Abstract
Seventeen yearling Quarter Horses were used in a randomized complete block design for a 56-d trial to determine ability of dietary CLA to mitigate joint inflammation and alter cartilage turnover following an inflammatory insult. Horses were blocked by age, sex, and BW, and randomly assigned to dietary treatments consisting of commercial concentrate offered at 1% BW (as-fed) supplemented with either 1% soybean oil (CON; n = 6), 0.5% soybean oil and 0.5% CLA (LOW; n = 5; 55% purity; Lutalin, BASF Corp., Florham Park, NJ), or 1% CLA (HIGH; n = 6) top-dressed daily. Horses were fed individually every 12 h and offered 1% BW (as-fed) coastal bermudagrass (Cynodon dactylon) hay daily. This study was performed in 2 phases: phase I (d 0 to d 41) determined incorporation of CLA into plasma and synovial fluid; phase II (d 42 to d 56) evaluated potential of CLA to mitigate intra-articular inflammation and alter cartilage metabolism. Blood and synovial fluid were collected at 7- and 14-d intervals, respectively, to determine fatty acid concentrations. On d 42, carpal joints within each horse were randomly assigned to receive intra-articular injections of 0.5 ng lipopolysaccharide (LPS) derived from Escherichia coli 055:B5 or sterile lactated Ringer’s solution. Synovial fluid samples were obtained at preinjection h 0 and 6, 12, 24, 168, and 336 h postinjection, and analyzed for prostaglandin E2 (PGE2), carboxypeptide of type II collagen (CPII), and collagenase cleavage neopeptide (C2C). Data were analyzed using PROC MIXED procedure of SAS. Horses receiving the CON diet had undetectable levels of CLA for the duration of the study. A quadratic dose response was observed in concentrations of CLA in plasma and synovial fluid (P < 0.01). A negative quadratic dose response was observed for plasma arachidonic acid (20:4) with a reduction in concentration to d 14 in HIGH horses (P = 0.04). Synovial fluid 20:4 tended to decrease in horses receiving the HIGH diet (P = 0.06). Post LPS injection, synovial PGE2 was not affected by dietary treatment (P = 0.15). Synovial C2C was lower in HIGH horses (P = 0.05), and synovial CPII tended to be greater in LOW horses than HIGH and CON horses (P = 0.10). In conclusion, dietary CLA incorporated into plasma and synovial fluid prior to LPS challenge. Dietary CLA did not influence inflammation; however, there was a reduction in cartilage degradation and an increase in cartilage regeneration.
Keywords: carboxypeptide of type II collagen, collagenase cleavage neoepitope, conjugated linoleic acid, lipopolysaccharide, prostaglandin E2, synovial fluid
INTRODUCTION
Osteoarthritis (OA) is the leading cause of lameness in horses and is characterized by degradation of articular cartilage, often by recurring inflammation (McIlwraith et al., 2012; Schlueter and Orth, 2004). Using synovial fluid biomarkers, current research has focused on methods to mitigate inflammation through various nutraceuticals. Supplementation of ω-3 fatty acids, most commonly in the form of fish oil, is unpalatable to horses and contains elevated levels of arachidonic acid (20:4; Hall et al., 2004). Glucosamine, a common dietary supplement, is known to have poor bioavailability and thus requires higher rates of supplementation for anti-inflammatory effects to occur in young horses (Leatherwood et al., 2016). Therefore, it is desirable to find a dietary supplement that is both palatable and bioavailable.
Exogenous CLA delivered to growing pigs via dietary supplementation resulted in increased plasma concentrations of CLA, followed by incorporation of CLA into muscle tissue (Kramer et al., 1998). Conjugated linoleic acid’s anti-inflammatory effects (Battacharya et al., 2006) were studied in weaned pigs. Specifically, a systemic lipopolysaccharide (LPS) challenge conducted in weaned pigs following dietary supplementation of CLA found reduced plasma prostaglandin E2 (PGE2) levels relative to pigs fed corn oil (Changhua et al., 2005). No publications describe the potential of CLA to incorporate into the synovium and reduce joint inflammation in any species.
Dietary CLA does reach measureable circulating levels in equine plasma after 14 d of supplementation (Headley et al., 2011). This indicates that dietary supplementation of CLA may be beneficial in mitigating inflammation and minimizing cartilage turnover in the horse. Therefore, the objective of this study was to evaluate the potential of dietary CLA to reach synovial tissue, mitigate intra-articular inflammation, and minimize cartilage metabolism following an intra-articular LPS challenge.
MATERIALS AND METHODS
All procedures and handling of horses were approved by the Texas A&M University Institutional Animal Care and Use Committee.
Horses and Dietary Treatments
Horses assigned to the control dietary treatment in the current study were used in conjunction with a previous study investigating the age-related effects on joint inflammation and cartilage metabolism (Kahn et al., 2017). Seventeen Quarter Horse yearlings (initial BW 354.0 ± 16.0 kg; 470 ± 26 d of age; 8 geldings and 9 fillies) were used in a randomized complete block design for a 56-d trial. The experimental period was separated into 2 phases. Phase I consisted of 41 d and evaluated the incorporation of dietary CLA into plasma and synovial tissues. Phase II began on d 42, continued to d 56, and consisted of an intra-articular LPS challenge to evaluate inflammation and cartilage turnover in response to dietary CLA. All diets were formulated to be isocaloric and isonitrogenous, and horses were fed individually in 3 × 3 m stalls at 1% BW (as-fed) commercial concentrate feed (14% CP textured feed, Producers Cooperative Association, Bryan, TX) and 1% BW (as-fed) coastal bermudagrass (Cynodon dactylon) hay daily that was separated into 2 feedings at 0630 and 1830 h. Diets were formulated to meet or exceed NRC requirements, and were adjusted weekly for changes in BW (NRC, 2007). All dietary treatments were top-dressed on the concentrate feed and fed as a percent of total diet. Horses received either a control diet containing 1% soybean oil (CON; n = 6), 0.5% soybean oil + 0.5% CLA (LOW; n = 5), or 1% CLA (HIGH; n = 6). The CLA supplement contained 55% CLA with a mixture of cis-9, trans-11 and trans-10, cis-12 isomers (Lutalin, BASF Corp., Florham Park, NJ). Composited samples of concentrate, hay, soybean oil, and CLA were all analyzed by a commercial laboratory for nutrient content (Table 1) and fatty acid concentrations (Table 2).
Table 1.
Nutrient composition of concentrate and hay (DM basis) fed to yearling horses
| Concentratea | Coastal bermudagrass hay | |
|---|---|---|
| DM, % | 92.35 | 92.45 |
| CP, % | 18.82 | 11.26 |
| NDF, % | 16.83 | 61.05 |
| ADF, % | 9.47 | 33.87 |
| DE, Mcal/kg | 3.42 | 2.51 |
| Ether extract, % | 6.84 | 3.53 |
| Ca, % | 1.14 | 0.60 |
| P, % | 0.73 | 0.22 |
| K, % | 1.35 | 1.12 |
| Mg, % | 0.26 | 0.34 |
a14% CP textured feed (Producer’s Cooperative, Bryan, TX) offered at 1% BW (as-fed basis).
Table 2.
Concentration of CLA isomers and arachidonic acid in feedstuffs and supplements
| Fatty acid | Concentratea | Coastal bermudagrass hay | Soybean oil | CLAb |
|---|---|---|---|---|
| cis-9, trans-11 CLA, % | NDc | ND | ND | 26.18 |
| trans-10, cis-12 CLA, % | ND | ND | ND | 26.12 |
| Arachidonic acid, % | 0.25 | 0.52 | 0.32 | 0.57 |
a14% CP textured feed (Producer’s Cooperative, Bryan, TX).
bLutalin (BASF Corp., Florham Park, NJ).
cND, not detectable (minimum detectable level 0.003%).
Phase I: Physical Measurements, Blood, and Synovial Fluid Samples
Weekly, beginning at d 0, physical growth measurements were recorded including BW, wither and hip heights, body length, heart girth circumference, rump fat thickness, and BCS. The BCS was determined using the 1 to 9 scale outlined by Henneke et al. (1983). Rump fat thickness was measured via ultrasound images on the left hip at a point 5 cm dorsal of halfway between the first coccygeal vertebrae and the ischium (Westervelt et al., 1976) using an ultrasound instrument (Aloka SSD-500V, Aloka Inc., Tokyo, Japan). Blood samples (10 mL) were collected weekly via jugular venipuncture into evacuated tubes containing K3 EDTA (BD Vacutainer, Franklin Lakes, NJ). Samples were immediately placed on ice and centrifuged at 2,000 × g for 20 min at 4 °C. Plasma was collected into pour-off tubes and stored at −20 °C for subsequent analysis. Every 2 wk, synovial fluid was collected on both carpal joints via sterile arthrocentesis. Samples within horse were then pooled for each time point as a representative sample, and stored at −20 °C until further analysis. The pooled sample for each time point provided the necessary 3 mL synovial fluid for fatty acid analysis.
Phase II: Intra-articular LPS Challenge
On d 42, an intra-articular LPS challenge was conducted. For each horse, radial carpal joints were randomly assigned as either treatment (LPS) or contralateral control (lactated Ringer’s solution [LRS]). At preinjection h 0 (PIH 0), carpal joints were aseptically prepared and horses were sedated with 1.5 mL Sedivet (romifidine hydrochloride 1% injection, Boehringer Ingelheim, Fremont, CA). Synovial fluid (1 to 4 mL) was collected aseptically via arthrocentesis. Immediately following PIH 0 sample collection, 0.5 ng Escherichia coli O55:B5 derived LPS (Sigma-Aldrich, St Louis, MO) was injected to the randomly assigned LPS joint. The 0.5 ng LPS was reconstituted in 0.8 mL sterile lactated Ringer’s solution. The LRS joint received 0.8 mL sterile lactated Ringer’s solution only. Injections were given at a location medial to the extensor carpi radialis tendon in the palpable depression between the radial carpal bone and the third carpal bone to a depth of approximately 12.7 mm to avoid unnecessary contact with articular cartilage (Trotter and McIlwraith, 1996).
Following LPS injection, synovial fluid samples were collected via arthrocentesis at 6, 12, 24, 168, and 336 h. Synovial fluid samples were transferred to nonadditive 10-mL vacutainers (serum blood collection tubes, BD Vacutainer, Franklin Lakes, NJ) and immediately placed on ice. Synovial fluid was then divided into small aliquots in 1.5-mL snaplock microtubes and stored at −20 °C for later analysis. Prior to collection times PIH 0, and 6, 12, and 24 h postinjection, rectal temperature (RT; °C), heart rate (HR; beats per min), and respiration rate (RR; breaths per min) were recorded. Prior to each collection time, carpal circumference was measured at the level of the accessory carpal bone using a soft tape measure, and surface temperature of each carpal joint was determined at the same location and from a distance of 1.2 m using an infrared camera (FLIR E60 Series, Flir Systems Inc., Wilsonville, OR).
Sample Analyses
Total lipids were extracted by a modification of the method of Folch et al. (1957). In brief, 1 mL of plasma or 3 mL of synovial fluid were extracted in chloroform:methanol (2:1, vol/vol) and fatty acid methyl esters (FAME) were prepared as described by Morrison and Smith (1964), modified to include an additional saponification step (Archibeque et al., 2005). The FAME were analyzed using a Varian gas chromatograph (model CP-3800 fixed with a CP-8200 autosampler, Varian Inc., Walnut Creek, CA). Separation of FAME was accomplished on a fused silica capillary column CP-Sil88 [100 m × 0.25 mm (i.d.)] (Chrompack Inc., Middleburg, The Netherlands), with hydrogen as the carrier gas (flow rate = 35 mL/min). After 32 min at 180 °C, oven temperature was increased at 20 °C/min to 225 °C and held for 13.75 min. Total run time was 48 min. Injector and detector temperatures were at 270 °C and 300 °C, respectively. Individual fatty acids were identified using genuine external standards (Nu-Chek Prep, Inc., Elysian, MN).
Synovial fluid collected during the LPS challenge was analyzed for PGE2, CPII, and C2C using ELISA kits previously validated for use in horses (Bertone et al., 2001; de Grauw et al., 2006). Synovial fluid samples for PGE2 analysis were diluted at 2:1, 1:2, 1:3, or 1:4 depending on time postinjection using calibrator diluent provided in the kit (R&D Systems, Inc., Minneapolis, MN). Samples for CPII analysis were diluted at 1:4, 1:5, 1:6, or 1:7 using buffer III provided by the kit, and samples for C2C analysis were diluted at 1:3 using buffer III (IBEX Pharmaceuticals Inc., Montreal, Quebec, Canada).
Final concentrations of all markers were read using a microplate reader with optical density set at 450 nm (BioRad 680 Microplate Reader, BioRad Laboratories, Hercules, CA). The PGE2 ELISA had intra-assay precision between 5.0% and 9.0% and inter-assay precision between 9.0% and 12.9%. The CPII plates ran with inter-assay precision within plates between 0.5% and 9.5% and intra-assay precision between plates between 0% and 8.8%. The C2C commercial kits used a mouse monoclonal antibody and the assay had an inter-assay precision between 0.6% and 9.5% and intra-assay precision between 1.0% and 6.0%.
Statistical Analysis
Samples were numerically coded in a blinded fashion and were processed randomly to eliminate systematic error. All data were analyzed using the PROC MIXED method of SAS (SAS Inst. Inc., Cary, NC). The model contained effects for treatment, time, and treatment by time by knee interactions to determine the incorporation of CLA into plasma and synovial fluid. Orthogonal contrasts were implemented for means separation. When all horses were challenged with LPS, the model statement included diet, injection type, time, and their respective interactions. Linear and cubic effects were tested in the form of orthogonal contrasts. Block was initially added as a main effect for all analyses, but removed from the model if insignificant. Significance was established for all variables at P ≤ 0.05, and P ≤ 0.10 was defined as a trend toward significance.
RESULTS
Phase I: Physical Measurements, Plasma, and Synovial Fluid Fatty Acid Concentrations
Horses readily consumed all diets and there were no significant refusals across dietary treatment groups for the entire 56-d experimental period. Wither height, hip height, BW, BCS, length, heart girth circumference, and rump fat thickness were not affected (P > 0.40) by dietary treatment; however, all physical measurements increased over time (P < 0.01; Table 3).
Table 3.
Effect of dietary CLA supplementation on yearling physical measurements
| Item | Time | Treatmenta | SEM | P-values | ||||
|---|---|---|---|---|---|---|---|---|
| CON | LOW | HIGH | Trt | Time | Trt * time | |||
| BW, kg | Initial | 353.0 | 353.0 | 354.8 | 10.4 | 0.97 | <0.01 | 0.93 |
| Final | 373.0 | 375.0 | 373.2 | |||||
| Wither height, cm | Initial | 137.3 | 139.3 | 138.1 | 1.9 | 0.81 | <0.01 | 0.42 |
| Final | 140.7 | 141.4 | 141.9 | |||||
| Hip height, cm | Initial | 143.5 | 144.7 | 143.8 | 1.5 | 0.93 | <0.01 | 0.89 |
| Final | 147.9 | 147.7 | 147.6 | |||||
| Length, cm | Initial | 147.2 | 146.2 | 145.2 | 1.7 | 0.97 | <0.01 | 0.73 |
| Final | 153.3 | 153.7 | 153.6 | |||||
| Heart girth, cm | Initial | 159.8 | 157.5 | 160.5 | 1.9 | 0.87 | <0.01 | 0.71 |
| Final | 164.0 | 163.6 | 164.0 | |||||
| RFTb, cm | Initial | 0.28 | 0.29 | 0.28 | 0.01 | 0.78 | <0.01 | 0.79 |
| Final | 0.33 | 0.34 | 0.34 | |||||
| BCS | Initial | 5.5 | 5.8 | 5.4 | 0.2 | 0.81 | <0.01 | 0.72 |
| Final | 5.8 | 5.9 | 5.8 | |||||
aCON = 1% diet soybean oil; LOW = 0.5% diet soybean oil, 0.5% diet CLA; HIGH = 1% diet CLA.
bRFT = rump fat thickness measured by ultrasound (Westervelt et al., 1976).
A quadratic dose response was observed between concentrations of CLA isomers in plasma and synovial fluid (c9, t11; t10, c12; Tables 4 and 5). In plasma, CLA concentrations increased in horses receiving the HIGH dose of dietary CLA by d 28 continuing to d 42 above LOW. Horses receiving the LOW dose of dietary CLA also exhibited an increase at d 28 where concentrations plateaued, but were lower than horses receiving the HIGH dietary treatment. Concentrations of CLA isomers in synovial fluid illustrate an increase in horses receiving the HIGH dose of CLA to d 28 above LOW, and continue to increase to d 56. Horses receiving the LOW dose also increased CLA concentrations in synovial fluid to d 56 with lower concentrations than HIGH.
Table 4.
Effect of CLA supplementation on concentrations of CLA and arachidonic acid in plasma
| Fatty acid, mg/100 mg total fatty acids | Day | Treatmenta | SEM | P-values | ||||
|---|---|---|---|---|---|---|---|---|
| CON | LOW | HIGH | Trtb | Time | Contrastc | |||
| cis-9, trans-11 CLA | 0 | 0.00 | 0.00 | 0.00 | 0.10 | <0.01 | <0.01 | <0.01 |
| 28 | 0.00a | 0.92b | 1.96c | |||||
| 42 | 0.00a | 0.80b | 2.47c | |||||
| 56 | 0.00a | 0.92b | 1.69c | |||||
| trans-10, cis-12 CLA | 0 | 0.00 | 0.00 | 0.00 | 0.11 | <0.01 | <0.01 | <0.01 |
| 28 | 0.00a | 0.90b | 1.75c | |||||
| 42 | 0.00a | 0.73b | 2.15c | |||||
| 56 | 0.00a | 0.90b | 1.62c | |||||
| Arachidonic acid | 0 | 0.87 | 0.83 | 0.79 | 0.09 | 0.02 | 0.50 | 0.04 |
| 28 | 0.94 | 0.77 | 0.74 | |||||
| 42 | 0.89a | 0.76a,b | 0.63b | |||||
| 56 | 0.82a | 0.82a | 0.54b | |||||
aCON = 1% diet soybean oil; LOW = 0.5% diet soybean oil, 0.5% diet CLA; HIGH = 1% diet CLA.
bTrt = main effect of treatment for the 56-d trial.
cContrast = quadratic contrast of dietary treatments to d 42, prior to the LPS challenge.
a–cSuperscripts denote differences between dietary treatments (P < 0.05).
Table 5.
Effect of CLA supplementation on concentrations of CLA and arachidonic acid in synovial fluid
| Fatty acid, mg/100 mg total fatty acids | Day | Treatmenta | SEM | P-values | ||||
|---|---|---|---|---|---|---|---|---|
| CON | LOW | HIGH | Trtb | Time | Contrastc | |||
| cis-9, trans-11 CLA | 0 | 0.00 | 0.00 | 0.00 | 0.08 | <0.01 | <0.01 | <0.01 |
| 28 | 0.00a | 0.22a | 0.73b | |||||
| 42 | 0.00a | 0.35b | 0.62c | |||||
| 56 | 0.00a | 0.48b | 0.79c | |||||
| trans-10, cis-12 CLA | 0 | 0.00 | 0.00 | 0.00 | 0.08 | <0.01 | <0.01 | <0.01 |
| 28 | 0.00a | 0.18a | 0.69b | |||||
| 42 | 0.00a | 0.35b | 0.62c | |||||
| 56 | 0.00a | 0.43b | 0.81c | |||||
| Arachidonic acid | 0 | 1.16 | 1.29 | 1.25 | 0.2 | 0.06 | <0.01 | 0.22 |
| 28 | 1.04 | 0.61 | 0.77 | |||||
| 42 | 1.47a | 0.93b | 0.8b | |||||
| 56 | 1.35a | 1.15a | 0.86b | |||||
aCON = 1% diet soybean oil; LOW = 0.5% diet soybean oil, 0.5% diet CLA; HIGH = 1% diet CLA.
bTrt = main effect of treatment for the 56-d trial.
cContrast = quadratic response of dietary treatments to d 42, prior to the LPS challenge.
a-cSuperscripts denote differences between dietary treatments (P < 0.05).
Plasma concentrations of 20:4 (Table 4) exhibited a negative quadratic dose relationship, where horses receiving the HIGH dose of dietary CLA had a marked decrease in 20:4 concentrations to d 14 followed by a plateau. Horses receiving the LOW dose of dietary CLA had a slower rate of 20:4 decrease to d 28, and horses on the CON diet had unchanging levels of 20:4 in plasma during the 56-d supplemental period. Synovial fluid concentrations of 20:4 (Table 5) tended to be influenced by treatment (P = 0.06) with horses receiving the HIGH CLA diet having lower concentrations than CON (P = 0.02), and LOW tending to have lower concentrations than CON (P = 0.10). Concentrations of 20:4 did not differ between LOW and HIGH treatment groups (P = 0.49).
Phase II: LPS Challenge
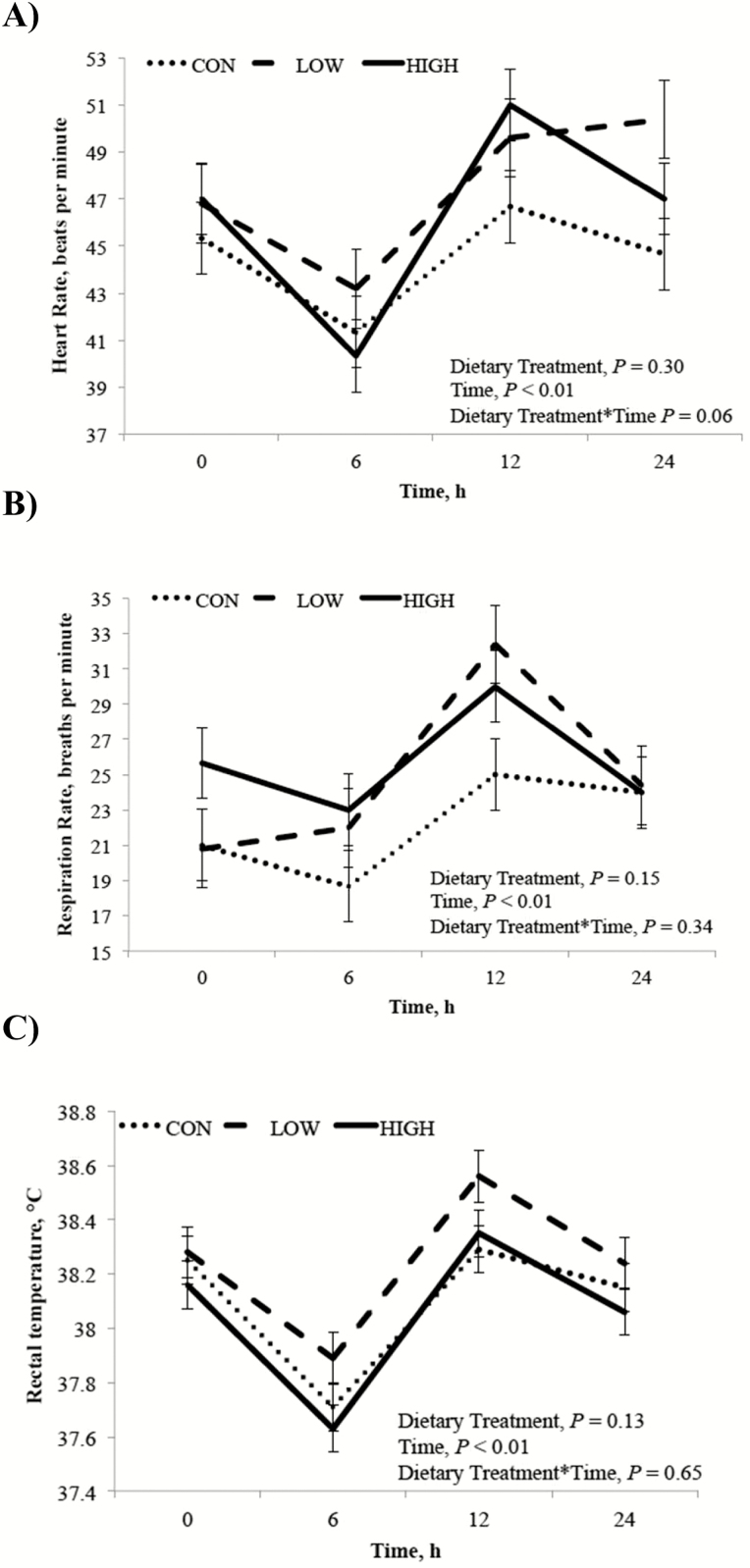
During the LPS challenge, dietary treatment did not affect HR, RR, or RT (P ≥ 0.13), but all measurements were influenced by time post LPS injection (P < 0.01; Fig. 1). Both HR and RR decreased (P < 0.01) from PIH 0 to 6 h postinjection, and an increase from baseline for all 3 vital signs was recorded at 12 h postinjection (P < 0.01). All vitals, however, returned to baseline levels by 24 h postinjection.
Figure 1.
Clinical assessment of HR (panel A), RR (panel B), and RT (panel C) after intra-articular LPS (derived from Escherichia coli O55:B5) injection. Treatments were 1% diet soybean oil (CON; n = 6), 0.5% diet soybean oil with 0.5% diet CLA (LOW; n = 5), and 1% diet CLA (HIGH; n = 6).
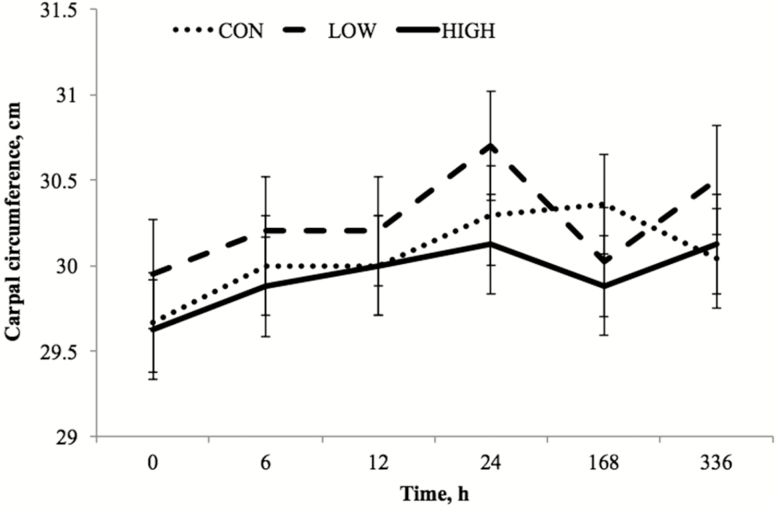
A treatment by time interaction (P < 0.01) for carpal circumference was observed and characterized by delayed peak values in CON horses to 168 h compared to peak values at 24 h by both the HIGH and LOW treatment groups. Carpal circumference was not affected by dietary treatment or knee (P = 0.74), and because carpal circumference was not influenced by knee (P < 0.74), combined mean carpal circumference for both the LRS and LPS joint is presented (Fig. 2). There was an effect of time (P < 0.01) with peak circumference values noted at 24 h postinjection, followed by a decrease at 168 h.
Figure 2.
Carpal joint circumference (cm; least squares mean ± SEM) after intra-articular LPS (derived from Escherichia coli O55:B5) injection at preinjection h 0 and 6, 12, 24, 168, and 336 h postinjection. Treatments were 1% diet soybean oil (CON; n = 6), 0.5% diet soybean oil with 0.5% diet CLA (LOW; n = 5), and 1% diet CLA (HIGH; n = 6). Main effects include dietary treatment (P = 0.74) and time (P < 0.01).
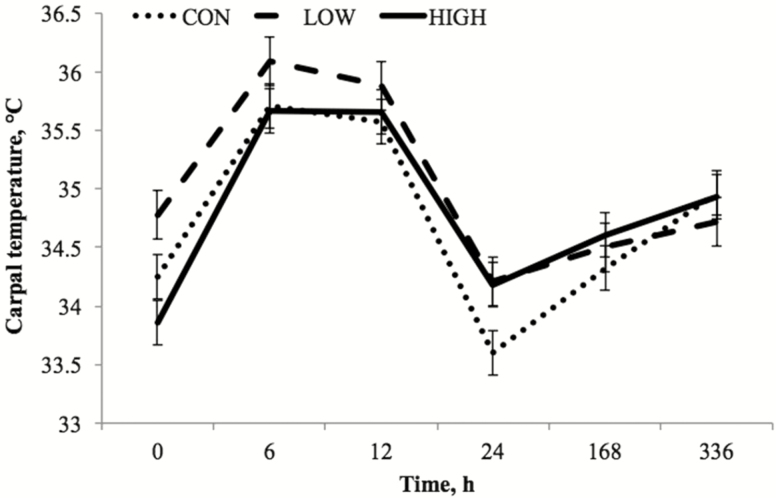
A tendency toward a dietary treatment by time interaction for carpal surface temperature (P = 0.10) was observed, which was evident at preinjection h 0 and postinjection h 24. Surface temperatures recorded at h 0 were greater (P < 0.01) for LOW horses vs. HIGH, and tended to be greater (P = 0.06) than CON horses. At 24 h postinjection, the CON group’s carpal surface temperatures were lower (P = 0.03) than both HIGH and LOW horses.
Carpal surface temperatures tended to be influenced by dietary treatment (P = 0.10) with horses fed the LOW diet having greater surface temperatures than CON (P = 0.04). Since no effect of knee was noted (P < 0.27), mean carpal surface temperatures for both carpal joints are presented (Fig. 3). Regardless of dietary treatment, carpal surface temperatures were influenced by time (P < 0.01) with peak values noted at 6 h postinjection with an increase from baseline levels at h 0 (P < 0.01). Values remained elevated at 12 h postinjection and fell from h 12 to 24 (P < 0.01) to levels tending to be below baseline (P = 0.06).
Figure 3.
Carpal joint surface temperature (°C; least squares mean ± SEM) at preinjection h 0 and 6, 12, 24, 168, and 336 h post LPS injection. Treatments were 1% diet soybean oil (CON; n = 6), 0.5% diet soybean oil with 0.5% diet CLA (LOW; n = 5), and 1% diet CLA (HIGH; n = 6). Main effects include dietary treatment (P = 0.12) and time (P < 0.01).
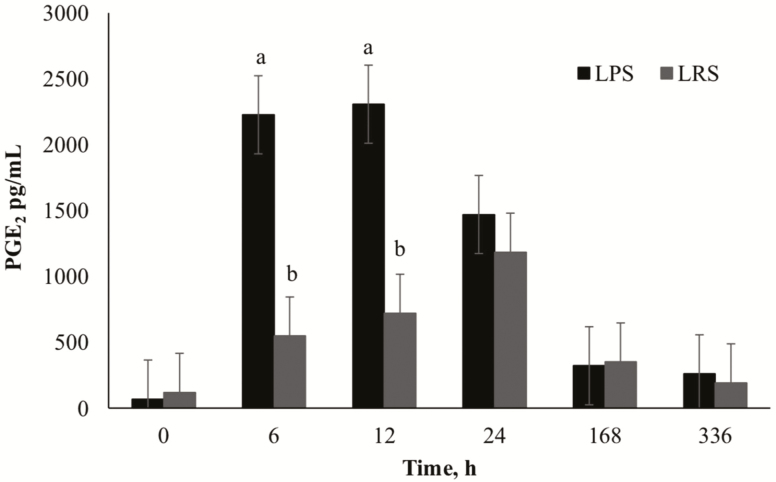
Synovial PGE2 concentrations exhibited a time by knee interaction (P < 0.01), with the LPS knee showing greater changes in concentration over time compared to the LRS knee (Fig. 4). Peak PGE2 concentrations were observed at 6 and 12 h postinjection with a return to baseline by 168 h postinjection in the LPS knee. In the LRS knee, concentrations peaked 24 h postinjection (P = 0.02) and returned to baseline by 168 h. There was a significant knee effect with the LPS knee having greater (P = 0.01) PGE2 concentrations than the LRS knee. Synovial PGE2 concentrations were not influenced by dietary treatment (P ≥ 0.15); however, PGE2 concentrations were affected by time (P < 0.01) with concentrations increasing to peak values at 6 h postinjection and decreasing to baseline by 336 h for all horses. Because PGE2 was not influenced by dietary treatment, the time * knee interaction is represented in Fig. 4 to validate that the LPS model stimulated an acute, transient inflammatory response.
Figure 4.
Mean synovial fluid concentrations (pg/mL) of PGE2 over time (h) after intra-articular injection of 0.5 ng LPS or 0.8 mL sterile lactated Ringer’s solution (LRS). Main effect includes LPS (P = 0.01). a,bSuperscript denotes difference between knee (P < 0.05).
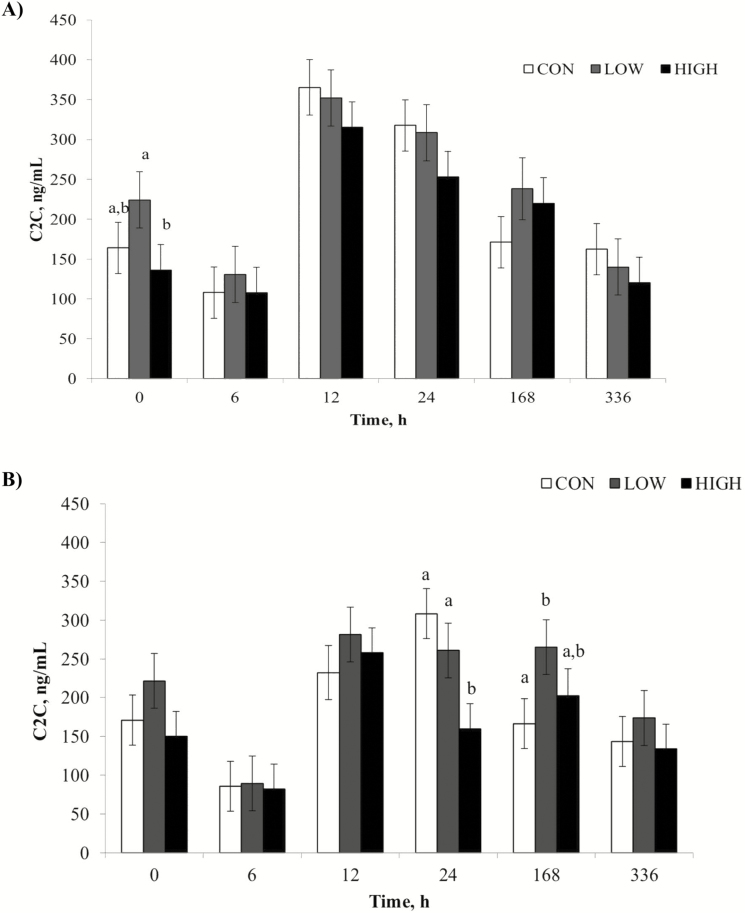
A treatment by time interaction (P = 0.03) was observed for synovial fluid C2C concentrations, where, at PIH 0, HIGH horses had lower (P = 0.02) values than LOW, and CON horses tended to have lower concentrations (P = 0.10) than LOW horses (Fig. 5). Also, at 24 h postinjection, HIGH CLA horses had lower concentrations than both the LOW and CON groups (P = 0.02 and P < 0.01, respectively). Synovial C2C concentrations were influenced by dietary treatment with horses in the HIGH group having lower (P = 0.01) concentrations than LOW. There was also an effect of time (P < 0.01) with peak values seen at 12 and 24 h postinjection and returning to baseline by 336 h. Concentrations of C2C tended to be influenced by knee (P = 0.08) with the LPS injected joint having greater concentrations than the LRS joint over the 24 h challenge.
Figure 5.
Mean synovial fluid concentrations (ng/mL) of C2C over time (h) after intra-articular injection of 0.5 ng LPS (panel A; LPS; derived from Escherichia coli 055:B5) or 0.8 mL sterile lactated Ringer’s solution (panel B; LRS). Treatments were 1% diet soybean oil (CON; n = 6), 0.5% diet soybean oil with 0.5% diet CLA (LOW; n = 5), and 1% diet CLA (HIGH; n = 6). Main effects include dietary treatment (P = 0.05), time (P < 0.01), and LPS (P = 0.08). a,bSupersripts denote differences between dietary treatments (P < 0.05).
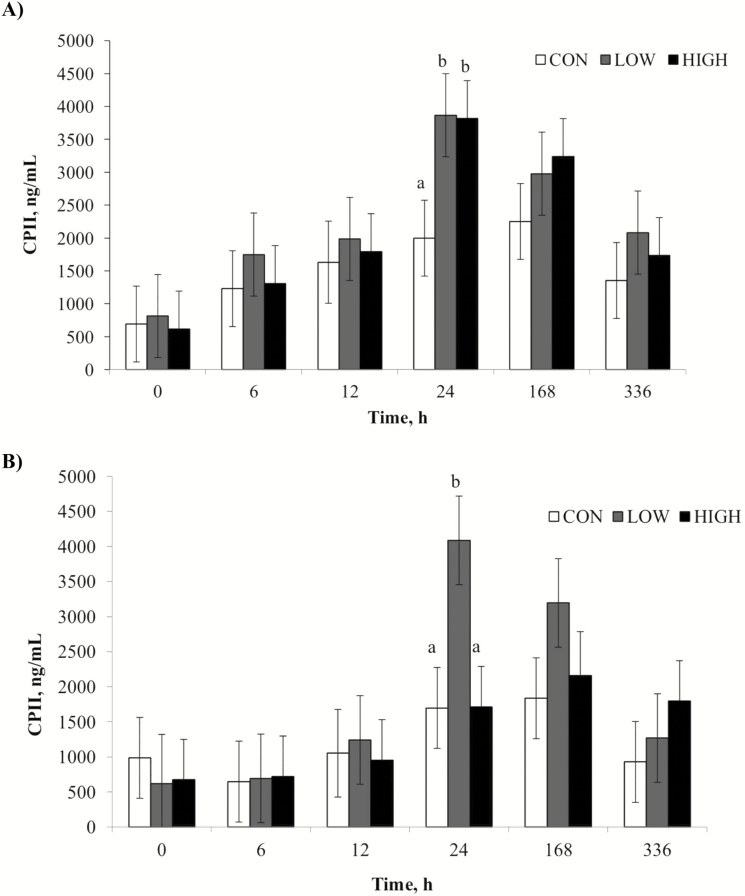
Horses receiving the LOW dose CLA treatment tended to have greater (P = 0.10) synovial CPII concentrations than the other treatment groups. Synovial samples from the LPS knee also tended to have greater CPII concentrations (P = 0.06) than samples from the LRS knee. There was an effect of time (P < 0.01) with concentrations increasing (P = 0.03) from PIH 0 to 12 h followed by a sharp increase (P < 0.01) to peak values from 12 to 24 h postinjection (Fig. 6). Concentrations of CPII decreased (P < 0.01) from 168 to 336 h but did not reach baseline values by the end of the trial (Fig. 6).
Figure 6.
Mean synovial fluid concentrations (ng/mL) of CPII after intra-articular injection of 0.5 ng LPS (panel A; LPS; derived from Escherichia coli O55:B5) or 0.8 mL sterile lactated Ringer’s solution (panel B; LRS). Treatments were 1% diet soybean oil (CON; n = 6), 0.5% diet soybean oil with 0.5% diet CLA (LOW; n = 5), and 1% diet CLA (HIGH; n = 6). Main effects include dietary treatment (P = 0.10), time (P < 0.01), and LPS (P = 0.06). a,bSupersripts denote differences between dietary treatments (P < 0.05).
DISCUSSION
This study assessed the ability of dietary CLA to mitigate joint inflammation and alter cartilage turnover following an inflammatory insult in horses. During the 56-d study of yearling horses, their BW, hip and wither heights, length, and heart girth circumference increased over time, which was expected. Plasma CLA concentrations were not detectable in the CON dietary treatment at any time, which was also expected since the CON diet contained no detectable levels of CLA. Changes in plasma CLA concentrations in the current study for yearling horses in the LOW group followed the same trend found by Headley et al. (2011), who reported measureable levels of CLA detected at d 14 with peak concentrations at d 28 in mature mares supplemented with dietary CLA. Interestingly though, in the current study, horses in the HIGH treatment group did not follow the same trend, and had steady increases in plasma CLA concentrations to d 42, followed by a notable decrease in circulating levels at d 56. The decrease in CLA concentrations from d 42 to 56 may be explained by the inflammatory response caused by LPS injection affecting circulating fatty acid concentrations for the final 14 d of the study. During peak inflammation, fatty acids are recruited from the phospholipid bilayer to produce proinflammatory prostaglandins. The re-filling of the phospholipid bilayer could potentially explain the reduction in circulating CLA levels in plasma. This is further supported by the subsequent increase in synovial fluid CLA concentrations from d 42 to d 56.
Arachidonic acid, the fatty acid precursor to prostaglandin production, was reduced in both plasma and synovial fluid of horses receiving the HIGH CLA diet. This corresponds to findings by Headley et al. (2011), in which lower concentrations of 20:4 were measured in plasma of horses supplemented to the same extent as in the current study (at 0.01% BW CLA) than in the corn oil-supplemented controls. In synovial fluid, 20:4 was less for both LOW and HIGH dietary treatment groups, indicating the potential for CLA to reduce inflammation during an acute inflammatory response. In regards to LOW horses having similar 20:4 levels as CON in plasma in the current study, it may be that 0.5% diet CLA may not be sufficient to reduce circulating 20:4 levels in the horse. During an inflammatory response, 20:4 present in cell membranes is converted to inflammatory prostaglandins, including PGE2, through the COX-2 pathway. A successful reduction in circulating and synovial 20:4 levels through 1% diet CLA supplementation reduces the availability of a direct precursor to inflammation.
In phase II of this study, clinical responses of HR, RR, and RT recorded prior to arthrocentesis in the first 24 h of the LPS challenge were unaffected by dietary treatment; however, the increased handling and stress of repeated arthrocentesis may explain the changes in vitals over the 24 h period. Values recorded at PIH 0 and postinjection h 24 were not different indicating that all vitals returned to baseline by 24 h post LPS injection. Residual effects from sedation prior to arthrocentesis may explain the decreased values of each vital sign at 6 h postinjection. Peak values at 12 h postinjection correspond to the increased inflammatory response as indicated by peak PGE2 concentrations, causing a slight, but noticeable increase in vital signs. It is important to note, however, that vitals were never outside the normal range confirming that no animal had an adverse, systemic reaction to the LPS injection.
Carpal circumference and surface temperature values were also measured during the LPS challenge on both the LPS and LRS knees, using the LRS knee as a contralateral control. The increase over time in carpal circumference without an effect of knee indicates increased joint effusion from the initiation of an inflammatory response in both carpal joints. The increased effusion reflected by increased carpal circumference in the LRS joint is likely due to repeated arthrocentesis and has been noted in a previous study (van den Boom et al., 2005). Both carpal joints had greatest values at 24 h postinjection, which is consistent with findings in a similar study performed by Lucia et al. (2013) using LPS in weanling horses.
Increased surface temperatures during an inflammatory response are caused by hyperemia at the site of inflammation due to increased metabolic waste accumulation (Bliss, 1998). In the current study, carpal surface temperatures peaked at 6 and 12 h postinjection, which were expected due to the inflammatory response caused by LPS injection. Surface temperatures in both knees returned to baseline levels by 24 h postinjection, which indicate a return to normal blood flow and reduction in inflammation. This is one of the first uses of infrared images during an intra-articular LPS challenge, and values appear to correspond closely with inflammatory markers in synovial fluid.
Analyses of synovial fluid biomarkers indicate time effects on PGE2, CPII, and C2C, validating the LPS model and the ability to stimulate an acute and transient inflammatory response. In addition, the LPS carpal joint had consistently greater concentrations of each biomarker compared to the LRS joint, further confirming the effectiveness of LPS to induce an acute inflammatory response. An increase in PGE2 and cartilage biomarkers was expected in the LRS knee due to repeated arthrocentesis. Furthermore, the delayed response of biomarkers in the LRS knee indicate that repeated arthrocentesis, compared to a single arthrocentesis, is required to initiate a measurable inflammatory response. Peak concentrations for PGE2, CPII, and C2C correspond to values previously recorded in studies performed by Lucia et al. (2013) and de Grauw et al. (2009) who measured similar parameters in carpal joints of weanling and mature horses following intra-articular LPS injection. Furthermore, both PGE2 and C2C returned to baseline prior to 336 h postinjection proving that inflammation caused by intra-articular LPS injection is transient.
Prostaglandins released during an immune response function to regulate the early phases of inflammation. Specifically, PGE2 promotes early stages of local inflammation through vasodilation, and activation of neutrophils and macrophages (Kalinski, 2012). Numerous studies have measured PGE2 concentrations in the horse, and have confirmed its use as an excellent biomarker of equine joint disease and inflammation (Bertone et al., 2001). In regards to the effect of CLA on synovial PGE2 concentrations, no treatment effect or treatment by time interaction was observed. The limited number of horses in each treatment group may explain not detecting an effect of treatment. Furthermore, the late increase in CLA concentrations in synovial fluid indicates that this study may not have captured the maximum effects of CLA. Complete incorporation of CLA into the phospholipid bilayers of joint tissue would allow CLA to reduce 20:4 concentrations via competitive inhibition, activate PPARγ, and suppress the COX-2 pathway of inflammation (Kramer et al., 1998; Thiel-Cooper et al., 2001; Yu et al., 2002). Following CLA supplementation to pigs, Changhua et al. (2005) found lower PGE2 concentrations in those supplemented with CLA (916.3 ± 53.5 pg/mL) than the control diet (1211.0 ± 53.5 pg/mL) following systemic LPS injection. Further research is needed to determine the temporal effects of CLA on the inflammatory eicosanoid PGE2 in the horse.
Although no dietary treatment effect was noted in synovial PGE2 concentrations, dietary CLA supplementation did have an effect on biomarkers of articular cartilage metabolism. Following an inflammatory insult, the fibrous type II collagen begins to unwind and reveal hidden epitopes, including C2C, which is used as a marker for articular cartilage degradation. This epitope has been previously measured following exercise, LPS injection, and osteochondral fragmentation in horses (Frisbie et al., 2008; Lucia et al., 2013). In the current study, there was an initial decrease in synovial C2C concentrations from PIH 0 to 6 h postinjection, which is likely due to a shift in cartilage metabolism at the onset of an inflammatory response. The same decrease from PIH 0 to 6 h postinjection was observed in a similar study performed by Lucia et al. (2013) following LPS injection in weanling horses. Peak C2C values were observed at 24 h postinjection, which is consistent with peak times recorded by Lucia et al. (2013) and de Grauw et al. (2009). Peak concentrations of C2C in the current study are similar to those recorded by Lucia et al. (2013) using weanling horses.
A reduction in type II collagen degrading biomarker, C2C, was observed in horses fed HIGH CLA when compared to the LOW dose. This demonstrates less cartilage degradation when horses received 1% diet CLA supplementation prior to LPS administration, indicating a potential reduction in inflammation not captured by the selected biomarker PGE2. At PIH 0, HIGH horses had lower C2C concentrations than LOW, which can be attributed to the previous 42 d of CLA supplementation reducing basal inflammatory levels. Furthermore, at 24 h postinjection when peak values were recorded, HIGH fed horses had lower concentrations of C2C compared to both LOW and CON horses. This indicates CLA supplemented at 1% diet is effective in reducing cartilage degradation both prior to and during an inflammatory insult.
When type II collagen degrades, there is an attempt to repair the type II collagen fibers (Frisbie et al., 2008). During this repair, CPII is released from the type II collagen precursor, procollagen, and can be used as a marker of type II collagen regeneration. This biomarker is often used as a measurement to counter the degradative marker C2C. Concentrations of CPII peaked at 24 h postinjection, which agrees with both Lucia et al. (2013) and de Grauw et al. (2009). Peak values recorded in the current study more closely resemble those seen by Lucia et al. (2013) in weanling horses, which are lower than those recorded by de Grauw et al. (2009) using mature horses. It is expected that yearling horses would have values similar to weanlings as opposed to mature horses (5 to 8 yr). Unlike PGE2 and C2C, concentrations of CPII in the current study did not return to baseline levels by 336 h postinjection, which was not anticipated since CPII concentrations in weanlings following LPS injection returned to baseline by 336 h postinjection (Lucia et al., 2013). This delay indicates recurring cartilage regeneration at 2 wk following LPS injection. An additional sample could have provided baseline measurements; however, concentrations of CPII were on the decline by 336 h postinjection.
With regard to the effect of CLA on cartilage metabolism, there was a tendency toward a dietary treatment effect on CPII, indicating that horses fed the LOW dose CLA had greater cartilage regeneration than CON fed horses. This likely relates to the increased cartilage degradation observed in LOW horses when measuring C2C. No previous studies have yet been performed to determine the effects of CLA on the metabolism of type II collagen.
In conclusion, dietary CLA incorporates into the target joint by 14 d of dietary supplementation. This incorporation into the target tissue provides the potential for CLA to compete with the proinflammatory 20:4, and reduce inflammation. Downstream, this would conserve the integrity of articular cartilage, potentially delaying the onset of OA as the horse matures. This is further supported through the use of LPS to induce an acute, transient inflammatory insult. Observations support the potential for dietary CLA to alter cartilage metabolism in favor of delaying the onset of OA; however, further studies are necessary to more precisely determine the effect of dietary CLA on inflammatory biomarkers and its mechanisms of action.
LITERATURE CITED
- Archibeque S. L., Lunt D. K., Gilbert C. D., Tume R. K., and Smith S. B.. 2005. Fatty acid indices of stearoyl-CoA desaturase do not reflect actual stearoyl-CoA desaturase enzyme activities in adipose tissues of beef steers finished with corn-, flaxseed-, or sorghum-based diets. J. Anim. Sci. 83:1153–1166. doi:10.2527/2005.8351153x [DOI] [PubMed] [Google Scholar]
- Battacharya A., Banu J., Rahman M., Causey J., and Fernandes G.. 2006. Biological effects of conjugated linoleic acids in health and disease. J. Nutr. Biochem. 17:789–810. doi:10.1016/j.jnutbio.200602.009 [DOI] [PubMed] [Google Scholar]
- Bertone A. L., Palmer J. L., and Jones J.. 2001. Synovial fluid cytokines and eicosanoids as markers of joint disease in horses. Vet. Surg. 30:528–531. doi:10.1053/jvet.2001.28430 [DOI] [PubMed] [Google Scholar]
- Bliss M. R. 1998. Hyperaemia. J. Tissue Viability. 8:4–13. doi:10.1016/S0965-206x(98)80028-4 [DOI] [PubMed] [Google Scholar]
- Changhua L., Jindong Y., Defa L., Lidan Z., Shiyan Q., and Jianjun X.. 2005. Conjugated linoleic acid attenuates the production and gene expression of proinflammatory cytokines in weaned pigs challenged with lipopolysaccharide. J. Nutr. 135:239–244. [DOI] [PubMed] [Google Scholar]
- De Grauw J. C., van de Lest C. H., Brama P. A., Rambags B. P., and van Weeren P. R.. 2009. In vivo effects of meloxicam on inflammatory mediators, MMP activity and cartilage biomarkers in equine joints with acute synovitis. Equine Vet. J. 41:693–699. doi:10.1186/ar2640 [DOI] [PubMed] [Google Scholar]
- De Grauw J. C., van de Lest C. H. A., van Weeren R., Brommer H., and Brama P. A. J.. 2006. Arthrogenic lameness of the fetlock: synovial fluid markers of inflammation and cartilage turnover in relation to clinical joint pain. Equine Vet. J. 38:305–311. doi:10.2746/042516406777749236 [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., and Sloane Stanley G. H.. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497–509. [PubMed] [Google Scholar]
- Frisbie D. D., Al-Sobayil F., Billinghurst R. C., Kawcak C. E., and McIlwraith C. W.. 2008. Changes in synovial fluid and serum biomarkers with exercise and early osteoarthritis in horses. Osteoarthr. Cartil. 16:1196–1204. doi:10.1016/j.joca.2008.03.008 [DOI] [PubMed] [Google Scholar]
- Hall J. A., van Saun R. J., and Wander R. C.. 2004. Dietary (n-3) fatty acids from menhaden fish oil alter plasma fatty acids and leukotriene B synthesis in healthy horses. J. Vet. Intern. Med. 18:871–879. doi:10.1111/j.1939-1676.2004.tb02635.x [DOI] [PubMed] [Google Scholar]
- Headley S., Coverdale J. A., Jenkins T. C., Klein C. M., Sharp J. L., and Vernon K. L.. 2011. Dietary supplementation of conjugated linoleic acid in horses increases plasma conjugated linoleic acid and decreases plasma arachidonic acid but does not alter body fat. J. Anim. Sci. 90:4876–4882. doi:10.2527/jas2011-4976 [DOI] [PubMed] [Google Scholar]
- Henneke D. R., Potter G. D., Kreider J. L., and Yeates B. F.. 1983. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 15:371–372. doi:10.111/j.2042-3306.1983.tb01826.x [DOI] [PubMed] [Google Scholar]
- Kahn M. K., Coverdale J. A., Leatherwood J. L., Arnold C. E., Dabareiner R. A., Bradbery A. N., Millican A. A., and Welsh T. H. Jr. 2017. Age-related effects on markers of inflammation and cartilage metabolism in response to an intra-articular lipopolysaccharide challenge in horses. J. Anim. Sci. 95:657–670. doi:10.2527/jas2016.1078 [DOI] [PubMed] [Google Scholar]
- Kalinski P. 2012. Regulation of immune responses by prostaglandin E2. J. Immunol. 188:21–28. doi:10.4049/jimmunol.1101029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. K. G., Sehat N., Dugan M. E. R., Mossoba M. M., Yurawecz M. P., Roach J. A. G., Eulitz K., Aalhus J. L., Schaefer A. L., and Ku Y.. 1998. Distributions of conjugated linoleic acid (CLA) isomers in tissue lipid classes of pigs fed a commercial CLA mixture determined by gas chromatography and silver ion-high-performance liquid chromatography. Lipids. 33:549–558. [DOI] [PubMed] [Google Scholar]
- Leatherwood J. L., Gehl K. L., Coverdale J. A., Arnold C. E., Dabareiner R. A., Walter K. N., and Lamprecht E. D.. 2016. Influence of oral glucosamine supplementation in young horses challenged with intra-articular lipopolysaccharide. J. Anim. Sci. 94:3294–3302. doi:10.2527/jas2016-0343 [DOI] [PubMed] [Google Scholar]
- Lucia J. L., Coverdale J. A., Arnold C. E., and Winsco K. N.. 2013. Influence of an intra-articular lipopolysaccharide challenge on markers of inflammation and cartilage metabolism in young horses. J. Anim. Sci. 91:2693–2699. doi:10.2527/jas.2012-5981 [DOI] [PubMed] [Google Scholar]
- McIlwraith C. W., Frisbie D. D., and Kawcak C. E.. 2012. The horse as a model of naturally occurring osteoarthritis. Bone Joint Res. 1:297–309. doi:10.1302/2046-3758.111.2000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison W. R., and Smith L. M.. 1964. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 5:600–608. [PubMed] [Google Scholar]
- NRC 2007. Nutrient requirements of horses. 6th rev. ed. National Academies Press, Washington, DC. [Google Scholar]
- Schlueter A. E., and Orth M. W.. 2004. Equine osteoarthritis: a brief review of the disease and its causes. Equine Comp. Exerc. Physiol. 1:221–231. doi:10.1079/ECEP200428 [Google Scholar]
- Thiel-Cooper R. L., Parrish F. C. Jr., Sparks J. C., Wiegand B. R., and Ewan R. C.. 2001. Conjugated linoleic acid changes swine performance and carcass characteristics. J. Anim. Sci. 79:1821–1828. doi:10.2527/2001.7971821x [DOI] [PubMed] [Google Scholar]
- Trotter G. W., and McIlwraith C. W.. 1996. Clinical features and diagnosis of equine joint disease. In: C. W. McIlwraith and G. W. Trotter, editors, Joint disease in the horse. W.B. Saunders Co, Philadelphia, PA: p. 120–145. [Google Scholar]
- van den Boom R., van de Lest C. H. A., Bull S., Brama P. A. J., van Weeren P. R., and Barneveld A.. 2005. Influence of repeated arthrocentesis and exercise on synovial fluid concentrations of nitric oxide, prostaglandin E2 and glycosaminoglycans in healthy equine joints. Equine Vet. J. 37:250–256. doi:10.2746/0425164054530740 [DOI] [PubMed] [Google Scholar]
- Westervelt R. G., Stouffer J. R., Hintz H. F., and Schryver H. F.. 1976. Estimating fatness in horses and ponies. J. Anim. Sci. 43:781–785. doi:10.2527/jas1976.434781x [Google Scholar]
- Yu Y., Correll P. H., and Vanden Heuvel J. P.. 2002. Conjugated linoleic acid decreases production of pro-inflammatory products in macrophages: evidence for a PPAR gamma-dependent mechanism. Biochem. Biophys. Acta. 1581(3):89–99. doi:10.1016/S1388-1981(02)00126-9 [DOI] [PubMed] [Google Scholar]