1796. [DOI] [PubMed] [Google Scholar]
- 7. Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175 (suppl 2):57-63. [DOI] [PubMed] [Google Scholar]
- 8. Hug EB, Munzenrider JE. Charged particle therapy for base of skull tumors: past accomplishments and future challenges. Int J Radiat Oncol Biol Phys. 1994;29(4):911-912; discussion 919. [DOI] [PubMed] [Google Scholar]
- 9. Austin-Seymour M, Munzenrider J, Goitein M et al. Fractionated proton radiation therapy of chordoma and low-grade chondrosarcoma of the base of the skull. J Neurosurg. 1989;70(1):13-17. [DOI] [PubMed] [Google Scholar]
- 10. Jahangiri A, Chin AT, Wagner JR et al. Factors predicting recurrence after resection of clival chordoma using variable surgical approaches and radiation modalities. Neurosurgery. 2015;76(2):179-185; discussion 185-186. [DOI] [PubMed] [Google Scholar]
- 11. Scheil-Bertram S, Kappler R, von Baer A et al. Molecular profiling of chordoma. Int J Oncol. 2014;44(4):1041-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gil Z, Fliss DM. Cytogenetic analysis of skull base tumors: Where do we stand? Curr Opin in Otolaryngol Head Neck Surg. 2012;20(2):130-136. [DOI] [PubMed] [Google Scholar]
- 13. Larizza L, Mortini P, Riva P. Update on the cytogenetics and molecular genetics of chordoma. Hered Cancer Clin Pract. 2005;3(1):29-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vujovic S, Henderson S, Presneau N et al. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209(2):157-165. [DOI] [PubMed] [Google Scholar]
- 15. Stacchiotti S, Marrari A, Tamborini E et al. Response to imatinib plus sirolimus in advanced chordoma. Ann Oncol. 2009;20(11):1886-1894. [DOI] [PubMed] [Google Scholar]
- 16. Sen CN, Sekhar LN, Schramm VL, Janecka IP. Chordoma and chondrosarcoma of the cranial base: an 8-year experience. Neurosurgery. 1989;25(6):931-940; discussion 940-941. [DOI] [PubMed] [Google Scholar]
- 17. Roberti F, Sekhar LN, Kalavakonda C, Wright DC. Posterior fossa meningiomas: surgical experience in 161 cases. Surg Neurol. 2001;56(1):8-20; discussion 20-21. [DOI] [PubMed] [Google Scholar]
- 18. Sekhar LN, Sen C, Snyderman CH, Janecka IP. Anterior, anterolateral, and lateral approaches to extradural petroclival tumors. In: Sekhar LN, ed. Surgery of Cranial Base Tumors. Raven Press; 1993:157-224. [Google Scholar]
- 19. Koutourousiou M, Gardner PA, Tormenti MJ et al. Endoscopic endonasal approach for resection of cranial base chordomas: outcomes and learning curve. Neurosurgery. 2012;71(3):614-624; discussion 624-625. [DOI] [PubMed] [Google Scholar]
- 20. Crockard HA, Steel T, Plowman N et al. A multidisciplinary team approach to skull base chordomas. J Neurosurg. 2001;95(2):175-183. [DOI] [PubMed] [Google Scholar]
- 21. Gui S, Zong X, Wang X et al. Classification and surgical approaches for transnasal endoscopic skull base chordoma resection: a 6-year experience with 161 cases. Neurosurg Rev. 2016;39(2):321-333. [DOI] [PubMed] [Google Scholar]
- 22. Singh H, Harrop J, Schiffmacher P, Rosen M, Evans J. Ventral surgical approaches to craniovertebral junction chordomas. Neurosurgery. 2010;66(3 suppl):96-103. [DOI] [PubMed] [Google Scholar]
- 23. al-Mefty O, Borba LA. Skull base chordomas: a management challenge. J Neurosurg. 1997;86(2):182-189. [DOI] [PubMed] [Google Scholar]