Abstract
This study evaluated the effects of gradual reduction in frequency of energy supplementation following vaccination on growth and measurements of innate and humoral immunity of beef steers. At 14-d postweaning (d 0), Angus steers (n = 42; 200 ± 5 kg of BW; 175 ± 4 d of age) were stratified by BW and age, and randomly assigned into 1 of 14 drylot pens (three steers/pen). From d 0 to 42, steers were provided ad libitum ground tall fescue hay (57% TDN, 13% CP of DM basis) and supplemented with concentrate at 1% of BW (50:50 soybean hulls and corn gluten feed; 71% TDN, 15% CP of DM basis). Treatments were randomly assigned to pens, and consisted of similar weekly concentrate DM supplementation (1% of BW multiplied by 7 d) that was divided and offered daily from d 0 to 42 (7X; 4 pens), 3 times weekly from d 0 to 42 (3X; Monday, Wednesday, and Friday; 5 pens), or daily from d 0 to 15 and then 3 times weekly from d 16 to 42 (7-3X; 5 pens). Steers were vaccinated against infectious bovine rhinotracheitis (IBR), bovine viral diarrhea virus (BVDV), parainfluenza-3 (PI-3), Mannheimia haemolytica, and Clostridium on d 0 and 15. Individual shrunk BW was collected on d 0 and 42, following 12 h of feed and water withdrawal. Blood samples were collected via jugular venipuncture 4 h after concentrate supplementation on d 0, 1, 2, 3, 7, 15, 16, 17, 18, 22, and 42. Mean BW, ADG, G:F, hay DMI, and total DMI over the 42-d period did not differ among treatments (P ≥ 0.26). Plasma concentrations of cortisol and mean serum BVDV-1a titers also did not differ among treatments (P ≥ 0.35), but overall plasma haptoglobin concentrations were greater for 3X vs. 7-3X and 7X steers (P ≤ 0.05; 0.44, 0.37, and 0.33 ± 0.026 mg/mL, respectively). Also, 3X steers had less mean serum IBR titers (P ≤ 0.05; 0.29 vs. 0.88 and 0.79 ± 0.179 log2, respectively) and less seroconversion to PI-3 virus on d 15 than 7-3X and 7X steers (P ≤ 0.05; 36.0 vs. 76.6 and 57.8 ± 8.24%, respectively). In summary, a gradual reduction in frequency of energy supplementation during a 42-d preconditioning period did not negatively impact growth, but alleviated indices of inflammation and prevented reductions in vaccine response against BVDV-1a and PI-3 viruses compared to steers offered concentrate 3 times weekly during the entire study.
Keywords: immune, steers, supplementation frequency, vaccination
INTRODUCTION
Beef calves that are preconditioned typically experience multiple management processes (i.e., weaning, vaccination, and feedlot entry) that elicit an acute-phase protein response (APR) and impair growth performance (Arthington et al., 2008, 2013). Recently, weaned beef calves also require energy supplementation to meet their nutrient requirements and improve BW gain (NRC, 2016), but daily concentrate supplementation increases production costs associated with labor, fuel, and equipment (Loy et al., 2007; Cooke et al., 2008). Decreasing the frequency of energy supplementation from daily to three times weekly reduced feeding costs without changing the weekly supplement amount (Drewnoski et al., 2014). However, this strategy leads to fluctuations in forage and nutrient intake, which further exacerbates the postvaccination APR and decreases growth and vaccine-induced production of antibodies against bovine viral diarrhea virus (BVDV) type 1b (Artioli et al., 2015) and 1a (Moriel et al., 2016a). The hypothesis of the current study was that gradually reducing the frequency of energy supplementation by offering daily concentrate supplementation during the vaccine-induced inflammatory response (first 14 d after the first round of vaccination), and then reducing concentrate supplementation to three feeding events weekly until the end of a 42-d preconditioning period, would prevent a heightened postvaccination APR and a reduction on BW gain. Consequently, negative impacts of reduced frequency of energy supplementation on immunity and growth of recently weaned beef steers would be prevented. Hence, this study evaluated growth performance and measurements of innate and humoral immunity of beef steers offered different frequencies of energy supplementation during a 42-d preconditioning period.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee of NC State University (protocol #16-032-A) approved all procedures for the experiment conducted at the Mountain Research Station (Waynesville, NC; 35.48°N, 82.99°W; elevation = 659 m) from May to July 2016.
Animals, Diets, and Sample Collection
Angus steers (n = 42; 200 ± 5 kg of BW; 175 ± 4 d of age) were weaned on d 14, immediately allocated into a single 22-ha tall fescue pasture (Lolium arundinaceum; 16% CP and 59% TDN; DM basis), and provided concentrate DM at 0.5% of BW (50:50 soybean hulls pellets and corn gluten pellets; Table 1) and free-choice access to white salt for 14 d. On d 0, steers were stratified by BW and age, and randomly assigned into 1 of 14 drylot pens (3 steers/pen; 18 × 4 m; 24 m2/steer) in a concrete floor, half-covered drylot feeding facility. From d 0 to 42, steers were provided free-choice access to ground tall fescue hay and supplemented with concentrate DM at 1% of BW (50:50 soybean hulls pellets and corn gluten pellets; Table 1). Treatments were randomly assigned to pens and consisted of similar weekly concentrate DM supplementation (1% of BW multiplied by 7 d) that was divided and offered daily from d 0 to 42 (7X; 4 pens), three times weekly from d 0 to 42 (3X; Monday, Wednesday, and Friday; 5 pens), or daily from d 0 to 15 and then three times weekly from d 16 to 42 (7-3X; 5 pens).
Table 1.
Average weekly chemical composition of ground tall fescue hay and concentrate (50% soy hulls and 50% corn gluten feed; as-fed basis) provided to steers from d 0 to 42*
| Item | Tall fescue hay | Concentrate† |
|---|---|---|
| DM, % | 90.2 | 93.4 |
| ------------ DM basis ------------- | ||
| CP, % | 13.5 | 15.3 |
| ADF, % | 36.8 | 31.1 |
| NDF, % | 62.7 | 53.3 |
| TDN‡, % | 56.5 | 71.0 |
| NEm§, Mcal/kg | 1.09 | 1.61 |
| NEg§, Mcal/kg | 0.54 | 1.01 |
*Hay and concentrate samples were collected daily, pooled within each wk, and sent in duplicate to a commercial laboratory for wet chemistry analysis (Dairy One Forage Laboratory, Ithaca, NY).
†Same concentrate was used from weaning to end of study (d 14 to 42) and consisted of 50% soybean hulls pellets and 50% corn gluten feed pellets (DM basis).
‡Calculated as described by Weiss et al. (1992).
§Calculated using the equations proposed by the NRC (2000).
Hay and concentrate were offered separately in the same concrete feed bunk at 0800 h. Weekly concentrate DM offered was estimated based on average shrunk BW of each pen on d 0, and readjusted on d 15 and 30 using average full BW of each pen obtained before feeding. Individual BW was measured before feeding on d 0 and 42, following 12 h of feed and water withdrawal. Shrunk BW was not obtained on d 15 and 30 to not disturb feeding behavior and avoid an unnecessary physiological stress response due to shrink that could interfere with plasma measurements and vaccine response (Marques et al., 2012). A complete mineral/vitamin mix (RU-MIN 1600, Southern States, Richmond, VA; average composition, DM basis: 18.2% Ca, 0.72% K, 0.88% Mg, 0.76% S, 7.0% Na, 10.8% Cl, 2.9 % P, 29 mg/kg Co, 1,220 mg/kg Cu, 2,130 mg/kg Mn, 29 mg/kg Se and 2,530 mg/kg Zn) was top-dressed daily over the supplement at a rate of 0.114 g/steer from d 0 to 42.
Steers consumed 100% of the concentrate DM offered within 6 h after supplementation. Hay DM offered and refused were obtained daily for each pen by drying samples of hay offered and refused in a forced-air oven at 56°C for 48 h. Daily DMI was determined by subtracting the daily hay DM refused from the daily hay DM offered. Immediately before feeding, samples of hay, concentrate, and mineral/vitamin mix offered to steers were collected daily from each pen (approximately 100 g of each sample/pen), pooled within each week (1 to 6), and then sent in duplicate (approximately 100 g of each pooled sample) to a commercial laboratory (Dairy One Forage Laboratory, Ithaca, NY) for wet chemistry analysis of all nutrients (Table 1). Samples were analyzed for concentrations of CP (method 984.13; AOAC, 2006), ADF (method 973.18 modified for use in an Ankom 200 fiber analyzer; Ankom Technology Corp., Fairport, NY; AOAC, 2006), and NDF (Van Soest et al., 1991; modified for use in an Ankom 200 fiber analyzer; Ankom Technology Corp.). Concentrations of TDN were calculated as proposed by Weiss et al. (1992), whereas NEm and NEg were calculated using equations from NRC (2000).
On d 0, all steers were treated with doramectin for internal and external parasites (5 mL s.c.; Dectomax injectable, Zoetis Inc., Kalamazoo, MI), and were vaccinated against infectious bovine rhinotracheitis (IBR), BVDV-1a and -2, parainfluenza-3 virus (PI-3), Mannheimia haemolytica (2 mL s.c; Bovi Shield Gold One Shot; Zoetis Inc., New York, NY), and clostridium (2 mL s.c; Ultrabac 7, Zoetis Inc., New York, NY). On d 15, steers received 2-mL s.c. boosters of Bovi Shield Gold 5 (Zoetis Inc., New York, NY) and Ultrabac 7. The vaccination protocol described above was chosen to replicate the protocol utilized by the local preconditioning alliance (Mountain Cattle Alliance, Canton, NC; Artioli et al., 2015; Moriel et al., 2015, 2016a).
Blood samples (10 mL) were collected via jugular venipuncture into sodium-heparin (158 USP) containing tubes (Vacutainer, Becton Dickinson, Franklin Lakes, NJ) for plasma harvest 4 h after concentrate supplementation on d 0, 1, 2, 3, 7, 15, 16, 17, 18, and 22. The approach of collecting blood samples 4 h after concentrate supplementation was utilized to correspond to the peak of ruminal fermentation and end products release after concentrate consumption (Moriel et al., 2012, 2015, 2016a; Artioli et al., 2015). Additional blood samples (10 mL) from jugular vein were collected into tubes containing no additives (Vacutainer, Becton Dickinson) for serum harvest on d 0, 15, and 42 to evaluate serum antibody titers against IBR, BVDV-1a, and PI-3 viruses. Blood samples were immediately placed on ice following collection and then centrifuged at 1,200 × g for 25 min at 4°C. Plasma and serum samples were stored at −20°C until later laboratory analysis.
Laboratory Analyses
Plasma concentrations of haptoglobin were determined in duplicate samples using a biochemical assay assessing haptoglobin-hemoglobin complex by the estimation of differences in peroxidase activity (Cooke and Arthington, 2013). Plasma concentrations of cortisol were determined using a single chemiluminescent enzyme immunoassay (Immulite 1000; Siemens Medical Solutions Diagnostics, Los Angeles, CA). Intra-assay and interassay CV for assays of haptoglobin and cortisol were 3.0 and 5.7, and 2.5 and 3.1%, respectively.
Serum antibody titers against IBR, BVDV-1a, and PI-3 viruses were determined by the Oklahoma Animal Disease and Diagnostic Laboratory using a virus neutralization test (Rosenbaum et al., 1970). Serum titers were reported as the log2 of the greatest dilution of serum that provided complete protection of the cells (lowest and greatest tested dilution = 1:4 and 1:256, respectively). For the seroconversion analyses, samples with serum neutralization value of <4 were considered negative and assigned a value of 0, whereas samples with serum neutralization value ≥4 were considered positive and assigned a value of 1. Then the assigned values (0 or 1) were used to calculate the positive seroconversion (% of steers with positive serum neutralization; Richeson et al., 2008; Artioli et al., 2015; Moriel et al., 2015, 2016a).
Statistical Analyses
Except for seroconversion, all data were analyzed as a completely randomized design using the MIXED procedure of SAS (SAS Institute Inc., Cary, NC, USA, version 9.4) with Satterthwaite approximation to determine the denominator degrees of freedom for the test of fixed effects. Pen was the experimental unit, and steer(pen) and pen(treatment) were included as random effects in all analyses, except for analyses of G:F, hay DMI, and total DMI that included only pen(treatment) as random effect. Overall G:F, ADG, hay, and total DMI (d 0 to 42) were tested for fixed effects of frequency of supplementation.
From d 0 to 15, 3X steers were supplemented three times weekly, whereas 7X and 7-3X steers where supplemented daily. From d 16 to 42, 3X and 7-3X steers received supplementation three times weekly, whereas only 7X steers received daily supplementation. Hence, in order to simplify data analyses and interpretation, daily hay and total DMI, and daily total intake of CP, and TDN of steers were divided into two periods: d 0 to 15 and 16 to 42. Daily hay DMI and total DMI, and total intake of CP and TDN (kg/d) were pooled by days that all steers were fed concentrate (Monday, Wednesday, and Friday), and days that concentrate supplementation was offered only to 7-3X and 7X steers (d 0 to 15) or only to 7X steers (d 16 to 42; Tuesday, Thursday, Saturday, and Sunday). Daily hay DMI and total DMI, and total intake of CP and TDN were analyzed as repeated measures and tested for fixed effects of supplementation frequency, week of the study, day of the week, and all resulting interactions, using steer(pen) as the subject. Body weight, plasma, and serum measurements were analyzed as repeated measures and tested for fixed effects of frequency, day of the study, week of the study (except for BW analyses), and all resulting interactions, using steer(pen) as the subject. Compound symmetry covariance structure was used for all the repeated measures analyses as this covariance structure generated the lowest Akaike information criterion. Positive seroconversion to IBR, BVDV-1a, and PI-3 viruses were analyzed using the GLIMMIX procedure of SAS with pen(treatment) and steer(pen) as random effects. All results are reported as least-squares means. Data were separated using PDIFF if a significant preliminary F-test was detected. Significance was set at P ≤ 0.05, and tendencies if P > 0.05 and ≤0.10.
RESULTS
Steer BW on d 0 did not differ among treatments (P ≥ 0.93; 202, 200, and 200 kg for 3X, 7-3X, and 7X steers, respectively), but was included as a covariate (P < 0.01) for the analyses of BW over time (Table 2). Effects of frequency of supplementation × day of study and frequency were not detected (P ≥ 0.19) for BW and ADG from d 0 to 42 (Table 2).
Table 2.
Growth performance of beef steers provided similar weekly concentrate DM that was divided and offered daily from d 0 to 42 (7X; four pens), three times weekly from d 0 to 42 (3X; Monday, Wednesday, and Friday; five pens), or daily from d 0 to 15 and then three times weekly from d 16 to 42 (7-3X; five pens)*
| Treatment | P value‡ | ||||
|---|---|---|---|---|---|
| Item | 3X | 7-3X | 7X | SEM† | Frequency |
| BW§, kg | |||||
| d 15 | 232 | 233 | 231 | 2.6 | 0.47 |
| d 30 | 240 | 243 | 237 | 2.6 | |
| d 42 | 242 | 243 | 238 | 2.6 | |
| d 0 to 42 | |||||
| ADG¶, kg/d | 0.85 | 0.96 | 0.89 | 0.062 | 0.44 |
| Total hay DMI, kg/steer | 99.1 | 110.0 | 104.3 | 4.11 | 0.27 |
| Total DMI$, kg/steer | 192.4 | 200.6 | 199.2 | 3.98 | 0.38 |
| G:F# | 0.196 | 0.212 | 0.191 | 0.0135 | 0.57 |
*From d 0 to 42, steers were provided daily free-choice access to ground tall fescue hay. Weekly concentrate DMI = 1% of BW multiplied by 7 d.
†Pen as the experimental unit (three steers/pen; four pens for 7X and five pens for 3X and 7-3X treatments).
‡ P value for the main effects of treatment.
§Least square means covariate-adjusted to BW on d 0 (P < 0.01). Individual BW was measured on d 0 and 42, following 12 h of feed and water withdrawal. Full BW was obtained on d 15 and 30 to not disturb feeding behavior and avoid an unnecessary physiological stress caused by feed and water withdrawal.
¶Calculated using shrink BW recorded on d 0 and 42. Calculations of ADG from d 0 to 15, 15 to 30, and 30 to 42 were not performed to avoid data misinterpretation due to gut fill effects of full BW recorded on d 15 and 30.
$Total DMI obtained from consumption of hay and concentrate.
#Calculated by dividing total BW gain by total DMI from d 0 to 42.
Effects of frequency of supplementation × day of the week (P < 0.01), but not frequency × day of the wk × wk of the study (P ≥ 0.12), were detected for hay and total DMI (Table 3). From d 0 to 15, hay and total DMI of 7-3X and 7X steers did not differ throughout the wk (P ≥ 0.67), whereas 3X steers had less hay DMI and greater total DMI (P < 0.01) on days that they received supplementation compared to days of no concentrate supplementation. From d 16 to 42, hay and total DMI of 7X steers did not differ throughout the wk (P ≥ 0.12), whereas 3X and 7-3X steers had less hay DMI and greater total DMI (P < 0.01) on days that they received supplementation compared to days of no concentrate supplementation. However, despite the differences in hay and total DMI, the total amount of hay DM and total DM consumed from d 0 to 15, 16 to 42, and 0 to 42 did not differ among treatments (P ≥ 0.27; Table 2).
Table 3.
Dry matter intake of beef steers provided similar weekly concentrate DM that was divided and offered daily from d 0 to 42 (7X; four pens), three times weekly from d 0 to 42 (3X; Monday, Wednesday, and Friday; five pens), or daily from d 0 to 15 and then three times weekly from d 16 to 42 (7-3X; five pens)
| Treatment | P value‡ | ||||
|---|---|---|---|---|---|
| Item* | 3X | 7-3X | 7X | SEM† | Freq. × day |
| Hay DMI, kg/d | |||||
| d 0 to 15 | |||||
| Mon, Wed, Fri | 1.83a | 2.55b | 2.46b | 0.323 | <0.01 |
| Tues, Thurs, Sat, Sun | 3.03a | 2.57a | 2.76a | 0.323 | |
| P value§ | <0.01 | 0.87 | 0.77 | ||
| d 16 to 42 | |||||
| Mon, Wed, Fri | 2.47a | 2.33a | 3.24b | 0.261 | <0.01 |
| Tues, Thurs, Sat, Sun | 3.18a | 3.27a | 2.87a | 0.261 | |
| P value§ | <0.01 | <0.01 | 0.12 | ||
| Total DMI, kg/d | |||||
| d 0 to 15 | |||||
| Mon, Wed, Fri | 6.49b | 5.03a | 4.47a | 0.384 | <0.01 |
| Tues, Thurs, Sat, Sun | 3.03a | 5.01b | 4.77b | 0.384 | |
| P value§ | <0.01 | 0.93 | 0.67 | ||
| d 16 to 42 | |||||
| Mon, Wed, Fri | 7.54b | 7.79b | 5.55a | 0.344 | <0.01 |
| Tues, Thurs, Sat, Sun | 3.18a | 3.52a | 5.18b | 0.344 | |
| P value§ | <0.01 | <0.01 | 0.15 | ||
a-bWithin a row, means without a common superscript differ (P ≤ 0.05).
*Monday, Wednesday, and Friday = days when all steers received concentrate supplementation; Tuesday, Thursday, Saturday, and Sunday = days that concentrate supplementation was offered to 7-3X and 7X steers (d 0 to 15), or only to 7X steers (d 16 to 42).
†Pen as the experimental unit (three steers/pen; four pens for 7X and five pens for 3X and 7-3X treatments).
‡ P value for effects of supplementation frequency × day of supplementation.
§Comparison of day within each treatment.
Effects of frequency of supplementation × day of the wk (P < 0.01), but not frequency × day of the wk × wk of the study (P ≥ 0.17), were detected for daily total intake of CP and TDN (Table 4). From d 0 to 15, daily total intake of CP and TDN of 7-3X and 7X steers did not differ throughout the wk (P ≥ 0.91), whereas 3X steers had greater total intake of CP and TDN (P < 0.01) on days that they received supplementation compared to days of no concentrate supplementation. From d 16 to 42, daily total intake of CP and TDN of 7X steers did not differ throughout the wk (P ≥ 0.15), whereas 3X and 7-3X steers had greater total intake of CP and TDN (P < 0.01) on days that they received supplementation compared to days of no concentrate supplementation. However, despite the differences in total CP and TDN, the average total amount of CP and TDN consumed from d 0 to 15 and 16 to 42 did not differ among treatments (P ≥ 0.91). Average total intake of TDN from d 0 to 15 and 16 to 42 were 3.03 and 3.40, 2.87 and 3.33, and 2.90 and 3.37 kg/d for 3X, 7-3X, and 7X steers, respectively. Average total intake of CP from d 0 to 15 and 16 to 42 were 0.68 and 0.77, 0.65 and 0.76, and 0.66 and 0.77 kg/d for 3X, 7-3X, and 7X steers, respectively.
Table 4.
Total CP and TDN intake (kg/d) of beef steers provided similar weekly concentrate DM that was divided and offered daily from d 0 to 42 (7X; four pens), three times weekly from d 0 to 42 (3X; Monday, Wednesday, and Friday; five pens), or daily from d 0 to 15 and then three times weekly from d 16 to 42 (7-3X; five pens)
| Treatment | P value‡ | ||||
|---|---|---|---|---|---|
| Item* | 3X | 7-3X | 7X | SEM† | Freq. × day |
| Total CP intake, kg/d | |||||
| d 0 to 15 | |||||
| Mon, Wed, Fri | 0.96b | 0.65a | 0.64a | 0.067 | <0.01 |
| Tues, Thurs, Sat, Sun | 0.41a | 0.65b | 0.68b | 0.067 | |
| P value§ | <0.01 | 0.91 | 0.19 | ||
| d 16 to 42 | |||||
| Mon, Wed, Fri | 1.11b | 1.07b | 0.79a | 0.060 | <0.01 |
| Tues, Thurs, Sat, Sun | 0.43a | 0.44a | 0.74b | 0.060 | |
| P value§ | <0.01 | <0.01 | 0.22 | ||
| Total TDN intake, kg/d | |||||
| d 0 to 15 | |||||
| Mon, Wed, Fri | 4.34b | 2.86a | 2.82a | 0.263 | <0.01 |
| Tues, Thurs, Sat, Sun | 1.71a | 2.87b | 2.99b | 0.263 | |
| P value§ | <0.01 | 0.91 | 0.33 | ||
| d 16 to 42 | |||||
| Mon, Wed, Fri | 4.99b | 4.82b | 3.47a | 0.235 | <0.01 |
| Tues, Thurs, Sat, Sun | 1.80a | 1.85a | 3.27b | 0.235 | |
| P value§ | <0.01 | <0.01 | 0.15 | ||
a-bWithin a row, means without a common superscript differ (P ≤ 0.05).
*Monday, Wednesday, and Friday = days when all steers received concentrate supplementation; Tuesday, Thursday, Saturday, and Sunday = days that concentrate supplementation was offered to 7-3X and 7X steers (d 0 to 15), or only to 7X steers (d 16 to 42).
†Pen as the experimental unit (three steers/pen; four pens for 7X and five pens for 3X and 7-3X treatments).
‡ P value for effects of supplementation frequency × day of supplementation.
§Comparison of day within each treatment.
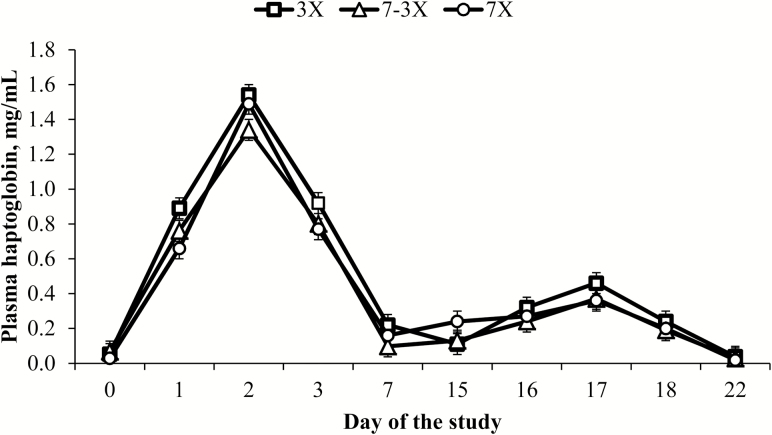
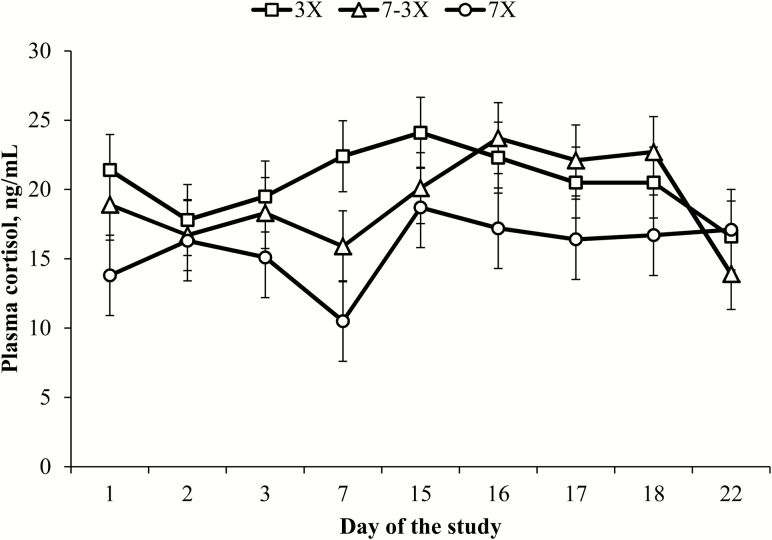
Plasma concentrations of cortisol and seroconversion to PI-3 virus on d 0 did not differ among treatments (P ≥ 0.18), but were included as covariates (P ≤ 0.02) in their respective statistical analyses. Effects of frequency of supplementation × day of the study (P ≥ 0.57) were not detected for plasma concentrations of haptoglobin and cortisol. However, effects of day of the study (P < 0.01) were detected for plasma concentrations of haptoglobin and cortisol (Figures 1 and 2). Effects of frequency of supplementation were detected for plasma concentrations of haptoglobin (P = 0.04) and tended to differ for plasma concentrations of cortisol (P = 0.10). Overall plasma haptoglobin concentrations were greatest for 3X steers (P ≤ 0.05) and did not differ between 7-3X and 7X steers (P = 0.92; Table 5), whereas plasma concentrations of cortisol were greater for 3X vs. 7X steers (P = 0.04) and were intermediate for 7-3X steers (P ≥ 0.14; Table 5).
Figure 1.
Plasma haptoglobin concentrations of beef steers provided similar weekly concentrate DM that was divided and offered daily from d 0 to 42 (7X; four pens), three times weekly from d 0 to 42 (3X; Monday, Wednesday, and Friday; five pens), or daily from d 0 to 15 and then three times weekly from d 16 to 42 (7-3X; five pens). Steers were vaccinated with Bovi Shield Gold One Shot and Ultrabac 7 (Zoetis Inc.) on d 0, and Bovi Shield Gold 5 (Zoetis Inc.) and Ultrabac 7 on d 15. Effects of day (P < 0.01), but not frequency of supplementation × day (P = 0.94), were detected for plasma haptoglobin concentrations.
Figure 2.
Plasma cortisol concentrations of beef steers provided similar weekly concentrate DM that was divided and offered daily from d 0 to 42 (7X; four pens), three times weekly from d 0 to 42 (3X; Monday, Wednesday, and Friday; five pens), or daily from d 0 to 15 and then three times weekly from d 16 to 42 (7-3X; five pens). Steers were vaccinated with Bovi Shield Gold One Shot and Ultrabac 7 (Zoetis Inc.) on d 0, and Bovi Shield Gold 5 (Zoetis Inc.) and Ultrabac 7 on d 15. Effects of day (P < 0.01), but not frequency of supplementation × day (P = 0.57), were detected for plasma cortisol concentrations. Least square means were covariate-adjusted to measurements obtained on d 0 (P = 0.02).
Table 5.
Plasma and serum measurements of beef steers provided similar weekly concentrate DM that was divided and offered daily from d 0 to 42 (7X; four pens), three times weekly from d 0 to 42 (3X; Monday, Wednesday, and Friday; five pens), or daily from d 0 to 15 and then three times weekly from d 16 to 42 (7-3X; 5 pens)*
| Treatment | P value | ||||||
|---|---|---|---|---|---|---|---|
| Item | 3X | 7-3X | 7X | SEM† | Freq. | Day | Freq. × day |
| Plasma haptoglobin, mg/dL | 0.44a | 0.37b | 0.37b | 0.026 | 0.04 | <0.01 | 0.94 |
| Plasma cortisol‡, ng/mL | 20.6 | 19.2 | 15.7 | 1.68 | 0.10 | 0.040 | 0.57 |
| Bovine viral diarrhea virus-1a | |||||||
| Serum titers, log2 | 2.07 | 2.19 | 1.97 | 0.217 | 0.76 | <0.01 | 0.34 |
| Seroconversion, % | 42.2 | 38.1 | 38.9 | 4.50 | 0.75 | <0.01 | 0.64 |
| Infectious bovine rhinotracheitis virus | |||||||
| Serum titers, log2 | 0.29a | 0.88b | 0.79b | 0.179 | 0.05 | <0.01 | 0.24 |
| Seroconversion, % | 22.2 | 33.1 | 30.6 | 8.51 | 0.60 | <0.01 | 0.76 |
| Parainfluenza-3 virus | |||||||
| Serum titers, log2 | 3.54 | 4.46 | 3.66 | 0.606 | 0.52 | <0.01 | 0.81 |
| Seroconversion‡, % | |||||||
| d 15 | 36.0 | 76.6 | 57.0 | 8.24 | 0.09 | <0.01 | 0.04 |
| d 42 | 100.0 | 98.0 | 98.9 | ||||
a-bWithin a row, means without a common superscript differ (P ≤ 0.05).
*Steers were vaccinated with Bovi Shield Gold One Shot and Ultrabac 7 (Zoetis Inc., New York, NY) on d 0, and Bovi Shield Gold 5 (Zoetis Inc., New York, NY) and Ultrabac 7 on d 15.
†Pen as the experimental unit (three steers/pen; four pens for 7X and five pens for 3X and 7-3X treatments).
‡Least square means covariate-adjusted to the respective measurements obtained on d 0 (P ≤ 0.02).
Effects of frequency of supplementation × day of the study, and frequency of supplementation were not detected (P ≥ 0.34) for serum titers and positive seroconversion to BVDV-1a virus (Table 5). However, effects of frequency of supplementation × day of study and frequency of supplementation were detected (P ≤ 0.05) for positive seroconversion to PI-3 virus on d 15 and serum IBR titers, respectively (Table 5). Positive seroconversion to IBR virus did not differ among treatments (P ≥ 0.24), whereas serum IBR titers were the least for 3X steers (P ≤ 0.05), and did not differ between 7-3X and 7X steers (P = 0.72; Table 5). In contrast, serum PI-3 titers did not differ among treatments (P ≥ 0.52), whereas positive seroconversion to PI-3 was the least for 3X steers (P ≤ 0.05), and did not differ between 7-3X and 7X steers (P = 0.13; Table 5). Seroconversion to PI-3 virus on d 42 did not differ among treatments (P ≥ 0.70).
DISCUSSION
Previous studies have reported that reducing the frequency of energy supplementation from daily to three times weekly decreased ADG by 10 to 21% (Cooke et al., 2008; Loy et al., 2008; Artioli et al., 2015) or had no impact on ADG of preconditioning calves (Drewnoski et al., 2011; Moriel et al., 2016a). Discrepancies among these results can be associated to differences in supplement composition (high-moisture vs. grain pellet-based supplements), animal breed and gender, location of the study, forage species and quality, and the potential interactions among those factors (Artioli et al., 2015). Artioli et al. (2015) reported that calves supplemented with concentrate daily were 9 kg heavier compared to calves offered concentrate supplementation three times weekly during the entire 42-d preconditioning period. The similar overall ADG and final BW between 7-3X and 7X calves suggests that a gradual reduction in frequency of concentrate supplementation did not negatively impact growth performance of preconditioning calves, and could be used as a strategy to decrease labor and feeding costs compared to daily supplementation. However, in the current study, growth performance of steers receiving supplementation three times weekly during the entire preconditioning period also did not differ compared with steers that received the 7-3X and 7X treatments. This response was unexpected and in contrast to our previous study (Artioli et al., 2015), but it indicates that in the current study, providing concentrate supplementation three times weekly would be the best alternative to minimize labor and feeding costs. The reasons for the lack of treatment effects on growth performance in the current study are unknown, but further research is warranted to identify the potential reasons for the discrepancy between these studies.
Regardless of treatment, steers consumed 100% of concentrate DM offered within 6 h after supplementation, as reported by Artioli et al. (2015). From d 0 to 15, hay DMI of 3X steers on days that all steers received supplementation (Monday, Wednesday, and Friday) decreased by 26 to 28% compared to 7X and 7-3X steers. On days that 3X steers did not receive concentrate supplementation (Tuesday, Thursday, Saturday, and Sunday) hay DMI of 3X steers increased by 10 to 18% compared to 7X and 7-3X steers. Likewise, hay DMI (d 16 to 42) of 3X and 7-3X steers on days that all steers received supplementation decreased by 24 to 28% compared to 7X steers, and increased by 11 to 14% compared to 7X steers on days that 3X and 7-3X steers did not receive concentrate supplementation. Total intake of CP and TDN from d 0 to 15 and 16 to 42 followed the same pattern as observed for total DMI described above. Despite the low starch concentration of supplements used in this study, this response on daily hay DMI was expected because supplements often decrease forage DMI when supplemental TDN intake is greater than 0.7% of BW (Moore et al.,1999). However, total hay DMI (and total intake of CP and TDN) did not differ among treatments, which could partially explain the lack of differences on calf overall ADG and final BW among treatments. Artioli et al. (2015) reported that daily hay DMI of steers supplemented three times weekly decreased by 53% on the days of concentrate supplementation and increased by 10% on days that concentrate was not offered compared to steers receiving daily concentrate supplementation, leading to an overall reduction in intake of hay DM, CP, and NEg. It has been suggested that the reduction in forage DMI after concentrate supplementation increases as forage nutritive value increases (Horn and McCollum, 1987). Although the 1.5% difference in forage TDN value reported by current study and Artioli et al. (2015) is narrower than the range evaluated by Horn and McCollum (1987), the greater reduction in hay DMI after concentrate supplementation reported by Artioli et al. (2015) may be attributed to the greater forage nutritive value in that study compared to the forage nutritive value used in the present study (17.4 vs. 13.5% CP; 58.0 vs. 56.5% TDN; 34.4 vs. 36.8% ADF; 57.7 vs. 62.7% NDF of DM, respectively).
In the current and previous study (Artioli et al., 2015), protein intake of steers was in excess of requirements irrespective of treatment (NRC, 2016). However, during an immunological challenge, the immune system has a greater priority for nutrients than tissue growth, and nutrients are partitioned to support multiple immune responses and ensure the organism survival (Elsasser et al., 2008). In addition, AA profile needed to support the immune system might not be supported by the excess dietary intake of protein, and specific AA may need to be mobilized to support several physiological responses. For instance, acute-phase proteins in humans contain increased amounts of Phe, Trp, Lys, Cys, and Ser compared with skeletal muscle (Reeds and Jahoor, 2001), and lymphocytes preferentially utilize the branched-chain AA during inflammation as substrates for protein synthesis (i.e., antibody production) or energy production (Calder, 2006). Thus, although protein intake was in excess of daily protein requirements, the greater reduction in hay DM and CP intake by steers supplemented three times weekly vs. daily reported by Artioli et al. (2015) possibly induced a greater muscle protein mobilization to support a specific AA profile for the immune system, which could be the cause for the reduced ADG and final BW of 3X vs. 7X steers. Increasing the metabolizable protein supply to preconditioning beef steers corrected imbalances between amino acids supplied and required by the immune system, which consequently alleviated muscle protein mobilization, leading to a greater growth performance during a 42-d preconditioning period (Moriel et al., 2015). Hence, the similar total DMI (and consequently, CP and TDN intake) among treatments reported in the present study, may have contributed to equalize the rate of muscle protein mobilization among treatments, which may partially explain the lack of differences on steer overall ADG and final BW among treatments. Further studies are needed to directly measure indices of muscle protein mobilization of beef cattle supplemented at different frequencies of supplementation.
Weaning, feedlot entry, and vaccination of beef cattle elicit an APR that leads to increased hepatic synthesis of acute-phase proteins (i.e., haptoglobin), reduced feed intake and ADG (Arthington et al., 2013; Artioli et al., 2015; Moriel et al., 2015, 2016a), enhanced mobilization of muscle and fat tissues (Johnson, 1997), and consequently, decreased feed efficiency (Arthington et al., 2013). Haptoglobin is one of the major proteins associated with APR in cattle (Carroll and Forsberg, 2007) that is detectable in high concentrations in injured or stressed animals (Petersen et al., 2004). Thus, haptoglobin can be used as an indicator of bovine acute and chronic inflammation when plasma concentrations are ≥0.11 mg/mL (Tourlomoussis et al., 2004). In addition, an increase in plasma concentrations of cortisol reduced DMI (Allen et al., 2009), elicited an APR, and increased plasma haptoglobin concentrations (Cooke and Bohnert, 2011). In the present study, overall plasma haptoglobin concentrations were 19% greater for 3X vs. 7-3X and 7X steers, whereas overall plasma cortisol concentrations were greatest for 3X, least for 7X steers, and intermediate for 7-3X steers. These results are in agreement with previous studies (Artioli et al., 2015; Moriel et al. 2016a), and reinforce previous evidence that reducing the frequency of energy supplementation exacerbates the vaccine-induced APR. Furthermore, the results of the present study support our hypothesis that gradually reducing the frequency of energy supplementation prevented further increments on vaccine-induced APR of preconditioning beef calves.
Although APR is an essential early defense mechanism in response to cellular injury (Eckersall and Conner, 1988), nutrient demand is increased to support the synthesis of acute-phase proteins, immune cells, and gluconeogenic precursors (Reeds and Jahoor, 2001). Consequently, muscle protein and fat reserves are mobilized (Jahoor et al., 1999), and absorbed AA are shifted from growth toward hepatic uptake (Reeds et al., 1994) leading to a negative correlation with growth performance (Qiu et al., 2007). In the present study, overall plasma haptoglobin concentrations ranged between 0.38 to 0.44 mg/mL, whereas overall plasma cortisol concentrations ranged between 15.7 and 20.6 ng/mL. Artioli et al. (2015) observed that plasma haptoglobin concentrations ranged between 0.69 and 0.91 mg/mL, whereas overall plasma cortisol concentrations ranged between 19.6 and 23.4 ng/mL. Hence, the average plasma concentrations of cortisol and haptoglobin of all treatments were 18 and 95%, respectively, greater in the study of Artioli compared to the present study. In addition, the magnitude of differences in plasma concentrations of haptoglobin between 3X vs. 7X steers was greater in the study of Artioli et al. (2015) vs. in the current study (32 vs. 19%, respectively). Together, these differences in plasma concentrations of cortisol and haptoglobin between studies and among treatments within these studies may indicate that steers experienced greater physiological stress and inflammatory responses in the study of Artioli et al. (2015). It is possible that the differences in muscle protein mobilization among treatments was alleviated because the APR was not as severely exacerbated as expected after reducing the frequency of concentrate supplementation, which may partially explain the lack of differences on calf ADG and final BW between 3X and 7X steers observed in the present study.
Vaccination is one of the most useful management practices to protect animals from different pathogens (Carrol and Forsberg, 2007), and conferring successful vaccine protection in cattle is important in preventing new or recurring infections (Langel et al., 2016). Antigens present in the vaccine stimulate the humoral response (i.e., antibody production) without causing disease (Kindt et al., 2007). The ability of an animal to respond to vaccination varies from animal to animal and depends on environmental and genetic factors, timing of vaccination after feedlot entry, maternal antibody concentrations (Downey et al., 2013), stress and previous exposure to pathogens (Loerch and Fluharty, 1999), frequency of supplementation (Artioli et al., 2015), metabolizable protein supply (Moriel et al., 2015), and maternal energy-restriction during late gestation (Moriel et al.., 2016b). Neutralizing antibody titers provides an indication of immune protection (Bolin and Ridpath, 1990) and response to vaccination (Richeson et al., 2008), and it has been correlated positively with disease prevention (Howard et al., 1989).
In the present study, mean serum titers and positive seroconversion to BVDV-1a did not differ among treatments. However, mean serum titers against IBR and positive seroconversion to PI-3 virus on d 15, but not on d 42, were greater for 7-3X and 7X vs. 3X steers, which is in agreement with previous studies (Artioli et al., 2015; Moriel et al., 2016a). These responses provide further evidence that steers offered reduced frequency of concentrate supplementation during the entire study had altered postvaccination antibody production. It remains unknown if such alterations in antibody production means that the humoral immunity was impaired by the reduced frequency of supplementation and became more susceptible to pathogen invasions. Nonetheless, elevated cortisol causes immune suppressive effects (Salak-Johnson and McGlone, 2007), excessive protein catabolism (Friend, 1991), weakens the innate immune system (Dai and McMurray,1998), and decreases cytokine secretion by TH1 and TH2 cells (Carroll and Forsberg, 2007). Perhaps, the tendency for greater mean plasma cortisol concentrations of 3X vs. 7-3X and 7X steers may have impacted the communication between innate and humoral immune system (Artioli et al., 2015), which may have reduced mean serum IBR titers and increased the time to achieve similar serum PI-3 titers compared to 7X and 7-3X steers. Nonetheless, mean serum titers and positive seroconversion against BVDV-1a, PI-3, and IBR viruses did not differ between 7X and 7-3X steers, which supports our hypothesis and indicates that a gradual reduction in frequency of concentrate supplementation prevented the APR exacerbation and reductions on antibody production of preconditioning beef steers.
In summary, the present study provides further evidence that steers offered reduced frequency of concentrate supplementation during the entire 42-d preconditioning period may have an exacerbated acute-phase response, as well as, reduced vaccine-induced antibody production compared to steers receiving daily concentrate supplementation. More importantly, the present study demonstrated that delaying the reduction in frequency of concentrate supplementation from daily to 3 times weekly by 15 d, relative to first vaccination, did not negatively impact growth, but alleviated inflammation and prevented reductions in vaccine-induced antibody response compared to steers supplemented three times weekly during the entire 42-d preconditioning period.
Footnotes
Financial support for this research was provided by the Beef Assessment Program from the NC Cattlemen’s Association and Zoetis Animal Health. Appreciation is expressed to William Hyatt, Noah Henson, and Kaleb Rathbone (Mountain Research Station, Waynesville, NC) and Julie Warren (University of Florida) for their assistance during this study.
LITERATURE CITED
- Allen M. S., Bradford B. J., and Oba M.. 2009. The hepatic oxidation theory of the control of feed intake and its application to ruminants. J. Anim. Sci. 87:3317–3334. [DOI] [PubMed] [Google Scholar]
- AOAC 2006. Official methods of analysis. 18th ed Arlington (VA): AOAC Int. [Google Scholar]
- Arthington J. D., Qiu X., Cooke R. F., Vendramini J. M. B., Araujo D. B., Chase C. C. Jr., and Coleman S. W.. 2008. Effects of preshipping management on measures of stress and performance of beef steers during feedlot receiving. J. Anim. Sci. 86:2016–2023. [DOI] [PubMed] [Google Scholar]
- Arthington J. D., Cooke R. F., Maddock T. D., Araujo D. B., Moriel P., DiLorenzo N., and Lamb G. C.. 2013. Effects of vaccination on the acute-phase protein response and measures of performance in growing beef calves. J. Anim. Sci. 91:1831–1837. [DOI] [PubMed] [Google Scholar]
- Bolin S. R., and Ridpath J. F.. 1990. Range of viral neutralizing activity and molecular specificity of antibodies induced in cattle by inactivated bovine viral diarrhea virus-vaccines. Am. J. Vet. Res. 51:703–707. [PubMed] [Google Scholar]
- Calder P. C. 2006. Branched-chain amino acids and immunity. J. Nutr. 136:288S–293S. [DOI] [PubMed] [Google Scholar]
- Carroll J. A., and Forsberg N. E.. 2007. Influence of stress and nutrition on cattle immunity. Vet. Clin. Food. Anim. 23:105–149. [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Arthington J. D., Araujo D. B., Lamb G. C., and Ealy A. D.. 2008. Effects of supplementation frequency on performance, reproductive, and metabolic responses of Brahman-crossbred females. J. Anim. Sci. 86:2296–2309. [DOI] [PubMed] [Google Scholar]
- Cooke R. F., and Bohnert D. W.. 2011. Technical note: Bovine acute-phase response after corticotropin-release hormone challenge. J. Anim. Sci. 89:252–257. [DOI] [PubMed] [Google Scholar]
- Cooke R. F., and Arthington J. D.. 2013. Concentrations of haptoglobin in bovine plasma determined by ELISA or a colorimetric method based on peroxidase activity. J. Anim. Physiol. Anim. Nutr. 97:531–536. [DOI] [PubMed] [Google Scholar]
- Dai G., and McMurray D. N.. 1998. Altered cytokine production and impaired antimycobacterial immunity in protein-malnourished guinea pigs. Infect. Immun. 66:3562–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey E. D., Tait R. G. Jr., Mayes M. S., Park C. A., Ridpath J. F., Garrick D. J., and Reecy J. M.. 2013. An evaluation of circulating bovine viral diarrhea virus type 2 maternal antibody level and response to vaccination in Angus calves. J. Anim. Sci. 91:4440–4450. [DOI] [PubMed] [Google Scholar]
- Drewnoski M. E., Benson G. A., and Poore M. H.. 2011. Effect of frequency of supplementation of a soyhulls and corn gluten feed blend on hay intake and performance of growing steers. Anim. Feed Sci. Technol. 164:38–44. [Google Scholar]
- Drewnoski M. E., Huntington G. B., and Poore M. H.. 2014. Reduced supplementation frequency increased insulin-like growth factor 1 in beef steers fed medium quality hay and supplemented with a soybean hull and corn gluten feed blend. J. Anim. Sci. 92:2546–2553. [DOI] [PubMed] [Google Scholar]
- Eckersall P. D., and Conner J. G.. 1988. Bovine and canine acute-phase proteins. Vet. Res. Communic. 12:169–178. [DOI] [PubMed] [Google Scholar]
- Elsasser T. H., Caperna T. J., Li C. J., Kahl S., and Sartin J. L.. 2008. Critical points in the impact of the proinflammatory immune response on growth and metabolism. J. Anim. Sci. 86:E105–E125. [DOI] [PubMed] [Google Scholar]
- Friend T. H. 1991. Behavioral aspects of stress. J. Dairy. Sci. 74:292–303. [DOI] [PubMed] [Google Scholar]
- Horn G. W., and McCollum F. T.. 1987. Energy supplementation of grazing ruminants. In Proc. Grazing Livest. Nutr. Conf, Jackson Hole (WY); p. 125–136. [Google Scholar]
- Howard C. J., Clarke M. C., and Brownlie J.. 1989. Protection against respiratory infection with bovine virus diarrhea virus by passively acquired antibody. Vet. Microbiol. 19:195–203. [DOI] [PubMed] [Google Scholar]
- Johnson R. W. 1997. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 75:1244–1255. [DOI] [PubMed] [Google Scholar]
- Jahoor F., Wykes L., Del Rosario M., Frazer M., and Reeds P. J.. 1999. Chronic protein undernutrition and an acute inflammatory stimulus elicit different protein kinetic responses in plasma but not in muscle of piglets. J. Nutr. 129:693–699. [DOI] [PubMed] [Google Scholar]
- Kindt T. J. 2007. Kuby Immunology ( Richard A., Goldsby Barbara A., Osborne, Ed.). 6th ed New York (NY): W. H. Freeman and Company. [Google Scholar]
- Langel S. N., Wark W. A., Garst S. N., James R. E., McGilliard M. L., Petersson-Wolfe C. S., and Kanevsky-Mullarky I.. 2016. Effect of feeding whole compared with cell-free colostrum on calf immune status: vaccination response. J. Dairy Sci. 99:3979–3994. [DOI] [PubMed] [Google Scholar]
- Loerch S. C., and Fluharty F. L.. 1999. Physiological changes and digestive capabilities of newly received feedlot cattle. J. Anim. Sci. 77:1113–1119. [DOI] [PubMed] [Google Scholar]
- Loy T. W., MacDonald J. C., Klopfenstein T. J., and Erickson G. E.. 2007. Effect of distillers grains or corn supplementation frequency on forage intake and digestibility. J. Anim. Sci. 10:2625–2630. [DOI] [PubMed] [Google Scholar]
- Loy T. W., Klopfenstein T. J., Erickson G. E., Macken C. N., and McDonald J. C.. 2008. Effect of supplemental energy source and frequency on growing calf performance. J. Anim. Sci. 86:3504–3510. [DOI] [PubMed] [Google Scholar]
- Marques R. S., Cooke R. F., Francisco C. L., and Bohnert D. W.. 2012. Effects of twenty-four hour transport or twenty-four hour feed and water deprivation on physiologic and performance responses of feeder cattle. J. Anim. Sci. 90:5040–5046. [DOI] [PubMed] [Google Scholar]
- Moore J. E., Brant M. H., Kunkle W. E., and Hopkins D. I.. 1999. Effects of supplementation on voluntary forage intake, diet digestibility, and animal performance. J. Anim. Sci. 77(Suppl. 2):122–135. [DOI] [PubMed] [Google Scholar]
- Moriel P., Cooke R. F., Bohnert D. W., Vendramini J. M. B., and Arthington J. D.. 2012. Effects of energy supplementation frequency and forage quality on performance, reproductive, and physiological responses of replacement beef heifers. J. Anim. Sci. 90:23712380. [DOI] [PubMed] [Google Scholar]
- Moriel P., Artioli L. F. A., Poore M. H., Confer A. W., Marques R. S., and Cooke R. F.. 2015. Increasing the metabolizable protein supply enhanced growth performance and led to variable results on innate and humoral immune response of preconditioning beef steers. J. Anim. Sci. 93:4473–4485. [DOI] [PubMed] [Google Scholar]
- Moriel P., Artioli L. F. A., Piccolo M. B., Poore M. H., Marques R. S., and Cooke R. F.. 2016a. Decreasing the frequency and rate of wet brewers grains supplementation did not impact growth but reduced humoral immune response of preconditioning beef heifers. J. Anim. Sci. 94:3030–3041. [DOI] [PubMed] [Google Scholar]
- Moriel P., Artioli L. F. A., Piccolo M. B., Poore M. H., Marques R. S., and Cooke R. F.. 2016b. Short-term energy restriction during late gestation and subsequent effects on postnatal growth performance, and innate and humoral immune responses of beef calves. J. Anim. Sci. 94:2542–2552. [DOI] [PubMed] [Google Scholar]
- NRC 2000. Nutrient Requirements of Beef Cattle. Revised 7th ed. Washington (DC): National Academies Press. [Google Scholar]
- NRC 2016. Nutrient Requirements of Beef Cattle. Revised 8th ed Washington (DC): National Academies Press. [Google Scholar]
- Petersen H. H., Nielsen J. P., and Heegaard P. M. H.. 2004. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 35:163–187. [DOI] [PubMed] [Google Scholar]
- Qiu X., Arthington J. D., Riley D. G., Chase C. C. Jr., Phillips W. A., Coleman S. W., and Olson T. A.. 2007. Genetic effects on acute phase protein response to the stresses of weaning and transportation in beef calves. J. Anim. Sci. 85:2367–2374. [DOI] [PubMed] [Google Scholar]
- Reeds P. J., Fjeld C. R., and Jahoor F.. 1994. Do the differences in the amino acid composition of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states. J. Nutr. 124:906–910. [DOI] [PubMed] [Google Scholar]
- Reeds P. J., and Jahoor F.. 2001. The amino acid requirements of disease. Clin. Nutr. 20 (Suppl. 1):15–22. [Google Scholar]
- Richeson J. T., Beck P. A., Gadberry M. S., Gunter S. A., Hess T. W., Hubbell D. S. III, and Jones C.. 2008. Effects of on arrival versus delayed modified live virus vaccination on health, performance, and serum infectious bovine rhinotracheitis titers of newly received beef calves. J. Anim. Sci. 86:999–1005. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M. J., Edwards E. A., and Sullivan E. V.. 1970. Micromethods for respiratory virus sero-epidemiology. Health Lab. Sci. 7:42–52. [PubMed] [Google Scholar]
- Salak-Johnson J. L., and McGlone J. J.. 2007. Making sense of apparently conflicting data: stress and immunity in swine and cattle. J. Anim. Sci. 85:E81–E88. [DOI] [PubMed] [Google Scholar]
- Tourlomoussis P., Eckersall P. D., Waterson M. M., and Buncic S.. 2004. A comparison of acute phase protein measurements and meat inspection findings in cattle. Foodborne Pathog. Dis. 1:281–290. [DOI] [PubMed] [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to nutrition animal. J. Dairy Sci. 74:3583–3597. [DOI] [PubMed] [Google Scholar]
- Weiss W. P., Conrad H. R., and St N. R..Pierre. 1992. A theoretically-based model for predicting total digestible nutrient values of forages and concentrates. Anim. Feed Sci. Technol. 39:95–110. [Google Scholar]