Abstract
A 3 yr study evaluated the effects of three preweaning injections of bovine ST, administered 14 d apart, on growth and reproductive performance of Bos indicus-influenced beef heifers. On d 0 of each year, suckling Angus × Brangus heifers (n = 15 heifers/treatment/yr) were stratified by BW (147 ± 20 kg) and age (134 ± 11 d) and randomly assigned to receive an s.c. injection of saline (SAL; 5 mL; 0.9% NaCl) or 250 mg of sometribove zinc (BST; Posilac, Elanco, Greenfield, IN) on d 0, 14, and 28. Heifers and respective dams were managed as a single group on bahiagrass (Paspalum notatum) pastures from d 0 until weaning (d 127). From d 127 to 346, heifers were grouped by treatment, allocated to bahiagrass pastures (1 pasture/treatment/yr) and fed a molasses-based supplement (2.9 kg/heifer daily; DM basis) until d 346. Blood samples were collected on d 0, 14, 28, 42, and then every 9–10 d from d 179 to 346. In yr 3, liver biopsy samples were collected on d 0, 42, and 263. Heifers were exposed to mature Angus bulls from d 263 to 346. Growth performance and physiological parameters were analyzed using the MIXED procedure, whereas reproductive variables were analyzed using the GLIMMIX procedure of SAS. Effects of treatment × year and treatment × year × time were not detected for any variable measured in this study (P ≥ 0.14), except for calving percentage (P = 0.03). Heifers assigned to BST injections had greater overall plasma concentrations of IGF-1 and ADG from d 0 to 42 (P ≤ 0.05), less ADG from d 42 to 127 (P = 0.04), but had similar BW at weaning and postweaning ADG (P ≥ 0.25) compared to SAL heifers. Heifers assigned to BST tended to achieve puberty 26 d earlier (P = 0.10), had greater percentage of pubertal heifers on d 244, 263, 284, and 296 (P ≤ 0.04), tended to have greater overall pregnancy percentage (P = 0.10), and had greater (P ≤ 0.05) calving percentages in yr 1 and 2 (but not yr 3; P = 0.68) compared to SAL heifers. Liver mRNA expression of GHR-1B and IGF-1 on d 0 and 42 did not differ between treatments (P ≥ 0.15), but was greater for BST vs. SAL heifers on d 263 (P ≤ 0.02). Hence, administering three injections containing 250 mg of sometribove zinc at 14 d intervals before weaning (between 135 and 163 d of age) induced long-term impacts on liver gene expression and may be a feasible management practice to enhance puberty and pregnancy attainment in B. indicus-influenced replacement beef heifers.
Keywords: beef heifers, Bos indicus, IGF-1, pregnancy, puberty, ST
INTRODUCTION
A major determinant of lifetime productivity of beef heifers is the age at attainment of puberty (Lesmeister et al., 1973; Day and Nogueira, 2013). Day and Anderson (1998) proposed that the period from birth to puberty in beef heifers could be divided into infantile, developmental, static, and peripubertal periods. Enhancing ADG and nutrient intake of beef heifers during the developmental phase (60 to 180 d of age) hastened follicle size (Gasser et al., 2006a, 2006b) and puberty attainment (Moriel et al., 2014). Circulating IGF-I impacts the gonadotropin activity required for the first ovulation in beef heifers by influencing hypothalamic–pituitary secretory activity (Schillo et al., 1992) and augmenting the effects of gonadotropins in ovarian follicular cells (Spicer and Echternkamp, 1995). In agreement, heifer ADG and plasma IGF-1 concentrations from 70 to 160 d of age explained approximately 34% of the variability of age at puberty (Moriel et al., 2014). These responses may be attributed to metabolic imprinting, which is the concept that body physiological responses to early-life nutritional challenges can persist for long periods, even after the removal of such challenges (Lucas, 1991). Thus, metabolic imprinting may be explored to optimize reproductive performance of beef heifers.
Postweaning injections of bovine ST hastened puberty attainment of Bos taurus heifers (Cooke et al., 2013). However, less emphasis has been placed on pre- vs. post-weaning management strategies, despite their greater impact on attainment of puberty in beef heifers (Roberts et al., 2007). It was hypothesized that preweaning injections of bovine ST would enhance growth and percentage of pubertal and pregnant beef heifers compared to saline injections. Hence, this 3 yr study evaluated the effects of preweaning injections of bovine ST on blood parameters and liver gene expression measurements associated with somatotropic axis, growth, and reproduction of Bos indicus-influenced beef heifers.
MATERIALS AND METHODS
The 3 yr experiment described herein was conducted at the University of Florida, Institute of Food and Agricultural Sciences, Range Cattle Research and Education Center (RCREC), Ona, Florida (27°23′N and 81°56′W) from March 2014 to December 2017. Heifers used in these experiments were cared for by acceptable practices as outlined in the Guide for the Care and Use of Agricultural Animals in Research and Teaching (FASS, 2010) and approved by the IFAS-Animal Research Committee.
Animals and Diets
On d 0 of each year (n = 3 yr), 30 cow–calf pairs were selected from four herds of mature, lactating, Angus × Brahman crossbred beef cows (10 ± 3 yr of age). Only cow–calf pairs with heifer calves of similar BW and approximately 120 to 150 d of age (147 ± 20 kg; 134 ± 11 d) were selected for the study. The age criterion was based on previous results indicating that beef heifers of approximately 70 to 180 d of age were susceptible to long-term, nutrition-induced impacts on postweaning puberty attainment (Moriel et al., 2014). Immediately after selection, cow–calf pairs were stratified by heifer BW and age, and heifers were randomly assigned to receive an s.c. injection of a saline solution (SAL; 5 mL; 0.9% NaCl) or 250 mg of sometribove zinc (BST; Posilac, Elanco, Greenfield, IN) on d 0, 14, and 28. Injections were always administered in the neck, alternating between the right and left side of the heifer. The interval between injections (every 14 d) and dosage (250 mg) of sometribove zinc was chosen according to Buskirk et al. (1996) who successfully reported an increase in plasma IGF-1 concentrations after similar dosage and interval between bovine ST injections, without any detrimental effects to heifer growth and physiological parameters. The injections containing 250 mg of sometribove zinc were prepared by transferring the contents of a standard, commercially available injection of Posilac (500 mg of sometribove zinc) into a sterile container and determining its total volume, and then, the total volume was split in half to achieve the 250 mg dosage, which was administered to each heifer using sterile syringes. The number of injections (n = 3 injections) was selected so that the plasma IGF-1 concentrations remained increased for a total period of 42 d after the first bovine ST injection, which corresponds to the age window (70 to 180 d) that heifers were susceptible to nutrition-induced impacts on puberty (Moriel et al., 2014).
All cow–calf pairs were managed as a single group, without access to concentrate, and grazed the same bahiagrass (Paspalum notatum) pastures (4 pastures/yr; 4 ha/pasture) from d 0 until weaning (d 127). Immediately after weaning, cows returned to their original herds, whereas heifers were sorted by treatment, transferred into one of eight bahiagrass pastures (one pasture per treatment; 0.8 ha/pasture), and offered the same concentrate supplementation strategy until the end of the study (d 346). Treatment groups were rotated among the eight bahiagrass pastures every 9 to 10 d throughout the study to prevent any potential confounding effects of pasture on the variables investigated herein. Postweaning concentrate was formulated using NRC (2000) and designed to allow heifers to achieve 60% of their mature BW at the initiation of breeding season (assuming a mature BW of 499 kg; based on average BW of mature cows in the same location; Moriel et al., 2017). Concentrate supplementation was offered three times weekly (Monday, Wednesday, and Friday) at 0800 h to achieve an average daily intake of 2.9 kg of supplement DM per heifer daily from d 127 to 346. Nutritional composition of concentrate supplement is shown in Table 1. All cows and heifers were provided free-choice access to water and a salt-based trace mineral and vitamin mix during the entire study (University of Florida, Institute of Food and Agricultural Sciences, Cattle Research Mineral, Brookville, OH; 16.8% Ca, 4% P, 20.7% NaCl, 1.0% Mg, 60 mg/kg Co, 1,750 mg/kg Cu, 350 mg/kg I, 60 mg/kg Se, 5,000 mg/kg Zn, 441 IU/g of vitamin A, 33 IU/g of vitamin D3, and 0.44 IU/g of vitamin E). In each year, free-choice access to long-stem stargrass (Cynodon nlemfuensis) hay was offered when pasture availability was limited (d 263 to 346). Heifers were exposed to mature Angus bulls from d 263 to 346 (one bull per group). Bulls passed the breeding soundness exam 90 d before the start of the study and were rotated between treatment groups every 9 to 10 d during the breeding season to remove any potential effects of bull on the variables investigated herein.
Table 1.
Average nutritional composition of concentrate offered to heifers during the postweaning phase (d 127 to 346)
| Item | Postweaning concentrate1 |
|---|---|
| Ingredient2, kg DM daily | |
| Molasses | 1.0 |
| Crude glycerin | 1.0 |
| Dried distillers grains | 0.59 |
| Soybean meal | 0.30 |
| Ca carbonate | 0.009 |
| Phosphoric acid | 0.009 |
| TDN3, % of DM | 81.3 |
| CP, % of DM | 15.4 |
| NEm4, Mcal/kg of DM | 2.05 |
| NEg4, Mcal/kg of DM | 1.39 |
| Ca, % of DM | 0.55 |
| P, % of DM | 0.40 |
1Concentrate samples were collected monthly from weaning (d 127) until the end of the study (d 346) for wet chemistry analysis of all nutrients.
2Ingredients were hand-mixed immediately before feeding. Concentrate was provided three times weekly (Monday, Wednesday, and Friday) at 0800 h in amounts to achieve a target daily DM intake of 2.9 kg/heifer from day 127 to 346.
3Calculated as described by Weiss et al. (1992).
4Calculated using the equations proposed by the NRC (2000).
Sample and Data Collection
Individual heifer shrunk BW was recorded on d 0 and 42, after 6 h of feed and water withdrawal, and then approximately every 28 d from d 127 to 346, after 16 h of feed and water withdrawal. Full BW of heifers were recorded on d 14 and 28 to avoid any potential impacts of shrink-induced stress on blood metabolites and hormones during the period of treatment injections. Hip height of heifers was assessed on d 179 and 346.
Blood samples (10 mL) were collected from all heifers via jugular venipuncture into tubes (Vacutainer, Becton Dickinson) containing sodium-heparin (158 United States Pharmacopeia units) for plasma harvest on d 0, 14, 28, 42, 127, 234, 263, and 296 to determine the plasma concentrations of IGF-1. Blood samples (10 mL) also were collected from all heifers via jugular venipuncture into tubes (Vacutainer, Becton Dickinson) containing no additives for serum harvest at 9 to 10 d intervals from d 179 to 346 to determine the serum concentrations of progesterone (P4). Blood samples were immediately placed on ice following collection and then centrifuged at 1,200 × g for 25 min at 4 °C. Plasma and serum samples were stored frozen at −20 °C until later laboratory analysis. Onset of puberty in this study was defined as the first increase in concentrations of P4 greater than 1.0 ng/mL. The first increase in concentrations of P4 that exceeds 1.0 ng/mL is associated with females containing a luteal structure because of ovulation or luteinization suggesting onset of puberty (Perry et al., 1991). Body weight at puberty was determined using the ADG, and initial and final BW measurements of the respective 28 d interval when puberty was attained (BW at puberty = initial BW of the respective 28 d interval + [ADG of the respective 28 d interval × number of days between the day at puberty attainment and initial BW collection]).
Percentages of pregnant heifers were determined by palpation of the uterus and its contents per rectum by a trained veterinarian approximately 45 d after the end of the breeding season. Heifers were checked twice daily for calving. Calving date was converted to Julian date, and calf birth BW was obtained within 12 h of birth. Pregnancy loss was calculated as percentage of heifers diagnosed pregnant at approximately 45 d after the end of breeding season, but failed to calve. Calving distribution was reported as the percentage of heifers that calved weekly relative to the total number of heifers per treatment.
Hand-plucked samples of pastures, concentrate, and hay were collected every 56 d from d 0 to 346, dried in a forced-air oven at 56 °C for 72 h, ground in a Wiley mill (Model 4, Thomas-Wiley Laboratory Mill, Thomas Scientific, Swedesboro, NJ) to pass a 4 mm stainless steel screen, and pooled across month within each year. The pooled concentrate samples were analyzed in duplicates by a commercial laboratory (Dairy One Forage Laboratory, Ithaca, NY) for concentrations of CP (method 984.13; AOAC, 2006), TDN (Weiss et al., 1992), and NEm and NEg (NRC, 2000). Nutritive analyses of pooled samples of pastures and hay were performed at the University of Florida Forage Evaluation Support Laboratory using the micro-Kjeldahl technique for N (Gallaher et al., 1975) and the two-stage technique for in vitro organic matter digestibility (IVDOM; Moore and Mott, 1974). Average nutritional composition of pastures during the preweaning phase was 42.0% IVDOM and 10.9% CP (DM basis), whereas the average nutritional composition (DM basis) of pastures and hay during the postweaning phase was 35.3% and 46.3% IVDOM and 9.6% and 7.2% CP NDF, respectively.
Based on the results obtained in yr 1 and 2, liver biopsy sample collections (six heifers per treatment) were performed on d 0, 42, and 263 of yr 3 to determine the long-term impact of treatments on liver mRNA expression of genes associated with energy metabolism. All liver samples were collected via needle biopsy, following the procedure described by Arthington and Corah (1995). Immediately following collection, 100 mg of wet liver tissue per heifer was stored into 1.5 mL of RNA stabilization solution (RNAlater, Ambion Inc., Austin, TX), kept on ice for 8 h, and stored at −80 °C until later analyses of mRNA expression of cyclophilin, GH receptor 1A and 1B (GHR-1A and GHR-1B), IGF-1, IGF binding protein 3 (IGFBP-3), and 40S ribosomal protein S9 (RSP9). Primer sequence for each gene is shown in Table 2.
Table 2.
Primer sequences and accession number for all gene transcripts analyzed by quantitative real-time PCR1
| Target gene | Primer sequence | Accession number |
|---|---|---|
| Cyclophilin | ||
| Forward | 5′-GGTACTGGTGGCAAGTCCAT-3′ | NM_178320.2 |
| Reverse | 5′-GCCATCCAACCACTCAGTCT-3′ | |
| IGF-1 | ||
| Forward | 5′-CTCCTCGCATCTCTTCTATCT-3′ | NM_001077828 |
| Reverse | 5′-ACTCATCCACGATTCCTGTCT-3′ | |
| IGFBP-3 | ||
| Forward | 5′-AATGGCAGTGAGTCGGAAGA-3′ | NM_174556.1 |
| Reverse | 5′-AAGTTCTGGGTGTCTGTGCT-3′ | |
| GHR-1A | ||
| Forward | 5′-CCAGCCTCTGTTTCAGGAGTGT-3′ | AY748827 |
| Reverse | 5′-TGCCACTGCCAAGGTCAAC-3′ | |
| GHR-1B | ||
| Forward | 5′-AGCCTGGAGGAACCATACGA-3′ | - |
| Reverse | 5′-TAGCCCCATCTGTCCAGTGA-3′ | |
| RSP9 | ||
| Forward | 5′-CCTCGACCAAGAGCTGAAG-3′ | DT860044 |
| Reverse | 5′-CCTCCAGACCTCACGTTTGTTC-3′ | |
1Primer sequence for IGF-1, IGFBP-3, and GHR-1A genes was obtained from Coyne et al. (2011), whereas the primer sequence for Cyclophilin and RSP9 genes was obtained from Cooke et al. (2008) and Janovick-Guretzky et al. (2007), respectively. Primer sequence for GHR-1B gene was designed based on the bovine gene sequences deposited in the National Center for Biotechnology Information and using the Primer Express v. 3.0.1 software (Applied Biosystems, Foster City, CA).
Laboratory Analyses
Plasma concentrations of IGF-I were determined using a human-specific commercial ELISA kit (SG100; R&D Systems, Inc., Minneapolis, MN) with 100% cross-reactivity with bovine IGF-I and previously validated for bovine samples (Moriel et al., 2012). Intra- and inter-assay CV for IGF-1 assay were 1.60% and 3.65%, respectively. Plasma P4 concentrations were determined using a solid-phase, competitive, chemiluminescent enzyme immunoassay (Immulite 1000, Diagnostics Products Corp.) previously validated for bovine samples (Martin et al., 2007). Detectable range and intra-assay for plasma P4 concentrations were, respectively, 0.2 to 40 ng/mL and 4.38%.
A detailed description of procedures for mRNA isolation and tissue gene expression was described by Cappellozza et al. (2014). Briefly, total RNA was extracted from liver tissue samples using the TRIzol Plus RNA Purification Kit (Invitrogen, Carlsbad, CA). Extracted RNA was quantified via UV absorbance (UV Mini 1240; Shimadzu Scientific Instruments, Inc., Columbia, MD) at 260 nm, incubated (2.5 µg) at 37 °C for 30 min in the presence of RNase-free (DNase; New England Biolabs Inc., Ipswich, MA) and reverse transcribed using the High Capacity cDNA Reverse Transcription Kit with random hexamers (Applied Biosystems, Foster City, CA). Real-time PCR was completed using the SYBR Green PCR Master Mix (Applied Biosystems) and gene-specific primers (20 pM each) with the StepOne Real-Time PCR system (Applied Biosystems). At the end of each real-time PCR, amplified products were subjected to a dissociation gradient (95 °C for 15 s, 60 °C for 30 s, and 95 °C for 15 s) to verify the amplification of a single product by denaturation at the anticipated temperature. A portion of the amplified products was purified with the QIAquick PCR purification kit (Qiagen Inc., Valencia, CA) and sequenced at the Oregon State University Center for Genome Research and Biocomputing (Corvallis, OR), whereas the remaining portion was sequenced at the Department of Animal Science from University of Florida to verify the specificity of amplification. All amplified products represented only the genes of interest. Primer sequence of target genes was validated by previous studies, except for GHR-1B, which was designed based on the bovine gene sequences deposited in the National Center for Biotechnology Information and using the Primer Express v.3.0.1 software (Applied Biosystems, Foster City, CA). Responses were quantified based on the threshold cycle (CT) and were normalized to geometrical mean of CT values from cyclophilin and RSP9 (ΔCT) examined in the same sample and assessed at the same time as the targets. Within each target gene, results are expressed as relative fold change (2−ΔΔCT) using the average ΔCT of all samples (Ocón-Grove et al., 2008).
Statistical Analyses
All data were analyzed as a complete randomized design using SAS (SAS Institute Inc., Cary, NC, USA, version 9.4) with Satterthwaite approximation to determine the denominator degrees of freedom for the test of fixed effects. Heifer was the experimental unit, whereas heifer within treatment × year was included as random effect in all analyses. Growth and physiological results were analyzed using the MIXED procedure, whereas reproductive binary data (puberty attainment, percentage of heifers that became pregnant and calved, pregnancy loss, and weekly calving distribution) were analyzed using the GLIMMIX procedure. Heifer ADG, BW and age at puberty, and mature BW on d 263 were tested for fixed effects of preweaning treatment, year, and treatment × year. Heifer BW, plasma IGF-1, liver mRNA expression, puberty attainment, and calving distribution were tested for fixed effects of treatment, day of the study (or week of calving season), year, and all resulting interactions, using heifer within treatment × year as the subject. Results from d 0 were included as covariates in each respective analysis but removed from the model when P > 0.10. Proper covariance structure for each repeated measure analysis was selected based on the lowest Akaike information criterion. Compound symmetry covariance structure was used for statistical analyses of postweaning heifer BW, plasma IGF-1 concentrations, and liver mRNA expression of IGFBP-3. Autoregressive 1 covariance structure was used for the analyses of preweaning heifer BW, liver mRNA expression of IGF-1, calving distribution, and puberty attainment. Unstructured covariance structure was used for the statistical analyses of liver mRNA expression of GHR-1A and GHR-1B. All results are reported as least squares means. Data were separated using PDIFF if a significant F-test was detected. Significance was set at P ≤ 0.05 and tendencies at 0.05 < P ≤ 0.10.
RESULTS
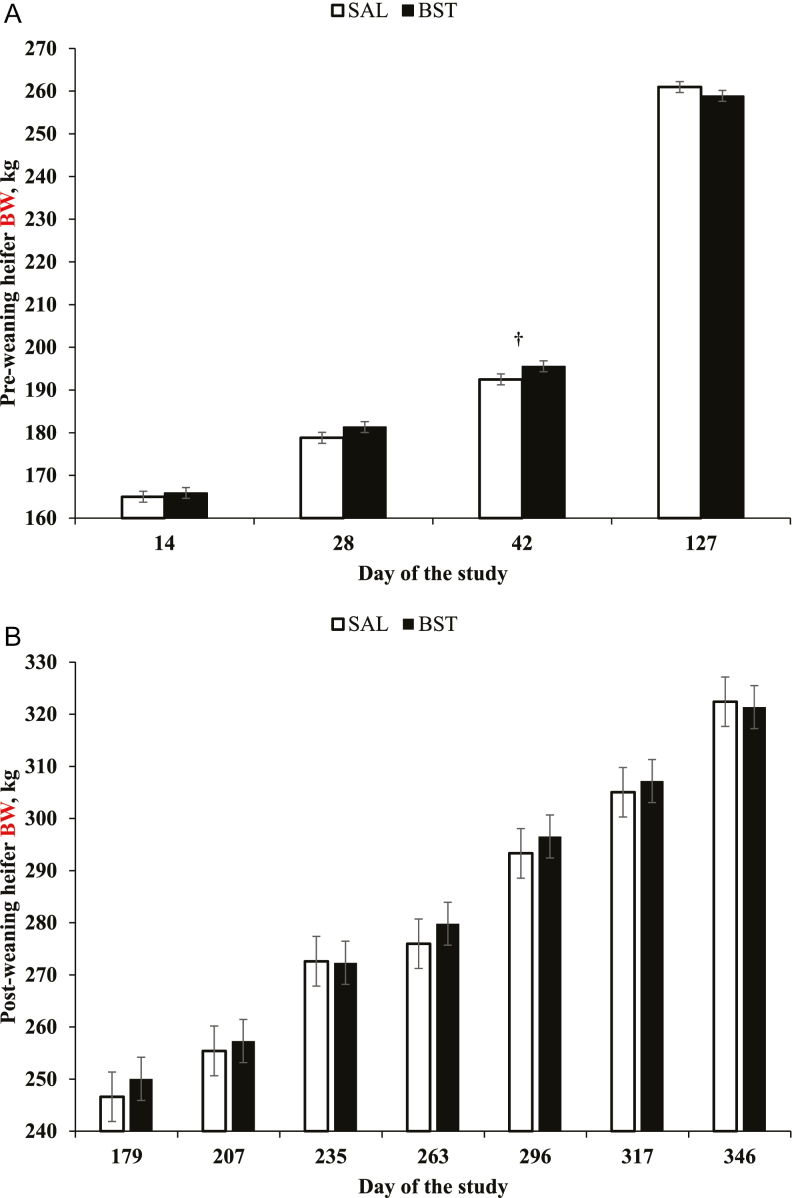
Heifer BW on d 0 did not differ (P ≥ 0.90) between treatments but was included as covariate (P < 0.0001) in the statistical analyses of preweaning heifer BW. Effects of treatment × year × day of the study, and treatment × year were not detected (P ≥ 0.30) for pre- and post-weaning heifer BW. Effects of treatment × day of the study were detected for preweaning (P = 0.01; Figure 1a), but not for postweaning BW of heifers (P = 0.50; Figure 1b). Heifers administered preweaning injections of bovine ST tended (P = 0.09) to be heavier on d 42, but had BW on d 14 and 28 and from d 127 to 346 that did not differ (P ≥ 0.17) compared with SAL heifers. Effects of year were detected (P = 0.0007) for mean postweaning BW of heifers, which was greatest in yr 2, least in yr 3, and intermediate in yr 1 (P ≤ 0.05; 294, 266, and 279 ± 5.3 kg, respectively).
Figure 1.
Pre- (a) and post-weaning (b) BW of beef heifers that received an s.c. injection of saline solution (SAL; 5 mL; 0.9% NaCl) or 250 mg of sometribove zinc (BST; Posilac, Elanco) on d 0, 14, and 28 (n = 15 heifers per treatment annually; 3 yr). Heifers were weaned on d 127. Body weight on d 0 did not differ (P ≥ 0.91) between treatments, but was included as covariate in the BW analyses (P < 0.0001). Effects of treatment × day of the study were detected (P = 0.01; SEM = 1.28) for preweaning BW, but not (P = 0.50; SEM = 4.75) for postweaning BW of heifers. †P = 0.09.
Effects of treatment × year were not detected (P ≥ 0.14) for ADG of heifers during the pre- and post-weaning phases, age and BW at puberty attainment, and percentage of mature BW on d 263 (Table 3). Heifers assigned to BST had greater (P = 0.03) ADG from d 14 to 28 and 0 to 42, less ADG from d 127 to 346 (P = 0.04), and ADG from d 0 to 127 and 127 to 346 that did not differ (P ≥ 0.50) compared to SAL heifers (Table 3). Hip height on d 179 and 346, and hip height change from d 179 to 346 did not differ (P ≥ 0.41) between treatments (Table 3).
Table 3.
Pre- and post-weaning growth performance of beef heifers that received an s.c. injection of saline solution (SAL; 5 mL; 0.9% NaCl) or 250 mg of sometribove zinc (BST; Posilac, Elanco, Greenfield, IN) on d 0, 14, and 28 (n = 15 heifers per treatment annually; 3 yr)1
| Item | Treatment | P-value | ||||
|---|---|---|---|---|---|---|
| SAL | BST | SEM | Treatment × year | Treatment | year | |
| Preweaning (d 0 to 127) | ||||||
| ADG, kg/d | ||||||
| d 0 to 14 | 1.26 | 1.35 | 0.06 | 0.46 | 0.18 | <0.01 |
| d 14 to 28 | 0.99 | 1.09 | 0.04 | 0.78 | 0.03 | <0.01 |
| d 28 to 42 | 1.00 | 0.97 | 0.04 | 0.27 | 0.40 | <0.01 |
| d 0 to 42 | 1.07 | 1.15 | 0.03 | 0.56 | 0.03 | 0.07 |
| d 42 to 127 | 0.80 | 0.74 | 0.02 | 0.48 | 0.04 | <0.01 |
| d 0 to 127 | 0.89 | 0.88 | 0.02 | 0.66 | 0.50 | <0.01 |
| Postweaning (d 127 to 346) | ||||||
| ADG, kg/d | ||||||
| d 127 to 262 | 0.12 | 0.13 | 0.03 | 0.36 | 0.71 | 0.02 |
| d 127 to 346 | 0.30 | 0.28 | 0.02 | 0.14 | 0.61 | 0.20 |
| Hip height, cm | ||||||
| d 179 | 115 | 116 | 0.60 | 0.66 | 0.66 | <0.01 |
| d 346 | 123 | 123 | 0.60 | 0.48 | 0.87 | 0.11 |
| Hip height change, cm | 7.8 | 7.4 | 0.37 | 0.79 | 0.41 | <0.01 |
1Individual heifer shrunk BW was assessed on d 0, 14, 28, and 42, after 6 h of feed and water withdrawal, and then every 28 d from d 127 to 346, after 16 h of feed and water withdrawal. Heifers and their dams were managed as a single group without access to concentrate supplementation during the preweaning phase (d 0 to 127). After weaning (d 127), heifers were sorted by treatment, allocated to one of eight bahiagrass pastures (one pasture per treatment), and offered the same concentrate supplementation strategy until d 346.
Effects of treatment × year and treatment × year × day of the study were not detected (P ≥ 0.14) for pre- and post-weaning plasma concentrations of IGF-1. Effect of treatment × day of the study was not detected (P = 0.83) for preweaning plasma concentrations of IGF-1, but BST heifers had greater (P = 0.05) overall plasma IGF-1 concentrations from d 0 to 42 compared to SAL heifers, after covariate adjusted for plasma IGF-1 concentrations obtained on d 0 (P < 0.0001; Table 4). Effect of treatment × day of the study was detected (P = 0.04) for postweaning plasma concentrations of IGF-1. Heifers treated with BST had greater (P = 0.008) plasma concentrations of IGF-1 on d 234, but plasma concentrations of IGF-1 on d 263 and 296 did not differ (P ≥ 0.82) compared to SAL heifers (Table 4).
Table 4.
Pre- and post-weaning plasma IGF-1 concentrations of beef heifers that received an s.c. injection of saline solution (SAL; 5 mL; 0.9% NaCl) or 250 mg of sometribove zinc (BST; Posilac, Elanco, Greenfield, IN) on d 0, 14, and 28 (n = 15 heifers per treatment annually; 3 yr)1
| Item | Treatment | P-value2 | ||||
|---|---|---|---|---|---|---|
| SAL | BST | SEM | P 3 | Treatment × day | Treatment | |
| Preweaning plasma IGF-1, ng/mL | ||||||
| Overall (d 0 to 42)4 | 94.8 | 103.4 | 3.16 | – | 0.83 | 0.05 |
| Postweaning plasma IGF-1, ng/mL | ||||||
| d 234 | 166.9 | 197.2 | 8.00 | <0.01 | 0.04 | 0.19 |
| d 263 | 181.9 | 184.5 | 8.00 | 0.82 | ||
| d 296 | 181.0 | 181.0 | 8.00 | 0.98 | ||
1Blood samples were collected from jugular vein from all heifers on d 0, 14, 28, 42, 127, and then every 9 to 10 d from d 179 to 346. Blood samples for the assessment of plasma IGF-1 concentrations were selected to represent the period of preweaning injections (d 0, 14, 28, and 42), day of weaning (d 127), and then 28 d before (d 235), immediately at (d 263), and 33 d after (d 296) the start of the breeding season.
2Effects of day (P < 0.0001), but not treatment × year and treatment × year × day of the study (P ≥ 0.14), were detected for pre- and post-weaning plasma IGF-1 concentrations.
3 P-value for the comparison of treatment within day.
4Average plasma IGF-1 concentrations of blood samples collected on d 14, 28, and 42, after covariate adjusted for plasma IGF-1 concentrations obtained on d 0 (P < 0.0001).
Liver mRNA expression of GHR-1A, GHR-1B, and IGF-1, but not IGFBP-3 (P = 0.77), was covariate adjusted (P ≤ 0.06) to respective mRNA expression obtained on d 0. Effects of treatment × day of the study were detected (P ≤ 0.02) for liver mRNA expression of GHR-1B and IGF-1, but not for GHR-1A and IGFBP-3 (P ≥ 0.15; Table 5). Liver mRNA expression of GHR-1B and IGF-1 did not differ (P ≥ 0.15) between treatments on d 42 but was greater (P ≤ 0.02) for BST vs. SAL heifers on d 263 (Table 5). Overall liver mRNA expression of GHR-1A and IGFBP-3 did not differ (P ≥ 0.12) between treatments (Table 5).
Table 5.
Liver mRNA expression (fold increase1; yr 3 only) of beef heifers that received an s.c. injection of saline solution (SAL; 5 mL; 0.9% NaCl) or 250 mg of sometribove zinc (BST; Posilac, Elanco, Greenfield, IN) on d 0, 14, and 28 (n = 15 heifers per treatment)2
| Treatment | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Gene3 | SAL | BST | SEM | P 4 | Treatment × day | Day | Treatment |
| Fold increase | |||||||
| GHR-1A 5 | 1.77 | 1.84 | 0.18 | – | 0.15 | <0.01 | 0.79 |
| IGFBP-3 5 | 1.58 | 1.96 | 0.15 | – | 0.44 | <0.01 | 0.12 |
| GHR-1B | |||||||
| d 42 | 3.59 | 4.36 | 0.41 | 0.22 | 0.02 | <0.01 | 0.76 |
| d 263 | 0.72 | 1.70 | 0.41 | 0.02 | |||
| IGF-1 | |||||||
| d 42 | 1.50 | 1.87 | 0.17 | 0.15 | <0.01 | 0.04 | 0.22 |
| d 263 | 0.93 | 1.85 | 0.17 | <0.01 | |||
1Responses were quantified based on the threshold cycle (CT) and were normalized to average CT of Cyclophilin and RSP9 (ΔCT) examined in the same sample and assessed at the same time as the targets. Within each target gene, results are expressed as relative fold change (2–ΔΔCT) using the average ΔCT of all samples as reference, as described by Ocón-Grove et al. (2008).
2Heifers and their dams were managed as a single group without access to concentrate supplementation during the preweaning phase (d 0 to 127). After weaning (d 127), heifers were sorted by treatment, allocated into bahiagrass pastures (one pasture per treatment), and offered the same concentrate supplementation strategy until d 346.
3Liver mRNA expression of GHR-1A, GHR-1B, and IGF-1, but not IGFBP-3 (P = 0.77), were covariate adjusted to respective mRNA expression obtained on d 0 (P ≤ 0.06).
4 P-value for the comparison of treatment within day.
5Average liver mRNA expression of GHR-1A and IGFBP-3 obtained on d 0, 42, and 263.
Heifers assigned to BST had BW at puberty and percentage of mature BW on d 263 did not differ (P ≥ 0.16) compared to SAL heifers, but tended (P = 0.10) to achieve puberty 26 d earlier than SAL heifers (Table 6). Effects of treatment × year were not detected (P ≥ 0.17) for pregnancy percentage, pregnancy loss, calving date, and calf BW at birth, except for overall calving percentage (P = 0.03; Table 6). Heifers assigned to BST tended (P ≤ 0.10) to have greater overall pregnancy percentage and less pregnancy loss compared to SAL heifers (Table 6). Overall calving percentage was greater (P ≤ 0.05) for BST vs. SAL heifers in yr 1 and 2, but did not differ (P = 0.68) between treatments in yr 3 (Table 6). Calving date and calf BW at birth did not differ (P ≥ 0.34) between treatments.
Table 6.
Reproductive performance of beef heifers that received an s.c. injection of saline solution (SAL; 5 mL; 0.9% NaCl) or 250 mg of sometribove zinc (BST; Posilac, Elanco, Greenfield, IN) on d 0, 14, and 28 (n = 15 heifers per treatment annually; 3 yr)1
| Item | Treatment | P-value | |||||
|---|---|---|---|---|---|---|---|
| SAL | BST | SEM | P 2 | Treatment× year | Treatment | Year | |
| Age at puberty3, d | 414 | 388 | 12.9 | – | 0.45 | 0.10 | 0.48 |
| BW at puberty4, kg | 291 | 285 | 5.70 | – | 0.46 | 0.34 | 0.37 |
| Mature BW d 2635, % | 54.7 | 56.2 | 1.22 | – | 0.69 | 0.16 | <0.01 |
| Overall pregnancy6, % | 68.9 | 82.2 | 6.11 | – | 0.17 | 0.10 | 0.01 |
| Overall calving, % | |||||||
| Yr 1 | 73.3 | 93.3 | 6.48 | 0.05 | 0.03 | 0.02 | 0.08 |
| Yr 2 | 33.3 | 86.7 | 6.48 | <0.01 | |||
| Yr 3 | 66.7 | 60.0 | 6.48 | 0.68 | |||
| Pregnancy loss6, % | 11.1 | 2.2 | 3.66 | – | 0.28 | 0.08 | 0.20 |
| Calving date6, Julian d | 277 | 284 | 5.80 | – | 0.25 | 0.34 | 0.38 |
| Calf birth BW6, kg | 26.1 | 25.0 | 1.00 | – | 0.57 | 0.41 | <0.01 |
1After weaning (d 127), heifers were sorted by treatment, allocated to one of eight bahiagrass pastures (one pasture per treatment), and offered the same concentrate supplementation strategy until d 346. Heifers were exposed to mature Angus bulls from d 263 to 346 (one bull per group). Every 9 to 10 d, heifers were rotated among the same eight bahiagrass pastures from d 127 to 346 and bulls rotated among heifer treatment groups from d 263 to 346.
2 P-value for the comparison of treatment within day.
3Heifers were considered pubertal after the first increase in plasma progesterone concentrations that exceeded 1.0 ng/mL (Perry et al., 1991).
4Body weight at puberty = initial BW of the respective 28 d interval + (ADG of the respective 28 d interval × number of days between the day at puberty attainment and initial BW collection).
5Assuming a cow herd mature body weight of 499 kg (Moriel et al., 2017).
6Pregnancy rates were determined via rectal palpation at approximately 45 d after the end of the breeding season. Pregnancy loss calculated as percentage of heifers that were categorized as pregnant at approximately 45 d after the end of breeding season, but did not calve. Heifers were observed twice daily for calving. Calving date was determined using Julian date, and calf birth BW was obtained within 12 h of birth.
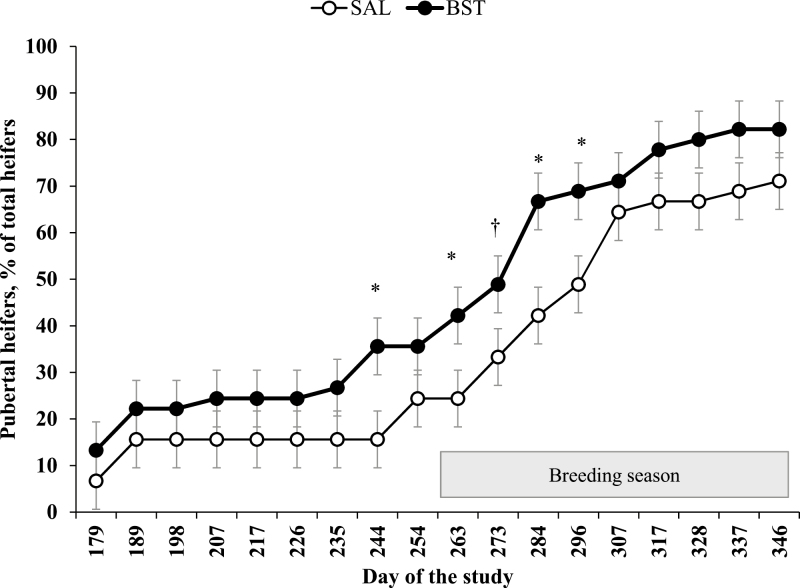
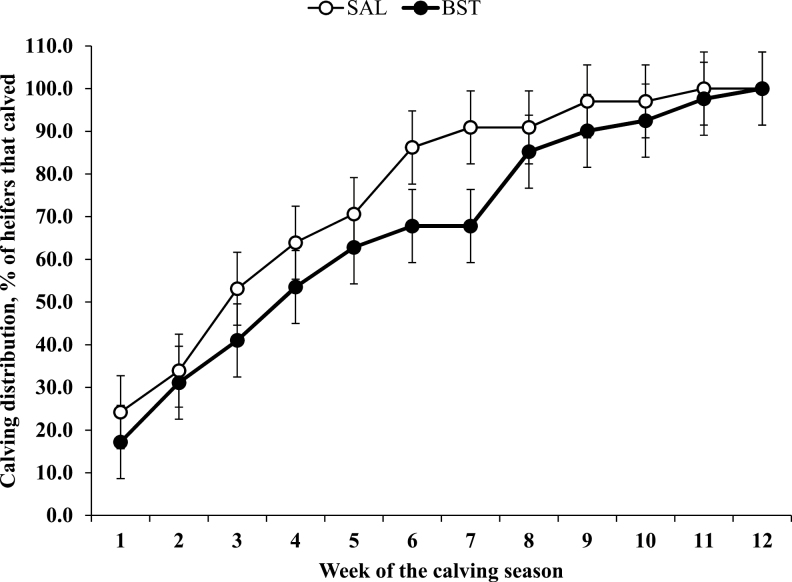
Effects of treatment × year and treatment × year × day of the study (or week of the calving season) were not detected (P ≥ 0.28) for puberty attainment (Figure 2) or calving distribution (Figure 3). Effect of treatment × day of the study was detected (P = 0.03) for puberty attainment. The percentage of pubertal heifers was greater (P ≤ 0.04) for BST vs. SAL heifers on d 244, 263, 284, and 296 and tended (P = 0.08) to be greater for BST vs. SAL heifers on d 273 (Figure 2). Effect of treatment × week was not detected (P = 0.78) for calving distribution (Figure 3).
Figure 2.
Percentage of pubertal beef heifers that received an s.c. injection of saline solution (SAL; 5 mL; 0.9% NaCl) or 250 mg of sometribove zinc (BST; Posilac, Elanco) on d 0, 14, and 28 (n = 15 heifers per treatment annually; 3 yr). Heifers were weaned on d 127. Heifers were considered pubertal after the first increase in serum progesterone concentrations that exceeded 1.0 ng/mL (Perry et al., 1991). Heifers were exposed to mature Angus bulls from d 263 to 346 (one bull per treatment). Effects of treatment × day of the study were detected (P = 0.03; SEM = 6.09) for puberty achievement from d 179 to 346. *P ≤ 0.05 and †P = 0.08.
Figure 3.
Calving distribution (% of heifers that calved) of beef heifers that received an s.c. injection of saline solution (SAL; 5 mL; 0.9% NaCl) or 250 mg of sometribove zinc (BST; Posilac, Elanco) on d 0, 14, and 28 (n = 15 heifers per treatment annually; 3 yr). Heifers were weaned on d 127 and exposed to mature Angus bulls from d 263 to 346 (one bull per treatment). Every 9 to 10 d, heifers were rotated among the same bahiagrass pastures from d 127 to 346 and bulls rotated among heifer groups from d 263 to 346. Effects of year, treatment, treatment × year, treatment × week of calving season, and treatment × week of calving season × year were not detected (P ≥ 0.27) for calving distribution.
DISCUSSION
Beef heifers early-weaned at 70 d of age and limit-fed a high concentrate diet for 90 d after weaning had similar BW and ADG during breeding season, but hastened puberty attainment compared to heifers that were weaned at 270 d of age and provided similar postweaning management (Moriel et al., 2014). The exact nutrition-mediated mechanisms involved in this early activation of the reproductive axis in beef heifers are unknown. However, circulating IGF-I impacts gonadotropin activity required for puberty achievement in beef heifers (Butler and Smith, 1989; Schillo et al., 1992; Spicer and Echternkamp, 1995). Thus, metabolic imprinting may be explored by identifying strategies that can increase heifer ADG and plasma IGF-1 during the developmental phase leading to optimized future reproductive performance.
In the present study, heifers administered preweaning injections of bovine ST had an 8.6 ng/mL increase in mean plasma IGF-1 concentrations, a 7.5% increase in ADG from d 0 to 42, and tended to be heavier on d 42 compared to heifers administered saline solution. Other studies demonstrated that postweaning bovine ST injections increased plasma IGF-1 concentrations (Cooke et al., 2013), but did not increase postweaning ADG of Angus × Holstein heifers administered 500 mg of sometribove zinc every 14 d from 6 to 10 mo of age (Carstens et al., 1997) and Angus × Hereford heifers injected with 250 mg of sometribove zinc every 14 d from 6 to 13 mo of age (Cooke et al., 2013). The increase in BW gain and circulating IGF-1 concentrations following bovine ST injections varied from 0% to 45% compared to control treatments (Dalke et al., 1992; Houseknecht et al., 1992), and several factors, such as plane of nutrition, age, and animal size, may explain this large variation (Rausch et al., 2002). Body weight gain and circulating IGF-1 response to bovine ST are positively influenced by cattle age and nutritional status (Rausch et al., 2002; Radcliff et al., 2004). Cattle somatotropic axis is functional at birth (Granz et al., 1997), and the response to ST begins as early as 1 d of age (Govoni et al., 2004), gradually increasing as age increases (Velayudhan et al., 2007). Likewise, plasma IGF-1 concentrations following bovine ST injection were greater for Holstein heifers gaining 1.2 vs. 0.8 kg/d (Radcliff et al., 2004).
Multiple mechanisms may be involved in the BW gain of cattle following bovine ST injections, including the repartitioning of nutrients toward muscle rather than adipose tissue deposition (Breier, 1999), enhanced long-bone growth (Buskirk et al., 1996), improved nitrogen retention (Eisemann et al., 1986), and increased circulating IGF-1-induced synthesis of muscle (Jiang and Ge, 2014) and noncarcass tissues (Early et al., 1990). Multiple 14 d apart administrations of bovine ST during the postweaning phase reduced subcutaneous fat thickness by 9.2% without impacting LM depth, marbling scores, and BW gain (Cooke et al., 2013). Although body composition was not evaluated in the present study, it is unlikely that three 14 d apart injections of bovine ST substantially affected body composition and nutrient requirements of heifers, leading to similar overall pre- and post-weaning growth performance. Hip height throughout the postweaning phase did not differ between treatments, indicating that bone growth did not differ. Our results perhaps indicate that the increment on bovine ST-induced ADG from d 0 to 42 may be the result of increased feed intake and gut fill, as reported by Enright et al. (1990). Heifer BW on d 0 and 42 were recorded after shrink, but it is possible that gut fill was not completely eliminated after shrink. The less ADG from d 42 to 127 for BST vs. SAL heifers, and lack of differences on overall preweaning ADG from d 0 to 127, supports this rationale of potential gut fill effects. In addition, muscle protein deposition from d 0 to 42 perhaps was not sufficient to dramatically impact heifer BW at weaning. Nevertheless, preweaning bovine ST injections in the present study successfully increased plasma IGF-1 concentrations and ADG of heifers during the developmental phase of the reproductive axis in beef heifers (Day and Anderson, 1998).
The binding of GH to GHR-1A stimulates hepatic synthesis of IGF-1 (Smith et al., 2002) and is highly correlated with liver mRNA expression of GHR-1A and IGF-1 (Lucy et al., 2001). Transcription of the growth hormone receptor gene (GHR) is initiated from multiple transcription start sites, generating GHR-1A, GHR-1B, and GHR-1C mRNA that differ in the 5′-untranslated region, but still encode the same amino acid sequence (Jiang and Lucy, 2001). The GHR-1A mRNA is only expressed in the liver (Lucy et al., 1998), whereas GHR-1B and GHR-1C mRNA are expressed in a wide array of tissues, including liver, skeletal muscle, adipose tissue, and mammary gland (Jiang et al., 1999; Jiang and Lucy, 2001). Hepatic synthesis of IGF-1 is regulated primarily at the transcriptional level (Thissen et al., 1994) and is the major source of circulating IGF-1 (Yakar et al., 1999), which is also responsible for stimulating the hepatic expression of IGFBP-3 mRNA (Thissen et al., 1994). Thus, an increased hepatic expression of GHR-1A mRNA enhances the capacity for GH binding (Lapierre et al., 1992) and the hepatic synthesis of IGF-1 (Radcliff et al., 2004). Nutrient intake and BW gain positively affect the abundance of GHR-1A, IGF-1, and IGFBP-3 in the liver (Thissen et al., 1994; Smith et al., 2002; Radcliff et al., 2004). Holstein heifers administered daily injections of bovine ST (25 µg/kg of BW from 120 to 247 d of age) had greater liver mRNA expression of IGF-1, but similar liver mRNA expression of GHR-1A and IGFBP-3 (Radcliff et al., 2004).
In the present study, preweaning injections of bovine ST did not impact liver mRNA expression of GHR-1A and IGFBP-3 throughout the study, and GHR-1B and IGF-1 mRNA on d 42. Following bovine ST administration to lactating and nonlactating dairy cattle, plasma IGF-1 concentrations increase after 3 d, peak at approximately 7 to 8 d, and gradually return to baseline concentrations starting at 12 d postinjection (Bilby et al., 1999, 2004). Hence, it is possible that the timing of liver sample collection was not optimal to detect the peak expression of liver mRNA of IGFBP-3, IGF-1, GHR-1B, and GHR-1A. Detection of greater mean plasma IGF-1 concentrations from d 0 and 42, but similar liver mRNA expression of IGF-1 on d 42 between BST vs. saline heifers support this rationale. Nevertheless, the primary goal for the collection of liver mRNA expression data was to evaluate any potential carryover effects of preweaning injections of bovine ST on postweaning liver gene expression. Preweaning injections of bovine ST increased liver mRNA expression of GHR-1B and IGF-1 approximately 221 d after the last injection of bovine ST, despite the similar postweaning nutritional management and ADG between treatments, which may be an evidence that preweaning injections of bovine ST caused metabolic imprinting effects. Similarly, Moriel et al. (2014) reported that beef heifers early-weaned at 70 d of age and limit-fed a high concentrate diet for 90 d after weaning had similar BW and ADG during breeding season compared to heifers normally weaned at 270 d of age, but had increased liver IGF-1 mRNA expression 70 d after all heifer groups were allocated to the same postweaning nutritional management (Moriel et al., 2014). Further studies are required to identify the metabolic imprinting mechanisms influencing the postweaning gene expression and reproduction of BST-injected heifers.
Despite the greater liver mRNA expression of IGF-1 at the start of the breeding season, postweaning plasma IGF-1 concentrations were greater for BST heifers on d 234, but not at the start and 33 d after the start of the breeding season. This response indicates that the greater liver mRNA expression of IGF-1 on d 263 did not translate into greater systemic concentrations of IGF-1 on that same day. The greater plasma IGF-1 concentrations of BST heifers on d 234, however, may indicate that liver mRNA expression of IGF-1 was likely greater for BST vs. SAL heifers before the start of the breeding season and that the magnitude of differences on liver mRNA expression of IGF-1 was declining during the postweaning phase leading to similar plasma IGF-1 concentrations on d 263 and 296. Therefore, one could speculate that the potential metabolic imprinting effects of preweaning bovine ST injections on liver metabolism of IGF-1 may not have persisted after d 263.
The impact of bovine ST injections on puberty attainment of beef heifers has been variable. Injections of bovine ST (250 mg every 14 d from 120 to 232 d of age) did not impact attainment of puberty of Angus × Simmental crossbred heifers (Buskirk et al., 1996). Postweaning bovine ST injections (250 mg of bovine ST every 14 d from 6 to 13 mo of age) increased the percentage of Angus × Hereford heifers attaining puberty at the start of breeding season (Cooke et al., 2013), but had no impact on attainment of puberty of Angus heifers administered bovine ST (350 mg every 14 d from 7 to 14.5 mo of age) compared to control-treated heifers (Hall et al., 1994). In the present study, preweaning injections of bovine ST tended to decrease age at puberty by 26 d and hastened the percentage of pubertal heifers immediately at and during the first 33 d after the initiation of the breeding season compared to saline injections, despite their similar nutritional management, ADG, and BW during breeding season. The tendency to advance puberty supports our hypothesis and is likely a result of the increased plasma IGF-1 concentrations and ADG during the developmental phase of the reproductive axis in beef heifers (Day and Anderson, 1998). The exact mechanism for such responses on puberty attainment of these heifers cannot be determined in the present study, and the discussion of all potential mechanism leading to the enhanced puberty attainment is beyond the scope of this article.
Heifers should attain puberty before the initiation of the breeding season because the percentage of pregnant heifers was 21% greater in heifers mated on third vs. first postpubertal estrous cycle (Byerley et al., 1987; Perry et al., 1991), and the timing of conception in the first breeding season impacts lifetime productivity (Lesmeister et al., 1973). Heifers classified as cyclic at the initiation of breeding season had greater overall pregnancy and calving percentages to the first breeding season (Moriel et al., 2017). Beef heifers that conceived early during their initial breeding season and calved within the first 21 d period of the calving season had greater overall pregnancy percentage and calf weaning weights for the first six parturitions (Cushman et al., 2013). They also remained in the herd longer compared to females that calved during the second and third 21 d period of calving season (Cushman et al., 2013). In agreement with our hypothesis and the rationale described above, preweaning injections of bovine ST increased the overall pregnancy percentages in yr 1, 2, and 3, and calving percentages in yr 1 and 2. Calving distribution, however, did not differ between treatments, which was unexpected. Thus, the greater overall reproductive efficiency of BST heifers is likely because of the greater percentage of pubertal heifers at the initiation and during the first 30 d of the breeding season, highlighting the importance of age at puberty attainment for B. indicus-influenced heifers. Taken together, the results of the current study indicate that major limiting factor for reproductive success of B. indicus-influenced heifers is the delayed attainment of puberty because of poor environment-induced growth performance of heifers. It also suggests that successful pregnancy and calving percentages can occur even under situations of poor growth performance, if heifers are administered preweaning injections of bovine ST and become pubertal before the initiation of breeding season.
It is important to note that heifers were slightly lighter than expected at the initiation of the breeding season (% of mature BW), and for that reason, only 20% to 40% of all heifers were considered pubertal at the start of the breeding season. This is likely a result of the impacts of environmental conditions reducing postweaning growth performance of heifers, as reported previously in cohorts at the same location (Moriel et al., 2014). In addition, the fact that only 20% to 40% of heifers were pubertal at the initiation of the breeding season indicates that bull breeding power was not a limiting factor for the reproductive performance of heifers.
Calving percentages in yr 3 did not differ between BST and SAL heifers. This response was surprising considering that this was the only variable measured in the present study that demonstrated an effect of treatment × year. Further evaluation revealed that heifers in yr 3, regardless of treatment, had the lightest mean BW during the postweaning phase compared to heifers in yr 1 and 2, which could be attributed to differences in environmental conditions as postweaning nutritional management of heifers was similar across year. Likewise, overall puberty attainment was less for yr 3 vs. 1 and 2 (30% vs. 42% and 52%, respectively), and overall pregnancy percentage was less in yr 2 and 3 vs. 1 (60% and 63% vs. 90%, respectively). Hence, the similar postweaning nutritional management and ADG of heifers among year (P = 0.19), and lack of treatment and treatment × year interactions for postweaning BW and ADG, indicates that the similar calving percentage between treatments in yr 3 may be attributed to heifers being the lightest, which limited the overall puberty attainment and pregnancy percentage, and prevented similar treatment effects observed for calving percentages in yr 1 and 2.
In conclusion, preweaning injections of bovine ST (250 mg every 14 d between 135 and 163 d of age) increased puberty attainment of beef heifers at the initiation of their first breeding season, overall pregnancy percentage in all 3 yr, and calving percentage in 2 of 3 yr. In addition, preweaning injections of bovine ST led to long-term effects on plasma IGF-1 concentrations and liver mRNA expression of genes associated with energy metabolism and known for positively influencing reproduction in beef cattle. These latter responses may be indicators of metabolic imprinting, but further measures are warranted to elucidate the actual metabolic imprinting mechanisms that may be occurring.
LITERATURE CITED
- AOAC.. 2006. Official methods of analysis. 18th ed Arlington (VA): AOAC Int. [Google Scholar]
- Arthington J. D., and Corah L. R.. 1995. Liver biopsy procedures for determining the trace mineral status in beef cows. Part II. (Video, AI 9134). Manhattan (KS): Extension TV, Dept. Commun. Coop. Ext. Serv., Kansas State Univ. [Google Scholar]
- Bilby C. R., Bader J. F., Salfen B. E., Youngquist R. S., Murphy C. N., Garverick H. A., Crooker B. A., and Lucy M. C.. 1999. Plasma GH, IGF-I, and conception rate in cattle treated with low doses of recombinant bovine GH. Theriogenology 51:1285–1296. [DOI] [PubMed] [Google Scholar]
- Bilby T. T., Guzeloglu A., Kamimura S., Pancarci S. M., Michel F., Head H. H., and Thatcher W. W.. 2004. Pregnancy and bovine somatotropin in nonlactating dairy cows: I. Ovarian, conceptus, and insulin-like growth factor system responses. J. Dairy. Sci. 87:3256–3267. [DOI] [PubMed] [Google Scholar]
- Breier B. H. 1999. Regulation of protein and energy metabolism by the somatotropic axis. Domest. Anim. Endocrinol. 17:209–218. [DOI] [PubMed] [Google Scholar]
- Buskirk D. D., Faulkner D. B., Hurley W. L., Kesler D. J., Ireland F. A., Nash T. G., Castree J. C., and Vicini J. L.. 1996. Growth, reproductive performance, mammary development, and milk production of beef heifers as influenced by prepubertal dietary energy and administration of bovine somatotropin. J. Anim. Sci. 74:2649–2662. [DOI] [PubMed] [Google Scholar]
- Butler W. R., and Smith R. D.. 1989. Interrelationships between energy balance on postpartum reproductive function in dairy cattle. J. Dairy Sci. 72:767–783. [DOI] [PubMed] [Google Scholar]
- Byerley D. J., Staigmiller R. B., Berardinelli J. B., and Short R. E.. 1987. Pregnancy rates of beef heifers bred either on puberal or third estrus. J. Anim. Sci. 65:645–650. [DOI] [PubMed] [Google Scholar]
- Cappellozza B. I., Cooke R. F., Reis M. M., Moriel P., Keisler D. H., and Bohnert D. W.. 2014. Supplementation based on protein or energy ingredients to beef cattle consuming low-quality cool season forages: II. Performance, reproductive, and metabolic responses of replacement heifers. J. Anim. Sci. 92:2725–2734. [DOI] [PubMed] [Google Scholar]
- Carstens G. E., Glaser D. E., Byers F. M., Greene L. W., and Lunt D. K.. 1997. Effects of bovine somatotropin treatment and intermittent growth pattern on mammary gland development in heifers. J. Anim. Sci. 75:2378–2388. [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Arthington J. D., Araujo D. B., Lamb G. C., and Ealy A. D.. 2008. Effects of supplementation frequency on performance, reproductive, and metabolic responses of Brahman-crossbred females. J. Anim. Sci. 86:2296–2309. [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Bohnert D. W., Francisco C. L., Marques R. S., Mueller C. J., and Keisler D. H.. 2013. Effects of bovine somatotropin administration on growth, physiological, and reproductive responses of replacement beef heifers. J. Anim. Sci. 91:2894–2901. [DOI] [PubMed] [Google Scholar]
- Coyne G. S., Kenny D. A., and Waters S. M.. 2011. Effect of dietary n-3 polyunsaturated fatty acid supplementation on bovine uterine endometrial and hepatic gene expression of the insulin-like growth factor system. Theriogenology 75:500–512. [DOI] [PubMed] [Google Scholar]
- Cushman R. A., Kill L. K., Funston R. N., Mousel E. M., and Perry G. A.. 2013. Heifer calving date positively influences calf weaning weights through six parturitions. J. Anim. Sci. 91:4486–4491. [DOI] [PubMed] [Google Scholar]
- Dalke B. S., Roeder R. A., Kasser T. R., Veenhuizen J. J., Hunt C. W., Hinman D. D., and Schelling G. T.. 1992. Dose–response effects of recombinant bovine somatotropin implants on feedlot performance in steers. J. Anim. Sci. 70:2130–2137. [DOI] [PubMed] [Google Scholar]
- Day M. L., and Anderson L. H.. 1998. Current concepts on the control of puberty in cattle. J. Anim. Sci. 76:1–15. [Google Scholar]
- Day M. L., and Nogueira G. P.. 2013. Management of age at puberty in beef heifers to optimize efficiency of beef production. Anim. Front. 3:6–11. [Google Scholar]
- Early R. J., McBride B. W., and Ball R. O.. 1990. Growth and metabolism in somatotropin-treated steers: II. Carcass and noncarcass tissue components and chemical composition. J. Anim. Sci. 68:4144–4152. [DOI] [PubMed] [Google Scholar]
- Eisemann J. H., Hammond A. C., Bauman D. E., Reynolds P. J., McCutcheon S. N., Tyrrell H. F., and Haaland G. L.. 1986. Effect of bovine growth hormone administration on metabolism of growing Hereford heifers: dietary digestibility, energy and nitrogen balance. J. Nutr. 116:157–163. [DOI] [PubMed] [Google Scholar]
- Enright W. J., Quirke J. F., Gluckman P. D., Breier B. H., Kennedy L. G., Hart I. C., Roche J. F., Coert A., and Allen P.. 1990. Effects of long-term administration of pituitary-derived bovine growth hormone and estradiol on growth in steers. J. Anim. Sci. 68:2345–2356. [DOI] [PubMed] [Google Scholar]
- Federation of Animal Science Societies (FASS).. 2010. Guide for the care and use of agricultural animals in research and teaching, 3rd ed. Champaign , IL: FASS. [Google Scholar]
- Gallaher R. N., Weldon C. O., and Futral J. G.. 1975. An aluminum block digester for plant and soil analysis. Soil Sci. Soc. Am. J. 39:803–806. [Google Scholar]
- Gasser C. L., Burke C. R., Mussard M. L., Behlke E. J., Grum D. E., Kinder J. E., and Day M. L.. 2006a. Induction of precocious puberty in heifers II: advanced ovarian follicular development. J. Anim. Sci. 84:2042–2049. [DOI] [PubMed] [Google Scholar]
- Gasser C. L., Grum D. E., Mussard M. L., Fluharty F. L., Kinder J. E., and Day M. L.. 2006b. Induction of precocious puberty in heifers I: enhanced secretion of luteinizing hormone. J. Anim. Sci. 84:2035–2041. [DOI] [PubMed] [Google Scholar]
- Govoni K. E., Hoagland T. A., and Zinn S. A.. 2004. The ontogeny of the somatotropic axis in male and female Hereford calves from birth to one year of age and its response to administration of exogenous bovine somatotropin. J. Anim. Sci. 82:1646–1655. [DOI] [PubMed] [Google Scholar]
- Granz S., Ellendorff F., Grossmann R., Kato Y., Muhlbauer E., and Elsaesser F.. 1997. Ontogeny of growth hormone and LH beta-, FSH beta- and alpha-subunit mRNA levels in the porcine fetal and neonatal anterior pituitary. J. Neuroendocrinol. 9:439–449. [DOI] [PubMed] [Google Scholar]
- Hall J. B., Schillo K. K., Fitzgerald B. P., and Bradley N. W.. 1994. Effects of recombinant bovine somatotropin and dietary energy intake on growth, secretion of luteinizing hormone, follicular development, and onset of puberty in beef heifers. J. Anim. Sci. 72:709–718. [DOI] [PubMed] [Google Scholar]
- Houseknecht K. L., Bauman D. E., Fox D. G., and Smith D. F.. 1992. Abomasal infusion of casein enhances nitrogen retention in somatotropin-treated steers. J. Nutr. 122:1717–1725. [DOI] [PubMed] [Google Scholar]
- Janovick-Guretzky N. A., Dann H. M., Carlson D. B., Murphy M. R., Loor J. J., Drackley J. K.. 2007. Housekeeping gene expression in bovine liver is affected by physiological state, feed intake, and dietary treatment. J. Dairy Sci. 90:2246–2252. [DOI] [PubMed] [Google Scholar]
- Jiang H., and Ge X.. 2014. Mechanism of growth hormone stimulation of skeletal muscle growth in cattle. J. Anim. Sci. 92:21–29. [DOI] [PubMed] [Google Scholar]
- Jiang H., and Lucy M. C.. 2001. Variants of the 5′-untranslated region of the bovine growth hormone receptor mRNA: isolation, expression and effects on translational efficiency. Gene 265:45–53. [DOI] [PubMed] [Google Scholar]
- Jiang H., Okamura C. S., and Lucy M. C.. 1999. Isolation and characterization of a novel promoter for the bovine growth hormone receptor gene. J. Biol. Chem. 274:7893–7900. [DOI] [PubMed] [Google Scholar]
- Lapierre H., Reynolds C. K., Elsasser T. H., Gaudreau P., Brazeau P., and Tyrrell H. F.. 1992. Effects of growth hormone-releasing factor and feed intake on energy metabolism in growing beef steers: net hormone metabolism by portal-drained viscera and liver. J. Anim. Sci. 70:742–751. [DOI] [PubMed] [Google Scholar]
- Lesmeister J. L., Burfening P. J., and Blackwell R. L.. 1973. Date of first calving in beef cows and subsequent calf production. J. Anim. Sci. 36:1–6. doi:10.2527/jas1973.3611 [Google Scholar]
- Lucas A. 1991. Programming by early nutrition in man. Ciba Found. Symp. 156:38–50. [PubMed] [Google Scholar]
- Lucy M. C., Boyd C. K., Koenigsfeld A. T., and Okamura C. S.. 1998. Expression of somatotropin receptor messenger ribonucleic acid in bovine tissues. J. Dairy Sci. 81:1889–1895. [DOI] [PubMed] [Google Scholar]
- Lucy M. C., Jiang H., and Kobayashi Y.. 2001. Changes in the somatotrophic axis associated with the initiation of lactation. J. Dairy Sci. 84:E113–E119. [Google Scholar]
- Martin J. L., Vonnahme K. A., Adams D. C., Lardy G. P., and Funston R. N.. 2007. Effects of dam nutrition on growth and reproductive performance of heifer calves. J. Anim. Sci. 85:841–847. [DOI] [PubMed] [Google Scholar]
- Moore J. E., and Mott G. O.. 1974. Recovery of residual organic matter from “in vitro” digestion of forages. J. Dairy Sci. 57:1258–1259. [Google Scholar]
- Moriel P., Cooke R. F., Bohnert D. W., Vendramini J. M. B., and Arthington J. D.. 2012. Effects of energy supplementation frequency and forage quality on performance, reproductive, and physiological responses of replacement beef heifers. J. Anim. Sci. 90:2371–2380. [DOI] [PubMed] [Google Scholar]
- Moriel P., Johnson S. E., Vendramini J. M. B., Mercadante V. R. G., Hersom M. J., and Arthington J. D.. 2014. Effects of calf weaning age and subsequent management system on growth and reproductive performance of beef heifers. J. Anim. Sci. 92:3096–3107. [DOI] [PubMed] [Google Scholar]
- Moriel P., Lancaster P., Lamb G. C., Vendramini J. M. B., and Arthington J. D.. 2017. Effects of post-weaning growth rate and puberty induction protocol on reproductive performance of Bos indicus-influenced beef heifers. J. Anim. Sci. 95:3523–3531. [DOI] [PubMed] [Google Scholar]
- NRC 2000. Nutrient requirements of beef cattle. Rev. 7th ed Washington (DC): Natl. Acad. Press. [Google Scholar]
- Ocón-Grove O. M., Cooke F. N. T., Alvarez I. M., Johnson S. E., Ott T. L., and Ealy A. D.. 2008. Ovine endometrial expression of fibroblast growth factor (FGF) 2 and conceptus expression of FGF receptors during early pregnancy. Domest. Anim. Endocrinol. 34:135–145. [DOI] [PubMed] [Google Scholar]
- Perry R. C., Corah L. R., Cochran R. C., Brethour J. R., Olson K. C., and Higgins J. J.. 1991. Effects of hay quality, breed, and ovarian development on onset of puberty and reproductive performance of beef heifers. J. Prod. Agric. 4:13–18. [Google Scholar]
- Radcliff R. P., Vandehaar M. J., Kobayashi Y., Sharma B. K., Tucker H. A., and Lucy M. C.. 2004. Effect of dietary energy and somatotropin on components of the somatotropic axis in Holstein heifer. J. Dairy Sci. 87:1229–1235. [DOI] [PubMed] [Google Scholar]
- Rausch M. I., Tripp M. W., Govoni K. E., Zang W., Weber W. J., Crooker B. A., Hoagland T. A., and Zinn S. A.. 2002. The influence of level of feeding on growth and serum insulin-like growth factor I and insulin-like growth factor-binding proteins in growing beef cattle supplemented with somatotropin. J. Anim. Sci. 80:94–100. [DOI] [PubMed] [Google Scholar]
- Roberts A. J., Paisley S. I., Geary T. W., Grings E. E., Waterman R. C., and MacNeil M. D.. 2007. Effects of restricted feeding of beef heifers during the postweaning period on growth, efficiency, and ultrasound carcass characteristics. J. Anim. Sci. 85:2740–2745. [DOI] [PubMed] [Google Scholar]
- Schillo K. K., Hall J. B., and Hileman S. M.. 1992. Effects of nutrition and season on the onset of puberty in the beef heifer. J. Anim. Sci. 70:3994–4005. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Van Amburgh M. E., Diaz M. C., Lucy M. C., and Bauman D. E.. 2002. Effect of nutrient intake on the development of the somatotropic axis and its responsiveness to GH in Holstein bull calves. J. Anim. Sci. 80:1528–1537. [DOI] [PubMed] [Google Scholar]
- Spicer L. J., and Echternkamp S. E.. 1995. The ovarian insulin and insulin-like growth factor system with an emphasis on domestic animals. Domest. Anim. Endocrinol. 12:223–245. [DOI] [PubMed] [Google Scholar]
- Thissen J. P., Ketelslegers J. M., and Underwood L. E.. 1994. Nutritional regulation of the insulin-like growth factors. Endocr. Rev. 15:80–101. [DOI] [PubMed] [Google Scholar]
- Velayudhan B. T., Govoni K. E., Hoagland T. A., and Zinn S. A.. 2007. Growth rate and changes of the somatotropic axis in beef cattle administered exogenous bovine somatotropin beginning at two hundred, two hundred fifty, and three hundred days of age. J. Anim. Sci. 85:2866–2872. [DOI] [PubMed] [Google Scholar]
- Weiss W. P., Conrad H. R., and St. Pierre N. R.. 1992. A theoretically-based model for predicting total digestible nutrient values of forages and concentrates. Anim. Feed Sci. Technol. 39:95–110. [Google Scholar]
- Yakar S., Liu J., Stannard B., Butler A., Accili D., Sauer B., and LeRoith D.. 1999. Normal growth and development in the absence of hepatic insulin-like growth factor. Proc. Natl. Acad. Sci. U.S.A. 96:7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]