Abstract
This experiment evaluated the impacts of estrus expression and intensity, estimated by physical activity during a timed-AI protocol, on reproductive performance of Bos indicus-influenced beef cows. A total of 290 lactating, primiparous, and multiparous nonpregnant Nelore × Angus cows received a 2 mg injection of estradiol benzoate and an intravaginal progesterone (P4) releasing device (CIDR) on d −11, a 12.5 mg injection of PGF2α on d −4, CIDR removal in addition to 0.6 mg injection of estradiol cypionate and 300 IU injection of eCG on d −2, and timed-AI on d 0. Cows were fitted with a pedometer behind their left shoulder on d −4. An estrus detection patch was attached to the tail-head of each cow on d −2. Pedometer results were recorded on d −2 and 0. Estrus expression was defined as removal of >50% of the rub-off coating from the patch on d 0. Net physical activity during estrus was calculated by subtracting total steps from d −4 to −2 (nonestrus basal activity) from total steps from d −2 to 0 (proestrus + estrus period) of each cow. Cows that did not express estrus were classified as NOESTR. Cows that expressed estrus were ranked by net physical activity; those above the median were classified as HIESTR and the remaining cows as LWESTR. Ovarian ultrasonography was performed on d 0 and 7. Blood was collected on d 0, 7, 20, and 30. Pregnancy status was verified by ultrasonography on d 30. Only data from cows responsive to the estrus synchronization protocol were utilized (NOESTR, n = 59; LWESTR, n = 100; HIESTR, n = 98). Diameter of dominant follicle on d 0, corpus luteum volume on d 7, and plasma P4 concentrations on d 7 were greater (P ≤ 0.05) in HIESTR vs. LWESTR and NOESTR and also greater (P ≤ 0.05) for LWESTR vs. NOESTR. Plasma P4 concentrations on d 0 were greater (P < 0.01) in NOESTR vs. HIESTR and LWESTR and similar (P = 0.93) between HIESTR and LWESTR. Whole blood mRNA expression of myxovirus resistance 2 on d 20 was greater (P ≤ 0.05) in HIESTR vs. LWESTR and NOESTR, and similar (P = 0.72) between LWESTR and NOESTR. Pregnancy rates were less (P ≤ 0.04) in NOESTR vs. HIESTR and LWESTR (52.4%, 68.9%, and 73.5%, SEM = 7.2), and similar (P = 0.57) between HIESTR and LWESTR. Hence, expression of estrus during a timed-AI protocol improved ovarian dynamics and pregnancy success, whereas estrus intensity modulated key biological markers associated with fertility but not pregnancy rates in B. indicus-influenced cows beef cows.
Keywords: beef cows, estrus expression, estrus intensity, physical activity, pregnancy
INTRODUCTION
Reproductive management is a critical component of cow–calf systems and directly contributes to the number of cattle available for harvest. With the continuing increase in worldwide beef demand, strategies to maximize the reproductive efficiency of beef females are warranted to ensure profitability of cattle producers (Lamb et al., 2008) and address the global demand for animal protein (FAO, 2009). One strategy is the adoption of timed-AI protocols, which directly contributed to increased efficiency in U.S. beef production over the last decades (Patterson et al., 2016). Yet, alternatives to maximize pregnancy success to timed-AI programs are warranted to further enhance reproductive efficiency of cow–calf systems and encourage its adoption by commercial producers (Lamb et al., 2010).
Expression of estrus near the time of AI is associated with improved ovarian function, resulting in enhanced fertility, embryo development, and pregnancy establishment in cattle (Davoodi et al., 2016; Pereira et al., 2016). Recent research also demonstrated that intensity of estrus expression, assessed by activation level of estrus detection patches, was positively associated with conceptus viability in beef cows (Pohler et al., 2016b). However, research is warranted to further understand fertility and pregnancy establishment parameters associated with estrus and to characterize the impacts of estrus intensity on reproductive function of beef females. One alternative for the latter is the use of pedometers to estimate cow physical activity (Schubach et al., 2017), which has been used as measurement for estrus intensity in dairy cattle (Silper et al., 2015). Therefore, we hypothesized that estrus expression and intensity will be positively associated with fertility variables and pregnancy rates in beef cows. To test this hypothesis, this experiment evaluated the impacts of estrus expression and intensity, assessed via physical activity during a timed-AI protocol, on reproductive responses and performance of Bos indicus-influenced beef females.
MATERIALS AND METHODS
This experiment was conducted from January to March 2017 at a commercial cow–calf operation located in Aruanã/GO, Brazil. The animals utilized herein were cared for in accordance with the practices outlined in the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (FASS, 2010).
Animals and Treatments
Two hundred and ninety lactating, nonpregnant, primiparous (n = 95), and multiparous (n = 195) Nelore × Angus cows (mean ± SEM; BW = 428 ± 4 kg; BCS = 4.72 ± 0.02 according to Wagner et al., 1988; days postpartum = 134 ± 1 d) were assigned to this experiment. For at least 60 d prior to and during the experimental period, cows were managed in three experimental groups of approximately 96 cows each according to the general management scheme of the operation. Experimental groups were maintained in individual Panicum maximum pastures averaging 35 ha each, with ad libitum access to water and a commercial mineral–vitamin mix (M. Cassab Tecnologia Animal, São Paulo, Brazil). The walking distance between pastures and the working facility ranged from 360 to 420 m, and pastures were at least 300 m apart from each other. To facilitate cattle management and sampling procedures, experimental groups started the experiment 5 d apart but followed the same experimental schedule (d −11 to 30).
Cows were enrolled in an estrus synchronization + fixed-time AI protocol (Meneghetti et al., 2009) from d −11 to 0. More specifically, cows received a 2 mg injection of estradiol benzoate and an intravaginal progesterone (P4) releasing device (CIDR) on d −11, a 12.5 mg injection of PGF2α on d −4, CIDR removal in addition to 0.6 mg injection of estradiol cypionate and 300 IU injection of eCG on d −2, and fixed-time AI on d 0. All cows were inseminated by the same technician, using semen from the same bull and batch. At the time of PGF2α on d −4, cows were fitted with a pedometer (HJ-321; Omron Healthcare, Inc., Bannockburn, IL) placed inside a polyester patch (Heat Watch II; Cow Chips, LLC, Manalapan, NJ) fixed behind their left shoulder to assess physical activity (Haley et al., 2005; Knight et al., 2015; Schubach et al., 2017). Pedometers had the capability to store daily data for seven consecutive days. Pedometer results were recorded concurrently with handling for estrus synchronization and timed-AI on d −2 and 0, respectively. Estrus detection patches (Estrotect; Rockway Inc., Spring Valley, WI) were applied to the tail-head of each cow during handling on d −2, and occurrence of estrus was recorded at timed-AI on d 0. Estrus was defined as removal of >50% of the rub-off coating on the estrus detection patch (Thomas et al., 2014), whereas no visual assessment of estrus behavior was conducted herein..
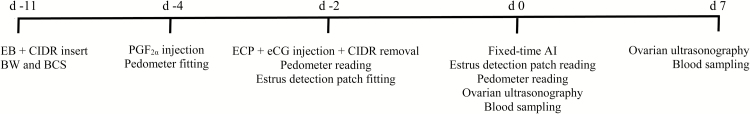
Total steps recorded from d −4 to −2 were considered the basal physical activity of each cow, as estrus expression was not expected during this period due to CIDR insert (Lamb et al., 2001). Total steps recorded from d −2 to 0 were considered physical activity associated with estrus expression, due to CIDR removal and hormonal treatments applied to cows on d −2 (Meneghetti et al., 2009). To account for individual variations in basal physical activity, d −4 to −2 total steps were subtracted from d −2 to 0 total steps to calculate net physical activity. Cows that did not express estrus from d −2 to 0, according to the estrus detection patch, were classified as NOESTR regardless of their net physical activity. Cows that expressed estrus were ranked by net physical activity; those above the median were classified as HIESTR and the remaining cows as LWESTR. Cows that were inserted with a pedometer that malfunctioned or it was lost were not included in this classification and removed from further analysis (n = 9). An outline of the experimental schedule is described in Figure 1.
Figure 1.
Outline of the experimental protocol assigned to 290 lactating, primiparous, and multiparous nonpregnant Nelore × Angus cows. Blood samples were also collected on d 20 and 30, and pregnancy status was verified by transrectal ultrasonography on d 30. EB = estradiol benzoate injection; CIDR = intravaginal progesterone-releasing device; ECP = estradiol cypionate injection.
Sampling
Cow BW and BCS (Wagner et al., 1988) were recorded on d −11. Blood samples were collected immediately before timed-AI (d 0) and on d 7 of the experiment from either the coccygeal vein or artery into blood collection tubes (Vacutainer, 10 mL; Becton Dickinson, Franklin Lakes, NJ). After collection, blood samples were placed immediately on ice, allowed to clot for 24 h at 4 °C, centrifuged at 3,000 × g at room temperature for 15 min for serum collection, and stored at −20 °C. Transrectal ultrasonography (7.5 MHz transducer; 500 V, Aloka, Wallingford, CT) was performed concurrently with blood sampling on d 0 and 7 to verify dominant follicle diameter (DFD; d 0) and determine corpus luteum (CL) presence (d 0) and volume (d 7). Corpus luteum volume was estimated using the formula for the volume of a sphere; volume = 4/3π × (D/2)3, where D is the maximum luteal diameter (Cooke et al., 2009). When the CL had a cavity, the cavity volume was also calculated as a sphere and subtracted from the CL volume.
Cows diagnosed without the presence of a CL on d 0, but with a CL greater than 0.38 cm3 in volume on d 7 were classified as responsive to the estrus synchronization protocol (Cooke et al., 2014; Cipriano et al., 2016). This criterion was based on the smallest diameter of a functional CL detected in B. indicus-influenced cows following induced ovulation, as reported by Figueiredo et al. (1997). Hence, only cows that responded to the estrus synchronization protocol (n = 260; synchronization rate = 89.6% [260/290 total cows]) and that were successfully classified according to estrus characteristics were maintained in the experiment (NOESTR, n = 59; LWESTR, n = 100; HIESTR, n = 98). Within cows classified as responsive to the estrus synchronization protocol, those without a dominant follicle on d 0 were considered as having ovulated prior to timed-AI.
On d 20, whole blood samples were collected from 11 cows randomly selected from each estrus characteristics (NOESTR, LWESTR, and HIESTR) within each experimental group into PAXgene tubes (BD Diagnostics, Sparks, MD) for whole blood RNA extraction and subsequent mRNA expression analysis of interferon-stimulated genes (ISG). On d 30, blood samples were collected from all cows and processed for serum as described for d 0 and 7, whereas cow pregnancy status was verified by detecting a viable conceptus via transrectal ultrasonography (7.5 MHz transducer; 500 V, Aloka).
Laboratory Analysis
Serum samples from d 0 and 7 were analyzed for P4 concentrations using a chemiluminescent enzyme immunoassay (Immulite 1000; Siemens Medical Solutions Diagnostics, Los Angeles, CA). Serum samples from d 0 were analyzed for estradiol-17β using an RIA as described by Kirby et al. (1997). Serum samples collected on d 30 from cows diagnosed as pregnant (HIESTR, n = 71; LWESTR, n = 69, NOESTR, n = 31) were analyzed for concentrations of bovine pregnancy-associated glycoproteins (bPAG) using a polyclonal antibody ELISA as described in Pohler et al. (2016a). All serum samples were analyzed for P4 and bPAGs within single assays, with an intra-assay CV of 2.0 and <10% and minimum detectable concentration of 0.05 ng/mL and 0.28 ng/mL, respectively. The intra- and inter-assay CV for the estradiol-17β procedure were 4.8% and 14.3% and minimum detectable concentration of 0.5 pg/mL.
Total RNA was extracted from whole blood samples collected on d 20 using the PAXgene Blood RNA Kit (Qiagen, Valencia, CA). Only samples from cows diagnosed as pregnant on d 30 were utilized for mRNA expression analysis of the ISGs interferon-stimulated gene 15, 20,50-oligoadenylate synthetase, and myxovirus resistance 2 (HIESTR, n = 26; LWESTR, n = 30, NOESTR, n = 20). Quantity and quality of isolated RNA were assessed via UV absorbance (NanoDrop Lite; Thermo Fisher Scientific, Wilmington, DE) at 260 nm and 260/280 nm ratio, respectively (Fleige and Pfaffl, 2006). All samples had a 260/280 nm ratio between 1.8 and 2.0, hence appropriate for cDNA synthesis (Fleige and Pfaffl, 2006). Extracted RNA (120 ng) was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit with random hexamers (Applied Biosystems, Foster City, CA). Real-time RT-PCR was completed using the Fast SYBR Green Master Mix (Applied Biosystems) and gene-specific primers for the ISGs (20 pM each; Table 1) with the StepOne Real-Time PCR system (Applied Biosystems), according to procedures described by Cooke et al. (2008). At the end of each RT-PCR, amplified products were subjected to a dissociation gradient (95 °C for 15 s, 60 °C for 30 s, and 95 °C for 15 s) to verify the amplification of a single product by denaturation at the anticipated temperature. Responses were quantified based on the threshold cycle (CT), which is the number of PCR cycles required for target amplification to reach a predetermined threshold. Responses from ISGs were quantified based on CT and normalized to the geometrical mean of CT values from β2-microglobulin and β-actin (Vandesompele et al., 2002). The CV for the geometrical mean of β2-microglobulin and β-actin CT values across all samples was 2.4%. Results are expressed as relative fold change (2−ΔΔCT) as described by Ocón-Grove et al. (2008).
Table 1.
Primer sequences, accession number, and reference for all gene transcripts analyzed by real-time RT-PCR
| Target gene | Primer sequence | Accession number | Source |
|---|---|---|---|
| 20,50-oligoadenylate synthetase | NM 001040606 | Fricke et al. (2016) | |
| Forward | ACCCTCTCCAGGAATCCAGT | ||
| Reverse | GATTCTGGTCCCAGGTCTGA | ||
| Interferon-stimulated gene 15 | NM_174366 | Fricke et al. (2016) | |
| Forward | GGTATGAGCTGAAGCAGTT | ||
| Reverse | ACCTCCCTGCTGTCAAGGT | ||
| Myxovirus resistance 2 | NM 173941 | Fricke et al. (2016) | |
| Forward | CTTCAGAGACGCCTCAGTCG | ||
| Reverse | TGAAGCAGCCAGGAATAGTG | ||
| β-Actin | AY141970 | Gifford et al. (2007) | |
| Forward | CTGGACTTCGAGCAGGAGAT | ||
| Reverse | GGATGTCGACGTCACACTTC | ||
| β2-microglobulin | NM_173893 | Silva et al. (2008) | |
| Forward | GGGCTGCTGTCGCTGTCT | ||
| Reverse | TCTTCTGGTGGGTGTCTTGAGT | ||
Statistical Analyses
Quantitative and binary data were analyzed, respectively, with the MIXED and GLIMMIX procedures of SAS (SAS Inst., Inc., Cary, NC) and Satterthwaite approximation to determine the denominator df for the tests of fixed effects. All data were analyzed using cow as the experimental unit. Model statements used for cow BCS, BW, days postpartum on d (11), as well as physical activity parameters contained the effects of estrus characteristics (NOESTR, LWESTR, and HIESTR), parity, and the interaction. All other model statements contained the effects of estrus characteristics (NOESTR, LWESTR, and HIESTR), parity, the interaction, and cow BCS (d −11) as independent covariate. All random statements contained the effects of cow (estrus characteristics × parity × experimental group) and experimental group. Given that walking distance between pastures and the working facility was similar across experimental groups, which contained cows from all estrus characteristics, the activity associated with cattle gathering and handling for experimental procedures was not discounted from net physical activity, but was statistically accounted for by the random effect of experimental group. Results are reported as least square means or covariately adjusted least square means to BCS.
The probability of cows to become pregnant to timed-AI was evaluated according to cow BW and BCS on d −11, DFD and serum estradiol-17β concentrations on d 0, serum P4 concentrations on d 0 and 7, as well as CL volume and CL volume:serum P4 ratio on d 7. The GLM procedure of SAS was initially used to determine if each individual measurement influenced pregnancy maintenance linearly, quadratically, or cubically. The LOGISTIC procedure was used to generate the regression model and determine the intercept and slope(s) values according to maximum likelihood estimates from each significant continuous order effect, and the probability of pregnancy was determined according to the following equation: Probability = (elogistic equation)/(1 + elogistic equation). Logistic curves were constructed according to the values detected for each variable.
For all analyses, significance was set at P ≤ 0.05 and tendencies were determined if P > 0.05 and ≤ 0.10. Results are reported according to estrus characteristics if no interaction containing this variable was significant, or according to highest-order interaction detected. Least square means were separated using PDIFF when the P-value for the main effect, such as estrus characteristics, was ≤0.10.
RESULTS AND DISCUSSION
The estrus characteristics × parity interaction was not significant (P ≥ 0.25) for any of the variables evaluated herein; hence, all results are reported across parities. Moreover, 77% of cows expressed estrus (198/257 of LWESTR and HIESTR/total cows classified as responsive to the estrus synchronization protocol) according to the estrus detection patch (Thomas et al., 2014). This outcome is greater than the results reported by a meta-analysis conducted by Richardson et al. (2016) and research with B. indicus cattle utilizing estradiol cypionate as ovulatory stimulus (Sá Filho et al., 2010). Different from these authors, this experiment evaluated only cows classified as responsive to the synchronization protocol, thus removing from analyses those that did not express estrus due to lack of estrus synchronization. If cows that failed to respond to the synchronization protocol were included into the experiment, estrus expression would be observed in 68% of cows (198/290 of LWESTR and HIESTR/total cows assigned to the experiment on d −11), which is coherent to values reported by Carvalho et al. (2016) in B. indicus cattle receiving estradiol cypionate to stimulate ovulation. The estrus synchronization protocol utilized herein (Meneghetti et al., 2009) is commonly used in B. indicus-influenced cattle in South America and other parts of the world, and likely contributed to the elevated incidence of estrus expression due to the use of exogenous eCG and estradiol (Vasconcelos et al., 2014). Yet, all cows received the exact same dosage of exogenous hormones for estrus synchronization. Hence, reproductive differences among NOESTR, LWESTR, and HIESTR cows should be attributed to physiology and endocrinology variances among these groups, similar to research investigating estrus behavior in beef (Carvalho et al., 2016; Davoodi et al., 2016; Pohler et al., 2016b) and dairy cows (Madureira et al., 2015b; Pereira et al., 2016; Silper et al., 2017) administered eCG and estradiol for estrus synchronization.
Estrus expression is primarily determined by circulating concentrations of estradiol, which triggers the hypothalamus to initiate estrus behavior (Vailes et al., 1992; Allrich, 1994). In turn, intensity of estrus expression has been associated, although weakly, with preovulatory concentrations of estradiol in lactating dairy cows (Madureira et al., 2015a), whereas estrus expression is considered a biomarker for estradiol concentrations in beef females (Perry et al., 2005; Larimore et al., 2015). Estrus intensity has been quantified in dairy cattle by physical activity using pedometers, with their activity during diestrus serving as baseline (Madureira et al., 2015a; Silper et al., 2015). In view of that, cows utilized in this experiment were classified by net physical activity during the proestrus + estrus period with pedometers (Schubach et al., 2017), which accounted for individual basal physical activity when cows were inserted with a CIDR (Lamb et al., 2001). Accordingly, net physical activity was greater (P < 0.01) in HIESTR and LWESTR vs. NOESTR cows (Table 2), corroborating with the increased physical activity triggered by estrus behavior (Kiddy, 1977). By design, net physical activity was also greater (P < 0.01) in HIESTR compared with LWESTR cows (Table 2). Lastly, total steps from d −4 to −2 were similar (P = 0.97) among estrus characteristic groups (Table 2), representing similar baseline physical activity in NOESTR, LWESTR, and HIESTR cows. Hence, differences in net physical activity between estrus characteristic groups should be mainly attributed to changes in physical activity during the proestrus + estrus period (total steps from d −2 to 0), which were greater (P ≤ 0.03) in HIESTR vs. LWESTR and NOESTR and in LWESTR vs. NOESTR cows (Table 2).
Table 2.
Physical activity, BW, BCS, and days postpartum in Nelore × Angus beef cows according to estrus expression and intensity1,2,3
| Item | NOESTR | LWESTR | HIESTR | SEM | P |
|---|---|---|---|---|---|
| Activity variables | |||||
| Total steps, d −4 to −2 | 10,946 | 10,926 | 11,030 | 541 | 0.97 |
| Total steps, d −2 to 0 | 13,737c | 15,248b | 21,504a | 593 | <0.01 |
| Net physical activity, steps | 2,779 | 4,273 | 10,475 | 375 | <0.01 |
| Cow variables | |||||
| Days postpartum (d −11), d | 136 | 135 | 137 | 2 | 0.75 |
| BW (d −11), kg | 412b | 426ab | 436a | 9 | 0.02 |
| BCS (d −11)2 | 4.56c | 4.71b | 4.81a | 0.06 | <0.01 |
1Cows were assigned to an estrus synchronization + timed-AI protocol (Meneghetti et al., 2009) from d −11 to 0. Cows were fitted with a pedometer (HJ-321; Omron Healthcare, Inc., Bannockburn, IL) behind their left shoulder on d −4 (Schubach et al., 2017). An estrus detection patch (Estrotect; Rockway Inc., Spring Valley, WI) was attached to the tail-head of each cow on d −2. Pedometer results were recorded on d −2 and 0. Estrus expression was defined as removal of >50% of the rub-off coating from the patch on d 0 (Thomas et al., 2014). Net physical activity during estrus was calculated by subtracting total steps from d −4 to −2 (nonestrus basal activity) from total steps from d −2 to 0 (expected estrus period) of each cow. Only data from cows responsive to the estrus synchronization protocol and with physical activity recorded on d −2 and 0 were utilized. Cows that did not express estrus were classified as NOESTR (n = 59). Cows that expressed estrus were ranked by net physical activity; those above the median were classified as HIESTR (n = 98) and the remaining cows as LWESTR (n = 100).
2According to Wagner et al. (1988).
3Within rows, values with different superscripts (a, b, c) differ (P ≤ 0.05).
No differences in days postpartum were detected (P = 0.75) among estrus characteristics groups on d −11 of the experiment (Table 2), which were adequate for optimal pregnancy rates of B. indicus-influenced cattle to timed-AI (Vasconcelos et al., 2014). However, BW on d −11 was greater (P < 0.01) in HIESTR vs. NOESTR and similar (P ≥ 0.12) between these groups compared with LWESTR (Table 2). Cow BCS on d −11 was greater (P < 0.01) in HIESTR vs. LWESTR and NOESTR cows and in LWESTR vs. NOESTR cows (Table 2). Similar to these findings, others have also documented a positive association between BW and BCS with estrus expression and intensity (Madureira et al., 2015a; Richardson et al., 2016). Moreover, Aungier et al. (2015) reported that a 0.25 increase in BCS was positively correlated with an increase in physical activity prior to ovulation in dairy cows. The importance of nutrient status for adequate reproductive function of beef cows, including fertility and pregnancy maintenance, is well known (Hess et al., 2005). In contrast, the specific mechanism by which nutritional status modulates estrogen-dependent estrus behavior, particularly in cows that successfully responded to an estrus synchronization protocol as herein, is still unclear. To account for differences in nutritional status among NOESTR, LWESTR, and HIESTR cows during the timed-AI protocol, all reproductive variables evaluated herein were covariately adjusted to cow BCS on d −11. Yet, research is warranted to determine the mechanisms underlying a cause–effect relationship between cow nutritional status and estrus expression, which could result in management strategies to increase the incidence of females displaying high-intensity estrus.
A greater (P < 0.01) percentage of HIESTR cows had no dominant follicle on d 0, hence were considered as having ovulated prior to timed-AI, compared with LWESTR and NOESTR cows, which was similar (P = 0.91) between LWESTR and NOESTR cows (Table 3). This outcome suggests an anticipated ovulatory LH surge due to increased preovulatory estradiol concentrations, or perhaps reduced estradiol threshold for estrus expression and ovulation, in HIESTR compared with LWESTR and NOESTR cows (Chenault et al., 1975). However, no differences in serum estradiol-17β concentrations on d 0 were detected (P = 0.73) among estrus characteristics groups (Table 3), which does not corroborate with the rationale of estrus expression and intensity being driven by circulating estradiol concentration (Perry et al., 2005) but can be explained by the sampling schedule and timed-AI protocol adopted herein. Not only LWESTR and HIESTR cows likely experienced an estradiol surge that elicited estrus expression prior to sampling on d 0 (Larimore et al., 2015), an individual collection only provided a snapshot of circulating estradiol at the time of AI. Moreover, the estradiol cypionate administered on d −2 may have cleaved and cross-reacted with the estradiol-17β RIA utilized herein (Kirby et al., 1997). Hence, additional research is warranted to characterize serum estradiol-17β concentrations and further assess time of ovulation in beef cows according to estrus intensity. These should include collection of serial blood samples and follicle assessment beginning at CIDR removal in timed-AI protocols, as well as protocols based on spontaneous ovulation or that use GnRH as ovulatory stimulus.
Table 3.
Cow and physical activity variables in Nelore × Angus beef cows according to estrus expression and intensity1,2,3
| Item | NOESTR | LWESTR | HIESTR | SEM | P |
|---|---|---|---|---|---|
| Ovarian variables | |||||
| Cows with no dominant follicle at timed-AI (d 0), % | 2.29b | 2.77b | 17.22a | 3.09 | <0.01 |
| Dominant follicle at timed-AI (d 0), mm | 12.6c | 13.3b | 14.0a | 0.3 | <0.01 |
| Corpus luteum volume (d 7), cm3 | 3.72b | 4.89a | 5.42a | 0.47 | <0.01 |
| Physiological variables | |||||
| Plasma progesterone, ng/mL | |||||
| d 0 | 0.48b | 0.26a | 0.30a | 0.06 | <0.01 |
| d 7 | 3.04a | 4.03b | 4.74c | 0.19 | <0.01 |
| P4 to CL ratio (d 7) | 1.07 | 1.04 | 1.02 | 0.10 | 0.91 |
| Estradiol-17β (d 0), pg/mL | 7.64 | 7.46 | 7.29 | 0.45 | 0.73 |
1Cows were assigned to an estrus synchronization + timed-AI protocol (Meneghetti et al., 2009) from d −11 to 0. Cows were fitted with a pedometer (HJ-321; Omron Healthcare, Inc., Bannockburn, IL) behind their left shoulder on d −4 (Schubach et al., 2017). An estrus detection patch (Estrotect; Rockway Inc., Spring Valley, WI) was attached to the tail-head of each cow on d −2. Pedometer results were recorded on d −2 and 0. Estrus expression was defined as removal of >50% of the rub-off coating from the patch on d 0 (Thomas et al., 2014). Net physical activity during estrus was calculated by subtracting total steps from d −4 to −2 (nonestrus basal activity) from total steps from d −2 to 0 (expected estrus period) of each cow. Only data from cows responsive to the estrus synchronization protocol and with physical activity recorded on d −2 and 0 were utilized. Cows that did not express estrus were classified as NOESTR (n = 59). Cows that expressed estrus were ranked by net physical activity; those above the median were classified as HIESTR (n = 98) and the remaining cows as LWESTR (n = 100).
2Transrectal ultrasonography (7.5 MHz transducer; 500 V, Aloka, Wallingford, CT) was performed concurrently with blood sampling on d 0 and 7. Corpus luteum volume was estimated using the formula for the volume of a sphere (Cooke et al., 2009).
3Within rows, values with different superscripts (a, b, c) differ (P ≤ 0.05). All values reported are covariately adjusted to cow BCS (Wagner et al., 1988) recorded on d −11.
On d 0, DFD was greater (P ≤ 0.05) in HIESTR vs. LWESTR and NOESTR cows and in LWESTR vs. NOESTR cows (Table 3), whereas serum P4 concentrations were greater (P < 0.01) in NOESTR vs. HIESTR and LWESTR cows and similar (P = 0.38) between HIESTR and LWESTR cows (Table 3). Supporting our findings, Carvalho et al. (2016) also reported that beef cows that expressed estrus had increased DFD and decreased serum P4 concentrations at timed-AI compared with cohorts that did not express estrus. Similar outcomes were reported in dairy cattle by Pereira et al. (2016). To our knowledge, this is the first research to report a positive relationship among estrus intensity and follicle size in beef cows at timed-AI, which can be attributed to increased circulating estradiol concentrations as DFD increases (Perry et al., 2007; Jinks et al., 2013) although such relationship was not observed herein as discussed in the previous paragraph. Circulating P4 during proestrus and estrus suppresses LH pulsatility, dominant follicular growth, estradiol synthesis, and estrus behavior (Allrich, 1994; Kinder et al., 1996); hence, reduced serum P4 in HIESTR and LWESTR vs. NOESTR corroborates with estrus expression and DFD results. Despite the lack of a visible CL on d 0 in the cows utilized herein, serum P4 can be originated from residual luteal structures due to incomplete luteolysis (Pereira et al., 2013), as well as the adrenal gland if cows are exposed to stressful conditions (Cooke and Arthington, 2008). Conversely, differences in DFD between LWESTR and HIESTR cows were not complemented by equivalent changes in P4 concentration, suggesting that serum P4 levels in HIESTR and LWESTR cows on d 0 were at negligible levels that did not hinder follicle growth.
Corpus luteum volume on d 7 was greater (P < 0.01) in HIESTR and LWESTR vs. NOESTR cows and similar (P = 0.16) between HIESTR and LWESTR cows (Table 3). Serum P4 concentrations on d 7 were also greater (P < 0.01) in HIESTR vs. LWESTR and NOESTR cows and in LWESTR vs. NOESTR cows (Table 3). These outcomes can be directly associated with corresponding DFD results on d 0, as ovulation of larger follicles yields larger CLs and greater P4 synthesis (Vasconcelos et al., 2001). Madureira et al. (2015b) also reported that circulating P4 concentrations 10 d after AI was greater in cows displaying high-intensity estrus, although these authors did not report an equivalent effect on DFD at AI. Preovulatory estradiol also modulates proliferation of follicular granulosa cells, which after ovulation differentiate into luteal cells responsible for 80% of progesterone secretion by the CL (Donaldson and Hansel, 1965; Murdoch and Van Kirk, 1998). However, no differences in CL volume to serum P4 ratio on d 7 were detected (P = 0.91) among estrus characteristics groups (Table 3), indicating that serum P4 concentration on d 7 increased according to estrus expression and intensity due to increasing CL volume rather than CL efficiency in P4 synthesis (Cipriano et al., 2016).
No differences in mRNA expression of interferon-stimulated gene 15 and 20,50-oligoadenylate synthetase in whole blood cells were detected (P ≥ 0.67) among estrus characteristics groups (Table 4). Expression of myxovirus resistance 2 mRNA, however, was greater (P ≤ 0.05) in HIESTR vs. LWESTR and NOESTR cows and similar (P = 0.72) between LWESTR and NOESTR cows (Table 4; main estrus characteristics effect, P = 0.08). Expression of ISGs in whole blood can be used to assess conceptus development from d 15 to 22 of gestation, as well as pregnancy diagnosis on d 18 of gestation (Fricke et al., 2016). Interferon-tau production by the conceptus upregulates mRNA expression of myxovirus resistance 2 in body tissues (Forde et al., 2011), including whole blood cells (Gifford et al., 2007; Stevenson et al., 2007). Moreover, expression of estrus near the time of AI have been associated with enhanced uterine environment for embryo development (Larimore et al., 2015; Davoodi et al., 2016), whereas estradiol appears to modulate secretion of oviductal glycoproteins (Buhi, 2002) and endometrial nutrients that contribute to early conceptus growth (Geisert et al., 1992; Gray et al., 2001). Larimore et al. (2015) reported that heifers that exhibited estrus yielded embryos on d 6 of gestation that were more advanced in stage and had improved quality when compared with embryos recovered from heifers not exhibiting estrus. Davoodi et al. (2016) reported that estrus expression favored expression of endometrial genes that suppress the local maternal immune system and modulate adhesion between endometrium epithelial cells and conceptus. These same authors reported that cows that displayed estrus yielded longer conceptuses on d 19 of gestation, which can be associated with better chances of pregnancy establishment (Davoodi et al., 2016). Data from the present experiment partially corroborates with these findings, as myxovirus resistance 2 mRNA results suggest enhanced conceptus development in cows that expressed high-intensity estrus, without equivalent outcomes on interferon-stimulated gene 15 and 20,50-oligoadenylate synthetase. Nonetheless, research is warranted to further elucidate the relationship among estrus expression, estrus intensity, and early pregnancy development.
Table 4.
Expression of genes associated with pregnancy establishment in whole blood, serum concentrations of bovine pregnancy-associated glycoproteins (bPAGs), and pregnancy rates of Nelore × Angus beef cows according to estrus expression and intensity1,2,3
| Item | NOESTR | LWESTR | HIESTR | SEM | P |
|---|---|---|---|---|---|
| mRNA expression, fold effect | |||||
| Interferon-stimulated gene 15 | 4.54 | 4.27 | 3.94 | 0.83 | 0.67 |
| Myxovirus resistance 2 | 4.75b | 5.30b | 7.89a | 1.12 | 0.08 |
| 20,50-oligoadenylate synthetase | 4.99 | 5.35 | 5.10 | 1.00 | 0.91 |
| Serum bPAGS, ng/mL | 11.5 | 12.3 | 13.2 | 1.1 | 0.46 |
| Pregnancy rates,4 % | 52.4b (31/59) | 68.9a (69/100) | 73.5a (71/98) | 7.2 | 0.03 |
1Cows were assigned to an estrus synchronization + timed-AI protocol (Meneghetti et al., 2009) from d −11 to 0. Cows were fitted with a pedometer (HJ-321; Omron Healthcare, Inc., Bannockburn, IL) behind their left shoulder on d −4 (Schubach et al., 2017). An estrus detection patch (Estrotect; Rockway Inc., Spring Valley, WI) was attached to the tail-head of each cow on d −2. Pedometer results were recorded on d −2 and 0. Estrus expression was defined as removal of >50% of the rub-off coating from the patch on d 0 (Thomas et al., 2014). Net physical activity during estrus was calculated by subtracting total steps from d −4 to −2 (nonestrus basal activity) from total steps from d −2 to 0 (expected estrus period) of each cow. Only data from cows responsive to the estrus synchronization protocol and with physical activity recorded on d −2 and 0 were utilized. Cows that did not express estrus were classified as NOESTR (n = 59). Cows that expressed estrus were ranked by net physical activity; those above the median were classified as HIESTR (n = 98) and the remaining cows as LWESTR (n = 100).
2On d 20, blood samples were collected from 33 cows randomly selected from each estrus characteristics (NOESTR, LWESTR, HIESTR) into PAXgene tubes (BD Diagnostics, Sparks, MD) for whole blood RNA extraction. Values are expressed as relative fold change compared to threshold cycle of reference genes analyzed within the same sample (Ocón-Grove et al., 2008). On d 30, blood samples were collected for the analysis of serum bPAGs and pregnancy status was verified by detecting a viable conceptus via transrectal ultrasonography (5.0 MHz transducer; 500 V, Aloka, Wallingford, CT). Only data from cows diagnosed as pregnant were analyzed for whole blood mRNA expression and serum bPAGs concentrations.
3Within rows, values with different superscripts (a, b, c) differ (P ≤ 0.05). All values reported are covariately adjusted to cow BCS (Wagner et al., 1988) recorded on d −11.
4Values reported within parenthesis correspond to number of pregnant cows/total cows responsive to the estrus synchronization protocol and classified based on estrus characteristics.
No differences in serum bPAGs concentrations on d 30 were detected (P = 0.46) among HIESTR, LWESTR, and NOESTR cows (Table 4), whereas bPAG is an accurate predictor of late embryonic mortality in cattle (Pohler et al., 2016b). These latter authors assessed estrus intensity prior to timed-AI according to the level of activation of estrus detection patches during the same timed-AI protocol utilized herein. As intensity of estrus expression increased, circulating bPAG concentrations on 28 d after AI also increased, suggesting decreased likelihood of late embryonic mortality (Pohler et al., 2016b). In the present experiment, however, estrus expression and intensity failed to impact serum concentrations of bPAGs and perhaps late embryonic survival as in Pohler et al. (2016b), although pregnancy diagnosis was only performed on d 30. Nonetheless, Pohler et al. (2013) reported that serum bPAG concentrations were not influenced by ovulatory follicle size or serum P4 concentration on d 7 of gestation in beef cows, which does not support a direct relationship among bPAGs with estrus expression and intensity as reported herein.
Pregnancy status to timed-AI on d 30 was greater (P ≤ 0.04) in HIESTR and LWESTR vs. NOESTR cows (Table 4), which corroborate with a multitude of research efforts documenting that beef females expressing estrus between CIDR removal and timed-AI have greater pregnancy success compared with cohorts that do not express estrus (Perry et al., 2005; Whittier et al., 2013; Thomas et al., 2014). In fact, a meta-analysis conducted by Richardson et al. (2016) reported a 27% overall increase in pregnancies per AI in beef females detected in estrus. The positive effects of estrus expression on pregnancy success in timed-AI protocols are mainly attributed to increased circulating estradiol prior to ovulation, which coordinates a series of physiological events that regulate fertility and pregnancy establishment (Perry and Perry, 2008a, 2008b; Pohler et al., 2012). Contrary to our hypothesis, however, pregnancy rates to timed-AI were similar (P = 0.56) between HIESTR and LWESTR cows (Table 4). In dairy cattle, a positive association among estrus intensity and pregnancy success to AI has been reported, representing a 35% improvement in fertility (Madureira et al., 2015a, 2015b). The exact reasons for these outcomes are yet to be clarified, but may be associated with increased preovulatory concentrations of circulating estradiol (Perry et al., 2005), lower rates of ovulation failure (Silper et al., 2017), and a more favorable P4 profile around the time of AI (Madureira et al., 2015b). In the present experiment, one could attribute similar pregnancy rates between HIESTR and LWESTR cows to the elevated incidence of HIESTR cows with no dominant follicle on d 0 and considered having ovulated prior to timed-AI. If these cows are removed from the analysis, pregnancy rates to AI remained similar (P = 0.42) between HIESTR and LWESTR cows (75.0% vs. 68.9% of pregnant cows/total cows, respectively; SEM = 5.7). Alternatively, pregnancy rates to timed-AI were already elevated in LWESTR cows based on research efforts using similar cattle and timed-AI protocol as herein (Vasconcelos et al., 2017), which could have limited the fertility benefits of high-intensity estrus. The 6% to 8% improvement in pregnancy rate to timed-AI in HIESTR cows could be considered biologically relevant, but would require at least 400 cows/estrous characteristic group (G*power 3 software; Faul et al., 2007) to yield a statistical difference (P ≤ 0.05) compared with LWESTR cows.
Across estrus characteristic groups, the probability of cows becoming pregnant to timed-AI decreased as serum P4 concentrations on d 0 increased (linear effect, P < 0.01; Figure 2), and was affected quadratically P < 0.01; Figure 2) by DFD on d 0 and serum P4 concentrations on d 7. No other variables from the present experiment affected the probability of pregnancy to timed-AI (P ≥ 0.15; data not shown) including BW and BCS on d −11, corroborating that none of the reproductive differences between estrous characteristics groups should be associated with BW and BCS differences on d −11. Accordingly, others have reported the importance of low progesterone concentrations on d 0 for pregnancy success (Pereira et al., 2013; Carvalho et al., 2016) due to the deleterious effects of elevated P4 during the estrus period on female fertility (Kinder et al., 1996). A quadratic relationship among DFD on d 0 and serum P4 concentrations on d 7 with pregnancy success has also been documented in cattle (Perry et al., 2005; Diskin et al., 2006; McNeill et al., 2006), denoting an optimal range of these variables to maximize pregnancy to timed-AI. Moreover, values of DFD and serum P4 on d 7 that yielded the maximum probability of pregnancy to timed-AI were, respectively, 14.3 mm and 4.5 ng/mL. Caution should be adopted when interpreting these results, which can vary according to the population of cows evaluated and laboratory procedures for serum P4 analysis.
Figure 2.
Probability of pregnancy to timed-AI (d 0) in Nelore × Angus beef cows (n = 257) according to the diameter of dominant follicle on d 0 (A) and serum P4 concentrations on d 0 (B) and d 7 (C). Pregnancy status was verified 30 d after timed-AI via transrectal ultrasonography (5.0 MHz transducer; 500 V, Aloka, Wallingford, CT).
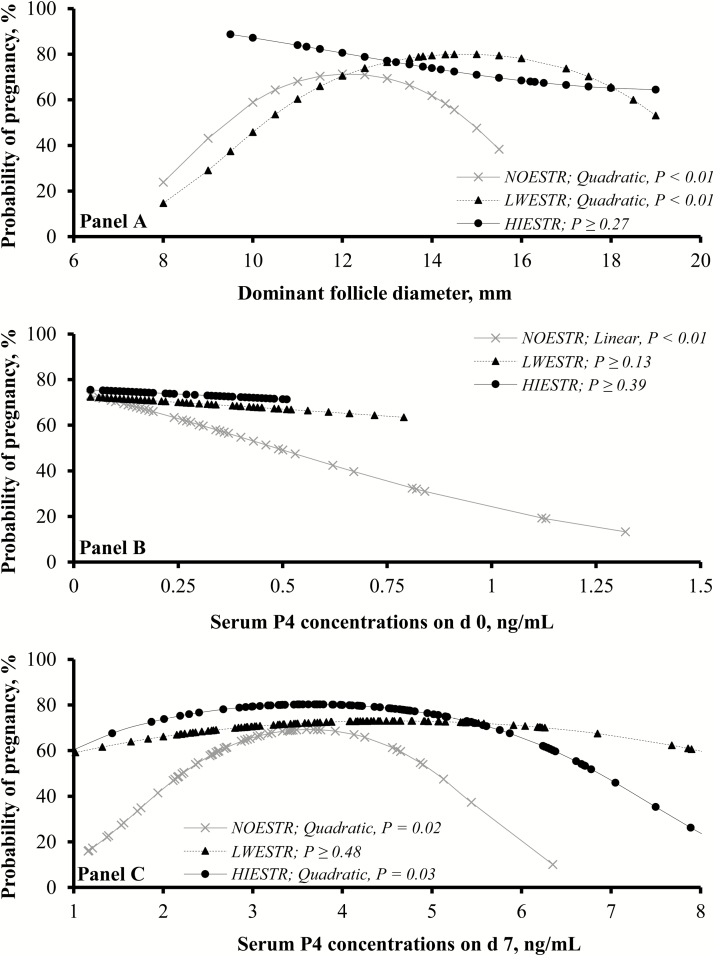
It should be noted, however, that the probability of cows becoming pregnant to timed-AI according to DFD and serum P4 concentrations on d 0 and 7 differed among estrus characteristic groups (Figure 3). More specifically, DFD affected the probability of pregnancy in LWESTR and NOESTR (quadratically; P < 0.01) but not in HIESTR cows (P ≥ 0.27), suggesting that DFD on d 0 does not impact pregnancy risk when cows express high-intensity estrus. Serum P4 concentrations on d 7 affected probability of pregnancy to timed-AI in HIESTR and NOESTR (quadratically; P ≤ 0.02), but not in LWESTR cows (P ≥ 0.48). This outcome should be associated with the majority of LWESTR cows having serum P4 concentrations near the population average; hence, not enough cows were in both extremes of the curve as noted for HIESTR and NOESTR cows (Figure 3). Lastly, serum P4 concentrations on d 0 affected probability of pregnancy to timed-AI in NOESTR (linear; P < 0.01), but not in LWESTR and HIESTR cows (P ≥ 0.13). Again, a limited number of HIESTR and LWESTR cows had elevated serum P4 concentrations on d 0, corroborating with estrus characteristics effects on this variable (Table 3). Still, it seems that when cows exhibit estrus during a timed-AI protocol, the detrimental effects of elevated serum P4 during proestrus and estrus on pregnancy success are mostly eliminated (Wiltbank et al., 2012; Pereira et al., 2013). Collectively, these results propose that expression of estrus during a timed-AI protocol, particularly high-intensity estrus behavior, overcomes the impacts of DFD and serum P4 on pregnancy success in beef cows.
Figure 3.
Probability of pregnancy to timed-AI (d 0) in Nelore × Angus beef cows according to the diameter of dominant follicle on d 0 (A) and serum progesterone (P4) on d 0 (B) and d 7 (C). Cows were fitted with a pedometer behind their left shoulder on d −4. An estrus detection patch was attached to the tail-head of each cow on d −2. Estrus expression was defined as removal of >50% of the rub-off coating from the patch on d 0. Net physical activity was calculated by subtracting total steps from d −4 to −2 from total steps from d −2 to 0 of each cow. Cows that did not express estrus were classified as NOESTR (n = 59). Cows that expressed estrus were ranked by net physical activity; those above the median were classified as HIESTR (n = 98) and the remaining as LWESTR (n = 100). All cows were assigned and effectively responded to an estrus synchronization protocol (Meneghetti et al., 2009; d −11 to 0). Pregnancy status was verified 30 d after timed-AI via transrectal ultrasonography (5.0 MHz transducer; 500 V, Aloka, Wallingford, CT).
In summary, physical activity during the proestrus + estrus period, DFD and serum P4 concentrations at timed-AI, CL volume and serum P4 concentration 7 d after timed-AI, and pregnancy rates to timed-AI were greater in cows that expressed estrus during a timed-AI protocol (LWESTR and HIESTR) compared to cohorts that did not express estrus (NOESTR). In turn, physical activity during the proestrus + estrus period, DFD on d 0, and CL volume and serum P4 concentrations on d 7 were further increased in cows that expressed high-intensity estrus (HIESTR). Whole blood mRNA expression of myxovirus resistance 2 mRNA on d 20 of gestation, an indicator of conceptus development and interferon-tau production (Gifford et al., 2007; Stevenson et al., 2007; Forde et al., 2011), was also greater in HIESTR cows compared with the other estrous characteristics groups. Pregnancy rates to timed-AI were not impacted by estrus intensity, although HIESTR had improved indicators of fertility such as DFD on d 0 and serum P4 concentration on d 7 compared with LWESTR cows (Perry et al., 2005; McNeill et al., 2006). Cow BCS on d −11 was positively associated with estrus expression and intensity; hence, it was included as independent covariate into all reproductive analyses and did not impact probability of pregnancy to timed-AI. It should be noted that the estrus synchronization protocol utilized herein likely increased the incidence of pharmacologically induced estrus compared with protocols based on spontaneous ovulation or using GnRH as ovulatory stimulus (Vasconcelos et al., 2014), whereas the impacts of estrus expression and intensity should also be evaluated in beef cows assigned to these last two ovulation strategies. Collectively, expression of estrus during a timed-AI protocol improved reproductive function and pregnancy success, whereas estrus intensity modulated key biological markers associated with fertility but not pregnancy rates in B. indicus-influenced cows beef cows.
Footnotes
Financial support for this research was provided by Fundação de Amparo à Pesquisa do Estado de São Paulo (Brazil; grants nos 2016/18216-8 and 2016/18460-6).
LITERATURE CITED
- Allrich R. D. 1994. Endocrine and neural control of estrus in dairy cows. J. Dairy Sci. 77:2738–2744. [DOI] [PubMed] [Google Scholar]
- Aungier S. P. M., Roche J. F., Duffy P., Scully S., and Crowe M. A.. 2015. The relationship between activity clusters detected by an automatic activity monitor and endocrine changes during the periestrous period in lactating dairy cows. J. Dairy Sci. 98:1666–1684. [DOI] [PubMed] [Google Scholar]
- Buhi W. C. 2002. Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction 123:355–362. [DOI] [PubMed] [Google Scholar]
- Carvalho E. R., Martins T., Lamb G. C., and Vasconcelos J. L. M.. 2016. Ovulation time in suckled beef cows is anticipated by use of low doses of progesterone and temporary calf removal on fixed timed AI protocol. Theriogenology 86:2238–2243. [DOI] [PubMed] [Google Scholar]
- Chenault J. R., Thatcher W. W., Kalra P. S., Abrams R. M., and Wilcox C. J.. 1975. Transitory changes in plasma progestins, estradiol, and luteinizing hormone approaching ovulation in the bovine. J. Dairy Sci. 58:709–717. [DOI] [PubMed] [Google Scholar]
- Cipriano R. S., Cooke R. F., Rodrigues A. D., Silva L. G. T., Bohnert D. W., Marques R. S., Vasconcelos J. L. M., Pires A. V., and Cerri R. L. A.. 2016. Post-AI supplementation with Ca salts of soybean oil influences pregnancy establishment factors in Bos indicus beef cows. J. Anim. Sci. 94:4892–4902. [DOI] [PubMed] [Google Scholar]
- Cooke R. F., and Arthington J. D.. 2008. Plasma progesterone concentrations determined by commercial radioimmunoassay kit as puberty criteria for Brahman-crossbred heifers. Livest. Sci. 123:101–105. [Google Scholar]
- Cooke R. F., Arthington J. D., Araujo D. B., and Lamb G. C.. 2009. Effects of acclimation to human interaction on performance, temperament, physiological, and pregnancy rates of Brahman-crossbred cows. J. Anim. Sci. 87:4125–4132. [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Arthington J. D., Araujo D. B., Lamb G. C., and Ealy A. D.. 2008. Effects of supplementation frequency on performance, reproductive, and metabolic responses of Brahman-crossbred females. J. Anim. Sci. 86:2296–2309. [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Cappellozza B. I., Guarnieri Filho T. A., Bohnert D. W., Depner C. M., Lytle K. A., Jump D. B., Cerri R. L. A., and Vasconcelos J. L. M.. 2014. Effects of calcium salts of soybean oil on factors that influence pregnancy establishment in Bos indicus beef cows. J. Anim. Sci. 92:2239–2250. [DOI] [PubMed] [Google Scholar]
- Davoodi S., Cooke R. F., Fernandes A. C. C., Cappellozza B. I., Vasconcelos J. L. M., and Cerri R. L. A.. 2016. Expression of estrus modifies the gene expression profile in reproductive tissues on day 19 of gestation in beef cows. Theriogenology 85:645–655. [DOI] [PubMed] [Google Scholar]
- Diskin M. G., Murphy J. J., and Sreenan J. M.. 2006. Embryo survival in dairy cows managed under pastoral conditions. Anim. Reprod. Sci. 96:297–311. [DOI] [PubMed] [Google Scholar]
- Donaldson L., and Hansel W.. 1965. Histological study of bovine corpora lutea. J. Dairy Sci. 48:905–909. [DOI] [PubMed] [Google Scholar]
- FASS.. 2010. Guide for the care and use of agricultural animals in agricultural research and reaching. 3rd ed Savoy (IL): Federation of Animal Science Societies. [Google Scholar]
- Faul F., Erdfelder E., Lang A. G., and Buchner A.. 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39:175–191. [DOI] [PubMed] [Google Scholar]
- Figueiredo R. A., Barros C. M., Pinheiro O. L., and Soler J. M. P.. 1997. Ovarian follicular dynamics in Nelore breed (Bos indicus) cattle. Theriogenology 47:1489–1505. [DOI] [PubMed] [Google Scholar]
- Fleige S., and Pfaffl M. W.. 2006. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Aspects Med. 27:126–139. [DOI] [PubMed] [Google Scholar]
- Food and Agricultural Organization (FAO).. 2009. How to feed the world in 2050. Proc. Expert Meeting on How to Feed the World in 2050. Rome (Italy): FAO Headquarters. [Google Scholar]
- Forde N., Carter F., Spencer T. E., Bazer F. W., Sandra O., Mansouri-Attia N., Okumu L. A., McGettingan P. A., Mehta J. P., McBride R.,. et al. 2011. Conceptusinduced changes in endometrial transcriptome: how soon does the cow know she is pregnant?Biol. Reprod. 85:144–156. [DOI] [PubMed] [Google Scholar]
- Fricke P. M., Carvalho P. D., Lucy M. C., Curran F., Herlihy M. M., Waters S. M., Larkin J. A., Crowe M. A., and Butler S. T.. 2016. Effect of manipulating progesterone before timed artificial insemination on reproductive and endocrine parameters in seasonal-calving, pasture-based Holstein-Friesian cows. J. Dairy. Sci. 99:6780–6792. [DOI] [PubMed] [Google Scholar]
- Geisert R. D., Morgan G. L., Short E. C. Jr., and Zavy M. T.. 1992. Endocrine events associated with endometrial function and conceptus development in cattle. Reprod. Fertil. Dev. 4:301–305. [DOI] [PubMed] [Google Scholar]
- Gifford C. A., Racicot K., Clark D. S., Austin K. J., Hansen T. R., Lucy M. C., Davies C. J., and Ott T. L.. 2007. Regulation of interferon-stimulated genes in peripheral blood leukocytes in pregnant and bred, nonpregnant dairy cows. J. Dairy Sci. 90:274–280. [DOI] [PubMed] [Google Scholar]
- Gray C. A., Taylor K. M., Ramsey W. S., Hill J. R., Bazer F. W., Bartol F. F., and Spencer T. E.. 2001. Endometrial glands are required for preimplantation conceptus elongation and survival. Biol. Reprod. 64:1608–1613. [DOI] [PubMed] [Google Scholar]
- Haley D. B., Bailey D. W., and Stookey J. M.. 2005. The effects of weaning beef calves in two stages on their behavior and growth rate. J. Anim. Sci. 83:2205–2214. [DOI] [PubMed] [Google Scholar]
- Hess B. W., Lake S. L., Scholljegerdes E. J., Weston T. R., Nayigihugu V., Molle J. D. C., and Moss G. E.. 2005. Nutritional controls of beef cow reproduction. J. Anim. Sci. 83(E. Suppl):E90–E106. [Google Scholar]
- Jinks E. M., Smith M. F., Atkins J. A., Pohler K. G., Perry G. A., MacNeil M. D., Roberts A. J., Waterman R. C., Alexander L. J., and Geary T. W.. 2013. Preovulatory estradiol and the establishment and maintenance of pregnancy in suckled beef cows. J. Anim. Sci. 91:1176–1185. [DOI] [PubMed] [Google Scholar]
- Kiddy C. A. 1977. Variation in physical activity as an indication of estrus in dairy cows. J. Dairy Sci. 60:235–243. [DOI] [PubMed] [Google Scholar]
- Kinder J. E., Kojima F. N., Bergfeld E. G. M., Wehrman M. E., and Fike K. E.. 1996. Progestin and estrogen regulation of pulsatile LH release and development of persistent ovarian follicles in cattle. J. Anim. Sci. 74:1424–1440. [DOI] [PubMed] [Google Scholar]
- Kirby C. J., Smith M. F., Keisler D. H., and Lucy M. C.. 1997. Follicular function in lactating dairy cows treated with sustained-release bovine somatotropin. J. Dairy Sci. 80:273–285. [DOI] [PubMed] [Google Scholar]
- Knight C. W., Bailey D. W., Faulkner D., and Schafer D. W.. 2015. Intake and grazing activity of mature range cows on Arizona rangelands. Proc. West. Sec. Am. Soc. Anim. Sci. 66:222–224. [Google Scholar]
- Lamb G. C., Dahlen C. R., Lason J. E., Marquezini G., and Stevenson J. S.. 2010. Control of the estrous cycle to improve fertility for fixed-time artificial insemination in beef cattle: a review. J. Anim. Sci. 88(E-Suppl):E181–E192. [DOI] [PubMed] [Google Scholar]
- Lamb G. C., Dahlen C. R., and Maddox M.. 2008. What is the economic impact of infertility in beef cattle? Available from http://edis.ifas.ufl.edu/an208 [accessed September 2, 2017].
- Lamb G. C., Stevenson J. S., Kesler D. J., Garverick H. A., Brown D. R., and Salfen B. E.. 2001. Inclusion of an intravaginal progesterone insert plus GnRH and prostaglandin F2 alpha for ovulation control in postpartum suckled beef cows. J. Anim. Sci. 79:2253–2259. [DOI] [PubMed] [Google Scholar]
- Larimore E. L., Amundson O. L., Bird S. L., Funnell B. J., Kruse S. G., Bridges G. A., and Perry G. A.. 2015. Influence of estrus at fixed-time artificial insemination on early embryonic development in beef cattle. J. Anim. Sci. 93:2806–2812. [DOI] [PubMed] [Google Scholar]
- Madureira A. M. L., Silper B. F., Burnett T. A., Polsky L. B., Cruppe L. H., Vasconcelos J. L. M., and Cerri R. L. A.. 2015a. Factors affecting expression of estrus measured by activity monitors and conception risk of lactating dairy cows. J. Dairy Sci. 98:7003–7014. [DOI] [PubMed] [Google Scholar]
- Madureira A. M. L., Silper B. F., Burnett T. A., Polsky L. B., Drago Filho E. L., Soriano S., Sica A. F., Vasconcelos J. L. M., and Cerri R. L. A.. 2015b. Effects of expression of estrus measured by activity monitors on ovarian dynamics and conception risk in Holstein cows. J. Dairy Sci. 98(Suppl. 1):875. [DOI] [PubMed] [Google Scholar]
- McNeill R. E., Diskin M. G., Sreenan J. M., and Morris D. G.. 2006. Associations between milk progesterone concentration on different days and with embryo survival during the early luteal phase in dairy cows. Theriogenology 65:1435–1441. [DOI] [PubMed] [Google Scholar]
- Meneghetti M., Sá Filho O. G., Peres R. F. G., Lamb G. C., and Vasconcelos J. L. M.. 2009. Fixed-time artificial insemination with estradiol and progesterone for B. indicus cows I: basis for development of protocols. Theriogenology 72:179–189. [DOI] [PubMed] [Google Scholar]
- Murdoch W. J., and Van Kirk E. A.. 1998. Luteal dysfunction in ewes induced to ovulate early in the follicular phase. Endocrinology 139:3480–3484. [DOI] [PubMed] [Google Scholar]
- Ocón-Grove O. M., Cooke F. N. T., Alvarez I. M., Johnson S. E., Ott T. L., and Ealy A. D.. 2008. Ovine endometrial expression of fibroblast growth factor (FGF) 2 and conceptus expression of FGF receptors during early pregnancy. Domest. Anim. Endocrinol. 34:135–145. [DOI] [PubMed] [Google Scholar]
- Patterson D. J., Cooke R. F., Dahlke G. R., Funston R. N., Hall J. B., Lamb G. C., Lauderdale J., Perry G. A., and Van Eenennaam A. L.. 2016. Physiological and management advances enhancing adoption of applied reproductive management procedures in beef cattle. J. Anim. Sci. 94(E-Suppl. 5):550. [Google Scholar]
- Pereira M. H., Sanches C. P., Guida T. G., Rodrigues A. D., Aragon F. L., Veras M. B., Borges P. T., Wiltbank M. C., and Vasconcelos J. L. M.. 2013. Timing of prostaglandin F2α treatment in an estrogen-based protocol for timed artificial insemination or timed embryo transfer in lactating dairy cows. J. Dairy Sci. 96:2837–2846. [DOI] [PubMed] [Google Scholar]
- Pereira M. H. C., Wiltbank M. C., and Vasconcelos J. L. M.. 2016. Expression of estrus improves fertility and decreases pregnancy losses in lactating dairy cows that receive artificial insemination or embryo transfer. J. Dairy Sci. 99:2237–2247. [DOI] [PubMed] [Google Scholar]
- Perry G. A., and Perry B. L.. 2008a. Effect of preovulatory concentrations of estradiol and initiation of standing estrus on uterine pH in beef cows. Domest. Anim. Endocrinol. 34:333–338. [DOI] [PubMed] [Google Scholar]
- Perry G. A., and Perry B. L.. 2008b. Effects of standing estrusand supplemental estradiol on changes in uterine pH during a fixed-time artificial insemination protocol. J. Anim. Sci. 86:2928–2935. [DOI] [PubMed] [Google Scholar]
- Perry G. A., Smith M. F., Lucy M. C., Green J. A., Parks T. E., MacNeil M. D., Roberts A. J., and Geary T. W.. 2005. Relationship between follicle size at insemination and pregnancy success. Proc. Natl. Acad. Sci. U.S.A. 102:5268–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G. A., Smith M. F., Roberts A. J., MacNeil M. D., and Geary T. W.. 2007. Relationship between size of ovulatory follicle and pregnancy success in beef heifers. J. Anim. Sci. 85:684–689. [DOI] [PubMed] [Google Scholar]
- Pohler K. G., Geary T. W., Atkins J. A., Perry G. A., Jinks E. M., and Smith M. F.. 2012. Follicular determinants of pregnancy establishment and maintenance. Cell Tissue Res. 349:649–664. [DOI] [PubMed] [Google Scholar]
- Pohler K. G., Geary T. W., Johnson C. L., Atkins J. A., Jinks E. M., Busch D. C., Green J. A., MacNeil M. D., and Smith M. F.. 2013. Circulating bovine pregnancy associated glycoproteins are associated with late embryonic/fetal survival but not ovulatory follicle size in suckled beef cows. J. Anim. Sci. 91:4158–4167. [DOI] [PubMed] [Google Scholar]
- Pohler K. G., Pereira M. H. C., Lopes F. R., Lawrence J. C., Keisler D. H., Smith M. F., Vasconcelos J. L. M., and Green J. A.. 2016a. Circulating concentrations of bovine pregnancy associated glycoproteins and late embryonic mortality in lactating dairy herds. J. Dairy Sci. 99:1584–1594. [DOI] [PubMed] [Google Scholar]
- Pohler K. G., Peres R. F. G., Green J. A., Graff H., Martins T., Vasconcelos J. L. M., and Smith M. F.. 2016b. Use of bovine pregnancy-associated glycoproteins to predict late embryonic mortality in postpartum Nelore beef cows. Theriogenology 85:1652–1659. [DOI] [PubMed] [Google Scholar]
- Richardson B. N., Hill S. L., Stevenson J. S., Gemechis G. D., and Perry G. A.. 2016. Expression of estrus before fixed-time AI affects conception rates and factors that impact expression of estrus and the repeatability of expression of estrus in sequential breeding seasons. Anim. Reprod. Sci. 166:133–140. [DOI] [PubMed] [Google Scholar]
- Sá Filho M. F., Crespilho A. M., Santos J. E., Perry G. A., and Baruselli P. S.. 2010. Ovarian follicle diameter at timed insemination and estrous response influence likelihood of ovulation and pregnancy after estrous synchronization with progesterone or progestin-based protocols in suckled Bos indicus cows. Anim. Reprod. Sci. 120:23–30. [DOI] [PubMed] [Google Scholar]
- Schubach K. M., Cooke R. F., Brandão A. P., Lippolis K. D., da Silva L. G. T., Marques R. S., and Bohnert D. W.. 2017. Impacts of stocking density on development and puberty attainment of replacement beef heifers. Animal. doi:10.1017/S1751731117001070 [DOI] [PubMed] [Google Scholar]
- Silper B. F., Madureira A. M. L., Kaur M., Burnett T. A., and Cerri R. L. A.. 2015. Short communication: Comparison of estrus characteristics in Holstein heifers by 2 activity monitoring systems. J. Dairy Sci. 98:3158–3165. [DOI] [PubMed] [Google Scholar]
- Silper B. F., Madureira A. M. L., Polsky L. B., Soriano S., Sica A. F., Vasconcelos J. L. M., and Cerri R. L. A.. 2017. Daily lying behavior of lactating Holstein cows during an estrus synchronization protocol and its associations with fertility. J. Dairy Sci. 100:8484–8495. [DOI] [PubMed] [Google Scholar]
- Silva E., Gaivão M., Leitão S., Amaro A., Lopes da Costa L., and Mateus L.. 2008. Blood COX-2 and PGES gene transcription during the peripartum period of dairy cows with normal puerperium or with uterine infection. Domest. Anim. Endocrinol. 35:314–323. [DOI] [PubMed] [Google Scholar]
- Stevenson J. L., Dalton J. C., Ott T. L., Racicot K. E., and Chebel R. C.. 2007. Correlation between reproductive status and steady-state messenger ribonucleic acid levels of the myxovirus resistance gene, MX2, in peripheral blood leukocytes of dairy heifers. J. Anim. Sci. 85:2163–2172. [DOI] [PubMed] [Google Scholar]
- Thomas J. M., Poock S. E., Ellersieck M. R., Smith M. F., and Patterson D. J.. 2014. Delayed insemination of non-estrous heifers and cows when using conventional semen in timed artificial insemination. J. Anim. Sci. 92:4189–4197. [DOI] [PubMed] [Google Scholar]
- Vailes L. D., Washburn S. P., and Britt J. H.. 1992. Effects of various steroid milieus or physiological states on sexual behavior of Holstein cows. J. Anim. Sci. 70:2094–2103. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., and Speleman F.. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos J. L. M., Carvalho R., Peres R. F. G., Rodrigues A. D. P., Meneghetti M., Junior I. C., Aono F. H., Costa W., Lopes C. N., Cooke R. F., and Pohler K. G.. 2017. Reproductive programs for beef cattle: incorporating management and reproductive techniques for better fertility. Anim. Reprod. 14:547–557. [Google Scholar]
- Vasconcelos J. L. M., de Sá Filho O. G., and Cooke R. F.. 2014. Impacts of reproductive technologies on beef production in South America. Adv. Exp. Med. Biol. 752:161–180. [DOI] [PubMed] [Google Scholar]
- Vasconcelos J. L., Sartori R., Oliveira H. N., Guenther J. G., and Wiltbank M. C.. 2001. Reduction in size of the ovulatory follicle reduces subsequent luteal size and pregnancy rate. Theriogenology 56:307–314. [DOI] [PubMed] [Google Scholar]
- Wagner J. J., Lusby K. S., Oltjen J. W., Rakestraw J., Wettemann R. P., and Walters L. E.. 1988. Carcass composition in mature Hereford cows: estimation and effect on daily metabolizable energy requirement during winter. J. Anim. Sci. 66:603–612. [DOI] [PubMed] [Google Scholar]
- Whittier W. D., Currin J. F., Schramm H., Holland S., and Kasimanickam R. K.. 2013. Fertility in Angus cross beef cows following 5-day CO-Synch + CIDR or 7-day CO-Synch + CIDR estrus synchronization and timed artificial insemination. Theriogenology 80:963–969. [DOI] [PubMed] [Google Scholar]
- Wiltbank M. C., Souza A. H., Giordano J. O., Nascimento A. B., Vasconcelos J. M., Pereira M. H. C., Fricke P. M., Surjus R. S., Zinsly F. C. S., Carvalho P. D.,. et al. 2012. Positive and negative effects of progesterone during timed AI protocols in lactating dairy cattle. Anim. Reprod. 9:231–241. [Google Scholar]