Abstract
Study objectives were to evaluate the impact of early life thermal stress (ELTS) on thermoregulation, stress response, and intestinal health of piglets subjected to a future heat stress (HS) challenge during simulated transport. From d 7 to 9 post-farrowing, 12 first-parity sows and their litters were exposed to thermoneutral (ELTN; 25.4 ± 1.1 °C w/heat lamp; n = 4), HS (ELHS; cycling 32–38 °C w/heat lamp; n = 4), or cold stress (ELCS; 25.4 ± 1.1 °C w/no heat lamp; n = 4) conditions, and then from d 10 until weaning all piglets were exposed to thermoneutral (TN) conditions (25.3 ± 1.9 °C w/heat lamp). During the ELTS period, respiration rate, rectal temperature (TR), and skin temperature (TS) of three mixed-sex piglets per dam were monitored daily (0800, 1200, 1600, 2000 h). At 13 ± 1.3 d of age, temperature recorders were implanted intra-abdominally into all piglets. At weaning (20.0 ± 1.3 d of age), piglets were bled and then herded up a ramp into a simulated transport trailer and exposed to HS conditions (cycling 32–38 °C) for 8 h. During the 8 h simulated transport, core body temperature (TC) and TS were assessed every 15 min. After the simulated transport, piglets were unloaded from the trailer, bled, weighed, and then housed individually in TN conditions (28.5 ± 0.7 °C) for 7 d. During this time, ADFI and ADG were monitored, blood samples were taken on d 1, 4, and 7, and piglets were video-recorded to assess behavior. Piglets were sacrificed on d 8 post-simulated transport and intestinal morphology was assessed. Data were analyzed using PROC MIXED in SAS 9.4. In the ELTS period, piglet TR was increased overall (P = 0.01) in ELHS (39.77 ± 0.05 °C) compared to ELTN (39.34 ± 0.05 °C) and ELCS (39.40 ± 0.05 °C) litters. During simulated transport, TC was greater (P = 0.02) in ELHS (40.84 ± 0.12 °C) compared to ELTN (40.49 ± 0.12 °C) and ELCS (40.39 ± 0.12 °C) pigs. Following simulated transport, BW loss was greater (P = 0.01; 40%) for ELHS compared to ELTN and ELCS pigs and ADFI was reduced (P = 0.05; 28.6%) in ELHS compared to ELTN pigs. Sitting behavior tended to be increased (P = 0.06; 47.4%) in ELHS vs. ELCS or ELTN pigs. Overall, circulating cortisol was greater for ELHS (P ≤ 0.01; 38.8%) compared to ELCS and ELTN pigs. Goblet cells per villi were reduced (P = 0.02; 20%) in the jejunum of ELHS vs. ELCS and ELTN pigs. In summary, ELHS reduced thermotolerance and increased the future stress response of piglets compared to ELCS and ELTN.
Keywords: pigs, thermal stress, thermotolerance, transport
INTRODUCTION
Transporting newly weaned pigs can reduce well-being (Johnson and Lay, 2017), and ambient temperature may contribute to piglets total stress load (Lambooy, 1988). Unfortunately, newly weaned pigs are often transported through wide temperature ranges and for extended durations (Lewis et al., 2005). As global temperatures rise (NOAA, 2016), heat stress (HS)-related welfare issues will likely increase. Therefore, it’s imperative that management strategies are developed to improve piglet well-being during incidences of simultaneous production stress and HS.
Weaning causes intestinal barrier deterioration due to chronically elevated stress hormones (Smith et al., 2010) and reduced feed intake (Wijtten et al., 2011). Moreover, HS exposure increases intestinal damage due to ischemia and hypoxia of the gut (Lambert, 2009; Liu et al., 2011; Pearce et al., 2012). Furthermore, transport during times of HS can prolong post-transport recovery rate (Lewis, 2008) and increase BW loss due to dehydration (Berry and Lewis, 2001). Taken together, this implies that the well-being of newly weaned pigs is compromised due to the additive effects of weaning, transport, and HS.
Prior HS exposure may improve future thermotolerance and provide protection against unrelated stressors such as hypoxia, ischemia/reperfusion, and traumatic injuries (as reviewed by Horowitz, 2007). Because HS and weaning cause intestinal injury characterized by hypoxia and ischemia, it is possible that early life HS (ELHS) exposure may improve future piglet well-being in response to weaning and transport during HS. Consequently, because cold stress (CS) acclimation is characterized by increased thermogenesis and vasoconstriction (Tipton et al., 2008), it is possible that early life CS (ELCS) would decrease piglets’ ability to adapt to a future HS challenge. Therefore, study objectives were to evaluate the effects of early life thermal stress (ELTS) on the future thermoregulation, productivity, and intestinal morphology of newly weaned piglets exposed to simulated transport and HS. We hypothesized that ELHS would be beneficial and ELCS would be detrimental to future piglet well-being when exposed to a HS challenge during simulated transport compared to early life thermoneutral (ELTN) exposure.
MATERIALS AND METHODS
Early Life Thermal Stress
All procedures involving animal use were approved by the Purdue University Animal Care and Use Committee (protocol #1701001525), and animal care and use standards were based upon the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Science Societies, 2010). Twelve first-parity sows with similar sized litters [n = 11.8 piglets/litter; Duroc × (Landrace × Yorkshire)] were selected and exposed to ELTN [n = 4 sows and litters; 25.4 ± 1.1 °C with heat lamp; 56.1 ± 8.1% relative humidity (RH)], ELHS (n = 4 sows and litters; cycling 32–38 °C with heat lamp; 41.2 ± 10.2% RH), or ELCS (n = 4 sows and litters; 25.4 ± 1.1 °C with no heat lamp; 56.1 ± 8.1% RH) on d 7 ± 1.3 d, 8 ± 1.3 d, and 9 ± 1.3 d post-farrowing. The HS temperature was selected based on the Guide for the Care and Use of Agricultural Animals in Research and Teaching recommended thermal conditions for swine (Federation of Animal Science Societies, 2010). Cycling HS was achieved by setting the ambient temperature (TA) to 32 °C as a baseline, and then over a 4 h period it was gradually increased until 38 °C was achieved, which was then held constant for a second 4 h period. Following the second 4 h period, TA was gradually reduced over 4 h until 32 °C was achieved and this temperature was held overnight for 12 h. Litters were weighed on d 6, 10, and at weaning (20.0 ± 1.3 d of age) to calculate ADG during the ELTS testing period and from d 10 to weaning. Thermal measurements were performed at 0800, 1200, 1600, and 2000 h on d 6, 7, 8, and 9 post-farrowing on each sow as well as three piglets per sow (n = 2 barrows and 1 gilt per 2 sows and 1 barrow and 2 gilts per 2 sows) that represented the mean pig BW within litter. Thermal measures for piglets included respiration rate (RR), skin temperature (TS), and rectal temperature (TR). In addition, sow TR was measured at 0800, 1200, 1600, and 2000 h daily. Respiration rate (breaths per minute; bpm) of piglets was determined by counting flank movement for 15 s then multiplying by four. Skin temperature was measured by taking a broadside photo of individual piglets using an infrared camera (FLIR-T62101; accuracy ±0.1 °C; FLIR Systems Inc.; Wilsonville, OR), and photos were analyzed with FLIR Tools software (version 2.1). Rectal temperature of piglets was measured with a calibrated and lubricated thermometer (ReliOn model #144-MT-118-BF; accuracy ± 0.2 °C; Mabis Healthcare Inc.; Waukegan, IL) inserted approximately 2.5 cm into the rectum, and sow TR was measured using a calibrated and lubricated thermometer (Cooper Atkins model# TM99A; accuracy ± 0.2 °C; Middlefield, CT) inserted approximately 10 cm into the rectum.
On 13 ± 1.3 d of age, calibrated thermochron temperature recorders (iButton model 1921H; accuracy ± 0.2 °C; Dallas Semiconductor, Maxim, Irving, TX) were implanted intra-abdominally into the 36 selected piglets to measure core body temperature (TC). For thermochron implantation, piglets were anesthetized using 1 to 4% isoflurane, and then a 6-cm incision was made on the abdomen, 2 cm lateral to the linea alba. Sterile temperature recorders were then placed in between the peritoneum and abdominal muscle and the incision site was closed. After surgery, all piglets were administered a broad spectrum antibiotic (5 mg/kg IM every 3 d; Ceftiofur; Zoetis; Florham Park, NJ) to prevent infection at the surgical site, as well as analgesia (2.2 mg/kg IM; Flunixin meglumine; Merck Animal Health; Madison, NJ) immediately after surgery and 24 h post-surgery to control pain. Piglets were immediately returned to the sow after surgery where they remained in thermoneutral (TN) conditions (25.3 ± 1.9 °C with heat lamp; 48.3 ± 9.8% RH) until weaning. Following weaning, the wean to estrus interval and total pigs weaned per sow in the next parity was recorded for all sows exposed to TN, CS, or HS conditions.
Simulated Transport
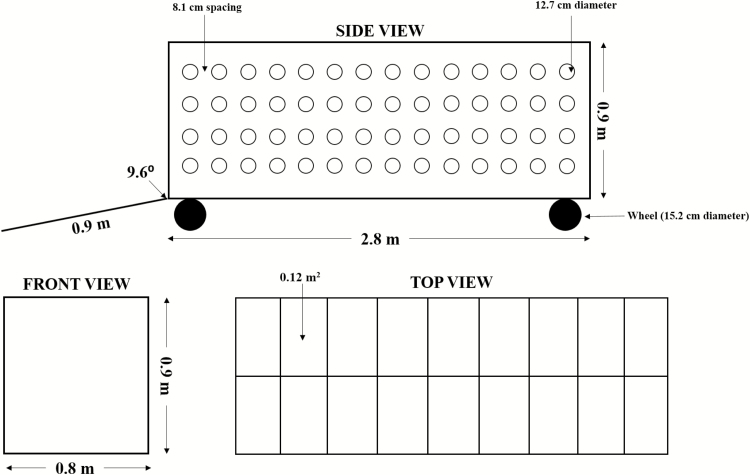
At weaning, the three selected piglets were removed from each sow, weighed, and then herded up a ramp (0.9 m; 9.6° angle) into a simulated transport trailer where they were placed into 18 total pens (n = 2 pigs/pen; Fig. 1). Pigs were then exposed to cycling HS (cyclical 32–38 °C; 18.9 ± 2.9% RH) for 8 h. For cyclical HS, the TA was set at 32 °C as a baseline, and then over a 2 h period it was gradually increased to 38 °C where it was held for 4 h before being reduced to 32 °C over the final 2 h period. Design of the trailer was adapted from previous work by our lab (Johnson and Lay, 2017). Briefly, the simulated trailer (3 m × 0.8 m × 0.9 m) was constructed out of plywood coated in corrugated plastic sheeting and provided 0.06 m2/piglet based on the Guide for the Care and Use of Agricultural Animals in Research and Teaching recommendations for minimum area allowances in transportation of 4.5 to 9.0 kg piglets (Federation of Animal Science Societies, 2010; Fig. 1). The trailer did not have a roof so that TS could be measured without the need to enter the trailer. The trailer was divided into 18 pens (0.12 m2/pen) to separate the piglets for TS measurements and to ensure that piglets were randomly distributed within the trailer throughout the entire transport (Fig. 1). Piglets were blocked into pens by ELTS treatment and sex (n = 6 pens/ELTS treatment), and pigs within each pen were randomly selected from different litters to simulate piglet mixing. Pen walls were constructed out of wire mesh so as to not obstruct airflow through the trailer. Two data loggers (Hobo; data logger temperature/RH; Onset; Bourne, MA) were evenly spaced within the trailer to measure TA and RH% in 5-min intervals. Four rows of holes (12.7 cm diameter) spaced 8.1 cm apart were drilled into the side panels of the trailer and two fans were positioned toward the front corners at a 45° angle to simulate air flow through a moving trailer. As previously described (Johnson and Lay, 2017), this procedure is referred to as simulated transport as in piglets are weaned from the sow, and then moved down an alley and up a ramp then mixed inside a trailer. The movement of the trailer is not simulated, only the mixing, isolation, duration, feed and water withdrawal, and thermal stress component of the procedure.
Figure 1.
Simulated transport trailer schematic.
In 15-min intervals throughout the entire 8 h simulated transport, TC was measured by implanted iButtons, and TS was determined using a FLIR-T62101 infrared camera. At the conclusion of the 8 h simulated transport, piglets were herded out of the simulated trailer, weighed, blood samples were collected, and piglets were placed into individual pens (0.5 × 0.9 m) in TN conditions (28.5 ± 0.7 °C; 24.6 ± 9.5% RH) for 7 d. During the trial, four piglets were euthanized due to illness unrelated to the ELTS treatments leaving 10 piglets in the ELHS, 11 piglets in the ELCS, and 11 piglets in the ELTN groups, respectively.
Following simulated transport, all piglets were housed in individual pens (0.86 × 0.41 m) with rubber-coated wire flooring. All pens were connected to each other and were separated by vertical bars allowing for auditory, olfactory, visual, and nose-to-nose contact between pigs. Pigs were provided feed and water ad libitum with individual feeders and nipple waterers within each pen. ADFI, ADG, and gain:feed were determined for individual piglets throughout the 7 d post-simulated transport period. In addition, piglets were video-recorded 23 h/d (0900–0800 h) using ceiling-mounted cameras (Panasonic WV-CP254H, Matsushita Electric Industrial Co. Ltd., Osaka, Japan) attached to a digital video recorder system. Video was recorded both during the 12 h light (0900–2000 h) and the 12 h dark (2000–0800 h) periods. Only 23 h were analyzed because animal caretakers entered the room between 0800 and 0900 h daily, which disturbed the piglets’ natural behavior. Video files were later analyzed in Observer XT 11.5 (Noldus; The Netherlands) by two trained individuals that were blind to the treatments and maintained an agreement of 90% or greater. Behaviors were determined using an instantaneous scan-sampling technique in 10-min intervals and included posture and consumption. Posture behaviors included lying, sitting, and standing and then percent of total observations each pig displayed the behavior was calculated. Consumption behaviors included feeding (head in feeder) and drinking (snout in contact with waterer), or other (anything other than head in feeder or snout in contact with waterer) and then percent of total observations each pig displayed the behavior was calculated. In addition, time to first feeding bout (head in feeder) and time to first drinking bout (snout in contact with waterer) was determined immediately after pigs were placed into their respective pens following simulated transport. On d 7 post-simulated transport, all piglets were weighed and then on d 8 they were euthanized and a section of the jejunum (0.7 m distal to the pyloric sphincter), and ileum (0.2 m anterior to the ileal–cecal junction) were collected and placed into 10% formalin for histology.
Blood Collection and Analyses
Blood samples were collected (BD vacutainers; Franklin Lakes, NJ; serum; 5 mL) 1 d prior to transport, immediately post-transport, and on d 1, 4, and 7 post-transport. All blood samples except for the sample taken at 1600 h immediately post-transport were obtained at 0800 h to correspond with the daily feeding and animal care schedule so that pigs were not disturbed more than once a day. Serum was collected by centrifugation at 4 °C and 1,900 × g for 15 min, aliquoted, and stored at −80 °C. A commercially available ELISA kit was used to determine serum heat shock protein 70 (HSP70; normal range = 4.49 ± 1.36 ng/mL; Johnson et al. 2015a) concentrations (minimum detectable level: 0.8 ng/mL; Porcine HSP70 ELISA Kit, NEO Biolab, Cambridge, MA) following the manufacturer’s instructions. Serum cortisol concentrations (normal range = 41.0 ± 2.3 ng/mL; Marchant-Forde et al., 2012) were analyzed using a commercially available RIA kit (minimum detectable level: 0.9 ng/mL; Cortisol RIA, Tecan Trading AG, Mannedorf, Switzerland) according to manufacturer’s instructions. The intra-assay coefficients of variation were 3.78 and 7.14 for HSP70 and cortisol, respectively. The interassay coefficients of variation were 8.31 and 10.11 for HSP70 and cortisol, respectively. Plate was included in the statistical model to account for interplate variation.
Histology
Histological analyses were performed as previously described (Johnson and Lay, 2017). Briefly, jejunum and ileum samples were fixed in a 10% formalin solution and then referred to the Purdue University Histology and Phenotyping Laboratory for sectioning (5-µm thickness) and staining using toluidine blue and periodic acid-Schiff stain. Each slide contained two sections/tissue/piglet, and each section was imaged three times (n = 6 images/tissue/piglet; 20×) using Q-capture Pro 6.0 software (Qimaging, Surrey, British Columbia, Canada). Mean villus height (µm), mean crypt depth (µm), and mean goblet cell count per villi within each image were determined by one individual using ImageJ 1.47v software (National Institutes of Health; Bethesda, MD). Mean villus height, crypt depth, villus height to crypt depth ratio, and mean goblet cell count per villi for each image were used in the final analysis.
Statistics
Data were analyzed using the PROC MIXED procedure in SAS 9.4 (SAS Institute Inc., Cary, NC). The assumptions of normality of error, homogeneity of variance, and linearity were confirmed post-hoc. All behavioral data (standing%, sitting%, lying%, feeding%, drinking%, other%) were log-transformed to meet assumptions of normality; however, all log-transformed data are presented as arithmetic means for ease of interpretation. During the ELTS phase, litter was the experimental unit and fixed effects included ELTS treatment (ELTN, ELHS, ELCS), day of age (d 7, 8, 9), and all interactions. Average TR, TS, and RR of the litter at 6 d of age was used as a covariate for the analysis of TR, TS, and RR, respectively, during the ELTS phase. For repeated analyses during the ELTS phase, each litter’s respective parameter was analyzed using repeated measures with an auto-regressive covariance structure with day as the repeated effect when required. In the simulated transport period, pen was the experimental unit and fixed effects included ELTS treatment (ELTN, ELHS, ELCS), time (hours 1 to 8), sex (barrow, gilt), and all interactions. No sex differences were observed with any analyses so it was removed from the final model. Average TC of the pen for 4 d prior to transport was used as a covariate for the analysis of TC during simulated transport. For repeated analyses during the simulated transport period, each pen’s respective parameter was analyzed using repeated measures with an auto-regressive covariance structure with time as the repeated effect when required. For the analysis of data in the post-simulated transport phase, fixed effects included ELTS treatment (ELTN, ELHS, ELCS), post-transport day (d 1 to 7), sex (barrow, gilt), and their interaction. No sex differences were observed with any analyses, so it was removed from the final model. Sow was included as a random effect for all analyses in the post-simulated transport phase and plate was included as a random effect for blood parameter analyses in the post-simulated transport phase. Weaning weight was used as a covariate in the analysis of ADG, ADFI, gain:feed, and d 7 BW during the post-simulated transport phase. Preplanned statistical comparisons were conducted for ELTN vs. ELHS or ELCS pigs, and ELHS vs. ELTN or ELCS pigs using the CONTRAST statement of SAS. For repeated analyses during the post-transport period, each pig’s respective parameter was analyzed using repeated measures with an auto-regressive covariance structure with day as the repeated effect when required. Statistical significance was defined as P ≤0.05 and a tendency was defined as 0.05 < P ≤ 0.10.
RESULTS
Thermoregulation
Early life thermal stress phase.
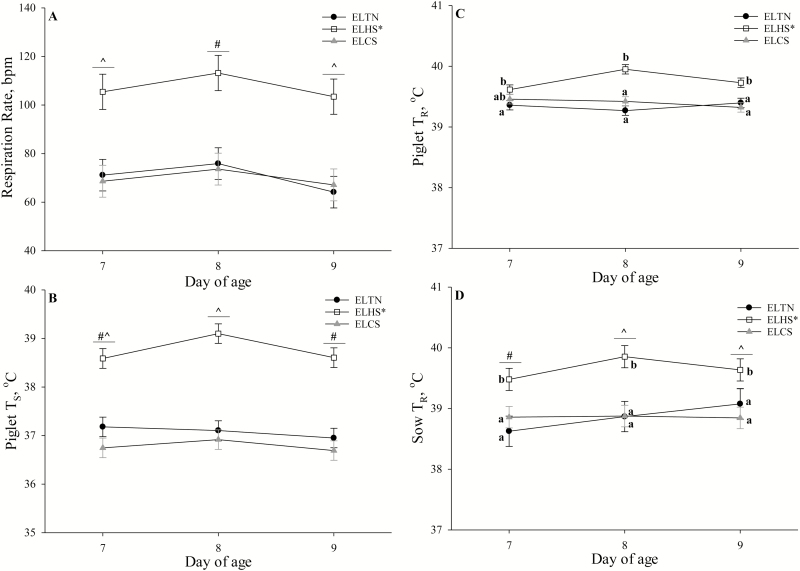
RR was increased overall (P = 0.01; 52.3%) in litters exposed to ELHS compared to ELCS and ELTN, but no differences were detected between litters exposed to ELCS or ELTN (Fig. 2A). A day effect was detected where RR was greatest (P = 0.01; 10%) on d 8 of age compared to d 7 and 9 regardless of early life thermal treatment (Fig. 2A). No ELTS treatment by day effect was detected for RR (P = 0.91; Fig. 2A).
Figure 2.
The effects of early life thermoneutral (ELTN), early life heat stress (ELHS), and early life cold stress (ELCS) conditions on (A) piglet respiration rate by day of age, (B) piglet skin temperature (TS) by day of age, (C) piglet rectal temperature (TR) by day of age, and (D) sow TR by day of age. Error bars indicate ± 1 SE. An asterisk (*) on the legend indicates overall early life thermal stress (ELTS) treatment differences (P ≤ 0.05). #,^Symbols indicate overall day of age differences (P ≤ 0.05). a,bLetters indicate ELTS treatment by day interactions (P ≤ 0.05).
Skin temperature was increased overall (P = 0.01) in ELHS (38.77 ± 0.10 °C) compared to ELCS (36.78 ± 0.10 °C) and ELTN (37.70 ± 0.10 °C) litters, and tended (P = 0.10) to be reduced in ELCS compared to ELTN litters (Fig. 2B). Overall, TS was greatest (P = 0.02) on d 8 but did not differ when comparing d 7 and 9 (Fig. 2B). No ELTS treatment by day interaction was detected for TS (P = 0.24; Fig. 2B).
Piglet TR was increased overall (P = 0.01) in ELHS (39.77 ± 0.05 °C) compared to ELTN (39.34 ± 0.05 °C) and ELCS (39.40 ± 0.05 °C) litters, but no differences were detected between ELTN and ELCS litters (Fig. 2C). Although no day effect was detected (P = 0.44), an ELTS treatment by day effect was observed (P = 0.04) where TR of ELHS and ELTN litters were similar to ELCS pigs but greater in ELHS compared to ELTN litters (Fig 2C).
Sow TR was greater overall (P = 0.01) in ELHS litters (39.66 ± 0.17 °C) compared to ELCS (38.86 ± 0.16 °C) and ELTN (38.86 ± 0.24 °C) litters (Fig. 2D). A day effect was observed (P = 0.01) where sow TR was greater on d 8 and d 9 compared to d 7, but no differences were detected between d 8 and 9 (Fig. 2D). A ELTS treatment by day effect was observed (P = 0.03) where sow TR was increased in ELHS litters on d 7, 8, and 9 compared to ELTN and ELCS litters, but no differences were detected between ELTN and ELCS litters (Fig. 2D).
Simulated transport phase.
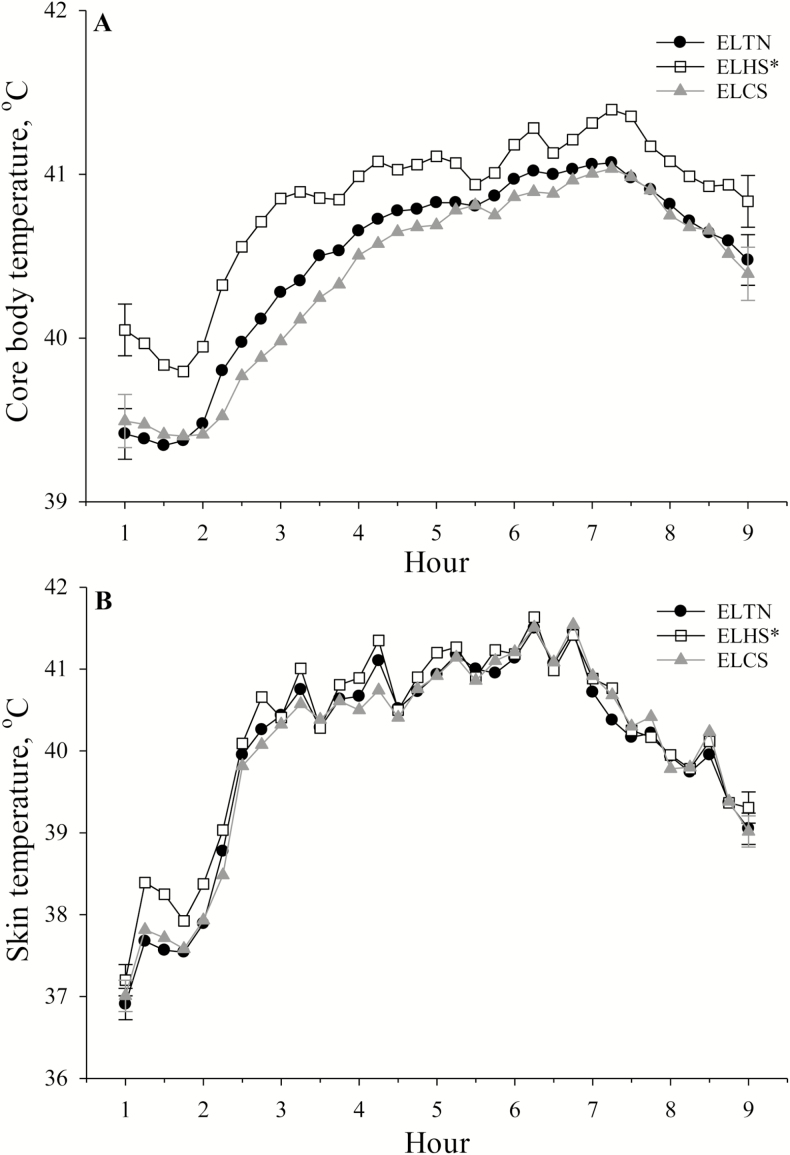
During simulated transport, piglet TC tended to be increased overall (P = 0.06) in ELHS (40.84 ± 0.12 °C) compared to ELTN (40.49 ± 0.12 °C) and ELCS (40.39 ± 0.12 °C) pigs, but no differences were detected between ELTN and ELCS pigs (Fig. 3A). Overall, TC was greater (P = 0.02) in ELHS compared to ELCS or ELTN pigs (Fig. 3A). An overall time effect was observed where TC was increased as time progressed throughout the simulated transport phase and reached a peak at 7 h (Fig. 3A). No other differences were detected for TC.
Figure 3.
The effects of early life thermoneutral (ELTN), early life heat stress (ELHS), and early life cold stress (ELCS) conditions on (A) piglet core body temperature by hour during simulated transport, and (B) piglet skin temperature by hour during simulated transport. Error bars at h 1 and h 9 indicate ± 1 SEM. An asterisk (*) on the legend indicates overall early life thermal stress treatment differences for core body temperature (P = 0.02) and skin temperature (P = 0.03).
Piglet TS tended to be greater overall (P = 0.09) in ELHS (40.20 ± 0.06 °C) when compared with ELTN (40.01 ± 0.06 °C) and ELCS (40.02 ± 0.06 °C) pigs (Fig. 3B). Skin temperature was increased (P = 0.03) in ELHS vs. ELTN or ELCS pigs (Fig. 3B). An overall time effect was detected (P < 0.01) where TS was increased as time progressed throughout the simulated transport phase and reached a peak at 6 h (Fig. 3B). No other differences were detected for TS.
Future Sow Performance
No differences in d to estrus or percent pigs weaned per sow in the next parity were detected following the ELTS phase (Table 1).
Table 1.
Effects of early life thermal stress (ELTS) on piglet performance during the ELTS and post-simulated transport phases and subsequent sow performance following the ELTS treatment
| Parameter | Environmental treatment | SEM | P | ||||
|---|---|---|---|---|---|---|---|
| ELTN* | ELHS† | ELCS‡ | E§ | Contrast 1¶ | Contrast 2║ | ||
| ELTS phase | |||||||
| Initial BW, kg | 2.02 | 1.86 | 1.91 | 0.21 | 0.84 | 0.68 | 0.59 |
| Weaning weight, kg | 5.42 | 5.01 | 4.89 | 0.19 | 0.26 | 0.48 | 0.11 |
| ADG,@ g/d | 230 | 210 | 200 | 1 | 0.15 | 0.52 | 0.06 |
| ELTS ADG,# g/d | 220 | 170 | 170 | 2 | 0.11 | 0.49 | 0.04 |
| Post-simulated transport | |||||||
| BW loss,$ g | 240a | 320b | 210a | 2 | 0.01 | 0.01 | 0.26 |
| ADFI, g/d | 171.8a | 122.7b | 142.6ab | 13.8 | 0.05 | 0.05 | 0.03 |
| ADG, g/d | 160 | 120 | 150 | 2 | 0.29 | 0.15 | 0.28 |
| Gain:feed | 0.98 | 0.86 | 0.97 | 0.12 | 0.73 | 0.44 | 0.65 |
| d 7 BW, kg | 6.65 | 6.27 | 6.57 | 0.18 | 0.29 | 0.15 | 0.28 |
| Sow performance | |||||||
| Days to estrus^ | 5.5 | 6 | 5.8 | 0.5 | 0.84 | 0.60 | 0.64 |
| Pigs weaned,+ % | 96.2 | 100 | 90.1 | 5.1 | 0.37 | 0.31 | 0.88 |
*Pigs exposed to early life thermoneutral conditions on d 7, 8, and 9 post-farrowing.
†Pigs exposed to early life heat stress conditions on d 7, 8, and 9 post-farrowing.
‡Pigs exposed to early life cold stress conditions on d 7, 8, and 9 post-farrowing.
§Environmental treatment.
¶Early life heat stress vs. early life thermoneutral or early life cold stress conditions.
║Early life thermoneutral vs. early life heat stress or early life cold stress conditions.
@ADG of piglets from d 10 to 20 of age.
#ADG of piglets during the ELTS phase (d 7, 8, and 9 of age).
$BW loss during the 8 h simulated transport.
^Weaning to estrus interval following the early life thermal stress treatment.
+Percent of pigs weaned per sow following the early life thermal stress treatment.
a,bLetters indicate significant differences (P ≤ 0.05) within a row.
Growth performance.
Early life thermal stress phase.
Initial BW and weaning weight were similar (P > 0.25; 1.93 ± 0.21 kg and 5.11 ± 0.19 kg, respectively), regardless of ELTS treatment (Table 1). Overall ADG and ADG during the ELTS phase were similar (P > 0.10; 210 ± 1 g/d and 190 ± 2 kg/d, respectively), regardless of ELTS treatment (Table 1). Overall ADG tended to be reduced (P = 0.06; 10.9%) in ELHS or ELCS vs. ELTN pigs, and ADG from d 7 to 9 of the ELTS phase was reduced (P = 0.04; 22.7%) in ELHS or ELCS vs. ELTN pigs (Table 1). No other growth performance differences were detected during the ELTS phase.
Post-simulated transport phase.
BW loss following simulated transport was greater (P = 0.01; 40%) in ELHS when compared with ELCS and ELTN pigs, but no differences were detected between ELCS and ELTN pigs (Table 1).
During the post-simulated transport phase, ADFI was reduced overall (P = 0.05; 28.6%) in ELHS compared to ELTN pigs (Table 1). ADFI was reduced overall (P < 0.05) in ELHS or ELCS vs. ELTN pigs, and in ELHS vs. ELTN or ELCS pigs (Table 1). No other post-simulated transport growth performance differences were detected (Table 1).
Blood parameters.
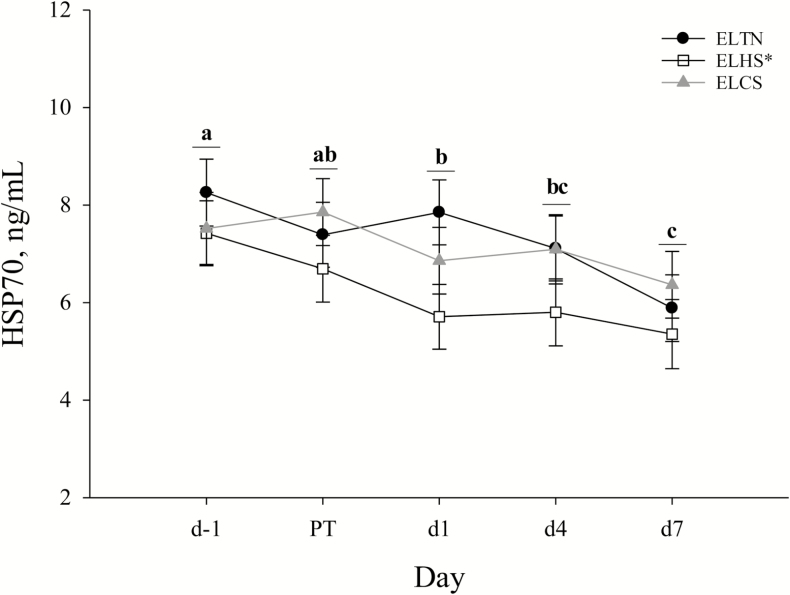
Overall, circulating HSP70 was reduced (P < 0.01) in ELHS (6.20 ± 0.49 ng/mL) compared to ELCS (7.14 ± 0.49 ng/mL) and ELTN (7.30 ± 0.48 ng/mL) pigs (Fig. 4). An overall day effect was detected where circulating HSP70 was reduced (P < 0.01) as days progressed regardless of ELTS treatment (Fig. 4). No ELTS treatment by day differences (P = 0.73) were detected for circulating HSP70 (Fig. 4).
Figure 4.
The effects of early life thermoneutral (ELTN), early life heat stress (ELHS), and early life cold stress (ELCS) conditions on circulating heat shock protein 70 (HSP70) by day. Pre-transport (d−1), immediately post-transport (PT), 24 h post-transport (d1), 4 d post-transport (d4), and 7 d post-transport (d7). Error bars indicate ±1 SE. a,bLetters indicate overall day differences (P ≤ 0.01), and an asterisk (*) on the legend indicates overall early life thermal stress treatment differences (P ≤ 0.05).
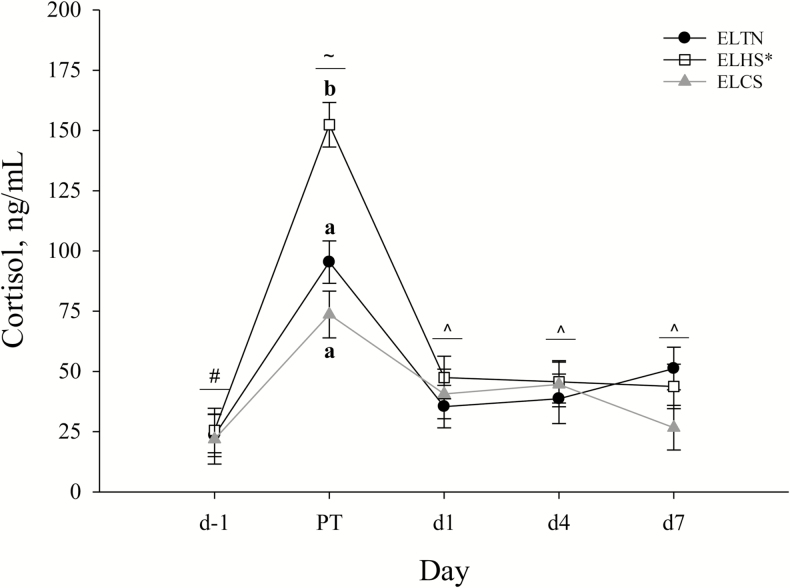
Circulating cortisol was increased overall (P = 0.01) in ELHS (62.97 ± 4.62 ng/mL) compared to ELTN (48.82 ± 4.64 ng/mL) and ELCS (41.48 ± 4.94 ng/mL) pigs, but no differences were detected between ELTN and ELCS pigs (Fig. 5). An overall day effect was observed where cortisol was greater (P < 0.01) for all pigs during PT, d1, d4, and d7 compared to d−1, and was greater during PT compared to all other time points regardless of ELTS treatment (Fig. 5). An ELTS treatment by day effect was observed where circulating cortisol was greater (P < 0.01) immediately post-simulated transport for ELHS (152.42 ± 9.23 ng/mL) compared to ELTN (95.39 ± 8.81 ng/mL) and ELCS (73.64 ± 9.72 ng/mL) pigs (Fig. 5). No other differences in circulating cortisol were observed (Fig. 5).
Figure 5.
The effects of early life thermoneutral (ELTN), early life heat stress (ELHS), and early life cold stress (ELCS) conditions on circulating cortisol by day. Pre-transport (d−1), immediately post transport (PT), 24 h post-transport (d1), 4 d post-transport (d4), and 7 d post-transport (d7). Error bars indicate ±1 SE. a,bLetters indicate early life thermal stress (ELTS) treatment by day differences (P < 0.01), an asterisk (*) on the legend indicates overall ELTS treatment differences (P = 0.01), and #,~,^symbols indicate overall day differences (P < 0.01).
Behavior.
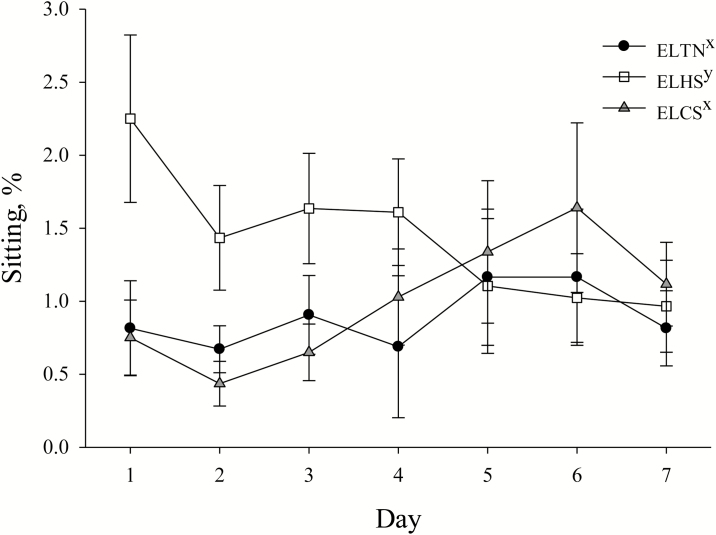
Overall, sitting behavior tended to be increased (P = 0.06; 47.4%) in ELHS vs. ELCS or ELTN pigs (Table 2; Fig. 6). During the dark period (2000–0800 h), sitting behavior was increased (P = 0.01; 188.9%) in ELHS compared to ELCS and ELTN pigs, but no differences were detected between ELCS and ELTN pigs (Table 2). Lying behaviors were increased (P = 0.01) and standing behaviors were reduced (P = 0.01) overall as days progressed, regardless of ELTS treatment (data not presented). Sitting behavior tended to be reduced overall (P = 0.07) during the dark period (2000–0800 h) regardless of ELTS treatment (data not presented). No other posture behavior differences were detected with any analysis (Table 2).
Table 2.
Effects of early life thermal stress on pig posture and consumption behaviors for 7 d post-simulated transport and heat stress exposure
| Parameter | Environmental treatment | SEM | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ELTN* | ELHS† | ELCS‡ | E§ | D¶ | E × D | Contrast 1║ | Contrast 2@ | ||
| Posture | |||||||||
| 0900–0800 h | |||||||||
| Lying, % | 82.9 | 81.3 | 83.6 | 0.5 | 0.23 | 0.01 | 0.51 | 0.10 | 0.69 |
| Standing, % | 16.2 | 17.2 | 15.3 | 0.5 | 0.41 | 0.01 | 0.57 | 0.24 | 0.98 |
| Sitting, % | 0.9 | 1.4 | 1.0 | 0.1 | 0.15 | 0.60 | 0.56 | 0.06 | 0.20 |
| 0900–2000 h | |||||||||
| Lying, % | 77.4 | 76.8 | 79.4 | 0.5 | 0.41 | 0.01 | 0.13 | 0.35 | 0.72 |
| Standing, % | 21.1 | 21.6 | 19.2 | 0.5 | 0.47 | 0.01 | 0.14 | 0.38 | 0.74 |
| Sitting, % | 1.5 | 1.6 | 1.4 | 0.1 | 0.92 | 0.11 | 0.74 | 0.69 | 0.92 |
| 2000–0800 h | |||||||||
| Lying, % | 87.9 | 85.6 | 87.5 | 0.4 | 0.35 | 0.01 | 0.05 | 0.16 | 0.31 |
| Standing, % | 11.7 | 13.1 | 11.8 | 0.4 | 0.68 | 0.01 | 0.14 | 0.38 | 0.57 |
| Sitting, % | 0.3a | 1.3b | 0.6a | 0.1 | 0.01 | 0.07 | 0.11 | 0.01 | 0.03 |
| Consumption | |||||||||
| 0900–0800 h | |||||||||
| Feeding, %# | 8.8 | 8.7 | 8.0 | 0.3 | 0.56 | 0.01 | 0.58 | 0.66 | 0.52 |
| Drinking, %$ | 1.6 | 1.6 | 1.4 | 0.1 | 0.67 | 0.08 | 0.49 | 0.66 | 0.65 |
| Other, %^ | 89.6 | 89.7 | 90.6 | 0.4 | 0.44 | 0.01 | 0.34 | 0.54 | 0.48 |
| 0900–2000 h | |||||||||
| Feeding, % | 10.9 | 10.2 | 9.1 | 0.3 | 0.24 | 0.01 | 0.26 | 0.80 | 0.18 |
| Drinking, % | 2.2 | 2.1 | 2.0 | 0.1 | 0.89 | 0.21 | 0.83 | 0.90 | 0.74 |
| Other, % | 86.9 | 87.7 | 88.9 | 0.3 | 0.20 | 0.01 | 0.22 | 0.75 | 0.16 |
| 2000–0800 h | |||||||||
| Feeding, % | 6.9 | 7.4 | 6.9 | 0.3 | 0.91 | 0.01 | 0.73 | 0.67 | 0.78 |
| Drinking, % | 1.0 | 1.0 | 0.9 | 0.1 | 0.69 | 0.21 | 0.14 | 0.57 | 0.77 |
| Other, % | 92.1 | 91.6 | 92.2 | 0.3 | 0.92 | 0.01 | 0.25 | 0.68 | 0.80 |
| Latency to feed, min:s& | 3:10 | 4:06 | 5:14 | 1:16 | 0.41 | - | - | 0.94 | 0.30 |
| Latency to drink, min:s+ | 1:05 | 1:06 | 1:30 | 0:20 | 0.60 | - | - | 0.65 | 0.58 |
*Pigs exposed to early life thermoneutral conditions on d 7, 8, and 9 post-farrowing.
†Pigs exposed to early life heat stress conditions on d 7, 8, and 9 post-farrowing.
‡Pigs exposed to early life cold stress conditions on d 7, 8, and 9 post-farrowing.
§Environmental treatment.
¶Day.
║Early life heat stress vs. early life thermoneutral or early life cold stress conditions.
@Early life thermoneutral vs. early life heat stress or early life cold stress conditions.
#Head in feeder.
$Snout in contact with waterer.
^Anything other than head in feeder or snout in contact with waterer.
&Time to first head in feeder behavior following simulated transport.
+Time to first snout in contact with waterer behavior following simulated transport.
a,bLetters indicate significant differences (P ≤ 0.05) within a row.
Figure 6.
The effects of early life thermoneutral (ELTN), early life heat stress (ELHS), and early life cold stress (ELCS) conditions on sitting behavior as a percent of a 23 h period (0900–0800 h) on d 1 to 7 post-simulated transport in heat stress (HS) conditions. Error bars indicate ±1 SEM. x,yLetters on the legend indicate overall early life thermal stress treatment tendencies (P = 0.06).
No ELTS treatment differences were detected for consumption behaviors (Table 2). As days progressed, feeding behavior increased (P = 0.01), drinking tended to be increased, and other behavior was reduced (P = 0.01) regardless of ELTS treatment (data not presented). No other consumption behavior differences were detected (Table 2).
Histology.
In the jejunum, no ELTS treatment differences (P > 0.47) were detected for villus height (330.8 ± 30.5 µm), crypt depth (316.1 ± 42.3 µm), or the villus height to crypt depth ratio (1.20 ± 0.17; Table 3). However, an ELTS treatment difference was detected in the jejunum where the number of goblet cells per villi were decreased (P = 0.02; 20%) for ELHS compared to ELCS and ELTN pigs (Table 3). No morphological differences in villus height (351.7 ± 19.2 µm), crypt depth (291.5 ± 31.9 µm), the villus height to crypt depth ratio (1.34 ± 0.19), or goblet cells per villi (17.5 ± 1.2) were detected in the ileum (Table 3).
Table 3.
Effects of early life thermal stress on pig intestinal morphology at 8 d post-weaning and simulated transport in heat stress conditions
| Parameter | Environmental treatment | SEM | P | ||||
|---|---|---|---|---|---|---|---|
| ELTN* | ELHS† | ELCS‡ | E§ | Contrast 1¶ | Contrast 2║ | ||
| Jejunum | |||||||
| Villus height, µm | 340.2 | 322.7 | 329.4 | 30.5 | 0.88 | 0.61 | 0.77 |
| Crypt depth, µm | 352.2 | 279.9 | 316.2 | 42.3 | 0.48 | 0.30 | 0.30 |
| Villus height: crypt depth | 1.11 | 1.28 | 1.20 | 0.17 | 0.78 | 0.55 | 0.53 |
| Goblet cells@ | 13.9a | 11.0b | 13.6a | 0.8 | 0.02 | 0.01 | 0.08 |
| Ileum | |||||||
| Villus height, µm | 347.5 | 364.5 | 343.2 | 19.2 | 0.71 | 0.42 | 0.80 |
| Crypt depth, µm | 313.2 | 261.3 | 299.9 | 31.9 | 0.49 | 0.25 | 0.41 |
| Villus height: crypt depth | 1.23 | 1.52 | 1.28 | 0.19 | 0.51 | 0.25 | 0.45 |
| Goblet cells | 17.7 | 16.6 | 18.3 | 1.2 | 0.59 | 0.34 | 0.88 |
*Pigs exposed to early life thermoneutral conditions on d 7, 8, and 9 post-farrowing.
†Pigs exposed to early life heat stress conditions on d 7, 8, and 9 post-farrowing.
‡Pigs exposed to early life cold stress conditions on d 7, 8, and 9 post-farrowing.
§Environmental treatment.
¶Early life heat stress vs. early life thermoneutral or early life cold stress conditions.
║Early life thermoneutral vs. early life heat stress or early life cold stress conditions.
@Mean number of goblet cells per villi.
a,bLetters indicate significant differences (P ≤ 0.05) within a row.
DISCUSSION
Newly weaned piglets transported under HS conditions incur more stress compared to those transported in TN conditions (Berry and Lewis, 2001; Johnson and Lay, 2017). While completely preventing simultaneous weaning, transport, and HS may improve swine welfare, it is not a feasible solution in modern swine production systems. As such, developing or improving management practices to reduce stress and promote recovery following common production stressors are essential to enhance swine well-being. Although previous studies have attempted to improve thermotolerance in pigs through intrauterine imprinting (Johnson et al. 2013, 2015a), it had the undesired effect of permanently increasing postnatal body temperature regardless of ambient temperature. Despite this, however, studies in rodent and poultry species indicate that ELHS exposure imprints future thermotolerance and may protect against unrelated stressors (Yahav and Hurwitz, 1996; Horowitz, 2007). Therefore, in the present study, lactating sows and their litters were exposed to either TN, HS, or CS conditions on d 7, 8, and 9 post-farrowing. As a result, ELHS litters had an overall increase in RR, TS, and TR compared to ELCS and ELTN litters and an overall reduction in ADG during the ELTS phase compared to ELTN litters indicating that piglets were suffering from hyperthermia (Johnson et al. 2015a, 2015b) while the ELTS treatments were applied. Although ELCS litters were maintained at their lower critical TA (Federation of Animal Science Societies, 2010) without a supplementary heat source, no thermoregulatory differences were detected between ELCS and ELTN piglets. Regardless of the lack of body temperature differences, ADG was reduced in ELCS pigs during the ELTS phase and tended to be decreased overall compared to ELTN piglets. This may imply that ELCS piglets had a greater energy requirement due to increased thermogenesis needed to maintain euthermia resulting in reduced BW gain (Lopez et al., 1991; Johnson et al., 2015b). Furthermore, while the HS treatment resulted in hyperthermia in the sows, no adverse effects on future reproductive efficiency were observed likely due to the short-term cyclical nature of the HS challenge.
Early life HS exposure can improve future thermotolerance and may provide cross-tolerance against unrelated novel stressors (as reviewed by Horowitz, 2007). Specifically, early life heat-acclimated rodents display reduced TC and increased cytoprotective molecule (i.e., heat shock proteins) production in response to a subsequent HS exposure (Horowitz, 2007; Tetievsky and Horowitz, 2010). Furthermore, ELHS poultry species (Arjona et al., 1988; Yahav and Hurwitz, 1996) have improved thermotolerance and performance and reduced mortality in response to a HS challenge later in life. However, in contrast to these reports, ELHS pigs in the present study had increased TC and TS during simulated transport in HS conditions when compared to ELCS and ELTN pigs indicating a greater hyperthermic response. This was surprising considering the aforementioned reports in rodent and poultry species. However, these data were similar to reports in prenatally heat-stressed pigs in which TC was increased during postnatal HS exposure when compared to controls (Johnson et al., 2013). Although, it is tempting to speculate that similar mechanisms are responsible for the effects of prenatal HS and ELHS on future body temperature, the influence of the placental barrier on prenatal HS imprinting in pigs is currently unknown making it difficult to directly compare prenatal HS and ELHS. Furthermore, while reasons for the discrepancy between pigs and other species are currently unclear, differences in future thermotolerance may be due to either the length or timing of ELHS exposure in the present study. It is possible that if piglets had been exposed to ELHS for a longer period of time similar to the aforementioned study in rodents (Tetievsky and Horowitz, 2010) or earlier in their life similar to the aforementioned studies in poultry (Arjona et al., 1988; Yahav and Hurwitz, 1996) that they may have become more thermotolerant later in life. Regardless, it is likely that the greater hyperthermic response of ELHS pigs contributed to reduced performance, increased stress response, and reduced intestinal health of ELHS pigs in the present study. Consequently, no thermoregulatory differences were detected between ELCS and ELTN pigs during the simulated transport; however, this may be due to the fact that the ELCS treatment was not severe enough during the ELTS phase given the lack of thermoregulatory differences when compared to ELTN pigs.
A well-described consequence of HS is an overall reduction in growth performance of all livestock species (as reviewed by Johnson et al., 2015b). In the present study, all pigs had a reduction in BW following the 8 h simulated transport in HS conditions, which was likely due to dehydration (Berry and Lewis, 2001; Johnson and Lay, 2017). However, ELHS pigs had a 40% greater loss in BW following simulated transport compared to ELCS and ELTN pigs and this was likely due to increased hyperthermia because greater TC is associated with an increase in BW loss following transport stress (Berry and Lewis, 2001; Johnson and Lay, 2017). The greater BW loss of ELHS pigs may have negative implications toward future pig welfare and performance as previous reports (Lewis, 2008) suggest that high TA and greater BW loss during transport can reduce the rate at which piglets regain weaning weight. Despite this, no ELTS treatment ADG differences were detected during the post-simulated transport phase. This was surprising considering that ADFI was reduced in ELHS compared to ELTN pigs in the post-simulated transport phase; however, it is possible that ADG and BW differences would have become more apparent had the study been continued for longer as there was a numerical trend for reduced ADG, gain:feed, and decreased d 7 BW for ELHS pigs. Furthermore, because no ADFI differences were detected between ELCS and ELTN pigs, the ELHS or ELCS vs. ELTN difference that was detected by the CONTRAST statement was likely driven by the overall reduction in ADFI for ELHS pigs.
During times of HS, HSP70 production is increased proportionally to hyperthermia severity (Mizzen and Welch, 1988). Heat shock proteins act as cytoprotective molecules that reduce HS-induced cellular damage, play an important role in cellular homeostasis, and are essential for survival during times of elevated ambient temperatures (Kregel, 2002). Although previous studies in heat-stressed pigs have determined that HSP70 gene expression is increased in tissue during times of HS (Pearce et al., 2013b), others have described a reduction in circulating levels during acute HS that was hypothesized to be associated with an increase in cellular uptake to perform cytoprotective functions (Johnson et al., 2015a). In the present study, ELHS pigs had an overall reduction in circulating HSP70 concentrations compared to ELTN and ELCS pigs, which may be indicative of the increased hyperthermic response as previously described (Johnson et al., 2015a). Despite the presence of ELTS differences, no significant difference in circulating HSP70 was detected immediately post-transport compared to d−1. While the lack of HSP70 differences are surprising considering that all pigs were suffering from hyperthermia, it is possible that the blood sample timing was either too late or too early relative to the HS treatment to detect differences since single blood samples are only a snapshot in time.
In addition to the reduction in circulating HSP70, circulating cortisol was greater overall (39%) in ELHS compared to ELTN and ELCS pigs, and this response was magnified (i.e., 80% increase) when compared to ELTN and ELCS pigs immediately post-simulated transport. Cortisol production is increased in response to stress and is often used to quantify stress levels in pigs (i.e., increased cortisol = greater stress response; Averós et al., 2009; Marchant-Forde et al., 2012). Additionally, previous reports in heat-stressed livestock species have described an increase in glucocorticoid production (Collier et al., 1982; Baumgard and Rhoads, 2013). Therefore, it is likely that the greater hyperthermic response of the ELHS piglets resulted in greater circulating levels of cortisol and an increase in their overall stress response. Furthermore, this increase may have negative implications toward the long-term well-being of piglets since increased cortisol production can have deleterious effects on metabolism, gastrointestinal function, growth, and immune function (Chrousos, 2009).
Increased stress can induce behavioral changes in pigs that are indicative of reduced well-being. A reduction in activity and greater lying frequency may suggest an increase in illness (Johnson and von Borell, 1994; Johnson and Lay, 2017), while a greater prevalence of motionless sitting has been associated with a greater incidence of stress. Specifically, pigs that are handled roughly (Pearce et al., 1989) or kept in barren environments (Wood-Gush and Beilharz, 1983) have been shown to have an increase in sitting behavior and it has been suggested that increased sitting behavior is a strategy that enables pigs to cope with greater stress levels (Pearce and Paterson, 1993). In the present study, although no differences in lying, standing, feeding, or drinking behaviors were observed, ELHS pigs tended to have an overall increase in sitting behavior and this was likely driven by a 189% increase in nighttime sitting behavior compared to ELCS and ELTN pigs. In addition, sitting behavior differences were greatest for ELHS compared to ELTN and ELCS pigs in the first 3 d post-weaning and simulated transport in HS conditions and this difference was likely related to the greater stress incurred by ELHS pigs as a result of increased hyperthermia. Furthermore, this behavioral response may be beneficial for producers to identify and treat stressed pigs following weaning and transport.
Hyperthermia causes an increase in intestinal damage due to ischemic injury (Arieli et al., 2003) and hypoxia (Horowitz, 2007) resulting in greater permeability and endotoxin translocation (Lambert, 2009; Johnson et al., 2016) and morphological alterations such as reduced villus height and crypt depth, and a reduction in the villus height to crypt depth ratio (Pearce et al., 2013a; Johnson et al., 2016). Although ELHS pigs had a greater hyperthermic response during simulated transport, no changes in villus height, crypt depth, or the villus height to crypt depth ratio were detected compared to ELCS and ELTN pigs. This result was surprising, however, it may be due to the fact that morphological measures were not quantified until 8 d after the HS challenge and it is likely that greater damage would have been detected in the ELHS pigs had tissue samples been taken closer to the HS challenge. Nevertheless, a reduction in goblet cells per villi was detected in the jejunum of ELHS compared to ELCS and ELTN pigs on d 8 post-weaning and simulated transport. Goblet cells are responsible for producing mucus forming mucins, which is the primary defense mechanism of the intestinal tract (Kim and Ho, 2010). Mucus production lubricates the intestine and prevents bacterial adhesion that can activate the immune system and trigger inflammation (Johansson et al., 2013). Unfortunately, stressors such as weaning (Dunsford et al., 1991), underfeeding (Nuñez et al., 1996), and infection (Jung and Saif, 2017) can reduce goblet cell counts in the villi leading to an impaired mucus layer and increased susceptibility to infection. Furthermore, reductions in goblet cell counts due to stress and infection may be more pronounced in the jejunum compared to the ileum (Boshuizen et al., 2005). Therefore, it is likely that the greater hyperthermic and stress response in ELHS pigs led to a reduction in goblet cell counts compared to ELCS and ELTN pigs, and this may have caused a greater rate of infection and illness had the trial been conducted for a greater length of time.
CONCLUSIONS
We hypothesized that ELHS would improve a pig’s ability to remain euthermic, reduce the stress response, and decrease intestinal damage associated with a HS challenge during simulated transport. However, contrary to our hypothesis, we determined that pigs subjected to ELHS had increased body temperature during HS that likely contributed to a greater stress response when compared to ELCS and ELTN pigs. Although these data have improved our understanding of the impact of ELHS on the future response of piglets, the mechanisms by which ELHS reduces thermotolerance in pigs have yet to be elucidated.
Footnotes
1The authors would like to thank the swine farm staff at Purdue University for animal care. Additionally, thank you to the employees at the USDA-ARS Livestock Behavior Research Unit for assistance in daily animal care and data collection. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. No conflicts of interest, financial, or otherwise are declared by the author(s).
LITERATURE CITED
- Arieli Y., M. Eynan H. Gancz R. Arieli, and Kashi Y.. 2003. Heat acclimation prolongs the time to central nervous system oxygen toxicity in the rat. Possible involvement of HSP72. Brain Res. 962:15–20. doi:10.1016/S0006-8993(02)03681-8 [DOI] [PubMed] [Google Scholar]
- Arjona A. A., Denbow D. M., and Weaver W. D. Jr. 1988. Effect of heat stress early in life on mortality of broilers exposed to high environmental temperatures just prior to marketing. Poult. Sci. 67:226–231. doi:10.3382/ps.0670226 [DOI] [PubMed] [Google Scholar]
- Averós X., Herranz A., Sánchez R., and Gosálvez L. F.. 2009. Effect of duration of commercial journeys between rearing farms and growing-finishing farms on the physiological stress response of weaned pigs. Livestock Sci. 122:339–344. doi:10.1016/j.livsci.2008.09.019 [Google Scholar]
- Baumgard L. H. and Rhoads R. P. Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi:10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Berry R. J., and Lewis N. J.. 2001. The effect of duration and temperature of simulated transport on the performance of early-weaned piglets. Can. J. Anim. Sci. 81:199–204. doi:10.4141/A00-069 [Google Scholar]
- Boshuizen J. A., J. H. Reimerink A. M. Korteland-van Male V. J. van Ham J. Bouma G. J. Gerwig M. P. Koopmans H. A. Büller J. Dekker, and Einerhand A. W.. 2005. Homeostasis and function of goblet cells during rotavirus infection in mice. Virology. 337:210–221. doi:10.1016/j.virol.2005.03.039 [DOI] [PubMed] [Google Scholar]
- Chrousos G. P. 2009. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 5:374–381. doi:10.1038/nrendo.2009.106 [DOI] [PubMed] [Google Scholar]
- Collier R. J., Doelger S. G., Head H. H., Thatcher W. W., and Wilcox C. J.. 1982. Effects of heat stress during pregnancy on maternal hormone concentrations, calf birth weight and postpartum milk yield of Holstein cows. J. Anim. Sci. 54:309–319. [DOI] [PubMed] [Google Scholar]
- Dunsford B. R., Haensly W. E., and Knabe D. A.. 1991. Effects of diet on acidic and neutral goblet cell populations in the small intestine of early weaned pigs. Am. J. Vet. Res. 52:1743–1746. [PubMed] [Google Scholar]
- Federation of Animal Science Societies 2010. Guide for the care and use of agricultural animals in research and teaching. 3rd ed Champaign (IL): Fed. Anim. Sci. Soc. Chap. 11. [Google Scholar]
- Horowitz M. and Robinson S. D.. 2007. Heat shock proteins and the heat shock response during hyperthermia and its modulation by altered physiological conditions. Prog. Brain Res. 162:433–446. doi:10.1016/S0079-6123(06)62021-9 [DOI] [PubMed] [Google Scholar]
- Johansson M. E., Sjövall H., and Hansson G. C.. 2013. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10:352–361. doi:10.1038/nrgastro.2013.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. S., Abuajamieh M., Sanz Fernandez M. V., Seibert J. T., Stoakes S. K., Nteeba J., Keating A. F., Ross J. W., Rhoads R. P., and Baumgard L. H.. 2015b. Thermal stress alters postabsorptive metabolism during pre- and postnatal development. In: Sejian V., J. Gaughan L. Baumgard, eds. Climate change impact on livestock: adaptation and mitigation. India: Springer, pp. 61–80. [Google Scholar]
- Johnson J. S., Boddicker R. L., Sanz-Fernandez M. V., Ross J. W., Selsby J. T., Lucy M. C., Safranski T. J., Rhoads R. P., and Baumgard L. H.. 2013. Effects of mammalian in utero heat stress on adolescent body temperature. Int. J. Hyperthermia. 29:696–702. doi:10.3109/02656736.2013.843723 [DOI] [PubMed] [Google Scholar]
- Johnson J. S. and Lay D. C.. 2017. Evaluating the behavior, growth performance, immune parameters, and intestinal morphology of weaned piglets after simulated transport and heat stress when antibiotics are eliminated from the diet or replaced with L-glutamine. J. Anim. Sci. 95:91–102. doi:10.2527/jas.2016.1070 [DOI] [PubMed] [Google Scholar]
- Johnson J. S., Sanz Fernandez M. V., Seibert J. T., Ross J. W., Lucy M. C., Safranski T. J., Elsasser T. H., Kahl S., Rhoads R. P., and Baumgard L. H.. 2015a. In utero heat stress increases postnatal core body temperature in pigs. J. Anim. Sci. 93:4312–4322. doi:10.2527/jas.2015-9112 [DOI] [PubMed] [Google Scholar]
- Johnson J. S., Sapkota A., and Lay D. C. Jr. 2016. Rapid cooling after acute hyperthermia alters intestinal morphology and increases the systemic inflammatory response in pigs. J. Appl. Physiol. 120:1249–1259. doi.10.1152/japplphysiol.00685.2015 [DOI] [PubMed] [Google Scholar]
- Johnson R. W. and von Borell E.. 1994. Lipopolysaccharide-induced sickness behavior in pigs is inhibited by pretreatment with indomethacin. J. Anim. Sci. 72:309–314.doi:10.2527/1994.722309x [DOI] [PubMed] [Google Scholar]
- Jung K., and Saif L. J.. 2017. Goblet cell depletion in small intestinal villous and crypt epithelium of conventional nursing and weaned pigs infected with porcine epidemic diarrhea virus. Res Vet Sci. 110:12–15. doi:10.1016/j.rvsc.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Kim Y. S. and Ho S. B.. 2010. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 12:319–330. doi:10.1007/s11894-010-0131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel K. C. 2002. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. (1985). 92:2177–2186. doi:10.1152/japplphysiol.01267.2001 [DOI] [PubMed] [Google Scholar]
- Lambert G. P. 2009. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci. 87(14 Suppl):E101–E108. doi:10.2527/jas.2008-1339 [DOI] [PubMed] [Google Scholar]
- Lambooy E. 1988. Road transport of pigs over a long distance: some aspects of behavior, temperature and humidity during transport and some effects of the last two factors. Anim. Prod. 46:257–263. doi:10.1017/S000335610004232X [Google Scholar]
- Lewis N. J. 2008. Transport of early weaned piglets. Appl. Anim. Behav. Sci. 110:128–135. doi:10.1016/j.applanim.2007.03.027 [Google Scholar]
- Lewis N. J., Berry R. J., Crow G., and Wamnes S.. 2005. Assessment of the effects of the season of transport on the performance of early-weaned piglets. Can. J. Anim. Sci. 85:449–454. doi:10.4141/A05-015 [Google Scholar]
- Liu Z., X. Sun J. Tang Y. Tang H. Tong Q. Wen Y. Liu, and Su L.. 2011. Intestinal inflammation and tissue injury in response to heat stress and cooling treatment in mice. Mol. Med. Rep. 4:437–443. doi:10.3892/mmr.2011.461 [DOI] [PubMed] [Google Scholar]
- Lopez J., Jesse G. W., Becker B. A., and Ellersieck M. R.. 1991. Effects of temperature on the performance of finishing swine: II. Effects of a cold, diurnal temperature on average daily gain, feed intake, and feed efficiency. J. Anim. Sci. 69:1850–1855. doi:10.2527/1991.6951850x [DOI] [PubMed] [Google Scholar]
- Marchant-Forde J. N., Matthews D. L., Poletto R., McCain R. R., Mann D. D., DeGraw R. T., Hampsch J. M., Peters S., Knipp G. T., and Kissinger C. B.. 2012. Plasma cortisol and noradrenalin concentrations in pigs: automated sampling of freely moving pigs housed in the PigTurn® versus manually sampled and restrained pigs. Anim. Welfare. 21:197–205. doi:10.7120/09627286.21.2.197 [Google Scholar]
- Mizzen L. A. and Welch W. J.. 1988. Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J. Cell Biol. 106:1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration (NOAA) 2016. National centers for environmental information global analysis [accessed October 31, 2017]. https://www.ncdc.noaa.gov/sotc/global/201702.
- Nuñez M. C., J. D. Bueno M. V. Ayudarte A. Almendros A. Ríos M. D. Suárez, and Gil A.. 1996. Dietary restriction induces biochemical and morphometric changes in the small intestine of nursing piglets. J. Nutr. 126:933–944. [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Gabler N. K., Ross J. W., Escobar J., Patience J. F., Rhoads R. P., and Baumgard L. H.. 2013b. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 91:2108–2118. doi:10.2527/jas.2012–5738 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Mani V., Boddicker R. L., Johnson J. S., Weber T. E., Ross J. W., Baumgard L. H., and Gabler N. K.. 2012. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. PLoS ONE. 8:e70215. doi:10.2527/jas.52339 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Mani V., Weber T. E., Rhoads R. P., Patience J. F., Baumgard L. H., and Gabler N. K.. 2013a. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 91:5183–5193. doi:10.2527/jas.2013-6759 [DOI] [PubMed] [Google Scholar]
- Pearce G. P. and Paterson A. M.. 1993. The effect of space restriction and provision of toys during rearing on the behaviour, productivity and physiology of male pigs. Appl. Anim. Behav. Sci. 36:11–28. doi:10.1016/0168-1591(93)90095–7 [Google Scholar]
- Pearce G. P., Paterson A. M., and Pearce A. N.. 1989. The influence of pleasant and unpleasant handling and the provision of toys on the growth and behaviour of male pigs. Appl. Anim. Behav. Sci. 23:27–37. doi:10.1016/0168-1591(89)90004-X [Google Scholar]
- Smith F., Clark J. E., Overman B. L., Tozel C. C., Huang J. H., Rivier J. E., Blikslager A. T., and Moeser A. J.. 2010. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 298:G352–G363. doi:10.1152/ajpgi.00081.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetievsky A. and Horowitz M.. 2010. Posttranslational modifications in histones underlie heat acclimation-mediated cytoprotective memory. J. Appl. Physiol. (1985). 109:1552–1561. doi:10.1152/japplphysiol.00469.2010 [DOI] [PubMed] [Google Scholar]
- Tipton M. J., Pandolf K. B., Sawka M. N., Werner J., and Taylor N. A. S.. 2008. Physiological adaptation to hot and cold environments. In: N. Taylor, and H. Grollier, eds. Physiological bases of human performance during work and exercise. London, UK: Churchill Livingstone; pp. 379–400. [Google Scholar]
- Wijtten P. J., J. van der Meulen, and Verstegen M. W.. 2011. Intestinal barrier function and absorption in pigs after weaning: a review. Br. J. Nutr. 105:967–981. doi:10.1017/S0007114510005660 [DOI] [PubMed] [Google Scholar]
- Wood-Gush D. G. M., and Beilharz R. G.. 1983. The enrichment of a bare environment for animals in confined conditions. Appl. Anim. Behav. Sci. 10:209–217. [Google Scholar]
- Yahav S. and Hurwitz S.. 1996. Induction of thermotolerance in male broiler chickens by temperature conditioning at an early age. Poult. Sci. 75:402–406. doi:10.3382/ps.0750402 [DOI] [PubMed] [Google Scholar]