Abstract
The aim of this study was to investigate how dietary supplementation of tea polyphenols (TP) and tea catechins (TC) affect laying performance, albumen quality, ovomucin composition, and magnum morphology of laying hens in the late phase of production. Two hundred seventy Hy-Line Brown laying hens (64 wk old) were assigned to a basal diet (the control), the basal diet supplemented with 200 mg/kg tea polyphenols (TP200) or 200 mg/kg tea catechins (TC200). Each treatment had 6 replicates with 15 hens each. The feeding trial lasted 10 wks. Over the course of the trial, dietary supplementation with TP200 significantly increased the egg production (EP) and improved the feed conversion ratio (FCR) in wk 6 to 10 and wk 1 to 10 (P < 0.05). The albumen height and the Haugh unit (HU) of hens fed TP200 were higher than those of hens fed the control diet at wks 8 and 10 (P < 0.05). However, there were no significant differences in the albumen height and the HU between the TP200 and TC200 groups (P > 0.05). The SDS-PAGE analysis indicated that bands of the ovomucin fractions in the TP200 group had the highest intensity compared with those of the control and TC200 groups. Compared with the control, there was a significant increase in protein sulfhydryl (SH) content of the albumen in the TP200 group at the end of experiment, while a significant decrease in protein carbonyl content and protein surface hydrophobicity (P < 0.05). There were also obvious increase in the height and width of the primary folds, epithelial cell height, and cilia height of the simple columnar epithelium in the TP200 group compared with the control and TC200 groups (P < 0.05). In conclusion, dietary supplementation with 200 mg/kg TP can improve performance, albumen quality, and magnum morphology of aged hens. In addition, TP rather than TC could improve the health status of the magnum for aged layers.
Keywords: albumen quality, laying hen, magnum morphology, tea catechin, tea polyphenol
INTRODUCTION
Egg internal quality continues to be of concern to the poultry industry around the world. The albumen quality is of great importance for customer preference. Reactive oxygen species (ROS) are excessively produced in the state of aging, which results in damage to proteins (Stadtman, 2001). It is clear that there is a decrease in albumen height and the Haugh unit (HU) with the increasing of layers’ age (Al-Batshan et al., 1994; Silversides and Scott, 2001; Roberts, 2005). Thinning of the thick albumen can be essentially described by degradation of β-ovomucin, which causes a decrease in the thick albumen proportion followed by an increase of the thin albumen proportion (Kato et al., 1970). Moreover, the production of watery eggs has been associated with morphological changes in the luminal and glandular epithelia of the magnum (Chousalkar and Robert, 2007; Kimaro et al., 2013). Hence, improving the ovomucin composition and health status of the magnum can help improve the albumen quality of eggs.
With the increasing of laying hens’ age, the albumen height and HU decreases and the albumen height of the egg increases when hens were fed a diet supplemented with vitamins C and E (Franchini et al., 2002) or green tea (Biswas et al., 2000; Ariana et al., 2011; Yuan et al., 2016). Green tea contains polyphenols, and these compounds account for up to 30% of the dry weight. Tea polyphenols (TP), a natural antioxidant of typical flavonoids, can scavenge active oxygen free radicals produced in many systems and protect cells from damage (Frei and Higdon, 2003). Most polyphenols are catechins, which make up close to 70% of total flavonoids (Kim et al., 2000; Higdon and Frei, 2003). It is important that, to our knowledge, no study has been conducted in layers on the effects of TP or tea catechins (TC) on albumen quality in the late laying period. Therefore, the objective of this study was to investigate whether TP or TC could be used to improve albumen quality and magnum morphology in the late laying phase of laying hens.
MATERIALS AND METHODS
This study was conducted in accordance with the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, P.R. China) and the criteria outlined in the Guide for the Care and Use of Laboratory Animals of Feed Research Institute, Chinese Academy of Agricultural Sciences (FRICAAS). All animal experimental protocols were approved by the Animal Care and Use Committee of FRICAAS.
Experimental Birds, Design, and Feed Preparation
Two hundred seventy 64-wk-old Hy-Line Brown laying hens with initial egg production (EP) of 82.3 ± 1.0% were randomly allocated into one of the three dietary treatments. Each treatment had 6 replicates with 15 hens each. Replicates were equally distributed into upper and lower cage levels to minimize the replicate level effect. Three hens were housed in a 45-by-45-by-45-cm cage and five neighboring galvanized steel cages as a replicate. Ambient temperature and humidity in the laying hen house were maintained at 23 ± 2 °C and 50% to approximately 65%, respectively. The photoperiod was set to 16L:8D throughout the study. All hens were supplied with ad libitum feed and water. Prior to the feeding trial, all the birds were fed a basal diet (Table 1) for 1 wk (64 wk of age). The experimental period lasted 10 wks (65–74 wk of age). Animal housing and handling procedures during experimentation were in accordance with the recommendations of the Hy-Line International Online Management Guide (Hy-Line International, 2011).
Table 1.
Dietary composition and nutrient levels of the experimental dietsa (as-fed basis)
| Item | Control | TP200 | TC200 |
|---|---|---|---|
| Ingredients, % | |||
| Corn | 63.30 | 63.30 | 63.30 |
| Soybean oil | 5.00 | 5.00 | 5.00 |
| Soybean meal | 24.65 | 24.65 | 24.65 |
| Calcium carbonate | 9.60 | 9.60 | 9.60 |
| Calcium hydrophosphate | 0.90 | 0.90 | 0.90 |
| NaCl | 0.30 | 0.30 | 0.30 |
| Tea polyphenols | 0.02 | ||
| Tea catechins | 0.02 | ||
| Choline chloride | 0.11 | 0.11 | 0.11 |
| dl-Met | 0.11 | 0.11 | 0.11 |
| Premixb | 0.53 | 0.53 | 0.53 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient content,c % | |||
| ME, MJ/kg | 11.20 | 11.20 | 11.20 |
| CP | 17.04 | 17.04 | 17.04 |
| Calcium | 3.64 | 3.64 | 3.64 |
| Total phosphorus | 0.47 | 0.47 | 0.47 |
| Available phosphorus | 0.26 | 0.26 | 0.26 |
| Lysine | 0.85 | 0.85 | 0.85 |
| Methionine | 0.37 | 0.37 | 0.37 |
aControl = basal diet; TP200 = basal diet supplemented with 200 mg/kg tea polyphenols; TC200 = basal diet supplemented with 200 mg/kg tea catechins.
bProvided the following per kilogram of diet: 12,200 IU vitamin A; 4,125 IU vitamin D3; 30 IU vitamin E; 4.5 mg vitamin K; 1.00 mg vitamin B1; 8.5 mg vitamin B2; 50 mg calcium pantothenate; 32.5 mg niacin; 8 mg pyridoxine; 2 mg biotin; 5 mg folic acid; 5 mg vitamin B12; 8 mg copper; 1 mg iodine; 60 mg iron; 0.3 mg selenium; 80 mg manganese; 80 mg zinc; 100 mg phytase; and 3.0 g yeast culture.
cData for the nutrients were analyzed values with the exception of ME and available phosphorus.
TP, epicatechin (EC > 90%), epigallocatechin (EGC > 95%), epicatechin gallate (ECG > 95%), and epigallocatechin-3-gallate (EGCG > 80%) were obtained from a commercial supplier (Wuxi Taiyo Green Power Technology Co., Ltd., Jiangsu, P.R. China). The polyphenols composition (66.73%) of the TP used in this study was assayed by HPLC and found to the total catechins (37.25%), contains EC (11.35%), EGC (8.41%), ECG (5.00%), and EGCG (12.49%). The hens were fed diets in mash form during the feeding experiment. The basal diet (Table 1) was formulated to meet or exceed the NRC (1994) recommended nutrient requirements. Treatments included a basal diet (the control), the basal diet supplemented with 200 mg/kg tea polyphenols (TP200) or 200 mg/kg tea catechins (TC200). The TC200 diet was supplemented with EC, ECG, EGC, and EGCG to meet the total catechins profile of TP.
Performance Parameters
Eggs were collected daily at 14:00 h, and the number of eggs and egg weight in each replicate were recorded. Feed consumption by each replicate was recorded weekly. Average egg weight (AEW) was calculated as the mean weight of all eggs from each replicate. The feed conversion ratio (FCR) was calculated as feed consumption divided by the total egg weight (feed/egg, g/g). Mortality was recorded daily as it occurred. Daily feed intake (DFI) was adjusted for mortalities and was calculated using the following equation: DFI = feed consumption (g)/(hen number × d). EP, DFI, AEW, and FCR were calculated for wk 1 to 5, wk 6 to 10, and wk 1 to 10 of the experiment.
Albumen Quality Parameters
At the end of wks 4, 6, 8 and 10, five eggs from each replicate with weights closest to the replicate average were collected for albumen quality and albumen proportion tests (30 eggs from each treatment). First, each egg was individually weighed and broken, and the albumen height and HU were measured using an Egg Analyzer (Orka Food Technology Ltd, Ramat Hasharon, Israel). Second, each yolk was separated from the albumen with an egg separator. The thick and thin albumen was homogenized using a stirrer (Jintan Ronghua Instrument Manufacture Co., Ltd., Jiangsu, P.R. China) for 30 min at room temperature (22 °C). Stirring speed was set at 350 rpm to avoid foaminess. The albumen pH was measured using a pH meter (Testo 205, Lenzkirch, Germany). Third, each yolk weight was determined after the chalazae were removed with forceps. Each yolk was rolled on a paper towel to remove adhering albumen. The eggshell was washed, air-dried, and weighed. Albumen weight was calculated by subtracting the yolk and eggshell weight from the weight of the individual egg. The albumen (yolk or eggshell) proportion (%) = albumen (yolk or eggshell) weight/egg weight × 100. The average value of the five egg samples was regarded as the replicate value.
Protein Carbonyl Content, Sulfhydryl Groups, and Surface Hydrophobicity in Albumen
Three eggs from each replicate with weights closest to the replicate average were collected for albumen antioxidative indices test at the end of the feeding trial. Briefly, each egg was individually weighed and broken, and the albumen was separated from the yolk with an egg separator. The thick and thin albumen was thoroughly mixed and freeze-dried using freeze-drying equipment (FD-12; Beijing Huichengjia Scientific Instrument Factory Co., Ltd., Beijing, P.R. China). After freeze-drying, the albumen samples were weighed and carefully ground to pass through a 40-mesh sieve. The albumen samples were stored at −20 °C until analysis.
Colorimetric kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, P.R. China) were used to measure protein carbonyl content and sulfhydryl (SH) groups in albumen samples. The determinations were performed strictly according to the manufacturer’s instruction. And the protein surface hydrophobicity of albumen was measured according to Yuksel et al. (2012). The average value of the three egg samples was regarded as the replicate value.
Separation and Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) of Ovomucin
In each replicate, three eggs based on the AEW were collected on the last day of the feeding trial for measurement of ovomucin composition in the albumen within 24 h of being laid (18 eggs for each treatment). Six eggs, three from each replicate, were considered a pooled sample for the SDS-PAGE assay of ovomucin.
Ovomucin was extracted according to the method of Omana and Wu (2009). Briefly, the eggs were manually broken, and the albumen was separated from the egg yolk. After removing the chalazae, the albumen was homogenized using a stirrer (Jintan Ronghua Instrument Manufacture Co., Ltd., Jiangsu, P.R. China) for 30 min at room temperature (22 °C). Stirring speed was set at 350 rpm to avoid foaminess. Ovomucin was first prepared using isoelectric precipitation in the presence of 100 mM NaCl solution. The dispersion was kept overnight at 4 °C and separated by centrifugation at 15,300 × g for 10 min at 4 °C. The precipitate was further suspended in 500 mM NaCl solution and stirred for 4 h followed by overnight settling at 4 °C. After centrifugation at 15,300 × g for 10 min at 4 °C, the precipitate was freeze-dried, weighed, and stored at −20 °C until analysis. The SDS-PAGE of ovomucin was carried out according to Itoh et al. (1987).
Light Microscopy Assay of Magnum
By the end of the feeding trial, two hens from each replicate were randomly chosen, weighed, and sacrificed by exsanguination via the left jugular vein. The whole length of the oviduct was removed, and a length of approximately 2 cm of the tissue samples from the midpoint of the magnum was obtained. The samples were gently flushed several times with physiological saline (0.1% NaCl) to remove their contents and placed in 10% formalin in 0.1 M phosphate buffer (pH = 7.0) for fixation (48 h). The samples were processed for 24 h in a tissue processor with anhydrous ethanol as the dehydrant, and the samples were embedded in paraffin. Three 4 μm slices were made from the tissue and stained with hematoxylin and eosin.
Histopathological changes of cross sections were examined under a bright field light microscope (Nikon DS-U3, Tokyo, Japan) coupled with a digital camera (BX51; Olympus Corp., Tokyo, Japan) at magnifications of 100× and 200×. The cross section morphometry contained the height and width of primary folds. In addition, the magnum longitudinal sections were evaluated at magnifications of 200× and 400× using an analyzer (Nikon DS-U3, Tokyo, Japan) coupled with a digital camera (BX51; Olympus Corp., Tokyo, Japan). The longitudinal section morphometry contained the epithelial cell height and cilia height of the simple columnar epithelium. The height of a primary fold was measured by drawing a vertical line from the base to the luminal end (Kimaro et al., 2013). Epithelial cell height was determined by measuring the height of 15 cells in 5 different primary folds (Berg et al., 2001). Each treatment had six replicates with two birds each, and three samples were examined for each bird, with two images taken of each sample. The average value of the two birds was regarded as the replicate value.
Statistical Analysis
The laying performance, albumen quality, protein antioxidation indices of albumen, and magnum morphometrical data are presented as means (SD) and analyzed using a one-way ANOVA (SPSS 19.0 for windows, SPSS Inc., Chicago, IL). Replicates were the experimental units for all analyses. When significant differences were found, a 5% of variance average was used, with a Tukey’s multiple comparison tests for comparisons among the groups. P-value ˂ 0.05 was considered significant.
RESULTS
Laying Performance
Table 2 shows the effects of dietary supplementation with TP200 or TC200 on laying performance of layers (65 to 74 wk of age) during the 10 wks of the treatment period. No significant differences in laying performance were observed among all treatments in wk 1 to 5 (P > 0.05). During wk 6 to 10, dietary TP200 or TC200 supplementation did not affect the AEW and DFI but significantly affected the EP and FCR (P < 0.05). The TP200 group showed higher EP and FCR compared with the control (P < 0.05). Hens fed TP200 had no difference in the AEW and DFI but had higher EP and FCR during wk 1 to 10 compared with hens fed the control diet (P < 0.05).
Table 2.
Effect of the basal diet (control) supplemented with TP200 or TC200 on performance of laying hens (wks 65 to 74) during 10 wks of treatment period. Data are presented as means (SD)a
| Items | Treatment | P-value | ||
|---|---|---|---|---|
| Control | TP200 | TC200 | ||
| Wk 1 to 5 | ||||
| Egg production, % | 81.05 (4.04) | 83.62 (3.73) | 82.49 (4.02) | 0.330 |
| Average egg weight, g | 64.17 (0.89) | 64.62 (0.81) | 64.65 (0.84) | 0.307 |
| Daily feed intake, g/hen per day | 114.09 (2.54) | 115.86 (1.45) | 112.32 (1.40) | 0.066 |
| Feed conversion ratio (F:Eb), g/g | 2.19 (0.03) | 2.17 (0.08) | 2.13 (0.09) | 0.060 |
| Wk 6 to 10 | ||||
| Egg production, % | 80.14 (4.32)d | 85.84 (2.56)c | 84.03 (3.27)c,d | 0.045 |
| Average egg weight, g | 63.75 (0.92) | 64.98 (1.83) | 64.60 (1.82) | 0.105 |
| Daily feed intake, g/hen per day | 118.08 (3.24) | 120.76 (4.38) | 119.93 (4.63) | 0.557 |
| Feed conversion ratio (F:E), g/g | 2.26 (0.08)c | 2.07 (0.11)d | 2.13 (0.16)c,d | 0.047 |
| Wk 1 to 10 | ||||
| Egg production, % | 80.60 (4.19)d | 84.73 (3.15)c | 83.26 (3.65)c | 0.014 |
| Average egg weight, g | 63.96 (0.91) | 64.80 (1.32) | 64.63 (1.33) | 0.650 |
| Daily feed intake, g/hen per day | 116.09 (2.89) | 118.31 (2.92) | 116.13 (3.02) | 0.454 |
| Feed conversion ratio (F:E), g/g | 2.23 (0.06)c | 2.12 (0.10)d | 2.13 (0.13)c,d | 0.039 |
aMeans were obtained from 6 replicate cages of 15 birds each.
bF:E = feed:egg ratio.
c,dMeans with a row with different superscripts significantly differ (P < 0.05).
Albumen Quality and SDS-PAGE of Ovomucin
No differences were found for albumen pH or albumen proportion among all treatments at wks 4, 6, 8, and 10 (Tables 3 and 4). There were no significant differences regarding yolk proportion and eggshell proportion among all treatments at wks 4, 6, 8, and 10 (Table 4). However, dietary TP200 or TC200 supplementation partially affected albumen height and HU when examined at wks 6, 8, and 10 (P < 0.05). Significant increases were also observed in albumen height and HU in the TP200 group at wks 6, 8, and 10 (P < 0.05).
Table 3.
Effect of the basal diet (control) supplemented with TP200 or TC200 on albumen quality of laying hens (wks 65 to 74) during 10 wks of treatment period. Data are presented as means (SD)a
| Items | Time, wk | Treatment | P-value | ||
|---|---|---|---|---|---|
| Control | TP200 | TC200 | |||
| Albumen height, mm | 4 | 5.13 (0.47) | 5.65 (0.56) | 5.49 (0.52) | 0.409 |
| 6 | 6.38 (0.47)c | 7.55 (0.43)b | 6.72 (0.36)c | 0.001 | |
| 8 | 6.47 (0.49)c | 7.33 (0.48)b | 6.80 (0.58)b,c | 0.049 | |
| 10 | 5.93 (0.22)c | 6.78 (0.47)b | 6.40 (0.48)b,c | 0.011 | |
| Haugh unit | 4 | 69.26 (4.7) | 71.41 (4.61) | 71.43 (4.51) | 0.896 |
| 6 | 77.49 (3.06)c | 85.42 (2.49)b | 79.89 (2.34)c | 0.001 | |
| 8 | 77.52 (4.82)c | 84.19 (2.79)b | 79.94 (4.41)b,c | 0.029 | |
| 10 | 72.94 (4.26)c | 81.60 (3.04)b | 77.72 (3.23)b,c | 0.008 | |
| Albumen pH | 4 | 8.34 (0.1) | 8.37 (0.12) | 8.36 (0.03) | 0.959 |
| 6 | 7.78 (0.06) | 7.77 (0.10) | 7.81 (0.08) | 0.826 | |
| 8 | 7.81 (0.07) | 7.80 (0.13) | 7.80 (0.09) | 0.805 | |
| 10 | 8.13 (0.09) | 8.09 (0.13) | 8.10 (0.09) | 0.656 | |
aMeans were calculated on n = 6 replicates (five eggs per replicate) per treatment.
b,cMeans with a row with different superscripts significantly differ (P < 0.05).
Table 4.
Effect of the basal diet (control) supplemented with TP200 or TC200 on egg composition of laying hens (wks 65 to 74) during 10 wks of treatment period. Data are presented as means (SD)a
| Items | Time, wk | Treatment | P-value | ||
|---|---|---|---|---|---|
| Control | TP200 | TC200 | |||
| Albumen proportion, % | 4 | 0.64 (0.02) | 0.64 (0.01) | 0.64 (0.03) | 0.917 |
| 6 | 0.65 (0.01) | 0.66 (0.03) | 0.65 (0.02) | 0.533 | |
| 8 | 0.65 (0.02) | 0.65 (0.01) | 0.64 (0.02) | 0.892 | |
| 10 | 0.64 (0.01) | 0.63 (0.02) | 0.63 (0.01) | 0.511 | |
| Eggshell proportion, % | 4 | 0.11 (0.01) | 0.10 (0.01) | 0.11 (0.00) | 0.125 |
| 6 | 0.10 (0.01) | 0.10 (0.01) | 0.10 (0.01) | 0.575 | |
| 8 | 0.10 (0.01) | 0.10 (0.01) | 0.10 (0.01) | 0.567 | |
| 10 | 0.10 (0.01) | 0.10 (0.01) | 0.10 (0.01) | 0.577 | |
| Yolk proportion, % | 4 | 0.25 (0.01) | 0.26 (0.01) | 0.25 (0.01) | 0.657 |
| 6 | 0.25 (0.01) | 0.24 (0.02) | 0.25 (0.02) | 0.508 | |
| 8 | 0.25 (0.01) | 0.25 (0.01) | 0.26 (0.01) | 0.212 | |
| 10 | 0.26 (0.01) | 0.27 (0.12) | 0.27 (0.01) | 0.626 | |
aMeans were calculated on n = 6 replicates (five eggs per replicate) per treatment.
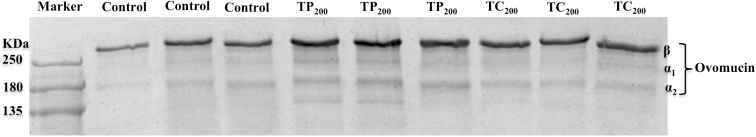
Figure 1 shows the electropherograms obtained from the ovomucin samples using the SDS-PAGE technique. As shown, the ovomucin complex in the albumen samples displayed three bands among all treatments. Ovomucin contains β, α1, and α2 subunits. As was expected, in the SDS-PAGE analysis, it was found that the β subunit of ovomucin in the TP200 group had the highest intensity compared with the control and TC200 samples.
Figure 1.
Sodium dodecyl sulfate–PAGE electrophoretograms of ovomucin in laying hens (74 wk) after a 10 wk treatment period. (a) Means were calculated on n = 3 replicates (six eggs per replicate) per treatment. Control = basal diet; TP200 = basal diet supplemented with 200 mg/kg tea polyphenols; TC200 = basal diet supplemented with 200 mg/kg tea catechins.
Albumen Antioxidative Indices
The albumen oxidative indices of eggs from laying hens fed the TP200 or TC200 are shown in Table 5. The TP200 group had lower protein carbonyl content and protein surface hydrophobicity in the albumen than the control group at the end of the feeding trial (P < 0.05). Compared with the control, the albumen SH content in the TP200 group was increased by 10.71% at the end of the feeding trial.
Table 5.
Effect of the basal diet (control) supplemented with TP200 or TC200 on protein oxidation indices of the albumen in laying hens (74 wk) after 10 wks of treatment period. Data are presented as means (SD)a
| Items | Treatment | P-value | ||
|---|---|---|---|---|
| 0 | TP200 | TC200 | ||
| Protein carbonyl content, nmol/mg protein | 0.69 (0.11)b | 0.52 (0.10)c | 0.55 (0.07)c | 0.025 |
| Sulfhydryl content, mmol/g protein | 0.112 (0.007)c | 0.124 (0.006)b | 0.117 (0.009)b,c | 0.034 |
| Protein surface hydrophobicity | 1,182.05 (92.13)b | 1,001.06 (90.50)c | 1,091.27 (92.20)b,c | 0.017 |
aMeans were calculated on n = 6 replicates (three eggs per replicate) per treatment.
b,cMeans with a row with different superscripts significantly differ (P < 0.05).
Tissue Morphometry of the Magnum
Table 6 and Figure 2 show the effects of TP200 or TC200 on the morphometric analysis of the magnum at the end of the feeding trial. Significant increases in the height and the width of primary folds were observed in the TP200 group compared with the control and TC200 groups (P < 0.05; Table 6; Figure 2A). Moreover, there were significant increases in the epithelial cell height and cilia height of the simple columnar epithelium in the TP200 group compared with the other groups (P < 0.05; Table 6; Figure 2B).
Table 6.
Effect of the basal diet (control) supplemented with TP200 or TC200 on magnum morphology of laying hens (74 wk) after 10 wks of treatments period. Data are presented as means (SD)a
| Items | Treatment | P-value | |||
|---|---|---|---|---|---|
| Control | TP200 | TC200 | |||
| Primary folds | Fold height, μm | 2,949.38 (298.66)c | 3,700.28 (176.08)b | 2,365.33 (467.97)d | <0.001 |
| Fold width, μm | 711.39 (130.50)c | 838.89 (58.47)b | 616.61 (106.16)c | 0.007 | |
| Simple columnar epithelium | Epithelium height, μm | 12.63 (1.63)c | 26.58 (8.84)b | 13.79 (2.99)c | 0.001 |
| Cilia height, μm | 7.05 (0.60)c | 10.72 (1.63)b | 8.31 (0.65)c | <0.001 | |
aMeans were calculated on n = 6 replicates (two hens per replicate) per treatment.
b–dMeans with a row with different superscripts significantly differ (P < 0.05).
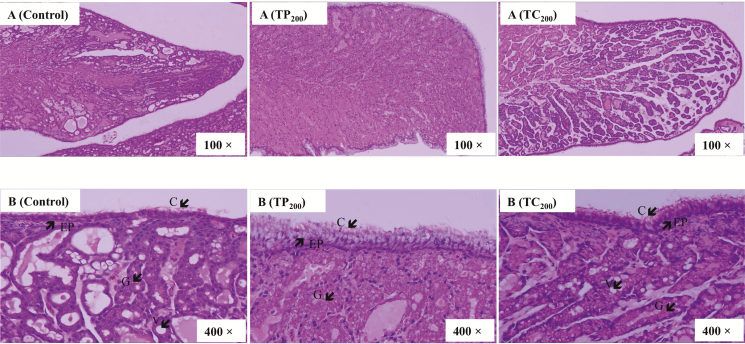
Figure 2.
Hematoxylin and eosin–stained images of the cross section (100× magnification, A) or longitudinal section (400× magnification, B) of the magnum in laying hens (74 wk) after a 10 wks treatment period. EP = epithelium line of the mucosa; C = cilia; G = tubular glands; V = vacuoles; Control = basal diet; TP200 = basal diet supplemented with 200 mg/kg tea polyphenols; TC200 = basal diet supplemented with 200 mg/kg tea catechins.
Hematoxylin and eosin–stained images of the cross section (100×; Figure 2A) or longitudinal section (400×; Figure 2B) of the magnum are shown in Figure 2. Compared with the TP200 group, there were numerous vacuoles in degenerating gland cells as well as the degenerating luminal epithelial cells in the control and TC200 groups (Figure 2B). In contrast, it can be seen clearly that the simple columnar epithelium consisted of ciliated cells and nonciliated granular cells lining the mucosal layer in the TP200 group and that the gland cells contained round nuclei and eosinophilic granular cytoplasm.
DISCUSSION
Numerous studies indicated that laying hens fed a 500 to approximately 600 mg/kg green tea extract diet had increased feed efficiency and EP in the late phase (Ariana et al., 2011; Yuan et al., 2016). Sahin et al. (2010) also noted that feeding 200 to 400 mg/kg epigallocatechin-3-gallate diets increased EP in heat-stressed quails. In a previous work, we studied the effect of dietary TP supplementation on the performance, egg quality, and antioxidant ability of laying hens and determined an optimum level of 200 mg/kg TP, among 40, 80, and 400 mg/kg; moreover, 200 mg/kg TP increased feed efficiency and antioxidant ability (Wang et al., 2017). Indeed, it demonstrated that 200 mg/kg TP significantly reduced oxidative stress (Lv et al., 2013). Therefore, dietary supplementation with 200 mg/kg TP or TC was used in this study. We found that diet supplemented with 200 mg/kg TP did significantly increase the EP and FCR of laying hens under the experimental conditions. To sum up, feeding a diet supplemented with 200 mg/kg TP could improve hen performance of layers in the late phase.
Albumen quality is a standard measure of egg internal quality that is most often measured as the albumen height and HU. The HU was calculated using the height of the thick albumen and the weight of an egg (Haugh, 1937). In the present study, we found that dietary supplementation with 200 mg/kg TP elevated the albumen height and HU in hens of late laying period. Our present study is consistent with a previous study that showed albumen height and HU were decreased in aged layers as a result of vanadium administration, which can be overcome by 600 to approximately 1,000 mg/kg TP (Yuan et al., 2016). The ovomucin, accounting for approximately 3.5% of total egg white protein, is important in determining the height of the thick albumen, and it is responsible for the thick gel characteristics of liquid albumen (Toussant and Latshaw, 1999; Omana et al., 2010). Therefore, the albumen height and HU were influenced by the ovomucin content of the thick albumen. Ovomucin mainly consists of two types of subunits, α and β; α-ovomucin is homogeneous, and its molecular weight varies from 150 to 220 kDa; whereas, β-ovomucin is heterogeneous, and its molecular weight varies from 400 to 523 kDa. α-Ovomucin has two subunits called α1 and α2, which have less carbohydrates than β-ovomucin, which is rich in carbohydrates (Hiidenhovi et al., 1999; Omana et al., 2010). It has been reported that the β subunit is major subunit of ovomucin in the albumen. Thinning albumen was caused by changes in physical and chemical properties of the ovomucin complex, that is, breakdown of hexose-rich β-ovomucin forming an ionic complex with lysozyme, leading to less viscosity of the thick albumen and more carbohydrates in the sol portion of the thick albumen (Kato et al., 1970; Robinson and Monsey, 1972). At present, there is no commercially available ovomucin standard and antiovomucin antibody to identify ovomucin using western blotting, so only SDS-PAGE results are shown. We found that β-ovomucin in the TP200 group had the highest intensity, compared with that of the control and TC200 samples. This finding was consistent with results observed for TP200 could improve the albumen height and HU of egg. Moreover, a previous study (Biswas et al., 2000) reported that there was a higher β-ovomucin content of the thick albumen when laying hens were supplemented with a green tea powder diet. It may be because the polyphenols can form complexes with proteins and polysaccharides (Bravo, 1998), and another study also reported that tea extracts could increase the strength of albumen gels (Hatanaka et al., 2009).
Oxidative stress is caused by an imbalance between oxidants and antioxidants at either the cellular or the individual level (Voljč et al., 2011; Yu et al., 2015). Furthermore, protein is one of the main targets of ROS and can be provoked by free radicals into the process of protein peroxidation, which results in oxidative modifications of amino acid residues, altering protein structure and function (Cecarini et al., 2007). Carbonylation of proteins is an irreversible oxidative damage, often leading to a loss of protein function, which is considered a widespread indicator of severe oxidative damage and protein dysfunction (Agarwal et al., 2005; Trombetta et al., 2006). The introduction of carbonyl groups into proteins can be triggered by ROS or secondary byproducts of oxidative stress and can arise at different sites and by different mechanisms (Stadtman and Levine, 2003; Dalle-Donne et al., 2006). The protein surface hydrophobicity and SH content could reflect the tertiary structure of the proteins (Yeksel et al., 2012; Cao et al., 2015). A previous study confirmed that oxidative damage of proteins has caused increased protein carbonyl content and surface hydrophobicity as well as decreased SH content (Kholari et al., 2016). We further explored the effect of dietary supplementation with TP200 or TC200 on the content of oxidation protein products in the albumen. The current study showed that hens fed TP200 had significantly increased protein SH content in the albumen but decreased protein carbonyl content and surface hydrophobicity in aged hens compared with the control group. These results are consistent with those of a previous study that suggested that the protein carbonyl levels in the heart and liver were decreased when aged mice were supplemented with 500 mg/kg green tea extract (Wang, 2013). Moreover, the increase of SH groups in proteins may be related to enhanced antioxidant properties (Taylor and Richardson, 1980). It was demonstrated that dietary green tea extract supplementation of aged animal results in a significant reduction of protein oxidation and improvement of the antioxidant defense system (Skrzydlewska et al., 2002; Khan et al., 2007). Therefore, it was concluded that TP could help aged hens balance their pro-oxidant/antioxidant system and that the increased antioxidant properties of proteins may be due to polyphenol–protein interactions (Wang et al., 2014). However, there are few studies related to whether TP improved albumen quality through increasing protein SH content and decreasing protein carbonyl content and surface hydrophobicity. The exact mechanism of TP-enhanced protein antioxidant properties in the albumen needs to be further studied.
Furthermore, the albumen proteins are synthesized and secreted in the magnum segment of the oviductal lumen during the 24 h EP cycle. Therefore, the health status of this organ can influence albumen quality of egg. The present study has showed the effect of TP or TC on the morphology of the magnum in the Hy-Line Brown hens during the late laying period. Our study confirmed that the magnum fold height and surface epithelium height were significantly increased in the TP200 group compared with the control and TC200 groups. This increase could be due to improvement of the health status of the magnum as a result of TP antioxidant properties. This finding was consistent with results observed for the higher albumen height and HU in the TP200 group. This may be because the magnum fold height and magnum surface epithelium height may be important factors affecting egg albumen quality (Toussant et al., 1995; Kimaro et al., 2013), and deterioration of internal egg quality may be mediated by an inhibition of magnum motility during egg formation (Eyal and Moran, 1984). On the other hand, we found higher cilia height in the TP200 group compared with other groups in the current study, but the role of cilia of magnum in the oviduct during EP is still speculative, and further investigations are essential to better explain this phenomenon. Further investigation is required to fully study the effects of TP on the mucopolysaccharides in the magnum of laying hens during the late laying period.
These study results have confirmed that albumen quality is associated with the magnum health status and will serve as theoretical support and empirical evidence to improve the albumen quality of aged layers. Furthermore, these novel data provided further evidence that 200 mg/kg TP rather than 200 mg/kg TC may have a positive affect on magnum morphology and result in improved albumen quality of egg during the late laying period in hens. In general, TC is a major component of TP that has potent antioxidative and radical-scavenging activities (Skrzydlewska et al., 2002). However, it was demonstrated that (+)-catechin appeared to exhibit a strong prooxidant effect on hemoglobin oxidative damage at lower concentrations (Lu et al., 2011). The present study found that the TC200 did not improve the laying performance, albumen quality, and magnum morphology compared with the control diet. The reason may be because the supplemental dosage of TC had no obvious antioxidant effects under the current experimental conditions. However, there is a need for more detailed studies on TP improving albumen quality of egg in hens in the late laying phase, which is stated to play a significant role for laying hens’ producers to increase economic benefits.
In conclusion, this study demonstrated that supplementation of 200 mg/kg TP can improve hen performance and albumen quality of laying hens during the late laying period. In addition, our study provides evidence that the positive effect of TP on albumen quality may be associated with improvement of the magnum health status and ovomucin composition. Further studies should be carried out on the mechanism underlying TP’s improvement of albumen quality of laying hens in late laying phase.
Footnotes
The authors are grateful for the financial support provided by the China Postdoctoral Science Foundation (2017M611073), the earmarked fund for Modern Agroindustry Technology Research System (CARS-40-K12), the China Agriculture Research System Poultry-Related Science and Technology Innovation Team of Peking (CARS-PSTP), and the Agricultural Science and Technology Innovation Program (ASTIP).
LITERATURE CITED
- Agarwal A., Gupta S., and Sharma R. K.. 2005. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 14:3–28. doi:10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Batshan H. A., Scheideler S. E., Black B. L., Garlich J. D., and Anderson K. E.. 1994. Duodenal calcium uptake, femur ash, and eggshell quality decline with age and increase following molt. Poult. Sci. 73:1590–1596. doi:10.3382/ps.0731590. [DOI] [PubMed] [Google Scholar]
- Ariana M., Samie A., Edriss M., and Jahanian R.. 2011. Effects of powder and extract form of green tea and marigold, and α-tocopheryl acetate on performance, egg quality and egg yolk cholesterol levels of laying hens in late phase of production. J. Med. Plants Res. 5:2710–2716. Available at http://www.academicjournals.org/JMPR [Google Scholar]
- Berg C., Holm L., Brandt I., and Brunstrom B.. 2001. Anatomical and histological changes in the oviducts of Japanese quail, Coturnix japonica, after embryonic exposure to ethynyloestradiol. Reproduction 121:155–165. doi:10.1530/rep.0.1210155. [DOI] [PubMed] [Google Scholar]
- Biswas, Md A. H., Miyazaki Y., Nomura K., and Wakita M.. 2000. Influences of long-term feeding of Japanese green tea powder on laying performance and egg quality in hens. Asian-Australas. J. Anim. Sci. 13:980–985. doi:10.5713/ajas.2000.980. [Google Scholar]
- Bravo L. 1998. Polyphenols: chemistry, dietary sources, metabolism and nutritional significance. Nutr. Rev. 56:317–333. doi:10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Cao J. L., Zhang W., Wu S. Z., Liu C., Li Y., Li H. M., and Zhang L. B.. 2015. Effects of nanofiltration and evaporation on the physiochemical properties of milk protein during processing of milk protein concentrate. J. Dairy Sci. 98:100–105. doi:10.3168/jds.2014-8619. [DOI] [PubMed] [Google Scholar]
- Cecarini V., Gee J., Fioretti E., Amici M., Ahgeletti M., Eleuteri A. M., and Keller J. N.. 2007. Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim. Biophys. Acta. 1773:93–104. doi:10.1016/j.bbamcr.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Chousalkar K. K. and Roberts J. R.. 2007. Ultrastructural study of infectious bronchitis virus infection in infundibulum and magnum of commercial laying hens. Vet. Microbiol. 122:223–236. doi:10.1016/j.vetmic.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I., Aldini G., Carini M., Colombo R., Rossi R., and Milzani A.. 2006. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell Mol. Med. 10:389–406. doi:10.1111/j.1582–4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyal A. and Moran E.. 1984. Egg changes associated with reduced interior quality because of dietary vanadium toxicity in the hen. Poult. Sci. 63:1378–1385. doi:10.3382/ps.0631378. [Google Scholar]
- Franchini A., Sirri F., Tallarico N., Minelli G., Iaffaldano N., and Meluzzi A.. 2002. Oxidative stability and sensory and functional properties of eggs from laying hens fed supranutritional doses of vitamins E and C. Poult. Sci. 81:1744–1750. doi:10.1093/ps/81.11.1744. [DOI] [PubMed] [Google Scholar]
- Frei B. and Higdon J. V.. 2003. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J. Nutr. 133:3275S–3284S. [DOI] [PubMed] [Google Scholar]
- Hatanaka Y., Yamauchi A., Kobayashi O., and Muro T.. 2009. Electron microscopic analysis of the effects of tea extracts on strength improvement of egg white gels. Food Sci. Technol. Res. 15:5–10. doi:10.3136/fstr.15.5. [Google Scholar]
- Haugh R. R. 1937. The Haugh unit for measuring egg quality. U.S. Egg Poult. Mag. 43:552–555. [Google Scholar]
- Higdon J. V. and Frei B.. 2003. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit. Rev. Food. Sci. 43:89–143. doi:10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Hiidenhovi J., Aro H. S., and Kankare V.. 1999. Separation of ovomucin subunits by gel filtration: enhanced resolution of subunits by using a dual-column system. J. Agric. Food Chem. 47:1004–1008. doi:10.1021/jf9811774. [DOI] [PubMed] [Google Scholar]
- Hy-Line International 2011. Hy-Line International online management guide: management guide for all Hy-Line varieties of laying hens Available at http://www.hyline.com/Redbook/New.html. Accessed February22, 2012.
- Itoh T., Miyazaki J., Sugawara H., and Adachi S.. 1987. Studies on the characterization of ovomucin and chalaza of the hen’s egg. J. Food Sci. 52:1518–1521. doi:10.1111/j.1365-2621.1987.tb05868.x. [Google Scholar]
- Kato A., Nakamura R., and Sato Y.. 1970. Studies on change in stored shell egg. Part VI. Changes in the chemical composition of ovomucin during storage. Biosci. Biotech. Bioch. 34:1009–1013. doi:10.1271/bbb1961.34.1009. [Google Scholar]
- Khan S. A., Priyavada S., Arivarasu N. A., Khan S., and Yusufi A. N.. 2007. Influence of green tea on enzymes of carbohydrate metabolism, antioxidant defense, and plasma membrane in rat tissues. Nutrition 23:687–695. doi:10.1016/j.nut.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Kholari F. S., Dehpour A. R., Nourbakhsh M., Doustimotlagh A. H., Bagherieh M., and Golestani A.. 2016. Erythrocytes membrane alterations reflecting liver damage in CCl4-induced cirrhotic rats: the ameliorative effect of naltrexone. Acta Med. Iran. 54:631–639. PMID:27888590. [PubMed] [Google Scholar]
- Kim B., Lee M. J., Hong J., Li C., Smith T. J., Yang G. Y., Seril D. N., and Yang C. S.. 2000. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr. Cancer. 37:41–48. doi:10.1207/S15327914NC3701_5. [DOI] [PubMed] [Google Scholar]
- Kimaro W. H., Madekurozwa M. C., and Groenewald H. B.. 2013. Histomorphometrical and ultrastructural study of the effects of carbendazim on the magnum of the Japanese quail (Coturnix coturnix japonica). Onderstepoort J. Vet. Res. 80:579–586. doi:10.4102/ojvr.v80il.579. [DOI] [PubMed] [Google Scholar]
- Lu N. H., Chen P. Q., Yang Q., and Peng Y. Y.. 2011. Anti- and pro-oxidant effects of (+)-catechin on hemoglobin-induced protein oxidative damage. Toxicol. In Vitro. 25:833–838. doi:10.1016/j.tiv.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Lv J. R., Xue R. L., Zhao J., Wei X., Gao H., Fu R. G., Wu G., Li W., Lei X. M., and Tian J. B.. 2013. An optimal dose of tea polyphenols protects against global cerebral ischemia/reperfusion injury. Neural Regen. Res. 8:783–791. doi:10.3969/j.issn.1673-5374.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC 1994. Nutrient requirements of poultry. 9th rev. ed. Washington (DC): National Academies Press. [Google Scholar]
- Omana D. A., Wang J. P., and Wu J. P.. 2010. Ovomucin—a glycoprotein with promising potential. Trends Food Sci. Technol. 21:455–463. doi:10.1016/j.tifs.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omana D. A., and Wu J. P.. 2009. A new method of separating ovomucin from egg white. J. Agric. Food Chem. 579:3596–3603. doi:10.1021/jf8030937. [DOI] [PubMed] [Google Scholar]
- Roberts J. R. 2005. Egg quality guidelines for the Australian egg industry. In: Proceedings of the 6th European Symposium on the Quality of Eggs and Egg Products, World’s Poultry Science Association, May 23 to 26;Doorwerth, the Netherlands. World Poult. Sci. J. 61(Suppl. 1):326–330. [Google Scholar]
- Robinson D. S. and Monsey J. B.. 1972. Change in the composition of ovomucin during liquefaction of thick egg white: the effect of ionic strength and magnesium salts. J. Sci. Food Agric. 23:29–38. doi:10.1002/jsfa.2740230710. [DOI] [PubMed] [Google Scholar]
- Sahin K., Orhan C., Tuzcu M., Ali S., Sahin N., and Hayirli A.. 2010. Epigallocatechin-3-gallate prevents lipid peroxidation and enhances antioxidant defense system via modulating hepatic nuclear transcription factors in heat-stressed quails. Poult. Sci. 89:2251–2258. doi:10.3382/ps/2010-00749. [DOI] [PubMed] [Google Scholar]
- Silversides F. G. and Scott T. A.. 2001. Effect of storage and layer age on quality of eggs from two lines of hens. Poult. Sci. 80:1240–1245. doi:10.1093/ps/80.8.1240. [DOI] [PubMed] [Google Scholar]
- Skrzydlewska E., Ostrowstry J., Farbiszewski R., and Michalak K.. 2002. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine 9:232–238. doi:10.1078/0944-7113-00119. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. 2001. Protein oxidation in aging and age-related diseases. Ann. N. Y. Acad. Sci. 928:22–38. doi:10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. and Levine R. L.. 2003. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25:207–218. doi:10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Taylor M. J. and Richardson T.. 1980. Antioxidant activity of skim milk: effect of heat and resultant sulfhydryl groups. J. Dairy Sci. 63:1783–1795. doi:10.3168/jds.S0022-0302(80)83140–7. [DOI] [PubMed] [Google Scholar]
- Toussant M. J. and Latshaw J. D.. 1999. Ovomucin content and composition in chicken eggs with different interior quality. J. Sci. Food Agric. 79:1666–1670. doi:10.1002/(SICI)1097-0010(199909)79:12<1666::AID-JSFA416>3.0.CO;2-H. [Google Scholar]
- Toussant M. J., Swayne D. E., and Latshaw J. D.. 1995. Morphologic characteristics of oviducts from hens producing eggs of different Haugh units caused by genetics and by feeding vanadium as determined with computer software-integrated digitizing technology. Poult. Sci. 74:1671–1676. doi:10.3382/ps.0741671. [DOI] [PubMed] [Google Scholar]
- Trombetta D., Gangemi S., Saijia A., Minciulo P. L., Cimino F., Cristani M., Briuglia B., Piraino B., Isola S., and Salpietro C. D.. 2006. Increase protein carbonyl groups in the serum of the patients affected by thalassemia major. Ann. Hematol. 85:520–522. doi:10.1007/s00277-006-0115-3. [DOI] [PubMed] [Google Scholar]
- Voljč M., Frankič T., Levart M., Nemec M., and Salobir J.. 2011. Evaluation of different vitamin E recommendations and bioactivity of α-tocopherol isomers in broiler nutrition by measuring oxidative stress in vivo and the oxidative stability of meat. Poult. Sci. 90:1478–1488. doi:10.3382/ps.2010-01223. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang J., Liang C., Yuan F., and Gao Y.. 2014. Covalent complication and functional evaluation of (−)-epigallocatechin gallate and α-lactalbumen. Food Chem. 150:341–347. doi:10.1016/j.foodchem.2013.09.127. [DOI] [PubMed] [Google Scholar]
- Wang X. H., Wu S. G., Cui Y. M., Qi G. H., Wang J., and Zhang H. J.. 2017. Effects of dietary tea polyphenols on performance, egg quality and antioxidant ability of laying hens. Chinese. J. Anim. Nutr. 29:193–201. doi:10.3969/j.issn.1006-267x.2017.01.022. [Google Scholar]
- Wang Y. C. 2013. Supplementation of green tea attenuates protein carbonyls formation in aged mice. Life Sci. J. 10:1034–1037. [Google Scholar]
- Yu J., Chen Y., Zhai L., Zhang L., Xu Y., Wang S., and Hu S.. 2015. Antioxidative effect of ginseng stem-leaf saponins on oxidative stress induced by cyclophosphamide in chickens. Poult. Sci. 94:927–933. doi:10.3382/ps/pev055. [DOI] [PubMed] [Google Scholar]
- Yuan Z. H., Zhang K. Y., Ding X. M., Luo Y. H., Bai S. P., Zeng Q. F., and Wang J. P.. 2016. Effect of tea polyphenols on production performance, egg quality, and hepatic antioxidant status of laying hens in vanadium-containing diets. Poult. Sci. 95:1709–1717. doi:10.3382/ps/pew097. [DOI] [PubMed] [Google Scholar]
- Yuksel A., Avci E., Uymaz B., and Erdem Y. K.. 2012. General composition and protein surface hydrophobicity of goat, sheep and cow milk in the region of Mount Ida. Small Ruminant Res. 106:137–144. doi:10.1016/j.smallrumres.2012.03.022. [Google Scholar]