Abstract
The objective of this study was to determine effect of ruminal acidosis (RA) and low feed intake [LFI] on the regional barrier function of the gastrointestinal tract. Twenty-one Holstein steers were fed for ad libitum intake for 5 d (control [CON]), fed at 25% of ad libitum intake for 5 d (LFI), or provided 2 d of ad libitum intake followed by 1-d of feed restriction (25% of ad libitum intake), 1 d where 30% of ad libitum dry matter intake (DMI) was provided as pelleted barley followed by the full allocation (RA) and fed for ad libitum intake the following day. Tissues and digesta from the rumen, omasum, duodenum, jejunum, ileum, cecum, proximal, and distal colon were collected. Permeability was assessed using the mucosal-to-serosal flux of inulin (JMS-inulin) and mannitol (JMS-mannitol). Digesta pH was 0.81, 0.63, and 0.42 pH units less for RA than CON in the rumen, cecum, and proximal colon; while, LFI had pH that was 0.47 and 0.36 pH units greater in the rumen and proximal colon compared to CON. Total ruminal short-chain fatty acid (SCFA) concentration were less for LFI (92 mM; P = 0.010) and RA (87 mM; P = 0.007) than CON (172 mM) steers. In the proximal colon, the proportion of butyrate (P = 0.025 and P = 0.022) and isobutyrate (P = 0.019 and P = 0.019) were greater, and acetate (P = 0.028 and P = 0.028) was less for LFI and RA, respectively, when compared to CON steers. Ruminal papillae length, width, perimeter, and surface area were 1.21 mm, 0.78 mm, 3.84 mm, and 11.15 mm2 less for LFI than CON; while, RA decreased papillae width by 0.52 mm relative to CON. The JMS-mannitol was less for LFI steers than CON in the proximal colon (P = 0.041) and in the distal colon (P = 0.015). Increased gene expression for claudin 1, occludin, tight-cell junction protein 1 and 2, and toll-like receptor 4 were detected for LFI relative to CON in the rumen, jejunum, and proximal colon. For RA steers, expression of toll-like receptor 4 in the rumen, and occludin and tight-cell junction protein 1 were greater in the jejunum than CON. An acute RA challenge decreased pH in the rumen and large intestine but did not increase tissue permeability due to increases in the expression of genes related to barrier function within 1 d of the challenge. Steers exposed to LFI for 5 d had reduced ruminal SCFA concentrations, smaller ruminal papillae dimensions, and increased tissue permeability in the proximal and distal colon despite increases for genes related to barrier function and immune function.
Keywords: barrier function, cattle, low feed intake, ruminal acidosis, tissue permeability
INTRODUCTION
Despite best management, there is the potential for cattle to be exposed to nutritional challenges such as low dry matter intake (DMI) and provision of highly fermentable diets. For example, marked reductions in DMI occur in association with parturition (Hayirli et al., 2002), weaning (Boland et al., 2008), heat stress (Rhoads et al., 2009), and transportation (Loerch and Fluharty, 1999; González et al., 2012a,b). Moreover, cattle fed highly fermentable diets are at risk for ruminal acidosis (RA; Penner et al., 2007; Castillo-Lopez et al., 2014). Both challenge scenarios listed above alter the luminal environment and may affect functionality of the gastrointestinal tract (GIT).
The cells lining the GIT promote the absorption of nutrients, prevent the translocation of non-desired molecules, sense and potentially regulate the luminal microbiota, and initiate an immune response (Jutfelt, 2011). Given the rapid turnover rate of GIT cells, it is not surprising that absorptive and barrier functions adapt acutely when exposed to low feed intake (LFI; Gäbel et al., 1993; Albornoz et al., 2013; Zhang et al., 2013) and increased diet fermentability (Etschmann et al., 2009; Schurmann et al., 2014). Based on previous studies, RA (Minuti et al., 2014), weaning (Wood et al., 2014), and LFI (Zhang et al., 2013) increase total GIT permeability. In addition to the rumen, RA also reduces pH in the large intestine, and LFI increases intestinal permeability in pigs (Pearce et al., 2013a). It is known that the effects of RA extend beyond the rumen but it is not known which regions are impacted. Likewise, for LFI, it is not clear which regions of the GIT are most affected.
Based on the above listed information, it was hypothesized that RA would negatively affect indicators for GIT barrier function in the rumen and large intestine, while LFI would have a negative effect across all regions of the GIT.
MATERIALS AND METHODS
The steers used in this study were cared for in accordance with the guidelines of the Canadian Council on Animal Care (Ottawa, ON, Canada) and all procedures were pre-approved by the University of Saskatchewan Animal Research Ethics Board (protocol 20100021). A companion study evaluating short-chain fatty acid flux in response to the challenge treatments has been published (Laarman et al., 2016).
Experimental Design
Twenty-one Holstein steers (body weight [BW] = 213 kg ± 13 kg and 6 to 8 mo of age) were housed at the Livestock Research Building at the University of Saskatchewan (Saskatoon, Saskatchewan, Canada). Steers were blocked by BW and, within block, randomly assigned to one of three treatments: control (CON); RA; or LFI. Prior to the start of the study, all steers were group-housed and fed a common diet consisting of 50% grass hay, 38% rolled barley grain, 8% of a pelleted vitamin and mineral supplement, and 4% canola meal on a dry matter (DM) basis. The pelleted vitamin and mineral supplement supplied monensin to achieve a dietary concentration of 33 mg/kg. Throughout the study, steers were fed once daily at 0800 h.
Initiation of the treatments was staggered to balance the day of killing among treatments within each block as only one steer could be handled each day for ex vivo measurements. At the time of treatment initiation, steers were individually housed in pens with rubber mats on the floor. Steers were fed a common total mixed ration (Table 1) at 0800 h daily and were exposed to a 14-d dietary and environmental adaptation period followed by a 5-d duration for measurement of voluntary DMI. Subsequently, CON steers were provided an additional 4 d of ad libitum intake. Steers on the RA treatment were provided 2 d of ad libitum feed intake, 1 d of feed restriction (restricted to 25% of ad libitum intake), and 1 d where steers were fed 30% of their ad libitum DMI as pelleted barley (0800 h) along with by their full ad libitum ration allocation at 1200 h. The RA induction protocol was similar to that used by Schwaiger et al. (2014). For the LFI treatment, steers were exposed to a 4-d duration of LFI by restricting the amount of DM offered to 25% of their voluntary DMI. On the day following the completion of 4 d of ad libitum feeding (CON), 4 d of feed restriction (LFI), and the RA challenge (4 d), CON and RA steers were provided their full ration allocation (targeting ad libitum feeding) at 0800 h and killed at 1000 h using captive bolt stunning, pithing, and exsanguination. The LFI steers were also fed at 0800 h and killed at 1000 h, but the amount of feed offered was restricted to 25% of their ad libitum intake. The mean ± SD for DMI on the day of kill were 3.5 ± 1.1, 1.7 ± 0.2, and 3.2 ± 1.8 for CON, LFI, and RA steers, respectively.
Table 1.
Chemical composition of the ingredients, dietary inclusion rate, and analyzed dietary chemical composition of the total mixed ration fed to all Holstein steers
| Item | Barley silage | Grass hay | Barley grain | Pelleted barley grain | Canola meal | Mineral and vitamin pelleta |
|---|---|---|---|---|---|---|
| DM, % | 31.3 ± 2.2 | 89.5 ± 2.0 | 88.9 ± 1.3 | 91.4 ± 0.72 | 92.3 ± 0.54 | 92.8 ± 1.1 |
| OM | 91.7 ± 0.47 | 92.5 ± 0.64 | 97.7 ± 0.61 | 96.8 ± 0.61 | 92.6 ± 0.18 | 73.5 ± 1.1 |
| CP | 10.7 ± 0.53 | 9.7 ± 0.79 | 12.3 ± 0.33 | 14.8 ± 0.31 | 39.5 ± 0.29 | 14.7 ± 0.32 |
| EE | 3.3 ± 0.11 | 1.8 ± 0.15 | 2.6 ± 0.17 | 4.0 ± 0.08 | 4.0 ± 0.24 | 2.5 ± 0.08 |
| ADF | 29.3 ± 2.2 | 42.0 ± 0.62 | 9.1 ± 0.77 | 7.4 ± 0.25 | 20.5 ± 0.53 | 7.8 ± 0.77 |
| NDF | 44.9 ± 2.1 | 58.8 ± 0.97 | 19.2 ± 0.90 | 17.2 ± 0.71 | 27.8 ± 0.55 | 16.7 ± 1.44 |
| Starch | 21.7 ± 3.1 | 0.48 ± 0.24 | 56.2 ± 1.8 | 51 ± 0.96 | 1.6 ± 0.60 | 30.4 ± 1.53 |
| Calcium | 0.41 ± 0.02 | 0.69 ± 0.06 | 0.09 ± 0.02 | 0.29 ± 0.03 | 0.85 ± 0.03 | 5.4 ± 0.19 |
| Phosphorus | 0.32 ± 0.01 | 0.14 ± 0.01 | 0.34 ± 0.02 | 0.49 ± 0.02 | 1.23 ± 0.05 | 0.51 ± 0.03 |
| Dietary inclusion rate, % DM basis | 25.0 | 25.0 | 28.0 | 5.0 | 9.0 | 8.0 |
| Dietary chemical composition | ||||||
| DM, % | 61.3 | |||||
| OM, % of DM | 92.0 | |||||
| CP, % of DM | 13.6 | |||||
| EE, % of DM | 2.7 | |||||
| NDF, % of DM | 33.0 | |||||
| ADF, % of DM | 15.0 | |||||
| Starch, % of DM | 27.2 | |||||
OM, organic matter; ADF, acid detergent fiber; NDF, neutral detergent fiber; CP, crude protein; EE, ether extract.
aThe pellet contained (% DM) barley grain (48.4%), corn dried distillers grains plus solubles (19.8%), canola meal (7.4%), potassium-magnesium-sulfate (7.5%; Dynamate, The Mosaic Company, Plymouth, MN), salt (2.3%), limestone (12.9%), magnesium oxide (1.23%), and a trace mineral and vitamin supplement (0.61%) providing 4.6 mg/kg Co from cobalt carbonate, 146 mg/kg Cu from copper sulfate, 8.0 mg/kg I from ethylenediamine dihydriodide, 452 mg/kg Fe from ferrous carbonate, 336.0 mg/kg of Mn from manganese oxide, 2.26 mg/kg of Se, 319 of Zn from zinc oxide, 40,000 IU/ kg vitamin A, 15,000 IU/kg vitamin D3, and 300 IU/kg of vitamin E. Rumensin (Elanco Animal Health, Greenfield, IN) was included to achieve final monensin concentrations of 33 ppm in the final diet.
Data and Sample Collection
DMI, and feed sampling and analysis.
The amount of feed offered and refused was recorded daily. The platform scale used for weighing the amount of feed offered and refused measured to the nearest gram (Model M1, Western Scales Co. Ltd., Port Coquitlam, BC, Canada). Forage ingredients (barley silage and grass hay) were sampled twice weekly while concentrates were sampled once weekly. All samples were placed in a forced air oven at 55 °C until achieving a constant weight in order to determine DM content. The weekly samples were then stored until grinding and further analysis. All feed ingredient samples were ground with a hammer mill with a 2-mm sieve prior to being ground to pass through a 1-mm sieve (Christy and Norris, Christy Turner, Ltd., Chelmsford, UK). To determine the DM content of the feed refused, 5-d composite samples were used. Feed refusals were dried in a forced-air oven as described above. Feed ingredient samples were analyzed for DM, organic matter (OM), acid detergent fiber (ADF), neutral detergent fiber (NDF), crude protein (CP), ether extract (EE), starch, calcium and phosphorus by Cumberland Valley Analytical Services, and the dietary composition is reported in Table 1 (Hagerstown, MD, United States).
Measurement of reticulo-ruminal pH.
Reticulo-ruminal pH was recorded every 1 min using the Small Ruminant pH Measurement System (Dascor, Escondido, CA; Penner et al., 2009). The pH meter was orally dosed 5 d prior to killing and recovered post-mortem (48% recovered in the reticulum and 52% recovered in the rumen). The daily reticulo-ruminal pH was summarized as minimum, mean, and maximum values. In addition, the duration (min/d) and area (pH × min/d) that pH was < 5.5 were determined (Penner et al., 2007). The pH systems were standardized before and after incubation in the reticulo-rumen using pH buffers 7 and 4 at 39 °C and the two linear relationships (starting and ending) derived were used to calculate reticular pH with the assumption of linear drift over the duration of incubation.
Blood sampling and analysis.
Blood samples were collected from the jugular vein into two 10-mL evacuated sampling containers with one container coated with 158 IU of lithium heparin (Beckton Dickinson, Franklin Lakes, NJ) and the other coated with a clot activator and silica gel (Beckton Dickinson, Franklin Lakes, NJ). Blood collection occurred at 1800 h on the day prior to killing. Plasma samples were centrifuged immediately after collection at 2,500 × g for 15 min at 4 °C, whereas serum samples were allowed to clot at room temperature before being centrifuged. All samples were stored at −20 °C until being analyzed. Plasma samples were used for glucose, insulin, serum amyloid A (SAA) and haptoglobin concentrations, and serum was used for NEFA and β-hydroxybutyrate (BHBA).
All blood analyses were conducted with standard curves on each microplate. All microplates were read on a SpectraMax Plus Microplate Reader (Molecular Devices, LCC, Sunnyvale, CA, United States). Plasma glucose was measured using the glucose oxidase-peroxidase method (Sigma Aldrich, Oakville, ON, Canada). Plasma insulin concentrations were measured in duplicate using a bovine-specific ELISA (Mercodia, Uppsala, Sweden) with an intra assay CV of 2.9% and an inter assay CV of 2.1%. The Tridelta Phase ELISA was used to measure the SAA concentrations in duplicate (Tridelta Development Limited, Maynooth, CO; Kildare, Ireland) with an intra assay CV of 4.8% and an inter assay CV of 19.8%. The haptoglobin concentrations were measured in duplicate with a sandwich ELISA (Genway, San Diego, CA, United States) with an intra assay CV of 2.2% and an inter assay CV of 4.1%. In serum, the concentration NEFA was measured in triplicate using a commercial kit (NEFA-HR (2) kit, Wako Diagnostics, Mountain View, CA, United States) and absorbance was measured at 550 nm. Serum BHBA was measured in triplicate using methodology based on Williamson et al. (1962) with BHBA dehydrogenase sourced from Roche Diagnostics (Mississauga, ON, Canada).
Tissue and digesta collection.
The GIT was removed from each steer immediately after killing. The rumen (ventral sac), omasum (laminae from the central region), duodenum (proximal to the duodenal colic fold), jejunum (middle point), ileum (proximal to the ileocecal junction), cecum (apical region), proximal colon (end of the centripetal turns), and distal colon (mid-point between the duodenal colic fold and rectum) were collected as described by Penner et al. (2014).
After removing the GIT, digesta was collected from the reticulo-rumen, duodenum, jejunum, ileum, cecum, proximal colon, and distal colon. The entire reticulo-ruminal contents were emptied and mixed in a clean container. Subsequently, pH was measured in duplicate using a portable hand-held pH device (AP110, Fisher Scientific, Ottawa, ON, Canada). In addition, a representative 1-liter sample was collected from the mixed digesta and was strained through two layers of cheesecloth. Ten milliliters of strained reticulo-ruminal fluid were mixed with 2 mL of 25% [wt/v] metaphosphoric acid. For all other regions of the GIT, 10 g of digesta was collected and 10 g of double distilled water was added and mixed to enable pH measurements. After recording pH, 10 mL of the diluted digesta was then mixed with 2 mL of 25% metaphosphoric acid as described for reticulo-ruminal fluid. Ileal digesta was not included in analysis due to the inability to consistently collect samples from all calves. Samples of the diluted and acidified digesta were stored at −20 °C until being analyzed for SCFA concentration. Reticulo-ruminal fluid and the digesta samples were analyzed for SCFA concentrations using gas chromatography (Agilent 6890 Series GC System, Santa Clara, CA, United States) as described by Khorasani et al. (1996). For all regions other than the rumen, digesta was filtered through a 25 μm syringe filter (Fisher Scientific, Ottawa, ON, Canada) prior to preparation. The SCFA measured included acetic acid, propionic acid, iso-butyric acid, butyric acid, valeric acid, isovaleric acid and caproic acid using isocaproic as an internal standard.
Tissue samples from the rumen and omasum were collected for gross morphology and histology. The rumen and omasal tissues for gross morphology and histology were stored in PBS with 0.901 mM Ca2+, 0.493 mM Mg2+, 25 mM glycine and 0.1% sodium azide at 4 °C. Additional tissue samples from the rumen, jejunum, and colon were collected for gene expression measurements. Tissues for gene expression were stored in RNA-later (Qiagen, Toronto, ON, Canada) for 24 h at 4 °C and then transferred to −20 °C until analysis.
After collection of tissues for morphology, histology, and gene expression, tissues from the rumen, jejunum, and distal colon were collected for measurement of barrier function ex vivo. Tissues were thoroughly cleaned using a pre-heated (38 °C) incubation buffer (pH 7.4; Table 2) saturated with 95% O2 and 5% CO2. The submuocosal tissues from the rumen were gently removed by hand stripping. The intestinal tissues were split along the mesenteric connection. Tissues were then placed in oxygen saturated pre-heated buffer (38 °C) for transport back to the laboratory for ex vivo measurements of tissue permeability. Once in the lab, the submucosal tissues from the intestinal regions were gently removed by hand stripping.
Table 2.
Body weight, DMI, and reticulo-ruminal pH data for CON (n = 7), RA (n = 7), and LFI (n = 7) steers
| Treatment | P value | |||||
|---|---|---|---|---|---|---|
| Item | CON | RA | LFI | SEM | CON vs. RA | CON vs. LFI |
| BW, kg | 253.8 | 253.7 | 229.6 | 5.49 | 0.62 | 0.01 |
| DMI, kg | 6.64 | 4.86 | 1.74 | 0.24 | <0.001 | <0.001 |
| Reticulo-ruminal pH | ||||||
| Minimum pH | 5.74 | 5.24 | 6.00 | 0.20 | 0.10 | 0.39 |
| Mean pH | 6.55 | 5.86 | 6.94 | 0.21 | 0.039 | 0.21 |
| Maximum pH | 7.23 | 6.77 | 7.48 | 0.23 | 0.18 | 0.47 |
| Duration pH < 5.5, min/d | 4.43 | 538.00 | 47.43 | 147.03 | 0.025 | 0.84 |
| Area pH < 5.5, pH × min/d | 0.12 | 352.84 | 7.28 | 100.12 | 0.028 | 0.96 |
Data represent means from the last 4 d when fed ad libitum (CON) when fed at 25% of ad libitum intake (LFI) or when fed ad libitum for 2 d, restricted to 25% of ad libitum intake for 1 d, and provided 30% of ad libitum DMI as pelleted barley coupled with the ad libitum provision of the complete ration.
Ussing chamber measurements.
In the laboratory, tissues were mounted between two halves of an Ussing chamber (Free University of Berlin, Germany; n = 3 per region) with an exposed surface area of 3.14 cm2 for the rumen and omasum, and 1 cm2 for all other regions. Differences in the exposed surface area were based on a previous study (Penner et al., 2014) and that the intestinal tissues are more homogenous and less able to tolerate hydrostratic pressure. Tissues with visible lesions were avoided in order to measure paracellular movement. While it is acknowledged that tissue damage induced by the treatment may be an important outcome, use of tissues with visible lesions would directly compromise barrier function properties and do not reflect paracellular movement (Clarke, 2009). Moreover, use of tissues with visible lesions would increase variability. Among regions, tissues were incubated using one of two buffer solutions differing primarily in the energy source (Supplementary Table 1). The rumen, omasum, cecum, and colon tissues were incubated with 15 mL of buffer containing SCFA on the luminal side and 15 mL of buffer containing glucose on the serosal side, while tissues from the small intestine were bathed in buffer containing glucose on both sides (15 mL each). All buffers had a pH of 7.4. This approach was used to mimic the luminal energy sources present in vivo. The time from killing to mounting tissues in the Ussing chambers was completed within 40 min.
All tissues were incubated under short-circuit conditions as previously described by Aschenbach and Gäbel (2000). Argenthal reference electrodes (Mettler Toledo, Urdorf, Switzerland) were used to measure the potential difference across the epithelia via agar bridges (4% agar in 3 M KCl) with voltage measured using a computer controlled voltage clamp device (K. Mussler Scientific Instruments, Aachen, Germany). Based on the voltage measured, current was passed in the opposite direction such that the transepithelial potential difference was equal to 0 mV. The inverse value of the clamp current represents the short-circuit current (Isc), which is indicative of transcellular charge transfer across the epithelium. Tissue conductance (Gt) was determined every 6 s according to Ohm’s law by measuring the impulse-induced change in the transepithelial potential difference following the application of short bipolar current impulses. Tissues were provided with 20 min for stabilization of electrophysiology before being used for flux measurements.
The mucosal-to-serosal flux of mannitol (JMS-mannitol) and inulin (JMS-inulin) were measured in parallel in the absence of a concentration gradient. The buffers contained mannitol (2.5 mM) and inulin (2.5 mM), and the mucosal side of each column was spiked with D-1-14C-mannitol (74 kBq; Perkin Elmer, Waltham, MA) and 3H-inulin (148 kBq of methoxy-3H-inulin; Perkin Elmer). The mannitol and inulin were both purchased from Sigma Aldrich (Oakville, ON, Canada). A 45-min duration was provided prior to the start of the flux measurements to allow for equilibration of the radio-labeled compounds within the tissues. Samples of the mucosal buffer (100 μL) were collected at the beginning and the end of the experiment while the serosal buffer samples (500 μL) were collected at 0, 60, and 120 min, representing the two 1-h flux periods. Samples collected from the serosal side were replaced with an equivalent volume of fresh buffer to avoid changes in hydrostatic pressure. All samples were placed in 7-mL scintillation vials (Perkin Elmer, Waltham, MA, United States) and for mucosal samples, an additional 400 μL of fresh buffer was added to the scintillation vial to equalize the volume between the serosal and mucosal samples. Subsequently, 5 mL of scintillation cocktail (Ultima Gold, Perkin Elmer) was added to each sample prior to counting on a liquid scintillation counter (Tricarb 2910, Perkin Elmer) accounting for the overlap in the 3H and 14C spectrums. The decays per minute from the serosal and mucosal samples were used to determine the specific activity and the serosal appearance, respectively of 14C-mannitol and 3H-inulin. These data were then used to calculate the JMS-3H-mannitol and JMS-14C-inulin according to Gäbel et al. (1991). Data from the two consecutive flux periods were averaged for each chamber and the three chambers per region were considered as technical replicates. For the chambers incubating rumen and omasal tissue, 100 μM of ouabain (Sigma Aldrich, Oakville, ON, Canada) was added and for post-omasal tissues, 10 mM forskolin (Sigma Aldrich, Oakville, ON, Canada) was added to verify tissue viability once sampling was complete. Both ouabain and forskolin were dissolved in dimethyl sulfoxide.
Gross morphology and histology measurements.
The ruminal papillae width, length, perimeter, and surface area were measured in 15 papillae from a 1-cm2 tissue sample and the average was calculated for each steer. The papillae were cut at the basal attachment using an Olympus S761 dissecting microscope (Olympus Corporation, Tokyo, Japan). The software used for taking the ruminal papillae measurements consisted of Olympus Controller 3.2.1.276 (Olympus Canada Inc., Toronto, ON, Canada) and Olympus Manager 3.1.1.208 (Olympus Canada Inc.). The total epithelial thickness as well as individual strata thickness within the rumen and omasum epithelium was also measured using the software Image Pro Premier 9.1.4 (Media Cybernetics, Inc., Rockville, MD, United States). Hematoxylin and eosin staining was used for all slides (Prairie Diagnostic Services Inc., Saskatoon, SK, Canada) and slides were viewed on an Olympus BX41 microscope (Olympus Corporation, Tokyo, Japan).
Quantitative real-time PCR.
Tissues samples from the rumen, jejunum, and distal colon of each steer were used for quantitative real-time PCR (qPCR). All samples were ground by hand using a mortar and pestle on dry ice and RNA was extracted using TRIzol reagent (Invitrogen, Burlington, ON, Canada). A Nanodrop 2000 Spectrophotometer (Thermo Scientific Inc., Waltham, MA, United States) was used to quantify the RNA concentration in each sample. All samples were treated with DNase (Ambion DNA-free, Life Technologies Corporation, Carlsbad, CA) using the protocol provided in the kit. One modification to the protocol included preparing each sample with 22 μL of RNA, 2.5 μL of TURBO DNase buffer, and 0.5 μL of TURBO DNase. The protocol was then followed where samples were incubated at 37 °C for 30 min before adding in 2.5 μL of DNase Inactivation Reagent and incubating for 5 min at room temperature while mixing occasionally. The samples were then centrifuged at 10,000 × g for 1.5 min and the RNA was collected. The average RNA concentration after DNase treatment was 459.0 ng/µL ± 177.7 ng/µL for rumen samples, 936.7 ng/µL ± 350.2 ng/µL for jejunum samples, and 594.0 ng/µL ± 257.4 ng/µL for distal colon samples. The average ratio of absorbance at 260:280 nm was 1.94 ± 0.027 for rumen samples, 1.98 ± 0.027 for jejunum samples, and 1.97 ± 0.020 for distal colon samples, which was used to initially assess the quality of RNA present in each sample. All samples were evaluated via gel electrophoresis to analyze RNA integrity before shipping to the Advanced Analysis Center Genomics Laboratory at the University of Guelph (Guelph, ON, Canada) for qPCR. Gene expression for claudin 1 (CLDN1), occludin 1 (OCLN), zonula occludens 1 (TJP1), zonula occludens 2 (TJP2), toll-like receptor 2 (TLR2), toll-like receptor 4 (TLR4), and the immunoglobin A receptor (FCAR) as well as for the reference genes, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), beta-actin (ACTB), and 60s acidic ribosomal protein P0 (RPLP0) was measured. For each tissue region, reference genes were tested and the best individual or combination of genes were selected using NormFinder (Andersen et al., 2004). Once arriving at Guelph, samples were analyzed using an Agilent Bioanalyzer 2100 (Agilent Techonolgies, Santa Clara, CA, United States) to further assess RNA quality. All samples had an RNA integrity number > 5 with the mean RIN values for the rumen, jejunum, and distal colon being 7.6, 7.5, and 7.4. The forward and reverse primers used in this study were designed using the National Center for Biotechnology Information (NCBI) Primer-BLAST (NCBI, Bethesda, MD, United States) and ordered from Integrated DNA Technologies (Coralville, IA, United States). Primer efficiency was determined for each gene of interest by using a pooled sample of cDNA and serial dilutions to create a standard curve (R2 > 98%). Target genes of interest, the NCBI accession number for each gene, the forward and reverse primer sequences, gene function and amplicon size are shown in Supplementary Table 2. All samples were run in triplicate using a StepOnePlus qPCR system (Applied Biosystem, Thermo Fisher Scientific Inc.) and Power SYBR green Master Mix (Thermo Fisher Scientific Inc.). There was a 30 s holding stage prior to the qPCR cycles starting at 95 °C. There were 40 cycles for each sample with a 3 s denaturing stage (95 °C) and a 30 s annealing stage (60 °C). A melting curve was performed to confirm specificity of the PCR amplicon with three steps. Step 1 consisted of 15 s at 95 °C, step 2 consisted of 1 min at 60 °C and step 3 consisted of 15 s at 95 °C. The PCR products were also sequenced to further confirm gene of interest amplification (data not shown). The cycle threshold (CT) for the reference gene (GAPDH, RPLP10 and ACBT) was subtracted from the CT for the target gene to calculate ΔCT. The mean and median Ct values are shown in Supplementary Table 3. The ΔCT was then normalized to the CON by calculating ΔΔCT (ΔCT − ΔCT calibrator where ΔCT calibrator was the average value from the CON steers for that particular gene). This approach allowed for the calculation of a ΔΔCT value for each of the steers including the CON. The fold change for gene expression was then determined for each gene and each treatment according to Livak and Schmittgen (2001). To ensure consistency, each qPCR plate was designed with reference genes and all regions from one steer were measured on one plate to reduce the effect of errors among plates.
Statistical Analysis
Data for DMI and ruminal pH were used to calculate a 5-d average for each steer. Subsequently, data were analyzed using the Mixed or Glimmix procedures of SAS (version 9.4, SAS Institute Inc., Cary, NC) with steer considered as the experimental unit. All data were analyzed as a randomized complete block design with treatment as a fixed effect and block as a random effect. Data for mannitol flux, inulin flux, and Gt were analyzed independently by region. For mannitol flux, data from the rumen, omasum, duodenum, cecum, and proximal colon were transformed, as data were not normally distributed. For inulin flux, the rumen and omasum were analyzed using the Glimmix procedure and contrasts, as the data were not normally distributed and it was not corrected by transformation. Flux data in the jejunum were transformed for analysis by taking the square root of the data, the cecum data was transformed by taking the 5.5 root, and the proximal colon was analyzed by taking the 1.1 root. For the Isc data, the omasum data was transformed by taking the fifth root while the ileum data was transformed by taking the fourth root and the cecum was transformed by taking the 1.1 root. All data were re-transformed for presentation in the tables. Contrasts were used to compare the CON to the RA and the CON to the LFI treatment. Differences were declared significant when P < 0.05. Data are reported as means ± SEM.
The relationship between the indwelling pH system measurements in the reticulo-rumen at the time of killing and the pH measured in the reticulo-rumen digesta after killing using a hand-held pH meter was assessed using PROC REG in SAS (version 9.4).
RESULTS
BW, DMI, and Digesta pH
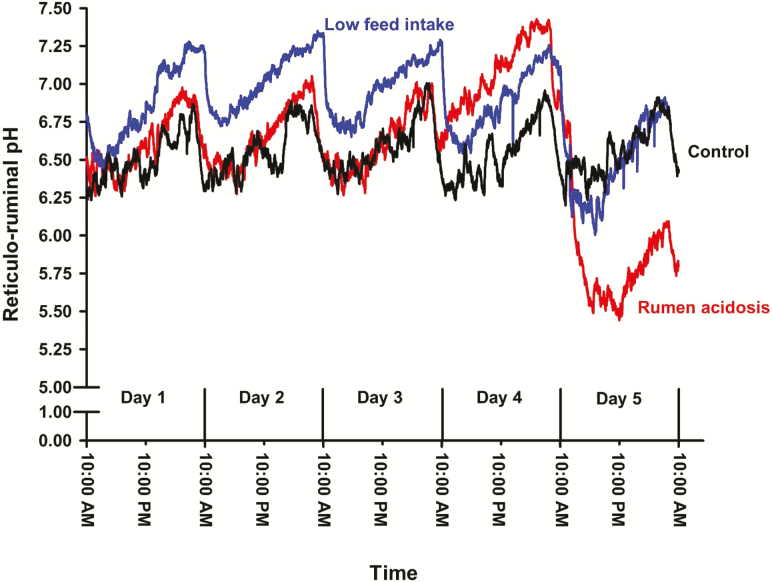
Initial BW did not differ among treatments (CON vs. RA, P = 0.69 and CON vs. LFI, P = 0.63; data not shown). Likewise, voluntary DMI prior to the challenge did not differ among treatments averaging 6.64, 7.32, and 7.44 kg/d for CON, RA, and LFI, respectively (P = 0.16; data not shown). However, in response to the nutritional challenges, the BW of LFI steers was 24 kg lighter than the CON steers (Table 2) while there was no difference between the CON and RA steers. DMI differed between CON and RA steers as well as between CON and LFI steers. As imposed by treatment design, the LFI calves had DMI that was 23.4% relative to that during the voluntary intake measurement period and was 26.2% relative to CON. The lower than targeted DMI for LFI was unexpectedly due to some steers having small quantities of refusals during the LFI challenge. The RA steers had a lower mean reticulo-ruminal pH when compared to the CON. The minimum reticulo-ruminal pH ranged from 5.24 to 6.00 but did not differ based on the contrasts used, and the maximum pH ranged from 6.77 to 7.48. The duration and area that ruminal pH was below 5.5 was greater for RA steers than CON. The duration of the ruminal pH depression for RA steers was indicative of RA (Gozho et al., 2005; Penner et al., 2007). The variability in reticulo-ruminal pH within a day over the 5-d measurement period are shown in Figure 1.
Figure 1.
One-minute averages for reticulo-ruminal pH for steers exposed to a 5-d period of ad libitum intake (n = 7), steers exposed to ruminal acidosis by reducing feed intake followed by provision of pelleted barley (n = 7), and when exposed to low feed intake (n = 7) for 5 d prior to killing.
At the time of killing, spot pH measurements were conducted for the reticulo-rumen, duodenum, jejunum, ileum, cecum, proximal colon, and distal colon (Table 3). Supporting the indwelling pH measurements, reticulo-ruminal pH was less for RA than CON. However, CON steers had reduced ruminal pH compared to LFI, which was not detected using continuous pH measurement. There were no treatment differences for pH among regions of the small intestine (P ≥ 0.22), but cecal pH was less for RA than CON steers. In the proximal colon, both the RA and LFI differed from the CON steers with RA steers having reduced pH and LFI steers having greater pH than CON. There were no differences in the distal colon pH.
Table 3.
Spot pH measurements in digesta at time of killing for CON (n = 7), RA (n = 7), and LFI (n = 7) steers
| Treatment | P value | |||||
|---|---|---|---|---|---|---|
| Regiona | CON | RA | LFI | SEM | CON vs. RA | CON vs. LFI |
| Reticulo-rumen | 6.14 | 5.33 | 6.61 | 0.14 | 0.001 | 0.032 |
| Duodenum | 5.28 | 4.99 | 5.18 | 0.35 | 0.71 | 0.82 |
| Jejunum | 7.03 | 6.98 | 7.31 | 0.16 | 0.82 | 0.22 |
| Cecum | 6.96 | 6.33 | 7.05 | 0.15 | 0.012 | 0.68 |
| Proximal colon | 6.94 | 6.52 | 7.30 | 0.10 | 0.010 | 0.023 |
| Distal colon | 6.90 | 6.52 | 7.13 | 0.14 | 0.06 | 0.26 |
apH was measured using a ratio of 1:1 g/g of digesta and double distilled water.
The Pearson correlation between the reticulo-ruminal pH measurements obtained using the orally dosed indwelling pH measurement system and a hand-held pH meter was 0.71 (P < 0.001) for all steers. The resulting equation to predict indwelling pH from the spot sample was .
Blood Metabolites, Insulin, and Acute Phase Proteins
The CON steers had greater plasma glucose (Table 4) than LFI steers, but plasma glucose did not differ among CON and RA steers. For insulin, RA steers had greater concentration than CON. Insulin concentration was less LFI than CON. For haptoglobin, concentrations varied between 39.4 ng/mL and 60.7 ng/mL but did not differ among treatments. Similarly, serum amyloid A concentrations varied between 22.5 μg/mL and 53.8 μg/mL, but did not differ among treatments. Serum NEFA concentrations were over four times greater in LFI steers when compared to CON steers, but RA and CON did not differ. Serum BHBA concentrations were not different between CON and RA, but BHBA tended to be greater for LFI than CON steers.
Table 4.
Plasma glucose, insulin, NEFA, haptoglobin, and serum amyloid A, and serum BHBA concentrations for CON (n = 7), RA (n = 7), and LFI (n = 7) steers
| Treatment | P value | |||||
|---|---|---|---|---|---|---|
| Item | CON | RA | LFI | SEM | CON vs. RA | CON vs. LFI |
| Plasma | ||||||
| Glucose, mg/dL | 98.8 | 98.3 | 84.6 | 2.2 | 0.87 | <0.001 |
| Insulin, µg/liter | 0.57 | 1.0 | 0.22 | 0.11 | 0.014 | 0.050 |
| NEFA, mEq/liter | 133.7 | 139.9 | 560.1 | 34.2 | 0.91 | <0.001 |
| Haptoglobin, ng/mL | 60.7 | 67.7 | 39.4 | 15.7 | 0.76 | 0.33 |
| Serum amyloid A, µg/mL | 34.1 | 53.8 | 22.5 | 15.0 | 0.37 | 0.59 |
| Serum | ||||||
| BHBA, mg/dL | 13.7 | 12.4 | 9.4 | 1.43 | 0.52 | 0.055 |
Gross Morphology and Histology
The LFI had shorter and smaller measurements than CON steers for ruminal papillae length, width, perimeter, and surface area; while the RA only had reduced ruminal papillae width than CON (Table 5). For histology, no differences (Table 6) were detected for total epithelial thickness or strata thickness (μm) in ruminal epithelia. Likewise, no differences, other than for granulosum strata thickness, was detected for the omasal epithelia. In the latter case, RA steers had a thicker granulosum than CON steers.
Table 5.
Rumen papillae length, width, perimeter, and surface area measurements for CON (n = 7), RA (n = 7), and LFI (n = 7) steers
| Treatment | P value | |||||
|---|---|---|---|---|---|---|
| Item | CON | RA | LFI | SEM | CON vs. RA | CON vs. LFI |
| Length, mm | 5.11 | 4.33 | 3.90 | 0.44 | 0.17 | 0.043 |
| Width, mm | 2.37 | 1.85 | 1.59 | 0.13 | 0.026 | 0.002 |
| Perimeter, mm | 13.81 | 11.43 | 9.97 | 0.98 | 0.09 | 0.012 |
| Surface areaa, mm2 | 18.71 | 13.18 | 7.72 | 1.86 | 0.08 | 0.002 |
aSurface area is the surface area of one side of the papillae multiplied by 2.
Table 6.
Total epithelial thickness and strata thickness for the rumen and omasum regions for CON (n = 7), RA (n = 7), and LFI (n = 7) steers
| Treatment | P value | |||||
|---|---|---|---|---|---|---|
| Region | CON | RA | LFI | SEM | CON vs. RA | CON vs. LFI |
| Rumen | ||||||
| Total epithelial thickness, µm | 8.54 | 8.90 | 8.00 | 0.75 | 0.73 | 0.60 |
| Basale strata thickness, µm | 1.92 | 1.93 | 1.92 | 0.09 | 0.90 | 0.98 |
| Spinosum strata thickness, µm | 3.12 | 3.06 | 2.67 | 0.43 | 0.91 | 0.45 |
| Granulosum strata thickness, µm | 2.68 | 3.28 | 2.87 | 0.44 | 0.33 | 0.75 |
| Corneum strata thickness, µm | 0.82 | 0.63 | 0.54 | 0.12 | 0.28 | 0.11 |
| Omasum | ||||||
| Total epithelial thickness, µm | 8.87 | 10.73 | 7.51 | 0.84 | 0.11 | 0.18 |
| Basale strata thickness, µm | 1.93 | 2.06 | 1.93 | 0.12 | 0.42 | 1.00 |
| Spinosum strata thickness, µm | 3.38 | 4.43 | 3.02 | 0.58 | 0.18 | 0.60 |
| Granulosum strata thickness, µm | 2.04 | 3.05 | 1.42 | 0.31 | 0.029 | 0.11 |
| Corneum strata thickness, µm | 1.51 | 1.18 | 1.13 | 0.27 | 0.36 | 0.23 |
Digesta SCFA Concentrations
The concentration of total SCFA only differed for the reticulo-rumen (Table 7). The RA and LFI steers had total SCFA concentrations that were less than 92 mM while for CON it was greater than 170 mM. The molar proportion of isobutyrate in ruminal fluid was less for RA steers in comparison with the CON steers and between CON and LFI steers. The molar proportion of SCFA did not differ between RA and CON steers in the small intestine with the exception of greater isobutyrate and valerate for RA than CON. For LFI, the proportion of acetate in the duodenum was reduced for LFI and the proportions of propionate and butyrate were increased relative to CON. In the jejunum, propionate was also greater for LFI steers than CON. In the cecum, isovalerate and valerate proportions were less for RA than CON while LFI concentrations were greater than CON. In the proximal colon, CON had greater molar proportions of acetate than both the RA and LFI steers. In contrast, the molar proportion of isobutyrate and butyrate were less for CON than both RA and LFI steers. The molar proportion of valerate was less for RA than CON, but was not different between LFI and CON. In the distal colon, isobutyrate proportions were greater for CON than RA, but the molar proportion of isobutyrate was less for CON than LFI. Isovalerate followed the same trend as isobutyrate with concentrations being lower in RA than CON and greater in LFI than CON.
Table 7.
Total short-chain fatty acid (SFCA, mM) and percentage of individual SCFA concentrations in digesta at the time of killing for CON (n = 7), RA (n = 7), and LFI (n = 7) steers
| Treatment | P value | |||||
|---|---|---|---|---|---|---|
| Item | CON | RA | LFI | SEM | CON vs. RA | CON vs. LFI |
| Rumen | ||||||
| Total, mM | 170.8 | 91.6 | 86.7 | 18.26 | 0.010 | 0.007 |
| Acetate, % | 58.8 | 66.5 | 61.7 | 3.96 | 0.20 | 0.61 |
| Propionate, % | 24.9 | 23.4 | 24.1 | 1.98 | 0.60 | 0.77 |
| Isobutyrate, % | 0.82 | 0.36 | 1.33 | 0.10 | 0.004 | 0.002 |
| Butyrate, % | 12.5 | 7.4 | 9.2 | 1.91 | 0.09 | 0.25 |
| Isovalerate, % | 1.47 | 1.34 | 2.13 | 0.23 | 0.07 | 0.06 |
| Valerate, % | 1.52 | 1.00 | 1.53 | 0.22 | 0.12 | 0.97 |
| Duodenum | ||||||
| Total, mM | 6.41 | 2.26 | 0.48 | 2.26 | 0.18 | 0.08 |
| Acetate, % | 80.6 | 52.5 | 24.6 | 13.35 | 0.11 | 0.007 |
| Propionate, % | 16.1 | 12.7 | 48.3 | 9.23 | 0.78 | 0.025 |
| Isobutyrate, % | 0.25 | 11.2 | 6.1 | 3.99 | 0.05 | 0.30 |
| Butyrate, % | 2.75 | 0.74 | 9.2 | 2.03 | 0.45 | 0.036 |
| Isovalerate, % | 0.16 | 5.47 | 2.72 | 8.36 | 0.22 | 0.56 |
| Valerate, % | 0.19 | 17.4 | 9.0 | 6.19 | 0.05 | 0.31 |
| Jejunum | ||||||
| Total, mM | 9.7 | 6.4 | 14.3 | 3.71 | 0.50 | 0.37 |
| Acetate, % | 82.9 | 76.4 | 80.4 | 8.13 | 0.54 | 0.82 |
| Propionate, % | 2.07 | 0.99 | 5.57 | 1.13 | 0.47 | 0.042 |
| Isobutyrate, % | 0.83 | 0.88 | 2.85 | 0.95 | 0.97 | 0.14 |
| Butyrate, % | 7.8 | 5.2 | 2.64 | 2.52 | 0.44 | 0.16 |
| Isovalerate, % | 6.4 | 2.18 | 9.7 | 2.84 | 0.26 | 0.40 |
| Valerate, % | 0.00 | 0.00 | 0.00 | - | - | - |
| Cecum | ||||||
| Total, mM | 46.2 | 42.7 | 36.3 | 6.63 | 0.72 | 0.31 |
| Acetate, % | 68.9 | 76.7 | 72.2 | 5.11 | 0.30 | 0.65 |
| Propionate, % | 14.8 | 17.6 | 16.9 | 2.94 | 0.52 | 0.63 |
| Isobutyrate, % | 5.5 | 0.15 | 1.83 | 2.55 | 0.16 | 0.32 |
| Butyrate, % | 8.5 | 4.76 | 5.41 | 2.59 | 0.33 | 0.42 |
| Isovalerate, % | 0.98 | 0.22 | 1.79 | 0.10 | <0.001 | <0.001 |
| Valerate, % | 1.29 | 0.64 | 1.85 | 0.12 | 0.003 | <0.001 |
| Proximal colon | ||||||
| Total, mM | 59.8 | 76.0 | 51.3 | 12.35 | 0.37 | 0.63 |
| Acetate, % | 56.8 | 37.0 | 37.1 | 5.60 | 0.028 | 0.028 |
| Propionate, % | 11.4 | 9.29 | 7.1 | 1.62 | 0.39 | 0.09 |
| Isobutyrate, % | 14.0 | 26.8 | 26.8 | 3.33 | 0.019 | 0.019 |
| Butyrate, % | 16.0 | 26.8 | 27.0 | 2.98 | 0.025 | 0.022 |
| Isovalerate, % | 0.83 | 0.39 | 1.25 | 0.15 | 0.05 | 0.07 |
| Valerate, % | 1.02 | 0.26 | 0.73 | 0.14 | 0.002 | 0.16 |
| Distal colon | ||||||
| Total, mM | 31.4 | 31.9 | 22.0 | 4.50 | 0.92 | 0.16 |
| Acetate, % | 77.1 | 74.4 | 70.4 | 3.24 | 0.53 | 0.16 |
| Propionate, % | 14.0 | 18.0 | 14.5 | 2.31 | 0.74 | 0.32 |
| Isobutyrate, % | 1.10 | 0.26 | 2.90 | 0.21 | 0.012 | <0.001 |
| Butyrate, % | 5.29 | 5.93 | 8.15 | 1.60 | 0.76 | 0.22 |
| Isovalerate, % | 1.14 | 0.26 | 2.45 | 0.18 | 0.004 | <0.001 |
| Valerate, % | 1.34 | 1.12 | 1.62 | 0.52 | 0.75 | 0.70 |
Inulin and Mannitol Flux, and Gt
For mannitol flux (Table 8), there was a difference detected in the proximal (33.83 µmol/(cm2 × h); P = 0.041) and distal colon (22.92 µmol/(cm2 × h); P = 0.015) for the LFI steers in comparison with the CON steers (46.82 µmol/(cm2 × h)). For each other region, there were no differences or general trends detected amongst the treatments. The inulin flux and the Gt were not different among treatments in any of the regions.
Table 8.
Mucosal to serosal inulin (JMS-14C-inulin) and mannitol (JMS-3H-mannitol) flux rate, and Gt data for CON (n = 7), RA (n = 7), and LFI (n = 7) steers
| Treatment | P value | |||||
|---|---|---|---|---|---|---|
| Item | CON | RA | LFI | SEM | CON vs. RA | CON vs. LFI |
| Rumen | ||||||
| JMS-14C-inulin, µmol/(cm2 × h) | 21.48 | 16.82 | 25.28 | 3.62 | 0.27 | 0.37 |
| JMS-3H-mannitol, µmol/(cm2 × h) | 43.50 | 35.56 | 30.75 | 2.01 | 0.23 | 0.07 |
| Gta, mS/cm2 | 9.51 | 10.8 | 8.6 | 1.05 | 0.41 | 0.55 |
| Omasum | ||||||
| JMS-14C-inulin, µmol/(cm2 × h) | 26.98 | 24.89 | 28.96 | 3.76 | 0.62 | 0.64 |
| JMS-3H-mannitol, µmol/(cm2 × h) | 22.98 | 32.87 | 20.56 | 2.54 | 0.20 | 0.73 |
| Gt, mS/cm2 | 9.80 | 9.58 | 6.41 | 0.47 | 0.95 | 0.30 |
| Duodenum | ||||||
| JMS-14C-inulin, µmol/(cm2 × h) | 18.31 | 24.01 | 21.59 | 4.40 | 0.38 | 0.61 |
| JMS-3H-mannitol, µmol/(cm2 × h) | 57.78 | 61.72 | 56.77 | 9.00 | 0.22 | 0.95 |
| Gt, mS/cm2 | 28.57 | 28.00 | 27.65 | 4.77 | 0.93 | 0.89 |
| Jejunum | ||||||
| JMS -14C-inulin, µmol/(cm2 × h) | 16.35 | 14.49 | 15.07 | 0.42 | 0.80 | 0.86 |
| JMS -3H-mannitol, µmol/(cm2 × h) | 52.85 | 48.76 | 42.99 | 11.49 | 0.81 | 0.56 |
| Gt, mS/cm2 | 22.31 | 24.26 | 23.24 | 4.2 | 0.74 | 0.86 |
| Ileum | ||||||
| JMS -14C-inulin, µmol/(cm2 × h) | 5.41 | 3.03 | 4.30 | 1.08 | 0.15 | 0.48 |
| JMS-3H-mannitol, µmol/(cm2 × h) | 15.44 | 10.94 | 12.69 | 2.42 | 0.21 | 0.44 |
| Gt, mS/cm2 | 11.36 | 9.60 | 12.30 | 0.61 | 0.60 | 0.80 |
| Cecum | ||||||
| JMS -14C-inulin, µmol/(cm2 × h) | 3.89 | 5.73 | 5.15 | 0.40 | 0.51 | 0.59 |
| JMS-3H-mannitol, µmol/(cm2 × h) | 15.07 | 12.44 | 11.20 | 0.66 | 0.71 | 0.55 |
| Gt, mS/cm2 | 15.62 | 13.31 | 12.59 | 1.67 | 0.48 | 0.36 |
| Proximal colon | ||||||
| JMS-14C-inulin, µmol/(cm2 × h) | 14.62 | 12.14 | 11.83 | 0.94 | 0.21 | 0.16 |
| JMS-3H-mannitol, µmol/(cm2 × h) | 48.86 | 38.17 | 33.83 | 1.54 | 0.14 | 0.041 |
| Gt, mS/cm2 | 24.97 | 25.68 | 24.27 | 2.61 | 0.85 | 0.85 |
| Distal colon | ||||||
| JMS-14C-inulin, µmol/(cm2 × h) | 12.66 | 9.26 | 9.01 | 1.24 | 0.08 | 0.06 |
| JMS-3H-mannitol, µmol/(cm2 × h) | 46.82 | 29.29 | 22.92 | 5.97 | 0.06 | 0.015 |
| Gt, mS/cm2 | 22.65 | 17.86 | 16.14 | 2.40 | 0.17 | 0.07 |
aGt, tissue conductance.
Quantitative Real-Time PCR
In the rumen, LFI steers had increased gene expression for CLDN1 (2.12), OCLN (1.79), TJP1 (1.89), TJP2 (2.04), and TLR4 (1.98) when compared to CON (1.02, P = 0.0002; 1.05, P = 0.006; 1.12, P = 0.042; 1.15, P = 0.016 and 1.03, P = 0.001 respectively; Table 9). For RA, TLR4 expression was increased (1.62) compared to CON (P = 0.028) in the rumen. In the jejunum, LFI steers had increased gene expression for CLDN1 (1.84), OCLN (1.84), TJP1 (2.39), TJP2 (2.06), TLR4 (1.74), and FCAR (4.43) when compared to CON (1.05, P = 0.009; 1.08, P = 0.004; 1.05, P = 0.014; 1.06, P = 0.002; 1.08, P = 0.031; and 1.27, P = 0.003, respectively). For RA, OCLN (1.84; P = 0.004), TJP1 (2.20; P = 0.030), and TJP2 (2.16; P = 0.0009) had increased expression compared to CON steers. In the distal colon, CLDN1 for RA (0.66) had decreased expression compared to CON (1.05; P = 0.036). For LFI, OCLN (2.13), TJP1 (1.76), TJP2 (1.89), and TLR4 (1.57) had increased expression compared to CON steers where OCLN was 1.15 (P = 0.011), TJP1 was 1.12 (P = 0.062), TJP2 was 1.07 (P = 0.020), and TLR4 was 1.03 (P = 0.045).
Table 9.
Relative expression of genes in the rumen, jejunum, and distal colon from CON (n = 7), RA (n = 7), and LFI (n = 7) steers
| Treatment | P value | |||||
|---|---|---|---|---|---|---|
| Genea | CON | RA | LFI | SEM | CON vs. RA | CON vs. LFI |
| Rumen | ||||||
| CLDN1 | 1.02 | 1.21 | 2.12 | 0.16 | 0.39 | < 0.001 |
| OCLN | 1.05 | 1.07 | 1.79 | 0.17 | 0.97 | 0.006 |
| TJP1 | 1.12 | 1.10 | 1.89 | 0.26 | 0.74 | 0.042 |
| TJP2 | 1.15 | 1.49 | 2.04 | 0.27 | 0.39 | 0.016 |
| TLR2 | 1.11 | 1.42 | 2.43 | 0.66 | 0.81 | 0.14 |
| TLR4 | 1.03 | 1.62 | 1.98 | 0.16 | 0.028 | 0.001 |
| FCAR | 1.26 | 7.10 | 17.46 | 7.08 | 0.87 | 0.20 |
| Jejunum | ||||||
| CLDN1 | 1.05 | 1.07 | 1.84 | 0.18 | 0.95 | 0.009 |
| OCLN | 1.08 | 1.84 | 1.84 | 0.15 | 0.004 | 0.004 |
| TJP1 | 1.05 | 2.20 | 2.39 | 0.30 | 0.030 | 0.014 |
| TJP2 | 1.06 | 2.16 | 2.06 | 0.18 | < 0.001 | 0.002 |
| TLR2 | 1.10 | 1.00 | 1.34 | 0.15 | 0.69 | 0.35 |
| TLR4 | 1.08 | 1.19 | 1.74 | 0.17 | 0.68 | 0.031 |
| FCAR | 1.27 | 1.57 | 4.43 | 0.57 | 0.73 | 0.003 |
| Distal colon | ||||||
| CLDN1 | 1.05 | 0.66 | 1.23 | 0.11 | 0.036 | 0.31 |
| OCLN | 1.15 | 1.25 | 2.13 | 0.25 | 0.77 | 0.011 |
| TJP1 | 1.12 | 1.19 | 1.76 | 0.20 | 0.82 | 0.062 |
| TJP2 | 1.07 | 1.65 | 1.89 | 0.21 | 0.09 | 0.020 |
| TLR2 | 1.06 | 1.19 | 1.15 | 0.16 | 0.57 | 0.69 |
| TLR4 | 1.03 | 1.16 | 1.57 | 0.15 | 0.61 | 0.045 |
| FCAR | 1.42 | 1.93 | 2.99 | 0.80 | 0.64 | 0.16 |
aCLDN1, Claudin; OCLN, Occludin; TJP1, Tight-cell junction protein 1; TJP2, Tight-cell junction protein 2; TLR2, Toll-like receptor 2; TLR4, Toll-like receptor 4; FCAR, Immunoglobin A receptor.
DISCUSSION
LFI and RA are two nutritional challenges that can occur under conventional beef and dairy cattle production; however, these challenges differ with respect to predisposing factors and, in some cases, the arising consequences on health and productivity. For example, LFI may occur as a physiological response to parturition (Bertics et al., 1992; Hayirli et al., 2002), infection (Jackson et al., 2016), heat stress (Huber, 1996; Rhoads et al., 2009; Pearce et al., 2012), social and physical stress as occurs for newly received feedlot calves (Loerch and Fluharty, 1999), or due to limited ability to access feed. Consequences of LFI include reduced nutrient supply, reduced SCFA concentration in the rumen (Albornoz et al., 2013; Zhang et al., 2013), elevated NEFA (Albornoz et al., 2013; Zhang et al., 2013), reduced rates of SCFA absorption across the reticulo-rumen (Gäbel et al., 1993; Albornoz et al., 2013), and increased permeability of the GIT (Zhang et al., 2013). It should be noted that changes imposed by LFI can be detected within 48 h (Gäbel et al., 1993). RA occurs when fermentation acid production exceeds acid removal strategies (Allen, 1997; Aschenbach et al., 2011) resulting in elevated SCFA and lactate concentrations during the progression of RA (Schwaiger et al., 2014), reduced rates of SCFA absorption across the rumen (Harmon, 1985; Schwaiger et al., 2014), and compromised barrier function of the ruminal epithelium (Gäbel et al., 1989; Gäbel and Aschenbach, 2002; Penner et al., 2010). LFI followed by re-feeding is also a predisposing factor for RA (Albornoz et al., 2013; Zhang et al., 2013) and that a transient reduction in feed provision is often used in RA induction models (Dohme et al., 2008; Schwaiger et al., 2014). Thus, LFI and RA are two differing nutritional challenges that have the potential to affect luminal conditions and potentially the function of the GIT. However, no studies to date have evaluated which regions of the GIT may be affected or are most affected by LFI and RA.
Barrier function of the GIT encompasses paracellular tissue permeability, the mucosal immune system (innate immunity), the commensal microbial populations, and the adaptive immune system (Jutfelt, 2011; Peterson and Artis, 2014). In the present study, we focused on tissue permeability using ex vivo measurements of mannitol and inulin movement across regions of the GIT, morphological and histological measurements for the rumen and omasum, and expression of genes in the rumen, jejunum, and distal colon that are associated with tight-cell junctions as well as innate and adaptive immunity. A challenge with such measurements is that they represent tissue functionality at a single point in time. Tissue selection was based on the focus of this study (paracellular transport of molecules), leaving tissue with visible lesions to be avoided in order to enable viable tissue preparations. This approach was used to improve the understanding for how LFI and RA affect the GIT.
Regional changes in SCFA concentration for LFI.
In the present study we imposed a 5-d LFI period with the amount of feed consumed equating to 23.4% of ad libitum DMI. In response to the LFI, there were marked reductions in BW, reduced reticulo-ruminal SCFA concentrations, and reduced plasma glucose and increased NEFA concentrations. All of the aforementioned variables are supportive of reduced nutrient supply as imposed in our experimental model. Others have also reported similar results when LFI has been imposed using a feed restriction model (Albornoz et al., 2013; Zhang et al., 2013). While we hypothesized that LFI would induce negative effects on the barrier function of the entire GIT epithelium, we did not observe such a response. We had further hypothesized that the outcomes arising from LFI would be mediated by reduced nutrient supply (as indicated by SCFA concentration) throughout the GIT; again, our results do not support this hypothesis. In fact, total SCFA concentration in the duodenum, jejunum, cecum, proximal colon, and distal colon were not affected by the LFI treatment although there were changes in the molar proportion of individual SCFA. Of the SCFA measured, butyrate is known to promote tight-cell junction formation (Plöger et al., 2012; Wang et al., 2012) and we observed that the proportion of butyrate increased in the duodenum and the proximal colon relative to that for CON steers. In addition, we observed that molar proportion of propionate was greater for LFI steers in the duodenum and jejunum than CON steers and that the concentrations of isovalerate was increased in the cecum, and proximal and distal colon. The lack of a reduction in SCFA concentration among regions may be in part due to reduced passage rate with LFI and increased digestibility (Colucci et al., 1982; Church, 1988; Kim et al., 2007). In addition, the shift in fermentation suggests that substrate availability was altered by region of the GIT and this assumed change in substrate availability may have resulted in changes in microbial profile, although we cannot confirm this speculation. Regardless of the mechanisms involved, we did observe an increase in the proportion of butyrate in the duodenum and proximal colon for LFI relative to CON. Although concentration and supply are not equivalent, the increase in butyrate concentration for LFI steers was not sufficient to completely compensate for the numerically lower SCFA concentration measured in those regions. That said, previous research has demonstrated increasing the butyrate supply may stimulate rumen papillae proliferation (Sakata and Tamate, 1978) and tight-cell junction assembly and expression (Plöger et al., 2012; Wang et al., 2012) and it is possible that subtle changes in supply among days may also elicit such a response. We also observed increases in isovalerate concentration in the cecum and distal colon for LFI steers relative to CON suggesting that a portion of the amino acids present in the digesta were being used as a carbon skeleton source to support microbial fermentation.
Effect of LFI on barrier function of the GIT.
In the present study, we used the flux of mannitol and inulin as markers for the potential for small and large molecules to cross the GIT epithelium, respectively. This approach has been used previously and has provided new evidence on regional permeability of the GIT under a non-challenged state (Penner et al., 2014). Regions were not compared in this study due to the variance in data transformation performed for mannitol and inulin flux, and Gt data. The mucosal-to-serosal flux of inulin and mannitol across the rumen, omasum, duodenum, jejunum, ileum, and cecum was not affected by LFI. Moreover, the flux of inulin was not affected in the proximal or distal colon, but we observed a reduction in mannitol flux for both regions. The reduction for mannitol flux is in contrast to previous studies showing that complete feed deprivation (Gäbel et al., 1993) and LFI (Zhang et al., 2013) increased permeability of the total GIT. The discrepancy for the results of the current study and previous studies may be related to the timing for when measurements were conducted. Previous studies have evaluated permeability immediately after 48 h of complete feed deprivation or initiated the measurement of total GIT permeability in vivo on the second day after imposing feed restriction protocol. In contrast, we conducted our measurements on the fifth day of LFI and hence the timing of our measurements differ from previous studies. Past research by Pearce et al. (2012; 2013a,b) has demonstrated that exposed of gilts to heat stress for 1, 3, or 7 d resulted in a 45% increase in plasma endotoxin concentration, a 30% decrease for transepithelial resistance (decreased barrier function), and a 2-fold increase for the LPS apparent permeability coefficient. In addition, Pearce et al. (2013a) observed that day 7 heat stress gilts tended to have a 41% increase for the LPS apparent permeability coefficient when compared to day 7 pair fed thermo neutral gilts, which was not observed for the other exposure durations. Overall, this may imply that timing of measurement is critical in order to detect changes in permeability and that, in our study, the epithelium may have initiated compensatory mechanisms to prevent consequences arising from increased permeability.
Zhang et al. (2013) suggested that in response to LFI, tissue morphology and histology may be altered as a mechanism to reduce GIT energy expenditure. We observed that ruminal papillae width, perimeter, and surface area were reduced for LFI steers relative to CON. This reduction in papillae dimensions is in support of retrogressive adaptation reported by Dirksen et al. (1985) when dairy cattle were exposed to a reduced plane of nutrition. Interestingly, we did not detect differences in the total epithelial thickness or the thickness of the stratum basale, spinosum, granulosum, or corneum in the rumen. The reduction in ruminal papillae dimensions without a corresponding change in cell strata thickness suggest that changes may occur in the lamina propria.
We observed that the expression of CLDN1, OCLN, TJP1, and TJP2 were upregulated in the rumen and jejunum and OCLN, TJP1, and TJP2 were upregulated in the distal colon for LFI relative to CON. This upregulation would be expected to result in increased barrier function as these are critical tight-cell junction proteins that help regulate paracellular movement of molecules; however, no differences in the flux rate of mannitol or inulin were detected between CON and LFI steers. The increase in expression of genes related to tight-cell junctions is supported by previous work where treatment application and measurement of the responses occurred over a 7-d period in swine (Pearce et al., 2013a). Thus, it is possible that the increased gene expression represents an adaptive response to prevent a reduction in permeability during LFI.
Toll-like receptors 2 and 4, and FCAR were evaluated as components of the innate and adaptive immune responses. We observed that TLR4 was greater in the rumen, jejunum, and distal colon and that FCAR was greater in the jejunum for LFI than CON suggesting that the innate immune system was stimulated in all three regions, where the adaptive immune system was stimulated only in the jejunum. Considering the jejunum has been shown to be an area with greater tissue permeability (Penner et al., 2014; Wood et al., 2014), the adaptive immune system would seem to be more likely stimulated in this area if barrier function was compromised. Toll-like receptors are pattern recognition receptors for microbial products (Ignacio et al., 2016). Toll-like receptor 2 recognizes pathogen associated microbial patterns from Gram-positive bacteria while TLR4 recognizes pathogen associated microbial patterns from Gram-negative bacteria, such as LPS (Ignacio et al., 2016). No LPS was measured in the digesta from this study, so it can not be concluded that the reason for increased TLR4 expression was from an increased amount of LPS throughout the GIT from the LFI challenge.
Regional changes in SCFA concentration for RA.
Regional differences for SCFA concentration were not detected in all the fermentative regions of the GIT as hypothesized, but could have been due to the combined effect of the feed restriction and grain overload used to induce RA coupled with numerically less DMI on the day of sampling. The only difference found was for the rumen, but proportions of the SCFA measured did differ between the main fermentative regions of the GIT (rumen, cecum, and proximal and distal colon) as hypothesized. The shift to a lower isobutyrate in the rumen and distal colon, and the shift to lower isovalerate in the cecum and distal colon was significant, but at such a small proportion, it is unlikely to have a biologically relevant effect on fermentation. Lower proportions of isovalerate and valerate in the cecum were detected, and again they are small proportions, indicating it is unlikely to have had a large impact on microbial fermentation in these regions. Butyrate proportions increased in the proximal colon, possibly as a contributing factor to improve barrier function as mentioned above with LFI.
Effect of RA on barrier function of the GIT.
The RA challenge model in the present study included 1 d of LFI coupled by over-feeding pelleted barley and full allocation of the regular TMR. This model has been proven to be effective to induce subacute RA (Dohme et al., 2008; Schwaiger et al., 2014). Steele et al. (2009) also demonstrated acute changes in mRNA abundance with the onset of RA suggesting that changes could be detectable within a short timeframe. In the present study, we observed that mean reticulo-ruminal pH was reduced for RA relative to the CON and the duration and area that pH was below 5.5 increased. While overall, it appears that subacute RA was induced, there was substantial variability in the response to the RA induction protocol. For example, of the seven RA steers the minimum pH ranged from 4.50 to 6.06 with only four of the steers had a minimum pH that was below the threshold of 5.5. For three of the four steers that did decrease below 5.5, the RA induction was severe with steers experiencing reticulo-ruminal pH below 5.5 for over 1,100 min/d. This magnitude of variation has been reported previously with RA induction models (Brown et al., 2006; Penner et al., 2009).
The reduction in reticulo-ruminal pH was again detected with the spot samples. Moreover, we observed that induction of RA decreased digesta pH in the cecum and proximal colon. Others have suggested that RA is associated with hind-gut acidosis and the consequences arising from RA such as increased plasma concentrations of acute phase proteins may be a combination of the RA and hind-gut acidosis (Gressley et al., 2011; Li et al., 2012; Plaizier et al., 2012). Despite induction of RA, we did not observe an increase in SAA or haptoglobin for RA relative to CON. Other studies have reported an increase in SAA and haptoglobin concentration with the use of a barley-pellet induced RA (Gozho et al., 2005, 2006; Khafipour et al., 2009), but blood collection was also performed at later times than during our study, which was collected at one time-point (10 h) following the RA challenge.
Induction of RA decreased papillae width despite the short duration of exposure (26 h relative to the grain overload). Although we did not detect significant differences, there was also a numerical reduction for total surface area. RA has previously been reported to reduce the depth of the stratum basale, stratum spinosum, and stratum granulosum. In contrast to Steele et al. (2009), we were not able to detect an effect of RA on cell strata thickness for the ruminal or omasal epithelia. Differences between the results of our study and that of Steele et al. (2009) may be related to the challenge model where Steele et al. (2009) imposed marked increases in the dietary concentrate over time. Thus, it is not clear whether the results of Steele et al. (2009) truly reflect RA or adaptation to a greater proportion of dietary grain.
Past research evaluating acute effects of RA ex vivo have demonstrated that exposing tissues to low pH (pH 5.1 to 5.2) increases permeability to histamine (Gäbel and Aschenbach, 2002) and mannitol (Penner et al., 2010; Wilson et al., 2012). In the present study, we imposed a RA challenge in vivo and evaluated the carry-over effect ex vivo. We were not able to detect any differences in the flux of mannitol or inulin due to the RA challenge relative to CON. Penner et al. (2010) also reported that mannitol flux across the isolated ruminal epithelium did not differ when evaluating carry-over effects of an in vivo subacute RA treatment. Differences between in vivo and ex vivo treatment application may be related to compensatory mechanisms available under in vivo conditions but not ex vivo conditions. For example, alterations in blood flow (Storm et al., 2012) and arterial buffer supply (HCO3−; Sehested et al., 1999) may help to mitigate the negative effect of RA on the epithelium allowing for regulation of intracellular pH at the expense of ruminal pH (Schwaiger et al., 2014). However, it should be acknowledged that use of Ussing chambers relies on viable tissue preparations as a strategy to measure paracellular movement of molecules and occurrence of lesions is not represented with this model. That said, it appears that permeability of the GIT is not compromised acutely in association with RA.
Supporting the permeability measurements, we did not detect differences in the expression of tight-cell junction proteins evaluated in the rumen or distal colon. However, we observed an increase in OCLN, TJP1, and TJP2 in the jejunum, and CLDN1 in the distal colon. Factors such as enteric pathogens (e.g., Clostridium perfringens; Berkes et al., 2003) and basolateral inflammatory cytokines (e.g., interleukin-13; Heller et al., 2005) stimulate an increase in the expression of tight-cell junction genes by activating protein kinase C, mitogen activated protein kinases, and Rho GTPases signaling pathways to modulate barrier function (Liang and Weber, 2014). With the jejunum having a greater amount of permeability (Penner et al., 2014; Wood et al., 2014), the risk of translocated pathogens under a nutritional challenge is greater in this region. However, the increase in gene expression for OCLN, TJP1, and TJP2 did not translate into reduced permeability. Evidence for a stimulated immune response was provided with greater TLR4 expression in the rumen. The increase in TLR4 may have caused by an increase in LPS in the digesta, as indicated already with LFI.
This study confirms that exposing steers to a 5-d period of LFI (25% of voluntary DMI) reduces SCFA concentration in the rumen, decreases plasma glucose, and increases plasma NEFA. Our data suggest that the GIT epithelium adapts to the reduced plane of nutrition by up-regulating expression of genes related to barrier function such that GIT permeability reverts to a pre-challenged state within 5 d. We further demonstrated that an acute RA challenge had no effects on GIT permeability 26 h after a grain overload challenge, but that genes related to barrier function were up-regulated in the jejunum and distal colon. This study shows that LFI and RA alter the luminal conditions and have direct impacts on the GIT in terms of SCFA concentrations and barrier function.
SUPPLEMENTARY DATA
Supplementary data are available at Animal Frontiers online.
ACKNOWLEDGMENTS
Funding support for the project was provided by the Canadian Beef Cattle Research Council (BCRC). Salary support for R.A.P. was provided in part from the National Sciences and Engineering Research Council. The authors thank R. Kanafany Guzman, G. Gratton, A.H. Laarman, B. Wiese, F. Joy, K. Burakowska, and B. Sutherland for assistance during the study.
LITERATURE CITED
- Albornoz R. I., Aschenbach J. R., Barreda D. R., and Penner G. B.. 2013. Feed restriction reduces short-chain fatty acid absorption across the reticulorumen of beef cattle independent of diet. J. Anim. Sci. 91:4730–4738. doi:10.2527/jas.2012-6223 [DOI] [PubMed] [Google Scholar]
- Allen M. S. 1997. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J. Dairy Sci. 80:1447–1462. doi:10.3168/jds.S0022-0302(97)76074-0 [DOI] [PubMed] [Google Scholar]
- Andersen C. L., Jensen J. L., and Orntoft T. F.. 2004. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64:5245–5250. doi:10.1158/0008-5472.CAN-04-0496 [DOI] [PubMed] [Google Scholar]
- Aschenbach J. R., and Gäbel G.. 2000. Effect and absorption of histamine in sheep rumen: significance of acidotic epithelial damage. J. Anim. Sci. 78:464–470. doi:10.2527/2000.782464x [DOI] [PubMed] [Google Scholar]
- Aschenbach J. R., Penner G. B., Stumpff F., and Gäbel G.. 2011. Ruminant nutrition symposium: role of fermentation acid absorption in the regulation of ruminal pH. J. Anim. Sci. 89:1092–3301. doi: doi:10.2527/jas.2010-3301 [DOI] [PubMed] [Google Scholar]
- Bertics S. J., Grummer R. R., Cadorniga-valino C., and Stoddard E. E.. 1992. Effect of prepartum dry matter intake on liver triglyceride concentration and early lactation. J. Dairy Sci. 75:1914–1922. doi:10.3168/jds.S0022-0302(92)77951-X [DOI] [PubMed] [Google Scholar]
- Berkes J., Viswanathan V. K., Savkovic S. D., and Hecht G.. 2003. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 52:439–451. doi:10.1136/gut.52.3.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland H. T., Scaglia G., Swecker W. S. Jr., and Burke N. C.. 2008. Effects of alternate weaning methods on behavior, performance, and blood metabolites of beef calves. Prof. Anim. Sci. 24:539–551. doi:10.15232/S1080-7446(15)30903-7 [Google Scholar]
- Brown M. S., Ponce C. H., and Pulikanti R.. 2006. Adaptation of beef cattle to high-concentrate diets: Performance and ruminal metabolism. J. Anim. Sci. 84(E. Suppl):E25–E33. doi:10.2527/2006.8413_supplE25x [DOI] [PubMed] [Google Scholar]
- Castillo-Lopez E., Wiese B. I., Hendrick S., McKinnon J. J., McAllister T. A., Beauchemin K. A., and Penner G. B.. 2014. Incidence, prevalence, severity, and risk factors for ruminal acidosis in feedlot steers during backgrounding, diet transition, and finishing. J. Anim. Sci. 92:3053–3063. doi:10.2527/jas.2014-7599 [DOI] [PubMed] [Google Scholar]
- Church D. C. 1988. The ruminant animal: digestive physiology and nutrition. Englewood Cliffs (NJ): Prentice-Hall, Inc. [Google Scholar]
- Clarke L. L. 2009. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 296:G1151–G1166. doi:10.1152/ajpgi.90649.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci P. E., Chase L. E., and Van Soest P. J.. 1982. Feed intake, apparent diet digestibility, and rate of particulate passage in dairy cattle. J. Dairy Sci. 65:1445–1456. doi:10.3168/jds.S0022-0302(82)82367-9 [Google Scholar]
- Dirksen G. L., Liebich H. G., and Mayer E.. 1985. Adaptive changes of the ruminal mucosa and functional and clinical significance. Bovine Pract. 20:116–120. [Google Scholar]
- Dohme F., DeVries T. J., and Beauchemin K. A.. 2008. Repeated ruminal acidosis challenges in lactating dairy cows at high and low risk for developing acidosis: Ruminal pH. J. Dairy Sci. 91:3554–3567. doi:10.3168/jds.2008-1264 [DOI] [PubMed] [Google Scholar]
- Etschmann B., Suplie A., Martens H.. 2009. Change of ruminal sodium transport in sheep during dietary adaptation. Arch. Anim. Nutr. 63:26–38. [DOI] [PubMed] [Google Scholar]
- Gäbel G., and Aschenbach J. R.. 2002. Influece of food deprivation on the transport of 3-O-methyl-α-D-glucose across the isolated ruminal epithelium of sheep. J. Anim. Sci. 80:2740–2746. doi:10.2527/2002.80102740x [DOI] [PubMed] [Google Scholar]
- Gäbel G., Bell M., and Martens H.. 1989. The effect of low mucosal pH on sodium and chloride movement across the isolated rumen mucosa of sheep. Q. J. Exp. Physiol. 74:35–44. doi:10.1113/expphysiol.1989.sp003237 [DOI] [PubMed] [Google Scholar]
- Gäbel G., Bestmann M., and Martens H.. 1991. Influences of diet, short-chain fatty acids, lactate, and chloride on bicarbonate movement across the reticulo-rumen wall of sheep. Zentralbl. Veterinarmed. A 38:523–529. doi:10.1111/j.1439-0442.1991.tb01043.x [DOI] [PubMed] [Google Scholar]
- Gäbel G., Marek M., and Martens H.. 1993. Influence of food deprivation on SCFA and electrolyte transport across sheep reticulo-rumen. Zentralbl. Veterinarmed. A. 40:339–344. doi:10.1111/j.1439-0442.1993.tb00637.x [DOI] [PubMed] [Google Scholar]
- González L. A., Schwartzkopf-Genswein K. S., Bryan M., Silasi R., and Brown F.. 2012a. Factors affecting body weight loss during commercial long haul transport of cattle in North America. J. Anim. Sci. 90:3630–3639. doi:10.2527/jas.2011-4786 [DOI] [PubMed] [Google Scholar]
- González L. A., Schwartzkopf-Genswein K. S., Bryan M., Silasi R., and Brown F.. 2012b. Relationships between transport conditions and welfare outcomes during commercial long haul transport of cattle in North America. J. Anim. Sci. 90:3640–3651. doi:10.2527/jas.2011-4796 [DOI] [PubMed] [Google Scholar]
- Gozho G. N., Krause D. O., and Plaizier J. C.. 2006. Rumen lipopolysaccharide and inflammation during grain adaptation and subacute ruminal acidosis in steers. J. Dairy Sci. 89:4404–4413. doi:10.3168/jds.S0022-0302(06)72487-0 [DOI] [PubMed] [Google Scholar]
- Gozho G. N., Plaizier J. C., Krause D. O., Kennedy A. D., and Wittenberg K. M.. 2005. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J. Dairy Sci. 88:1399–1403. doi:10.3168/jds.S0022-0302(05)72807-1 [DOI] [PubMed] [Google Scholar]
- Gressley T. F., Hall M. B., and Armentano L. E.. 2011. Ruminant nutrition symposium: productivity, digestion, and health responses to hindgut acidosis in ruminants. J. Anim. Sci. 89:1120–1130. doi:10.2527/jas.2010-3460 [DOI] [PubMed] [Google Scholar]
- Hayirli A., Grummer R. R., Nordheim E. V., and Crump P. M.. 2002. Animal and dietary factors affecting feed intake during the prefresh transition period in Holsteins. J. Dairy Sci. 85:3430–3443. doi:10.3168/jds.S0022-0302(02)74431-7 [DOI] [PubMed] [Google Scholar]
- Harmon D. L., Britton R. A., Prior R. L., and Stock R. A.. 1985. Net portal absorption of lactate and volatile fatty acids in steers experiencing glucose-induced acidosis or fed a 70% concentrate diet ad libitum. J. Anim. Sci. 60:560–569. doi:10.2527/jas1985.602560x [DOI] [PubMed] [Google Scholar]
- Heller F., Florian P., Bojarski C., Richter J., Christ M., Hillenbrand B., Mankertz J., Gitter A. H., Bürgel N., Fromm M.,. et al. 2005. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterol. 129:550–564. doi:10.1016/j.gastro.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Huber J. T. 1996. Amelioration of heat stress in dairy cattle. In: C. J. C., Philips, editor. Progress in dairy science. Oxon (UK): CAB Int; p. 211–243. [Google Scholar]
- Ignacio A., Morales C. I., Câmara N. O. S., and Almeida R. R.. 2016. Innate sensing of the gut microbiota: modulation of inflammatory and autoimmune disease. Front. Immun. 7:1–11. doi:10.3389/fimmu.2016.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson K. S., Carstens G. E., Tedeschi L. O., and Pinchak W. E.. 2016. Changes in feeding behavior patterns and dry matter intake before clinical symptoms associated with bovine respiratory disease in growing bulls. J. Anim. Sci. 94:1644–1652. doi:10.2527/jas.2015-9993 [DOI] [PubMed] [Google Scholar]
- Jutfelt F. 2011. Barrier function of the gut. Encyclopedia of Fish Physiology. 2:1322–1331. doi:10.1016/B978-0-1237-4553-8.00068-X [Google Scholar]
- Khafipour E., Krause D. O., and Plaizier J. C.. 2009. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 92:1060–1070. doi:10.3168/jds.2008-1389 [DOI] [PubMed] [Google Scholar]
- Khorasani G. R., Okine E. K., and Kennelly J. J.. 1996. Forage source alters nutrient supply to the intestine without influencing milk yield. J. Dairy. Sci. 79:862–872. doi:10.3168/jds.S0022-0302(96)76435-4 [DOI] [PubMed] [Google Scholar]
- Kim B. G., Lindemann M. D., Cromwell G. L., Balfagon A., and Agudelo J. H.. 2007. The correlation between passage rate of digesta and dry matter digestibility in various stages of swine. Livestock Sci. 109:81–84. doi:10.1016/j.livsci.2007.01.082 [Google Scholar]
- Laarman A. R., Pederzolli L., Wood K., Penner G. B., and McBride B. W.. 2016. Effects of Subacute Ruminal Acidosis and Feed Restriction on SCFA Transporters and Flux Pathways in Holstein Steers. J. Anim. Sci. 94:3729–3737. doi:10.2527/jas.2016-0638 [DOI] [PubMed] [Google Scholar]
- Li S., Khafipour E., Krause D. O., Kroeker A., Rodriguez-Lecompte J. C., Gozho G. N., and Plaizier J. C.. 2012. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J. Dairy Sci. 95:294–303. doi:10.3168/jds.2011-4447 [DOI] [PubMed] [Google Scholar]
- Liang G. H., and Weber C. R.. 2014. Molecular aspects of tight junction barrier function. Curr. Opin. Pharmacol. 19:84–89. doi:10.1016/j.coph.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. doi:10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Loerch S. C., and Fluharty F. L.. 1999. Physiological changes and digestive capabilities of newly received feedlot cattle. J. Anim. Sci. 77:1113–1119. doi:10.2527/1999.7751113x [DOI] [PubMed] [Google Scholar]
- Minuti A., Ahmed S., Trevisi E., Piccioli-Cappelli F., Bertoni G., Jahan N., and Bani P.. 2014. Experimental acute rumen acidosis in sheep: consequences on clinical, rumen, and gastrointestinal permeability conditions and blood chemistry. J. Anim. Sci. 92:3966–3977. doi:10.2527/jas.2014-7594 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Mani V., Boddicker R. L., Johnson J. S., Weber T. E., Ross J. W., Baumgard L. H., and Gabler N. K.. 2012. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J. Anim. Sci. 90:257–259. doi:10.2527/jas.52339 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Mani V., Weber T. E., Rhoads R. P., Patience J. F., Baumgard L. H., and Gabler N. K.. 2013a. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 91:5183–5193. doi:10.2527/jas.2013-6759 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Mani V., Boddicker R. L., Johnson J. S., Weber T. E., Ross J. W., Rhoads R. P., Baumgard L. H., and Gabler N. K.. 2013b. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLOS One. 8:1–8. doi:10.1371/journal.pone.0070215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner G. B., Aschenbach J. R., Wood K., Walpole M. E., Kanafany-Guzman R., Hendrick S., and Campbell J.. 2014. Characterising barrier function among regions of the gastrointestinal tract in Holstein steers. Anim. Prod. Sci. 54:1282–1287. doi:10.1071/AN14285 [Google Scholar]
- Penner G. B., Aschenbach J. R., Gäbel G., and Oba M.. 2009. Technical note: Evaluation of a continuous ruminal pH measurement system for use in noncannulated small ruminants. J. Anim. Sci. 87:2363–2366. doi:10.2527/jas.2008-1665 [DOI] [PubMed] [Google Scholar]
- Penner G. B., Beauchemin K. A., and Mutsvangwa T.. 2007. Severity of ruminal acidosis in primiparous Holstein cows during periparturient period. J. Dairy Sci. 90:365–375. doi:10.3168/jds.S0022-0302(07)72638-3 [DOI] [PubMed] [Google Scholar]
- Penner G. B., Oba M., Gäbel G., and Aschenbach J. R.. 2010. A single mild episode of subacute ruminal acidosis does not affect ruminal barrier function in the short term. J. Dairy Sci. 93:4838–4845. doi:10.3168/jds.2010-3406 [DOI] [PubMed] [Google Scholar]
- Peterson L. W., and Artis D.. 2014. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nature Reviews Immunol. 14:141–153. doi:10.1038/nri3608 [DOI] [PubMed] [Google Scholar]
- Plaizier J. C., Khafipour E., Li S., Gozho G. N., and Krause D. O.. 2012. Subacute ruminal acidosis (SARA), endotoxins, and health consequences. Anim. Feed Sci. and Tech. 172:9–21. doi:10.1016/j.anifeedsci.2011.12.004 [Google Scholar]
- Plöger S., Stumpff F., Penner G. B., Schulzke J., Gäbel G., Martens H., Shen Z., Günzel D., and Aschenbach J. R.. 2012. Micobial butyrate and its role for barrier function in the gastrointestinal tract. Ann. N. Y. Acad. Sci. 1258:52–59. doi:10.1111/j.1749-6632.2012.06553.x [DOI] [PubMed] [Google Scholar]
- Rhoads M. L., Rhoads R. P., VanBaale M. J., Collier R. J., Sanders S. R., Weber W. J., Crooker B. A., and Baumgard L. H.. 2009. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 92:1986–1997. doi:10.3168/jds.2008-1641 [DOI] [PubMed] [Google Scholar]
- Sakata T., and Tamate H.. 1978. Rumen epithelial cell proliferation accelerated by rapid increase in intraruminal butyrate. J. Dairy Sci. 61:1109–1113. doi:10.3168/jds.S0022-0302(78)83694-7 [DOI] [PubMed] [Google Scholar]
- Schurmann B. L., Walpole M. E., Górka P., Ching J. C. H., Loewen M. E., and Penner G. B.. 2014. Short-term adaptation of the ruminal epithelium involves abrupt changes in sodium and short-chain fatty acid transport. Am. J. Phiol. Regul. Integr. Comp. Physiol. 307:R802–R816. doi:10.1152/ajpregu.00035.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger T., Beauchemin K. A., and Penner G. B.. 2014. The duration of time that beef cattle are fed a high-grain diet affects the recovery from a bout of ruminal acidosis: dry matter intake and ruminal fermentation. J. Anim. Sci. 91:5729–5742. doi:10.2527/jas.2013-6471 [DOI] [PubMed] [Google Scholar]
- Sehested J., Diernæs L., Møller P. D., and Skadhauge E.. 1999. Ruminal transport and metabolism of short-chain fatty acids (SCFA) in vitro: effect of SCFA chain length and pH. Comp. Biochem. Physiol. A. 123:359–368. doi:10.1016/S1095-6433(99)00074-4 [DOI] [PubMed] [Google Scholar]
- Steele M. A., AlZahal O., Hook S. E., Croom J., and McBride B. W.. 2009. Ruminal acidosis and the rapid onset of ruminal parakeratosis in a mature dairy cow: a case report. Acta Vet. Scand. 51:39–45. doi:10.1186/1751-0147-51-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm A. C., Kristensen N. B., and Hanigan M. D.. 2012. A model of ruminal volatile fatty acid absorption kinetics and rumen epithelial blood flow in lactating Holstein cows. J. Dairy Sci. 95:2919–2934. doi:10.3168/jds.2011-4239 [DOI] [PubMed] [Google Scholar]
- Wang H., Wang P., Wang X., Wan Y., and Liu Y.. 2012. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig. Dis. Sci. 57:3126–3135. doi:10.1007/s10620-012-2259-4 [DOI] [PubMed] [Google Scholar]
- Wilson D. J., Mutsvangwa T., and Penner G. B.. 2012. Supplemental butyrate does not enhance the absorptive or barrier functions of the isolated ovine ruminal epithelia. J. Anim. Sci. 90:3153–3161. doi:10.2527/jas.2011-4315 [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Mellanby J., and Krebs H. A.. 1962. Enzymic determination of D(-)-b-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 82:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. M., Palmer S. I., Steele M. A., Metcalf J. A., and Penner G. B.. 2014. The influence of age and weaning on permeability of the gastrointestinal tract in Holstein bull calves. J. Dairy Sci. 98:7226–7237. doi:10.3168/jds.2015-9393 [DOI] [PubMed] [Google Scholar]
- Zhang S., Aschenbach J. R., Barreda D. R., and Penner G. B.. 2013. Recovery of absorptive function of the reticulo-rumen and total tract barrier function in beef cattle after short-term feed restriction. J. Anim. Sci. 91:1696–1706. doi:10.2527/jas.2012-5774 [DOI] [PubMed] [Google Scholar]