Abstract
Livestock on the Qinghai-Tibetan Plateau are faced with extreme harsh winters and are often in negative energy balance during this period. Dietary supplementation can improve growth performance of Tibetan sheep and, consequently, we hypothesized that it would also increase microbial abundance and rumen epithelium development. To test this hypothesis, we examined the effect of feed supplementation during the cold season on rumen microbes, fermentation, epithelium development, and absorptive capability in Tibetan sheep. Eighteen 1-yr-old ewes (BW = 29.4 ± 1.79, kg) were offered oat hay ad libitum for 60 d and divided randomly into three groups: 1) no supplement; control group (CON); 2) urea-molasses lick block supplement (BS); and 3) concentrate feed supplement (CS). The ADG of CS ewes (143.3, g/d) was greater (P < 0.05) than BS ewes (87.9, g/d), which was greater (P < 0.05) than CON ewes (44.5, g/d). Serum concentrations of GH, IGF-1, and IGF-2 in the CS and BS groups were greater than in the CON group (P < 0.05). Greater relative abundance of protozoa, Ruminococcus albus, Fibrobacter succinogenes, Streptococcus bovis, and Ruminobacter amylophilus was observed in the CS and BS groups than in the CON group (P < 0.05), and relative abundances of rumen fungi, Butyrivibrio fibrisolvens, and Prevotella ruminicola in the CS group were greater than in the BS and CON groups (P < 0.05). Ruminal total VFA, ammonia, and microbial protein concentrations in the CS and BS groups were greater than in the CON group (P < 0.05), and in the CS group were greater than in the BS group (P < 0.05). Ruminal papillae width and surface area in the CS and BS groups were greater than in the CON group (P < 0.05), while in the CS group were greater than in the BS group (P < 0.05). The mRNA expressions of IGFBP5, NHE1 (sodium/hydrogen antiporter, isoform 1), DRA (downregulated in adenoma), and Na+/K+-ATPase (sodium/potassium ATPase pump) in ruminal epithelium were greater in the CS and BS groups than in the CON group (P < 0.05), and in the CS group was greater than in the BS group (P < 0.05), while NHE3 (sodium/hydrogen antiporter, isoform 3), MCT1 (monocarboxylate transporter 1), and MCT4 (monocarboxylate transporter 4) mRNA expressions in the CS group were greater than in the BS and CON groups (P < 0.05). It was concluded that supplementing Tibetan sheep during the cold season increases rumen microbial abundance and improves fermentation parameters, rumen epithelium development, and absorptive capability.
Keywords: absorptive capability, rumen morphology, ruminal microbes, supplementation in cold season, Tibetan sheep
INTRODUCTION
Tibetan sheep (Ovies aries), numbering approximately 50 million animals, are well-adapted to the conditions of the Qinghai-Tibetan Plateau (Zhou et al., 2015) and play a vital role in the livelihoods of Tibetan pastoralists (Li et al., 2015). Under traditional management, Tibetan sheep graze on rangeland all year-round without receiving feed supplements. The large increase in livestock numbers over the years has led to overgrazing of the pasture and degradation of the grasslands (Shang et al., 2014). During the long cold season, there generally is a severe shortage of herbage, and Tibetan sheep often lose BW (Dong et al., 2003; Xin et al., 2011; Sun et al., 2015). Severe snowfalls can prevent the livestock from grazing and can cause the death of large numbers of animals (Shang et al., 2012). Supplementary feed can improve the reproductive capability of Tibetan sheep (Jing et al., 2017) and can also improve their ADG or reduce their weight loss during the harsh winter (Liu et al., 2007; Feng et al., 2013; Jing et al., 2017). Dietary nutrients intakes not only affect animal performance, but also affect the rumen microbial community, epithelial development, and absorptive capabilities of the rumen in ruminants (Shen et al., 2004; Kamra, 2005; Pitta et al., 2010). However, little is known of how feed supplementation during the cold season affects the rumen microbial community and rumen epithelium development in Tibetan sheep. We hypothesize that feed supplementation to Tibetan sheep during the cold season would improve their rumen microbial community structure and rumen epithelial development, and, consequently, increase the ruminal fermentative and absorptive capabilities. To test this hypothesis, Tibetan sheep were offered oat hay with or without supplementary feed during the cold season, and BW change, the abundance of ruminal microbes, rumen fermentation, rumen epithelium morphology, and absorptive capability were examined.
MATERIALS AND METHODS
The experimental procedures in this research were approved by the Institutional Animal Care Unit of Sichuan Agricultural University.
Study Location
The experiment was conducted at the Haibei Demonstration Zone of the Plateau Modern Ecological Husbandry Science and Technology (6°44′–7°39′N, 100°23′–101°20′E; 3,200 m a.s.l.), Xihai Town, Haiyan County, Haibei Tibetan Autonomous Prefecture of Qinghai Province, China. Traditional husbandry is practiced in this area in which the livestock, mainly Tibetan sheep and yaks, only graze the grasslands. In this area, overgrazing over an extended period resulted in rangeland degradation, a process which has been increasing in recent years. The site is characterized by extreme harsh, cold conditions, with a semiarid plateau monsoon climate, having an average annual precipitation of about 400 mm and annual sunshine of about 2,980 h. During the experiment, the highest and lowest air temperatures in the sheep shelter were 12 °C and −25 °C, respectively.
Animals and Experimental Design
Eighteen healthy, 1-yr-old Tibetan ewes (29.4 ± 1.79, kg) were purchased from a Tibetan herder in the surrounding area of Haibei Demonstration Zone of Plateau Modern Ecological Husbandry Science and Technology. Before this study, the sheep grazed only natural pasture and were not offered supplements. The sheep were assigned randomly to one of three groups and were offered oat hay and water ad libitum throughout the experiment. The first group did not receive supplements and served as controls (CON); the second group was supplemented with a urea-molasses lick block (Table 1) ad libitum (BS); and the third group was supplemented with 200 g/d concentrate (Table 1) per sheep (CS). The composition of the oat hay, urea-molasses lick block, and concentrate is presented in Table 1. The experiment took place over 60 d (February 28, 2014 to April 28, 2014) after 15 d of adaptation to the experimental diets and conditions.
Table 1.
Composition of oat hay, urea-molasses lick block, and concentrate (DM basis)
| Item | Lick block | Concentrate | Oat hay |
|---|---|---|---|
| Ingredient, % of DM | |||
| Corn | 15.7 | 55.6 | - |
| Wheat bran | 6.50 | 17.5 | - |
| Rapeseed dregs | 5.00 | 16.6 | - |
| Soybean meal | 0 | 5.20 | - |
| Molasses | 13.5 | 0 | - |
| Urea | 8.00 | 0 | - |
| Rapeseed oil | 0 | 1.00 | - |
| Sodium chloride | 17.0 | 1.00 | - |
| Calcium carbonate | 2.50 | 0.6 | - |
| Calcium hydrophosphate | 1.00 | 0.4 | - |
| Sodium thiosulfate | 1.00 | 0 | - |
| Sodium bicarbonate | 5.00 | 1.00 | - |
| Compound vitamin | 0.90 | - | |
| Monensin | 0.50 | 0.1 | - |
| Commercial premixa | 1.00 | 1.00 | - |
| Bentonite | 8.40 | 0 | - |
| Portland cement | 14.0 | 0 | - |
| Composition, % of DM | |||
| DEb, MJ/kg | 7.24 | 14.05 | 10.4 |
| CP, g/kg | 325 | 171 | 100 |
| ADF, g/kg | 20.3 | 74.8 | 390 |
| NDF, g/kg | 46.7 | 167 | 630 |
aCommercial premix of lick block provided the following per kg of diets: Fe 524 mg; Cu 275 mg; Mn 1,288 mg; Zn 839 mg; I 10.3 mg; Se 1.10 mg; Co 2.20 mg; commercial premix of concentrate provided the following per kg of diets: Vitamin A 1700 IU; Vitamin D 190 IU; Vitamin E 18 IU; Fe 90 mg; Cu 20 mg; Mn 42 mg; Zn 48 mg; I 1.20 mg; Se 0.16 mg; Co 0.15 mg.
bDE = digestible energy, was a calculated value from the Feeding Standard of Meat-producing Sheep and Goats of China, NY/T 816-2004 (Ministry of Agriculture, MOA, PRC, 2004).
Animals Management
Each sheep was maintained in an individual pen (2 m × 4 m), equipped with a water tank and feed trough. The pens were under a three-sided roofed shelter, and thus the sheep were always exposed to outside temperatures. The sheep were fed twice daily, at 0800 and at 1600, and oat hay intake was recorded each day. The lick blocks were weighed every week to calculate the average daily intake. Concentrate feed (200, g/ewe) was offered the CS sheep at 0800 h each day. Sheep were weighed on d 0, 30, and 60 before feeding and ADG was calculated.
Hormones Determination
Ten milliliters of jugular blood were collected in glass tubes (Shanghai Kehua Bio-Engineering Co., Ltd, Shanghai, China) before feeding in the morning on d 0, 30, and 60. The tubes were maintained in a slanted position for 15 min, followed by centrifugation at 2,000 × g for 10 min at 4 °C. Serum samples were stored in 1.5 mL tubes (Eppendorf, GCS, New York, NY) at −80 °C till analysis. Concentrations of serum GH, IGF-1, and IGF-2 were determined by commercial ELISA kits (Shanghai Lengton Bioscience Co., Ltd, Shanghai, China).
Rumen Morphological Examination
At the end of the trial (on d 61), all sheep were slaughtered humanely 2 to 3 h after morning feeding (Metzler-Zebeli et al., 2013) and ruminal dorsal sac samples (2 × 2 cm) were collected immediately from each sheep. Samples were rinsed with physiological saline, then fixed in 4% (vol/vol) formalin solution, dehydrated with absolute ethanol (10%, 20%, 30%, 50%, 70%, 85%, 95%, and 100%), cleared with xylene (25%, 50%, 75%, and 100%, prepared with ethanol) twice, and saturated with and embedded in paraffin (Shen et al., 2004; Wang et al. 2009). The blocks were cut into 5-μm sections using a rotary microtome (RM2235, Leica, Germany) and the sections (four slices of each sample) were stained by hematoxylin and eosin (H&E). Ten images per slice in random fields were examined using a Nikon microscope (Eclipse E400, Tokyo, Japan). Average rumen papillae height, width, and area in each image were determined by Image-Pro Plus 6.0 software and the number of papillae in 1 cm2 was counted, according to the method of Wang et al. (2009) and Melo et al. (2013).
Rumen Fermentation Parameters
Rumen fluids were collected immediately after slaughter and filtered through four layers of cheesecloth (Hristov et al., 2001; Bailey et al., 2012). The pH was measured immediately using a pH electrode (Model PHS-3C, Shanghai Precision & Scientific Instrument Co., Ltd, Shanghai, China). Rumen fluid samples of 10 mL were snap-frozen in liquid nitrogen and then stored at −80 °C for subsequent analysis. For analysis of VFA concentrations, ruminal fluid samples were thawed and centrifuged at 20,000 × g for 10 min (Hristov et al., 2001), and then analyzed using a Varian CP-3800 gas chromatograph (Varian Inc., Palo Alto, CA). Samples were injected into a CP-FFAP capillary column (25 m × 0.32 mm i.d. × 0.3 μm film thickness, Varian Inc., Palo Alto, CA) and were run at a split vent flow of 40 mL/min, air flow of 450 mL/min, make-up gas flow of 35 mL/min, with a capillary column temperature of 100 °C for 1 min, and then increased to 190 °C at a rate of 20 °C/min and held for 3 min at 190 °C. The injection port temperature was 220 °C, and the flame ionization detector temperature was 250 °C. Crotonic acid was used as internal standard. Peak integration was determined using Galaxie Software (Varian Inc., Palo Alto, CA). The ammonia and microbial protein (MCP) content were quantified by commercial kits (Nanjing Jian Cheng Bioengineering Institute, China).
DNA Extraction and Abundance of Rumen Microbes
Rumen fluid samples were slow thawed at 4 °C, centrifuged at 500 × g for 15 min at 4 °C to remove feed particles while keeping bacterial cells, then the total genomic DNA was extracted using the TIANamp Bacterial DNA Kit (TIANGEN BIOTECH, Beijing, Co., Ltd, Beijing, China) according to the manufacturer’s protocol. DNA concentration was estimated by spectrophotometry at a wavelength of 260 and 280 nm. The quality of RNA was also checked by 1.0% agarose gel electrophoresis. The DNA was amplified by real-time PCR using a SYBR Green real-time PCR master mix kit (Takara Biotechnology Co., Ltd, Dalian, China) with the BIO-RAD CFX96 (Hercules, CA) in a total volume of 12.5 μL. The PCR plate was incubated at 95 °C for 2 min, followed by 39 cycles of 95 °C for 10 s, annealing at a temperature of each primer for 30 s and 72 °C for 30 s. Total microbial DNA was diluted to 1:10 before use in real-time PCR assays. The primers of total bacterial, total fungi, total protozoa, Ruminococcus albus, Ruminococcus flavefaciens, Fibrobacter succinogenes, Butyrivibrio fibrisolvens, Streptococcus bovis, Prevotella ruminicola, Ruminobacter amylophilus are shown in Table 2. The oligonucleotides were synthesized by Takara Biotechnology Co., Ltd, Dalian, China. Quantification for bacteria was expressed as a proportion of total rumen bacterial 16S rDNA according to the equation: relative quantification = 2−(Ct target − Ct total bacterial), where Ct represents threshold cycle (Chen et al., 2008).
Table 2.
Primer sequences and parameters
| Target | Primer sequences (5′→3′) | Products size, bp | T a a, °C | Reference |
|---|---|---|---|---|
| Total bacterial | Fb: CGGCAACGAGCGCAACCC | 130 | 60.0 | Denman and Mcsweeney (2006) |
| Rc: CCATTGTAGCACGTGTGTAGCC | ||||
| Total fungi | F: GCACTTCATTGTGTGTACTG | 120 | 60.0 | Denman and Mcsweeney (2006) |
| R: GGATGAAACTCGTTGACTTC | ||||
| Total protozoa | F: GCTTTCGWTGGTAGTGTATT | 223 | 55.0 | Sylvester et al. (2004) |
| R: CTTGCCCTCYAATCGTWCT | ||||
| Ruminococcus albus | F: CCCTAAAAGCAGTCTTAGTTCG | 176 | 55.0 | Koike and Kobayashi (2001) |
| R: CCTCCTTGCGGTTAGAACA | ||||
| Ruminococcus flavefaciens | F: CGAACGGAGATAATTTGAGTTTACTTAGG | 132 | 60.0 | Denman and Mcsweeney (2006) |
| R: CGGTCTCTGTATGTTATGAGGTATTACC | ||||
| Fibrobacter succinogenes | F: GTTCGGAATTACTGGGCGTAAA | 121 | 60.0 | Denman and Mcsweeney (2006) |
| R: CGCCTGCCCCTGAACTATC | ||||
| Butyrivibrio fibrisolvens | F: TCTGGAAACGGATGGTA | 295 | 55.0 | Koike and Kobayashi (2001) |
| R: CCTTTAAGACAGGAGTTTACAA | ||||
| Streptococcus bovis | F: TTCCTAGAGATAGGAAGTTTCTTCGG | 127 | 60.0 | Stevenson and Weimer (2007) |
| R: ATGATGGCAACTAACAATAGGGGT | ||||
| Prevotella ruminicola | F: GGTTATCTTGAGTGAGTT | 485 | 53.0 | Tajima et al. (2001) |
| R: CTGATGGCAACTAAAGAA | ||||
| Ruminobacter amylophilus | F: CAACCAGTCGCATTCAGA | 642 | 57.0 | Tajima et al. (2001) |
| R: CACTACTCATGGCAACAT |
a T a = optimal PCR annealing temperature.
bF = forward.
cR = reverse.
RNA Extraction and mRNA Expression Determination
Immediately after slaughter, epithelium samples of the ruminal dorsal sacs were collected, repeatedly rinsed with physiological saline, cut into small pieces, placed into 1.5 mL tubes (Eppendorf, GCS, New York, NY), snap-frozen in liquid nitrogen, and stored at −80 °C till RNA extraction. Total RNA was extracted using Trizol reagent (Takara Biotechnology Co., Ltd, Dalian, China) according to the manufacturer’s protocol, and measured spectrophotometrically at 260 and 280 nm. The ratio of light absorbance at 260 nm to that at 280 nm was between 1.8 and 2.0, indicating that samples were pure and clean. The quality of RNA was also checked by 1.0% agarose gel electrophoresis. RNA was extracted for reverse transcription reaction by Prime Script RT Reagent Kit (Takara Biotechnology Co., Ltd, Dalian, China) according to the manufacturer’s protocol. The resulting cDNA was diluted to 100 μL with RNase-free water and stored at −20 °C. The cDNA was amplified by real-time PCR using a SYBR Green real-time PCR master mix kit (Takara Biotechnology Co., Ltd, Dalian, China) with the BIO-RAD CFX96 (Hercules, CA) in a total volume of 12.5 μL. The PCR plate was incubated at 95 °C for 2 min, followed by 39 cycles of 95 °C for 10 s, annealing at a temperature of each primer for 30 s and 72 °C for 30 s. The IGFBP5, NHE1 (sodium/hydrogen antiporter, isoform 1), NHE3 (sodium/hydrogen antiporter, isoform 3), MCT1 (monocarboxylate transporter 1), MCT4 (monocarboxylate transporter 4), DRA (downregulated in adenoma), Na+/K+-ATPase (sodium/potassium ATPase pump), and β-actin primers were designed using Primer Premier 5.0 (PREMIER Biosoft International, Palo Alto, CA) (Table 3). The oligonucleotides were synthesized by Takara Biotechnology Co., Ltd, Dalian, China. The melting peaks of all samples were determined routinely by melting curve analysis to ascertain that only the expected products had been generated. Relative gene expression levels are presented as 2−∆∆Ct (Livak and Schmittgen, 2001).
Table 3.
Primers of fluorescent quantitation PCR
| Target genea | GenBank ID | Primer sequences (5′→3′) | Products size, bp | T a b, °C |
|---|---|---|---|---|
| IGFBP5 | NM-001129733.1 | Fc: CTGTGACCGCAAAGGGTTCTA | 160 | 56.0 |
| Rd: AACATTGCTGCTGTCAAAGGTG | ||||
| DRA | HM010763.1 | F: TCGTTGCCATCGGATTTCTCTT | 115 | 53.0 |
| R: CGCCACAATCTTCGTATTTCCAC | ||||
| MCT1 | AJ315929.1 | F: TGGCTGTCATGTACGGTGGA R: GCACAGTGTTACAGAAGGAAGCAG |
132 | 54.0 |
| MCT4 | XM-012186382.1 | F: TGGTGTCTGCGTCCTTCTGTG | 101 | 56.0 |
| R: GATGAGTGAGGGCTGGAAGTTG | ||||
| NHE1 | XM-004005085.2 | F: GCCGTCACTGTGGTCCTGTATC | 103 | 55.0 |
| R: ACACCACGAAGAAGCTCAGGAA | ||||
| NHE3 | XM-012097233.1 | F: GAAACAGCAGCATTCCCAACG | 130 | 55.0 |
| R: CCGCCACTCATCTCATCATCATA | ||||
| Na+/K+-ATPase | NM-001009360.1 | F: GAGAACGGCTTCCTCCCTAATC | 107 | 56.0 |
| R: TGTTCATAGGTCCACTGCTGCC | ||||
| β-actin | U39357.1 | F: ATCGGCAATGAGCGGTTCCR: GCGTAGAGGTCTTTGCGGATGT | 137 | 56.0 |
aDRA = downregulated in adenoma; MCT1 = monocarboxylate transporter 1; MCT4 = monocarboxylate transporter 4; NHE1 = sodium/hydrogen antiporter, isoform 1; NHE3 = sodium/hydrogen antiporter, isoform 3; Na+/K+-ATPase = sodium/potassium ATPase pump.
b T a = optimal PCR annealing temperature.
cF = forward.
dR = reverse.
Statistical Analysis
Data were analyzed with the Statistical Package for Social Science (SPSS, version 22.0, SPSS Inc., Chicago, IL). Single factor ANOVA was used to analyze the data, and Duncan’s multiple test was used to separate means where significant differences were found. A level of P < 0.05 was considered statistically significant.
RESULTS
Daily Intakes and BW Change
CP and DE intakes of the CS and BS groups were greater (P < 0.05) than the CON group, and the CS group was greater (P < 0.05) than the BS group (Table 4). The BW of the CS group was greater (P < 0.05) than both the CON and BS groups on d 60. The ADG of the CS group (143.3, g/d) was greater (P < 0.05) than the BS group (87.9, g/d) which was greater (P < 0.05) than the CON group (44.5, g/d) (Table 5).
Table 4.
The average daily intakes of Tibetan sheep
| Item | Treatmentsa | SEM | P-value | ||
|---|---|---|---|---|---|
| CON | BS | CS | |||
| Daily intake of dietsb | |||||
| Oat hay, kg | 1.09 | 1.12 | 0.98 | 0.039 | 0.169 |
| Block, g | 19.8 | ||||
| Concentrate, g | 185 | ||||
| Daily intake of chemical compositionc | |||||
| DM, kg/d | 0.96 | 1.07 | 1.17 | 0.041 | 0.112 |
| DEd, MJ/d | 9.94c | 11.07b | 12.89a | 0.390 | 0.001 |
| CP, kg/d | 0.10c | 0.12b | 0.14a | 0.011 | 0.007 |
| ADF, kg/d | 0.42 | 0.43 | 0.40 | 0.020 | 0.357 |
| NDF, kg/d | 0.68 | 0.70 | 0.65 | 0.021 | 0.453 |
a,b,cMeans in same row with different superscripts are different from each other (P < 0.05).
aTreatments: CON = control (no supplements); BS = supplemented with urea-molasses lick block; CS = supplemented with concentrate.
bDaily intake of diets were expressed as DM basis.
cDaily intake of chemical composition of DE, CP, ADF, and NDF were expressed as DM basis.
dDE = digestible energy, was a calculated value from the Feeding Standard of Meat-producing Sheep and Goats of China, NY/T 816-2004 (Ministry of Agriculture, MOA, PRC, 2004).
Table 5.
The growth performance of Tibetan sheep supplemented with urea-molasses lick block or concentrate in the cold season
| Item | Treatmentsa | SEM | P-value | ||
|---|---|---|---|---|---|
| CON | BS | CS | |||
| Initial BW, kg | 29.4 | 29.6 | 29.2 | 0.40 | 0.938 |
| Final BW, kg | 32.7b | 34.7b | 37.6a | 0.55 | 0.001 |
| ADG, g/d | 44.5c | 87.9b | 143.3a | 14.74 | <0.0001 |
a,b,cMeans in same row with different superscripts are different from each other (P < 0.05).
aTreatments: CON = control (no supplements); BS = supplemented with urea-molasses lick block; CS = supplemented with concentrate.
Serum Hormone Concentrations
The CS and BS groups had greater (P < 0.05) concentrations of GH, IGF-1, and IGF-2 than the CON group, on d 30 and d 60 (Table 6). In addition, the IGF-2 concentration in the CS group was greater (P < 0.05) than in the BS group on 60 d.
Table 6.
The serum GH, IGF-1, and IGF-2 concentrations in Tibetan sheep supplemented with urea-molasses lick block or concentrate in the cold season
| Item | Day | Treatmentsa | SEM | P-value | ||
|---|---|---|---|---|---|---|
| CON | BS | CS | ||||
| GH, ng/L | 0 | 316 | 312 | 315 | 3.42 | 0.916 |
| 30 | 264b | 337a | 321a | 11.63 | 0.001 | |
| 60 | 138b | 368a | 355a | 37.38 | <0.0001 | |
| IGF-1, ng/mL | 0 | 125 | 121 | 126 | 2.27 | 0.680 |
| 30 | 93b | 162a | 155a | 11.28 | <0.0001 | |
| 60 | 72b | 168a | 182a | 17.46 | <0.0001 | |
| IGF-2, ng/mL | 0 | 65 | 64 | 65 | 0.89 | 0.984 |
| 30 | 44b | 74a | 78a | 5.44 | <0.0001 | |
| 60 | 34c | 78b | 95a | 9.13 | <0.0001 | |
a,b,cMeans in same row with different superscripts are different from each other (P < 0.05).
aTreatments: CON = control (no supplements); BS = supplemented with urea-molasses lick block; CS = supplemented with concentrate.
The Abundance of Ruminal Microbes
Greater relative abundances of protozoa, R. albus, F. succinogenes, S. bovis, and R. amylophilus were observed in the CS and BS groups than in the CON group (P < 0.05); R. albus, S. bovis, and R. amylophilus in the CS group were greater (P < 0.05) than in the BS group, and protozoa in the BS group was greater (P < 0.05) than in the CS group (Table 7). Relative abundances of ruminal fungi, B. fibrisolvens, and P. ruminicola in the CS group were greater (P < 0.05) than in the BS and CON groups. The BS group had the greatest (P < 0.05) relative abundance of R. flavefaciens among groups.
Table 7.
The relative abundance of ruminal microbes in Tibetan sheep supplemented with urea-molasses lick block or concentrate in cold season.
| Item | Treatmentsa | SEM | P-value | ||
|---|---|---|---|---|---|
| CON | BS | CS | |||
| Rumen fungi | 0.0301b | 0.0257b | 0.0529a | 0.0045 | <0.0001 |
| Protozoa | 0.7781c | 1.7596a | 1.4387b | 0.1512 | 0.001 |
| Ruminococcus albus | 0.0041c | 0.0054b | 0.0069a | 0.0004 | 0.001 |
| Ruminococcus flavefaciens | 0.0157b | 0.0240a | 0.0123b | 0.0018 | 0.001 |
| Fibrobacter succinogenes | 0.0198b | 0.2683a | 0.3085a | 0.0456 | <0.0001 |
| Butyrivibrio fibrisolvens | 0.5698b | 0.6282b | 1.3083a | 0.1233 | <0.0001 |
| Streptococcus bovis | 0.1040c | 0.5379b | 1.5213a | 0.2129 | <0.0001 |
| Prevotella ruminicola | 0.0269b | 0.0337b | 2.2248a | 0.3671 | <0.0001 |
| Ruminobacter amylophilus | 0.5398c | 1.8825b | 2.3032a | 0.2685 | <0.0001 |
a,b,cMeans in same row with different superscripts are different from each other (P < 0.05).
aTreatments: CON = control (no supplements); BS = supplemented with urea-molasses lick block; CS = supplemented with concentrate.
Rumen Fermentation Parameters
Ruminal pH of the CS group was lowest (P < 0.05) among groups, but there was no difference between the BS and CON groups (Table 8). The CS and BS groups had greater (P < 0.05) concentrations of propionate, butyrate, total VFA, ammonia, and MCP than the CON group, and the CS group had greater (P < 0.05) concentrations than the BS group. The ratios of acetate/propionate in the CS and BS groups were lower (P < 0.05) than in the CON group, and the ratio in the CS group was lower (P < 0.05) than in the BS group.
Table 8.
The rumen fermentation parameters in Tibetan sheep supplemented with urea-molasses lick block or concentrate in the cold season
| Item | Treatmentsa | SEM | P-value | ||
|---|---|---|---|---|---|
| CON | BS | CS | |||
| pH | 6.94a | 6.80a | 6.55b | 0.060 | 0.004 |
| Acetate, mmol/L | 58. 0 | 61.8 | 60.6 | 1.440 | 0.615 |
| Propionate, mmol/L | 12.4c | 16.1b | 27.6a | 2.312 | <0.0001 |
| Butyrate, mmol/L | 6.23c | 8.59b | 9.84a | 0.551 | <0.0001 |
| Total VFA, mmol/L | 76.7c | 86.5b | 98.0a | 3.293 | <0.0001 |
| Acetate:propionate | 4.72a | 3.85b | 2.20c | 0.342 | <0.0001 |
| Ammonia, mmol/L | 3.39c | 5.29b | 8.43a | 0.761 | <0.0001 |
| MCPb, mg/100 mL | 116.3c | 136.0b | 163.5a | 7.651 | 0.002 |
a,b,cMeans in same row with different superscripts are different from each other (P < 0.05).
aTreatments: CON = control (no supplements); BS = supplemented with urea-molasses lick block; CS = supplemented with concentrate.
bMCP = microbial proteins.
Ruminal Morphology
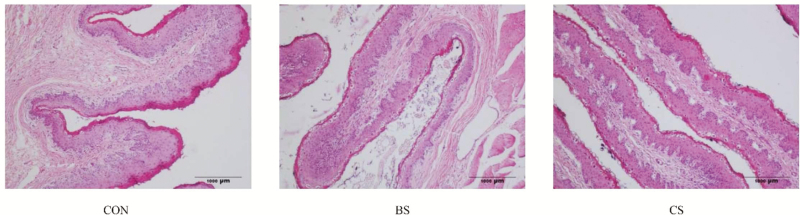
Morphology analysis of ruminal tissue is summarized in Table 9. Width and surface area of ruminal papillae in the CS and BS groups were greater (P < 0.05) than in the CON group, and greater (P < 0.05) in the CS group than in the BS group. In addition, the height of ruminal papillae in the CS group was greater (P < 0.05) than in the CON group. Representative micrographs are presented in Figure 1.
Table 9.
The rumen morphology in Tibetan sheep supplemented with urea-molasses lick block or concentrate in the cold season
| Item | Treatmentsa | SEM | P-value | ||
|---|---|---|---|---|---|
| CON | BS | CS | |||
| Papillae density, n/cm2 | 59.0 | 65.0 | 72.4 | 7.84 | 0.120 |
| Papillae height, μm | 1503.4b | 1587.3b | 1715.8a | 23.77 | 0.002 |
| Papillae width, μm | 338.9c | 376.1b | 432.8a | 9.65 | <0.0001 |
| Papillae surface, μm2 | 455,780c | 547,542b | 636,063a | 22,643.8 | <0.0001 |
a,b,cMeans in same row with different superscripts are different from each other (P < 0.05).
aTreatments: CON = control (no supplements); BS = supplemented with urea-molasses lick block; CS = supplemented with concentrate.
Figure 1.
Representative micrographs of H&E staining in the ruminal papillae of Tibetan sheep (10×). CON = controls (no supplements); BS = supplemented with urea-molasses lick block; CS = supplemented with concentrate.
The Gene Expression of Ruminal Epithelium
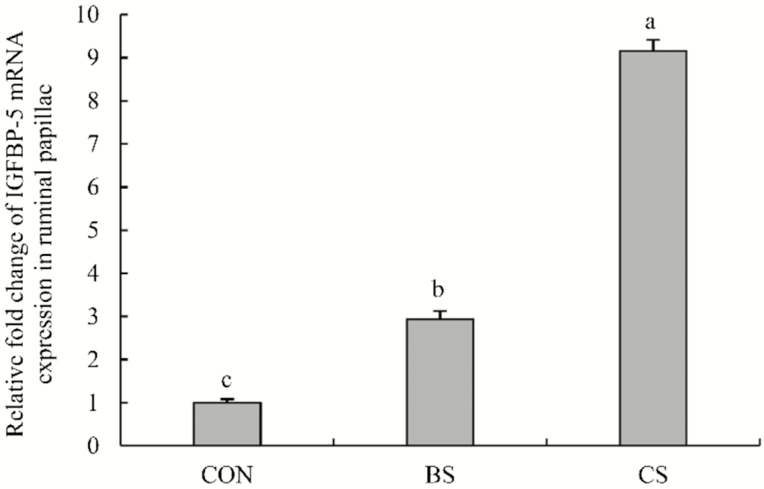
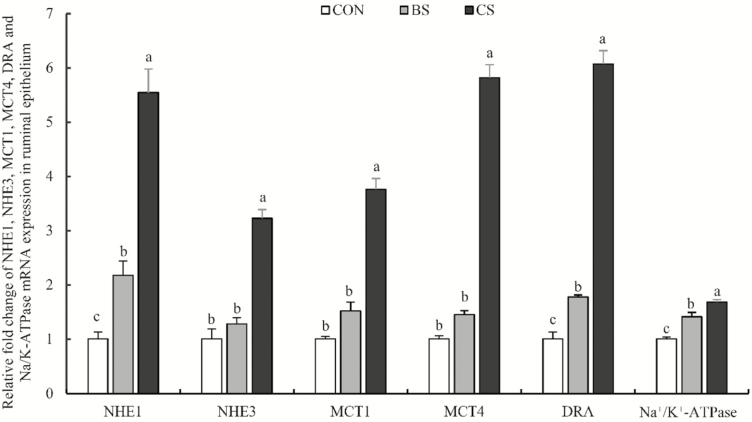
The mRNA relative expression levels of IGFBP5 were greater (P < 0.05) in the CS and BS groups than in the CON group, and in the CS group (P < 0.05) than in the BS group (Figure 2). Similarly, the NHE1, DRA, and Na+/K+-ATPase mRNA expressions in ruminal epithelium were greater (P < 0.05) in the CS and BS groups than in the CON group, and in the CS group (P < 0.05) than in the BS group (Figure 3). The NHE3, MCT1, and MCT4 mRNA expressions in the CS group were greater (P < 0.05) than in the BS and CON groups, but no difference was found between the BS and CON groups.
Figure 2.
The relative expression of IGFBP5 mRNA in ruminal papillae of Tibetan sheep supplemented with urea-molasses lick block or concentrate in the cold season. CON = controls (no supplements); BS = supplemented with urea-molasses lick block; CS = supplemented with concentrate. Different letters indicate a statistically significant difference at P < 0.05.
Figure 3.
The relative expression of NHE1, NHE3, MCT1, MCT4, DRA, and Na+/K+-ATPase mRNA in ruminal papillae of Tibetan sheep supplemented with urea-molasses lick block or concentrate in cold season. CON = controls (no supplements); BS = supplemented with urea-molasses lick block; CS = supplemented with concentrate. DRA = downregulated in adenoma; MCT1 = monocarboxylate transporter 1; MCT4 = monocarboxylate transporter 4; NHE1 = sodium/hydrogen antiporter, isoform 1; NHE3 = sodium/hydrogen antiporter, isoform 3; Na+/K+-ATPase = sodium/potassium ATPase pump. Different letters indicate a statistically significant difference at P < 0.05.
DISCUSSION
Rumen Microbial Abundance and Rumen Fermentation
Rumen microbial community structure and diversity are highly responsive to dietary nutrients, and increase in dietary nonstructural carbohydrate content (Kocherginskaya et al., 2001; Tajima et al., 2001; Kamra, 2005; Pitta et al., 2010). Corn-fed animals displayed more diverse and rich bacterial populations than did hay-fed animals (Kocherginskaya et al., 2001), and forage-fed cattle, supplemented with sucrose, increased rumen microbial growth (Kennedy, 1980). In the present study, greater relative abundances of protozoa, R. albus, F. succinogenes, S. bovis, and R. amylophilus were observed in the CS and BS groups than in the CON group, and rumen fungi, B. fibrisolvens and P. ruminicola in the CS group than in the BS and CON groups. Supplementations in the CS and BS groups increased substrates for rumen microbial fermentation, which promoted growth and proliferation of rumen microbes. The concentrate supplementation contained more starch and real protein than the urea-molasses lick block, and provided more protein and energy. Accordingly, the CS group had a greater relative abundance of rumen fungi, Butyrivibrio fibrisovens, and P. ruminicola, the main bacterial species for the catabolism of starch, protein, and peptides (Cotta, 1992; Attwood and Reilly, 1995). A greater relative abundance of protozoa was observed in the BS group than in the CS group. Protozoa can ferment soluble sugars very rapidly, as they possess a powerful glycolytic mechanism (Heald and Oxford, 1953; Denton et al., 2015; Teixeira et al., 2017). The urea-molasses lick block supplementation contained more molasses than the concentrate, and provided more soluble sugars to protozoa. It increased substrates for protozoa fermentation, which promoted their growth and multiplication.
VFA, consisting primarily of acetate, propionate, and butyrate are the most important end products of ruminal fermentation. Concentrate feed and urea-molasses lick block supplementation increased propionate, butyrate, and total VFA concentrations, and reduced acetate/propionate ratio, which is in agreement with previous studies (Getachew et al., 2004; Shen et al., 2004; Suárez et al., 2006) that reported diets rich in nonstructural carbohydrates increased propionate and butyrate production. Streptococcus bovis and R. amylophilus were found to be major nonstructural carbohydrate degradable bacterial species (Dehority, 1991; Cotta, 1992) and, accordingly, were greater in the supplemented groups than in the CON group. The greater VFA concentration in the CS group than in the other two groups resulted in the lowest pH in this group, as ruminal pH is maintained by a balance between acid production and its removal (Allen, 1997).
Ammonia, an important N source for MCP synthesis, is produced by rumen microbial fermentation and degradation of protein or urea in the rumen. In the present study, the supplemented groups consumed more CP than the CON group, which lead to greater ammonia concentrations in the supplemented groups. Ammonia and MCP increased with CP intakes, which was consistent with the finding of Bandyk et al. (2001).
Ruminal Morphology and Absorptive Capability
The rumen epithelium is essential for the absorption of fermentation end products as 50% to 85% of VFA is absorbed directly across the rumen epithelium. Absorption pathways by passive diffusion of undissociated VFA and by facilitated diffusion of dissociated VFA via carrier-mediated transport proteins across the rumen epithelium have been described (Kirat et al., 2006; Graham et al., 2007; Connor et al., 2010; Aschenbach et al., 2011). Consequently, the absorption rate of VFA by rumen epithelium is highly dependent on the papillae surface area and the availability of transport proteins (Bannink et al., 2008; Yang et al., 2012; Melo et al., 2013). In studies on goats and sheep, the width and surface area of ruminal papillae increased when energy-rich concentrate rations were offered (Shen et al., 2004; Odongo et al., 2006; Wang et al., 2009). In the present study, the ruminal papillae width and surface area were greater in the supplemented groups than in the CON group, which was consistent with their greater intakes of energy and protein. In addition, the fermentation end products of VFA were greater in the supplemented groups. The larger surface area of the ruminal papillae in the supplemented groups when compared to controls increased the absorption capacity and, ultimately, allowed for a faster absorption rate of VFA in the supplemented groups than in the controls (Bannink et al., 2008; Melo et al., 2013).
The VFA, in particular butyrate, stimulate the growth and development of ruminal epithelium (Sander et al., 1959; Sakata and Tamate, 1978; Shen et al., 2005). Malhi et al. (2013) reported that transient increases in cyclin D1 transcription contribute to butyrate-induced papillae growth and subsequently to increased absorption of VFA by the ruminal epithelium and Mentschel et al. (2001) found that butyrate stimulated ruminal papillae development. The CS and BS groups had greater concentrations of butyrate than the CON group, which could explain the greater ruminal papillae growth and surface area in these groups when compared to controls. In addition, Shen et al. (2004) observed that an energy-rich diet altered rumen morphology and function, which was associated with changes in systemic IGF-1. In the present study, on d 30 and d 60, the CS and BS groups exhibited greater concentrations of serum GH, IGF-1, and IGF-2 than the CON group. Firth and Baxter (2002) reported that the cellular events of IGF-1 stimulated rumen morphology development, and that it was modulated by IGFBP. In the present study, the mRNA expression of IGFBP5 was upregulated in the ruminal papillae in the CS and BS group when compared with the CON group, as also occurred in cattle during the transition to a high-grain diet (Steele et al., 2011; Steele et al., 2012).
Monocarboxylate transporter 1 (MCT1) and monocarboxylate transporter 4 (MCT4) are transporters for dissociated VFA across the ruminal epithelium (Müller et al., 2002; Graham et al., 2007; Kirat et al., 2007). In addition, VFA−/HCO3− exchange has been identified as a pathway for absorption of dissociated VFA (Bilk et al., 2005; Aschenbach et al., 2009). Moreover, downregulated in adenoma (DRA) participates in the transport of HCO3− and is responsible for movement of HCO3− from the rumen epithelium to the ruminal lumen for neutralizing acid and importing dissociated VFA (Bilk et al., 2005). Müller et al. (2002) reported that absorption of VFA across the ruminal epithelium is associated with a decreased intracellular pH; other studies demonstrated that exporting protons back into the lumen via Na+/H+ exchange (NHE) (Müller et al., 2000; Gäbel et al., 2002; Graham et al., 2007), energized by Na+/K+-ATPase (Aschenbach et al., 2011), plays a significant role in intracellular pH regulation. MCT, DRA, NHE, and Na+/K+-ATPase act in harmony to promote the absorption of VFA in the rumen epithelium. In the present study, relative expression levels of NHE1, DRA, and Na+/K+-ATPase mRNA of ruminal epithelium in the CS and BS groups were greater than in the CON group, and in the CS group were greater than in the BS group. Furthermore, the relative expression levels of NHE3, MCT1, and MCT4 mRNA in the CS group were greater than in the BS and CON groups. These results indicated that the supplements increased VFA absorption capacity of the ruminal epitheliums in the CS and BS groups, more so in the CS group than in the BS group. Yang et al. (2012) reported that NHE1 and NHE3 mRNA expression levels in the rumen epithelium were enhanced by a concentrate diet when compared with a forage diet, and observed positive correlations with these levels and VFA concentrations, but negative correlations with pH. Shen et al. (2004) observed similar results in that an energy-rich diet enhanced NHE activity in the rumen epithelium. Kuzinski et al. (2011) reported that the expression of Na+/K+-ATPase mRNA in rumen epithelium was upregulated in sheep fed a hay-concentrate diet when compared to hay-feeding alone, and, ruminants fed with highly fermentable diets demonstrated a potential increase in bicarbonate-dependent transport mediated via DRA (Penner et al., 2009; Connor et al., 2010; Aschenbach et al., 2011). In the present study, energy and the VFA concentrations in the CS and BS groups increased, which could explain the increase in the relative expression levels of NHE1, DRA, and Na+/K+-ATPase mRNA in the ruminal epithelium. In addition, Kiela et al. (2007) demonstrated that the expression of NHE3 mRNA increased with butyrate. The CS group had a greater concentration of butyrate than the BS and CS groups, and the relative expression of NHE3 mRNA in the CS group was also greater than in the BS and CON groups. It was reported that a high-grain diet upregulated MCT1 mRNA expression in ruminal epithelium (Metzler-Zebeli et al., 2013), and MCT4 was stably upregulated by ruminal butyrate infusion (Malhi et al., 2013). Consequently, the relative expression levels of MCT1 and MCT4 mRNA in ruminal epithelium were greater in the CS group than in the BS and CON groups.
Results suggested that Tibetan sheep with dietary supplements had a greater capacity for bicarbonate-dependent (VFA−/HCO3− exchange) absorption of VFA. Moreover, the CS group had a greater capacity for MCT-dependent absorption of VFA than the CON group. Supplementation with concentrate or urea-molasses lick block resulted in differences in ruminal papillae morphology and in concentration and composition of VFA and, ultimately, in the VFA absorption capacity between the two supplements.
CONCLUSIONS
Tibetan sheep, supplemented with energy and protein via concentrate feed or urea-molasses lick block in the cold season, increased ADG, rumen microbial abundance, and VFA production while improving fermentation parameters, rumen epithelium development, and absorptive capability. Consequently, nutrient supplementation to Tibetan sheep in the cold season could be an effective strategy to prevent BW loss and improve digestive processes. Adoption of this practice could decrease grazing pressures and promote a sustainable livestock production system on the grasslands, which is crucial for pastoralists to improve their livelihood on the Qinghai-Tibetan Plateau.
ACKNOWLEDGMENTS
This work was funded by the projects of the key technologies of the high efficiency conversion of grass and livestock in Qinghai Tibet Plateau community (201203008) and National Key Research and Development Plan – the research and development of key technologies for improving the efficiency of cattle and sheep (2017YFD0502005). We would like to thank two anonymous reviewers for their suggestions and all staff members of Haibei Demonstration Zone of Plateau Modern Ecological Husbandry Science and Technology for animal care.
LITERATURE CITED
- Allen M.S. 1997. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J. Dairy. Sci. 80:1447–1462. doi:10.3168/jds.S0022-0302(97)76074-0 [DOI] [PubMed] [Google Scholar]
- Aschenbach J.R., Bilk S., Tadesse G., Stumpff F., and Gäbel G.. 2009. Bicarbonate-dependent and bicarbonate-independent mechanisms contribute to nondiffusive uptake of acetate in the ruminal epithelium of sheep. Am. J. Physiol. Gastrointest. Liver 296:G1098–G1107. doi:10.1152/ajpgi.90442.2008 [DOI] [PubMed] [Google Scholar]
- Aschenbach J., Penner G., Stumpff F., and Gäbel G.. 2011. Ruminant nutrition symposium: role of fermentation acid absorption in the regulation of ruminal pH. J. Anim. Sci. 89:1092–1107. doi:10.2527/jas.2010-3301 [DOI] [PubMed] [Google Scholar]
- Attwood G., and Reilly K.. 1995. Identification of proteolytic rumen bacteria isolated from New Zealand cattle. J. Appl. Bacteriol. 79:22–29. doi:10.1111/j.1365-2672.1995.tb03119.x [DOI] [PubMed] [Google Scholar]
- Bailey E.A., Titgemeyer E.C., Olson K.C., Brake D.W., Jones M.L., and Anderson D.E.. 2012. Effects of supplemental energy and protein on forage digestion and urea kinetics in growing beef cattle. J. Anim. Sci. 90:3492–3504. doi:10.2527/jas.2011-4458 [DOI] [PubMed] [Google Scholar]
- Bandyk C.A., Cochran R.C., Wickersham T.A., Titgemeyer E.C., Farmer C.G., and Higgins J.J.. 2001. Effect of ruminal vs postruminal administration of degradable protein on utilization of low-quality forage by beef steers. J. Anim. Sci. 79:225–231. doi:10.2527/2001.791225x [DOI] [PubMed] [Google Scholar]
- Bannink A., France J., Lopez S., Gerrits W.J., Kebreab E., Tamminga S., and Dijkstra J.. 2008. Modelling the implications of feeding strategy on rumen fermentation and functioning of the rumen wall. Anim. Feed Sci. Technol. 143:3–26. doi:10.1016/j.anifeedsci.2007.05.002 [Google Scholar]
- Bilk S., Huhn K., Honscha K., Pfannkuche H., and Gäbel G.. 2005. Bicarbonate exporting transporters in the ovine ruminal epithelium. J. Comp. Physiol. B 175:365–374. doi:10.1007/s00360-005-0493-1 [DOI] [PubMed] [Google Scholar]
- Chen X., Wang J., Wu Y., and Liu J.. 2008. Effects of chemical treatments of rice straw on rumen fermentation characteristics, fibrolytic enzyme activities and populations of liquid- and solid-associated ruminal microbes in vitro. Anim. Feed Sci. Technol. 141:1–14. doi:10.1016/j.anifeedsci.2007.04.006 [Google Scholar]
- Connor E., Li R., Baldwin R., and Li C.. 2010. Gene expression in the digestive tissues of ruminants and their relationships with feeding and digestive processes. Animal 4:993–1007. doi:10.1017/s1751731109991285 [DOI] [PubMed] [Google Scholar]
- Cotta M.A. 1992. Interaction of ruminal bacteria in the production and utilization of maltooligosaccharides from starch. Appl. Environ. Microbiol. 58:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. 1991. Effects of microbial synergism on fibre digestion in the rumen. Proc. Nutr. Soc. 50:149–159. doi:10.1079/pns19910026 [DOI] [PubMed] [Google Scholar]
- Denman S., and Mcsweeney C.. 2006. Development of a real‐time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 58:572–582. doi:10.1111/j.1574-6941.2006.00190.x [DOI] [PubMed] [Google Scholar]
- Denton B.L., Diese L.E., Firkins J.L., and Hackmann T.J.. 2015. Accumulation of reserve carbohydrate by rumen protozoa and bacteria in competition for glucose. J. Appl. Environ. Microbiol. 81:1832–1838. doi:10.1128/aem.03736-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S., Long R., Kang M., Pu X., and Guo Y.. 2003. Effect of urea multinutritional molasses block supplementation on liveweight change of yak calves and productive and reproductive performances of yak cows. Can. J. Anim. Sci. 83:141–145. doi:10.4141/a01-097 [Google Scholar]
- Feng B.F., Zhao X.Q., Dong Q.M., Xu S.X., Zhao L., and Cao J.. 2013. The effect of feed supplementing and processing on the liveweight gain of Tibetan sheep during the cold season on the Qinghai-Tibetan Plateau. J. Anim. Vet. Adv. 12:312–315. doi:10.3923/javaa.2013.208.211 [Google Scholar]
- Firth S.M., and Baxter R.C.. 2002. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 23:824–854. doi:10.1210/er.2001-0033 [DOI] [PubMed] [Google Scholar]
- Gäbel G., Aschenbach J., and Müller F.. 2002. Transfer of energy substrates across the ruminal epithelium: implications and limitations. Anim. Health. Res. Rev. 3:15–30. doi:10.1079/ahrr200237 [DOI] [PubMed] [Google Scholar]
- Getachew G., Robinson P., DePeters E., and Taylor S.. 2004. Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim. Feed Sci. Technol. 111:57–71. doi:10.1016/s0377-8401(03)00217-7 [Google Scholar]
- Graham C., Gatherar I., Haslam I., Glanville M., and Simmons N.L.. 2007. Expression and localization of monocarboxylate transporters and sodium/proton exchangers in bovine rumen epithelium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292:R997–R1007. doi:10.1152/ajpregu.00343.2006 [DOI] [PubMed] [Google Scholar]
- Heald P.J., and Oxford A.E.. 1953. Fermentation of soluble sugars by anaerobic holotrich ciliate protozoa of the genera Isotricha and Dasytricha. Biochem. J. 53:506–512. doi:10.1042/bj0530506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov A.N., Ivan M., Rode L.M., and Mcallister T.A.. 2001. Fermentation characteristics and ruminal ciliate protozoal populations in cattle fed medium- or high-concentrate barley-based diets. J. Anim. Sci. 79:515–524. doi:10.2527/2001.792515x [DOI] [PubMed] [Google Scholar]
- Jing X.P., Peng Q.H., Rui H., Wang H.Z., Yu X.Q., Degen A.A., Zou H.W., Bao S.K., Zhao S.N., and Wang Z.S.. 2017. Effect of supplements during the cold season on the reproductive system in prepubertal Tibetan sheep ewes. Anim. Sci. J. 88:1269–1278. doi:10.1111/asj.12762 [DOI] [PubMed] [Google Scholar]
- Kamra D. 2005. Rumen microbial ecosystem. Curr. Sci. 89:124–135. [Google Scholar]
- Kennedy P. 1980. The effects of dietary sucrose and the concentrations of plasma urea and rumen ammonia on the degradation of urea in the gastrointestinal tract of cattle. Br. J. Nutr. 43:125–140. doi:10.1079/bjn19800072 [DOI] [PubMed] [Google Scholar]
- Kiela P.R., Kuscuoglu N., Midura A.J., Midurakiela M.T., Larmonier C.B., Lipko M.A., and Ghishan F.K.. 2007. Molecular mechanism of rat NHE3 gene promoter regulation by sodium butyrate. Am. J. Physiol., Cell Physiol. 293:C64–C74. doi:10.1152/ajpcell.00277.2006 [DOI] [PubMed] [Google Scholar]
- Kirat D., Masuoka J., Hayashi H., Iwano H., Yokota H., Taniyama H., and Kato S.. 2006. Monocarboxylate transporter 1 (MCT1) plays a direct role in short‐chain fatty acids absorption in caprine rumen. J. Physiol. (Lond). 576:635–647. doi:10.1113/jphysiol.2006.115931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirat D., Matsuda Y., Yamashiki N., Hayashi H., and Kato S.. 2007. Expression, cellular localization, and functional role of monocarboxylate transporter 4 (MCT4) in the gastrointestinal tract of ruminants. Gene 391:140–149. doi:10.1016/j.gene.2006.12.020 [DOI] [PubMed] [Google Scholar]
- Kocherginskaya S.A., Aminov R.I., and White B.A.. 2001. Analysis of the rumen bacterial diversity under two different diet conditions using denaturing gradient gel electrophoresis, random sequencing, and statistical ecology approaches. Anaerobe 7:119–134. doi:10.1006/anae.2001.0378 [Google Scholar]
- Koike S., and Kobayashi Y.. 2001. Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens. FEMS Microbiol. Lett. 204:361–366. doi:10.1016/s0378-1097(01)00428-1 [DOI] [PubMed] [Google Scholar]
- Kuzinski J., Zitnan R., Viergutz T., Legath J., and Schweigel M.. 2011. Altered Na+/K+-ATPase expression plays a role in rumen epithelium adaptation in sheep fed hay ad libitum or a mixed hay/concentrate diet. Vet. Med. (Praha). 56:35–47. [Google Scholar]
- Li J., et al. 2015. Molecular cloning, expression and purification of lactoferrin from Tibetan sheep mammary gland using a yeast expression system. Protein Expr. Purif. 109C:35–39. doi:10.1016/j.pep.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Liu J.X., Long R.J., Zhang D.. 2007. Feed supplementation blocks: experiences in China. In: Harinder P.S.M, editor. FAO animal production and health paper-feed supplementation blocks. Rome (Italy): FAO Press; p. 89–109 [Google Scholar]
- Livak K.J., and Schmittgen T.D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi:10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Malhi M., Gui H., Yao L., Aschenbach J.R., Gabel G., and Shen Z.. 2013. Increased papillae growth and enhanced short-chain fatty acid absorption in the rumen of goats are associated with transient increases in cyclin D1 expression after ruminal butyrate infusion. J. Dairy. Sci. 96:7603–7616. doi:10.3168/jds.2013-6700 [DOI] [PubMed] [Google Scholar]
- Melo L.Q., Costa S.F., Lopes F., Guerreiro M.C., Armentano L.E., and Pereira M.N.. 2013. Rumen morphometrics and the effect of digesta pH and volume on volatile fatty acid absorption. J. Anim. Sci. 91:1775–1783. doi:10.2527/jas.2011-4999 [DOI] [PubMed] [Google Scholar]
- Mentschel J., Leiser R., Mülling C., Pfarrer C., and Claus R.. 2001. Butyric acid stimulates rumen mucosa development in the calf mainly by a reduction of apoptosis. Arch. Anim. Nutr. 55:85–102. doi:10.1080/17450390109386185 [DOI] [PubMed] [Google Scholar]
- Metzler-Zebeli B.U., Hollmann M., Sabitzer S., Podstatzkylichtenstein L., Klein D., and Zebeli Q.. 2013. Epithelial response to high-grain diets involves alteration in nutrient transporters and Na/K-ATPase mRNA expression in rumen and colon of goats. J. Anim. Sci. 91:4256–4266. doi:10.2527/jas.2012-5570 [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture (MOA), PRC 2004. Feeding Standard of Meat-producing Sheep and Goats (NY/T 816–2004). Beijing (China): China Agricultural Press. [Google Scholar]
- Müller F., Aschenbach J., and Gäbel G.. 2000. Role of Na+/H+ exchange and HCO3− transport in pHi recovery from intracellular acid load in cultured epithelial cells of sheep rumen. J. Comp. Physiol. B 170:337–343. doi:10.1007/s003600000107 [DOI] [PubMed] [Google Scholar]
- Müller F. , K Huber, H Pfannkuche, J.R Aschenbach, G Breves, and G Gäbel. 2002. Transport of ketone bodies and lactate in the sheep ruminal epithelium by monocarboxylate transporter 1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283:G1139–G1146. doi:10.1152/ajpgi.00268.2001 [DOI] [PubMed] [Google Scholar]
- Odongo N.E., Alzahal O., Lindinger M.I., Duffield T.F., Valdes E.V., Terrell S.P., and Mcbride B.W.. 2006. Effects of mild heat stress and grain challenge on acid-base balance and rumen tissue histology in lambs. J. Anim. Sci. 84:447–455. doi:10.2527/2006.842447x [DOI] [PubMed] [Google Scholar]
- Penner G.B., Aschenbach J.R., Gäbel G., Rackwitz R., and Oba M.. 2009. Epithelial capacity for apical uptake of short chain fatty acids is a key determinant for intraruminal pH and the susceptibility to subacute ruminal acidosis in sheep. J. Nutr. 139:1714–1720. doi:10.3945/jn.109.108506 [DOI] [PubMed] [Google Scholar]
- Pitta D.W., Pinchak W.E., Dowd S.E., Osterstock J.B., Gontcharova V., Youn E., and Malinowski D.P.. 2010. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb. Ecol. 59:511–522. doi:10.1007/s00248-009-9609-6 [DOI] [PubMed] [Google Scholar]
- Sakata T., and Tamate H.. 1978. Rumen epithelial cell proliferation accelerated by rapid increase in intraruminal butyrate. J. Dairy. Sci. 61:1109–1113. doi:10.3168/jds.s0022-0302(78)83694-7 [DOI] [PubMed] [Google Scholar]
- Sander E., Warner R., Harrison H., and Loosli J.. 1959. The stimulatory effect of sodium butyrate and sodium propionate on the development of rumen mucosa in the young calf. J. Dairy. Sci. 42:1600–1605. doi:10.3168/jds.s0022-0302(59)90772-6 [Google Scholar]
- Shang Z.H., Gibb M.J., Leiber F., Ismail M., Ding L.M., Guo X.S., and Long R.J.. 2014. The sustainable development of grassland-livestock systems on the Tibetan plateau: problems, strategies and prospects. Rangeland J. 36:267–296. doi:10.1071/rj14008 [Google Scholar]
- Shang Z.H., Gibb M.J., and Long R.J.. 2012. Effect of snow disasters on livestock farming in some rangeland regions of China and mitigation strategies – a review. Rangeland J. 34:89–101. doi:10.1071/rj11052 [Google Scholar]
- Shen Z., Kuhla S., Zitnan R., Seyfert H., Schneider F., Hagemeister H., Chudy A., Lohrke B., Blum J.W., Hammon H.M., and Voigt J.. 2004. An energy-rich diet causes rumen papillae proliferation associated with more IGF type 1 receptors and increased plasma IGF-1 concentrations in young goats. J. Nutr. 134:11–17. doi:10.1093/jn/134.1.11 [DOI] [PubMed] [Google Scholar]
- Shen Z.M., D.S Kuhla, R Zitnan, H.M Seyfert, F Schneider, H Hagemeister, A Chudy, B Löhrke, J.W Blum, H.M Hammon, 2005. Intraruminal infusion of n-butyric acid induces an increase of ruminal papillae size independent of IGF-1 system in castrated bulls. Arch. Anim. Nutr. 59:213–225. doi:10.1080/17450390500216894 [DOI] [PubMed] [Google Scholar]
- Steele M.A., Croom J., Kahler M., Alzahal O., Hook S.E., Plaizier K., and Mcbride B.W.. 2011. Bovine rumen epithelium undergoes rapid structural adaptations during grain-induced subacute ruminal acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300:R1515–R1523. doi:10.1152/ajpregu.00120.2010 [DOI] [PubMed] [Google Scholar]
- Steele M., Dionissopoulos L., AlZahal O., Doelman J., and McBride B.. 2012. Rumen epithelial adaptation to ruminal acidosis in lactating cattle involves the coordinated expression of insulin-like growth factor-binding proteins and a cholesterolgenic enzyme. J. Dairy. Sci. 95:318–327. doi:10.3168/jds.2011-4465 [DOI] [PubMed] [Google Scholar]
- Stevenson D., and Weimer P.. 2007. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl. Microbiol. Biotechnol. 75:165–174. doi:10.1007/s00253-006-0802-y [DOI] [PubMed] [Google Scholar]
- Suárez B.J., Van Reenen C.G., Beldman G., Van Delen J., Dijkstra J., and Gerrits W.J.. 2006. Effects of supplementing concentrates differing in carbohydrate composition in veal calf diets: I. Animal performance and rumen fermentation characteristics. J. Dairy. Sci. 89:4365–4375. doi:10.3168/jds.s0022-0302(06)72483-3 [DOI] [PubMed] [Google Scholar]
- Sun Y., Angerer J., and Hou F.J.. 2015. Effects of grazing systems on herbage mass and liveweight gain of Tibetan sheep in Eastern Qinghai-Tibetan Plateau, China. Rangeland J. 37:181–190. doi:10.1071/RJ14062 [Google Scholar]
- Sylvester J., Karnati S., Yu Z., Morrison M., and Firkins J.. 2004. Development of an assay to quantify rumen ciliate protozoal biomass in cows using real-time PCR. J. Nutr. 134:3378–3384. doi:10.1093/jn/134.12.3378 [DOI] [PubMed] [Google Scholar]
- Tajima K., Aminov R.I., Nagamine T., Matsui H., Nakamura M., and Benno Y.. 2001. Diet-dependent shifts in the bacterial population of the rumen revealed with real-time PCR. Appl. Environ. Microbiol. 67:2766–2774. doi:10.1128/aem.67.6.2766-2774.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira C.R., Lana R.D.P., Tao J., and Hackmann T.J.. 2017. Comparing the responses of rumen ciliate protozoa and bacteria to excess carbohydrate. FEMS. Microbiol. Ecol. 93:fix060 doi:10.1093/femsec/fix060 [DOI] [PubMed] [Google Scholar]
- Wang Y.H., Xu M., Wang F., Yu Z.P., Yao J., Zan L., and Yang F.X.. 2009. Effect of dietary starch on rumen and small intestine morphology and digesta pH in goats. Livest. Sci. 122:48–52. doi:10.1016/j.livsci.2008.07.024 [Google Scholar]
- Xin G.S., Long R.J., Guo X.S., Irvine J., Ding L.M., Ding L.L., and Shang Z.H.. 2011. Blood mineral status of grazing Tibetan sheep in the Northeast of the Qinghai–Tibetan Plateau. Livest. Sci. 136:102–107. doi:10.1016/j.livsci.2010.08.007 [Google Scholar]
- Yang W., Shen Z., and Martens H.. 2012. An energy-rich diet enhances expression of Na/H exchanger isoform 1 and 3 messenger RNA in rumen epithelium of goat. J. Anim. Sci. 90:307–317. doi:10.2527/jas.2011-3854 [DOI] [PubMed] [Google Scholar]
- Zhou J.W., Guo X.S., Degen A.A., Zhang Y., Liu H., Mi J.D., Ding L.M., Wang H.C., Qiu Q., and Long R.J.. 2015. Urea kinetics and nitrogen balance and requirements for maintenance in Tibetan sheep when fed oat hay. Small Rumin. Res. 129:60–68. doi:10.1016/j.smallrumres.2015.05.009 [Google Scholar]