Abstract
It is known that physiological overproduction of nitric oxide (NO) contributes to oxidative stress and inflammation. Our published studies indicated that vitamin A (VA) reduces NO-induced oxidative stress in bovine mammary epithelial cells (BMECs) by increasing antioxidant enzyme activities. However, the precise mechanism is unclear. The present study was conducted to examine the protective effects of VA on NO-induced damage to BMECs in vitro using diethylenetriamine nitric oxide (DETA-NO) as the NO donor and to explore the intracellular signaling mechanisms of VA that involve nuclear factor erythroid 2-related factor (Nrf2) and nuclear factor kappa-B (NF-κB). Subconfluent BMECs were divided into 10 treatment groups with 6 replicates per treatment and were cultured with dimethyl sulfoxide (DMSO, vehicle negative control) or 0, 0.05, 0.1, 0.2, 0.5, 1, 2, 3, or 4 μg/mL of VA for 24 h and then incubated in the absence or presence of DETA-NO (1,000 μmol/liter) and VA for an additional 6 h. The results showed that exposure to DETA alone decreased cell proliferation compared with the negative control. Pretreatment with VA promoted the proliferation of BMECs, increased the activities of antioxidative enzymes including selenoprotein glutathione peroxidase (GPx) and thioredoxin reductase (TrxR) and their gene and protein expression but decreased NO and interleukin 1 (IL-1) contents in a quadratic manner (P < 0.05). In addition, the expression of mRNA and protein of factors that are related to NF-κB or Nrf2 signaling pathways in BMECs were regulated by VA in a quadratic dose-dependent manner; VA at a concentration of 1 μg/mL exhibited the strongest effect. Together, these results suggest that VA promotes antioxidant functions of BMECs by regulating the synthesis of selenoproteins including GPx and TrxR and by reducing concentrations of IL-1 and NO in vitro by modulating Nrf2 and NF-κB signaling pathways.
Keywords: bovine mammary epithelial cell, nitric oxide, nuclear factor erythroid 2-related factor, nuclear factor kappa-B, oxidative stress, vitamin A
INTRODUCTION
Oxidative stress is the primary factor that impairs the response to inflammatory conditions and ultimately leads to serious inflammation of mammary glands (Sharma et al., 2011). Our previous in vivo studies indicated that addition of vitamin A (VA) to the diet enhanced antioxidant activity in dairy cows (Jin et al., 2014a), and in vitro research showed similar results, indicating that VA prevents bovine mammary epithelial cells (BMECs) from oxidative stress (Jin et al., 2016). However, the exact mechanism is unclear. Nitric oxide (NO), a major secretory product of bioactive mammalian cells, has emerged as a fundamental signaling mechanism (Pacher et al., 2007). However, overproduction of NO synthesized by inducible nitric oxide synthase (iNOS) contributes to mitochondrial oxidative stress in adrenocortical cells, which is associated with inflammatory cytokines such as interleukin 1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α), which act by way of the nuclear factor kappa-B (NF-κB) pathway (Peng et al., 2015; Wang et al., 2015). Hence, high levels of NO are markers of oxidative stress. In addition, previous work from our group and others provided evidence that NO-induced cytokine production of IL-1, IL-6, and TNF-α was mediated by the NF-κB pathway (Sigala et al., 2012; Shi et al., 2016b).
Therefore, we concluded that the mechanism by which VA enhances the antioxidant function is likely to be related to reduced NO content (Shi et al., 2016b); however, there are few studies on this mechanism. Mann et al. (2007) noted that NO and reactive oxygen species (ROS) may induce activation of nuclear factor erythroid 2-related factor (Nrf2), which in turn triggers antioxidant defense enzymes in vascular cells. The diethylenetriamine nitric oxide adduct (DETA-NO) is a chemical-based NO releaser and can be used as a NO donor (Teng et al., 2016). In our previous study, a NO-induced oxidative stress model was created by using DETA as a NO donor (Guo et al., 2016). Thus, in the present study, we treated BMECs with DETA-NO to explore the possible mechanism by which VA protects BMECs from oxidative damage by modulating Nrf2 and NF-κB signaling pathways.
MATERIALS AND METHODS
All protocols for the projects using mammary glands were reviewed and approved by the Animal Care and Use Committee at the Inner Mongolia Agricultural University, Hohhot, China.
Preparation of the Working Solution
The DETA-NO (Sigma-Aldrich, Munich, Germany) working solution and the VA (all-trans retinoic acid [atRA]; Sigma-Aldrich, Munich, Germany) working solution were prepared as follows: DETA-NO was prepared in ultrapure water at room temperature at a concentration of 0.1 mol/liter. The resulting solution was added to the cell culture medium to obtain the desired concentration of 1,000 μmol/liter and the cell culture medium was sterile-filtered before the experiments. All-trans retinoic acid was dissolved in dimethyl sulfoxide (DMSO; Sigma, Munich, Germany), aliquoted in the dark and stored as 20 mg/mL stock solutions at −80 °C. Immediately before use, the stock solution was diluted to the desired concentration (0, 0.05, 0.1, 0.2, 0.5, 1, 2, 3, and 4 μg/mL) with Dulbecco’s Modified Eagle’s Medium/F12 (DMEM/F12; Gibco Laboratories, Grand Island, NY, USA) and subsequently sterile-filtered. The concentration of DMSO was always less than or equal to 0.5%, which is not toxic (Kurosawa et al., 2009). The 2 types of working solutions were stored at –4 °C until use.
Cell Culture and Treatments
Primary cells were isolated from the mammary glands of mid-lactation Holstein dairy cows at a local abattoir according to a modified procedure described by Wellnitz and Kerr (2004) and Qi et al. (2014). Briefly, after aseptically removing several pieces of mammary gland tissue (approximately 1 cm3), the tissue was washed with cold PBS (HyClone, Logan, UT, USA) containing 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, BRL, USA). The mammary tissue fragments were minced with sterile scissors and then digested by collagenase II (Gibco, BRL, USA) under 5% CO2 at 37 °C for 1 h with shaking at 20-min intervals. The digests were filtered through a 200 μm nylon mesh to remove the large tissue fragments, the filtered liquid was centrifuged at 179 × g for 5 min, and the supernatant was removed. The cell pellet was cultured in medium containing DMEM/F12 (Gibco, BRL, USA) media supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA), 0.5% insulin (Gibco, BRL, USA), 4 µg/mL prolactin (Sigma-Aldrich, St. Louis, MO, USA), 1 μg/mL hydrocortisone (Sigma-Aldrich, St. Louis, MO, USA), 10 ng/mL epithelial growth factor (Sigma-Aldrich, St. Louis, MO, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin under 5% CO2 and air at 37 °C. Cells were passaged twice and subsequently cryopreserved in DMEM/F12 containing 10% FBS and 10% DMSO. The normally growing BMECs showed an oval morphology in Fig. 1.
Figure 1.
Normal growth of BMECs (100×). Cells were cultured in DMEM/F12 media and showed an oval morphology
The cells were randomly divided into 10 groups with 6 replicates, and 3 independent experiments were performed. The first group served as the negative control: incubated without VA and DETA-NO for 30 h. Group 2 served as the DETA-NO-treated group: incubated without VA for 24 h before addition of DETA-NO (1,000 μmol/liter) for an additional 6 h. Groups 3 to 10 served as the VA plus DETA-NO-treated groups: each group was pretreated with 0.05, 0.1, 0.2, 0.5, 1, 2, 3, or 4 μg/mL of VA for 24 h and then incubated in the presence of 1,000 μmol/liter of DETA-NO and VA for a further 6 h. The DETA-NO concentration (1,000 μmol/liter) and its reaction time (6 h) were determined according to a previous test (Guo et al., 2016).
Cell Proliferation Assay
The susceptibility of cells to NO-mediated cell injury was evaluated by the methyl thiazolyl tetrazo-lium (MTT; Sigma, Munich, Germany) cytotoxicity assay using the following procedure: 5 × 104 cells/well were distributed in 96-well plates. Diethylenetriamine nitric oxide adduct and VA were added to the cells as stated in the experimental design. After the indicated 30 h incubation periods, 20 μL of MTT (5 mg/mL in 1× PBS) was added to each well and cells were incubated at 37 °C for 4 h. Next, formazan crystals dissolved in 100 μL DMSO were added to each well for 10 min with shaking. The absorbance (at 490 nm) was then immediately recorded for each well using the Synergy H4 ELISA microplate reader (BioTek, VT, USA). A high absorbance corresponds to high cell proliferation. The net absorbance in the wells containing cells cultured with control medium was considered to correspond to 100% viability. Cell proliferation was expressed as a relative growth rate (RGR): RGR = [OD490 nm (treatment group)/OD490 nm (control group)] × 100% (OD = optical density).
Preparation of Cell Lysates and Culture Supernatant
Cells from the treatment groups were lysed on ice for 30 min in lysis buffer (Beyotime, Nanjing, China). The lysates were centrifuged at 1,200 × g for 10 min at 4 °C to remove cell debris, and the supernatant was used for the analyses of the activities of GPx and ROS and the concentrations of malondialdehyde (MDA) and thioredoxin reductase (TrxR). The cell-free supernatant was collected for analysis of other parameters, including the activities of superoxide dismutase (SOD), hydrogen peroxide (CAT), total antioxidant capacity (T-AOC), and iNOS and concentrations of NO, IL-1, IL-6, and TNF-α.
Measurement of Enzyme Activity and Inflammatory Factor Content
The activities of GPx and TrxR in the cells were measured using a commercial colorimetric assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions, and absorbance was measured by a spectrophotometer at a wavelength of 412 nm (UN2CO-WFT2100; Aoyi Co. Ltd, Shanghai, China). The MDA concentrations and ROS activities were estimated with a thiobarbituric acid and chemical fluorescence analyzer test separately using a commercial kit (Nanjing Jiancheng Bioengineering Institute) following the manufacturer’s instructions and a Synergy H4 instrument (BioTek, VT, USA).
Enzyme-Linked Immunosorbent Assays
The activities of SOD, CAT, and iNOS and contents of T-AOC, NO, IL-1, IL-6, and TNF-α in culture supernatants were determined by ELISA, following their respective manufacturer’s instructions (R&D Systems, MN, USA) and using standard curves. Color changes were determined at 450 nm. The interassay coefficient of variation was lower than 11% and the intraassay coefficient of variation was lower than 9%.
RNA Extraction and Real-Time PCR
Total RNA was extracted from the cells using Trizol solution (TaKaRa, Dalian, China) according to the manufacturer’s instructions. Agarose gel electrophoresis (2% gel) and microplate reader were used to assess RNA integrity and purity. Then, cDNA was synthesized in a 10 μL reaction system using PrimeScript RT reagent Kit DRR036A (TaKaRa, Dalian, China). The reaction program of the cDNA synthesis was as follows: 37 °C for 15 min and 85 °C for 5 s. The reverse-transcribed products (cDNA) were stored at –20 °C for real-time PCR assay. Real-time PCR reactions were carried out in a final volume of 20 μL containing 10 μL of 2× SYBR Premix Ex TaqTMII (TaKaRa, Dalian, China), 2 μL of cDNA, 0.4 μL of each forward and reverse primer (10 μM each), and 7.2 μL of RNase-free water. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal controls. The primers are presented in Table 1. The reactions were performed in a LightCycler480 real-time PCR machine (Roche Diagnostics Ltd, Forrentrasse CH-6343 Rotkreuz, Switzerland) with an initial denaturing step of 95 °C for 30 s followed by 40 cycles consisting of 95 °C for 30 s (denaturation), 60 °C for 30 s (annealing), and 72 °C for 20 s (extension). The quality and specificity of the PCR products were assessed by a melt curve analysis and subsequent agarose gel electrophoresis. The quantitative real-time PCR data were analyzed using the 2−∆∆Ct method.
Table 1.
Sequences of primers used in real-time PCR
| Genesa | GenBank no. | Primer sequences (5′–3′)b | Length | Annealing temperature |
|---|---|---|---|---|
| GAPDH | NM_001034034 | F: ATCAAGTGGGGTGATGCTGG | 167 bp | 60 °C |
| R: TACTTCTCGTGGTTCACGCC | ||||
| GPx1 | NM_174076.3 | F: AGTGCGAGGTGAATGGCGAGAA | 328 bp | 60 °C |
| R: TGGGCAAAATCCCTGGAGAGCA | ||||
| GPx4 | NM_174770.3 | F: ATCAAAGAGTTCGCCGCTGGCT | 295 bp | 60 °C |
| R: TCGGAACACAGGCAACAGGCTT | ||||
| TrxR1 | NM_174625.3 | F: AGGAGAAAGCTGTGGAGAAA | 94 bp | 60 °C |
| R: TTATCCCTTGATGGAATCGT | ||||
| iNOS | NM_001076799.1 | F: TGTCAGCGGCAAGCACCACATT | 289 bp | 60 °C |
| R: CGGCTGGTTGCATGGGAAAACT | ||||
| IL-1β | NM_174093.1 | F: GCCTTGGGTATCAAGGACAA | 90 bp | 60 °C |
| R: TTTGGGGTCTACTTCCTCCA | ||||
| IL-6 | NM_173923.2 | F: ACAAGCGCCTTCACTCCATTCG | 242 bp | 60 °C |
| R: GCCAGTGTCTCCTTGCTGCTTT | ||||
| TNF-α | NM_173966.2 | F: TGCTTGTGCCTCAGCCTCTTCT | 254 bp | 60 °C |
| R: ACGAGGGCATTGGCATACGAGT | ||||
| NF-κBp50 | NM_001076409.1 | F: CAGATGGGCTACACTGAGGC | 179 bp | 60 °C |
| R: AAGGAGGTATCTACACCGCT | ||||
| NF-κBp65 | NM 001080242 | F: TCACAGACCTGGCATCTGTG | 123 bp | 60 °C |
| R: CCAGGCGAGTTATAGCCTCA | ||||
| Nrf2 | NM_0010111678.2 | F: GCAGAGACATTCCCGTTTGT | 115 bp | 60 °C |
| R: CCTGAGGAGGAGCAGTGAAG |
aGAPDH = glyceraldehyde-3-phosphate dehydrogenase; GPx1 = glutathione peroxidase 1; GPx4 = glutathione peroxidase 4; TrxR1 = thioredoxin reductase 1; SelP = selenoprotein P; NF-κBp50 = nuclear factor kappa-Bp50; NF-κBp65 = nuclear factor kappa-Bp65; Nrf2 = nuclear factor erythroid 2-related factor.
bF = forward primer; R = reverse primer.
Western Blot
Proteins were extracted from the experimental BMECs using a lysis buffer (RAPA; Beyotime, Beijing, China). After measurement of the protein concentration, the sample proteins were loaded and separated by 12% SDS–PAGE and then electrophoretically transferred (Mini Trans-Blot Cell; Bio-Rad, CA, USA) to polyvinylidene difluoride membranes (Merck Millipore, Billerica, MA, USA). The membranes were incubated overnight at 4 °C with primary polyclonal rabbit anti-GPx1 (22 kDa, 1:1,000; Abcam, Cambrige, MA, USA), anti-TrxR1 (55 kDa, 1:500; Sigma-Aldrich, Munich, Germany), anti-iNOS (130 kDa, 1:500; Novus, CO, USA), or anti-GAPDH (36 kDa, 1:2,000; Proteintech, Chicago, IL, USA) and polyclonal rabbit anti-RARα (55 kDa, 1:750), monoclonal rabbit anti-IL-1β (31 kDa, 1:750), anti-IKKβ (87 kDa, 1:750), monoclonal rat anti-IκBα (39 kDa, 1:1,000), monoclonal rabbit anti-NF-κBp65 antibody (65 kDa, 1:750), anti-phospho-IKKβ (87 kDa, 1:500, Ser180), anti-phospho-IκBα (40 kDa, 1:1,000, Ser32), or anti-phospho-NF-κBp65 (65 kDa, 1:500, Ser536) (all from Cell Signaling Technology, MA, USA). After washing 3 times with PBS/0.1% Tween 20 (PBS-T), membranes were incubated with goat anti-rabbit IgG (1:1,000) (KPL Laboratories, Gaithersburg, MD, USA) or goat anti-rat IgG labeled with horseradish peroxidase for 1 h at room temperature. After incubating with secondary antibodies and washing 3 times with PBS-T, the immunoreactive bands were visualized using an enhanced chemiluminescence system (Beyotime Institute of Biotechnology, Beyotime, Shanghai, China). Finally, images from the radiographic film were scanned and the integrated density was determined by the software Quantity One (Bio-Rad, CA, USA). Relative density was quantified by normalization of the integrated density of each protein to that of the corresponding GAPDH (used as an equal loading control) according to Kang et al. (2015) and our previous research in the study group (Jin et al., 2016). Relative protein levels were determined by ImageJ software (NIH).
Statistical Analysis
Data were analyzed using the General Linear Model procedure of SAS software (Statistical Analysis System, Version 9.2) to test the significance of the treatment groups by regression analysis. Regression analysis was conducted to evaluate linear and quadratic effects of VA on all response criteria except for negative controls. Differences between means were determined using Duncan’s multiple comparisons to test for significance. Data are presented in the tables as the means with SEM. A value of P < 0.05 was regarded as significant, whereas the differences were considered to be a statistical trend when 0.05 < P < 0.10.
RESULTS
Effect of VA on MTT of BMECs Induced by DETA
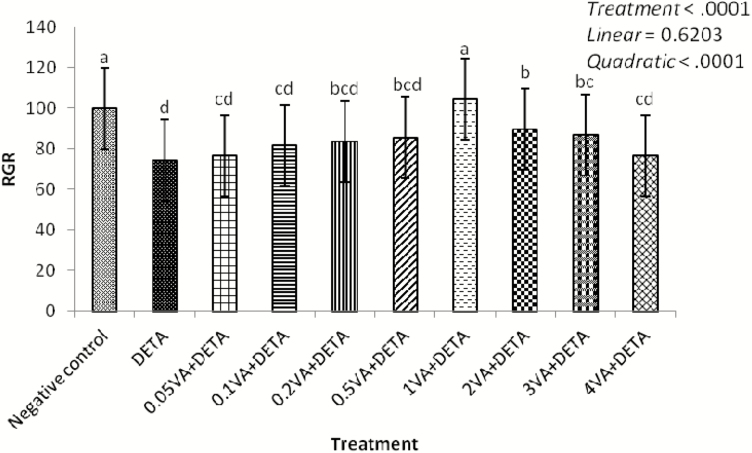
As shown in Fig. 2, exposure to DETA alone (1,000 μmol/liter) markedly decreased cell proliferation compared with the negative control (P < 0.0001). Compared with the DETA treatment group, cell proliferation increased in response to VA addition in a quadratic dose-dependent manner (P < 0.0001); the greatest value was observed with 1 μg/mL of VA.
Figure 2.
Effect of VA on MTT of BMEC disturbed by DETA. The BMEC were randomly divided into 10 groups with 6 replicates, and 3 independent experiments were performed. The first group was used as control (negative control): without VA (Sigma-Aldrich, Munich, Germany) and DETA-NO (Sigma-Aldrich, Munich, Germany) for 30 h. Group 2 was DETA-NO-treated group: without VA for 24 h before treated with DETA-NO (1,000 μmol/liter) for an additional 6 h. Groups 3–10 were 8 doses of VA plus DETA-NO-treated groups: pretreated BMEC with 0.05, 0.1, 0.2, 0.5, 1, 2, 3, or 4 μg/mL of VA for 24 h and then incubated in the presence of 1,000 μmol/liter of DETA-NO and Se for a further 6 h. The cell proliferation was detected by MTT (Sigma-Aldrich, Munich, Germany). a–dMeans in the treatment groups not followed by the same letter differ significantly (P < 0.05), whereas the differences were considered to be a statistical trend when 0.05 < P < 0.10.
Effect of VA on the Antioxidant Enzyme Activity and Inflammatory Cytokine Content of BMECs Induced by DETA
The antioxidant enzyme activities of GPx, T-AOC, SOD, CAT, and the TrxR content in the DETA treatment group were significantly lower than the negative controls (P < 0.0001). Compared with the DEAT treatment group, VA significantly reversed DETA-induced changes in a quadratic dose-dependent manner (P < 0.05); the greatest value was observed with 1 μg/mL of VA (Table 2).
Table 2.
Effect of VA on the antioxidant enzyme activity and inflammatory cytokines content in BMEC induced by DETA
| Index | Treatment | SEM | P-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative control | DETA | 0.05VA + DETA | 0.1VA + DETA | 0.2VA + DETA | 0.5VA + DETA | 1VA + DETA | 2VA + DETA | 3VA + DETA | 4VA + DETA | Treatment | Linear | Quadratic | ||
| GPx activity IU/mgPr | 40.538a | 18.644d | 20.950d | 24.743cd | 25.334cd | 26.180bc | 34.144bc | 33.332ab | 32.955bc | 32.091b | 2.377 | <0.0001 | <0.0001 | <0.0001 |
| TrxR content pg/mL | 78.870a | 35.470e | 38.470de | 47.170cde | 48.860cde | 62.450bc | 75.967ab | 59.152c | 54.216cd | 53.497cd | 4.683 | <0.0001 | 0.1640 | 0.0016 |
| T-AOC activity IU/ mL | 1.562a | 0.765c | 0.822c | 0.843c | 0.894c | 1.007bc | 1.283b | 1.048bc | 0.888c | 0.832c | 0.082 | <0.0001 | 0.8110 | 0.0039 |
| SOD activity U/mL | 28.714a | 10.251f | 11.784ef | 12.203e | 13.409e | 18.836d | 23.172b | 22.856bc | 21.938bc | 21.306c | 0.574 | <0.0001 | <0.0001 | <0.0001 |
| CAT activity U/mL | 1.716a | 0.623d | 0.685d | 0.926c | 1.054c | 1.066c | 1.400b | 1.084c | 1.012c | 0.994c | 0.063 | <0.0001 | 0.0671 | <0.0001 |
| MDA content nmol/ mgPr | 5.779c | 9.398a | 8.456ab | 7.963abc | 7.505abc | 7.031bc | 6.218c | 6.233c | 6.500bc | 6.851bc | 0.587 | 0.0061 | 0.0076 | 0.0007 |
| ROS activity fluorescence intensity/mL | 295.246d | 457.746a | 450.176a | 404.930b | 377.465b | 342.077c | 311.092cd | 324.120cd | 331.690c | 342.430c | 9.876 | <0.0001 | 0.0002 | <0.0001 |
| iNOS activity IU/mL | 17.702e | 42.361a | 39.555a | 34.598b | 29.243c | 21.674d | 18.084de | 19.213de | 20.051de | 19.698de | 1.113 | <0.0001 | <0.0001 | <0.0001 |
| NO content μmol/ liter | 131.348e | 173.137a | 165.417ab | 159.780abcd | 150.833cd | 147.279d | 131.961e | 146.258d | 159.044bcd | 161.127abc | 4.138 | <0.0001 | 0.7228 | 0.0007 |
| IL-1 content ng/liter | 33.196e | 132.553a | 77.652b | 45.730cde | 33.574e | 69.869bc | 32.472e | 42.746de | 68.595bcd | 60.285bcd | 7.911 | <0.0001 | 0.5266 | 0.1219 |
| IL-6 content ng/liter | 14.033a | 19.152a | 18.806a | 18.806a | 17.341a | 17.897a | 16.320a | 17.062a | 22.416a | 20.104a | 1.991 | 0.5056 | 0.2038 | 0.3149 |
| TNF-α content ng/ liter | 133.494a | 185.577a | 161.538a | 130.769a | 132.692a | 147.436a | 127.564a | 159.696a | 175.240a | 157.772a | 15.179 | 0.3810 | 0.5317 | 0.8249 |
The cells were randomly divided into 10 groups with 6 replicates, and 3 independent experiments were performed. a–fMeans in the same row not followed by the same lowercase superscript letters differ significantly (P < 0.05), whereas the differences were considered to be a statistical trend when 0.05 < P < 0.10. The number of observations for each mean value was 6 (n = 6).
The content of the lipid peroxide product MDA and the cellular metabolism intermediates for ROS and iNOS activity in the DETA treatment group were markedly greater than in the negative control (P < 0.05). Compared with the DETA treatment group, VA treatment significantly reversed DETA-induced changes in a quadratic manner (P < 0.05). The addition of VA at a concentration of 1 μg/mL achieved the greatest effect. The contents of NO and IL-1 were significantly increased in the DETA treatment group vs. the negative control (P < 0.0001). In comparison to the DETA-induced group, supplementation with VA decreased the NO content and IL-1 concentration in a quadratic manner (P = 0.0007); minimum values were reached with the addition of VA at a concentration of 1 μg/mL. The concentrations of inflammatory cytokines IL-6 and TNF-α exhibited the same trends as the IL-1 content; however, their differences were not significant.
Effect of VA on mRNA Expression of Selenoproteins, Inflammatory Cytokines, and Nrf2 and NF-κB Pathways in DETA-Induced BMECs
Compared with the negative control, the addition of DETA alone to the culture medium significantly downregulated mRNA expression of GPx1, GPx4, and TrxR1 (Table 3; P = 0.0097, P < 0.0001, and P < 0.0001, respectively). Vitamin A enhanced the mRNA expression of these 3 selenoprotein enzymes in a quadratic dose-dependent manner (P = 0.0110, P = 0.0068, and P = 0.0003, respectively) compared to the DETA group, and the addition of VA at a concentration of 1 μg/mL caused the greatest mRNA expression. Diethylenetriamine nitric oxide adduct group upregulated the mRNA expression of interleukin-1β (IL-1β), IL-6, iNOS, and TNF-α of BMECs significantly (P < 0.0001, P = 0.0004, and P < 0.0001, respectively) except TNF-α vs. the negative control group. In comparison to the DETA group, supplementation with VA decreased gene expression of IL-1β and iNOS in a quadratic manner (P = 0.0018 and P = 0.0039, respectively), and the addition of VA at a concentration of 1 μg/mL caused the lowest mRNA expression.
Table 3.
Effect of VA on selenoproteins, inflammatory cytokines, Nrf2 and NF-κB pathway mRNA expression in DETA-induced BMEC
| Index | Treatment | SEM | P-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative control | DETA | 0.05VA + DETA | 0.1VA + DETA | 0.2VA + DETA | 0.5VA + DETA | 1VA + DETA | 2VA + DETA | 3VA + DETA | 4VA + DETA | Treatment | Linear | Quadratic | ||
| GPx1 | 1.382ab | 0.510c | 1.136bc | 1.242bc | 1.600ab | 1.720ab | 2.110a | 1.738ab | 1.114bc | 1.001bc | 0.171 | 0.0097 | 0.4877 | 0.0110 |
| GPx4 | 0.950de | 0.610f | 1.064cde | 1.200bcd | 1.380abc | 1.461ab | 1.647a | 1.372abc | 0.893def | 0.789ef | 0.074 | <0.0001 | 0.2978 | 0.0068 |
| TrxR1 | 1.001c | 0.427d | 0.865cd | 1.512b | 1.701b | 1.929b | 2.469a | 2.010ab | 1.865b | 1.815b | 0.143 | <0.0001 | 0.0100 | 0.0003 |
| IL-1β | 0.969d | 4.404a | 4.242a | 3.004b | 2.724bc | 3.037b | 1.884c | 2.635bc | 2.687bc | 2.006c | 0.283 | <0.0001 | 0.0016 | 0.0018 |
| IL-6 | 1.011d | 2.013a | 1.509bc | 1.357bcd | 1.214cd | 1.240cd | 1.133cd | 1.195cd | 1.701ab | 1.378bcd | 0.131 | 0.0004 | 0.7827 | 0.2487 |
| TNF-α | 1.003abc | 1.207a | 1.015abc | 0.833bc | 0.831bc | 0.858abc | 0.706c | 0.872abc | 1.081ab | 0.869abc | 0.101 | 0.1045 | 0.7745 | 0.6732 |
| iNOS | 0.920ab | 1.104a | 0.641bc | 0.482cd | 0.470cd | 0.321d | 0.279d | 0.313d | 0.350cd | 0.318d | 0.080 | <0.0001 | 0.0223 | 0.0039 |
| NF-κBp50 | 1.021ab | 1.215a | 0.912ab | 0.818ab | 0.766ab | 0.754ab | 0.582b | 0.818ab | 0.652ab | 0.749ab | 0.138 | 0.2871 | 0.1205 | 0.2039 |
| NF-κBp65 | 1.021abc | 1.143a | 0.965abc | 0.875bc | 0.988abc | 0.986abc | 0.798c | 1.074ab | 1.109ab | 0.934abc | 0.078 | 0.1036 | 0.7018 | 0.9137 |
| Nrf2 | 1.038de | 0.592e | 1.305cd | 1.500cd | 1.697bc | 2.106ab | 2.531a | 2.347a | 2.096ab | 1.488cd | 0.123 | <0.0001 | 0.0743 | <0.0001 |
GPx1 = glutathione peroxidase 1; GPx4 = glutathione peroxidase 4; NF-κBp50 = nuclear factor kappa-Bp50; NF-κBp65= nuclear factor kappa-Bp65; Nrf2 = nuclear factor erythroid 2-related factor. The cells were randomly divided into 10 groups with 6 replicates, and 3 independent experiments were performed. a–fMeans in the same row not followed by the same lowercase superscript letters differ significantly (P < 0.05), whereas the differences were considered to be a statistical trend when 0.05 < P < 0.10. The number of observations for each mean value was 6 (n = 6).
The NF-κBp50 and NF-κBp65 mRNA expression showed trends that were similar to those of the inflammatory cytokines, but their differences were not significant. The gene expression of Nrf2, a key factor in the antioxidant signaling pathway, was lower in the DETA-damaged group than in the negative control but was not significantly different. Compared to the DETA group, increasing VA from 0.05 to 4 μg/mL resulted in greater Nrf2 mRNA expression in a quadratic dose-dependent manner (P < 0.0001); the addition of VA at a concentration of 1 μg/mL achieved the highest effect.
Effect of VA on Selenoproteins, Retinoic Acid Receptor α, and IL-1β Protein Expression and Phosphorylation of Nrf2 and NF-κB Pathways in DETA-Induced BMECs
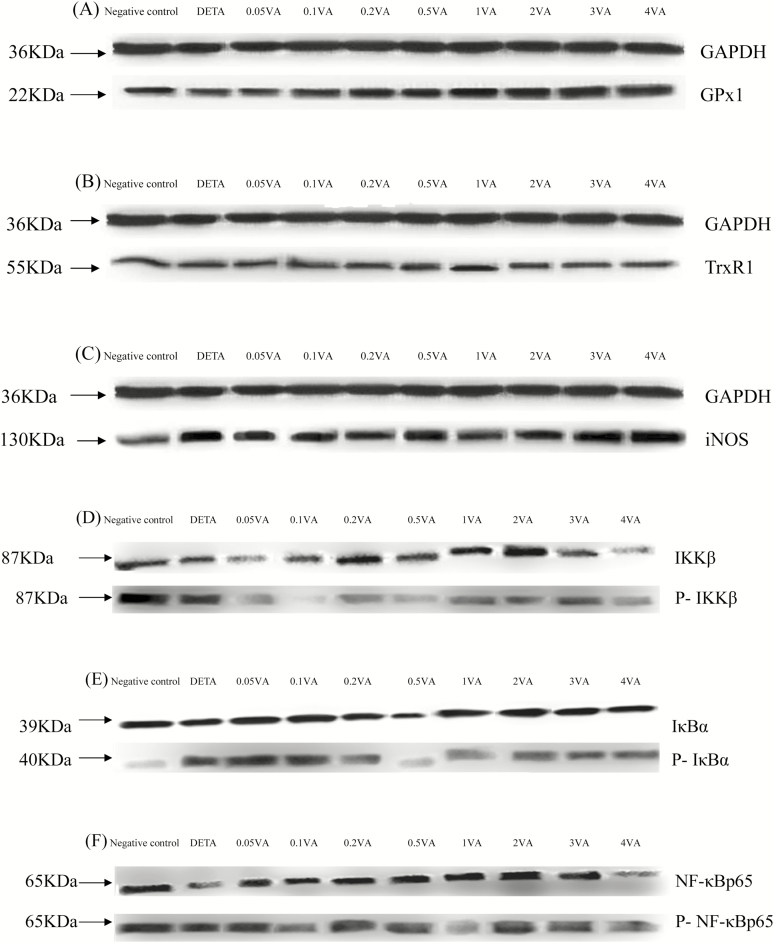
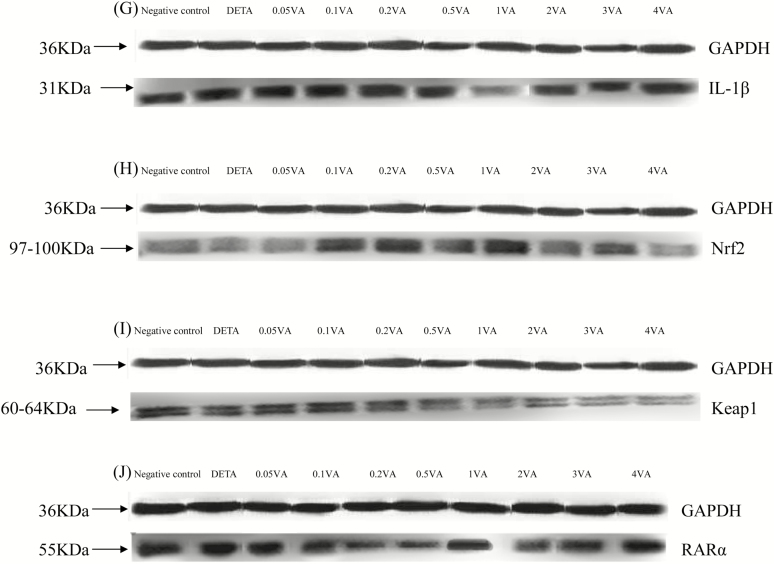
As shown in Table 4 and Fig. 3A, B, H, and J, DETA decreased the protein expression of GPx1, TrxR1, Nrf2, and retinoic acid receptor α (RARα) to levels that were significantly less than in the vehicle-treated cells (P < 0.0001). In comparison to the DETA group, supplementation with VA increased protein expression of GPx1, TrxR1, Nrf2, and RARα (P < 0.0001, P = 0.0007, P = 0.0098, and P < 0.0001, respectively) in BMECs in a quadratic manner and addition of VA at a concentration of 1 μg/mL achieved the highest effect. However, iNOS protein expression (Fig. 3C), phosphorylation levels of IκB kinase β (IKKβ; Fig. 3D), IκBα (Fig. 3E), and NF-κBp65 (Fig. 3F) and protein expression of IL-1β (Fig. 3G) and Keap1 (Fig. 3I) were opposite to levels of GPx1, TrxR1, Nrf2, and RARα with DETA and VA.
Table 4.
Effect of VA on DETA-induced selenoproteins, RARα, IL-1β protein expression, and phosphorylation of Nrf2 and NF-κB pathways in BMEC
| Items | Treatment | SEM | P-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative control | DETA | 0.05VA + DETA | 0.1VA + DETA | 0.2VA + DETA | 0.5VA + DETA | 1VA + DETA | 2VA + DETA | 3VA + DETA | 4VA + DETA | Treatment | Linear | Quadratic | ||
| GPx1 | 1.000fg | 0.668h | 0.825gh | 1.176ef | 1.369de | 1.481cd | 1.981a | 1.667b | 1.607bc | 1.519bcd | 0.077 | <0.0001 | 0.0026 | <0.0001 |
| TrxR1 | 1.000a | 0.591e | 0.633de | 0.723cd | 0.778bc | 0.806bc | 1.021a | 0.828b | 0.760bc | 0.707cd | 0.032 | <0.0001 | 0.5463 | 0.0007 |
| iNOS | 1.000e | 2.300a | 2.055abc | 1.870bcd | 1.685cd | 1.619d | 1.025e | 1.598d | 2.139ab | 2.365a | 0.134 | <0.0001 | 0.1846 | 0.0004 |
| IKKβ | 1.000a | 1.225a | 0.790a | 0.330b | 0.295b | 0.288b | 0.156b | 0.174b | 0.813a | 0.977a | 0.148 | 0.0003 | 0.1950 | 0.0047 |
| IkBα | 1.000d | 3.965a | 3.775a | 3.334b | 3.176b | 2.626c | 2.188c | 2.282c | 2.525c | 3.319b | 0.140 | <0.0001 | 0.0838 | <0.0001 |
| NF-κBp65 | 1.000b | 1.625a | 1.492a | 0.690cd | 0.607cd | 0.567cd | 0.254e | 0.404de | 0.751bc | 1.674a | 0.096 | <0.0001 | 0.5514 | <0.0001 |
| IL-1β | 1.000bc | 1.151a | 1.136a | 1.099ab | 0.950cd | 0.838de | 0.610f | 0.712ef | 0.768e | 0.809e | 0.040 | <0.0001 | 0.0019 | <0.0001 |
| Nrf2 | 1.000cde | 0.798ef | 0.690f | 1.181abc | 1.361ab | 1.150bcd | 1.386a | 0.943de | 1.030cd | 0.657f | 0.073 | <0.0001 | 0.1073 | 0.0098 |
| Keap1 | 1.000a | 0.503d | 0.637c | 0.716b | 0.425e | 0.254g | 0.122h | 0.352f | 0.554d | 0.548d | 0.019 | <0.0001 | 0.9262 | 0.0029 |
| RARα | 1.000ab | 0.779cd | 0.671de | 0.590e | 0.593e | 0.995ab | 1.122a | 1.012ab | 0.863bc | 0.359f | 0.048 | <0.0001 | 0.3710 | <0.0001 |
GPx1 = glutathione peroxidase 1; NF-κBp65 = nuclear factor kappa-Bp65; Nrf2 = nuclear factor erythroid 2-related factor; Keap1 = Kelch ECH associating protein 1. The cells were randomly divided into 10 groups with 6 replicates, and 3 independent experiments were performed. a–hMeans in the same row not followed by the same lowercase superscript letters differ significantly (P < 0.05), whereas the differences were considered to be a statistical trend when 0.05 < P < 0.10. The number of observations for each mean value was 6 (n = 6).
Figure 3.
Effect of VA on DETA-induced selenoproteins, RARα, IL-1β protein expression, and phosphorylation of Nrf2 and NF-κB pathways in BMEC. The cells were randomly divided into 10 groups with 6 replicates, and 3 independent experiments were performed. The first group was used as negative control: without VA (Sigma-Aldrich, Munich, Germany) and DETA-NO (Sigma-Aldrich, Munich, Germany) for 30 h. Group 2 was DETA-NO-treated group: without VA for 24 h before treated with DETA-NO (1,000 μmol/liter) for an additional 6 h. Groups 3–10 were 8 doses of VA plus DETA-NO-treated groups: pretreated BMEC with 0.05, 0.1, 0.2, 0.5, 1, 2, 3, or 4 μg/mL of VA for 24 h and then incubated in the presence of 1,000 μmol/liter of DETA-NO and VA for a further 6 h. Expressions of GPx1 (A), TrxR1 (B), iNOS (C), IL-1β (G), RARα (J), Nrf2 (H), Keap1 (I), and phosphorylated IKKβ (D), IκBα (E), NF-κBp65 (F) protein levels were detected by western blotting and normalized to GAPDH levels.
DISCUSSION
It has been reported that revulsives such as hydrogen peroxide, lipopolysaccharide (LPS), and DETA induce oxidative stress (Jin et al., 2014b; Guo et al., 2016; Shi et al., 2016a, 2016b). Changes in inflammatory factor concentrations are key to determining whether cells undergo oxidative stress. In addition to the cell survival rate, the activities of GPx, SOD, and CAT and the MDA content can also reflect the ability to eliminate free radicals and lipid peroxide damage in cells; therefore, they are indicators that can suggest whether or not cells undergo oxidative stress (Sinet et al., 1981). Nitric oxide, a short-lived radical molecule, which is an important biological mediator in the living organism, is synthesized from the oxidation of the terminal guanido-nitrogen atom of L-arginine by an enzyme termed NO synthase (NOS) (Jeronimo et al., 2016). However, the overproduction of NO synthesized by iNOS is cytotoxic (Peng et al., 2015). In the current study, the GPx, SOD, and CAT activities and gene and protein expression of GPx and TrxR in VA pretreated groups were improved in a quadratic dose-dependent manner compared with the DETA group. However, the NO concentrations as well as iNOS activity and gene and protein expression of BMECs were reduced, indicating that DETA-NO could cause BMECs oxidative stress, whereas VA supplementation protected BMECs against the DETA-induced injury by reducing NO production. Knowledge of the protection mechanism is limited.
There appear to be some relationships between NO overproduction synthesized by iNOS and the activation of NF-κB-mediated production of IL-1 (Jiang et al., 2017). Nuclear transcription factor NF-κB is the master regulator of expression of several genes involved in inflammation, immune responses, and apoptosis which are known to exacerbate inflammatory diseases. Nuclear factor kappa-B normally exists in the cytoplasm as an inactivated dimer composed of its p65 and p50 subunits (Viatour et al., 2005). In response to proinflammatory stimuli, inhibition kappa-B α (IκB) is phosphorylated and degraded by IKKβ, and NF-κB is released and translocated to the nucleus (Surh et al., 2001). The expression of many inflammatory cytokine genes, including iNOS and COX-2, are modulated by the binding of NF-κB to specific promoter regions (Limtrakul et al., 2015). Nuclear factor kappa-B is also required for maximal transcription of IL-1β, IL-6, and TNF-α (Wang et al., 2014). The present research found that the production of NO, IL-1β, and iNOS activity and the gene expression of IL-1β, IL-6, and iNOS, as well as the protein expression of IL-1β and iNOS, were significantly increased in DETA-induced BMECs, and phosphorylation of the NF-κB pathway showed the same trends as the parameters above. However, the VA treatment groups exhibited changes that were reversed compared to the changes associated with the parameters described above for the DETA treatment group. Hung et al. (2008) found that retinoid acid inhibited IL-1-induced iNOS, COX-2, and chemokine production in human chondrocytes, suggesting that the mechanism by which VA alleviates NO damage is by inhibition of IL-1 expression through the NF-κB pathway. On the other hand, Tran et al. (2016) demonstrated that GPx-1 overexpression can downregulate NF-κB DNA binding. Pineda-Molina et al. demonstrated for the first time, using an in vitro model of the C62S mutant, that the p50 subunit of NF-κB underwent S-glutathionylation in the Cys62 residue of its DNA-binding domain and that this modification reversibly inhibited its DNA-binding activity (Pinedamolina et al., 2001). This may explain why related factors in the NF-κB signaling pathway and its downstream IL-1, iNOS, and NO emissions were reduced while GPx1 expression was enhanced. Therefore, we inferred that VA might protect BMECs against DETA-induced damage and apoptosis because of the improved antioxidant enzyme activity that occurs with decreasing contents of IL-1β, IL-6, and TNF-α by inhibition of NF-κB activation resulting in decreased NO production, iNOS activity, and expression. Hong et al. (2014) suggested that atRA treatment in vitro dampens the LPS-induced NF-κB activation and TNF production in RAW 264.7 cells, which showed a crucial role of atRA in the progress of acute colitis through inhibition of NF-κB activation, and suggested that atRA represents a novel therapeutic management approach. The experimental results from Hong et al. are consistent with the results of the current study, and gene and protein expression of factors associated with the NF-κB signaling pathway show a quadratic dose–response relationship with VA addition.
To determine how VA inhibits NF-κB activation by antioxidant enzymes and decreases NO production, we studied the upregulation of Nrf2 signaling pathways by VA. Nuclear factor erythroid 2-related factor is a basic region-leucine zipper transcription factor that regulates the induction of phase II detoxification enzymes, such as HO-1, NQO1, GSH, GPx1, and SOD during stress conditions (Cho et al., 2005). Kelch ECH associating protein 1 (Keap1) is a cytoplasmic anchor for Nrf2 that tethers Nrf2 in the cytoplasm under basal unstressed conditions. Kelch ECH associating protein 1 prevents Nrf2 from translocating to the nucleus, where it would bind to the antioxidant response element and activate gene transcription (Kensler and Wakabayashi, 2010). It has been reported that Nrf2 is released from Keap1 under oxidative stress, resulting in an enhanced nuclear accumulation and transcriptional induction of target genes (phase II detoxification enzymes) that ensure cell survival (Motohashi et al., 2004). The present results showed that there were lower levels of Nrf2 gene and protein expression and Keap1 protein expression after DETA oxidative damage compared with the negative control. VA pretreatment enhanced Nrf2 expression and attenuated Keap1 expression, indicating that VA induced Nrf2 release from Keap1 and translocated nuclear protein. In our study, VA pretreatment not only suppressed NF-κB nuclear translocation and the NF-κB-targeted cytokines but also triggered Nrf2 nuclear translocation and elevated antioxidant enzyme activities and Nrf2-targeted protein expression. This is consistent with our previous study suggesting that one possible mechanism by which VA improves antioxidant capability is by way of increased GPx and TrxR activities (Shi et al., 2016b). Therefore, we speculate that VA attenuates DETA-induced oxidative stress in BMECs by upregulating the Nrf2-dependent GPx1 synthetic system, which downregulates phosphorylation of NF-κBp65.
To further prove a protective effect of VA, we measured the protein expression of the VA receptor. Retinoic acid, an active metabolite of VA, is a critical signaling molecule in various cell types (Osanai, 2017). Retinoic acid activity is mediated primarily by members of the retinoic acid receptor (RAR) subfamily, specifically RARα, retinoic acid receptor β (RARβ) and retinoic acid receptor γ (RARγ), which belong to the nuclear receptor (NR) superfamily of transcription factors. In other words, the effects of RA are mediated by RARs. The RAR is activated most efficiently by atRA and 9-cis RA. Retinoic acid receptor α appears to be the main contributor to reduced cell viability of the bovine mammary cell (Wang and Baumrucker, 2010). The present results showed that the level of RARα protein was markedly increased by VA pretreatment which succeeded in significantly reversing the inhibitory effects of DETA. Those results indicated that the RARα activated VA and also induced expression of Nrf2-regulated genes to protect BMECs against oxidative stress. Using in vitro cell and in vivo mouse models, Tan et al. (2008) also reported that RA, specifically atRA at suitable concentrations, activated Nrf2 and induced Nrf2 target genes, particularly the subunits of the rate-limiting enzyme of glutathione biosynthesis, glutamate cysteine ligase (GCLM/GCLC).
In conclusion, VA pretreatment improved, in a dose-dependent manner, the antioxidant capabilities of BMECs that were damaged by NO, and the addition of VA at a concentration of 1 μg/mL produced the greatest effect. The present results suggest that the protection by VA of NO-induced cell injury in BMECs can be attributed to the activated Nrf2 pathway, which triggers an adaptive response against subsequent oxidative stress through the NF-κB pathway. The complex scenario of VA redox regulation and the NF-κB and Nrf2 pathways require future studies.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation (Project No. 31160466) and the National Key Basic Research Program of China (Project No. 2011CB100800). The authors thank many colleagues, in particular, Yongmei Guo, and Boqi Zhang for their contribution to our study.
LITERATURE CITED
- Cho H. Y., Reddy S. P., Debiase A., Yamamoto M., and Kleeberger S. R.. 2005. Gene expression profiling of Nrf2-mediated protection against oxidative injury. Free Radic. Biol. Med. 38:325–343. doi:10.1016/j.freeradbiomed.2004.10.013 [DOI] [PubMed] [Google Scholar]
- Guo Y., Zhang B., Shi H., Yan S., Shi B., and Guo X.. 2016. Establishment of oxidative damage model of bovine mammary epithelial cells induced by diethylenetriamine/nitric oxide adduct. Chin. J. Anim. Nutr. 28:2378–2384. [Google Scholar]
- Hong K., Zhang Y., Guo Y., Xie J., Wang J., He X., Lu N., and Bai A.. 2014. All-trans retinoic acid attenuates experimental colitis through inhibition of NF-κB signaling. Immunol. Lett. 162:34–40. doi:10.1016/j.imlet.2014.06.011 [DOI] [PubMed] [Google Scholar]
- Hung L. F., Lai J. H., Lin L. C., Wang S. J., Hou T. Y., Chang D. M., Liang C. C., and Ho L. J.. 2008. Retinoid acid inhibits Il-1-induced iNOS, COX-2 and chemokine production in human chondrocytes. Immunol. Invest. 37:675–693. doi:10.1080/08820130802307237 [DOI] [PubMed] [Google Scholar]
- Jeronimo M. S., Barros A. D., Moritai V. E., Alves E. O., Souza N. L., Almeida R. M., Nobrega Y. K. M., Neto F. F. C., Amorin R., Borin M. F.,. et al. 2016. Oral or topical administration of l-arginine changes the expression of tgf and inos and results in early wounds healing. Acta Cir. Bras. 31:586–596. doi:10.1590/S0102-865020160090000003 [DOI] [PubMed] [Google Scholar]
- Jiang L., Li J., Zhou X., Bao J., and Wu L.. 2017. Paeonol inhibits IL-1β induced expression of iNOS, COX-2, and MMPs through NF-κB activation: an in vitro and in vivo study. Int. J. Clin. Exp. Med. 10:10010–10020. [Google Scholar]
- Jin L., Yan S., Shi B., Bao H., Gong J., Guo X., and Li J.. 2014a. Effects of vitamin A on the milk performance, antioxidant functions and immune functions of dairy cows. Anim. Feed Sci. Technol. 192:15–23. doi:10.1016/j.anifeedsci.2014.03.003 [Google Scholar]
- Jin L., Yan S., Shi B., Sheng R., Shi H., Zhao Y., and Li J.. 2016. Effects of retinoic acid on the synthesis of selenoprotein and the antioxidative indices of bovine mammary epithelial cells in vitro. Czech. J. Anim. Sci. 61:194–202. doi:10.17221/8851-CJAS [Google Scholar]
- Jin L., Yan S., Shi B., Shi H., Guo X., and Li J.. 2014b. Establishment of oxidative damage model of bovine mammary epithelial cells induced by hydrogen peroxide. Chin. J. Anim. Nutr. 26:3651–3658. [Google Scholar]
- Kang S., Lee J. S., Lee H. C., Petriello M. C., Kim B. Y., Do J. T., Lim D. S., Lee H. G., and Han S. G.. 2015. Phytoncide extracted from pinecone decreases LPS-induced inflammatory responses in bovine mammary epithelial cells. J. Microbiol. Biotechnol. 26:579–587. doi:10.4014/jmb.1510.10070 [DOI] [PubMed] [Google Scholar]
- Kensler T. W., and Wakabayashi N.. 2010. Nrf2: friend or foe for chemoprevention?Carcinogenesis 31:90–99. doi:10.1093/carcin/bgp231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa T., Nakamura H., Yamaura E., Fujino H., Matsuzawa Y., Kawashima T., and Murayama T.. 2009. Cytotoxicity induced by inhibition of thioredoxin reductases via multiple signaling pathways: role of cytosolic phospholipase A(2)α-dependent and -independent release of arachidonic acid. J. Cell. Physiol. 219:606–616. doi:10.1002/jcp.21703 [DOI] [PubMed] [Google Scholar]
- Limtrakul P., Yodkeeree S., Pitchakarn P., and Punfa W.. 2015. Suppression of inflammatory responses by black rice extract in raw 264.7 macrophage cells via downregulation of NF-kB and AP-1 signaling pathways. Asian Pac. J. Cancer Prev. 16(10):4277–4283. [DOI] [PubMed] [Google Scholar]
- Mann G. E., Rowlands D. J., Li F. Y. L., De Winter P., and Siow R. C. M.. 2007. Activation of endothelial nitric oxide synthase by dietary isoflavones: role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc. Res. 75:261–274. doi:10.1016/j.cardiores.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Motohashi H., and Yamamoto M.. 2004. Nrf2–Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 10:549–557. doi:10.1016/j.molmed.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Osanai M. 2017. Cellular retinoic acid bioavailability in various pathologies and its therapeutic implication. Pathol. Int. 67:281–291. doi:10.1111/pin.12532 [DOI] [PubMed] [Google Scholar]
- Pacher P., Beckman J. S., and Liaudet L.. 2007. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87:315–424. doi:10.1152/physrev.00029.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X. X., Zhang S. H., Wang X. L., Ye T. J., Li H., Yan X. F., Wei L., Wu Z. P., Hu J., Zou C. P.,. et al. 2015. Panax notoginseng flower saponins (PNFS) inhibit LPS-stimulated NO overproduction and iNOS gene overexpression via the suppression of TLR4-mediated MAPK/NF-kappa B signaling pathways in raw264.7 macrophages. Chin. Med. 10:15–26. doi:10.1186/s13020-015-0045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinedamolina E., Klatt P., Vazquez J., Marina A., de García L. M., Perezsala D., and Lamas S.. 2001. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry 40:14134–14142. doi:10.1021/bi0114590 [DOI] [PubMed] [Google Scholar]
- Qi L., Yan S., Sheng R., Zhao Y., and Guo X.. 2014. Effects of saturated long-chain fatty acid on mRNA expression of genes associated with milk fat and protein biosynthesis in bovine mammary epithelial cells. Asian-Australas. J. Anim. Sci. 27:414–421. doi:10.5713/ajas.2013.13499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma L., Verma A. K., Rahal A., Kumar A., and Nigam R.. 2011. Relationship between serum biomarkers and oxidative stress in dairy cattle and buffaloes with clinical and sub-clinical mastitis. Biotechnology 15:96–100. doi:10.3923/biotech.2016.96.100 [Google Scholar]
- Shi H., Guo Y., Liu Y., Shi B., Guo X., Li J., and Yan S.. 2016a. The in vitro effect of lipopolysaccharide on proliferation, inflammatory factors and antioxidant enzyme activity in bovine mammary epithelial cells. Anim. Nutr. 2:99–104. doi:10.1016/j.aninu.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Yan S., Jin L., Shi B., and Guo X.. 2016b. Vitamin A affects the expression of antioxidant genes in bovine mammary epithelial cells with oxidative stress induced by diethylene triamine-nitric oxide polymer. Czech. J. Anim. Sci. 61:117–126. doi:10.17221/8784-CJAS [Google Scholar]
- Sigala I., Zacharatos P., Boulia S., Toumpanakis D., Michailidou T., Parthenis D., Roussos C., Papapetropoulos A.. Hussain S. N., and Vassilakopoulos T.. 2012. Nitric oxide regulates cytokine induction in the diaphragm in response to inspiratory resistive breathing. J. Appl. Physiol. 113:1594–1603. doi:10.1152/japplphysiol.00233.2012 [DOI] [PubMed] [Google Scholar]
- Sinet P. M., and Garber P.. 1981. Inactivation of the human CuZn superoxide dismutase during exposure to O2− and H2O2. Arch. Biochem. Biophys. 212:411–416. [DOI] [PubMed] [Google Scholar]
- Surh Y. J., Chun K. S., Cha H. H., Han S. S., Keum Y. S., Park K. K., Lee S. S.. 2001. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappaB activation. Mutat. Res. 480–481:243–268. doi:10.1016/S0027-5107(01)00183-X [DOI] [PubMed] [Google Scholar]
- Tan K. P., Kosuge K., Yang M., and Ito S.. 2008. Nrf2 as a determinant of cellular resistance in retinoic acid cytotoxicity. Free Radic. Biol. Med. 45:1663–1673. doi:10.1016/j.freeradbiomed.2008.09.010 [DOI] [PubMed] [Google Scholar]
- Teng L., Bennett E., and Cai C.. 2016. Preconditioning c-kit positive human cardiac stem cells with a nitric oxide donor enhances cell survival through activation of survival signaling pathways. J. Biol. Chem. 291:9733–9747. doi:10.1074/jbc.M115.687806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T. V., Shin E. J., Jeong J. H., Lee J. W., Lee Y., Janq C. G., Nsh S. Y., Lei X. G., Toriumi K., Yamada K.,. et al. 2016. Protective potential of the glutathione peroxidase-1 gene in abnormal behaviors induced by phencyclidine in mice. Mol. Neurobiol. 9:1–21. doi:10.1007/s12035-016-0239-y [DOI] [PubMed] [Google Scholar]
- Viatour P., Merville M. P., Bours V., and Chariot A.. 2005. Phosphorylation of NF- -kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem. Sci. 30:43–52. doi:10.1016/j.tibs.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Wang C. N., Duan G. L., Liu Y. J., Yu Q., Tang X. L., Zhao W., Li X. H., Zhu X. Y., and Ni X.. 2015. Overproduction of nitric oxide by endothelial cells and macrophages contributes to mitochondrial oxidative stress in adrenocortical cells and adrenal insufficiency during endotoxemia. Free Radic. Biol. Med. 83:31–40. doi:10.1016/j.freeradbiomed.2015.02.024 [DOI] [PubMed] [Google Scholar]
- Wang Y., and Baumrucker C. R.. 2010. Retinoids, retinoid analogs, and lactoferrin interact and differentially affect cell viability of 2 bovine mammary cell types in vitro. Domest. Anim. Endocrin. 39:10–20. doi:10.1016/j.domaniend.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Wang J., Liu Y. T., Xiao L., Zhu L., Wang Q., and Yan T.. 2014. Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation 37:2085–2090. doi:10.1007/s10753-014-9942-x [DOI] [PubMed] [Google Scholar]
- Wellnitz O., and Kerr D. E.. 2004. Cryopreserved bovine mammary cells to model epithelial response to infection. Vet. Immunol Immunopathol. 101:191–202. doi:10.1016/j.vetimm.2004.04.019 [DOI] [PubMed] [Google Scholar]