Abstract
The goal of this study was to test the hypothesis that sodium selenite (ISe), SEL-PLEX (OSe), vs. an 1:1 blend (MIX) of ISe and OSe in a basal vitamin-mineral (VM) mix would differentially affect metabolic parameters and performance of growing steers grazing toxic endophyte-infected tall fescue mixed forage (E+) pasture. Predominately-Angus steers (BW = 183 ± 34 kg) were randomly selected from herds of fall-calving cows grazing E+ pasture and consuming VM mixes that contained 35 ppm Se as ISe, OSe, and MIX forms. Steers were weaned, depleted of Se for 98 d, and subjected to summer-long common grazing of an E+ pasture (0.51 ppm total ergovaline per ergovalinine; 10.1 ha). Steers were assigned (n = 8 per treatment) to the same Se-form treatments upon which they were raised. Selenium treatments were administered by daily top-dressing 85 g of VM mix onto 0.23 kg soyhulls, using in-pasture Calan gates. The PROC MIXED procedure of SAS was used to assess effect of Se-form treatments on whole blood Se (ng/mL) and serum prolactin (ng/mL) at day 0, 22, 43, 64, and 86, and caudal arterial area (mm2) at day −7, 43, and 86. The effect of Se treatment on ADG (day 86), and liver glutamine synthetase (GS) mRNA, protein, and activity (nmol/mg wet tissue/min) were assessed using the PROC GLM procedure of SAS. Fisher’s protected LSD procedure was used to separate treatment means. Whole blood Se increased (P < 0.01) for all treatments from day 0 to 22 and then did not change (P ≥ 0.17), and was greater (P ≤ 0.04) for MIX and OSe steers. Serum prolactin decreased (P < 0.01) over time and was greater (P < 0.05) for MIX and OSe steers. Liver GS mRNA content was 66% and 59% greater (P < 0.05) in MIX and OSe steers, respectively, than ISe steers. Liver GS protein content in MIX steers was 94% more (P < 0.01) than ISe steers. Moreover, MIX and OSe steers had 99% and 55% more (P ≤ 0.01) liver GS activity, respectively, than ISe steers. ADG was not affected (P = 0.36) by Se treatments. We conclude that consumption of 3 mg Se/d as OSe or MIX forms of Se in VM mixes increased 1) whole blood Se content, an indicator of greater whole-body Se assimilation; 2) serum prolactin, the reduction of which is a hallmark of fescue toxicosis; and 3) hepatic GS activity, indicating greater hepatic assimilation of acinar ammonia. However, 4) these positive effects on metabolic parameters were not accompanied by increased growth performance.
Keywords: fescue toxicosis, glutamine synthetase, prolactin, selenium supplementation, steer
INTRODUCTION
It is well established that cattle consuming tall fescue (Lolium arundinaceum) infected with a symbiont endophyte (Epichloë coenophiala) develop a syndrome of negatively altered physiological systems, collectively known as fescue toxicosis (Strickland et al., 2011). In addition to the classical decrease in prolactin, recent studies have identified that the hepatic metabolism of specific AA is altered in fescue toxicosis cattle (Brown et al., 2009; Liao et al., 2015), including a putative decrease in glutamine synthetase (GS) expression (Matthews and Bridges, 2014).
Another challenge to endophyte-infected tall fescue-based beef cattle operations is that the soils often are Se poor, necessitating the need to provide supplemental Se (Dargatz and Ross, 1996). Inorganic Se (ISe, sodium selenite) is the most common Se supplemented in cattle diets, whereas organic forms of Se (OSe), derived from specially cultivated Saccharomyces cerevisiae, also are available and approved for use in beef cattle diets. Supplementation (3 mg/d) with an 1:1 blend of ISe:OSe (MIX) or OSe vs. ISe results in greater amounts of Se in blood and liver of cattle (Brennan et al., 2011). Moreover, MIX stimulates expression of genes involved in hepatic selenoprotein synthesis and glutamate/glutamine metabolism relative to ISe or OSe (Matthews et al., 2014). Serendipitously, some of the genes upregulated by MIX were found downregulated in the liver (Liao et al., 2015) and pituitary (Li et al., 2017) of steers grazing high vs. low endophyte-infected forages.
The goal of this study was to test the general hypotheses that the form (ISe, OSe, and MIX) of supplemental Se in vitamin-mineral (VM) mixes consumed by growing beef steers grazing endophyte-infected tall fescue forage would ameliorate negative physiological parameters associated with fescue toxicosis, including depressed serum prolactin and hepatic GS.
MATERIALS AND METHODS
All experimental procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Animals
Twenty-four suckling, predominantly Angus, beef steers (BW: 182.6 ± 33.9 kg, age: 165.5 ± 14.2 day) were randomly selected (n = 8) from herds of fall-calving cows grazing toxic endophyte-infected tall fescue mixed forage pastures and consuming VM mixes that contained 35 mg/kg Se as ISe (sodium selenite, Prince Se Concentrate; Prince Agri Products, Inc., Quincy, IL), OSe (SEL-PLEX, Alltech Inc., Nicholasville, KY), or an 1:1 blend of ISe:OSe (MIX) forms.
Steers were removed from their dams; housed in feedlot pens of 4; fed (ad libitum access) a hay, soyhull, and DDGS-based “adaptation” diet; and had ad libitum access to a basal free-choice, VM mix (see below) that lacked Se for 40 d. Then, over a 39-d period (in consecutive groups of six, two steers from each treatment group, 2 wk/group), steers were moved to an in-pasture Calan gate facility and trained to eat the adaptation diet from individual in-pasture Calan gates. During this 39-d period, steers continued to have ad libitum access to the Se-free basal VM mix. Next, over a 19-d period, steers were transitioned from the adaptation diet to a common mixed-forage pasture that lacked endophyte-infected tall fescue and trained to consume the Se-free basal VM mix from individual Calan gates by top-dressing 85 g of the basal VM mix onto 227 g soyhulls. In summary, over a 98-d period (March 11 to June 17, 2015) steers were weaned, commonly depleted of Se, and trained to consume VM mix from in-pasture Calan gates. During this pretrial Se-depletion period, the ADG of steers was 0.82 ± 0.18 kg/d.
Steers then began (day 0) an 86-d (June 17 to September 9, 2015) period in which they commonly grazed a predominately endophyte-infected tall fescue-mixed pasture (10.1 ha) and individually consumed their respective Se-form-specific VM mix treatments, through the use of in-pasture Calan gates. During this 86-d period, two ISe steers were removed from the trial due to a bad hoof and failure to consume their mineral treatment. Thus, the final number of experimental observations was as follows: ISe = 6, and OSe and MIX = 8.
The composition (analyzed by Dairy One Cooperative, Inc., Ithaca, NY) of the basal VM mix was 11.62% Ca; 4.88% P; 1.05% S; 1.95% Mg; 0.88% K; 9.95% Na; 1,321 mg/kg Fe; 3,388 mg/kg Zn; 1,761 mg/kg Cu; 5,262 mg/kg Mn; 0.98 mg/kg Mo; 19.07 mg/kg Co; 550,000 IU/kg vitamin A; and 550 IU/kg vitamin E. The Se-specific mixes contained the basal mix plus 35 mg/kg Se as ISe, OSe, or MIX. As noted above, to ensure individual consumption of 3 mg Se/d·steer, 85 g of the specific VM mix (35 mg/kg Se) was top-dressed onto 227 g of soyhulls in each individual Calan gate feeder. The consumption of the mixture of soyhulls/VM mix was monitored daily, and all steers consumed all of the mixture every day. Thus, every steer consumed 3 mg of supplemental Se/d.
On day 0 and 86, shrunk BW (steers were denied access to water and forage for 12 h) was determined to calculate ADG. After day 86 shrunk BW determination, steers were then returned to pasture and, within their Se treatment, randomly assigned to 1 of 8 slaughter d (from day 93 to 119) and maintained on their Se treatments until their slaughter.
Pasture Sampling and Analysis
Pasture samples were collected on day 0, 22, 43, 64, 86, and 115 for determination of ergot alkaloid (ergovaline and ergovalinine) concentrations as previously described (Brown et al., 2009). Samples were stored on ice during transportation to the laboratory and then frozen and stored at −20 °C. Analysis of ergot alkaloids was performed by a high-performance liquid chromatography fluorescence procedure (Carter et al., 2010). Proximate analysis and mineral content were determined (http://dairyone.com/wp-content/uploads/2014/02/Forage-Lab-Analytical-Procedures-Listing-Alphabetical-July-2015.pdf) by Dairy One Cooperative, Inc.
Blood Collection
Jugular vein blood samples were collected by venipuncture on day 0, 22, 43, 64, and 86. For the preparation of whole blood, 8 mL of blood was collected in sodium heparin-containing blood collection tubes (Becton Dickinson, Franklin Lakes, NJ). For serum, 16 mL of blood was collected in serum blood collection tubes (Becton Dickinson) without an anticoagulant. Serum was recovered by centrifugation at 3,000 × g for 10 min at 4 °C and stored at −80 °C. Whole blood Se concentrations were analyzed by Michigan State University Diagnostic Center for Population and Animal Health (DCPAH) using an Agilent 7900 inductively coupled plasma-mass spectrometer as described previously (Wahlen et al., 2005). Serum prolactin concentrations were quantified by the laboratory of Dennis Hallford (New Mexico State University), using a double antibody RIA as described previously (Spoon and Hallford, 1989). The interassay CV was 9.5%, and the intra-assay CV was 6.6%.
Slaughter and Tissue Collection
Steers were slaughtered over a 26-d period, from day 93 to 119 (September 17 to October 13, 2015) of the study. Specifically, one OSe and one MIX steer were killed on the first two slaughter d. On the remaining six slaughter d, one steer each from ISe, OSe, and MIX treatment groups were killed. On any given slaughter day, steers were transported from the University of Kentucky Research and Education Center at Princeton, KY, to the University of Kentucky Meat Laboratory at Lexington, KY, and allowed to rest 1 to 2 h before slaughter and had ad libitum access to water. Body weight was determined, and steers were stunned by captive-bolt gun followed by exsanguination. Liver samples were collected from the mid-lower right lobe and used fresh for determination of GS activity. For real-time RT-PCR analysis, liver samples were placed in foil packs, snap-frozen in liquid nitrogen, and stored at −80 °C. Longissimus dorsi (LM) tissue was collected from between the 12th and 13th rib from the left side of the carcass and used fresh for determination of GS activity.
Analysis of GS Activity in Hepatic and LM Tissue Homogenates
After collection, fresh liver and LM muscle tissue were put on ice and immediately transferred to the laboratory. About 0.4 g of tissue was homogenized (30,000 rpm for two 15 s cycles) in five volumes of ice-cold extraction buffer (pH 7.9), containing 50 mM Tris and 2 mM EDTA, using a PowerGen 124 homogenizer (Thermo Fisher Scientific, Waltham, MA). The homogenate was centrifuged at 2,000 × g and 4 °C for 10 min. The derived supernatant was assayed for GS (EC 6.3.1.2) activity using a radiochemical method modified from James et al. (1998). A 50 µL aliquot of the supernatant was mixed with 200 µL of reaction medium which consisted of 0.25 µCi L-[1-14C]glutamate (ARC, Saint Louis, MO; specific activity: 50 to 60 mCi/mmol), 25 mM unlabeled glutamate, 25 mM MgCl2, 25 mM NH4Cl, 18.75 mM ATP, 12.5 mM phosphocreatine, 4 units creatine kinase, and 62.5 mM imidazole-HCl buffer, at pH 7.6. The mixture was incubated at 37 °C for 20 min, and the reaction was then terminated by adding 1 mL of ice-cold 20 mM imidazole-HCl buffer, at pH 7.5. A sample of the incubate (1 mL) was loaded into a 4 mL prefilled (formate resin) ion-exchange column (Bio-Rad, Hercules, CA) previously rinsed by distilled water. The column was washed with 4 mL of distilled water, and the effluent was collected. A sample of effluent (1 mL) was mixed with 4 mL of a ScintiSafe Plus 50% cocktail (Thermo Fisher Scientific), and the radioactivity was measured in a liquid scintillation analyzer TRI-CARB 2900TR (PerkinElmer, Downers Grove, IL). Assays were conducted in triplicate. Positive control contained 2.5 units of GS from Escherichia coli (Sigma, Saint Louis, MO) instead of supernatant from liver or LM muscle tissue. Negative control contained neither supernatant nor GS. GS activity is expressed as nmol/min/mg wet mass. The intra- and interassay CV were 8.5% and 8.8%, respectively.
Caudal Artery Area Measurement
Ultrasound measures of caudal artery area were performed on day −7, 43, and 86 of the experiment as previously described (Aiken et al., 2007). Briefly, caudal artery at the fourth coccygeal vertebra was scanned by an Aloka 3500 Ultrasound Unit (Aloka Inc., Wallingford, CT) with a UST-5542 (13 MHz) linear array transducer set to a depth of 2 cm. Three cross-sectional color Doppler flow scans were taken to determine the mean artery lumen area (mm2).
RNA Extraction and Analysis
For each animal, total RNA was extracted from 300 mg of frozen liver tissue using TRIzol Reagent (Invitrogen Corporation, Carlsbad, CA) following the manufacturer’s instructions. The purity and concentration of total RNA samples were analyzed using a NanoDrop ND-100 Spectrophotometer (NanaDrop Technologies, Wilmington, DE). All samples had an average concentration of 1.05 μg/μL and a high purity with 260/280 absorbance ratios of 1.98 to 2.03 and 260/230 absorbance ratios ranging from 1.89 to 2.18. The integrity of total RNA was examined by gel electrophoresis using an Agilent 2100 Bioanalyzer System (Agilent Technologies, Santa Clara, CA) at the University of Kentucky Microarray Core Facility. Visualization of gel images and electropherograms showed that all RNA samples were of high quality with RNA integrity numbers (RIN) being greater than 8.7 and 28S/18S rRNA absorbance ratios greater than 2.
Real-Time RT-PCR Analysis
The quantification of relative mRNA for genes of interest was performed using standard procedures in our laboratory, as previously described (Cerny et al., 2016). Briefly, 1 µg of each steer’s liver RNA was reversely transcribed to cDNA using the SuperScript III 1st Strand Synthesis System (Invitrogen). Real-time RT-PCR were performed using an Eppendorf Mastercycler ep realplex2 system (Eppendorf, Hamburg, Germany) with iQ SYBR Green Supermix (Bio-Rad). A total volume of 25 µL was used in each real-time RT-PCR reaction containing 5 µL of cDNA, 1 µL of a 10 µM stock of each primer (forward and reverse), 12.5 µL of 2× SYBR Green PCR Master Mix, and 5.5 µL of nuclease-free water. The relative amount of each transcript was calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001). Primer sets (Supplementary Table S1) for genes of interest were designed and obtained from NCBI Primer-BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) against RefSeq sequence (accessed February to April 2016).
All real-time RT-PCR cDNA products were validated by DNA sequencing verification and were 97% to 100% identical with their reference mRNA sequences (Supplementary Table S1). Briefly, the PCR-amplified cDNA products were electrophoresed in a 1.2% agarose (UltraPure Agarose, Invitrogen) slab gel. A single cDNA band at the desired size was identified under a UV light, excised from the gel, purified using the PureLink Quick Gel Extraction Kit (Invitrogen), then sequenced by Eurofins Genomics (Eurofins MWG Operon LLC, Louisville, KY). The resulting sequences (Supplementary Table S1) were compared with the NCBI RefSeq mRNA sequences used as templates for primer pair set design. Three constitutively expressed genes (GAPDH, PLOD1, and PCK2) were used, and their CT values were not affected (P > 0.43) by the Se-form treatment. Thus, the relative mRNA expression was normalized to the geometric means of three constitutively expressed genes. For the RT-PCR analysis, n = 6, 8, and 8 samples were used for ISe, OSe, and MIX treatments, respectively. RT-PCR reactions were performed in triplicate.
Immunoblot Analysis
All immunoblot and densitometric analyses for the relative expression of targeted proteins were performed using a standard protocol of our lab as previously described (Miles et al., 2015). Approximately 0.2 g of liver was homogenized on ice for 30 s (setting 11, Polytron Model PT10/35, Kinematic Inc., Lucerne, Switzerland) in 7.5 mL of 4 °C sample extraction buffer solution (0.25 mM sucrose, 10 mM HEPES-KOH pH 7.5, 1 mM EDTA, and 50 µL of protease inhibitor [Sigma]). Protein was quantified by a modified Lowry assay, using bovine serum albumin as a standard (Kilberg, 1989). Proteins were separated using 12% SDS-PAGE and electrotransferred onto a 0.45 μm nitrocellulose membrane (Bio-Rad) and then stained with Fast-Green (Fisher, Pittsburgh, PA). The relative amount of stained protein per lane per sample was determined by densitometric analysis and recorded as arbitrary units (Brown et al., 2009).
The relative content of GS in liver homogenate was evaluated using an antibody as previously described (Miles et al., 2015). Briefly, blots were hybridized with 1.25 µg of IgG anti-sheep polyclonal antibody (BD Biosciences, San Jose, CA) per milliliter of blocking solution (5% nonfat dry milk [wt/vol], 10 mM Tris-Cl [pH 7.5], 100 mM NaCl, 0.1% Tween 20 [vol/vol]) for 1 h at 37 °C with gentle rocking. Protein-primary antibody binding reactions were visualized with a chemiluminescence kit (Pierce, Rockford, IL) after hybridization of primary antibody with horseradish peroxidase–conjugated goat anti-mouse IgG (BD Biosciences; GS, 1:5000).
Densitometric analysis of immunoreactive products was performed as described previously (Miles et al., 2015). After exposure of auto-radiographic film (Amersham, Arlington Heights, IL), digital images of all observed immunoreactive species were recorded and quantified as described (Dehnes et al., 1998) using the Bio-Rad Versadoc imaging system and the Quantity One Program (Version 4.2.3, Bio-Rad). A single immunoreaction product (GS, 42.5 kDa) was assessed for treatment effect by densitometric analysis. The linearity of antibody-ligand immunoreactions and densitometry were validated using immunoblots containing protein gradients (data not shown). Data were collected as arbitrary densitometric units and then were corrected for unequal loading and/or transfer of proteins by normalization to densitometric values of Fast-Green-stained proteins common to all immunoblot lanes/samples. The range of Fast-Green normalization for GS was 6.9% to 8.2%. For all results, densitometric values were normalized to ISe steers by obtaining an average ISe steer densitometric value and dividing all results by this value.
Statistical Analysis
Data are presented as least square means (±SEM). Steers were the experimental units. The effect of Se supplementation on whole blood Se, serum prolactin, and caudal artery area was evaluated by ANOVA, using the PROC MIXED procedure of SAS (v 9.4, SAS Inst. Inc., Cary, NC). The statistical model included Se supplementation, time, and their interaction as fixed effects. class variables were Se supplementation and steer, with steer included in the random statement. The Kenward-Roger adjustment was used to calculate the denominator of df (Kenward and Roger, 1997). Within Se supplement treatment, nonorthogonal polynomial contrasts (linear, quadratic, cubic, and quartic) were used to characterize the effect of Se treatments over time on whole blood Se and serum prolactin concentration, using the PROC IML procedure of SAS to generate coefficients for unequally spaced contrasts. After slaughter, the effect of Se supplementation on hepatic GS activity, and the relative abundance of hepatic GS mRNA and protein was assessed by ANOVA, using the PROC GLM procedure of SAS. Fisher’s protected LSD procedure was used to separate treatment means of the time-course data.
RESULTS
Nutrient and Ergot Alkaloid Profiles of Forages
The composited means of proximate, mineral, and alkaloid analysis of pasture samples are presented in Table 1. The pasture samples from throughout the grazing periods had a CP of 16.3%, Se of 0.07 μg/g DM, and total ergot alkaloids of 0.51 μg/g.
Table 1.
Proximate, mineral, and alkaloid analysis of composited pasture samples (DM basis)1
| Item | Value |
|---|---|
| Proximate analysis, % | |
| DM | 28.05 |
| TDN | 59.42 |
| CP | 16.26 |
| Adjusted CP | 15.84 |
| ADF | 37.58 |
| aNDF | 60.08 |
| Crude fat | 2.62 |
| Lignin | 6.46 |
| Starch | 0.89 |
| Mineral analysis | |
| Ash, % | 10.32 |
| Calcium, % | 0.6 |
| Phosphorus, % | 0.25 |
| Magnesium, % | 0.23 |
| Potassium, % | 2.08 |
| Sodium, % | 0.01 |
| Sulfur, % | 0.28 |
| Iron, ppm | 438.67 |
| Zinc, ppm | 28.5 |
| Copper, ppm | 6.17 |
| Manganese, ppm | 63.5 |
| Molybdenum, ppm | 1.68 |
| Selenium, ppm | 0.07 |
| Ergot alkaloid analysis, µg/g | |
| Ergovaline | 0.34 |
| Ergovalinine | 0.17 |
| Total ergot alkaloids | 0.51 |
1Values are the average of samples collected on day 0, 22, 43, 64, 86, and 115, and presented on a DM basis. Samples were obtained systematically from approximately 30 sites from a common pasture, using a knife to cut the forage at approximately 2 cm above soil level.
Whole Blood Se Concentration
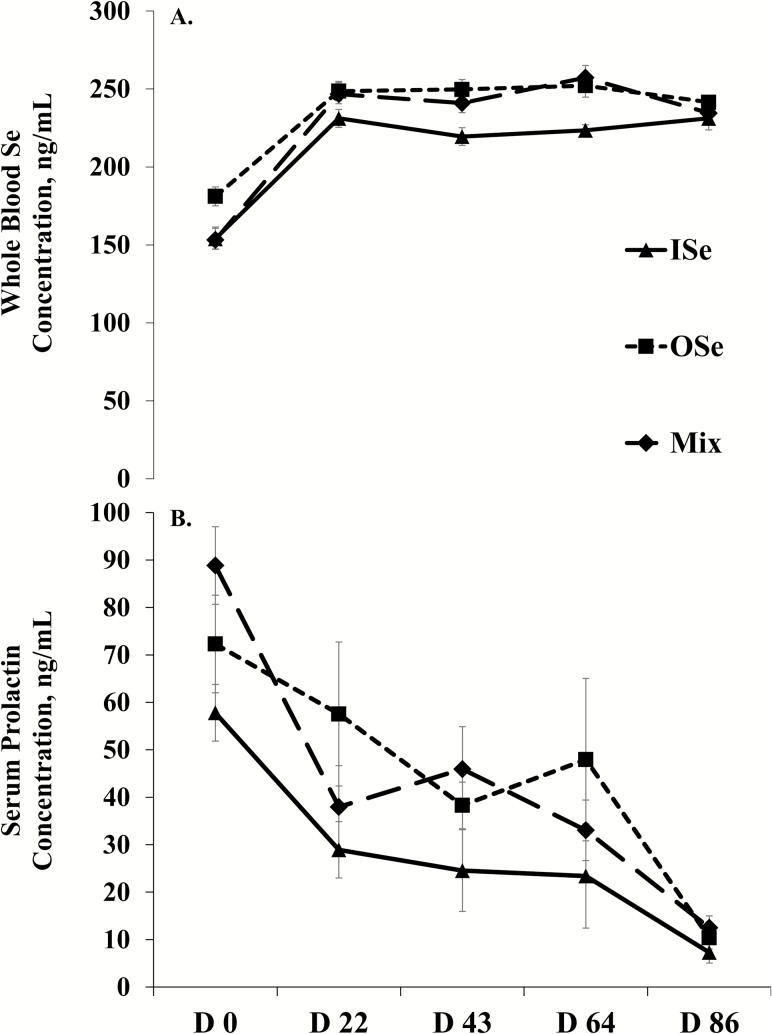
The concentration of Se in whole blood was affected by Se supplementation treatment (P = 0.01), time (P < 0.01), and their interaction (P < 0.01) (Figure 1A). Specifically, OSe steers and MIX steers had 11% (P < 0.01) and 7.5% (P = 0.04) more whole blood Se than ISe steers, respectively, and did not differ from each other (P = 0.30). Across Se supplementation treatments, the whole blood Se concentration increased (P < 0.01) 49% from day 0 to 22 and then did not change (P ≥ 0.17) throughout day 86 (Figure 1A). Within Se treatments, nonorthogonal polynomial contrast analysis showed that the concentrations of whole blood Se essentially increased in a quadratic manner (P < 0.01) over time for all three Se treatments.
Figure 1.
Whole blood selenium (Se) (A) and serum prolactin (B) concentrations in growing beef steers grazing endophyte-infected tall fescue and supplemented with 3 mg Se/d in VM mixes as sodium selenite (ISe), SEL-PLEX (OSe), or an 1:1 blend of ISe and OSe (MIX). Data are least squares means (n = 6 for ISe, n = 8 for OSe and MIX) ± SE. (A) Selenium form (P = 0.01), time (P < 0.01), and Se form by time interaction (P < 0.01) effects. Nonorthogonal polynomial contrasts: ISe, linear (P < 0.01), quadratic (P < 0.01), cubic (P < 0.01), and quartic (P = 0.03) effects; OSe, linear (P < 0.01), quadratic (P < 0.01), and cubic (P = 0.03) effects; MIX steers, linear (P < 0.01), quadratic (P < 0.01), and quartic (P = 0.04) effects. (B) Selenium form (P < 0.05) and time (P < 0.01) effects. Nonorthogonal polynomial contrasts: ISe, linear (P < 0.01) effect; OSe, linear (P < 0.01) effects; MIX, linear (P < 0.01) and cubic (P < 0.01) effects.
Serum Prolactin Concentration
The concentration of prolactin in serum was affected (P < 0.05) by Se treatment (Figure 1B). Across periods, OSe and MIX steers had 59% (P < 0.03) and 52% (P < 0.05) more serum prolactin than ISe steers, respectively. A time effect was found (P < 0.01) in serum prolactin concentration, but there was no (P = 0.12) Se treatment by time interaction. Across Se treatments, serum prolactin concentration decreased (P < 0.01) from day 0 to 22, plateaued (P > 0.25) from day 22 to 64, and then decreased (P < 0.01) again from day 64 to 84. Within Se treatments, nonorthogonal polynomial contrasts analysis revealed that serum prolactin essentially decreased in a linear (P < 0.01) manner for OSe and ISe steers. In contrast, serum prolactin essentially decreased in a cubic manner (P < 0.01) for MIX steers, apparently reflecting a decrease from day 0 to 22 and then from day 64 to 86.
GS Activity and Expression
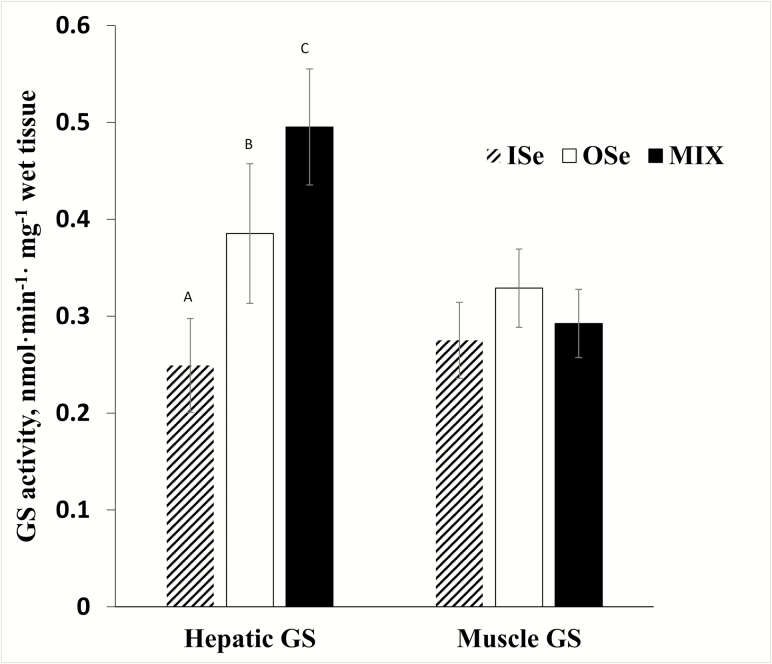
At slaughter, Se treatment affected (P < 0.01) hepatic GS activity of steers (Figure 2), where MIX steers had 99% (P < 0.01) and 29% (P = 0.03) more activity than ISe and OSe steers, respectively, and OSe steers had 55% more (P = 0.01) than ISe steers. In contrast, Se treatment did not affect (P = 0.21) GS activity in LM tissue (Figure 2).
Figure 2.
GS activity in liver and Longissimus dorsi (Muscle) homogenates of growing beef steers grazing endophyte-infected tall fescue and supplemented with 3 mg Se/d in VM mixes as either sodium selenite (ISe), SEL-PLEX (OSe), or an 1:1 blend of ISe and OSe (MIX). Data are least squares means (n = 6 for ISe, n = 8 for OSe and MIX) ± SE. Selenium form effect on liver GS activity (P < 0.01). Means with different letters differ (P < 0.05).
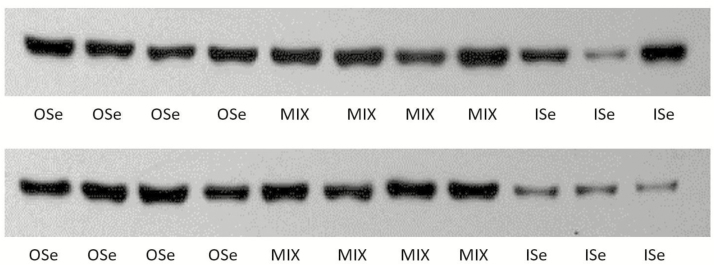
The relative expression of hepatic GS mRNA differed (P = 0.02) among Se treatment steers (Table 2). MIX steers had 66% more (P < 0.01) hepatic GS mRNA expression than did ISe steers, and there was 59% more (P < 0.05) hepatic GS mRNA in OSe than in ISe steers. However, hepatic GS mRNA content did not differ (P = 0.75) between OSe and MIX steers. Similarly, the relative hepatic GS protein content was affected (P = 0.02) by Se treatments (Table 2; Figure 3). Specifically, MIX steers had 94% more (P < 0.01) hepatic GS protein than ISe steers. However, the hepatic GS protein content of OSe steers did not differ (P > 0.05) with that of both ISe and MIX steers.
Table 2.
Comparison of glutamine synthetase mRNA and protein content in liver homogenates of growing beef steers grazing endophyte-infected tall fescue and supplemented with 3 mg Se/d in VM mixes as either sodium selenite (ISe), SEL-PLEX (OSe), or an 1:1 blend of ISe and OSe (MIX)1
| Glutamine synthetase | Treatment2 | ||||
|---|---|---|---|---|---|
| ISe | OSe | MIX | SEM3 | P value | |
| mRNA4 | 1.03a | 1.64b | 1.71b | 0.15 | 0.02 |
| Protein5 | 2,098a | 3,041ab | 3,632b | 331 | 0.02 |
1Values are least squares means (n = 6 for ISe, n = 8 for OSe and MIX) and pooled SEM.
2Means within a row that lack a common letter differ (P < 0.05).
3Most conservative error of the mean.
4Values are relative level of mRNA expression.
5Values (arbitrary densitometric units) were determined by densitometric evaluation of Western blot data (Figure 3).
Figure 3.
Western blot analysis of glutamine synthetase protein (42.5 kDa) content in liver homogenates (20 µg/lane) of growing beef steers grazing endophyte-infected tall fescue and supplemented with 3 mg Se/d in VM mixes as sodium selenite (ISe), SEL-PLEX (OSe), or an 1:1 blend of ISe and OSe (MIX).
Caudal Artery Area
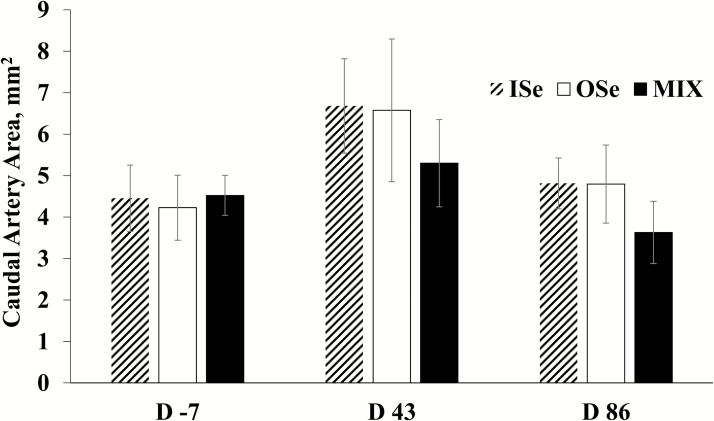
Selenium form did not affect (P = 0.65) caudal artery area, nor (P = 0.89) was there a Se form × time interaction (Figure 4). However, there was a time effect (P = 0.03). Specifically, across Se treatments, the caudal artery area at day 43 (6.19 ± 1.3 mm2) was 41% greater (P = 0.03) than day −7 (4.4 ± 0.69 mm2) and 40% greater (P = 0.02) than day 86 (4.41 ± 0.77 mm2), whereas the caudal area did not differ (P = 0.98) between day −7 and 86.
Figure 4.
Caudal artery area in growing beef steers grazing endophyte-infected tall fescue and supplemented with 3 mg Se/d in VM mixes as sodium selenite (ISe), SEL-PLEX (OSe), or an 1:1 blend of ISe and OSe (MIX). Data are least squares means (n = 6 for ISe, n = 8 for OSe and MIX) ± SE. Time (P = 0.03) effect.
Animal Performance
Final BW were 293.6, 293.5, and 272.0 kg, respectively, for ISe, OSe, and MIX steers, and did not differ (P = 0.34). Average daily gain was 0.43, 0.53, and 0.47 kg/day for ISe, OSe, and MIX steers, respectively, and was not affected by Se treatment (P = 0.36).
DISCUSSION
Animal Model
The goal of this study was to test the general hypotheses that the form of Se (ISe, OSe, and MIX) in VM mixes would affect the expression of important physiological parameters in growing beef steers commonly grazing an endophyte-infected tall fescue pasture. Steers had ad libitum access to their respective supplemental Se treatments as suckling calves before selection into the trial. During a pretrial Se-depletion period (98 d), steers received the basal VM mix that lacked any form of Se. From initiation of the trial to slaughter, steers individually consumed 85 g/d of a basal VM mix that contained 35 ppm Se supplied as ISe, OSe, or MIX forms. Thus, steers consumed 3 mg Se/d of their respective Se from initiation of the grazing period until their slaughter (up to 113 d) by use of Calan gates. The 3 mg Se/animal daily supplementation is the legal maximal limit for free-choice VM mixes allowed by the Food and Drug Administration and is considered adequate to achieve the NRC (NRC, 1996) recommendation of 0.1 mg/kg of diet. We chose to use a Se-adequate model to avoid confounding effects of Se deficiency and to legitimately compare the effect of Se form on experimental parameters.
Steers commonly grazed a single 10.1 ha predominately tall fescue-mixed pasture, which contained 0.51 ppm total ergot alkaloids (Table 1). In a previous study by our group (Brown et al., 2009), a similar level of ergot alkaloid challenge (0.52 ppm) induced fescue toxicosis in beef steers grazing endophyte-infected tall fescue and consuming ad libitum amounts of a similar VM mix that contained the ISe form of Se. Importantly, these steers had similar concentrations of serum prolactin and levels of ADG, as did the ISe steers of this study. The rapid increase then stabilization of Se assimilation in the whole blood of all three Se treatment groups is consistent with our previous Se depletion/repletion regimens with growing cattle (Brennan et al., 2011; Liao et al., 2011). Thus, the animal model of this study was successfully established to properly evaluate the potential effect of different Se-form treatments on physiological parameters of growing beef steers subjected to summer-long grazing of toxic endophyte-infected tall fescue pasture.
OSe and MIX Forms of Se Supplementation Resulted in Greater Whole Blood Se Than ISe
Selenium status is commonly determined by measuring the concentration of Se in blood or tissues, or the activity of glutathione peroxidase in blood. Whole blood Se concentrations are well correlated with Se intake (Patterson et al., 2013). Although there is no clear agreement in the literature as to what concentrations of whole blood Se are considered to be deficient or adequate, studies have reported that cattle have adequate levels of Se when whole blood Se is greater than 100 ng/mL (Gerloff, 1992) or between 81 to 160 ng/mL (Dargatz and Ross, 1996). In this study, across-periods, mean whole blood Se concentration ranged from 154 to 231 ± 5.4, 181 to 252 ± 4.7, and 153 to 257 ± 4.7 ng/mL in ISe, OSe, and MIX steers, respectively, indicating that all steers were Se adequate. Importantly, MIX and OSe steers had greater whole blood Se concentrations than did ISe steers. This finding is in agreement with previous studies, where greater whole blood Se was found in grazing cows supplemented with OSe at 3.3 mg/day·animal (Pehrson et al., 1999) or had ad libitum access to Se-containing mineral mixes (Gunter et al., 2013; Patterson et al., 2013). Of note, supplementation (3 mg/day) of slow maturing beef heifers with the MIX form of Se for 224 days resulted in similar Se concentrations in whole blood, red blood cells, serum, and liver compared with that supplemented with OSe (Brennan et al., 2011). Consistently, in this study with growing grazing steers, the whole blood Se concentration of MIX steers also was essentially the same as for OSe steers. This finding suggests that the MIX form of Se (1:1 equal blend of ISe and OSe) was assimilated as efficiently as was the 100% OSe.
Concentrations of Serum Prolactin Were Greater in MIX and OSe Steers
Reduced serum prolactin concentrations are a hallmark of fescue toxicosis (Goetsch et al., 1987; Davenport et al., 1993). One mechanism by which ergot alkaloids exert their inhibitory effect on serum prolactin is to bind dopamine receptor D2 (Larson et al., 1994), resulting in suppression of prolactin synthesis and release by lactotrophs within the anterior pituitary (Schillo et al., 1988; Li et al., 2017). In this study, OSe and MIX steers had greater serum prolactin concentrations than ISe steers throughout the grazing period. A priori, this novel finding suggests that supplemental OSe interacts in some manner with the prolactin synthetic cascade or enzymes involved in the degradation of circulating prolactin.
With regard to the first possibility, it may be reasonable to speculate that potential links exist between Se and prolactin given the critical roles of Se and selenoproteins in neurotransmission and brain function (Solovyev, 2015). More specifically, consumption (1 to 9 μg/g DM) of ISe (selenite), but not OSe resulted in increased dopamine concentrations in murine striatum (Tsunoda et al., 2000). As noted above, one of the key roles of dopamine is to negatively regulate prolactin secretion by binding and stimulating the dopamine receptor D2-mediated inhibition of prolactin synthesis (Fitzgerald and Dinan, 2008). The greater serum prolactin concentrations of MIX and OSe vs. ISe steers in this study appears consistent with the understanding that selenite supplementation increases dopamine content, accompanied by decreased prolactin synthesis in mouse brain models. Thus, it may be reasonable to speculate that the serum prolactin difference in this study may be due to the different dopamine concentrations induced by different form of Se. However, more research is needed about the expression and function of key enzyme/signaling proteins involved in lactotroph dopamine–prolactin interactions (such as dopamine receptor D2 (Beaulieu and Gainetdinov, 2011), prolactin receptor (Bernard et al., 2015), and Jak-Stat cascades (Rawlings et al., 2004)) to support this speculation.
As discussed early, consumption of endophyte-infected tall fescue suppresses serum prolactin. Unsurprisingly, in this study, the serum prolactin concentration decreased (86%) from day 0 to its nadir on day 86, regardless of the Se-form treatments of the steers. More specifically, from day 0 to 22, the serum prolactin concentration of all steers quickly dropped 43%, accounting for approximately half of the total reduction. The rapid decrease of serum prolactin concentrations after consumption of forages containing endophyte-infected tall fescue has been reported in steers (Aldrich et al., 1993; Jackson et al., 2015). The serum prolactin depression from day 0 to 84 observed in this study was approximately 86% (from 73.4 to 10.1 ng/mL). This magnitude of the reduction is in agreement with reported reductions of serum prolactin concentrations of 80% (Aldrich et al., 1993), 81% (Schillo et al., 1988), and 90% (Brown et al., 2009) in cattle consuming endophyte-infected vs. endophyte-free tall fescue.
This summer-long grazing study was conducted during June to September of 2015. Because reduction of photoperiod decreases concentrations of circulating prolactin in many species, including cattle (Dahl et al., 2000), it should be noted that the decrease in daylight also may have contributed to the depression of serum prolactin in steers from all three Se treatments.
Hepatic GS Activity, mRNA, and Protein Expression Are Greater in MIX Than ISe Steers
Part of the general hypotheses of this trial was that the form of supplemental Se would affect GS expression. This hypothesis was derived from a preliminary understanding (Matthews and Bridges, 2014) that the MIX form of Se increases the content of GS mRNA in steer liver, whereas the consumption of endophyte-infected tall fescue mixed forage decreases hepatic GS mRNA content. Thus, an important finding from this study was that hepatic GS activity was strongly affected (MIX > OSe > ISe) by the form of Se in the VM mix (Figure 2). In contrast, LM GS activity was not affected by Se-form treatment. Hepatic GS expression is restricted to pericentral hepatocytes (Haussinger, 1990). As such, GS is involved in scavenging ammonia that escapes periportal hepatocyte detoxification (urea cycle) by synthesizing glutamine from sinusoidal ammonia and glutamate (Wagenaar et al., 1994). Given the marked increase in hepatic GS activity by MIX and OSe steers, it is reasonable to expect that they possessed an increased N recycling capacity.
Although the regulatory mechanisms of GS have not been clearly defined, several studies indicate that GS activity is at least partially under transcriptional regulation (Gaunitz et al., 2001). More specifically, regulatory elements have been reported in the first intron (Fahrner et al., 1993) and in 5′ upstream regions (Gaunitz et al., 2001) of the GS gene. These regions contain binding sites for various transcription factors and have a functional role in the organ-specific expression patterns of GS, and in the restricted pericentral expression of GS within the liver (Lie-Venema et al., 1995). Moreover, it has been reported that posttranscriptional regulations such as alternative splicing also may contribute to the tissue-specific expression of GS (Shin et al., 2003). Although GS isolated from brain and other tissues of the same species have identical sequences, tissue-specific differences in substrate feedback regulation exist (Tate et al., 1972). Therefore, posttranslational modifications may exist (Eisenberg et al., 2000).
To gain insight into potential transcription, translational, or both, processes accounting for Se-form differences in hepatic GS activity, the relative mRNA, and protein abundance of GS was determined (Table 2). In agreement with the 99% greater hepatic GS activity, MIX steers had a 66% and 94% more hepatic GS mRNA and protein, respectively, than ISe steers. Although statistically not different from ISe steers, the 50% quantitatively greater GS protein content of OSe vs. ISe steers is consistent with the 59% greater mRNA content and 55% greater GS activity. Collectively, these data suggest that the MIX and OSe forms of Se affected at least the transcriptional regulation of hepatic GS.
Little is known about the potential for Se or selenoproteins to interact with GLUL (gene for GS) or GS. However, it has been reported that long-term supranutritional supplementation of sodium selenite to diabetic mice (vs. no supplementation) results in increased GS mRNA and protein (Wang et al., 2014). Although the specific mechanism(s) is unclear, our current finding in steer liver, coupled with this limited report in mouse liver, suggests that hepatic GS expression and activity may be sensitive to the form of Se and, possibly, the amount of supplemental Se.
Caudal Artery Area and Animal Performance Were Not Affected by Se Treatment
Caudal artery area.
Consumption of ergot alkaloids by cattle is known to constrict the caudal artery (Aiken et al., 2007), right ruminal artery and vein (Foote et al., 2011), and mesenteric artery and vein (Jia et al., 2015). Ergot alkaloid-induced vasoconstriction is thought to be a contributor to several symptoms of fescue toxicosis (Klotz and Nicol, 2016). Therefore, the caudal artery luminal area was determined in this study as a parameter to assess potential Se-form treatment differences. However, the caudal artery area was not affected by Se treatment. The range of the caudal artery areas was 4.40 to 6.19 mm2, which was higher (thus, less restricted) than the 2.0 to 3.8 mm2 (Aiken et al., 2007) and 1.2 to 5.0 mm2 (Aiken et al., 2009) reported for heifers (345 to 375 kg) consuming endophyte-infected tall fescue seed plus chopped alfalfa hay/concentrate diets. The difference in caudal artery areas in this study employing grazing steers and the earlier studies using confined heifers may be due to the difference of ergot alkaloids load. That is, in this study, the pasture contained 0.51 ppm ergovaline and ergovalinine, whereas endophyte-infected seed containing 1.17 (Aiken et al., 2009) to 1.21 ppm (Aiken et al., 2007) of ergovaline and ergovalinine were consumed by confined heifers. Another possible explanation for the relatively higher caudal artery area in this study may be an inherent difference between heifers and steers in artery size and luminal diameter. For example, intravascular ultrasound analysis has revealed that left main and proximal left anterior descending coronary arterial areas were smaller in women than in men (Sheifer et al., 2000).
Growth performance.
As noted above, the amount of ADG achieved by the ISe steers (0.43 kg/d) is almost identical to that achieved (0.40) by beef steers consuming endophyte-infected tall fescue pastures (0.52 ppm ergovaline/ergovalinine) and that had ad libitum access to ISe-containing (35 ppm) VM mix over a 85-d summer-long grazing period (Brown et al., 2009). In mammals, prolactin affects various physiologic parameters besides lactation, including osmoregulation, immunoregulation, and growth and development (Bole-Feysot et al., 1998). Moreover, prolactin has a somatogenic effect on postnatal body growth and BW of male rats (Perez-Villamil et al., 1992). However, the ADG by ISe steers did not differ from that by MIX or OSe steers over the 86-d grazing period. The form of Se (ISe, OSe, MIX) also does not affect the ADG of slow-growing beef heifers supplemented with 3 mg Se/day (Brennan et al., 2011). Similarly, no significant improvement on animal growth performances (ADG, ADI, or gain: feed ratio) has been reported by studies comparing the effects of OSe and ISe supplementation (Lawler et al., 2004; Brennan et al., 2011).
In summary, predominately Angus steers subjected to summer-long grazing of endophyte- infected pasture and supplemented (3 mg/d) with MIX or OSe forms of Se had greater whole blood Se, serum prolactin concentrations, and greater hepatic GS activity than ISe-supplemented steers. Despite these effects, no difference in growth performance was found.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Animal Science online.
Conflict of interest statement
None declared.
This is publication No. 17-07-115 of the Kentucky Agricultural Experiment Station and is published with the approval of the Director. This work is supported by a United States Department of Agriculture-Agricultural Research Service Cooperative Agreement (JCM) and by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project No. 1010352.
REFERENCES
- Aiken G.E., Kirch B.H., Strickland J.R., Bush L.P., Looper M.L., and Schrick F.N.. 2007. Hemodynamic responses of the caudal artery to toxic tall fescue in beef heifers. J. Anim. Sci. 85:2337–2345. doi:10.2527/jas.2006–821 [DOI] [PubMed] [Google Scholar]
- Aiken G.E., Strickland J.R., Looper M.L., Bush L.P., and Schrick F.N.. 2009. Hemodynamics are altered in the caudal artery of beef heifers fed different ergot alkaloid concentrations. J. Anim. Sci. 87:2142–2150. doi:10.2527/jas.2008-1562 [DOI] [PubMed] [Google Scholar]
- Aldrich C.G., Paterson J.A., Tate J.L., and Kerley M.S.. 1993. The effects of endophyte-infected tall fescue consumption on diet utilization and thermal regulation in cattle. J. Anim. Sci. 71:164–170. doi:10.2527/1993.711164x [DOI] [PubMed] [Google Scholar]
- Beaulieu J.M., and Gainetdinov R.R.. 2011. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 63:182–217. doi:10.1124/pr.110.002642 [DOI] [PubMed] [Google Scholar]
- Bernard V., Young J., Chanson P., and Binart N.. 2015. New insights in prolactin: pathological implications. Nat. Rev. Endocrinol. 11:265–275. doi:10.1038/nrendo.2015.36 [DOI] [PubMed] [Google Scholar]
- Bole-Feysot C., Goffin V., Edery M., Binart N., and Kelly P.A.. 1998. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 19:225–268. doi:10.1210/edrv.19.3.0334 [DOI] [PubMed] [Google Scholar]
- Brennan K.M., Burris W.R., Boling J.A., and Matthews J.C.. 2011. Selenium content in blood fractions and liver of beef heifers is greater with a mix of inorganic/organic or organic versus inorganic supplemental selenium but the time required for maximal assimilation is tissue-specific. Biol. Trace Elem. Res. 144:504–516. doi:10.1007/s12011-011-9069-y [DOI] [PubMed] [Google Scholar]
- Brown K.R., Anderson G.A., Son K., Rentfrow G., Bush L.P., Klotz J.L., Strickland J.R., Boling J.A., and Matthews J.C.. 2009. Growing steers grazing high versus low endophyte (Neotyphodium coenophialum)-infected tall fescue have reduced serum enzymes, increased hepatic glucogenic enzymes, and reduced liver and carcass mass. J. Anim. Sci. 87:748–760. doi:10.2527/jas.2008-1108 [DOI] [PubMed] [Google Scholar]
- Carter J.M., Aiken G.E., Dougherty C.T., and Schrick F.N.. 2010. Steer responses to feeding soybean hulls and steroid hormone implantation on toxic tall fescue pasture. J. Anim. Sci. 88:3759–3766. doi:10.2527/jas.2009–2536 [DOI] [PubMed] [Google Scholar]
- Cerny K.L., Garbacik S., Skees C., Burris W.R., Matthews J.C., and Bridges P.J.. 2016. Gestational form of selenium in free-choice mineral mixes affects transcriptome profiles of the neonatal calf testis, including those of steroidogenic and spermatogenic pathways. Biol. Trace Elem. Res. 169:56–68. doi: 10.1007/s12011-015-0386-4 [DOI] [PubMed] [Google Scholar]
- Dahl G.E., Buchanan B.A., and Tucker H.A.. 2000. Photoperiodic effects on dairy cattle: a review. J. Dairy Sci. 83:885–893. doi:10.3168/jds.S0022-0302(00)74952–6 [DOI] [PubMed] [Google Scholar]
- Dargatz D.A., and Ross P.F.. 1996. Blood selenium concentrations in cows and heifers on 253 cow-calf operations in 18 states. J. Anim. Sci. 74:2891–2895. doi:10.2527/1996.74122891x [DOI] [PubMed] [Google Scholar]
- Davenport G.M., Boling J.A., and Rahe C.H.. 1993. Growth and endocrine responses of cattle to implantation of estradiol-17 beta during continuous or discontinuous grazing of high- and low-endophyte-infected tall fescue. J. Anim. Sci. 71:757–764. doi:10.2527/1993.713757x [DOI] [PubMed] [Google Scholar]
- Dehnes Y., Chaudhry F.A., Ullensvang K., Lehre K.P., Storm-Mathisen J., and Danbolt N.C.. 1998. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J. Neurosci. 18:3606–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Gill H.S., Pfluegl G.M., and Rotstein S.H.. 2000. Structure-function relationships of glutamine synthetases. Biochim. Biophys. Acta 1477:122–145. doi:10.1016/S0167-4838(99)00270-8 [DOI] [PubMed] [Google Scholar]
- Fahrner J., Labruyere W.T., Gaunitz C., Moorman A.F., Gebhardt R., and Lamers W.H.. 1993. Identification and functional characterization of regulatory elements of the glutamine synthetase gene from rat liver. Eur. J. Biochem. 213:1067–1073. doi:10.1111/j.1432-1033.1993.tb17854.x [DOI] [PubMed] [Google Scholar]
- Fitzgerald P., and Dinan T.G.. 2008. Prolactin and dopamine: what is the connection? A review article. J. Psychopharmacol. 22:12–19. doi:10.1177/0269216307087148 [DOI] [PubMed] [Google Scholar]
- Foote A.P., Harmon D.L., Strickland J.R., Bush L.P., and Klotz J.L.. 2011. Effect of ergot alkaloids on contractility of bovine right ruminal artery and vein. J. Anim. Sci. 89:2944–2949. doi:10.2527/jas.2010–3626 [DOI] [PubMed] [Google Scholar]
- Gaunitz F., Weber S., Scheja L., and Gebhardt R.. 2001. Identification of a cis-acting element and a novel trans-acting factor of the glutamine synthetase gene in liver cells. Biochem. Biophys. Res. Commun. 284:377–383. doi:10.1006/bbrc.2001.4967 [DOI] [PubMed] [Google Scholar]
- Gerloff B.J. 1992. Effect of selenium supplementation on dairy cattle. J. Anim. Sci. 70:3934–3940. doi:10.2527/1992.70123934x [DOI] [PubMed] [Google Scholar]
- Goetsch A.L., Jones A.L., Stokes S.R., Beers K.W., and Piper E.L.. 1987. Intake, digestion, passage rate and serum prolactin in growing dairy steers fed endophyte-infected fescue with noninfected fescue, clover or wheat straw. J. Anim. Sci. 64:1759–1768. doi:10.2527/jas1987.6461759x [DOI] [PubMed] [Google Scholar]
- Gunter S.A., Beck P.A., and Hallford D.M.. 2013. Effects of supplementary selenium source on the blood parameters in beef cows and their nursing calves. Biol. Trace Elem. Res. 152:204–211. doi:10.1007/s12011-013-9620-0 [DOI] [PubMed] [Google Scholar]
- Haussinger D. 1990. Nitrogen metabolism in liver: structural and functional organization and physiological relevance. Biochem. J. 267:281–290. doi:10.1042/bj2670281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J.J., Lindemann M.D., Boling J.A., and Matthews J.C.. 2015. Summer-long grazing of high vs. low endophyte (Neotyphodium coenophialum)-infected tall fescue by growing beef steers results in distinct temporal blood analyte response patterns, with poor correlation to serum prolactin levels. Front. Vet. Sci. 2:77. doi:10.3389/fvets.2015.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James L.A., Lunn P.G., and Elia M.. 1998. Glutamine metabolism in the gastrointestinal tract of the rat assess by the relative activities of glutaminase (EC 3.5.1.2) and glutamine synthetase (EC 6.3.1.2). Br. J. Nutr. 79:365–372. doi:10.1079/BJN19980061 [DOI] [PubMed] [Google Scholar]
- Jia Y., Harmon D.L., Flythe M.D., and Klotz J.L.. 2015. Interaction of isoflavones and endophyte-infected tall fescue seed extract on vasoactivity of bovine mesenteric vasculature. Front. Nutr. 2:32. doi:10.3389/fnut.2015.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenward M.G., and Roger J.H.. 1997. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997. doi:10.2307/2533558 [PubMed] [Google Scholar]
- Kilberg M.S. 1989. Measurement of amino acid transport by hepatocytes in suspension or monolayer culture. Methods. Enzymol. 173:564–575. doi:10.1016/S0076-6879(89)73039-1 [DOI] [PubMed] [Google Scholar]
- Klotz J., and Nicol A.. 2016. Ergovaline, an endophytic alkaloid. 1. Animal physiology and metabolism. Anim. Prod. Sci. 56:1761–1774. doi:10.1071/AN14962 [Google Scholar]
- Larson B.T., Sullivan D.M., Samford M.D., Kerley M.S., Paterson J.A., and Turner J.T.. 1994. D2 dopamine receptor response to endophyte-infected tall fescue and an antagonist in the rat. J. Anim. Sci. 72:2905–2910. doi:10.2527/1994.72112905x [DOI] [PubMed] [Google Scholar]
- Lawler T.L., Taylor J.B., Finley J.W., and Caton J.S.. 2004. Effect of supranutritional and organically bound selenium on performance, carcass characteristics, and selenium distribution in finishing beef steers. J. Anim. Sci. 82:1488–1493. doi:10.2527/2004.8251488x [DOI] [PubMed] [Google Scholar]
- Li Q., Hegge R., Bridges P.J., and Matthews J.C.. 2017. Pituitary genomic expression profiles of steers are altered by grazing of high vs. low endophyte-infected tall fescue forages. PLoS ONE 12:e0184612. doi:10.1371/journal.pone.0184612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S.F., Boling J.A., and Matthews J.C.. 2015. Gene expression profiling indicates an increased capacity for proline, serine, and ATP synthesis and mitochondrial mass by the liver of steers grazing high vs. low endophyte-infected tall fescue. J. Anim. Sci. 93:5659–5671. doi:10.2527/jas.2015–9193 [DOI] [PubMed] [Google Scholar]
- Liao S.F., Brown K.R., Stromberg A.J., Burris W.R., Boling J.A., and Matthews J.C.. 2011. Dietary supplementation of selenium in inorganic and organic forms differentially and commonly alters blood and liver selenium concentrations and liver gene expression profiles of growing beef heifers. Biol. Trace Elem. Res. 140:151–169. doi:10.1007/s12011-010-8685-2 [DOI] [PubMed] [Google Scholar]
- Lie-Venema H., Labruyere W.T., van Roon M.A., de Boer P.A., Moorman A.F., Berns A.J., and Lamers W.H.. 1995. The spatio-temporal control of the expression of glutamine synthetase in the liver is mediated by its 5’-enhancer. J. Biol. Chem. 270:28251–28256. doi:10.1074/jbc.270.47.28251 [DOI] [PubMed] [Google Scholar]
- Livak K.J., and Schmittgen T.D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. doi:10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Matthews J.C., and Bridges P.J.. 2014. NutriPhysioGenomics applications to identify adaptations of cattle to consumption of ergot alkaloids and inorganic versus organic forms of selenium: altered nutritional, physiological and health states?Anim. Prod. Sci. 54:1594–1604. doi:10.1071/AN14274 [Google Scholar]
- Matthews J.C., Zhang Z., Patterson J.D., Bridges P.J., Stromberg A.J., and Boling J.A.. 2014. Hepatic transcriptome profiles differ among maturing beef heifers supplemented with inorganic, organic, or mixed (50% inorganic:50% organic) forms of dietary selenium. Biol. Trace Elem. Res. 160:321–339. doi:10.1007/s12011-014-0050-4 [DOI] [PubMed] [Google Scholar]
- Miles E.D., McBride B.W., Jia Y., Liao S.F., Boling J.A., Bridges P.J., and Matthews J.C.. 2015. Glutamine synthetase and alanine transaminase expression are decreased in livers of aged vs. young beef cows and GS can be upregulated by 17beta-estradiol implants. J. Anim. Sci. 93:4500–4509. doi:10.2527/jas.2015–9294 [DOI] [PubMed] [Google Scholar]
- NRC 1996. Minerals. In: Nutrient requirments of beef cattle. 7th rev. ed Washington (DC): National Academies Press; p. 54–74. [Google Scholar]
- Patterson J.D., Burris W.R., Boling J.A., and Matthews J.C.. 2013. Individual intake of free-choice mineral mix by grazing beef cows may be less than typical formulation assumptions and form of selenium in mineral mix affects blood Se concentrations of cows and their suckling calves. Biol. Trace Elem. Res. 155:38–48. doi:10.1007/s12011-013-9768-7 [DOI] [PubMed] [Google Scholar]
- Pehrson B., Ortman K., Madjid N., and Trafikowska U.. 1999. The influence of dietary selenium as selenium yeast or sodium selenite on the concentration of selenium in the milk of Suckler cows and on the selenium status of their calves. J. Anim. Sci. 77:3371–3376. doi:10.2527/1999.77123371x [DOI] [PubMed] [Google Scholar]
- Perez-Villamil B., Bordiu E., and Puente-Cueva M.. 1992. Involvement of physiological prolactin levels in growth and prolactin receptor content of prostate glands and testes in developing male rats. J. Endocrinol. 132:449–459. doi:10.1677/joe.0.1320449 [DOI] [PubMed] [Google Scholar]
- Rawlings J.S., Rosler K.M., and Harrison D.A.. 2004. The JAK/STAT signaling pathway. J. Cell Sci. 117:1281–1283. doi:10.1242/jcs.00963 [DOI] [PubMed] [Google Scholar]
- Schillo K.K., Leshin L.S., Boling J.A., and Gay N.. 1988. Effects of endophyte-infected fescue on concentrations of prolactin in blood sera and the anterior pituitary and concentrations of dopamine and dopamine metabolites in brains of steers. J. Anim. Sci. 66:713–718. doi:10.2527/jas1988.663713x [DOI] [PubMed] [Google Scholar]
- Sheifer S.E., Canos M.R., Weinfurt K.P., Arora U.K., Mendelsohn F.O., Gersh B.J., and Weissman N.J.. 2000. Sex differences in coronary artery size assessed by intravascular ultrasound. Am. Heart. J. 139:649–653. doi:10.1016/S0002-8703(00)90043–7 [DOI] [PubMed] [Google Scholar]
- Shin D., Park S., and Park C.. 2003. A splice variant acquiring an extra transcript leader region decreases the translation of glutamine synthetase gene. Biochem. J. 374:175–184. doi:10.1042/BJ20030132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovyev N.D. 2015. Importance of selenium and selenoprotein for brain function: from antioxidant protection to neuronal signalling. J. Inorg. Biochem. 153:1–12. doi:10.1016/j.jinorgbio.2015.09.003 [DOI] [PubMed] [Google Scholar]
- Spoon R.A., and Hallford D.M.. 1989. Growth response, endocrine profiles and reproductive performance of fine-wool ewe lambs treated with ovine prolactin before breeding. Theriogenology 32:45–53. doi:10.1016/0093-691X(89)90520–7 [DOI] [PubMed] [Google Scholar]
- Strickland J.R., Looper M.L., Matthews J.C., Rosenkrans C.F. Jr., Flythe M.D., and Brown K.R.. 2011. Board-invited review: St. Anthony’s Fire in livestock: causes, mechanisms, and potential solutions. J. Anim. Sci. 89:1603–1626. doi:10.2527/jas.2010–3478 [DOI] [PubMed] [Google Scholar]
- Tate S.S., Leu F.Y., and Meister A.. 1972. Rat liver glutamine synthetase. Preparation, properties, and mechanism of inhibition by carbamyl phosphate. J. Biol. Chem. 247:5312–5321. [PubMed] [Google Scholar]
- Tsunoda M., Johnson V.J., and Sharma R.P.. 2000. Increase in dopamine metabolites in murine striatum after oral exposure to inorganic but not organic form of selenium. Arch. Environ. Contam. Toxicol. 39:32–37. doi:10.1007/s002440010076 [DOI] [PubMed] [Google Scholar]
- Wagenaar G.T., Geerts W.J., Chamuleau R.A., Deutz N.E., and Lamers W.H.. 1994. Lobular patterns of expression and enzyme activities of glutamine synthase, carbamoylphosphate synthase and glutamate dehydrogenase during postnatal development of the porcine liver. Biochim. Biophys. Acta. 1200:265–270. doi:10.1016/0304-4165(94)90166-X [DOI] [PubMed] [Google Scholar]
- Wahlen R., Evans L., Turner J., and Hearn R.. 2005. The use of collision/reaction cell ICP-MS for the determination of elements in blood and serum samples. Spectroscopy 20:84–89. [Google Scholar]
- Wang C., Yang S., Zhang N., Mu Y., Ren H., Wang Y., and Li K.. 2014. Long-term supranutritional supplementation with selenate decreases hyperglycemia and promotes fatty liver degeneration by inducing hyperinsulinemia in diabetic db/db mice. PLoS One 9:e101315. doi:10.1371/journal.pone.0101315 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.