Abstract
The objective of the current study was to investigate the effect of supplementing a mono-component xylanase to a coarsely ground lactation diet (feed fraction particle size above 2 mm was more than 17%) based on wheat, barley, and soybean meal on nutrient digestibility and performance of lactating sows. A total of 30 cross-bred (Danish Landrace × Yorkshire) multiparous sows (parity 2 to 5) were used. Sows were fed a standard gestation diet from mating until day 108 of gestation, and then stratified for BW (295.4 ± 26.1 kg average BW) and parity to receive one of two dietary treatments (n = 15 per treatment), a lactation diet without (control diet) or with supplemented enzyme (enzyme diet). The enzyme applied was a mono-component xylanase dosed at 200 enzyme unit (FXU) per kg of feed, which corresponds to 200 g per ton of feed. The diets were fed until weaning at day 28 of lactation. On day 2 of lactation, litter size of each sow was adjusted to 14 piglets within treatment. Reproductive performance of the sows, growth of the piglets, yield and composition of sow milk, plasma metabolites and apparent total tract digestibility (ATTD) of nutrients were measured. Supplementation of xylanase had no effect (P > 0.05) on total born and live born piglets or stillbirth rate (%) at parturition. Initial parameters on day 2 of lactation including sow BW and back fat thickness, litter size, piglet weight, and litter weight were similar (P > 0.05) between treatments. Piglet weight gain, litter weight gain, litter size, and daily milk yield did not differ (P > 0.05) between treatments. The ADFI was increased by 4.5% (P < 0.01), and BW loss during the whole lactation was reduced from −13.6 to −5.2 kg (P = 0.04) with xylanase addition when compared to control sows. The ATTD of GE (83.9 vs. 82.9, P < 0.01), DM (84.2 vs. 83.4, P < 0.01), N (83.4 vs. 81.7, P = 0.02), OM (86.5 vs. 85.7, P < 0.01) and total nonstarch polysaccharides (NSP; 59.4 vs. 56.7, P = 0.02) were all increased by xylanase supplementation. Milk composition and plasma metabolites were not affected (P > 0.05), except that plasma triglycerides content was increased by xylanase addition (0.23 vs. 0.20 mM, P = 0.04). In conclusion, supplementing a mono-component xylanase to a coarsely ground lactating diet based on wheat, barley, and soybean meal improved sow feed intake and nutrient digestibility, thereby reducing sow BW loss throughout lactation, whereas milk yield and piglet performance were not affected.
Keywords: lactating sow, milk yield, milk composition, nutrient digestibility, piglet performance, xylanase
INTRODUCTION
In Europe, Australia, and western Canada, barley and wheat are the main ingredients used in pig diets (Nasir et al., 2015). However, barley contains 22%, while wheat contains 13%, dietary fiber (DF) content in their whole grains, which cannot be degraded by monogastric animals due to lack of corresponding digestive enzymes (Bach Knudsen, 1997). Arabinoxylans are particularly abundant both in wheat and barley (Bach Knudsen, 2014). DF and coarsely milled diets are beneficial factors to reduce the risk of gastric ulcers in sows (Millet et al., 2012; Möβeler et al., 2012) and DF are also known to enhance sow productivity by improving colostrum production (Theil et al., 2014) and reducing stillbirth rate (Feyera et al., 2017). However, both DF and coarse milling reduce digestibility of starch and protein by hampering the access to digestive enzymes and by increasing the viscosity of the digesta (Steenfeldt and Pettersson, 2001; Kim et al., 2005; Bach Knudsen, 2014).
Modern lactating sows have a huge demand for nutrients for the large litter size. The discrepancy between a large nutrient demand and insufficient nutrient intake leads to mobilization of their body reserves (Strathe et al., 2017). Supplementing nonstarch polysaccharides (NSP) degrading enzymes has been found to improve the nutrient digestibility, feed efficiency, and growth performance in poultry and young pigs (Patience et al., 1992; Diebold et al., 2004; Kiarie et al., 2007; Lordelo et al., 2008; Woyengo et al., 2008; Tsai et al., 2017), but has not been commonly tested in sows. Thus, the objective of this study was to determine effects of supplementing a mono-component xylanase on feed utilization, plasma metabolites and productivity of lactating sows. We hypothesized that the xylanase supplementation would improve the nutrient digestibility and milk production and/or reduce body mobilization of lactating sows.
MATERIALS AND METHODS
The experiment was carried out at Aarhus University, Foulum, Denmark, and was conducted in accordance with Danish laws and regulations for the humane care and use of animals in research (The Danish Ministry of Justice, 1995). Protocols were approved by The Danish Animal Experimentation Inspectorate, and the health of the animals was monitored during the experimental period.
Animals and Housing
A total of 30 cross-bred (Danish Landrace × Danish Yorkshire, DanAvl, Copenhagen, Denmark) multiparous sows were included in the experiment. The average parity for sows in control and enzyme group was 2.9 ± 0.3 and 3.0 ± 0.2, respectively. Sows were fed a standard gestation diet from mating until day 108 of gestation and then stratified for BW (295.4 ± 26.1 kg average BW) and parity to receive 1 of 2 dietary treatments (n = 15 per treatment) until weaning at day 28 of lactation. After farrowing, total born piglets, live born piglets, and stillborn piglets were recorded. On day 2 of lactation, litter size of each sow was adjusted to 13 or 14 piglets within treatment according to number of functional mammary glands. Sows and their litters were individually housed in fixed farrowing crates (2.7 m × 1.8 m). Pen floor consisted of one half concrete floor and one half iron slatted floor. Room temperature was kept at 20 °C before parturition, 24 °C after parturition and decreased to 18 °C before weaning. Piglets had access to a cave equipped with a heating lamp (it was turned on or off depending on piglet behavior) and sawdust as bedding, whereas the sow did not have access to bedding material to avoid intake of DF from straw. The light was on from 0700 to 1900 h and during feeding from 2300 to 2400 h. Sows and piglets were monitored daily for health condition and were treated in compliance with standard procedures.
Diets and Feeding
A standard lactation diet based on wheat, barley, and soybean meal was formulated to meet the nutrient requirements for lactating sows and regarded as the control diet (Tybirk et al., 2015). The enzyme diet was made by adding 200 FXU per kg of feed (corresponding to 200 g per ton of feed) to the control diet. The NSP enzyme, endo-1,4-β-xylanase, originated from a gene derived from Thermomyces lanuginosus expressed in Aspergillus oryzae (RONOZYME WX; a mono-component xylanase product; DSM Nutritional Products, Basel, Switzerland). The diets with and without enzyme were produced by Vestjyllands Andel (Borris, Denmark) using the same batch of cereals and protein sources. A premix of the control diet (all constituents except 13.8% of barley) was produced before the premix of the enzyme diet to ensure no carry-over of the enzymes. Both diets were heat treated at least at 81 °C (due to Danish legislation) and processed through an expander instead of pelleting because the pelleting process has been found to reduce the particle size of pig feed (Wolf et al., 2010). At Aarhus University, Foulum, 0.2% chromic oxide and 13.8% non-heat treated roller milled barley was mixed with the two premixes at the local feed mill and then the two diets were pelleted (cold pelleting) at 5 mm pellet size, to allow measurement of apparent total tract digestibility (ATTD) of nutrients using the external marker technique. The dietary ingredients of experimental diets are shown in Table 1.
Table 1.
Dietary ingredients of the experimental diets (as-fed basis)
| Ingredient, % | Diet | |
|---|---|---|
| Control | Enzyme | |
| Barley | 34.8 | 34.8 |
| Wheat | 35.4 | 35.4 |
| Oat | 5.00 | 5.00 |
| Soybean meal | 14.6 | 14.6 |
| Sunflower meal | 3.50 | 3.50 |
| Molasses | 0.50 | 0.50 |
| Lard (animal fat) | 1.80 | 1.80 |
| Leci E* | 0.50 | 0.50 |
| Calcium carbonate | 1.37 | 1.37 |
| Sodium chloride | 0.46 | 0.46 |
| Monocalcium phosphate | 0.85 | 0.85 |
| Chromium oxide | 0.20 | 0.20 |
| l-Lys sulfate, 70% | 0.39 | 0.39 |
| DL-Met, 99% | 0.06 | 0.06 |
| l-Thr, 98.5% | 0.09 | 0.09 |
| Phytase† | 0.02 | 0.02 |
| Xylanase added‡ | No | Yes |
| Bicco-LIN|| | 0.01 | 0.01 |
| Vitamin E premix$ | 0.28 | 0.28 |
| Vitamin-mineral premix¶ | 0.21 | 0.21 |
*Phospolipids, FFA, and triglycerides from rapeseed oil.
†1,500 U/kg.
‡200 enzyme unit (FXU) per kg of feed (corresponding to 200 g per ton of feed).
||Choline chloride in natural form from dried weeds.
$45.500 mg/kg.
¶Supplied per kilogram of diet: retinol 8694 IU, cholecalciferol 1242 IU, α- tocopherol 79.11 mg, phylloquinone 4.14 mg, thiamin 2.17 mg, cyanocobalamin 0.022 mg, riboflavin 5.43 mg, pyridoxine 3.26 mg, biotin 0.43 mg, d-pantothenic acid 16.3 mg, folic acid 1.63 mg, niacin 21.74 mg, 15.57 mg Cu as CuSO4 ∙ 5H2O, 103.5 mg Zn as ZnO, 86.94 mg Fe as FeSO4 ∙ 7 H2O, 2.07 mg I as CaI, 43.47 mg Mn as MnO, 0.31 mg Se as Na2SeO3.
Before parturition, sows were daily fed 3.5 Danish feed unit (FU) which is equivalent to approximately 3.3 kg/d (or 42 MJ ME/d), and from day 2 of lactation the feed supply increased daily with 0.5 FU (or 6.4 MJ ME/d) until reaching a maximum of 9.0 FU per day (equivalent to 108 MJ ME per day) for sows nursing 14 piglets. This feeding strategy is used as a guideline for the maximum daily allowance of the sows according to recommended Danish feeding practice. The feed supply was reduced by 3% for each piglet sows nursed below 14 littermates. Sows were fed automatically three times daily at 0730, 1430, and 2330 h. If sows had feed leftovers, the feed supply was reduced by 20% the next day and then by 10% the following day until the sow consumed the planned amount. Feed leftovers were collected daily to measure the daily intake of DM and nutrients. Sows had free access to water during the experimental period. Straw or rooting materials were not provided except that a rope was supplied to stimulate nursing and rooting behavior.
Measurements and Sample Collection
Sow BW and back fat thickness were measured on day 2, 7, 14, 21, and 28 of lactation. Back fat thickness was measured at 65 mm to the left side of the dorsal mid-line at the level of the last rib (known as P2 point) using an ultrasound (SonoGrader II, RENCO, MN, USA). Piglets were weighed individually on day 2, 7, 14, 21, and 28 of lactation to calculate piglet or litter weight gain, and to estimate milk yield. Milk and blood samples were collected at day 3, 10, 17, and 24 of lactation to analyze for milk composition and plasma metabolites, respectively. During milk sampling, piglets were removed from the sow, and the sow was injected 0.3 mL of oxytocin intravenously to induce milk letdown. A total of 45 to 50 mL milk was collected from two to five teats until milk letdown ceased and milk was filtered through gauze before storing at −20 °C. Blood samples were drawn by jugular vein puncture 4 h after morning meal and collected in heparinized tubes (Greiner BioOne GmbH, Kremsmünster, Austria). Plasma was obtained by centrifuging the blood sample at 1,558 × g at 4 °C for 12 min and stored at −20 °C until analysis. Fecal samples on day 3 and 17 of lactation were collected to determine nutrient ATTD using chromic oxide as the external marker. And feces on day 3 and 17 of lactation were scored by visual qualitative evaluation in the morning. Score values range from 0 to 5, with 0 being absence of feces, and 5 being very wet feces, unformed, and liquid (Oliviero et al., 2009).
Chemical Analyses
The diets were analyzed for GE, DM, ash, CP (N × 6.25), crude fat, total NSP, starch, Ca, P, AA, and chromic oxide. Feed analyses were carried out in triplicate except for GE, starch and total NSP, which were analyzed in duplicate. Fecal samples were freeze-dried and analyzed in duplicate for GE, DM, ash, CP, total NSP, and chromic oxide. Feed and fecal GE was determined using an adiabatic bomb calorimeter (Parr Instrument Company, Moline, Illinois, USA). The DM was determined by drying the samples at 105 °C to a constant weight, and ash was analyzed according to the AOAC method (AOAC, 1990). The CP (N × 6.25) was determined by the Dumas method (Hansen, 1989) while the HCl-fat was measured according to the Stoldt procedure (Stoldt, 1952). Starch and total NSP were measured by enzymatic-chemical methods as described by Bach Knudsen (1997) with the modification that 2M H2SO4 was applied in the acid hydrolysis procedure for total NSP analysis rather than 1M H2SO4. Klason lignin was measured by a modified method from Theander et al. (1993) as described by Bach Knudsen (1997). Dietary Ca and P were analyzed by spectrophotometer according to the Official Journal of the European Union (152/2009). Dietary AA was determined using an amino acid analyzer (Biochrom 20 plus; Biochrom Ltd., Cambridge, UK; [EC] 152/2009; European Commission 2009). The external marker chromic oxide in diets and fecal samples was measured spectrophotometrically according to Schürch et al. (1950).
Milk composition was analyzed in triplicate for DM, fat, protein, and lactose contents with infrared spectroscopy using a Milkoscan 4000 (Foss MilkoScan, Hillerød, Denmark).
The wet-sieve analysis was performed using an electronic wet-sieve (Retsch AS 200 basic, Retsch GmbH, Germany). The screen sizes applied were as follows: 3,150; 2,000; 1,400; 1,000; 500; and 355 μm. Briefly, feed samples were soaked in water for 1 h before wet-sieving with continuous waterflow. Each fraction obtained was then dried at 105 °C for 24 h to determine the DM weight. The percentage of the individual sieve fraction was obtained by dividing its DM weight by the DM weight of the total sample of feed before wet sieving calculated from the amount of feed weighed for this procedure and the analyzed DM content in the two diets. The fraction below 355 μm was estimated to be the difference between the DM weight of sample feed before sieving and the sum of the DM weight of all other fractions obtained.
Plasma was analyzed in duplicate for glucose, lactate, NEFA, triglycerides (TAG) and urea. The NEFA content was determined using the Wako, NEFA C ACS-ACOD assay method (Wako Chemicals GmbH, Neuss, Germany), and glucose, lactate, TAG, and urea were analyzed according to standard spectrophotometric procedures (Siemens Diagnostics Clinical Methods for ADVIA 1650) using an auto analyzer (ADVIA 1650 Chemistry System, Siemens Medical Solution, Tarrytown, NY).
Calculations
Sow milk yield was estimated according to Hansen et al. (2012) based on litter weight gain and litter size. Energy secreted in milk was calculated as the sum of energy secreted as milk protein with an energy value of 23.86 kJ/g, fat with an energy value of 39.76 kJ/g and lactose with an energy value of 16.51 kJ/g (Weast, 1984).
Nutrient ATTD was calculated using the following equation (Stein et al., 2007) with chromic oxide as the external marker:
Statistical Analysis
The experiment was regarded as a complete randomized design in which sows were stratified for BW and parity. Weekly recordings of sow feed intake, sow BW and back fat thickness changes, litter size, piglet and litter weight gain, milk yield and milk composition, as well as plasma metabolites, were applied to the following model to analyze data:
where Yijk is the observed trait, µ is the overall mean of the observations, αi is the main effect of the diet (i = control or enzyme), βj is the effect of week of lactation (j = 1, 2, 3, and 4), (αβ)ij is the interaction between diet and week of lactation, tk is the random effect of sows (k = 1, 2, 3, …, 30) to take into account repeated measurements within sow and εijk is the residual random error. Plasma NEFA was subjected to a logarithmic transformation to stabilize the residual variance.
Nutrient ATTD was tested with the same model where βj referred to day in lactation (j = 3, 17). Parameters other than the ones mentioned above were analyzed with a submodel without effects of week (or day) in lactation and (αβ)ij interaction and without the random effect of sows.
The statistical analysis was performed using the MIXED procedure of SAS (version 9.3), except for stillborn piglets and piglet mortality where odds ratios of these traits were analyzed using the GENMOD procedure of SAS (version 9.3). Mean values were presented as least square mean ± largest SEM, except for stillborn piglets, piglet mortality, and plasma NEFA which were reported as mean and their 95% confidence limits. All variables were considered significant when P ≤ 0.05 whereas P ≤ 0.1 was considered a tendency.
RESULTS
The analyzed nutrient composition of experimental diets is shown in Table 2. As expected, the control and enzyme diet were highly similar in nutrient composition and diet particle size. Due to the greater nutrient ATTD (Table 4), the calculated ME (MJ/kg DM) of the enzyme group was slightly greater (0.16 MJ/kg DM equivalent to ~1.1%) as compared with the control diet. The proportion of the feed fraction with particle size above 2 mm was 19.0% for the control and 17.7% for the enzyme group. The average enzyme activity (measured in triplicate) was 0 (zero) in the control group, while it was 124 ± 10.7 FXU per kg of feed in the enzyme group, corresponding to 62% of the supplemented level (200 FXU per kg of feed).
Table 2.
Analyzed chemical composition and diet particle size of the experimental diets
| Diet | ||
|---|---|---|
| Item | Control | Enzyme |
| GE, MJ/ kg DM | 18.29 | 18.28 |
| Calculated ME,* MJ/ kg DM | 14.62 | 14.78 |
| Calculated NE,† MJ/kg DM | 9.85 | 9.85 |
| Chemical composition, g/kg DM | ||
| DM, g/ kg as feed | 862 | 863 |
| CP | 162 | 163 |
| Crude fat | 52 | 53 |
| Starch | 499 | 504 |
| Ash | 53 | 54 |
| Total NSP | 132 | 133 |
| Dietary fiber | 152 | 153 |
| Ca | 9.1 | 9.0 |
| P | 6.1 | 6.4 |
| Cr2O3 | 2.3 | 2.3 |
| Indispensable amino acid | ||
| Lys | 9.5 | 9.5 |
| Met | 2.9 | 3.0 |
| Thr | 6.3 | 6.4 |
| Val | 7.2 | 7.2 |
| Particle size by wet sieve analysis,‡ % | ||
| ≤1 mm | 68.06 | 69.85 |
| 1 to 2 mm | 12.99 | 12.42 |
| >2 mm | 18.95 | 17.73 |
*Calculated ME = GE × GEATTD × 0.964 according to Theil et al. (2004).
†Calculated NE according to the EVAPig system (INRA, Saint-Gilles, France).
‡The wet-sieve analysis was performed using an electronic wet-sieve (Retsch AS 200 basic, Retsch GmbH, Germany). The screen sizes applied were as follow: 3150 μm, 2000 μm, 1400 μm, 1000 μm, 500 μm and 355 μm. Briefly, feed samples were soaked in water for 1 h before wet-sieving with continuous waterflow. Each fraction obtained was then dried at 105ºC for 24 h to determine the DM weight. The percentage of the individual sieve fraction was obtained by dividing its DM weight by the DM weight of the total sample of feed before wet sieving calculated from amount of feed weighed for this procedure and the analyzed DM content in the two diets. The fraction below 355 μm was estimated to be the difference between the DM weight of sample feed before sieving and the sum of the DM weight of all other fractions obtained.
Table 4.
Effects of xylanase supplementation on nutrient digestibility and fecal score of experimental sows during lactation
| Diet | DIL | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Control | Enzyme | SEM | 3 | 17 | SEM | Diet | DIL | Diet×DIL |
| GE in feces, MJ/kg DM | 18.8 | 18.6 | 0.1 | 18.7 | 18.8 | 0.1 | 0.41 | 0.45 | 0.34 |
| DM in feces, g/kg | 317.0 | 308.0 | 11 | 366a | 259b | 11 | 0.57 | <0.001 | 0.44 |
| N in feces, g/kg DM | 28.4 | 27.7 | 0.6 | 27.9 | 28.2 | 0.6 | 0.36 | 0.80 | 0.16 |
| OM in feces, g/kg DM | 816a | 806b | 3.5 | 806b | 816a | 3.2 | 0.05 | 0.02 | 0.62 |
| Total NSP in feces, g/kg DM | 344 | 342 | 5.9 | 337b | 350a | 5.9 | 0.74 | 0.03 | 0.27 |
| ATTD of GE, % | 82.9b | 83.9a | 0.2 | 84.2a | 82.6b | 0.2 | <0.01 | <0.001 | 0.22 |
| ATTD of DM, % | 83.4b | 84.2a | 0.2 | 84.5a | 83.0b | 0.2 | <0.01 | <0.001 | 0.06 |
| ATTD of N, % | 81.7b | 83.4a | 0.5 | 83.4a | 81.6b | 0.5 | 0.02 | <0.01 | 0.03 |
| ATTD of OM, % | 85.7b | 86.5a | 0.2 | 86.8a | 85.3b | 0.2 | <0.01 | <0.001 | 0.11 |
| ATTD of total NSP, % | 56.7b | 59.4a | 1.1 | 60.9a | 55.1b | 1.1 | 0.02 | <0.001 | 0.75 |
| Fecal score | 2.4 | 2.4 | 0.1 | 1.8b | 3.0a | 0.1 | 0.69 | <0.001 | 0.69 |
DIL, day in lactation.
a,bMean values with different letters within a row are significant different (P < 0.05).
Supplementation of the xylanase had no effect (P > 0.05) on total born, live born and stillborn piglets at parturition (Table 3). Initial parameters on day 2, including sow BW and back fat, piglet and litter weight and litter size, were not different (P > 0.05) between treatments. Sow BW loss decreased by xylanase supplementation compared with control sows (−5.2 vs. −13.6 kg, P = 0.04).
Table 3.
Characteristics of sows fed the experimental diets and their piglets during lactation
| Diet | ||||
|---|---|---|---|---|
| Item | Control | Enzyme | SEM | P-value |
| No. of sows | 15 | 15 | — | — |
| Sow BW on day 2, kg | 274 | 274 | 7 | 0.94 |
| Sow BW on day 28, kg | 261 | 268 | 7 | 0.42 |
| Sow BW change, kg | −13.6b | −5.2a | 2.7 | 0.04 |
| Sow back fat on day 2, mm | 16.7 | 17.8 | 0.9 | 0.41 |
| Sow back fat on day 28, mm | 13.3 | 15.3 | 0.9 | 0.14 |
| Sow back fat change, mm | −3.4 | −2.5 | 0.7 | 0.13 |
| Total born piglets | 20.2 | 20.1 | 0.9 | 0.92 |
| Live born piglets | 17.2 | 17.5 | 0.6 | 0.68 |
| Stillborn piglets,* % | 12.3 | 12.6 | 0.91 | |
| [8.9; 16.7] | [9.3; 16.9] | |||
| Piglet weight on day 2, kg | 1.62 | 1.61 | 0.04 | 0.92 |
| Piglet weight on day 28, kg | 8.21 | 8.18 | 0.17 | 0.88 |
| Litter weight on day 2, kg | 22.5 | 22.3 | 0.5 | 0.82 |
| Litter weight on day 28, kg | 105.4 | 103.3 | 2.9 | 0.61 |
| Litter size on day 2 | 13.9 | 13.9 | 0.1 | 0.60 |
| Litter size at weaning | 12.9 | 12.8 | 0.3 | 0.88 |
| Piglets mortality until day 28,* % | 7.7 | 7.7 | 0.99 | |
| [4.7; 12.1] | [4.8; 12.2] |
a,bMean values with different letters within a row are significantly different (P < 0.05).
*Data were binomially distributed, and hence confidence limits were given in brackets instead of SEM values.
The ATTD of GE (83.9 vs. 82.9, P < 0.01), DM (84.2 vs. 83.4, P < 0.01), N (83.4 vs. 81.7, P = 0.02), OM (86.5 vs. 85.7, P < 0.01) and total NSP (59.4 vs. 56.7, P = 0.02) were all increased by xylanase supplementation (Table 4). When considering the day of lactation, ATTD of all nutrients were all greater on day 3 compared with day 17 of lactation (P < 0.01). There was no difference in fecal score between treatments, but fecal score on day 17 of lactation was greater than that on day 3 of lactation (P < 0.001).
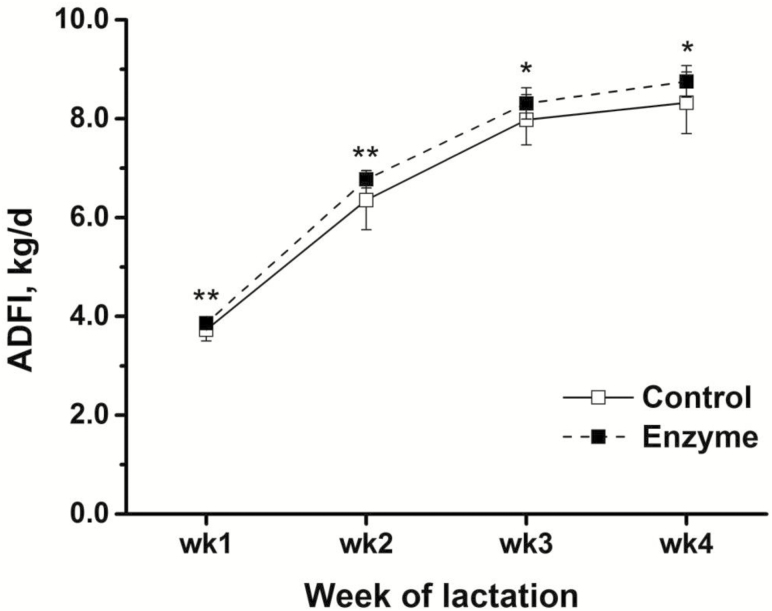
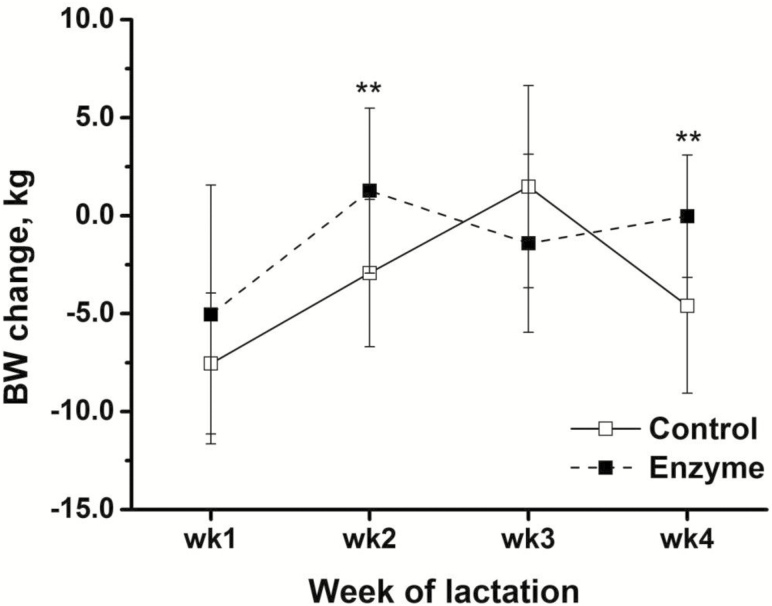
The effect of supplemental xylanase on the sow performance over the course of lactation is presented in Table 5. The ADFI of sows increased from weeks 1 to 4 of lactation (P < 0.001), and the ADFI of sows in the enzyme group was on average 4.5% greater compared with control group (6.9 vs. 6.6 kg/d, P < 0.01) (Figure 1). The sow BW loss was greatest in week 1 when compared with later stages of lactation (P < 0.001). The xylanase addition ameliorated the sow BW change compared with sows in control group (−1.30 vs. −3.38 kg/wk, P = 0.04). In addition, the sow BW change interacted with enzyme supplementation across the week of lactation (P < 0.01). Sows supplied feed with enzyme lost less BW (P < 0.01) in week 2 and 4 of lactation, whereas the weight loss was similar (P > 0.05) in week 1 and 3 of lactation (Figure 2). Individual piglet weight gain, litter weight gain, litter size, milk yield, and milk composition did not differ (P > 0.05) between treatments. The milk yield markedly increased from week 1 to week 3 and 4 of lactation (P < 0.001), whereas the milk contents of DM, fat, and protein decreased (P < 0.001) from week 1 to 4 of lactation. Concomitantly, milk lactose increased over the course of lactation with the greatest content found in week 4 of lactation (P < 0.001).
Table 5.
Effects of xylanase supplementation on sow performance over a 28 d lactation period
| Item | Diet | SEM | Week | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Enzyme | 1 | 2 | 3 | 4 | Diet | Week | Diet × week | |||
| ADFI, kg/d | 6.6b | 6.9a | 0.1 | 3.8d | 6.6c | 8.1b | 8.5a | 0.1 | <0.01 | <0.001 | 0.10 |
| Sow BW change, kg/wk | −3.38b | −1.30a | 0.69 | −6.28c | −0.82ab | 0.04a | −2.30b | 0.85 | 0.04 | <0.001 | <0.01 |
| Sow back fat change, mm/wk | −0.84 | −0.68 | 0.09 | −0.72 | −0.68 | −0.91 | −0.73 | 0.12 | 0.21 | 0.51 | 0.27 |
| Piglet weight gain, g/wk | 1579 | 1592 | 40 | 901c | 1754b | 1805ab | 1881a | 48 | 0.83 | <0.001 | 0.52 |
| Litter weight gain, kg/wk | 21.1 | 20.8 | 0.7 | 12.5b | 23.5a | 23.8a | 23.9a | 0.7 | 0.68 | <0.001 | 0.42 |
| Milk yield, kg/d | 13.3 | 13.0 | 0.3 | 8.6c | 13.7b | 15.3a | 15.0a | 0.3 | 0.66 | <0.001 | 0.34 |
| Milk DM, % | 17.8 | 17.6 | 0.2 | 19.3a | 17.4b | 17.3bc | 16.8c | 0.2 | 0.38 | <0.001 | 0.42 |
| Milk fat, % | 6.9 | 6.6 | 0.2 | 8.3a | 6.6b | 6.4b | 5.9c | 0.2 | 0.33 | <0.001 | 0.18 |
| Milk protein, % | 5.1 | 5.1 | 0.1 | 5.8a | 4.9b | 4.9b | 5.0b | 0.1 | 0.90 | <0.001 | 0.86 |
| Milk lactose, % | 5.1 | 5.2 | 0.1 | 4.8c | 5.2b | 5.2b | 5.3a | 0.1 | 0.25 | <0.001 | 0.58 |
| Milk energy, MJ/100 g | 0.48 | 0.47 | 0.01 | 0.55a | 0.46b | 0.46b | 0.44b | 0.01 | 0.36 | <0.001 | 0.32 |
a,bWithin a row and within a main effect, values with different letters are significantly different (P < 0.05).
Figure 1.
The weekly ADFI changes of sows from control and enzyme group during lactation. Thirty sows were fed either a wheat, barley, and soybean meal-based control diet or control diet supplemented with a mono-component xylanase dosed at 200 enzyme unit (FXU) per kg of feed. The ADFI for each week were subjected to a t-test using SAS. The ADFI of sows in enzyme group was greater than that of control group in each week of lactation (P < 0.05). *P < 0.05, **P < 0.01. Error bars indicate the SEM.
Figure 2.
The weekly BW changes of sows from control and enzyme group during lactation. Thirty sows were fed either a wheat, barley, and soybean meal-based control diet or control diet supplemented with a mono-component xylanase dosed at 200 enzyme unit (FXU) per kg of feed. The BW changes for each week were subjected to a t-test using SAS. Sows supplied feed with enzyme lost less BW (P < 0.01) in week 2 and 4 of lactation, whereas the weight loss was similar (P > 0.05) in week 1 and 3 of lactation. *P < 0.05, **P < 0.01. Error bars indicate the SEM.
Sow plasma metabolites including glucose, lactate, NEFA, and urea did not differ between treatments (P > 0.05; Table 6), except that TAG content was increased by xylanase addition (0.23 vs. 0.20 mM/liter, P = 0.04). Plasma glucose was not affected by week of lactation, whereas plasma contents of TAG, lactate, and NEFA were greater (P < 0.001) in week 1 when compared with any other week of lactation, and plasma urea content increased from week 1 to 4 of lactation (P < 0.001).
Table 6.
Effects of NSP-degrading enzyme supplementation on the concentration of plasma metabolites in lactating sows
| Diet | Week | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite | Control | Enzyme | SEM | 1 | 2 | 3 | 4 | SEM | Diet | Week | Diet × week |
| Glucose, mM | 5.49 | 5.48 | 0.12 | 5.44 | 5.45 | 5.54 | 5.51 | 0.12 | 0.93 | 0.69 | 0.16 |
| TAG, mM | 0.20b | 0.23a | 0.02 | 0.27a | 0.20bc | 0.21b | 0.18c | 0.02 | 0.04 | <0.001 | 0.75 |
| Lactate, mM | 2.49 | 2.22 | 0.30 | 3.15a | 2.05b | 2.14b | 2.07b | 0.31 | 0.42 | <0.001 | 0.64 |
| NEFA,* µekv/L1 | 143 | 140 | 1 | 315a | 188b | 94cd | 73d | 1 | 0.89 | <0.001 | 0.20 |
| [111; 185] | [109; 180] | [234; 423] | [140; 253] | [70; 126] | [54; 98] | ||||||
| Urea, mM | 3.40 | 3.43 | 0.19 | 2.51d | 3.27c | 3.73b | 4.14a | 0.20 | 0.86 | <0.001 | 0.62 |
a,bMean values with different letters within a row are significant different (P < 0.05).
*Plasma NEFA was log-transformed before statistical analysis, and hence confidence limits are given in brackets instead of SEM values.
DISCUSSION
Cereals such as wheat and barley are the commonly used ingredients in many swine and poultry diets (Ravn et al., 2016), and besides contributing with the dietary energy, they also increase the DF content. Barley is rich in DF (approximately 22%) whereas wheat has a lower content (approximately 13% DF; Bach Knudsen, 1997). Thus, most diets for lactating sows contain 15% to 17% DF, whereas diets for gestating sows have a slightly greater content (typically 17% to 19% DF). Arabinoxylans, which consist of a linear backbone of xylose substituted with arabinose, are the main structural NSP constituents of endosperm cell wall in most cereals representing about 65% of the total NSP in wheat and 45% of the total NSP in barley (Bach Knudsen, 2014). The arabinoxylans present in the cell walls could encapsulate potentially available nutrients; and the content of soluble NSP, in particular, can cause an increase in digesta viscosity; both reducing the access of digestive enzymes to their substrates (dietary nutrients) and these effects impair the digestibility of the nutrients (Campbell and Bedford, 1992; Steenfeldt and Pettersson, 2001; O’Connell et al., 2005).
Xylanase, which specifically degrades the arabinoxylans, has been intensively applied in diets for monogastric animals, particularly for poultry and young pigs. In contrast to poultry studies, pig studies have shown inconsistent results on improved digestibility among experiments. Increased energy digestibility and/or increased digestibility of DM, CP, crude fiber, and some AA have been reported with xylanase addition to wheat based diets (Yin et al., 2000; Barrera et al., 2004; Nortey et al., 2007, 2008; Kiarie et al., 2016), to barley based diets (Yin et al., 2001) or to corn-soybean meal based diets (Fang et al., 2007; Kiarie et al., 2016) fed to growing pigs. Beneficial effects of xylanase on digestibility have also been observed in weaned pigs fed a wheat based diet (Vahjen et al., 2007). In contrast to these studies, no response in performance to xylanase supplementation has been reported in nursery pigs fed wheat-based diets (Mavromichalis et al., 2000), growing pigs fed rye- or barley-based diets (Thacker et al., 1991) or a corn-based diet (Jang et al., 2016), nor in weaned pigs fed wheat- or barley-soybean meal based diets (Inborr et al., 1993). The reason for these discrepant results is likely related to the dietary ingredients, the carbohydrate composition, chemical structure, and dietary contents of arabinoxylans in the diets (Partridge et al., 2001; Kim et al., 2005). Indeed, low energy diets using poor quality fibrous materials with rather low fermentability were considered to provide more scope for the action of supplemented NSP degrading enzyme, thereby contributing to a more clear response to enzyme supplementation (Bach Knudsen and Hansen, 1991; Bedford et al., 1998; O’Connell et al., 2005). In addition, diet particle size may also account, at least in part, for the different impacts of supplemented NSP degrading enzyme across different studies. Given that Wolf et al. (2010) found the average proportion of feed fraction with particle size above 1.4 mm was only 14.8% in 29 commercial diets for lactating sows using wet-sieve analysis, our results indicated that the diets used in the present study were indeed coarsely processed as we expected. Besides, most of the studies mentioned above did not report the processing procedure of the feed, that is, heat-treatment and temperature when the diet was pelleted, nor report the enzyme activity of the feed after processing. The feed processing conditions are likely crucial to maintain a high enzyme activity and is a precondition to elicit a response on nutrient digestibility. Specifically, the reasonable explanation for the detected enzyme activity in the enzyme group (62% of the supplemented level) is likely related to the processing procedure of the feed. Particularly, after the enzyme was weighed and added to the enzyme diet, due to the addition of 0.2% chromic oxide for nutrient digestibility measurement, the feed was mixed again through a feed mill and then pelleted (cold pelleting) at 5 mm pellet size. During the cold pelleting process the feed may be heated because of heating of the matrice, but the temperature of the matrice will likely not exceed approximately 72°C.
Feeding late gestating sows with DF rich diet have been shown to favorably reduce stillbirth rate (Feyera et al., 2017) and increase colostrum yield of sows (Theil et al., 2014). DFs incorporation (Dirkzwager et al., 1998; Millet et al., 2012) and coarsely milling of feeds (Möβeler et al., 2012; Liermann et al., 2015) are important to reduce the occurrence of gastric ulcers. However, DFs and coarsely milling reduce the digestibility of energy and other nutrients (Zhang et al., 2013; Liermann et al., 2016; Saqui-Salces et al., 2017) and therefore it is of interest to evaluate whether enzyme supplementation indeed improved the nutrient digestibility of a fiber rich, coarsely milled lactation diet as mentioned above. In the present study, xylanase supplementation markedly improved the ATTD of total NSP, GE, DM, N, and OM of lactating sows. The xylanase used is known to be effective on both soluble and insoluble arabinoxylan fractions (Le et al., 2013). In line with our study, de Souza et al. (2007) found that ileal digestibility and the ATTD of DM and N increased by xylanase supplementation in lactating sows fed a corn–soybean meal diet. Unfortunately, de Souza et al. (2007) did not measure the digestibility of DF or the NSP. The NSP is believed to reduce the digestibility of other dietary nutrients (Bach Knudsen, 2014). The underlying mechanisms of xylanase on nutrient digestibility are considered to be dual, one is to decrease the viscosity of digesta caused by the soluble fractions of arabinoxylans (Vahjen et al., 2007; Francesch et al., 2012; Gonzalez-Ortiz et al., 2016); another is to cleave glycosidic bonds in the xylan main chain, randomly cutting the arabinoxylan backbone into smaller chains to liberate encapsulated nutrients such as protein and starch (Bedford and Schulze, 1998; Kim et al., 2005; Tapingkae et al., 2008). Improvements in the digestibility of GE, DM, N, and OM in the present study were mainly due to the successful hydrolysis of the NSP components by the xylanase addition. With the same xylanase product as we applied, improvements in apparent ileal disappearance of soluble (28%, P < 0.01) and insoluble (15%, P < 0.01) NSP as well as increased apparent ileal digestibility of N (2.0%, P < 0.001) and energy (2.9%, P < 0.001) have also been found in broiler chickens (Cowieson, 2016). Besides, a reasonable explanation for the greater nutrient digestibility on day 3 in comparison to day 17 of lactation could be related to the difference in feed intake between early and peak lactation. Thus, less time is available for nutrient digestion when feed intake is high at peak lactation, whereas delayed gastrointestinal emptying in early lactation likely allows increased nutrient digestion.
Feed intake of lactating sows is regarded being a limiting factor for their energy intake (Strathe et al., 2017) and this may be due to a limited gastric capacity or hormonal regulation of the appetite. In a previous study with a high supply of DF to lactating sows, it was demonstrated that the feed intake was compromised at peak lactation when sows were fed soluble DF from sugar beet pulp, but not when sows were fed insoluble DF from alfalfa (Krogh et al., 2017a). The possible explanation could be that diets high in soluble NSP increased digesta viscosity in the stomach of pigs and reduced gastric emptying (Johansen et al. 1996) and therefore lowered the digesta passage rate (Wenk 2001). An increased passage rate of digesta from the stomach and through the small intestine with NSP degrading enzyme was hypothesized to explain the increased feed intake by (partial) removal of inhibitory effects of NSP, specifically the soluble NSP arabinoxylans and β-glucans (Zijlstra et al., 2004), which is supported by the present study that increased feed intake and total NSP digestibility of lactating sows were consistently observed. The increased feed intake of sows during lactation in our study was consistent with that reported by Walsh et al. (2012) who showed that inclusion of an NSP-hydrolyzing enzyme multicomplex (xylanase and β-glucanase) from day 109 of gestation and throughout lactation increased the feed intake by 0.2 kg/d as a mean for the whole lactation period, although it only differed statistically in the third week of lactation in that study.
Excessive body mobilization and loss of back fat are problematic because they may reduce the subsequent reproductive performance by increasing the weaning to oestrus interval of sows (Vesseur et al., 1994; Thaker and Bilkei, 2005) and reducing ovulation rates and embryonic survivals (Zak et al., 1997; Van den Brand et al., 2000; Vinsky et al., 2006). Thus, the reduced body mobilization of lactating sows fed the xylanase supplemented diet in the present study is regarded being a clear benefit for the sows because the need for restoring body condition in the subsequent gestation period was almost eliminated, as only 5 kg of BW was lost. Recently it was shown that milk yield of high-producing sows was positively associated with a high weight loss (body mobilization), indicating that the milk yield could be compromised for sows in the enzyme group in the present study due to the low weight loss observed. However, the enzyme supplemented sows were indeed high-producers as they produced on average 13.0 kg/d of milk and this level was similar to that of the control sows (13.3 kg/d) which lost 14 kg BW during lactation. These levels are greater than any previous studies with high-producing DanAvl sows (range 10.8 to 12.2 kg/d of milk; Krogh et al., 2016, 2017b; Strathe et al. 2016, 2017), although their weight loss was minimal. For comparison, the previous studies reported sow weight losses in the range between 14 and 26 kg during lactation even though the feeding curves in all these experiments were similar. The results from the enzyme supplemented group in the present study suggest that body mobilization is not a prerequisite for a high milk yield, and that a high nutrient digestibility is a key factor for maximizing sow productivity during lactation.
Piglet performance including piglet weight gain, litter weight gain, or litter size was not affected in the present study and is in line with results obtained by Walsh et al. (2012). The lack of effects on piglet performance in the present study were not surprising because the similar milk yield between treatments and the fact that lactating sows prioritize nutrients for their milk production at the expense of their own body (Theil et al., 2012). In contrast to our results, Upadhaya et al. (2016) found increased ADG of piglets with increased supplementation of cellulase in corn–soybean meal-based lactation diet for sows. They attributed the increased piglet ADG to the improved sow milk yield caused by enhanced DM and N digestibility, however, milk production was not assessed in their study. The reason for the discrepancy between the current study and the study by Upadhaya and coworkers is not clear. One possible reason is the difference in diets and enzymes applied; another likely reason is the much greater litter size weaned in our study (12.9 vs. 10.5).
The xylanase supplementation increased the TAG concentration in lactating sows. The possible explanation could be that the fat digestibility was increased with xylanase addition, although this was not measured in this study. According to Kiarie et al. (2016), a greater apparent ileal digestibility (P < 0.05) of fat was observed in growing pigs fed either corn- or wheat-based diets supplemented with xylanase. Effects of xylanase supplementation on fat digestibility in lactating sows, therefore, warrant further scrutiny. Another possible explanation for the elevated TAG is that additional energy was net absorbed in sows fed the enzyme added diet and more de novo fat might be synthesized by the liver and released to peripheral blood.
In conclusion, the present study shows that supplementing a mono-component xylanase to wheat–barley based lactation diet improved sow feed intake and nutrient digestibility, thereby reducing sow BW loss throughout lactation, whereas no significant benefits were found on piglet performance or sow milk yield. This study may open up for opportunities to increase nutrient digestibility, feed efficiency, and performance, and to reduce nutrient loss of lactating sows using xylanase.
Footnotes
This research was funded by DSM Nutritional Products, Kaiseraugst, Switzerland. Pan Zhou received a scholarship provided by China Scholarship Council (CSC).
LITERATURE CITED
- AOAC 1990. Official methods of analysis. vol. 1, 15th ed. Arlington (VA): Association of Official Analytical Chemists. [Google Scholar]
- Bach Knudsen K. E. 1997. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol. 67:319–338. doi:10.1016/S0377-8401(97)00009-6 [Google Scholar]
- Bach Knudsen K. E. 2014. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 93:2380–2393. doi:10.3382/ps.2014–03902 [DOI] [PubMed] [Google Scholar]
- Bach Knudsen K. E., and Hansen I.. 1991. Gastrointestinal implications in pigs of wheat and oat fractions. Br. J. Nutr. 65:217–232. doi:10.1079/BJN19910083 [DOI] [PubMed] [Google Scholar]
- Barrera M., Cervantes M., Sauer W. C., Araiza A. B., and Torrentera N.. 2004. Ileal amino acid digestibility and performance of growing pigs fed wheat-based diets supplemented with xylanase. J. Anim. Sci. 82:1997–2003. doi:10.2527/2004.8271997x [DOI] [PubMed] [Google Scholar]
- Bedford M. R., and Schulze H.. 1998. Exogenous enzymes for pigs and poultry. Nutr. Res. Rev. 11:91–114. doi:10.1079/NRR19980007 [DOI] [PubMed] [Google Scholar]
- Bedford M. R., Scott T. A., Silversides F. G., Classen H. L., Swift M. L., and Pack M.. 1998. The effect of wheat cultivar, growing environment, and enzyme supplementation on digestibility of amino acids by broilers. Can. J. Anim. Sci. 78:335–342. doi:10.4141/A98-012 [Google Scholar]
- Campbell G. L., and Bedford M. R.. 1992. Enzyme applications for monogastric feeds: a review. Can. J. Anim. Sci. 72:449–466. doi:10.4141/cjas92-058 [Google Scholar]
- Cowieson A. J. 2016. Meta-analysis of effect of a mono-component xylanase on the nutritional value of wheat supplemented with exogenous phytase for broiler chickens. Anim. Prod. Sci. 56:2014–2022. doi:10.1071/AN15199 [Google Scholar]
- de Souza A. L. P., Lindemann M. D., and Cromwell G. L.. 2007. Supplementation of dietary enzymes has varying effects on apparent protein and amino acid digestibility in reproducing sows. Livest. Sci. 109:122–124. doi:10.1016/j.livsci.2007.01.113 [Google Scholar]
- Diebold G., Mosenthin R., Piepho H., and Sauer W. C.. 2004. Effect of supplementation of xylanase and phospholipase to a wheat-based diet for weanling pigs on nutrient digestibility and concentrations of microbial metabolites in ileal digesta and feces. J. Anim. Sci. 82:2647–2656. doi:10.2527/2004.8292647x [DOI] [PubMed] [Google Scholar]
- Dirkzwager A., Elbers A. R. W., Van der Aar P. J., and Vos J. H.. 1998. Effect of particle size and addition of sunflower hulls to diets on the occurrence of oesophagogastric lesions and performance in growing-finishing pigs. Livest. Prod. Sci. 56:53–60. doi:10.1016/S0301-6226(98)00143-2 [Google Scholar]
- Fang Z. F., Peng J., Liu Z. L., and Liu Y. G.. 2007. Responses of non-starch polysaccharide-degrading enzymes on digestibility and performance of growing pigs fed a diet based on corn, soya bean meal and Chinese double-low rapeseed meal. J. Anim. Physiol. Anim. Nutr. 91:361–368. doi:10.1111/j.1439-0396.2006.00664.x [DOI] [PubMed] [Google Scholar]
- Feyera T., Højgaard C. K., Vinther J., Bruun T. S., and Theil P. K.. 2017. Dietary supplement rich in fiber fed to late gestating sows during transition reduces rate of stillborn piglets. J. Anim. Sci. 95:5430–5438. doi:10.2527/jas2017.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesch M., Perez-Vendrell A. M., and Broz J.. 2012. Effects of a mono-component endo-xylanase supplementation on the nutritive value of wheat-based broiler diets. Br. Poult. Sci. 53:809–816. doi:10.1080/00071668.2012.750714 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ortiz G., Olukosi O., and Bedford M. R.. 2016. Evaluation of the effect of different wheats and xylanase supplementation on performance, nutrient and energy utilisation in broiler chicks. Anim. Nutr. 2:173–179. doi:10.1016/j.aninu.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen B. 1989. Determination of nitrogen as elementary N, an alternative to Kjeldahl. Acta Agric. Scand. 39:113–118. doi:10.1080/00015128909438504 [Google Scholar]
- Hansen A. V., Strathe A. B., Kebreab E., France J., and Theil P. K.. 2012. Predicting milk yield and composition in lactating sows: a Bayesian approach. J. Anim. Sci. 90:2285–2298. doi:10.2527/jas.2011–4788 [DOI] [PubMed] [Google Scholar]
- Inborr J., Schmitz M., and Ahrens F.. 1993. Effect of adding fibre and starch degrading enzymes to a barley/wheat based diet on performance and nutrient digestibility in different segments of the small intestine of early weaned pigs. Anim. Feed Sci. Technol. 44:113–127. doi:10.1016/0377-8401(93)90042-I [Google Scholar]
- Jang Y. D., Wilcock P., Boyd R. D., and Lindemann M. D.. 2016. Effect of combined xylanase and phytase supplementation on growth performance, carcass characteristics, and apparent total tract digestibility in pigs fed corn-based diets containing multiple by-products. J. Anim. Sci. 94:112. doi:10.2527/msasas2016-237 [DOI] [PubMed] [Google Scholar]
- Johansen H. N., Knudsen K. E. B., Sandstrom B., and Skjoth F.. 1996. Effects of varying content of soluble dietary fibre from wheat flour and oat milling fractions on gastric emptying in pigs. Br. J. Nutr. 75:339–351. doi:10.1079/BJN19960138 [DOI] [PubMed] [Google Scholar]
- Kiarie E., Nyachoti C. M., Slominski B. A., and Blank G.. 2007. Growth performance, gastrointestinal microbial activity, and nutrient digestibility in early-weaned pigs fed diets containing flaxseed and carbohydrase enzyme. J. Anim. Sci. 85:2982–2993. doi:10.2527/jas.2006–481 [DOI] [PubMed] [Google Scholar]
- Kiarie E., Walsh M. C., Romero L. F., and Baidoo S. K.. 2016. Digestibility responses of growing pigs fed corn plus corn distiller grains or wheat plus wheat coproduct-based diets without or with supplemental xylanase. J. Anim. Sci. 94:211–214. doi:10.2527/jas.2015–9736 [Google Scholar]
- Kim J. C., Simmins P. H., Mullan B. P., and Pluske J. R.. 2005. The digestible energy value of wheat for pigs, with special reference to the post-weaned animal. Anim. Feed Sci. Technol. 122:257–287. doi:10.1016/j.anifeedsci.2005.02.022 [Google Scholar]
- Krogh U., Bruun T. S., Poulsen J., and Theil P. K.. 2017a. Impact of fat source and dietary fibers on feed intake, plasma metabolites, litter gain and the yield and composition of milk in sows. Animal. 11:975–983. doi:10.1017/S1751731116002585 [DOI] [PubMed] [Google Scholar]
- Krogh U., Oksbjerg N., Purup S., Ramaekers P., and Theil P.. 2016. Colostrum and milk production in multiparous sows fed supplementary arginine during gestation and lactation. J. Anim. Sci. 94:22–25. doi:10.2527/jas.2015–9491 [DOI] [PubMed] [Google Scholar]
- Krogh U., Oksbjerg N., Storm A. C., Feyera T., and Theil P. K.. 2017b. Mammary nutrient uptake in multiparous sows fed supplementary arginine during gestation and lactation. J. Anim. Sci. 95:2517–2532. doi:10.2527/jas.2016.1291 [DOI] [PubMed] [Google Scholar]
- Le D. M., Fojan P., Azem E., Pettersson D., and Pedersen N. R.. 2013. Visualization of the anticaging effect of Ronozyme WX xylanase on wheat substrates. Cereal Chem. 90:439–444. doi:10.1094/CCHEM-10-12-0130-R [Google Scholar]
- Liermann W., Berk A., Böschen V., and Dänicke S.. 2015. Effects of particle size and hydro-thermal treatment of feed on performance and stomach health in fattening pigs. Arch. Anim. Nutr. 69:455–472. doi:10.1080/1745039X.2015.1087748 [DOI] [PubMed] [Google Scholar]
- Liermann W., Berk A., Böschen V., and Dänicke S.. 2016. Effects of diets differing in protein source and technical treatment on digestibility, performance, and visceral and biochemical parameters of fattening pigs. Arch. Anim. Nutr. 70:190–208. doi:10.1080/1745039X.2016.1157983 [DOI] [PubMed] [Google Scholar]
- Lordelo M. M., Cunha L. F., and Freire J. P. B.. 2008. Effects of dietary fibre source and enzyme supplementation on faecal apparent digestibility, short chain fatty acid production and activity of bacterial enzymes in the gut of piglets. Anim. Feed Sci. Technol. 146:124–136. doi:10.1016/j.anifeedsci.2007.12.001 [Google Scholar]
- Mavromichalis I., Hancock J. D., Senne B. W., Gugle T. L., Kennedy G. A., Hines R. H., and Wyatt C. L.. 2000. Enzyme supplementation and particle size of wheat in diets for nursery and finishing pigs. J. Anim. Sci. 78:3086–3095. doi:10.2527/2000.78123086x [DOI] [PubMed] [Google Scholar]
- Millet S., Kumar S., De Boever J., Meyns T., Aluwé M., De Brabander D., and Ducatelle R.. 2012. Effect of particle size distribution and dietary crude fibre content on growth performance and gastric mucosa integrity of growing-finishing pigs. Vet. J. 192:316–321. doi:10.1016/j.tvjl.2011.06.037 [DOI] [PubMed] [Google Scholar]
- Möβeler A., Wintermann M., Sander S. J., and Kamphues J.. 2012. Effect of diet grinding and pelleting fed either dry or liquid feed on dry matter and pH in the stomach of pigs and the development of gastric ulcers. J. Anim. Sci. 90:343–345. doi:10.2527/jas.53772 [DOI] [PubMed] [Google Scholar]
- Nasir Z., Wang L. F., Young M. G., Swift M. L., Beltranena E., and Zijlstra R. T.. 2015. The effect of feeding barley on diet nutrient digestibility and growth performance of starter pigs. Anim. Feed Sci. Technol. 210:287–294. doi:10.1016/j.anifeedsci.2015.10.014 [Google Scholar]
- Nortey T. N., Patience J. F., Sands J. S., Trottier N. L., and Zijlstra R. T.. 2008. Effects of xylanase supplementation on the apparent digestibility and digestible content of energy, amino acids, phosphorus, and calcium in wheat and wheat by-products from dry milling fed to grower pigs. J. Anim. Sci. 86:3450–3464. doi:10.2527/jas.2007-0472 [DOI] [PubMed] [Google Scholar]
- Nortey T. N., Patience J. F., Simmins P. H., Trottier N. L., and Zijlstra R. T.. 2007. Effects of individual or combined xylanase and phytase supplementation on energy, amino acid, and phosphorus digestibility and growth performance of grower pigs fed wheat-based diets containing wheat millrun. J. Anim. Sci. 85:1432–1443. doi:10.2527/jas.2006–613 [DOI] [PubMed] [Google Scholar]
- O’Connell J. M., Sweeney T., Callan J. J., and O’Doherty J. V.. 2005. The effect of cereal type and exogenous enzyme supplementation in pig diets on nutrient digestibility, intestinal microflora, volatile fatty acid concentration and manure ammonia emissions from finisher pigs. Anim. Sci. 81:357–364. doi:10.1079/ASC42040357 [Google Scholar]
- Oliviero C., Kokkonen T., Heinonen M., Sankari S., and Peltoniemi O.. 2009. Feeding sows with high fibre diet around farrowing and early lactation: impact on intestinal activity, energy balance related parameters and litter performance. Res. Vet. Sci. 86:314–319. doi: 10.1016/j.rvsc.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Partridge G. G., and Bedford M. R.. 2001. The role and efficacy of carbohydrase enzymes in pig nutrition. In: M. R. Bedford and G. G. Partridge, editors. Enzymes in Farm Animal Nutrition. Wallingford (UK): CABI Publishing; p. 161–198. [Google Scholar]
- Patience J. F., Bedford M. R., Classen H. L., and Inborr J.. 1992. The effect of dietary enzyme supplementation of rye-and barley-based diets on digestion and subsequent performance in weanling pigs. Can. J. Anim. Sci. 72:97–105. doi:10.4141/cjas92-011 [Google Scholar]
- Ravn J. L., Martens H. J., Pettersson D., and Pedersen N. R.. 2016. A commercial GH 11 xylanase mediates xylan solubilisation and degradation in wheat, rye and barley as demonstrated by microscopy techniques and wet chemistry methods. Anim. Feed Sci. Technol. 219:216–225. doi:10.1016/j.anifeedsci.2016.06.020 [Google Scholar]
- Saqui-Salces M., Luo Z., Urriola P. E., Kerr B. J., and Shurson G. C.. 2017. Effect of dietary fiber and diet particle size on nutrient digestibility and gastrointestinal secretory function in growing pigs. J. Anim. Sci. 95:2640–2648. doi:10.2527/jas2016.1249 [DOI] [PubMed] [Google Scholar]
- Schürch A. F., Lloyd L. E., and Crampton E. W.. 1950. The use of chromic oxide as an index for determining the digestibility of a diet. J. Nutr. 41:629–636. [DOI] [PubMed] [Google Scholar]
- Steenfeldt S., and Pettersson D.. 2001. Improvements in nutrient digestibility and performance of broiler chickens fed a wheat-and-rye based diet supplemented with enzymes. J. Anim. Feed Sci. 10:143–158. doi:10.22358/jafs/67952/2001 [Google Scholar]
- Stein H. H., Seve B., Fuller M. F., Moughan P. J., and De Lange C.. 2007. Invited review: Amino acid bioavailability and digestibility in pig feed ingredients: terminology and application. J. Anim. Sci. 85:172–180. doi:10.2527/jas.2005–742 [DOI] [PubMed] [Google Scholar]
- Stoldt W. 1952. Vorschlag zur vereinheitlichung der fettbestimmung in lebensmitteln. Eur. J Lipid Sci. Technol. 54:206–207. doi:10.1002/lipi.19520540406 [Google Scholar]
- Strathe A. V., Bruun T. S., and Hansen C. F.. 2017. Sows with high milk production had both a high feed intake and high body mobilization. Animal. 15:1–9. doi:10.1017/S1751731117000155 [DOI] [PubMed] [Google Scholar]
- Strathe A. V., Bruun T. S., Zerrahn J.-E., Tauson A.-H., and Hansen C. F.. 2016. The effect of increasing the dietary valine-to-lysine ratio on sow metabolism, milk production, and litter growth. J. Anim. Sci. 94:155–164. doi:10.2527/jas.2015–9267 [DOI] [PubMed] [Google Scholar]
- Tapingkae W., Yachai M., Visessanguan W., Pongtanya P., and Pongpiachan P.. 2008. Influence of crude xylanase from Aspergillus niger FAS128 on the in vitro digestibility and production performance of piglets. Anim. Feed Sci. Technol. 140:125–138. doi:10.1016/j.anifeedsci.2007.02.001 [Google Scholar]
- Thacker P. A., Campbell G. L., and GrootWassink J.. 1991. The effect of enzyme supplementation on the nutritive value of rye-based diets for swine. Can. J. Anim. Sci. 71:489–496. doi:10.4141/cjas91-058 [Google Scholar]
- Thaker M., and Bilkei G.. 2005. Lactation weight loss influences subsequent reproductive performance of sows. Anim. Reprod. Sci. 88:309–318. doi:10.1016/j.anireprosci.2004.10.001 [DOI] [PubMed] [Google Scholar]
- The Danish Ministry of Justice 1995. Animal Testing ACT, Consolidation ACT No. 726 of September 9, 1993 (as amended by Act No.1081 of December 20, 1995). [Google Scholar]
- Theander O., Aman P., Westerlund E., and Graham H.. 1993. Enzymatic/chemical analysis of dietary fiber. J. AOAC Int. 77:703–709. [PubMed] [Google Scholar]
- Theil P. K., Flummer C., Hurley W. L., Kristensen N. B., Labouriau R. L., and Sørensen M. T.. 2014. Mechanistic model to predict colostrum intake based on deuterium oxide dilution technique data and impact of gestation and pre-farrowing diets on piglet intake and sow yield of colostrum. J. Anim. Sci. 92:5507–5519. doi:10.2527/jas.2014–7841 [DOI] [PubMed] [Google Scholar]
- Theil P. K., Jørgensen H., Jakobsen K.. 2004. Energy and protein metabolism in lactating sows fed two levels of dietary fat. Livest. Prod. Sci. 89:265–276. doi:10.1016/j.livprodsci.2004.01.001 [Google Scholar]
- Theil P. K., Nielsen M. O., Sørensen M. T., and Lauridsen C.. 2012. Lactation, milk and suckling. Nutritional physiology of pigs. Copenhagen (Denmark): Danish Pig Research Centre, p. 1–47. [Google Scholar]
- Tsai T., Dove C. R., Cline P. M., Owusu-Asiedu A., Walsh M. C., and Azain M.. 2017. The effect of adding xylanase or β-glucanase to diets with corn distillers dried grains with solubles (CDDGS) on growth performance and nutrient digestibility in nursery pigs. Livest. Sci. 197:46–52. doi:10.1016/j.livsci.2017.01.008 [Google Scholar]
- Tybirk P., Sloth N. M., and Jørgensen L.. 2015. Danish nutrient requirement standards (In Danish: Normer for næringsstoffer). 20th ed. Axelborg (Denmark): SEGES Pig Research Centre. [Google Scholar]
- Upadhaya S. D., Lee S. I., and Kim I. H.. 2016. Effects of cellulase supplementation to corn soybean meal-based diet on the performance of sows and their piglets. Anim. Sci. J. 87:904–910. doi:10.1111/asj.12511 [DOI] [PubMed] [Google Scholar]
- Vahjen W., Osswald T., Schäfer K., and Simon O.. 2007. Comparison of a xylanase and a complex of non starch polysaccharide-degrading enzymes with regard to performance and bacterial metabolism in weaned piglets. Arch. Anim. Nutr. 61:90–102. doi:10.1080/17450390701203881 [DOI] [PubMed] [Google Scholar]
- Van den Brand H., Dieleman S. J., Soede N. M., and Kemp B.. 2000. Dietary energy source at two feeding levels during lactation of primiparous sows: I. Effects on glucose, insulin, and luteinizing hormone and on follicle development, weaning-to-estrus interval, and ovulation rate. J. Anim. Sci. 78:396–404. doi:10.2527/2000.782396x [DOI] [PubMed] [Google Scholar]
- Vesseur P. C., Kemp B., and den Hartog L. A.. 1994. Factors affecting the weaning-to-estrus interval in the sow. J. Anim. Physiol. Anim. Nutr. 72:225–233. doi:10.1111/j.1439-0396.1994.tb00391.x [Google Scholar]
- Vinsky M. D., Novak S., Dixon W. T., Dyck M. K., and Foxcroft G. R.. 2006. Nutritional restriction in lactating primiparous sows selectively affects female embryo survival and overall litter development. Reprod. Fertil. Dev. 18:347–355. doi:10.1071/RD05142 [DOI] [PubMed] [Google Scholar]
- Walsh M. C., Geraert P. A., Maillard R., Kluess J., and Lawlor P. G.. 2012. The effect of a non-starch polysaccharide-hydrolysing enzyme (Rovabio® Excel) on feed intake and body condition of sows during lactation and on progeny growth performance. Animal 6:1627–1633. doi:10.1017/S1751731112000237 [DOI] [PubMed] [Google Scholar]
- Weast R. C., Astle M. J., and Beyer W. H.. 1984. CRC handbook of chemistry and physics. Boca Raton (FL): CRC Press, Inc. [Google Scholar]
- Wenk C. 2001. The role of dietary fibre in the digestive physiology of the pig. Anim. Feed Sci. Technol. 90:21–33. doi:10.1016/S0377-8401(01)00194-8 [Google Scholar]
- Wolf P., Rust P., and Kamphues J.. 2010. How to assess particle size distribution in diets for pigs?Livest. Sci. 133:78–80. doi:10.1016/j.livsci.2010.06.030 [Google Scholar]
- Woyengo T. A., Sands J. S., Guenter W., and Nyachoti C. M.. 2008. Nutrient digestibility and performance responses of growing pigs fed phytase- and xylanase-supplemented wheat-based diets. J. Anim. Sci. 86:848–857. doi:10.2527/jas.2007-0018 [DOI] [PubMed] [Google Scholar]
- Yin Y., Baidoo S. K., Jin L. Z., Liu Y. G., Schulze H., and Simmins P. H.. 2001. The effect of different carbohydrase and protease supplementation on apparent (ileal and overall) digestibility of nutrients of five hulless barley varieties in young pigs. Livest. Prod. Sci. 71:109–120. doi:10.1016/S0301-6226(01)00215-9 [Google Scholar]
- Yin Y., McEvoy J. D. G., Schulze H., Hennig U., Souffrant W. -B., and McCracken K. J.. 2000. Apparent digestibility (ileal and overall) of nutrients and endogenous nitrogen losses in growing pigs fed wheat (var. Soissons) or its by-products without or with xylanase supplementation. Livest. Prod. Sci. 62:119–132. doi:10.1016/S0301-6226(99)00129-3 [Google Scholar]
- Zak L. J., Cosgrove J. R., Aherne F. X., and Foxcroft G. R.. 1997. Pattern of feed intake and associated metabolic and endocrine changes differentially affect postweaning fertility in primiparous lactating sows. J. Anim. Sci. 75:208–216. doi:10.2527/1997.751208x [DOI] [PubMed] [Google Scholar]
- Zijlstra R. T., Li S., Owusu-Asiedu A., and Patience J. F.. 2004. Effect of carbohydrase supplementation to wheat-canola meal diets on growth performance and nutrient digestibility in group-housed weaned pigs. Can. J. Anim. Sci. 84:689–695. doi:10.4141/A03-127 [Google Scholar]
- Zhang W., Li D., Liu L., Zang J., Duan Q., Yang W., and Zhang L.. 2013. The effects of dietary fiber level on nutrient digestibility in growing pigs. J. Anim. Sci. Biotechnol. 4:17. doi:10.1186/2049-1891-4-17 [DOI] [PMC free article] [PubMed] [Google Scholar]