Abstract
Effects of dietary energy level and intake of corn by-product-based diets on antibody production, acute phase protein response, stress, and immunocompetency of healthy and morbid newly received growing cattle were evaluated. Four dietary treatments were formulated to supply 0.99, 1.10, 1.21, and 1.32 Mcal NEg/ kg DM and were offered at 100%, 95%, 90%, and 85% of ad libitum based on 0.99/100 treatment intake, respectively. Thirty-two pens were utilized with approximately 12 animals/pen. Four animals from each pen (32/dietary treatment) were randomly selected and used to serve as a subset to monitor immune function and acute phase proteins following a split-plot design. In addition, two animals were randomly and independently selected from each pen (16/dietary treatment) and used to measure fecal cortisol metabolite. Additionally, animals removed from the pen one (M1), two (M2), or three (M3) times and classified as morbid were bled in conjunction with a healthy control (H) removed at the same time and the serum analyzed for the same parameters. A quadratic response to time (P < 0.01) was detected for haptoglobin concentrations and for antibody titers for bovine viral diarrhea type 1 (BVD-I) and infectious bovine rhinotracheitis (IBR; P < 0.01). Haptoglobin was lowest on arrival, highest on day 14, and similar to baseline levels by day 27. Titer levels for BVD-I and IBR were lowest on arrival, higher on day 14, and significantly higher on day 27. Titers for bovine viral diarrhea type 2 (BVD-II) responded linearly (P < 0.05) with lower levels on arrival and highest levels on day 27. Haptoglobin was elevated in morbid animals compared to healthy pen mates (P < 0.05). Titer levels for BVD-I and IBR were also higher in healthy animals compared to animals pulled for morbidity (P < 0.01). Fecal cortisol was higher on arrival than on day 14 (P < 0.05). Dietary treatment had no effect on any of the parameters investigated. In summary, high-energy receiving diets based on fermentable fiber from by-products can be fed to newly received growing cattle without negative effects on antibody production toward vaccines, inflammation, or overall stress. In addition, haptoglobin concentrations and titer levels for BVD-I and IBR viruses are higher in healthy animals compared to sick animals.
Keywords: antibody titers, growing cattle, haptoglobin, limit-feeding
INTRODUCTION
Newly received stocker cattle exposed to stress of marketing typically have low DMI on arrival to feeding facilities (Hutcheson and Cole, 1986). One strategy to mitigate the risk of dietary deficiencies from low intakes is to increase the concentration of dietary energy. Increasing dietary energy in receiving diets has been correlated in some research to increased morbidity, as defined as a higher percentage of animals in a group experiencing bovine respiratory disease (Lofgreen et al., 1975; Rivera et al., 2005). It is often thought the increased incidence of disease could be associated with metabolic disorders initiated by excessive carbohydrate fermentation from starch because cereal grains are often used to increase dietary energy. Restricting intake of high-energy diets for receiving and growing cattle to target specific gains has been shown to be a more efficient way of growing cattle (Schoonmaker et al., 2003), and limiting the amount of carbohydrate available for fermentation could also help to decrease metabolic disorders. Limit-feeding high-energy diets based primarily on fibrous by-products such as wet corn gluten feed (Sweet Bran; Cargill Animal Nutrition, NE) as the energy source (40% of the diet on DM basis) and not on cereal grains has not been studied to our knowledge in pen based experiments involving growing cattle. Therefore, there is little information of how limit-feeding diets of this nature could affect the animal’s immune system and stress level early in the feeding period.
The objectives of this experiment were to: 1) monitor immune function through the use of serological titer analysis; 2) characterize stress by measuring concentrations of glucocorticoid metabolite in feces; and 3) index the acute phase protein response using haptoglobin, across a broad range of dietary energy concentrations and intakes. Moreover, the trial was designed to identify differences in the serological and inflammatory parameters between healthy and morbid animals under the different dietary conditions.
MATERIALS AND METHODS
All procedures involving the use of animals were approved by the Kansas State University Institutional Animal Care and Use Committee (IACUC # 3745).
Arrival Management and Design
A total of 354 crossbred heifers (BW = 217 ± 4 kg) were purchased at auction markets in Alabama and Tennessee, assembled at an order buyer’s facility in Dickson, TN then shipped 1,086 km to the Kansas State University Beef Stocker Unit over a 10-d period from May 24 to June 3, 2016. The heifers were used in a randomized complete block design to analyze the effects of four energy levels and intakes of fibrous by-product-based diets on health and performance of stocker cattle in a 55-d receiving and growing study. Calves were blocked by load (4), stratified by individual arrival weight within load and assigned to pens containing 11 or 12 heifers. Pens within each block were randomly assigned to one of four treatments that equaled 8 pens/treatment for a total of 32 pens. The pens were soil surfaced and of equal size (9.1 × 15.2 m). Concrete bunks were 9.1 m in length and attached to a 3.6-m apron. Experimental diets (Table 1) were formulated to provide 0.99, 1.10, 1.21, or 1.32 Mcal NEg/kg DM and were offered for ad libitum intake (0.99/100), 95 (1.10/95), 90 (1.21/90), or 85% (1.32/85) of ad libitum intakes. All diets were formulated to contain 40% wet corn gluten feed (Sweet Bran; Cargill Animal Nutrition, Blair, NE) on a DM basis.
Table 1.
Composition of diets
| Dietsa | ||||
|---|---|---|---|---|
| Item | 0.99/100 | 1.10/95 | 1.21/90 | 1.32/85 |
| Ingredient, % DM | ||||
| Alfalfa | 22.50 | 17.00 | 12.00 | 6.50 |
| Prairie hay | 22.50 | 17.00 | 12.00 | 6.50 |
| Dry rolled corn | 8.57 | 19.08 | 28.50 | 38.82 |
| Wet corn gluten feedb | 40.00 | 40.00 | 40.00 | 40.00 |
| Supplementc | 6.43 | 6.92 | 7.50 | 8.18 |
aTreatment diets offered based on DMI of 0.99/100 treatment intake that was offered for ad libitum intake. First number = Mcal NEg/kg DM. Second number = % of 0.99/100 treatment offered on DM basis.
bCargill Animal Nutrition, Blair, NE.
cSupplement pellet was formulated to contain (DM basis) 10% CP, 8.0% Ca, 0.24% P, 5.0% salt, 0.55% potassium, 0.25% magnesium, 1.67% fat, 8.03% ADF, and as 367 mg/kg lasalocid (Bovatec; Zoetis, Parsippany, NJ).
At the time of arrival, calves were individually weighed, given an individual identification ear tag, and grossly assessed for disease and lameness. All animals were ear-notched, and the samples placed on ice until shipped following processing to the Kansas State University Veterinary Diagnostics Laboratory to identify animals persistently infected with Bovine Viral Diarrhea by a commercial kit utilizing a polymerase chain reaction assay (7500 Fast Real-Time PCR Systems, Applied Biosystems, Thermo Fisher Scientific, Austin, TX). Animals not demonstrating disease or lameness were assigned to 1 of 32 pens to stand overnight (11 or 12 heifers/pen). Each pen was provided long-stem hay and ad libitum access to water through automatic waterers.
The morning after arrival (day 0), calves were weighed, tagged with a pen number, and vaccinated for respiratory and clostridial disease. For clostridial pathogens, Vison 7 Somnus with Spur (Merck Animal Health, Omaha, NE) was used and for respiratory pathogens, Pyramid 5 + Presponse SQ (Boehringer Ingelheim Vetmedica Inc., St. Joseph, MO), a modified-live vaccine against infectious bovine rhinotracheitis (IBR), bovine viral diarrhea types 1 and 2 (BVDI-II), parainflueza 3 (PI3), and bovine respiratory syncytial virus (BRSV). Calves were also treated on day 0 for internal parasites with 10% Fenbendazole (Safe-Guard, Merck Animal Health) and administered enrofloxacin (Baytril 100, Bayer Animal Health, Shawnee Mission, KS). All animals were revaccinated on day 14 with Bovishield Gold 5, an additional modified-live virus against IBR, BVDI-II, BRSV and PI3 (Zoetis, Parsippany, NJ).
Animals were fed once daily at 0700 hours using a Roto-Mix feed wagon (model 414-14B, Dodge City, KS). Refusals were collected and weighed each day at 0600 hours immediately before feeding and those of pens offered the 0.99/100 treatment were used to calculate DMI each day and adjust feed delivery for the remaining treatments as described above. Refusals were targeted at 10% of feed delivery for the 0.99/100 treatment. Individual cattle weights were measured on day 0, at revaccination (day 14), and at conclusion of the study (day 55). A pen scale (Rice Lake Weighing Systems; Rice Lake, WI) was used to measure pen weights on days 27 and 42. After pen weights were measured on day 42, cattle were offered the 0.99/100 treatment for ad libitum intake through day 55 to equalize differences in gastrointestinal tract fill. Performance data was calculated from day 0 to each weigh period. Animals were observed twice daily for signs of morbidity that included overall depression, nasal and/or ocular discharge, and anorexia. Any animal displaying these symptoms was removed from the pen and taken to the hospital facilities. Once restrained in the chute, rectal temperature was measured and a clinical illness score (CIS) were recorded such that a CIS of 1 was a normal healthy animal; 2, slightly ill with mild depression or gauntness; 3, moderately ill demonstrating severe depression/labored breathing/and nasal or ocular discharge; and 4, severely ill and near death showing minimal response to human approach. Animals pulled from the pen with a rectal temperature ≥40 °C and demonstrating a CIS ≥ 2 were treated following label instructions. At first morbidity animals received florfenicol and flunixin meglumine (300 and 16.5 mg/mL, respectively; Resflor; Merck Animal Health, De Soto, KS). At second morbidity, ceftiofur (200 mg/mL; Excede; Zoetis, Parsippany, NJ), and at third, oxytetracycline (200 mg/mL; Bio-Mycin 200, Boehringer Ingelheim Vetmedica, Inc., St. Joeseph, MO). On the third treatment, animals were considered chronic and removed from the trial.
Blood Sampling and Analysis
Thirty-two animals from each dietary treatment (four from each pen) were randomly selected after arrival (day 1) and bled via a tail vein to serve as a subset for analysis of antibody production toward vaccines and the acute phase protein haptoglobin. Five animal samples were removed from the analysis due to labeling issues (three from 0.99/100 treatment, one from 1.21/90, and one from the 1.32/85 treatment) and four animal samples as a result of the animal testing positive as a BVD-PI and consequently being removed from the study (two animals from the 1.32/85 treatment and two from the 1.21/90 treatment). Animals were bled on days 0, 14, and 27 at 0700 hours immediately prior to feeding using venipuncture with an 18 gauge-bleeding needle. Blood was collected in glass 10-mL serum separator tubes (Monoject Blood Collection Tubes; Sherwood Medical, St. Louis, MO) and allowed to clot for 30 to 45 min in an upright position following collection. After the sample had clotted, the tubes were centrifuged at room temperature for 15 min at 2,000 × g. Immediately after being centrifuged, approximately 4 mL clear serum was pipetted from the top of the tubes with a transfer pipette and transferred to 5 mL glass tubes (Monoject Blood Collection Tubes; Sherwood Medical, St. Louis, MO) and kept frozen for analysis. Tubes containing serum were shipped to the Kansas State University Veterinary Diagnostic Laboratory and analyzed for antibody titers for bovine viral diarrhea I and II (BVD-I, BVD-II) and IBR as well as haptoglobin. Viral neutralizing antibody titers were determined using the American Association of Veterinary Laboratory Diagnosticians approved test procedure that is a varied serum-constant virus method and uses standardized specific viral strains as the indicator viruses. Haptoglobin concentrations were measured using a colorimetric assay (Smith et al., 1998).
Healthy and Morbid Animal Blood Analysis
Animals removed from the pen according to the protocol above for illness were also bled via a tail vein and the blood sample handled identically to the samples taken from the subset of cattle. In addition, a pre-determined random order of animals from each pen was generated on day 0 that served as a means to select a healthy control animal from each pen to obtain a blood sample following the same protocol and to use for pairwise comparisons. Animals that became morbid were permanently removed from the list of healthy candidates and therefore could never serve as a “healthy” animal for comparison.
Fecal Cortisol Metabolite Analysis and Sampling
Two randomly selected animals from each pen (16/dietary treatment) were used to determine fecal cortisol metabolite as a means of quantifying stress levels. Fecal grab samples (50 g) were obtained from the rectum of each of the selected animals on days 0 and 14 processing immediately prior to feeding using a different glove between samples. Samples were labeled by individual animal identification number and immediately frozen at −20 °C for analysis. All fecal samples were shipped to the Kansas State University Veterinary Diagnostic Laboratory to determine fecal cortisol metabolite concentrations by enzyme immunoassay to measure 11,17 dioxoandrostanes following the procedures of Montanholi et al. (2013).
Statistical Analysis
Serological and fecal cortisol data were analyzed using the MIXED procedure of SAS (ver. 9.4; SAS inst. Inc., Cary, NC), where sampling day, dietary treatment, and the interaction of sampling day × dietary treatment were fixed effects. The natural logarithm of titer levels for BVDI-II and IBR, and concentrations of haptoglobin were analyzed as the response variable. Contrast statements were used to detect linear, quadratic, and cubic effects of dietary treatment, sampling day, and their interactions when significant (α = 0.05). All values were back-transformed to normal scale and reported as such with accompanying back-transformed standard errors.
Data from the comparisons of healthy and sick animals was also transformed to the natural log scale and analyzed in the MIXED procedure of SAS as a split-plot design with dietary treatment as the whole plot treatment and health status as the sub-plot treatment. In this analysis, health status and the interaction of health status × dietary treatment were also used to test differences between healthy (H), animals pulled once (M1), animals pulled twice (M2), and animals pulled three times (M3). Orthogonal contrasts were used to detect linear, quadratic, and cubic effects of the fixed effects.
RESULTS AND DISCUSSION
Blood Analysis
Results from the analysis conducted on samples from the subset of cattle are in Tables 2 and 3. There were no effects of dietary treatment on titer levels for BVD-I (P = 0.89), BVD-II (P = 0.92), IBR (P = 0.62), or haptoglobin concentrations (P = 0.26). There were also no dietary treatment × sampling day interactions for BVD-I (P = 0.99), BVD-II (P = 0.99), IBR (P = 0.94), or haptoglobin (P = 0.64). In this trial, caloric intake was meant to be similar among treatments and all animals were theoretically programmed to gain weight similarly.
Table 2.
Effects of intake and energy level on log transformed haptoglobin and titer levels over time
| Dieta | P-valuec | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0.99/100 | 1.10/95 | 1.21/90 | 1.32/85 | SEMb | Diet | Day | Diet × Day |
| No. of pens | 8 | 8 | 8 | 8 | ||||
| No. of animals | 29 | 32 | 29 | 29 | ||||
| Haptoglobin,mg/dLg | 0.26 | <0.01 | 0.64 | |||||
| Day 0 | 2.7 | 2.6 | 3.2 | 2.6 | 0.26 | |||
| Day 14 | 3.6 | 3.0 | 3.5 | 3.3 | 0.26 | |||
| Day 27 | 3.1 | 3.0 | 3.1 | 3.0 | 0.27 | |||
| IBRd, titer levelf,g,i | 0.62 | <0.01 | 0.94 | |||||
| Day 0 | 0.3 | 0.7 | 0.4 | 0.3 | 0.30 | |||
| Day 14 | 2.5 | 2.9 | 2.2 | 2.5 | 0.30 | |||
| Day 27 | 2.8 | 3.0 | 2.9 | 2.7 | 0.30 | |||
| BVD-Ie, titer levelf,g,i | ||||||||
| Day 0 | 1.0 | 1.4 | 0.8 | 1.0 | 0.41 | 0.89 | <0.01 | 0.99 |
| Day 14 | 3.9 | 4.0 | 3.9 | 3.7 | 0.41 | |||
| Day 27 | 5.7 | 5.7 | 5.7 | 5.6 | 0.41 | |||
| BVD-IIe, titer levelf,i | 0.92 | <0.01 | 0.99 | |||||
| Day 0 | 1.4 | 1.3 | 1.1 | 1.2 | 0.59 | |||
| Day 14 | 3.1 | 3.0 | 2.6 | 3.2 | 0.59 | |||
| Day 27 | 4.0 | 4.3 | 3.8 | 4.2 | 0.59 | |||
aTreatment diets offered based on DMI of 0.99/100 treatment intake that was offered for ad libitum intake. First number = Mcal NEg/kg DM. Second number = % of 0.99/100 treatment offered on DM basis.
bLargest value across treatments for each time point is reported.
cFixed effects of dietary treatment, day, and dietary treatment × day interaction.
dStands for Infectious Bovine Rhinotracheitis.
eStands for Bovine Viral Diarrhea type 1 (BVD-I) and 2 (BVD-II).
fLinear effect of day (P < 0.0001).
gQuadratic effect of day (P < 0.01).
hQuadratic effect of day (P < 0.0001).
iDilution factor of serum at which viral neutralization was not detectable.
Table 3.
Effects of intake and energy level on haptoglobin and titer levels over time
| Dieta | P-valuec | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0.99/100 | 1.10/95 | 1.21/90 | 1.32/85 | SEMb | Diet | Day | Diet × Day |
| No. of pens | 8 | 8 | 8 | 8 | ||||
| No. of animals | 29 | 32 | 29 | 29 | ||||
| Haptoglobin, mg/dLg | 0.26 | <0.01 | 0.64 | |||||
| Day 0 | 15.2 | 13.3 | 25.8 | 13.8 | 6.8 | |||
| Day 14 | 35.8 | 19.3 | 32.5 | 27.2 | 9.5 | |||
| Day 27 | 22.1 | 19.8 | 21.5 | 19.6 | 5.9 | |||
| IBR,d titer levelf,h,i | 0.62 | <0.01 | 0.94 | |||||
| Day 0 | 0.3 | 1.0 | 0.5 | 0.3 | 0.6 | |||
| Day 14 | 11.6 | 16.6 | 8.2 | 10.7 | 5.1 | |||
| Day 27 | 15.7 | 19.1 | 17.3 | 14.4 | 5.9 | |||
| BVD-I,e titer levelf,g,i | ||||||||
| Day 0 | 1.7 | 2.9 | 1.2 | 1.6 | 1.1 | 0.89 | <0.01 | 0.99 |
| Day 14 | 48.6 | 51.7 | 46.5 | 40.8 | 20.1 | |||
| Day 27 | 286.2 | 303.1 | 284.7 | 257.9 | 121.6 | |||
| BVD-II,e titer levelf,i | 0.92 | <0.01 | 0.99 | |||||
| Day 0 | 3.0 | 2.8 | 1.9 | 2.4 | 2.8 | |||
| Day 14 | 20.6 | 18.4 | 12.5 | 24.4 | 15.0 | |||
| Day 27 | 55.7 | 75.4 | 45.3 | 68.4 | 44.5 | |||
aTreatment diets offered based on DMI of 0.99/100 treatment intake that was offered for ad libitum intake. First number = Mcal NEg/kg DM. Second number = % of 0.99/100 treatment offered on DM basis.
bLargest value across treatments for each time point is reported.
cFixed effects of dietary treatment, day, and dietary treatment × day interaction.
dStands for Infectious Bovine Rhinotracheitis.
eStands for Bovine Viral Diarrhea type 1 (BVD-I) and 2 (BVD-II).
fLinear effect of day (P < 0.0001).
gQuadratic effect of day (P < 0.01).
hQuadratic effect of day (P < 0.0001).
iDilution factor of serum at which viral neutralization was not detectable.
Pahlavani (2000) reviewed a large number of studies and determined the effects of caloric restriction on several aspects of the immune system. Results predominately favored a heightened innate and adaptive response in the face of pathogens and immunostimulants particularly increased IL-2 production. Pahlavani (2000) speculated the effects of caloric restriction could be due to altered transcription of IL-2, which aids in the maturation and activation of naive T-cells to become TH1 cells. Therefore, increased IL-2 could modulate adaptive immunity. Lymphocytes of the TH1 phenotype are central in the control and elimination of viruses. In partial agreement with Pahlavani (2000), Perkins et al. (2001) indicates that restriction of feed to 60% of maintenance had little effect on leukocytes or adhesion molecules in Holstein cows, but there were some instances of up-regulation. Whitney et al. (2006) observed that the febrile response was increased in steers on a high-forage diet compared to one of high concentrate and serum IgG was also decreased by the high concentrate diet after a Bovine Respiratory Syncytial Virus challenge. However, morbidity (number of animals initially being classified as ill) was not affected by dietary treatment. These authors speculated that the IgG produced in the calves fed the high concentrate diet could have been more effective at clearing the infection, and thus a more potent immune response was not necessary.
It is difficult to compare our results directly with these studies because our treatment altered dietary energy concentration, but total energy intakes were similar. Because our diets were not formulated to produce a state of energy deficiency, we did not expect differences in immune function due to dietary treatment as a result of energy restriction. We hypothesized that, if differences were detected in immune function, it would be due to increased incidence of disease, which is often times associated with decreased intakes and performance; that was not the case for any of those parameters. More research is warranted addressing the effects of how energy is delivered (programmed vs. full-fed) and its effects on the immune system of growing cattle.
We also hypothesized that if the high-energy diets that were limit-fed were increasing the incidence or severity of subacute ruminal acidosis (SARA), then there could be detectable differences in inflammation as measured by the acute phase protein response. Cattle experiencing acidotic conditions have ruminal epithelia that are more susceptible to damage caused by lipopolysaccharide and this damage could result in increased acute phase protein concentrations as endotoxin translocate into the bloodstream (Enemark et al., 2002). Dietary treatment did not affect haptoglobin concentrations in our study, which suggests no effects on the incidence of ruminal acidosis; research shows marked increases in acute phase proteins following SARA (Gozho et al., 2005). Although pH was lower for cattle fed the high-energy diets, it was not suggestive of being a cause of metabolic disorders, similar to other work involving Wet corn gluten feed (Montgomery et al., 2004).
Immunological parameters responded quadratically to sampling day (P < 0.01), except for BVD-II titers where only a linear effect was detected (P < 0.01; Table 1). For haptoglobin, concentrations were lowest on day 0, peaked at day 14, and were similar to base line levels by day 27. These results differ slightly from Berry et al. (2004) where peak levels of haptoglobin were realized on day 7 and returned to arrival levels by day 14. One discrepancy between our study and Berry et al. (2004) could be that we did not sample on day 7 and levels could have been higher than on day 14. The initial increase between day 0 and day 14 is most likely an effect of vaccination as Arthington et al. (2013) reported an acute phase protein response for 2 wk following vaccination against common respiratory and clostridial pathogens.
Titers for BVD-I and IBR viruses responded quadratically to sampling day. All animals were vaccinated on day 0 against both viruses and again on day 14. These results are a prime example of adaptive immunity as the immune system was primed and sensitized after day 0 vaccination and re-exposure on day 14 incited a much more robust response. This would explain the increase in titers between day 0 and day 14 and the large magnitude of increase between day 14 and day 27. More research is warranted which addresses the effects of programmed feeding on humoral immunity to vaccines.
The BVD-II titer response for sampling day was linear (P < 0.0001) and titer level numbers appeared in this study to be less than those for BVD-I. The lower titers for BVD type 2 compared to type 1 are in agreement with Fulton et al. (1997) and could be explained by lower immunomodulation from the type 2 antigen as evidence of antigen diversity between the two types.
Immuno-Characterization of Healthy and Morbid Animals
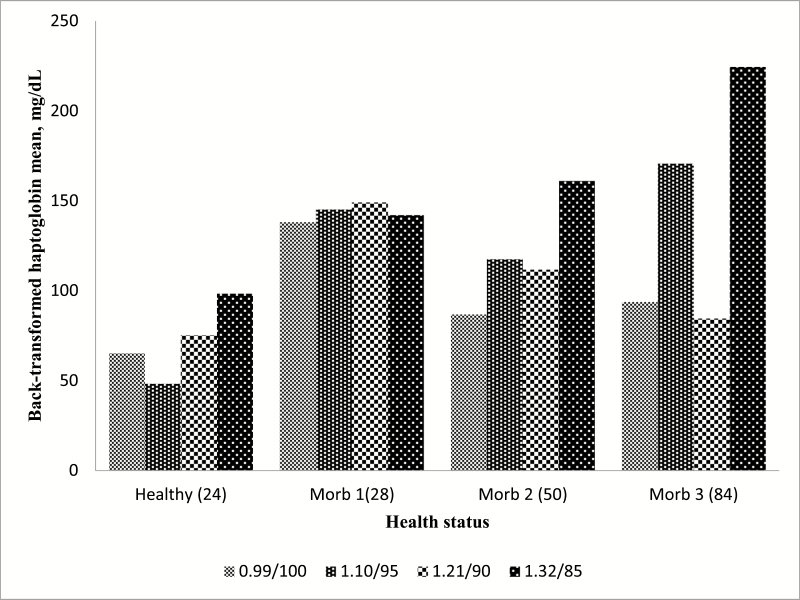
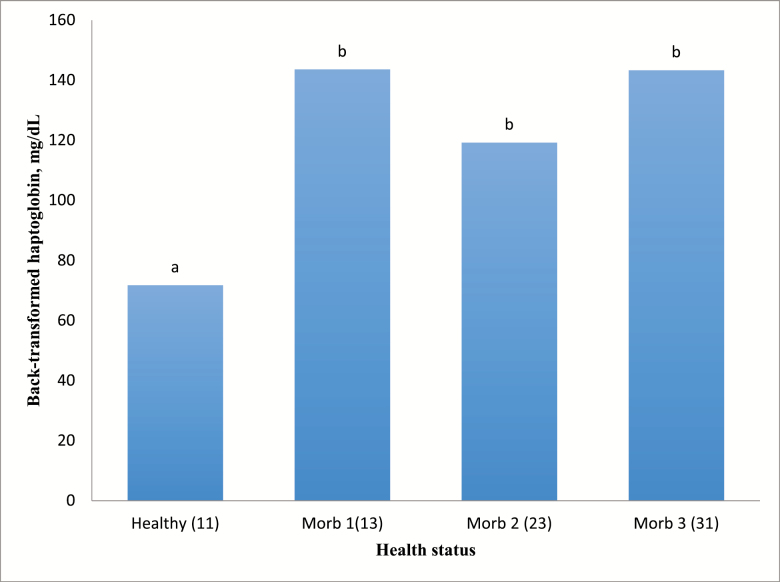
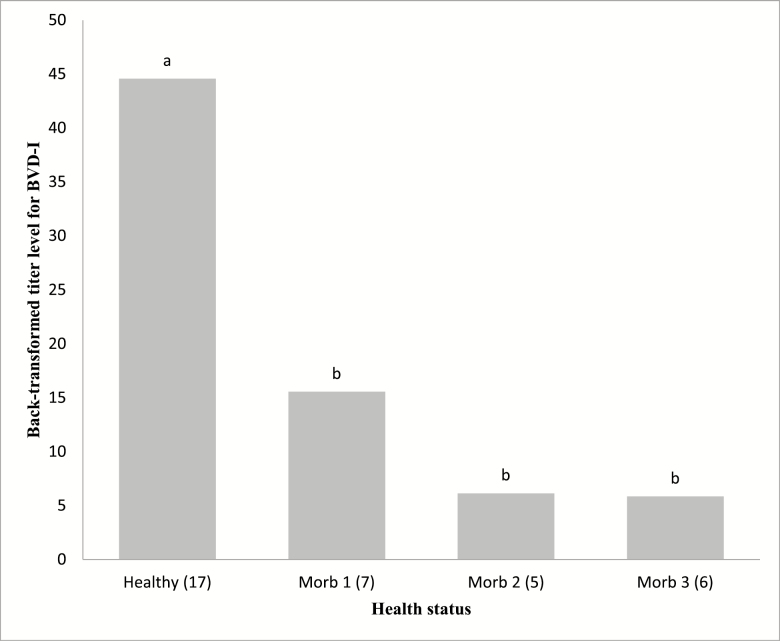
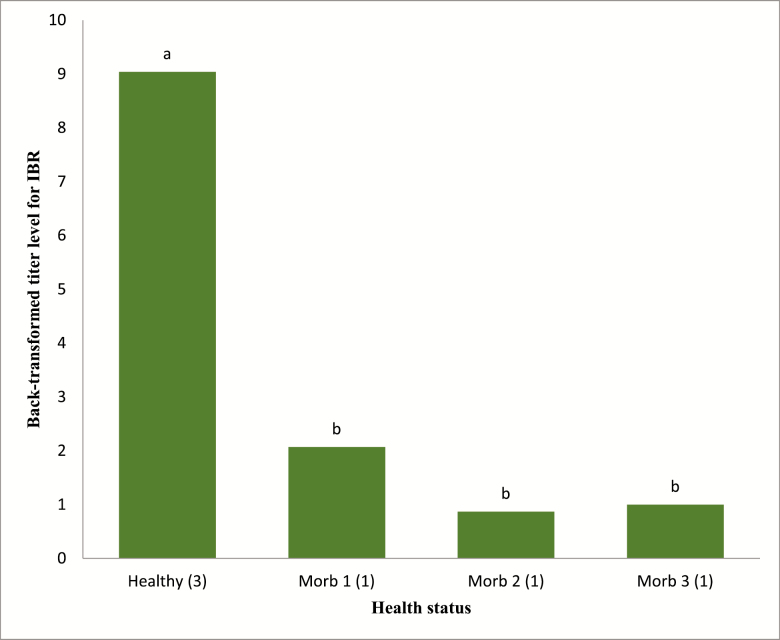
There were no interactions detected (P = 0.83) between dietary treatment and health status for haptoglobin concentrations (Figure 1). In addition, there were no interactions detected (P = 0.28) between dietary treatment and health status for titer levels. In contrast, health status was associated with haptoglobin concentrations, BVD-I, and IBR titers (P < 0.05; Figures 2–4; respectively).
Figure 1.
Effects of dietary energy level and intake on haptoglobin concentrations in healthy and morbid animals. 0.99/100 = 0.99 Mcal NEg/kg DM offered ad libitum intake (100%). 1.10/95 = 1.1 Mcal NEg/kg DM diet limit-fed at 95% of 0.99/100. 1.21/90 = 1.21 Mcal NEg/kg DM diet limit-fed at 90% of 0.99/100 diet. 1.32/85= 1.32 Mcal NEg/kg DM diet limit-fed at 85% of 0.99/100 diet. Healthy = healthy pen mate pulled with sick animal for comparison (n = 71; SE = 24). Morb 1 = first pull for illness (n = 46; SE = 28). Morb 2 = second pull for illness (n = 15; SE = 50). Morb 3 = third pull for illness (n = 10; SE = 84). Health status effect (P < 0.0001), diet effect (P = 0.28), diet × health status effect (P = 0.83). Largest standard error reported between treatments.
Figure 2.
Effects of health status on haptoglobin concentrations. Healthy = healthy pen mate pulled with sick animal for comparison (n = 71; SE = 11). Morb 1 = first pull for illness (n = 46; SE = 13). Morb 2 = second pull for illness (n = 15; SE = 23). Morb 3 = third pull for illness (n = 10; SE = 31). a,bUnlike superscripts above bars in chart differ (P < 0.05). Standard error reported after combining values between treatments.
Figure 3.
Effects of health status on antibody titers for BVD-I. Healthy = healthy pen mate pulled with sick animal for comparison (n = 71; SE = 17). Morb 1 = first pull for illness (n = 46; SE = 7). Morb 2 = second pull for illness (n = 15; SE = 5). Morb 3 = third pull for illness (n = 10; SE = 6). a,bUnlike superscripts above bars in chart differ (P < 0.05). Standard error reported after combining values between treatments.
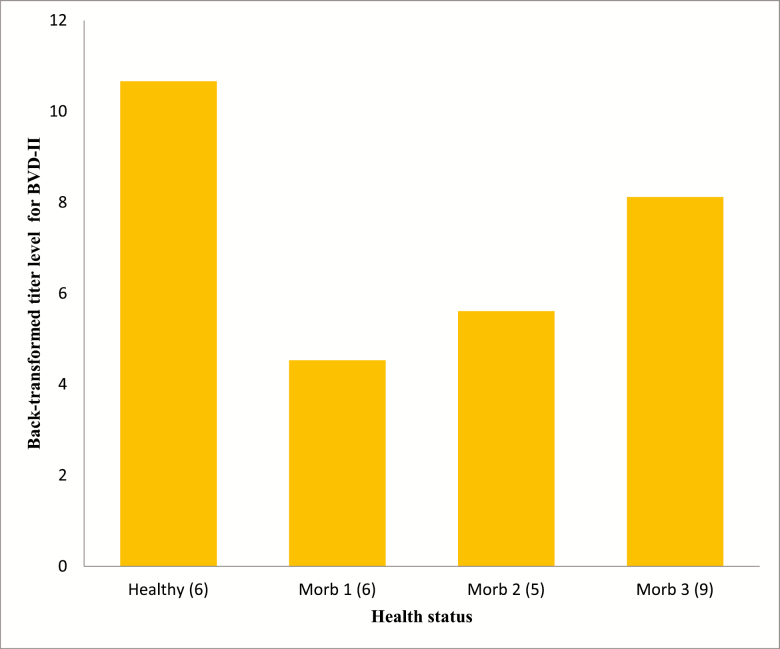
Figure 4.
Effects of health status on antibody titers for IBR. Healthy = healthy pen mate pulled with sick animal for comparison (n = 71; SE = 3). Morb 1 = first pull for illness (n = 46; SE = 1). Morb 2 = second pull for illness (n = 15; SE = 1). Morb 3 = third pull for illness (n = 10; SE = 1). a,bUnlike superscripts above bars in chart differ (P < 0.05). Standard error reported after combining values between treatments.
For haptoglobin, although concentrations in serum increased (P < 0.05) with increasing duration of morbidity, there were less cattle pulled twice (M2) and three times (M3) which greatly increases the variability as indicated in Table 4 and Figure 1. However, these results are in agreement with multiple studies involving haptoglobin concentrations between healthy and sick livestock (Berry et al., 2004; Humblet et al., 2004; El-Deeb, 2016) and would be expected if the animals being tested were truly suffering from infection. Titers for BVD-I and IBR titers were higher in healthy animals than in morbid animals (P < 0.05), but health status did not affect BVD-II titers (Figure 5). In this study, healthy animal titers were compared among cattle initially experiencing illness and those experiencing treatment failure and requiring further treatment. There are several possible reasons for the lower titers in cattle that were identified as morbid compared to healthy cattle sampled on multiple days of the study. One, the calves were from typical sale barn marketing protocols and arrived at the Kansas State University Beef Stocker Unit with little to no medical history. We do not know how many of the animals arrived already infected with a variety of different pathogens. The effects of initial sickness could have hindered the body’s immune system responses to the vaccines. Furthermore, the calves were transported for approximately 12 h before arriving at the receiving facilities and research has shown shipping could decrease immune response (Blecha et al., 1984). This theory would further advocate research into the field of delayed vaccination where vaccines are not administered on arrival but at some time later, when stress levels could have subsided and the immune system can mount a robust response to vaccines as well as to field pathogens.
Table 4.
Effects of intake and energy level on fecal cortisol over time
| Dieta | P-valuec | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0.99/100 | 1.10/95 | 1.21/90 | 1.32/85 | SEMb | Diet | Day | Diet × Day |
| No. of pens | 8 | 8 | 8 | 8 | ||||
| No. of animals | 16 | 15 | 16 | 15 | ||||
| Fecal cortisol, ng/g | 0.23 | <0.01 | 0.21 | |||||
| Day 0 | 45.2 | 72.0 | 52.6 | 37.4 | 7.5 | |||
| Day 14 | 16.2 | 13.7 | 17.7 | 11.6 | 9.6 | |||
aTreatment diets offered based on DMI of 0.99/100 treatment intake that was offered for ad libitum intake. First number = Mcal NEg/kg DM. Second number = % of 0.99/100 treatment offered on DM basis.
bLargest value between treatments at each time point reported.
cFixed effects of diet, day, and diet × day.
Figure 5.
Effects of health status on antibody titers for BVD-II. Healthy = healthy pen mate pulled with sick animal for comparison (n = 71; SE = 6). Morb 1 = first pull for illness (n = 46; SE = 6). Morb 2 = second pull for illness (n = 15; SE = 5). Morb 3 = third pull for illness (n = 10; SE = 9). Standard error reported after combining values between treatments.
Research which involves delayed vaccination has shown positive results for antibody titers against vaccines (Richeson et al., 2009). However, more research investigating the nutritional effects of such protocols should be conducted. Our results indicate immune function was depressed for morbid compared to healthy animals, but dietary treatment had no effect on these parameters.
Although no statistical differences were observed for BVD-II titers among morbidity groups, it should be noted that numerical patterns were similar between BVD-II titers and titers for BVD-I and IBR titers. It is possible that the immune system’s low affinity for the BVD-II antigen could be responsible for the very large standard errors associated with this specific analysis.
Fecal Cortisol
Fecal cortisol was unaffected by dietary treatment (P = 0.23) and there were no dietary treatment × sampling day interactions (P = 0.21), however sampling day did affect fecal cortisol (P < 0.05; Table 5). The results in regard to energy concentration are similar to those observed by Tolleson et al. (2013) where a low (9% CP, 58% TDN) and moderate (14% CP, 60% TDN) diet was fed and no differences in fecal cortisol were observed. One difference between this trial and ours includes how diets were offered. The current study used four diets; however, only one was offered for ad libitum intakes compared to all of them being fed for ad libitum intakes in Tolleson et al. (2013). Like Tolleson et al. (2013), we too used fecal cortisol as an index of overall stress. Fecal cortisol concentrations have been shown to increase following stressful events such as transportation (Morrow et al., 2002; Chen et al., 2015).
Table 5.
Number of animals experiencing illness and reoccurring illness by week of study
| Week of study | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Health statusa | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Total |
| H | 11 | 26 | 22 | 6 | 5 | 1 | 0 | 0 | 71 |
| M1 | 10 | 18 | 10 | 5 | 2 | 1 | 0 | 0 | 46 |
| M2 | 1 | 6 | 5 | 1 | 2 | 0 | 0 | 0 | 15 |
| M3 | 0 | 2 | 7 | 0 | 1 | 0 | 0 | 0 | 10 |
aH = healthy animal for comparison. M1 = first pull from pen for illness. M2 = second pull from pen for reoccurring illness. M3 = third pull from pen for reoccurring illness.
Results from this trial disagree with results from Bourguet et al. (2011) in terms of feed deprivation and stress. Their study showed increased levels of plasma cortisol in Holstein heifers when feed was deprived for 30 h. Heifers in the current study were fed every morning at approximately 0700 hours and the limit-fed rations were typically consumed within 3 h, which translates on most days to feed deprivation of 21 h. Further, cattle become quickly acclimated to stressors, and fecal cortisol can decline accordingly as reported by Andrade et al. (2001). These workers found that when cattle were restrained in a chute for 10 min repeatedly for 19 d, fecal cortisol gradually declined. Fecal cortisol samples were taken on days 0 and 14, which could explain the day effect detected in the current study. On day 0, fecal cortisol concentrations were much higher when compared to day 14 most likely as a result of stress from shipping and initial processing and acclimation by day 14. Measuring plasma cortisol concentrations may have been a more accurate or useful analysis to analyze the effects of diet deprivation; however, sampling can often confound results. Results from this trial indicate that limit-feeding and the increased energy in the limit-fed diets did not induce stress as measured by fecal cortisol metabolite.
IMPLICATIONS
Limit-feeding wet corn gluten feed based rations formulated to contain up to 1.32 Mcal NEg/kg DM does not affect stress or immune function in healthy or sick animals when compared to lower-energy high-roughage diets fed for ad libitum intake. This is important because limit-feeding to program gain is a efficient strategy for growing cattle and the higher energy in this case did not cause health issues or modulation of the immune system. Additionally, haptoglobin was increased and titer levels for BVD-I and IBR decreased in morbid animals compared to healthy pen mates. More research is warranted addressing the effects of programmed feeding systems based on by-products on the immune system and the subsequent effects on performance.
LITERATURE CITED
- Andrade O., Orihuela A., Solano J., and Galina C. S.. 2001. Some effects of repeated handling and the use of a mask on stress responses in zebu cattle during restraint. Appl. Anim. Behav. Sci. 71:175–181. doi:https://doi.org/10.1016/S0168-1591(00)00177-5 [DOI] [PubMed] [Google Scholar]
- Arthington J. D., Cooke R. F., Maddock T. D., Araujo D. B., Moriel P., DiLorenzo N., and Lamb G. C.. 2013. Effects of vaccination on the acute-phase protein response and measures of performance in growing beef calves1. J. Anim. Sci. 91:1831–1837. doi:10.2527/jas.2012-5724. [DOI] [PubMed] [Google Scholar]
- Berry B. A., Confer A. W., Krehbiel C. R., Gill D. R., Smith R. A., and Montelongo M.. 2004. Effects of dietary energy and starch concentrations for newly received feedlot calves: II. Acute-phase protein response. J. Anim. Sci. 82:845–850. doi:10.2527/2004-823845. [DOI] [PubMed] [Google Scholar]
- Blecha F., Boyles S. L., and Riley J. G.. 1984. Shipping suppresses lymphocyte blastogenic responses in Angus and Brahman × Angus feeder calves. J. Anim. Sci. 59:576–583. doi:10.2527/jas1984-593576. [DOI] [PubMed] [Google Scholar]
- Bourguet C., Deiss V., Boissy A., Andanson S., and Terlouw E. M. C.. 2011. Effects of feed deprivation on behavioral reactivity and physiological status in Holstein cattle. J. Anim. Sci. 89:3272–3285. doi:10.2527/jas.2010–3139. [DOI] [PubMed] [Google Scholar]
- Chen Y., Arsenault R., Napper S., and Griebel P.. 2015. Models and methods to investigate acute stress responses in cattle. Animals 5:1268–1295. doi:10.3390/ani5040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deeb W. M., and Elmoslemany A. M.. 2016. The diagnostic accuracy of acute phase proteins and proinflammatory cytokines in sheep with pneumonic pasteurellosis. Peer J. 4:e2161. doi:10.7717/peerj.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enemark J. M. D., Jorgenson R. J., and Enemark P.,. 2002. Rumen acidosis with special emphasis on diagnostic aspects of subclinical rumen acidosis: a review. Veterinarijair ir Zootechnika 20:16–29. [Google Scholar]
- Fulton R. W., Saliki J. T., Burge L. J., d’Offay J. M., Bolin S. R., Maes R. K., Baker J. C., and Frey M. I.. 1997. Neutralizing antibodies to type 1 and 2 bovine viral diarrhea viruses: detection by inhibition of viral cytopathology and Infectivity by immunoperoxidase assay. Clin. Diagn. Immunol. 4:380–383. doi:https://doi.org/10.1016/j.vaccine.2003.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozho G. N., Plaizier J. C., Krause D. O., Kennedy A. D., and Wittenburg K. M.. 2005. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J. Dairy. Sci. 88:1399–1403. doi:10.3168/jds.S0022-0302(05)72807-1. [DOI] [PubMed] [Google Scholar]
- Humblet M. F., Cogehe J., Lekeux P., and Godeu J. M.. 2004. Acute phase proteins assessment for an early selection of treatments in growing calves suffering from bronchopneumonia under field conditions. Res Vet Sci. 77:41–47. doi:https://doi.org/10.1016/j.rvsc.2004.02.009 [DOI] [PubMed] [Google Scholar]
- Hutcheson D. P., and Cole N. A.. 1986. Management of transit-stress syndrome in cattle: nutritional and environmental effects. J. Anim. Sci. 62:555–560. doi:10.2527/jas1986.622555. [Google Scholar]
- Lofgreen G. P., Dunbar J. R., Addis D. G., and Clark J. G.. 1975. Energy level in starting rations for calves subjected to marketing and shipping stress. J. Anim. Sci. 41:1256–1265. doi:10.2527/jas1975.4151256. [Google Scholar]
- Montanholi Y. R., Palme R., Haas L. S., Swanson K. C., Van der Voort G., and Miller S. P.. 2013. On the relationship between glucocorticoids and feed efficiency in beef cattle. Livestock Sci. 155:130–136. doi:10.1016/j.livsci.2013.04.002 [Google Scholar]
- Montgomery S. P., Drouillard J. S., Titgemeyer E. C., Sindt J. J., Farran T. B., Pike J. N., Coetzer C. M., Trater A. M., and Higgins J. J.. 2004. Effects of wet corn gluten feed and intake level on diet digestibility and ruminal passage rate in steers. J. Anim. Sci. 82:3526–3536. doi:10.2527/2004.82123526. [DOI] [PubMed] [Google Scholar]
- Morrow C. J., Kolver E. S., Verkerk G. A., and Mathews L. R.. 2002. Fecal glucocorticoid metabolites as a measure of adrenal activity in dairy cattle. Gen. Comp. Endo. 126:229–241. doi:10.1006/gcen.2002.7797. [DOI] [PubMed] [Google Scholar]
- Pahlavani M. 2000. Caloric restriction and immunosenescence: a current perspective. Fron. Bio. 5:580–587. doi:http://www.bioscience.org/2000/v5/d/pahlavan/pahlavan.pdf [DOI] [PubMed] [Google Scholar]
- Perkins K. H., VandeHaar M. J., Tempelman R. J., and Burton J. L.. 2001. Negative energy balance does not decrease expression of leukocyte adhesion or antigen-presenting molecules in cattle. J. Dairy Sci. 84:421–428. doi:https://doi.org/10.3168/jds.S0022-0302(01)74492-X [DOI] [PubMed] [Google Scholar]
- Richeson J. T., Kegley E. B., Gadberry M. S., Beck P. A., Powell J. G., and Jones C. A.. 2009. Effects of on-arrival versus delayed clostridial or modified live respiratory vaccinations on health, performance, bovine viral diarrhea virus type I titers, and stress and immune measures of newly received beef calves. J. Anim. Sci. 87:2409–2418. doi:10.2527/jas.2008-1484. [DOI] [PubMed] [Google Scholar]
- Rivera J. D., Galyean M. L., and Nichols W. T.. 2005. Review: dietary roughage concentration and health of newly received cattle. Prof. Anim. Sci. 21: 345–351. doi:https://doi.org/10.15232/S1080-7446(15)31231-6 [Google Scholar]
- Schoonmaker J. P., Cecava M. J., Faulkner D. B., Fluharty F. L., Zerby H. N., and Loerch S. C.. 2003. Effect of source of energy and rate of growth on performance, carcass characteristics, ruminal fermentation, and serum glucose and insulin of early-weaned steers. J. Anim. Sci. 81:843–855. doi:10.2527/2003.814843. [DOI] [PubMed] [Google Scholar]
- Smith J. E., Chavey P. S., and Andrews G. A.. 1998. Semiautomatic and robotic methods for determining serum haptoglobin levels. Vet. Clin. Pathol. 27: 11–14. doi:10.1111/j.1939-165X.1998.tb01073.x [DOI] [PubMed] [Google Scholar]
- Tolleson D. R., Prince S. D., Banik K. K., Welsh T. H., Carstens G. E., Strey O. F., Teel P. D., Willard S. T., and Longnecker M. T.. 2013. Plane of nutrition × tick burden interaction in cattle: effect on fecal composition. J. Anim. Sci. 91:3658–3665. doi:10.2527/jas.2013–6375. [DOI] [PubMed] [Google Scholar]
- Whitney T. R., Duff G. C., Collins J. K., Schafer D. W., and Hallford D. M.. 2006. Effects of diet for early-weaned crossbred beef steers on metabolic profiles and febrile response to an infectious bovine herpesvirus-1 challenge. Livst. Sci. 101:1–9. doi:10.1016/j.livprodsci.2005.04.011. [Google Scholar]