Abstract
Our objectives were to determine the effects of the administration of recombinant bovine somatotropin (bST) at the initiation of a fixed-time AI (TAI) protocol on concentrations of plasma IGF-1, follicle diameter, embryo/fetal size, and pregnancy rates in replacement beef heifers. Four hundred and fourteen Angus-based beef heifers were enrolled in a completely randomized design at 4 locations from January to July of 2016. All heifers were exposed to the 7-d CO-Synch + controlled internal drug release (CIDR) protocol where they received a 100-µg injection of GnRH and a CIDR insert on day −9, 25 mg of PGF2α at CIDR removal on day −2, followed by a 100-µg injection of GnRH and TAI 54 ± 2 h later on day 0. Within location, all heifers were randomly assigned to 1 of 2 treatments: 1) heifers that received 650 mg of bST on day −9 (BST; n = 191); or 2) heifers that did not receive bST on day −9 (CONTROL; n = 223). Blood samples were collected on day −9, 0, 28, and 60 to determine the plasma concentrations of IGF-1. Follicle diameter was determined on day −2 and 0 by transrectal ultrasonography. Pregnancy was diagnosed via transrectal ultrasonography on day 28 or 35, and again at least 30 d after the end of the breeding season. Embryo morphometry was assessed by measuring crown-to-rump length (CRL) on day 28, and fetal size was assessed by measuring crown-to-nose-length (CNL) on day 60. Concentrations of plasma IGF-1 did not differ between treatments on day −9 (P = 0.924), 28 (P = 0.075), and 60 (P = 0.792); however, concentrations of plasma IGF-1 were greater (P < 0.001) in BST-treated heifers at TAI (372.4 ± 16.6 vs. 193.7 ± 16.6 ng/ml). No differences (P = 0.191) were detected for follicle diameter between CONTROL and BST treatments on day −2 or 0. Pregnancy rates to TAI (PR/AI) were greater (P = 0.028) for CONTROL compared to BST heifers (42.5 ± 4.0 vs. 29.9 ± 4.1%). No differences (P = 0.536) in CRL were observed on day 28 between CONTROL and BST heifers. In addition, no difference (P = 0.890) was observed for CNL between CONTROL and BST treatments. Final pregnancy rates did not differ (P = 0.699) between treatments. The administration of bST to beef heifers at the initiation of a TAI protocol increased plasma concentrations of IGF-1 at TAI; however, failed to enhance follicle diameter, embryo/fetal size, and reduced PR/AI.
Keywords: beef heifers, fixed-time artificial insemination, insulin-like growth factor 1, recombinant bovine somatotropin
INTRODUCTION
Growth hormone, or somatotropin (ST), is a protein hormone produced by the anterior pituitary and, once released, transported to the liver where it induces the synthesis and secretion of IGF-1 (Lucy, 2000). Recombinant bovine ST (bST) is a biological equivalent to natural ST, and is commonly utilized in dairy operations to improve milk production of lactating dairy cows (Hartnell et al., 1991). The administration of bST increases plasma concentrations of IGF-1 in both beef and dairy cattle (Bilby et al., 2004; Cooke et al., 2013; Mercadante et al., 2016).
The use of bST has been reported to alter ovarian follicular development, and increased the number of recruited follicles in lactating dairy cows (De La Sota et al., 1993; Kirby et al., 1997) and beef heifers (Gong et al., 1991, 1993, 1997). Both bST and IGF-1 have been shown to stimulate embryonic development in bovines (Moreira et al., 2002) and the use of bST has increased pregnancy rates to AI (PR/AI) in lactating dairy cows (Moreira et al., 2000, 2001; Starbuck et al., 2006). Bovine ST supplementation enhanced conceptus development, reduced embryonic losses, and improved PR/AI in dairy cows administered at fixed-time AI (TAI) and 14 d later (Ribeiro et al., 2014). However, a similar study conducted in beef cows revealed no differences in fetal size or PR/AI (Mercadante et al., 2016).
Limited information exists on the effects of bST administration at the initiation of an estrus synchronization protocol on fertility in beef heifers. We hypothesized that an injection of bST at the initiation of a TAI protocol would increase concentrations of plasma IGF-1 at TAI, and consequently enhance follicle diameter, embryo/fetal size and improve PR/AI. Therefore, this study was performed to evaluate the effects of the administration of bST on plasma concentration of IGF-1, embryo/fetal size, and pregnancy rates of beef heifers exposed to TAI.
MATERIALS AND METHODS
All heifers were handled in accordance with procedures approved by each collaborating University’s Animal Care and Use Committee (IACUC #201509212).
Animals and Treatments
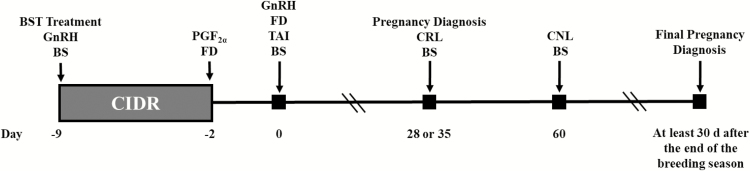
Four hundred and fourteen Angus-based, crossbred beef heifers were enrolled in the experiment at 4 locations in 2 states [Florida (FL-1, n = 84; and FL-2, n = 40) and Virginia (VA-1, n = 228; and VA-2, n = 62)]. All heifers were exposed to the 7-d CO-Synch + controlled internal drug release (CIDR) protocol where they received a 100-µg injection of GnRH (Factrel; gonadorelin hydrochloride; Zoetis Animal Health, Parssipany, NJ) and a CIDR (EAZI-BREED CIDR; 1.38 g of progesterone [P4]; Zoetis Animal Health) insert on day −9, a 25-mg injection of PGF2α (Lutalyse; dinoprost tromethamine; Zoetis Animal Health) at CIDR removal on day −2, and a 100-µg injection of GnRH and TAI 54 ± 2 h later on day 0. Within location, all heifers were randomly assigned to 1 of 2 treatments (Fig. 1): 1) BST (n = 191); heifers were administered 650 mg of bST (Posilac; sometribove zinc; Elanco Animal Health, Indianapolis, IN) on day −9; or 2) CONTROL (n = 223); heifers did not receive bST on day −9. Bovine ST was administered s.c. in the ischiorectal fossa area. The dose of bST was selected based on an unpublished preliminary study where beef heifers receiving 650 mg of bST tended to yield greater PR/AI. Heifers were evaluated for estrus activity between CIDR removal and TAI at 2 locations (FL-1 and VA-1) by determining the activation of estrus detection patches at TAI (Estrotect; Rockway Inc., Spring Valley, WI). Heifers were considered to be in estrus when at least 50% of the rub-off coating was removed from the patch, or when the patch was absent (Hill et al., 2016). Initially, collection of estrus detection data was not an objective of this study; however, this data was collected at the FL-1 and VA-1 locations. Heifer BW was recorded before the initiation of the experiment at 2 locations (FL-1 and VA-1). No less than 10 d after TAI, heifers were exposed to bulls for the remainder of the breeding season.
Figure 1.
Schematic of treatments. Heifers assigned to the BST (n = 191) treatment received a 650-mg injection of bovine somatotropin (bST; Posilac; sometribove zinc; Elanco Animal Health, Indianapolis, IN), an injection of GnRH (Factrel; gonadorelin hydrochloride; Zoetis Animal Health, Parssipany, NJ), and a controlled internal drug release (CIDR; EAZI-BREED CIDR; 1.38 g of progesterone; Zoetis Animal Health) insert on day -9; an injection of PGF2α (Lutalyse; dinoprost tromethamine; Zoetis Animal Health) at CIDR removal on day −2; and a second injection of GnRH concurrent with fixed-time AI (TAI) 54 ± 2 h later on day 0. CONTROL heifers (n = 223) were treated the same as BST; however, did not receive an injection of bST on day −9. Blood samples (BS) were collected on day −9, 0, 28, and 60. Follicle diameter (FD) was measured on day −2 and again on day 0. Pregnancy diagnosis was performed by transrectal ultrasonography on day 28 (FL-1, VA-1, and VA-2) or 35 (FL-2) and again at least 30 days after the end of the breeding season. Crown-to-rump length (CRL) was measured on day 28, and crown-to-nose length (CNL) was measured on day 60.
Ultrasonography
Transrectal ultrasonography (5.0-MHz linear array transducer, Aloka 500V, Instrument of Science and Medicine, Vancouver, BC, Canada; or Ibex portable ultrasound, 5.0-MHz linear multi-frequency transducer, Ibex, E.I. Medical Imaging, Loveland, CO) was performed on 28 (FL-1, VA-1, and VA-2) or 35 d (FL-2) after TAI to determine PR/AI. Embryo size was assessed by measuring crown-to-rump length (CRL) on day 28 at 3 locations (FL-1, VA-1, and VA-2), and fetal size was determined by measuring crown-to-nose length (CNL) on day 60 at 1 location (FL-1; Riding et al., 2008). A brief ultrasound recording was obtained at the first pregnancy diagnosis and the ideal position and orientation of the embryo was selected in a frame-by-frame manner to measure embryo CRL. A second video was recorded on d 60 and CNL was measured. The images were measured twice, each by a separate individual at each location. The final CRL and CNL were calculated as the mean of both measurements (Mercadante et al., 2016). Follicle diameter was determined on day −2, and 0 in a similar fashion to the CRL and CNL measurements. The length and width of the largest follicle was recorded, and the average of the 2 measurements was used to reflect the diameter of the follicle. Final pregnancy rates were determined by transrectal ultrasonography at least 30 d after the end of the breeding season at each location.
Blood Collection and Analysis
Blood samples were collected via jugular venipuncture into 10-mL evacuated tubes containing Na heparin (BD Vacutainer, Franklin Lakes, NJ), placed on ice after collection, and centrifuged for 15 min at 1,500 × g at 4°C. Blood samples were collected on day −9, 0, 28, and 60, to determine concentrations of plasma IGF-1 at 1 location (FL-1). After centrifugation, plasma was transferred into polypropylene vials (12 × 75 mm; Fisherbrand; Thermo Fisher Scientific Inc., Waltham, MA) and stored at −20°C for further analysis. Concentrations of total plasma IGF-1 were determined with an immunoassay system (Immulite 1000 Version 5.22; Siemens Healthcare Diagnostics, Malvern, PA) as previously used for bovine samples (Leiva et al., 2017; Lippolis et al., 2017). Retrospectively, it would have been desirable to collect blood samples from all 4 locations; however, it was not an initial objective, and thus blood samples were only collected at the FL-1 location.
Statistical Analyses
The SAS (version 9.4; SAS/STAT, SAS Inst. Inc., Cary, NC) statistical package was used for all statistical analyses. Heifer was considered the experimental unit. Body weight, CRL, CNL, PR/AI, and final pregnancy rates were analyzed using the GLIMMIX procedure of SAS. The model included the fixed effects of treatment, location, and the treatment × location interaction. Plasma concentrations of IGF-1 and follicle diameter were analyzed as repeated measures using the MIXED procedure of SAS. Both models included the fixed effects of treatment, day, and the treatment × day interaction. Individual heifer was considered the subject in both models; however, the covariance structure for plasma concentration of IGF-1 was unstructured, and for follicle diameter was autoregressive. The covariance structures were selected based on the lowest Akaike information criterion values. Location had no effect on follicle diameter (P = 0.301) and was therefore, excluded from the model. Artificial insemination sire and AI technician were distributed evenly among treatments; therefore, these variables were not included in the models. Not all sires were included at all locations; however, were evenly distributed within location between treatments. Statistical differences were considered significant at P ≤ 0.05.
RESULTS AND DISCUSSION
Body Weight
Body weight was recorded before the initiation of the study to ensure that heifers were evenly distributed between CONTROL and BST treatments at 2 locations (FL-1 and VA-1). Heifer BW differed (P < 0.001) between locations; however, did not differ (P = 0.443) between CONTROL and BST treatments (356.08 ± 2.56 vs. 353.23 ± 2.68 kg), or between treatments within location (P = 0.864).
Insulin-like Growth Factor 1
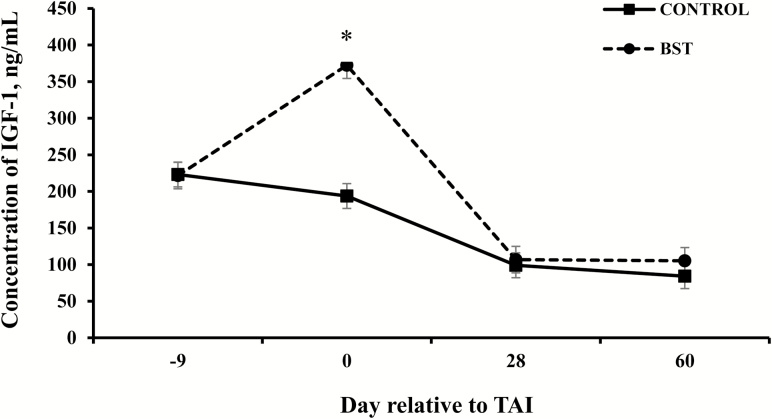
A treatment × day interaction (P < 0.001) was detected on plasma concentrations of IGF-1 (Fig. 2). Although plasma concentrations of IGF-1 were similar (P > 0.05) between CONTROL and BST heifers on day −9 (223.1 ± 10.4 vs. 221.7 ± 10.4 ng/mL, respectively), plasma concentrations of IGF-1 differed (P < 0.05) on day 0 at TAI, where BST heifers had greater plasma concentrations of IGF-1 than CONTROL heifers (372.4 ± 16.6 vs. 193.7 ± 16.6 ng/mL). Plasma concentrations of IGF-1 were similar between CONTROL and BST treatments on day 28 (P > 0.05; 99.0 ± 16.9 vs. 106.7 ± 18.1 ng/mL, respectively) and day 60 (P > 0.05; 84.2 ± 11.3 vs. 105.2 ± 12.1 ng/mL, respectively).
Figure 2.
Plasma concentrations of IGF-1 of beef heifers per day relative to fixed-time AI (TAI) by treatment. BST heifers received 650 mg of bovine somatotropin (bST; Posilac; sometribove zinc; Elanco Animal Health, Indianapolis, IN) on day −9, whereas CONTROL heifers did not receive bST. *Effect of treatment (P < 0.001); treatment × day interaction (P < 0.001).
The administration of bST increases plasma concentrations of ST, which stimulates the synthesis and release of IGF-1 from the liver (Lucy, 2000; Le Roith et al., 2001). Once released from the liver, IGF-1 acts on numerous tissues, where it influences the regulation of growth and differentiation of various cell types, and assists in the control of organ and tissue metabolic activity (Kuzmina et al., 2007). Bovine ST has been shown to increase the plasma concentrations of IGF-1 in both beef (Cooke et al., 2013; Mercadante et al., 2016) and dairy females (Bilby et al., 2004). In lactating dairy cows, bST is administered every 14 d to sustain elevated levels of IGF-1, therefore, maintaining increased milk production (Hartnell et al., 1991; Ribeiro et al., 2014). A study conducted in dairy cows indicated that a 325-mg injection of bST at TAI and again 14 d later increased plasma concentrations of GH and IGF-1 (Ribeiro et al., 2014). Similarly, plasma concentrations of IGF-1 were increased in beef cows receiving 325 mg of bST at TAI and again 14 d later (Mercadante et al., 2016). As expected, in the present study, plasma concentrations of IGF-1 were not different on day 28 or 60 between CONTROL and BST heifers, as concentrations of plasma IGF-1 typically peak 7 d after administration, and then gradually decline to baseline concentrations approximately 7 to 14 d later (Bilby et al., 2004; Ribeiro et al., 2014; Mercadante et al., 2016).
Follicle Diameter
Follicle diameter was measured on day −2, and again on day 0, and did not differ (P = 0.191) between CONTROL and BST treatments on either day. As expected, follicles on day 0 were greater (P < 0.001) in diameter than follicles on day −2 (12.59 ± 0.59 and 11.30 ± 0.58 mm, respectively); however, no treatment × day (P = 0.588) interaction was detected.
The bovine ovary contains receptors for both ST and IGF-1 (Lucy, 2000). Cumulus cells, mural granulosa cells, and oocytes of bovine follicles express receptors for ST (Izadyar et al., 1997); whereas granulosa cells of primary, secondary, and antral follicles express receptors for IGF-1 (Monget and Bondy, 2000). Bovine somatotropin has been shown to influence follicle development (Gong et al., 1993; Lucy et al., 1994), and the developmental competence of bovine oocytes (Kuzmina et al., 2007). A study conducted in dairy heifers showed that the number of small follicles present on the ovary was increased 72 h after treatment with 320 mg of bST, indicating that bST affected the recruitment of small follicles (Gong et al., 1993). Dairy heifers treated with bST had earlier emergence of their second follicular growth wave; however, had smaller dominant follicles in this wave when compared to the controls (Lucy et al., 1994). An experiment performed in vitro demonstrated that bST (10 ng/mL) interacts with granulosa cells and stimulates the oxidative activity of ooplasmic mitochondria, decreasing the intracellular stored calcium. As a result, the quality of bovine oocytes as well as the proportion that developed into blastocysts after in vitro fertilization, were greater in the bST treatment group (Kuzmina et al., 2007). Similar to the present study, a study utilizing both lactating and nonlactation dairy cows reported no differences in the size of the dominant follicles between cows receiving 25 mg bST or saline daily (De La Sota et al., 1993). Our original hypothesis was that administration of bST at the time of GnRH administration would stimulate follicular growth of the subsequent follicular wave as a result of increased IGF-1. Concentrations of plasma IGF-1 were increased in the BST heifers; however, it is possible that the baseline concentrations of IGF-1 were already sufficient, and therefore, treatment with bST had no additional effect on dominant follicle size.
Estrus Response
Estrus response differed by location (P = 0.003), yet was similar (P = 0.861) between CONTROL and BST treatments (Table 1). No treatment × location interaction was detected (P = 0.836).
Table 1.
Fertility and embryo/fetal size in beef heifers treated with recombinant bovine somatotropin
| Treatment1 | ||||
|---|---|---|---|---|
| Item | CONTROL | BST | SEM | P-value |
| Estrus Response, % | 56.2 | 51.1 | 6.23 | 0.861 |
| PR/AI2, % | 42.5 | 29.9 | 5.76 | 0.028 |
| Final PR3, % | 88.7 | 90.0 | 3.44 | 0.967 |
| Fetal size4, mm | ||||
| CRL day 28 | 9.1 | 9.3 | 0.40 | 0.536 |
| CNL day 60 | 27.2 | 27.1 | 0.82 | 0.890 |
1Heifers assigned to the BST (n = 191) treatment received a 650-mg injection of bovine somatotropin (bST; Posilac; sometribove zinc; Elanco Animal Health, Indianapolis, IN), an injection of GnRH (Factrel; gonadorelin hydrochloride; Zoetis Animal Health, Parssipany, NJ), and a controlled internal drug release (CIDR; EAZI-BREED CIDR; 1.38 g of progesterone; Zoetis Animal Health) insert on day −9; an injection of PGF2α (Lutalyse; dinoprost tromethamine; Zoetis Animal Health) at CIDR removal on day −2; and a second injection of GnRH concurrent with fixed-time AI (TAI) 54 ± 2 h later on day 0. CONTROL heifers (n = 223) were treated the same as BST; however, did not receive an injection of bST on day −9.
2Pregnancy diagnosis was performed by ultrasonography on day 28 or 35 to determine pregnancy rates to AI (PR/AI).
3Final pregnancy rates (PR). Pregnancy diagnosis was performed at least 30 days after the end of the breeding season.
4CRL = crown-to-rump length; CNL = crown-to-nose length.
Administration of bST has been shown to reduce estrus expression in dairy cows (Morbeck et al., 1991; Santos et al., 2004; Rivera et al., 2010). Lactating dairy cows that were administered with 500 mg of bST every 10 d for 6 wk had reduced estrus durations, fewer standing events, and reduced estrus expression when compared to control cows (Rivera et al., 2010). Dairy cows treated with different doses of bST (5.15, 10.3, or 16.5 mg/d) had lower rates of estrus detection, and thus longer intervals to first insemination compared with untreated controls (Morbeck et al., 1991). Similarly, there was a tendency for a reduction in the percentage of multiparous cows detected in estrus after treatment with 500 mg of bST every 14 d; however, detection of estrus in primiparous cows was unaffected by the bST treatment (Santos et al., 2004). However, in the current study, estrus expression between CIDR removal and TAI was similar between CONTROL and BST treatments. It is plausible that the reduction in estrus response in the abovementioned studies is a result of increased milk production in these lactating dairy cows receiving bST, as increased milk production has been associated with reduced estrus duration and estrus intensity (Wiltbank et al., 2006; Rivera et al., 2010). It is, therefore, possible that we did not see a difference in estrus response between the treatment groups, because of the physiological differences between nonlactating beef heifers and lactating dairy cows.
Fertility
Pregnancy rates to TAI were reduced (P = 0.040) in heifers from the BST compared to the CONTROL treatment (Table 1). In addition, there was an effect of location (P < 0.001) on PR/AI, which ranged from 20.8% to 46.2%. No treatment × location interaction was detected (P = 0.290). At the conclusion of the breeding season, final pregnancy rates did not differ (P = 0.699) between CONTROL and BST treatments; however, final pregnancy rates differed (P < 0.001) among location, and ranged from 76.5% to 96.8%.
The effects of bST on fertility in cows and heifers have been previously examined, and results have varied between beef and dairy cattle (Starbuck et al., 2006; Ribeiro et al., 2014; Mercadante et al., 2016), cow parity (Silvia et al., 2002; Starbuck et al., 2006), and timing of the bST injection (Bilby et al., 1999; Moreira et al., 2000; Ribeiro et al., 2014).
Pregnancy rates to AI have been improved in lactating dairy cows through the administration of bST (Moreira et al., 2000; Santos et al., 2004; Starbuck et al., 2006). Pregnancy rates to TAI were increased in lactating dairy cows that received 500 mg of bST at the initiation of a TAI protocol and every 14 d thereafter until 30 d before the dry period (Moreira et al., 2000, 2001). In addition, cyclic dairy cows treated with 500 mg of bST at 14-d intervals had improved conception rates to the first AI (Santos et al., 2004). When bST was injected at TAI and again 14 d later, lactating dairy cows had greater PR/AI, and had fewer pregnancies lost between day 31 and 66 compared with controls (Ribeiro et al., 2014). Furthermore, dairy cows receiving 500 mg bST at AI, 12 h after being observed in standing estrus, had improved conception rates compared to untreated controls (Starbuck et al., 2006). However, in the same study, beef and dairy heifers treated with bST had no differences in conception rates when compared to controls.
The improvements in fertility reported in previous studies may be dose dependent, as detrimental effects on fertility have been attained elsewhere through the use of bST (Downer et al., 1993; Bilby et al., 2004). Nonlactating dairy cows that were administered 500 mg bST at TAI and again 11 d later had reduced PR/AI compared to untreated controls (Bilby et al., 2004). Furthermore, a meta-analysis conducted on the effects of bST in dairy cattle indicated that the use of bST increased the risk of a cow not becoming pregnant by approximately 40% (Dohoo et al., 2003). Conception rates were decreased in lactating dairy cows that were administered 700 mg of bST every 14 d beginning from 14 wk postpartum for 30 wk (Downer et al., 1993). However, in the same experiment, conception rates were unaffected in cows receiving 350 mg every 14 d for 30 wk.
Treatment with bST has also been shown to have no effect on PR/AI in dairy cows (Rivera et al., 2010), beef cows (Rossetti et al., 2011), dairy heifers (Starbuck et al., 2006), and beef heifers (Bilby et al., 1999). Beef heifers and cows that were AI 8 to 12 h after observed estrus, and were administered 167 mg of bST at the time of AI, had no difference in conception rate when compared to the untreated females (Bilby et al., 1999).
It is, therefore, plausible that the dose of bST administered may be a determinant of the reproductive outcome. In the present study, 650 mg of bST significantly decreased PR/AI when administered at the initiation of the 7-d CO-Synch + CIDR estrus synchronization protocol, resulting in fewer established pregnancies. Excessive IGF-1 has been shown to increase early embryonic mortality in rats by creating a more unfavorable uterine environment (Katagiri et al., 1996). Concentrations of plasma IGF-1 in our study may have had a deleterious effect on the uterine environment, and been detrimental to embryo development. In addition, plasma concentrations of IGF-1 have been reported to have a quadratic effect on the probability of pregnancy in beef cows (Cooke et al., 2009). Therefore, it is plausible that the concentrations of plasma IGF-1 in our study may have been greater than what would be considered optimal, and as a result, reduced PR/AI.
Fetal Morphometry
Embryo and fetal size was determined by ultrasonography on day 28 and 60 after TAI (Table 1). Crown-to-rump length was measured on day 28 and did not differ (P = 0.536) between CONTROL and BST treatments; however, an effect of location was identified (P = 0.026) where location VA-2 had greater (P = 0.051) CRL than VA-1 (9.5 ± 0.3 vs. 8.5 ± 0.2 mm, respectively). No treatment × location interaction was detected (P = 0.425). Crown-to-nose length was recorded on day 60 and no differences (P = 0.890) were detected between CONTROL and BST treatments (27.2 ± 0.6 vs. 27.1 ± 0.6 mm, respectively).
The uterus and conceptus have receptors for ST and IGF-1 that mediate the actions of both hormones during the establishment of pregnancy (Kölle et al., 1997; Robinson et al., 2000; Rhoads et al., 2008). The administration of bST has been shown to enhance conceptus development in dairy cows (Bilby et al., 2004; Ribeiro et al., 2014); however, has also been shown to have no effect in beef cows (Starbuck et al., 2006; Mercadante et al., 2016) nor in dairy heifers (Starbuck et al., 2006).
A 500-mg injection of bST administered to nonlactating dairy cows at TAI and again 11 d later increased conceptus size on day 17 (Bilby et al., 2004). Furthermore, 325-mg injections of bST administered at TAI and 14 d later enhanced CRL on day 34 and day 48 after TAI in lactating dairy cows when compared to cows that only received an injection of bST at TAI, and compared to untreated controls (Ribeiro et al., 2014). Ewes treated with a single 500-mg dose of bST, tended to have greater CRL, and had greater lamb birth weights than untreated controls (Koch et al., 2010). Furthermore, ewes treated with bST at breeding had smaller but more efficient placentas, and gave birth to heavier lambs (Costine et al., 2005). In agreement with our findings, no differences were observed in conceptus size on day 17 in dairy cows administered with 25 mg per d of bST from first signs of estrus for 16 d (Lucy et al., 1995). In addition, bST treatment (500 mg) at AI, 12 h after detected estrus, had no effect on CRL in beef or dairy cows, nor in dairy heifers (Starbuck et al., 2006). Perhaps the dose and timing of the bST injection, as well as the physiological differences between dairy cows and beef heifers may be associated with the results observed in conceptus development in our study. The previously mentioned studies that reported a positive effect of bST on conceptus development administered bST at 2 different time points, with the initial injection administered at TAI (Bilby et al., 2004; Ribeiro et al., 2014). These 2 injections were successful at maintaining high concentrations of IGF-1 over a longer period, and as a result, may have stimulated conceptus development. When a single bST injection was administered at TAI, no difference in conceptus development was detected (Ribeiro et al., 2014). In addition, the bST injection in our study may have been administered too early, where concentrations of IGF-1 would have started to decline by TAI, resulting in similar concentrations of IGF-1 between treatments at the time of conceptus development.
Genotype has been shown to impact fetal size and rate of development in beef cows (Mercadante et al., 2013). The location effects observed on fertility and fetal size in the present study may have been due to the differences in herd genetics, in addition to differences in environmental conditions. Locations differed in sire and breed genetics, as well as in animal handling, facilities, and nutrition, which may have contributed to the reported ranges in estrus response, PR/AI, CRL, and final pregnancy rates.
Beef replacement heifers are a crucial part of any cow-calf enterprise; therefore, additional research for improving TAI protocols adjusted specifically for beef heifers is necessary. By improving beef heifer TAI protocols, greater pregnancy rates to AI may be achieved, resulting in an increase in reproductive efficiency. We conclude that a 650-mg injection of bST at the initiation of a TAI protocol was successful at increasing concentrations of plasma IGF-1 at TAI; however, failed to enhance follicle diameter, embryo/fetal size, and resulted in reduced PR/AI in beef heifers.
Conflict of interest statement. None declared.
Acknowledgments
The authors thank the Florida Cattle Enhancement Fund – DACS for providing the research funding; Zoetis Animal Health (Parsippany, NJ) for their donation of PGF2α (Lutalyse), GnRH (Factrel), and CIDR inserts (EAZI-BREED CIDR); as well as the Cherokee Ranch (Marianna, FL) and the Virginia Department of Corrections (Southhampton, VA) for providing heifers and labor for the implementation of the study. Sincere appreciation is expressed to T. Redifer, C. Joines, P. Folsom, M. Foran, D. Jones, C. Nowell, D. Thomas, and C. Wood for their assistance with data collection.
LITERATURE CITED
- Bilby C. R., Bader J. F., Salfen B. E., Youngquist R. S., Murphy C. N., Garverick H. A., Crooker B. A., and Lucy M. C.. 1999. Plasma GH, IGF-I, and conception rate in cattle treated with low doses of recombinant bovine GH. Theriogenology. 51:1285–1296. doi:10.1016/S0093-691X(99)00072-2 [DOI] [PubMed] [Google Scholar]
- Bilby T. R., Guzeloglu A., Kamimura S., Pancarci S. M., Michel F., Head H. H., and Thatcher W. W.. 2004. Pregnancy and bovine somatotropin in nonlactating dairy cows: I. ovarian, conceptus, and insulin-like growth factor system responses. J. Dairy Sci. 87:3256–3267. doi:10.3168/jds.S0022-0302(04)73462-1 [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Arthington J. D., Araujo D. B., and Lamb G. C.. 2009. Effects of acclimation to human interaction on performance, temperament, physiological responses, and pregnancy rates of brahman-crossbred cows. J. Anim. Sci. 87:4125–4132. doi:10.2527/jas.2009-2021 [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Bohnert D. W., Francisco C. L., Marques R. S., Mueller C. J., and Keisler D. H.. 2013. Effects of bovine somatotropin administration on growth, physiological, and reproductive responses of replacement beef heifers. J. Anim. Sci. 91:2894–2901. doi:10.2527/jas.2012–6082 [DOI] [PubMed] [Google Scholar]
- Costine B. A., Inskeep E. K., and Wilson M. E.. 2005. Growth hormone at breeding modifies conceptus development and postnatal growth in sheep. J. Anim. Sci. 83:810–815. doi:10.2527/2005.834810x [DOI] [PubMed] [Google Scholar]
- De La Sota R. L., Lucy M. C., Staples C. R., and Thatcher W. W.. 1993. Effects of recombinant bovine somatotropin (sometribove) on ovarian function in lactating and nonlactating dairy cows. J. Dairy Sci. 76:1002–1013. doi:10.3168/jds.S0022-0302(93)77428-7 [DOI] [PubMed] [Google Scholar]
- Dohoo I. R., DesCôteaux L., Leslie K., Fredeen A., Shewfelt W., Preston A., and Dowling P.. 2003. A meta-analysis review of the effects of recombinant bovine somatotropin. 2. Effects on animal health, reproductive performance, and culling. Can. J. Vet. Res. 67:252–264. [PMC free article] [PubMed] [Google Scholar]
- Downer J. V., Patterson D. L., and Rock D. W.. 1993. Dose titration of sustained-release recombinant bovine somatotropin in lactating dairy cows. J. Dairy Sci. 76:1125–1136. doi:10.3168/jds.S0022-0302(93)77441-X [DOI] [PubMed] [Google Scholar]
- Gong J. G., Baxter G., Bramley T. A., and Webb R.. 1997. Enhancement of ovarian follicle development in heifers by treatment with recombinant bovine somatotrophin: a dose-response study. J. Reprod. Fertil. 110:91–97. doi:10.1530/jrf.0.1100091 [DOI] [PubMed] [Google Scholar]
- Gong J. G., Bramley T., and Webb R.. 1991. The effect of recombinant follicular bovine somatotropin and on ovarian function in heifers: populations of obstetrics. Biol. Reprod. 949:941–949. [DOI] [PubMed] [Google Scholar]
- Gong J. G., Bramley T. A., and Webb R.. 1993. The effect of recombinant bovine somatotrophin on ovarian follicular growth and development in heifers. J. Endocrinol. 97:247–254. doi:10.1530/jrf.0.0970247 [DOI] [PubMed] [Google Scholar]
- Hartnell G. F., Franson S. E., Bauman D. E., Head H. H., Huber J. T., Lamb R. C., Madsen K. S., Cole W. J., and Hintz R. L.. 1991. Evaluation of sometribove in a prolonged-release system in lactating dairy cows–production responses. J. Dairy Sci. 74:2645–2663. doi:10.3168/jds.S0022-0302(91)78443-9 [DOI] [PubMed] [Google Scholar]
- Hill S. L., Grieger D. M., Olson K. C., Jaeger J. R., Dahlen C. R., Bridges G. A., Dantas F., Larson J. E., Muth-Spurlock A. M., Ahola J. K.,. et al. 2016. Using estrus detection patches to optimally time insemination improved pregnancy risk in suckled beef cows enrolled in a fixed-time artificial insemination program. J. Anim. Sci. 94:3703–3710. doi:10.2527/jas2016-0469 [DOI] [PubMed] [Google Scholar]
- Izadyar F., Vantol H. T. A., Colenbrander B., and Bevers M. M.. 1997. Stimulatory effect of growth hormone on in vitro maturation of bovine oocytes is exerted through cumulus cells and not mediated by IGF-I. Mol.Reprod.Dev. 47:175–180. doi:10.1002/(SICI)1098-2795(199706)47:2<175::AID-MRD8>3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- Katagiri S., Moon Y. S., and Yuen B. H.. 1996. The role for the uterine insulin-like growth factor I in early embryonic loss after superovulation in the rat. Fertil. Steril. 65:426–36. doi:10.1016/S0015-0282(16)58111–4 [DOI] [PubMed] [Google Scholar]
- Kirby C. J., Smith M. F., Keisler D. H., and Lucy M. C.. 1997. Follicular function in lactating dairy cows treated with sustained-release bovine somatotropin. J. Dairy Sci. 80:273–285. doi:10.3168/jds.S0022-0302(97)75935-6 [DOI] [PubMed] [Google Scholar]
- Koch J. M., Wilmoth T. A., and Wilson M. E.. 2010. Periconceptional growth hormone treatment alters fetal growth and development in lambs. J. Anim. Sci. 88:1619–1625. doi:10.2527/jas.2009-2392 [DOI] [PubMed] [Google Scholar]
- Kölle S., Sinowatz F., Boie G., Lincoln D., and Waters M. J.. 1997. Differential expression of the growth hormone receptor and its transcript in bovine uterus and placenta. Mol. Cell. Endocrinol. 131:127–136. doi:10.1016/S0303-7207(97)00097-X [DOI] [PubMed] [Google Scholar]
- Kuzmina T. I., Alm H., Denisenko V., Tuchscherer A., Kanitz W., and Torner H.. 2007. Effect of recombinant bovine somatotropin (rbST) on cytoplasmic maturation of bovine oocytes and their developmental competence in vitro. J. Reprod. Dev. 53:309–16. doi:10.1262/jrd.18116 [DOI] [PubMed] [Google Scholar]
- Leiva T., Cooke R. F., Brandão A. P., Schubach K. M., Batista L. F. D., Miranda M. F., Colombo E. A., Rodrigues R. O., Junior J. R. G., Cerri R. L. A.,. et al. 2017. Supplementing an immunomodulatory feed ingredient to modulate thermoregulation, physiologic, and production responses in lactating dairy cows under heat stress conditions. J. Dairy Sci. 100:4829–4838. doi:10.3168/jds.2016–12258 [DOI] [PubMed] [Google Scholar]
- Le Roith D., Bondy C., Yakar S., Liu J., and Butler A.. 2001. The somatomedin hypothesis: 2001. Endrocrine Rev. 22:53–74. doi:10.1210/edrv.22.1.0419 [DOI] [PubMed] [Google Scholar]
- Lippolis K. D., Cooke R. F., Schumaher T., Brandão A. P., Silva L. G. T., Schubach K. M., Marques R. S., and Bohnert D. W.. 2017. Physiologic, health, and performance responses of beef steers supplemented with an immunomodulatory feed ingredient during feedlot receiving. J. Anim. Sci. 95:4945–4957. doi:10.2527/jas2017.1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucy M. C. 2000. Regulation of ovarian follicular growth by somatotropin and insulin-like growth factors in cattle. J. Dairy Sci. 83:1635–1647. doi:10.3168/jds.S0022-0302(00)75032-6 [DOI] [PubMed] [Google Scholar]
- Lucy M. C., Curran T. L., Collier R. J., and Cole W. J.. 1994. Extended function of the corpus luteum and earlier development of the second follicular wave in heifers treated with bovine somatotropin. Theriogenology. 41:561–572. doi:10.1016/0093-691X(94)90091-V [DOI] [PubMed] [Google Scholar]
- Lucy M. C., Thatcher W. W., Collier R. J., Simmen F. A., Ko Y., Savio J. D., and Badinga L.. 1995. Effects of somatotropin on the conceptus, uterus, and ovary during maternal recognition of pregnancy in cattle. Domest. Anim. Endocrinol. 12:73–82. doi:10.1016/0739-7240(94)00010-X [DOI] [PubMed] [Google Scholar]
- Mercadante V. R. G., Fontes P. L. P., Ciriaco F. M., Henry D. D., Moriel P., Ealy A. D., Johnson S. E., Di Lorenzo N., and Lamb G. C.. 2016. Effects of recombinant bovine somatotropin administration at breeding on cow, conceptus, and subsequent offspring performance of beef cattle. J. Anim. Sci. 94:2128–2138. doi:10.2527/jas2015-0217 [DOI] [PubMed] [Google Scholar]
- Mercadante P. M., Waters K. M., Mercadante V. R., Lamb G. C., Elzo M. A., Johnson S. E., Rae D. O., Yelich J. V., and Ealy A. D.. 2013. Subspecies differences in early fetal development and plasma pregnancy-associated glycoprotein concentrations in cattle. J. Anim. Sci. 91:3693–3701. doi:10.2527/jas.2012-6130 [DOI] [PubMed] [Google Scholar]
- Monget P., and Bondy C.. 2000. Importance of the IGF system in early folliculogenesis. Mol. Cell. Endocrinol. 163:89–93. doi:10.1016/S0303-7207(99)00244-0 [DOI] [PubMed] [Google Scholar]
- Morbeck D. E., Britt J. H., and McDaniel B. T.. 1991. Relationships among milk yield, metabolism, and reproductive performance of primiparous holstein cows treated with somatotropin. J. Dairy Sci. 74:2153–2164. doi:10.3168/jds.S0022-0302(91)78388-4 [DOI] [PubMed] [Google Scholar]
- Moreira F., Orlandi C., Risco C. A., Mattos R., Lopes F., and Thatcher W. W.. 2001. Effects of presynchronization and bovine somatotropin on pregnancy rates to a timed artificial insemination protocol in lactating dairy cows. J. Dairy Sci. 84:1646–1659. doi:10.3168/jds.S0022-0302(01)74600-0 [DOI] [PubMed] [Google Scholar]
- Moreira F., Paula-Lopes F. F., Hansen P. J., Badinga L., and Thatcher W. W.. 2002. Effects of growth hormone and insulin-like growth factor-I on development of in vitro derived bovine embryos. Theriogenology. 57:895–907. doi:10.1016/S0093-691X(01)00694-X [DOI] [PubMed] [Google Scholar]
- Moreira F., Risco C. A., Pires M. F., Ambrose J. D., Drost M., and Thatcher W. W.. 2000. Use of bovine somatotropin in lactating dairy cows receiving timed artificial insemination. J. Dairy Sci. 83:1237–1247. doi:10.3168/jds.S0022-0302(00)74990-3 [DOI] [PubMed] [Google Scholar]
- Rhoads M. L., Meyer J. P., Kolath S. J., Lamberson W. R., and Lucy M. C.. 2008. Growth hormone receptor, insulin-like growth factor (igf)-1, and igf-binding protein-2 expression in the reproductive tissues of early postpartum dairy cows. j. Dairy Sci. 91:1802–1813. doi:10.3168/jds.2007-0664 [DOI] [PubMed] [Google Scholar]
- Ribeiro E. S., Bruno R. G. S., Farias A. M., Hernández-Rivera J. A., Gomes G. C., Surjus R., Becker L. F. V., Birt A., Ott T. L., Branen J. R.,. et al. 2014. Low doses of bovine somatotropin enhance conceptus development and fertility in lactating dairy cows. Biol. Reprod. 90:1–12. doi:10.1095/biolreprod.113.114694 [DOI] [PubMed] [Google Scholar]
- Riding G. A., Lehnert S. A., French A. J., and Hill J. R.. 2008. Conceptus-related measurements during the first trimester of bovine pregnancy. Vet. j. 175:266–272. doi:10.1016/j.tvjl.2007.01.022 [DOI] [PubMed] [Google Scholar]
- Rivera F., Narciso C., Oliveira R., Cerri R. L., Correa-Calderón A., Chebel R. C., and Santos J. E.. 2010. Effect of bovine somatotropin (500 mg) administered at ten-day intervals on ovulatory responses, expression of estrus, and fertility in dairy cows. j. Dairy Sci. 93:1500–1510. doi:10.3168/jds.2009-2489 [DOI] [PubMed] [Google Scholar]
- Robinson R. S., Mann G. E., Gadd T. S., Lamming G. E., and Wathes D. C.. 2000. The expression of the IGF system in the bovine uterus throughout the oestrous cycle and early pregnancy. J. Endocrinol. 165:231–243. doi:10.1677/joe.0.1650231 [DOI] [PubMed] [Google Scholar]
- Rossetti R. C., Perdigão A., Mesquita F. S., Filho M. S., and Nogueira G. P.. 2011. Effects of flunixin meglumine, recombinant bovine somatotropin and/or human chorionic gonadotropin on pregnancy rates in Nelore cows. Theriogenology. 76:751–758. doi:10.1016/j.theriogenology.2011.04.008 [DOI] [PubMed] [Google Scholar]
- Santos J. E., Juchem S. O., Cerri R. L., Galvão K. N., Chebel R. C., Thatcher W. W., Dei C. S., and Bilby C. R.. 2004. Effect of bst and reproductive management on reproductive performance of holstein dairy cows. J. Dairy Sci. 87:868–881. doi:10.3168/jds.S0022-0302(04)73231-2 [DOI] [PubMed] [Google Scholar]
- Silvia W. J., Hemken R. W., and Hatler T. B.. 2002. Timing of onset of somatotropin supplementation on reproductive performance in dairy cows. J. Dairy Sci. 85:384–389. doi:10.3168/jds.S0022-0302(02)74085-X [DOI] [PubMed] [Google Scholar]
- Starbuck M. J., Inskeep E. K., and Dailey R. A.. 2006. Effect of a single growth hormone (rbst) treatment at breeding on conception rates and pregnancy retention in dairy and beef cattle. Anim. Reprod. Sci. 93:349–359. doi:10.1016/j.anireprosci.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Wiltbank M., Lopez H., Sartori R., Sangsritavong S., and Gümen A.. 2006. Changes in reproductive physiology of lactating dairy cows due to elevated steroid metabolism. Theriogenology. 65:17–29. doi:10.1016/j.theriogenology.2005.10.003 [DOI] [PubMed] [Google Scholar]