Abstract
The precision of a dual-energy X-ray absorptiometry (DXA) device in terms of repeatability and reproducibility was evaluated on nine left half-carcasses from pigs with large variability in body weight and fat content. Repeatability was assessed by scanning each carcass 10 times sequentially in the same position. Reproducibility was assessed by scanning each carcass in 10 different positions. Images were analyzed with DXA software using a custom region of interest (ROI) and the standard head, trunk, arm, and leg ROI. Predicted values from the DEXA for bone mineral content (BMC), bone area, bone mineral density (BMD), total weight, soft-tissue weight, fat-tissue weight, and lean-tissue weight were considered. Repeatability was associated with the variance between measurements on the same carcass in the same position (repeatability conditions). An average variance value was obtained with all the carcasses combined, and the SD was calculated as the square root of this combined variance. The CV was the ratio between the SD of the measurements and its average value. Reproducibility was calculated for each carcass as the difference between the variance obtained under the reproducibility conditions and that obtained under the repeatability conditions. The effects of the ROI and conditions were evaluated by ANOVA and Tukey’s test. Means of BMC, bone area, BMD, fat tissue, and lean tissue differed among the ROI (P < 0.05) in both the repeatability and reproducibility conditions. The CV of DXA measurements under repeatability condition obtained in the head, arm, and leg ROI was lesser than 1%. Only the repeatability errors of fat tissue differed (P < 0.05) among the ROI, with the lowest precision found for the trunk ROI. The reproducibility errors of BMC, bone area, fat tissue, and lean tissue differed (P < 0.05) among the ROI. The custom ROI had reproducibility errors greater than 2% for fat tissue and greater than 3.5% for BMC and bone area. In addition, the trunk ROI had the highest reproducibility errors for fat tissue (20.7%) and lean tissue (6.2%) when compared to the other ROI. In conclusion, repeatability and reproducibility results obtained for most of the studied ROI indicate that DXA is a valuable tool for carcass evaluation. From a methodological viewpoint and considering the variations observed in this study, the ROI should be chosen based on the item to be evaluated or on the conditions in which the DXA measurements are to be taken.
Keywords: accuracy, body composition, dual-energy X-ray absorptiometry, precision, region of interest, pigs
INTRODUCTION
Precisely and accurately assessing body or carcass composition is essential in animal performance studies, genetic evaluations, and selection programs. Dual-energy X-ray absorptiometry (DXA) is a simple and suitable technology that can be used for this purpose.
A DXA device radiates high- or low-energy X-ray beams that are attenuated when they pass through the subject (Pietrobelli et al., 1996). The attenuation of each X-ray beam is different depending on the body tissues (bone, fat, or muscle), and the coefficient of attenuation can, therefore, be used to estimate the subject composition based on reference values (Tothill, 1995; Genton et al., 2002). Although usually developed to assess bone mineralization and body composition in human beings (Makovey et al., 2007), DXA equipment can also be used to assess companion animals (Jeusette et al., 2010), farm animals (Hunter et al., 2011), and carcasses (Ribeiro et al., 2011). These devices have great potential for applicability in animal research studies or genetic improvement programs because the nondestructive nature of DXA means that it can be used to evaluate the body composition of the same animal throughout its growth and before slaughter (Pomar et al., 2009).
In recent decades, DXA devices underwent major improvements that enhanced image quality and reduced radiation exposure. Improvements were also made to DXA algorithms, such as the development of specific adjustments for each region of the human body, which is divided into regions of interest (ROI) in the software used to analyze the DXA images (Nord and Payne, 1995). These updates were made mostly to improve the use of DXA in human health applications but may have implications for the accuracy of measurements obtained in animals, such as pigs. However, the proposed DXA human ROI cannot be used when scanning pork carcasses given the anatomical differences between these two subjects. Pig half-carcasses were used in this study to evaluate in terms of repeatability and reproducibility the precision of a modern DXA device by placing the entire half-carcass within the different DXA proposed ROI.
MATERIALS AND METHODS
Carcasses
Nine left half-carcasses of pigs (Table 1) were obtained from a local meat-packing plant in Quebec, Canada. Backfat thickness and weight were measured in 200 carcasses in a commercial slaughterhouse. Backfat thickness was measured at the Canadian grading site between the third- and fourth-last ribs, 7 cm off the midline, with a Destron grading probe (model PG-100; Anitech Identification System Inc., Markham, Ontario, Canada). Based on the obtained data, nine carcasses were selected to have large variability in terms of weight (“light,” “normal,” or “heavy” based on its weight), backfat thickness (“lean,” “normal,” or “fat” based on its backfat thickness) and sex.
Table 1.
Description of the pig carcasses used in the study
| Carcass | Sex | Hot carcass weight,a kg | Half-carcass weight,b kg | Backfat thickness,c mm |
|---|---|---|---|---|
| 1 | Barrow | 86.1 | 38.5 | 14.0 |
| 2 | Barrow | 99.6 | 44.7 | 16.5 |
| 3 | Barrow | 102.6 | 48.4 | 21.5 |
| 4 | Female | 102.8 | 46.2 | 18.5 |
| 5 | Barrow | 107.3 | 47.5 | 17.5 |
| 6 | Female | 108.6 | 39.0 | 15.5 |
| 7 | Barrow | 115.4 | 51.0 | 14.0 |
| 8 | Barrow | 115.8 | 51.4 | 20.0 |
| 9 | Female | 118.7 | 53.1 | 20.0 |
| Mean | 106.3 | 46.6 | 17.5 | |
| SEM | 3.4 | 1.7 | 0.9 | |
aWith head and without viscera.
bWithout head, kidneys, and leaf fat.
cMeasured at the Canadian grading site between the third- and fourth-last ribs, 7 cm off the midline, with a Destron PG-100 grading probe.
Standard commercial procedures were followed for carcass preparation, including the removal of the head, kidneys, and leaf fat (Canada Pork International, 1995). The carcasses were transported under refrigeration (4 °C) to Agriculture and Agri-Food Canada’s Sherbrooke Research and Development Centre, in Sherbrooke, Quebec, Canada, and stored thereafter in plastic bags at the same temperature. The carcasses were not frozen, to prevent water loss during thawing.
DXA Device
Carcass composition was assessed by DXA using the GE Lunar Prodigy Advance device equipped with the GE Lunar Encore (v. 13.40.038) software package, both from the same company (GE Healthcare, Madison, WI). The manufacturer’s recommended calibration procedure was performed daily before scanning. This DXA device generates 2D projected images in which pixels are classified as bone or as soft tissues (pixel segmentation) based on each pixel’s coefficient of attenuation. Because two X-ray beams are used, only two components can be quantified in the pixels. Thus, in pixels with bone, the amounts of bone and soft tissues are estimated. In pixels without bone (i.e., soft-tissue pixels), the amounts of fat and lean tissue are estimated. Finally, the composition of the soft tissue within the bone pixels is extrapolated from the composition of the soft-tissue pixels around the bone (Pietrobelli et al., 1996). For the scanned subject, this DXA device provides, for the total body or by ROI, the bone mineral content (BMC, g), the projected total bone area (cm2), and the soft-, fat-, and lean-tissue masses (kg). Bone mineral density (BMD, g/cm2) is calculated by the software as the ratio between BMC and bone area.
Repeatability and Reproducibility Tests
Half-carcasses were kept stored in plastic bags under refrigeration (4 °C) prior to the scans. Less than 5 min were spent to transport each carcass from the cold chamber to the DXA room, to position the piece on the table and to start the first scan in this piece. Each scan lasted for around 5 min, and all the scans of a carcass were taken subsequently, to reduce the manipulation and prevent weight loss. The temperature of the room where the scans were performed was maintained at 14 °C to retard the temperature increase in the pieces. The half-carcasses were weighed before and after the scanning procedures. Very low weight loss was observed during the scans, which was on average 54 g (maximum 70 g) per half-carcass.
Two sequential and complementary tests were performed to determine the precision of the DXA measurements in terms of repeatability and reproducibility. The total-body mode with the standard configuration of the software was used in all the scans. The “Smart Scan” feature was disabled during the study.
For the repeatability test, the nine half-carcasses were each scanned 10 times sequentially in the same position (the carcasses were skin up, centered on the table with the belly extended, and scanned from the head to the hind foot; position 1 in Table 2), and the subject (carcass) was not moved during the entire procedure. The number of sequential scans was defined based on a previous study developed using parts of pig body (Nielsen et al., 2004). These measuring conditions provided a single source of variation and were therefore used in this study to estimate the error inherent to the DXA device.
Table 2.
Positioning of the pig carcasses during scanning proceduresa
| Position | Skin | Carcass alignmentb | Belly position | Scan direction |
|---|---|---|---|---|
| 1 | Up | Centered | Extended | Head to hind foot |
| 2 | Up | Centered | Folded | Head to hind foot |
| 3 | Up | Diagonal | Extended | Head to hind foot |
| 4 | Up | Centered | Extended | Hind foot to head |
| 5 | Up | Centered | Folded | Hind foot to head |
| 6 | Down | Centered | Extended | Head to hind foot |
| 7 | Down | Centered | Folded | Head to hind foot |
| 8 | Down | Diagonal | Extended | Head to hind foot |
| 9 | Down | Centered | Extended | Hind foot to head |
| 10 | Down | Centered | Folded | Hind foot to head |
aRepeatability conditions: position 1; reproducibility conditions: positions 1 to 10.
bPosition of the carcass on the scan table of the DXA device.
For the reproducibility test, the nine half-carcasses were scanned once in each of 10 different positions described in Table 2, which were obtained by turning the carcass (skin up vs. skin down), changing the scanning direction (head to hind foot vs. hind foot to head), changing the carcass alignment (centered vs. diagonal), and changing the belly position (extended vs. folded). This design is not symmetrical, because the position was not the object of this study, but was developed to include the same reproducibility error in all carcasses. The design provided two sources of variation: the error inherent to the DXA device (i.e., repeatability) and the variation inherent to the carcass’s position on the scan table.
After the scanning procedure, the DXA system places the ROIs automatically over the image (Fig. 1). These ROI have been originally proposed to correspond to human anatomical landmarks, but the technician can adjust the ROI to other areas of the image under analysis. In the current study, all the images were analyzed with the same DXA software by placing the entire half-carcass within each of the ROI of the standard grid for the human body (head, trunk, arm, and leg; Fig. 2) and a custom ROI (a rectangular region traced manually over the DXA image). Because the scan table was narrower than the carcasses, the front foot was disarticulated at the carpometacarpal joint and positioned perpendicularly to the ventral face of the foreleg (not moved among reproducibility test).
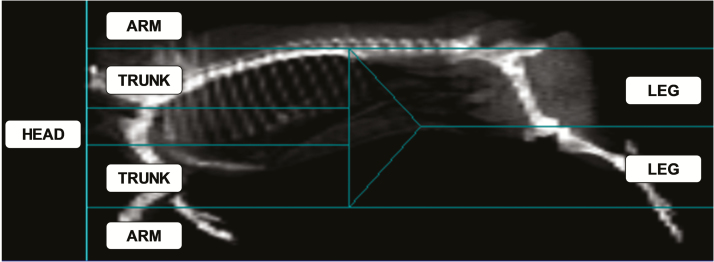
Figure 1.
Image of a pig half-carcass1 obtained by DXA and displayed under the standard grid for the human body with the ROI (head, trunk, arm, and leg) in the original positioning (before adjustment). To obtain the results presented in this study, the lines of each ROI were adjusted to encompass the entire carcass.
1The scan table was narrower than the carcasses, so the front foot was disarticulated at the carpometacarpal joint and positioned perpendicularly to the ventral face of the foreleg.
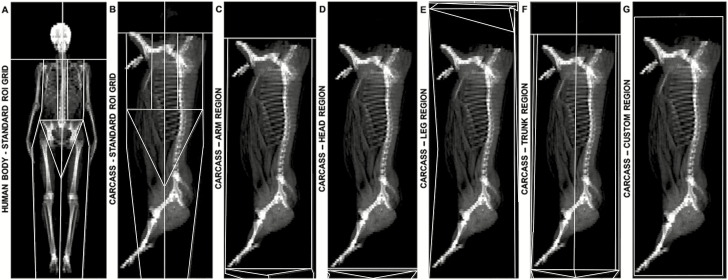
Figure 2.
Images of a human body1 (section A) and pig half-carcass obtained by DXA and displayed under the standard grid for the human body with the original ROI position (section B) or after adjusting the lines of each ROI to encompass the entire carcass (Arm, section C; Head, section D; Leg, section E; Trunk, section F; and Custom region, section G).
1Used to illustrate the adjustment of ROI to the human body. Not used in the study.
Statistical Analysis
The dispersion of the data collected under the repeatability and reproducibility conditions was calculated for each DXA measurement in each ROI. The guidelines of the International Organization for Standardization (ISO, 1993) were followed to estimate repeatability and were adapted for the reproducibility test. Repeatability was associated with the variance between measurements on the same carcass in the same position (repeatability conditions). An average variance value was obtained with all the carcasses combined, and the SD was calculated as the square root of this combined variance (Glüer et al., 1995). The CV was the ratio between the SD of the measurements and its average value. Reproducibility was calculated for each carcass as the difference between the variance obtained under the reproducibility conditions and that obtained under the repeatability conditions. In this approach, lesser values of error variance indicated better repeatability and reproducibility.
ANOVA was used to compare the ROI and the scan conditions (SAS Institute Inc., Cary, NC). The GLM procedure was applied to means of DXA measurements, in which the ROI was considered as fixed effect and the carcass was considered as a random effect. The GLIMMIX procedure was used for CV values, in which the scanning condition was considered as fixed effect and the carcass was considered as a random effect. Tukey’s test at 95% significance was used when necessary.
RESULTS
The carcasses were scanned under the repeatability and reproducibility conditions, and means and CV for BMD, BMC, bone area, total weight, soft-tissue weight, fat-tissue weight, and lean-tissue weight were then obtained using each available ROI in the DXA software (Tables 3 and 4). The DXA measurements were analyzed by first assessing the ROI effects and then comparing the repeatability and reproducibility conditions.
Table 3.
Measurements obtained by DXA in pig carcasses scanned under the repeatability and reproducibility conditions and analyzed using different regions of interest (ROI)
| Item | ROI† | Comparison among ROI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Custom | Head | Trunk | Arm | Leg | SEM | P | ||||||
| Repeatability conditions | ||||||||||||
| BMC, g | 949b | 883c | 983ab | 1008a | 995a | 10.3 | <0.001 | |||||
| Bone area, cm2 | 1045c | 963d | 1074bc | 1120a | 1087b | 8.2 | <0.001 | |||||
| BMD, g cm−2 | 0.907ab | 0.915a | 0.913a | 0.898b | 0.913a | 0.004 | 0.004 | |||||
| Total weight, kg | 46.98 | 47.24 | 46.92 | 46.87 | 46.87 | 0.536 | 0.988 | |||||
| Soft tissue, kg | 46.03 | 46.35 | 45.93 | 45.87 | 45.87 | 0.528 | 0.964 | |||||
| Fat tissue, kg | 9.65a | 5.27c | 8.09b | 8.54b | 8.51b | 0.236 | <0.001 | |||||
| Lean tissue, kg | 36.39b | 41.08a | 37.84b | 37.32b | 37.37b | 0.439 | <0.001 | |||||
| Reproducibility conditions | ||||||||||||
| BMC, g | 967a | 877b | 986a | 1004a | 990a | 10.6 | <0.001 | |||||
| Bone area, cm2 | 1073b | 964c | 1087b | 1125a | 1093ab | 8.8 | <0.001 | |||||
| BMD, g cm−2 | 0.900ab | 0.908a | 0.905a | 0.890b | 0.904a | 0.004 | 0.006 | |||||
| Total weight, kg | 46.91 | 47.21 | 46.65 | 46.82 | 46.82 | 0.539 | 0.966 | |||||
| Soft tissue, kg | 45.94 | 46.34 | 45.67 | 45.83 | 45.83 | 0.531 | 0.922 | |||||
| Fat tissue, kg | 9.79a | 5.21c | 9.84a | 8.68b | 8.64b | 0.247 | <0.001 | |||||
| Lean tissue, kg | 36.15b | 41.12a | 35.83b | 37.14b | 37.19b | 0.451 | <0.001 | |||||
| ROI † | ||||||||||||
| Custom | Head | Trunk | Arm | Leg | ||||||||
| SEM | P | SEM | P | SEM | P | SEM | P | SEM | P | |||
| Comparison between repeatability and reproducibility conditions‡,$ | ||||||||||||
| BMC, g | 2.7 | <0.001 | 0.7 | <0.001 | 1.7 | NS | 0.8 | <0.001 | 0.8 | <0.001 | ||
| Bone area, cm2 | 3.2 | <0.001 | 1.0 | 0.306 | 2.2 | <0.001 | 1.2 | 0.002 | 1.2 | 0.002 | ||
| BMD, g cm−2 | 0.001 | <0.001 | 0.001 | <0.001 | 0.001 | <0.001 | 0.001 | <0.001 | 0.001 | <0.001 | ||
| Total weight, kg | 0.010 | <0.001 | 0.009 | 0.151 | 0.025 | <0.001 | 0.008 | <0.001 | 0.008 | <0.001 | ||
| Soft tissue, kg | 0.011 | <0.001 | 0.009 | 0.329 | 0.026 | <0.001 | 0.008 | <0.001 | 0.008 | <0.001 | ||
| Fat tissue, kg | 0.018 | <0.001 | 0.012 | <0.001 | 0.138 | <0.001 | 0.018 | <0.001 | 0.018 | <0.001 | ||
| Lean tissue, kg | 0.023 | <0.001 | 0.011 | 0.011 | 0.161 | <0.001 | 0.018 | <0.001 | 0.018 | <0.001 | ||
†Means of 90 observations of the same item and conditions compared among the ROI. Within a row, means followed by same or no letter do not differ (P > 0.05) according to Tukey’s test.
‡Means of the same item and ROI were compared between conditions.
$NS = not significant.
Table 4.
Adjusted CV (%) of measurements obtained by DXA in pig carcasses scanned under the repeatability and reproducibility conditions and analyzed using different ROI
| Item1 | ROI† | Comparison among ROI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Custom | Head | Trunk | Arm | Leg | SEM | P | |||||||
| Repeatability conditions | |||||||||||||
| BMC, g | 0.68 | 0.56 | 0.68 | 0.56 | 0.55 | 0.021 | 0.992 | ||||||
| Bone area, cm2 | 1.06 | 0.72 | 0.91 | 0.78 | 0.81 | 0.042 | 0.969 | ||||||
| BMD, g/cm2 | 0.61 | 0.52 | 0.57 | 0.53 | 0.56 | 0.020 | 0.999 | ||||||
| Total weight, kg | 0.07 | 0.04 | 0.10 | 0.04 | 0.04 | 0.005 | 0.977 | ||||||
| Soft tissue, kg | 0.07 | 0.05 | 0.11 | 0.05 | 0.04 | 0.005 | 0.980 | ||||||
| Fat tissue, kg | 0.77b | 0.59b | 4.10a | 0.80b | 0.78b | 0.217 | <0.001 | ||||||
| Lean tissue, kg | 0.24 | 0.09 | 0.96 | 0.17 | 0.17 | 0.051 | 0.059 | ||||||
| Reproducibility conditions | |||||||||||||
| BMC, g | 3.59a | 0.59b | 2.20ab | 0.52b | 0.60b | 0.189 | <0.001 | ||||||
| Bone area, cm2 | 3.75a | 0.85b | 2.37ab | 0.79b | 0.85b | 0.190 | <0.001 | ||||||
| BMD, g/cm2 | 0.64 | 0.64 | 0.67 | 0.67 | 0.62 | 0.033 | 0.999 | ||||||
| Total weight, kg | 0.24 | 0.32 | 0.78 | 0.32 | 0.22 | 0.045 | 0.466 | ||||||
| Soft tissue, kg | 0.29 | 0.24 | 0.81 | 0.22 | 0.22 | 0.046 | 0.445 | ||||||
| Fat tissue, kg | 2.23b | 2.63b | 20.69a | 2.75b | 2.78b | 1.230 | <0.001 | ||||||
| Lean tissue, kg | 0.77b | 0.29b | 6.17a | 0.54b | 0.49b | 0.368 | <0.001 | ||||||
| ROI | |||||||||||||
| Custom | Head | Trunk | Arm | Leg | |||||||||
| SEM | P | SEM | P | SEM | P | SEM | P | SEM | P | ||||
| Comparison between repeatability and reproducibility conditions‡ | |||||||||||||
| BMC, g | 0.382 | 0.002 | 0.055 | 0.574 | 0.237 | 0.024 | 0.037 | 0.593 | 0.046 | 0.514 | |||
| Bone area, cm2 | 0.391 | 0.003 | 0.064 | 0.399 | 0.250 | 0.031 | 0.072 | 0.494 | 0.075 | 0.457 | |||
| BMD, g/cm2 | 0.056 | 0.529 | 0.055 | 0.437 | 0.061 | 0.464 | 0.055 | 0.456 | 0.051 | 0.507 | |||
| Total weight, kg | 0.027 | 0.398 | 0.028 | 0.390 | 0.106 | 0.134 | 0.028 | 0.388 | 0.028 | 0.394 | |||
| Soft tissue, kg | 0.031 | 0.330 | 0.029 | 0.396 | 0.110 | 0.127 | 0.027 | 0.407 | 0.028 | 0.400 | |||
| Fat tissue, kg | 0.240 | 0.026 | 0.310 | 0.010 | 2.690 | <0.001 | 0.30 | 0.012 | 0.310 | 0.010 | |||
| Lean tissue, kg | 0.078 | 0.130 | 0.028 | 0.346 | 0.804 | <0.001 | 0.054 | 0.221 | 0.047 | 0.249 | |||
†Means of 90 observations of the same item and conditions compared among the ROI. Within a row, means followed by same or no letter do not differ (P > 0.05) according to Tukey’s test.
‡Means of the same item and ROI were compared between conditions.
Comparing DXA Measurements Among ROI
Under the repeatability conditions, the BMC values provided by the arm and leg ROI were greater (P < 0.05) than the values obtained using the head and custom ROI. Under the reproducibility conditions, the BMC values obtained using the custom, trunk, arm, and leg ROI were greater (P < 0.05) than the value obtained using the head ROI for image analysis. The lowest and highest values (obtained using the head and arm ROI, respectively) differed from each other by 12.4% in the repeatability test and 12.7% in the reproducibility test. The adjusted CV did not differ (P > 0.05) among the ROI under the repeatability conditions. In the reproducibility test, however, the custom ROI produced measurements with greater (P < 0.05) CV than the head, arm, and leg ROI did, whereas the trunk ROI showed intermediate dispersion.
A similar pattern of ROI effects was observed for bone area, in which the highest (P < 0.05) values were obtained using the arm ROI in both the repeatability and reproducibility tests. These measurements differed by 14% from the lowest value, which was produced by the head ROI under both studied conditions. All ROI showed similar (P > 0.05) adjusted CV in the repeatability test. However, the custom ROI produced measurements with greater (P < 0.05) reproducibility errors than the head, arm, and leg ROI did, with intermediate CV value observed in the trunk ROI.
Similar BMD measurements were obtained under the repeatability and reproducibility conditions using the head, trunk, and leg ROI (P > 0.05). Analyzing the images using the arm ROI produced the lowest BMD (P < 0.05), whereas the custom ROI provided an intermediate value that did not differ from the values for all the other ROI under both measuring conditions. The adjusted CV did not differ among the studied ROI.
All ROI showed similar total and soft-tissue weights in both the repeatability and reproducibility tests. The adjusted CV obtained for these variables also did not differ among the ROI in either of the studied conditions.
The highest (P < 0.05) fat measurement was obtained in the custom ROI under the repeatability conditions, whereas the custom and trunk ROI showed the highest (P < 0.05) fat-tissue values in the reproducibility test. Analyzing the images using the head ROI produced the lowest (P < 0.05) fat estimate under both measuring conditions. The trunk ROI produced fat-tissue measurements with substantially greater (P < 0.05) CV than the other studied ROI had in both the repeatability and reproducibility tests.
Analyzing the carcass images using the head ROI generated greater (P < 0.05) lean-tissue measurements than those obtained in the other ROI under the repeatability and reproducibility conditions. The adjusted CV of lean-tissue measurements did not differ among the ROI in the repeatability test at 95% significance. However, the probability value may be interpreted as a tendency (P = 0.059) toward greater CV in the trunk ROI. In the same way, the trunk ROI produced lean-tissue measurements with substantially greater (P < 0.05) CV than the other studied ROI had in the reproducibility test.
Comparing DXA Measurements Between Repeatability and Reproducibility Conditions
The BMC values obtained using the head, arm, and leg ROI were greater (P < 0.05) under the repeatability conditions when compared to the values produced by the same ROI under the reproducibility conditions. However, similar BMC results were obtained under the repeatability and reproducibility conditions when the trunk ROI was used in the analysis. Finally, using the custom ROI produced lesser (P < 0.05) BMC measurements under the repeatability conditions than under the reproducibility conditions. The CV of the BMC measurements differed (P < 0.05) between the conditions only in the custom and trunk ROI, and as expected, greater error was found in the reproducibility test.
Similar bone area was estimated in the head ROI under the repeatability and reproducibility conditions. However, lesser (P < 0.05) estimates for this response were obtained in the custom, trunk, arm, and leg ROI under the repeatability conditions than under the reproducibility conditions. The CV of bone area showed the same effect pattern as described previously for BMC, with greater (P < 0.05) variation found in the reproducibility test when the custom and trunk ROI were used.
In all the studied ROI, the BMD values obtained under the repeatability conditions were greater (P < 0.05) than those obtained in the reproducibility test. However, the adjusted CV of the BMD measurements did not differ (P > 0.05) between the conditions.
Total and soft-tissue weights were greater (P < 0.05) under the repeatability conditions than under the reproducibility conditions in all ROI except for the head ROI, in which no differences (P > 0.05) were observed. The adjusted CV of the total weight and soft-tissue weight measurements did not differ between the repeatability and reproducibility tests.
The custom, trunk, arm, and leg ROI generated lesser (P < 0.05) fat-tissue weights in the carcasses under the repeatability conditions than under the reproducibility conditions. However, the fat tissue measured in the head ROI was greater (P < 0.05) in the repeatability test than in the reproducibility test. All the ROI produced means of fat tissue with greater (P < 0.05) CV under the reproducibility conditions than under the repeatability conditions.
Contrary to the effect observed in soft-tissue assessment, the lean-tissue values measured in the custom, trunk, arm, and leg ROI were greater (P < 0.05) under the repeatability conditions than under the reproducibility conditions, whereas the value measured in the head ROI was lesser (P < 0.05). The CV of the lean-tissue measurements differed (P < 0.05) between the conditions only in the trunk ROI, with greater variation found in the reproducibility test.
DISCUSSION
Methodology Evaluation
According to the guidelines of the International Organization for Standardization (ISO, 1993), evaluating the accuracy of an instrument involves evaluating the closeness between its measurements and the accepted reference values in terms of trueness and precision. The trueness of a measurement indicates the degree of agreement between the expected value and the reference value, and the precision indicates the degree of internal agreement between independent measurements made under specific conditions. A device is said to be accurate when it is true, i.e., when its measurements correspond to the true values, and to be precise when there is no spread around the true value.
The DXA devices may be very accurate assessing bone, fat, and even lean measurements (Mitchell et al., 1998). However, as outlined previously (Pomar et al., 2017) obtaining the other reference values in live animals or in carcasses is difficult. The morphological or chemical relationship that exists between DXA measurements and dissected carcass tissues or carcass chemical values is somewhat nonrepresentational because tissues that are obtained by dissection contain several chemical components (Marcoux et al., 2003; Pomar et al., 2017). Nonetheless, DXA measurements (i.e., BMC, fat and lean) are highly correlated with total ash, protein, and lipid chemical composition as well as with dissected adipose and muscle tissues (Marcoux et al., 2003). However, dissected bone does not correlate well with BMC or DXA lean mass because bones contain significant amounts of fat, protein, and water (Nielsen, 1973). Different regression techniques can be used to convert DXA measurements into body or carcass compositional measurements, but the regressions are specific to each DXA device, animal type, and body part (Mitchell et al., 1997). In addition, measurement errors in the independent variables can bias regression parameters unless more advanced procedures are used to account for the error variances (Hass et al., 2014).
Because of the complexity and uncertainty of the reference values required to evaluate the trueness of a DXA device, the objective of the present study was to evaluate only the precision of DXA within the different ROI in terms of repeatability and reproducibility. The repeatability error of the DXA equipment was assessed by determining the lack of precision in the measurements produced by the device under very specific measuring conditions. In the current study, results obtained using the same method, on identical test items (carcasses), in the same laboratory, by the same operator, using the same equipment, within short intervals of time are identified as repeatability conditions.
According to the International Organization for Standardization (ISO, 1993), the reproducibility error is the lack of precision in measurements that are obtained by applying the same method, on identical test items, in different laboratories, by different operators, using different equipment. However, the reproducibility conditions were modified in this study to improve our understanding of the implications of the conventional use of DXA devices in animal science, which involves the assessment of subjects (e.g., pigs or other animals scanned throughout their growth, or different carcasses obtained for a research project) by the same team, with the same equipment, using the same technique. Lösel et al. (2010) tested the reproducibility of DXA technology by comparing results obtained for the same pig in different devices. In the current study, the reproducibility conditions were designed to have the same method, test items, laboratory, operator, and equipment but different positioning of the carcasses on the scan table between scans. This method appeared to be valid, as the positioning changes were able to interfere with the obtained DXA results. An example was the skin positioning (up or down), which interfered in all DXA measurements (P < 0.05, data not shown).
Repeatability and Reproducibility
The DXA device produced measurements with very good repeatability in all the studied ROI, confirming that the technology can be used to assess pig carcass composition. Assessment by DXA is already being applied in animal studies and calibration assays (Nielsen et al., 2004; Pomar et al., 2009; Lösel et al., 2010; Ribeiro et al., 2011; Soladoye et al., 2016).
Repeatability is considered one of the most important parameters in the evaluation of a device, mostly because reducing the repeatability error would involve extensive modifications of the technique. However, reducing the reproducibility error is easier and can sometimes be achieved with minor methodological adjustments (Burdick et al., 2005). In addition, knowing the degree to which both repeatability and reproducibility contribute to the error is important for improving the precision of a method. The repeatability error should be lesser than the reproducibility error because more variability (due to carcass positioning) is included in the reproducibility conditions. Although some differences in CV values between the repeatability and reproducibility conditions were not statistically significant, this numerical variation was observed for all the studied items and ROI, which validates the methodology used in this study.
ROI
The software used in this study to evaluate the pig carcasses was developed to assess human body composition. In general, the DXA software divides the human body into four or five ROI (head, trunk, spine, arms, and legs). Each ROI presents particular anatomical characteristics, which are considered by the software by means of algorithms specifically developed to increase the trueness and precision of the measurements (Nord and Payne, 1995). However, these adjustments may not be effective or even helpful for assessing samples other than human beings. In addition, DXA measurements may not be essentially true in nonhuman subjects, and the DXA device, therefore, must be calibrated before use (Hunter et al., 2011; Ribeiro et al., 2011).
Adaptations of the standard ROI grid have been recently investigated as an approach to improve the evaluation of live animals (Suster et al., 2006; Hunter et al., 2011). Previous studies showed that analyzing images of growing pigs using adapted grids produced measurements with lesser CV, especially for fat tissue, compared to the values obtained using the standard grid proposed for human bodies (Suster et al., 2003, 2006). However, those studies used live animals (not carcasses) and other manufacturers’ equipment, differences that highlight the originality of the current study. Very little is known about the adjustments performed by the software in each ROI, not much information is publicly available due to commercial issues. The results of the present study indicate that the precision of DXA results could be modulated depending on the methodology (e.g., ROI) used for image analysis. The particularities of the findings in each studied ROI are discussed in the following sections.
Trunk ROI
The worst precision under the repeatability and reproducibility conditions in this study was found when fat tissues were evaluated using the trunk ROI. The variability of the results obtained for lean tissue under the reproducibility conditions was also greater when the carcass images were analyzed using the trunk ROI compared to the other ROI. These findings are in accordance with a previous study that showed increased variation in DXA measurements taken in live pigs and analyzed using the trunk ROI in comparison with analysis using the standard grid for human bodies (Suster et al., 2006). The lack of precision found in these data may be due to the algorithms used by the software to adjust the measurements in the trunk ROI. As previously stated, these algorithms were developed to assess the composition of the human thoracic region, considering the anatomical particularities of the area, but seem to be inappropriate for analyzing nonhuman subjects.
The soft-tissue content of a human thorax is difficult to estimate. The main reasons for this difficulty are the presence of air in the lungs, the great number of bones, and the overlapping of the bones. The precision of DXA measurements may be affected by the presence of bones because soft-tissue composition in bone pixels is estimated based on soft tissue at the edge of the bone area (Mazess et al., 1990; Jebb, 1997). Despite these issues, the trunk ROI showed good repeatability (0.11%) and good reproducibility (0.82%) for soft-tissue measurements, suggesting that the problems in this region lay not in the identification of soft tissue, but in its classification (fat or lean).
It is also important to point out that the trunk ROI has a subregion called the android. When DXA is used to evaluate human beings, this subregion is positioned outside the rib cage and comprises the abdominal/lumbar region. In this study, when the trunk ROI was expanded to encompass the entire carcass image, the android ROI was automatically positioned over the lesser portion of the image. The android ROI and its particular adjustments affected repeatability but mainly interfered with the reproducibility of the results. In the reproducibility conditions, the repositioning of the carcasses between scans changed the body parts that were placed into the android ROI, which generated an extra source of variation for the data.
Custom ROI
The custom ROI showed good repeatability (CV lesser or marginally greater than 1%) for all DXA measurements. However, this region showed reproducibility errors greater than 2% for fat tissue and greater than 3.5% for BMC and bone area. This lack of reproducibility can be related to the fact that the custom ROI is not adjusted to any specific part of the human body.
Small custom regions were used in several studies to evaluate specific areas of the body (Burkhart et al., 2009; Shepherd et al., 2010). Nielsen et al. (2004) developed a protocol to estimate bone mineralization in pigs based on DXA measurements taken in the front feet. In that study, the average CV of BMD measured in small regions involving phalanx and metacarpus bones was 0.6% with repositioning (which was defined as the removal of the feet from the scan table between scans) and 0.5% without repositioning. These findings are in agreement with the current study, in which similarly precise results were obtained for BMD.
Head, arm, and leg ROI
The head, arm, and leg ROI showed similar dispersion results for all variables. The repeatability errors were lesser than 1% for all the studied DXA responses. These ROI also showed good reproducibility results, supporting the great potential of DXA technology for carcass evaluation even if samples present large variability in terms of shape and the standardization of their positioning on the DXA table. Based on our results, the arm and leg ROI are highly indicated to assess pig half-carcasses using DXA technology.
Some software may have special corrections in the head ROI to consider the fat content of the brain (Hologic, 1996). These adjustments may be related to the greater reproducibility and reproducibility errors observed for fat measurements obtained using the head ROI in this study, in which the carcasses were evaluated without heads. A previous study used live pigs to analyze grid adaptations in the DXA software (enlarging the arm ROI to encompass the whole animal, with or without the head ROI over the skull) and indicated that the presence of the head ROI in the image analysis produced more precise results for fat and BMC (Suster et al., 2006). Therefore, the head ROI should be used with caution, considering the subject characteristics.
In conclusion, repeatability and reproducibility results obtained for most of the studied ROI indicate that DXA is a valuable tool for pork carcass evaluation. From a methodological viewpoint and considering the variations observed in this study, the ROI should be chosen based on the item to be evaluated or on the conditions in which the DXA measurements are to be taken. It is expected that similar results can be obtained in live animals. The DXA method may be less expensive than the traditional slaughter techniques, allows repeated measurements, reduces the errors due to variations in individual weight or composition, and removes operator biases. However, DXA values need to be converted to the true chemical or dissected values to be used in experimental research.
Footnotes
1This project is funded by Swine Innovation Porc within the Swine Cluster 2: Driving Results through Innovation research program. Funding is provided by Agriculture and Agri-Food Canada through the AgriInnovation Program as well as by provincial producer organizations and industry partners. The support of a PDSE fellowship (CAPES, Brazil) is also gratefully appreciated. Special thanks are addressed to N. Ouellet and the employees of the Agriculture and Agri-Food Canada swine complex in Sherbrooke, Quebec, Canada, for their assistance in the research.
LITERATURE CITED
- Burdick R. K., Borror C. M., and Montgomery D. C.. 2005. Design and analysis of gauge R&R studies: making decisions with confidence intervals in random and mixed ANOVA models. ASA and SIAM, Alexandria, VA, and Philadelphia, PA: p. 201. [Google Scholar]
- Burkhart T. A., Arthurs K. L., and Andrews D. M.. 2009. Manual segmentation of DXA scan images results in reliable upper and lower extremity soft and rigid tissue mass estimates. J. Biomech. 42:1138–1142. doi:10.1016/j.jbiomech.2009.02.017 [DOI] [PubMed] [Google Scholar]
- Canada Pork International.. 1995. Canadian Pork Buyer’s Manual. Canada Pork International, Ottawa, Ontario, Canada. [Google Scholar]
- Genton L., Hans D., Kyle U. G., and Pichard C.. 2002. Dual-energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition 18:66–70. [DOI] [PubMed] [Google Scholar]
- Glüer C.-C., Blake G., Lu Y., Blunt B. A., Jergas M., and Genant H. K.. 1995. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos. Int. 5:262–270. doi:10.1007/bf01774016 [DOI] [PubMed] [Google Scholar]
- Hass Z. J., Zhou Z., and Craig B. A.. 2014. Developing prediction equations for carcass lean mass in the presence of proportional measurement error. In: Conference on Applied Statistics in Agriculture, 26th Annual Conference Proceedings, Kansas State University, Manhattan, KS. p. 115–129. [Google Scholar]
- Hologic 1996. QDR 4500 fan beam X-ray bone densitometer user’s guide. Hologic, Waltham, MA: p. 203. [Google Scholar]
- Hunter T. E., Suster D., Dunshea F. R., Cummins L. J., Egan A. R., and Leury B. J.. 2011. Dual energy X-ray absorptiometry (DXA) can be used to predict live animal and whole carcass composition of sheep. Small Rumin. Res. 100:143–152. doi:10.1016/j.smallrumres.2011.07.003 [Google Scholar]
- ISO 1993. ISO 3534-1 Statistics – vocabulary and symbols. Part 1: probability and general statistical terms. ISO, Geneva, Switzerland, p. 47. [Google Scholar]
- Jebb S. A. 1997. Measurement of soft tissue composition by dual energy X-ray absorptiometry. Br. J. Nutr. 77:151–163. doi:10.1079/BJN19970021 [DOI] [PubMed] [Google Scholar]
- Jeusette I., Greco D., Aquino F., Detilleux J., Peterson M., Romano V., and Torre C.. 2010. Effect of breed on body composition and comparison between various methods to estimate body composition in dogs. Res. Vet. Sci. 88:227–232. doi:10.1016/j.rvsc.2009.07.009 [DOI] [PubMed] [Google Scholar]
- Lösel D., Kremer P., Albrecht E., and Scholz A. M.. 2010. Comparison of a GE Lunar DPX-IQ and a Norland XR-26 dual energy X-ray absorptiometry scanner for body composition measurements in pigs – in vivo. Arch. Tierzucht. 53:162–175. [Google Scholar]
- Makovey J., Nguyen T. V., Naganathan V., Wark J. D., and Sambrook P. N.. 2007. Genetic effects on bone loss in peri- and postmenopausal women: a longitudinal twin study. J. Bone Miner. Res. 22:1773–1780. [DOI] [PubMed] [Google Scholar]
- Marcoux M., Bernier J. F., and Pomar C.. 2003. Estimation of Canadian and European lean yields and composition of pig carcasses by dual-energy X-ray absorptiometry. Meat Sci. 63:359–365. doi:10.1016/S0309-1740(02)00094-3 [DOI] [PubMed] [Google Scholar]
- Mazess R. B., Barden H. S., Bisek J. P., and Hanson J.. 1990. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am. J. Clin. Nutr. 51:1106–1112. doi:10.1093/ajcn/51.6.1106 [DOI] [PubMed] [Google Scholar]
- Mitchell A. D., Rosebrough R. W., and Conway J. M.. 1997. Body composition analysis of chickens by dual energy x-ray absorptiometry. Poult. Sci. 76:1746–1752. doi:10.1093/ps/76.12.1746 [DOI] [PubMed] [Google Scholar]
- Mitchell A. D., Scholz A. M., and Conway J. M.. 1998. Body composition analysis of small pigs by dual energy x-ray absorptiometry. J. Anim. Sci. 76:2392–2398. doi:10.2527/1998.7692392x [DOI] [PubMed] [Google Scholar]
- Nielsen A. J. 1973. Anatomical and chemical composition of Danish Landrace pigs slaughtered at 90 kilograms live weight in relation to litter, sex and feed composition. J. Anim. Sci. 36:476–483. doi:10.2527/jas1973.363476x [Google Scholar]
- Nielsen D. H., McEvoy F. J., Poulsen H. L., Madsen M. T., Buelund L. E., and Svalastoga E.. 2004. Dual-energy X-ray absorptiometry of the pig: protocol development and evaluation. Meat Sci. 68:235–241. doi:10.1016/j.meatsci.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Nord R. H., and Payne R. K.. 1995. Body composition by dual-energy X-ray absorptiometry – a review of the technology. Asia Pac. J. Clin. Nutr. 4:167–171. [PubMed] [Google Scholar]
- Pietrobelli A., Formica C., Wang Z., and Heymsfield S. B.. 1996. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am. J. Physiol. Endocrinol. Metab. 271:E941–E951. doi:10.1152/ajpendo.1996.271.6.E941 [DOI] [PubMed] [Google Scholar]
- Pomar C., Kipper M., and Marcoux M.. 2017. Use of dual-energy x-ray absorptiometry in non-ruminant nutrition research. Rev. Bras. Zootec. 46:621–629. doi:10.1590/S1806-92902017000700010 [Google Scholar]
- Pomar C., Marcoux M., Gispert M., Font i Furnols M., and Daumas G.. 2009. Determining the lean content of pork carcasses. In: Kerry J. P., and D. Ledward, editors. Improving the sensory and nutritional quality of fresh meat. Woodhead Publishing, Cambridge, UK: p. 493–518. [Google Scholar]
- Ribeiro F. R. B., Tedeschi L. O., Rhoades R. D., Smith S. B., Martin S. E., and Crouse S. F.. 2011. Evaluating the application of dual X-ray energy absorptiometry to assess dissectible and chemical fat and muscle from the 9th-to-11th rib section of beef cattle. Prof. Anim. Sci. 27:472–476. doi:10.15232/S1080-7446(15)30521-0 [Google Scholar]
- Shepherd J. A., Fan B., Lu Y., Marquez L., Salama K., Hwang J., and Fung E. B.. 2010. Dual-energy X-ray absorptiometry with serum ferritin predicts liver iron concentration and changes in concentration better than ferritin alone. J. Clin. Densitom. 13:399–406. doi:10.1016/j.jocd.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soladoye O. P., López Campos Ó., Aalhusa J. L., Gariépy C., Shand P., and Juárez M.. 2016. Accuracy of dual energy X-ray absorptiometry (DXA) in assessing carcass composition from different pig populations. Meat Sci. 121:310–316. doi:10.1016/j.meatsci.2016.06.031 [DOI] [PubMed] [Google Scholar]
- Suster D., Leury B. J., Kerton D. J., and Dunshea F. R.. 2006. Repeatability of pig body composition measurements using dual energy X-ray absorptiometry and influence of animal size and subregional analyses. Aust. J. Exp. Agric. 46:1447–1454. doi:10.1071/EA04279 [Google Scholar]
- Suster D., Leury B. J., Ostrowska E., Butler K. L., Kerton D. J., Wark J. D., and Dunshea F. R.. 2003. Accuracy of dual energy X-ray absorptiometry (DXA), weight and P2 back fat to predict whole body and carcass composition in pigs within and across experiments. Livest. Prod. Sci. 84:231–242. doi:10.1016/S0301- 6226(03)00077-0 [Google Scholar]
- Tothill P. 1995. Dual-energy X-ray absorptiometry for the measurement of bone and soft tissue composition. Clin. Nutr. 14:263–268. doi:10.1016/S0261-5614(95)80062-X [DOI] [PubMed] [Google Scholar]