Abstract
Effects of live yeast (Saccharomyces cerevisiae) in steam-flaked corn-based diets fed to natural-program beef cattle on growth performance, total tract apparent digestibility, carcass characteristics, and feeding behavior were evaluated in a randomized block design experiment. Steers (n = 144; 341 ± 7.03 kg) were blocked by initial BW and assigned randomly to 1 of the 3 treatments (n = 12 pens per treatment with 4 steers per pen). Treatments included the following: 1) control (CTL; no yeast); 2) low yeast (LY; 1.5 g/animal daily [3 × 1010 CFU]); and 3) high yeast (HY; 3.0 g/animal daily [6 × 1010 CFU]). Technologies such as implants, ionophores, and antibiotics were not used, and the steam-flaked corn-based finishing diets were fed to provide ad libitum access to feed. Yeast was included in a cottonseed meal–based premix as 1% of the dietary DM. Spot fecal samples (twice daily for 5 consecutive days) and diets were composited by pen and analyzed for acid insoluble ash to estimate apparent total tract digestibility of nutrients. Cattle were slaughtered on days 183 (4 blocks) and 204 (8 blocks). Dry matter intake (P ≥ 0.29), ADG (P ≥ 0.17), and G:F (P ≥ 0.33) did not differ among treatments. The percentage of Premium Choice (P < 0.01) carcasses increased linearly with increasing yeast inclusion in the diet. A quadratic response was observed for total tract apparent digestibility, in which steers fed LY had greater digestibility (P < 0.01) of DM by 5.4%, OM by 4.8%, NDF by 15.2%, ADF by 20.2%, CP by 6.2%, and ether extract (EE) by 2.5% compared with steers fed CTL. Feeding behavior was not affected (P = 0.28) by treatments. Live yeast improved digestibility of DM, OM, CP, EE, and fiber, without changing feeding behavior and growth performance of natural-program steers fed steam-flaked corn-based finishing diets.
Keywords: behavior, cattle, digestibility, natural, yeast
INTRODUCTION
Growth-promoting technologies are used by the beef industry to improve the performance of feedlot cattle. Increasingly, consumers want to know more about their food and are willing to pay a premium for beef that matches their specifications (Sparling et al., 2002). Without the use of conventional growth-promoting technologies (natural programs), efficiency of feedlot cattle is decreased. Implants alone improve ADG by as much as 17% (Wileman et al., 2009). Labels such as “never ever” and “no antibiotics” can be used by producers to achieve a premium (USDA-APHIS, 2013). Unfortunately, there is conflicting evidence as to the value of yeast products in the diet with respect to ruminal fermentation, production, and digestibility in dairy cattle (Desnoyers et al., 2009) and a limited number of studies involving beef cattle (Zinn et al., 1999; Zerby et al., 2011; and Swyers et al., 2014). With increasing demand for beef produced in natural programs, research to identify products such as yeast that will improve growth and efficiency of feedlot production for natural production systems is justified.
Yeast is a product that potentially works through encouraging cellulolytic bacterial growth within the ruminal environment via a decrease in lactic acid production in the rumen (Swyers et al., 2014). Yeast supplements are included in the diet to stimulate fiber-digesting bacteria, enabling the animal to grow more efficiently with extra energy derived from the fiber (Wiedmeier et al., 1987). The effects of yeast in feedlot diets on the performance of growing steers have been varying, presumably as a result of differing experimental and environmental conditions among experiments. Our objective was to determine whether dietary supplementation of live yeast improved growth performance, digestibility of nutrients, behavioral measurements, and carcass characteristics of finishing feedlot cattle fed steam-flaked corn-based diets.
MATERIALS AND METHODS
All procedures involving live animals were approved by the Texas Tech University Institutional Animal Care and Use Committee (Protocol No. 15044-05).
Cattle Management and Experimental Design
On June 4, 2015, medium- to large-framed beef steers (n = 171; Black Angus crossbred) arrived at Texas Tech University Burnett Center. On arrival, the cattle were randomly allocated into 16 receiving pens, individually identified with a unique ear tag, treated with an internal and external parasiticide (Safe-guard, Intervet, Inc., Millsboro, DE; and Noromectin Pour-on, Norbrook Laboratories Ltd, Newry, Northern Ireland), vaccinated for 1) bovine rhinotracheitis virus, parainfluenza 3-respiratory syncytial virus, Mannheimia haemolytica, and Pasteurella multocida (Vista Once SQ, Intervet, Inc., Omaha, NE); 2) Mycoplasma bovis bacterin (Mycoplaz, Vetbio, Inc., San Angelo, TX); and 3) Clostridium chauvoei-septicum-novyl-sordellii-perfringens types C & D bacterin-toxoid (Vision 7 with SPUR, Intervet, Inc., Omaha, NE), and injected a vitamin A, D, and E solution (Vital E-A + D3, Stuart Products, Bedford, TX). From receiving until day 0 (31 d), animals were limit fed (2% of BW) with standard receiving diet (Table 1). From June 17 to 30 (before study initiation), cattle were weighed and sorted into 12 BW blocks, with 3 pens per block, totaling 36 pens of 4 animals each (randomized complete block design). Cattle within blocks were allocated randomly into concrete, partially slotted floor pens (3 m wide × 6 m long; linear bunk space = 2.5 m) at the Texas Tech Burnett Center on June 30. After 1 wk of acclimation, the study was initiated on July 8 (day 0), when an initial BW measurement was taken. An adaptation step-up diet 2 (which already contained yeast treatments) was fed for 7 d, followed by step-up diet 3 fed for another 7 d, with the cattle fed the final finishing diets (Table 2) on day 15 and thereafter.
Table 1.
Dietary ingredients and calculated nutritional composition of receiving and step-up diets fed to steers
| Adaptation diets | |||
|---|---|---|---|
| Items | Receiving | Step-up 2 | Step-up 3 |
| Days on feed | 8 | 7 | 7 |
| Period included in the study | No | Yes | Yes |
| Ingredient, % DM | |||
| Steam-flaked corn (330 g/L) | 22.00 | 36.00 | 50.10 |
| Wet corn gluten feed (sweet bran, cargill corn milling) | 54.00 | 36.85 | 27.25 |
| Cottonseed hulls | 20.00 | 10.00 | 5.00 |
| Corn silage | 10.00 | 10.00 | |
| Sorghum silage | 2.50 | 2.50 | |
| Supplementa | 2.00 | 2.00 | 2.00 |
| Urea | 0 | 0 | 0.50 |
| Limestone | 2.00 | 1.65 | 1.65 |
| Premixb | 0 | 1.00 | 1.00 |
| Calculated compositionc, DM basis | |||
| DM, % as-is | 66.45 | 62.89 | 64.74 |
| CP | 17.15 | 14.62 | 14.62 |
| Ca | 0.79 | 0.67 | 0.65 |
| P | 0.68 | 0.56 | 0.49 |
| K | 1.22 | 1.06 | 0.91 |
| S | 0.31 | 0.25 | 0.22 |
| NEm, Mcal/kgd | 1.68 | 1.83 | 1.94 |
| NEg, Mcal/kgd | 1.10 | 1.21 | 1.30 |
aSupplement contained (DM basis) as follows: carrier (cottonseed meal), 73.2699%; potassium chloride, 10%; sodium chloride, 15%; cobalt carbonate, 0.0022%; copper sulfate, 0.1965%; iron sulfate, 0.0833%; ethylenediamine dihydroiodide, 0.0031%; manganous oxide, 0.167%; selenium premix (0.2% Se), 0.125%; zinc sulfate, 0.9859%; vitamin A (1,000,000 IU/g), 0.0099%; and vitamin E (500 IU/g), 0.1575%.
bPremix contained cottonseed meal for control diet; 24.8% (DM basis) yeast commercial product (ABVista) for LY diet; and 49.59% (DM basis) yeast commercial product for HY diet. Cottonseed meal was used as a carrier for the yeast premixes. Inclusions were based on an expected DMI of 9.5 kg/d.
cCalculated composition based on regularly analyzed individual ingredients used at the Burnett Center.
dCalculated composition using tabular values (NRC, 2000).
Table 2.
Dietary ingredients and nutritional composition of steam-flaked corn-based finishing diets containing live yeast fed to natural-program beef steers
| Finishing diets | |||
|---|---|---|---|
| Item | Control | LY | HY |
| Ingredient, % DM | |||
| Steam-flaked corn | 62.35 | 62.35 | 62.35 |
| Wet corn gluten feed (sweet bran, cargill corn milling) | 20.00 | 20.00 | 20.00 |
| Corn silage | 10.00 | 10.00 | 10.00 |
| Sorghum silage | 2.50 | 2.50 | 2.50 |
| Supplementa | 2.00 | 2.00 | 2.00 |
| Limestone | 1.65 | 1.65 | 1.65 |
| Urea | 0.50 | 0.50 | 0.50 |
| Premixb | 1.00 | 1.00 | 1.00 |
| Analyzed compositionc, % DM | |||
| DM, % | 67.36 | 66.50 | 66.97 |
| CP | 14.71 | 14.58 | 14.94 |
| NDF | 18.39 | 19.60 | 19.79 |
| ADF | 5.90 | 6.14 | 6.33 |
| EE | 3.00 | 3.40 | 3.20 |
| Starch | 48.60 | 47.30 | 48.40 |
| NEm, Mcal/kgd | 1.96 | 1.98 | 1.96 |
| NEg, Mcal/kgd | 1.32 | 1.32 | 1.30 |
| Ca | 0.62 | 0.71 | 0.67 |
| P | 0.46 | 0.49 | 0.46 |
| K | 0.88 | 0.94 | 0.89 |
| S | 0.17 | 0.18 | 0.18 |
aSupplement composition described in Table 1.
bPremix contained cottonseed meal for control diet; 24.8% (DM basis) yeast commercial product (ABVista) for the LY diet; and 49.59% (DM basis) yeast commercial product for the HY diet. Cottonseed meal was used as a carrier for the yeast premixes. Inclusions were based on an expected DMI of 9.5 kg/d (CTL = 0; LY = 1.33; and HY = 2.66 g of live yeast per steer daily).
cAnalyzed composition from a commercial laboratory (Servi-Tech Laboratories, Amarillo, TX).
dCalculated from growth performance data (Vasconcelos and Galyean, 2008).
Dietary Treatments and Yeast Product
The 3 dietary treatments were assigned randomly to pens within each block and included the following: control (CTL; no yeast); low yeast (LY; targeted for a yeast intake of 1.5 g/animal daily [3 × 1010 CFU/animal daily]); and high yeast (HY; targeted for a yeast intake of 3 g/animal daily [6 × 1010 CFU/animal daily]). The product used was live yeast Saccharomyces cerevisiae provided by ABVista (United Kingdom). The product received contained 2 × 1010 CFU/g of pure yeast. The commercial blend contained yeast (6%, as-is basis) + a flour-based carrier; thus, the cattle on the 2 yeast treatments were fed 25 (1.5/0.06) and 50 (3/0.06) g of the blended product/day (as-is basis) for the LY and HY treatments, respectively. Because the commercial product contained 94.48% DM, the targets for intake of the product (DM basis) were 23.62 and 47.24 g/animal daily, for LY and HY, respectively. Current study DMI was 11.4% less than the initial target predicted; therefore, steers on LY and HY treatments consumed 1.33 and 2.66 g of pure yeast per animal daily, which represented 2.66 and 5.31 × 1010 CFU/animal daily, respectively. The yeast product was administered via premixes included at 1% (DM basis) in the diet (Tables 1 and 2). All premixes were made at the Texas Tech University Feed Mill in a ribbon type mixer (Marion Mixers, Inc., Marion, IA) using cottonseed meal as a carrier.
Management, Feeding, and Weighing Procedures
Diet and ingredient samples were taken once per week and dried at 100 °C for 24 h in a forced-air oven. Weekly diet samples were also frozen, and a composite from each period (35 d) was made throughout the entire study for subsequent determination of nutritional composition (dried at 55 °C for 48 h in forced-air oven). The collection of orts and deduction from dietary DM offered to the pen was used to calculate DMI.
Interim BW measurements were collected on days 0, 35, 70, 105, 140, 183, and 204 before the daily feeding at approximately 0630 h. Weights were taken using a large pen scale (Cardinal Scale Manufacturing Co., Webb City, MO; accuracy ± 2.7 kg), except on days 0, 183, and 204, when individual BW measurements were taken (Silencer Chute, Moly Manufacturing, Lorraine, KS, mounted on Avery Weigh-Tronix load cells, Fairmount, MN; readability ± 0.45 kg; before each use, the scale was validated with 454 kg of certified weights). Cattle BW measurements were shrunk 4% to account for gut fill. On the final day of the study, cattle were weighed individually (days 183 and 204) and shipped to Creekstone Packing Plant in Arkansas City, KS. Trained personnel from West Texas A&M University collected HCW, liver abscess score, KPH (%), and used in-plant camera data to determine quality grade, yield grade, marbling score, LM area, and fat thickness at the 12th rib. Liver abscesses were classified using the methods described by Brink et al. (1990). Dressing percent was calculated using HCW divided by the nonshrunk individual final BW. Carcass-adjusted final BW was calculated from HCW divided by the average dressing percent (63.01%) from all 3 dietary treatments and adjusting for 4% shrink. The carcass-adjusted BW data were then used to determine carcass-adjusted ADG and G:F. The interim BW data were used to calculate ADG and G:F during each period.
Apparent Total Tract Nutrient Digestibility
From days 110 to 116, a digestibility measurement period was conducted. On day 110, feed bunks were cleaned at 0700 h, and fecal samples were collected at 1600 h that evening. Feces were also collected fresh from the pen surface within each pen at 0700 and 1600 h through day 116 and composited per collection. Steers were watched to ensure that fecal samples came from at least 3 different animals within the pen. Orts were collected at 0700 h each day and subtracted from the previous day’s offered feed. Orts were proportionally sampled (10%) and frozen for further analyses. Diet samples were collected for all treatments when cattle were fed at 0900 h, and a daily composite representing each experimental unit was created. At the end of collections, fecal samples were composited by pen (n = 10 samples per pen), and 200 g (as-is basis) from each composite sample were mixed and dried (55 oC, forced-air oven, for 96 h). A similar composite approach was performed for diet and refusal samples. Refusal samples collected that were greater than 5% of total offered were kept for nutrient analyses and further used to correct nutrient intake of pens. Except for daily dietary subsamples dried at 100 °C in a forced-air oven for 24 h (used to calculate DMI), all feed samples to be used in the laboratory were dried at 55 °C in a forced-air oven for 48 h before analyses. Samples were ground to pass a 1-mm screen in a Wiley Mill (Thomas Scientific, Swedesboro, NJ) and analyzed in laboratory for DM, ash, NDF, ADF, acid insoluble ash (AIA), and CP. The AIA was used as an internal marker (Van Keulen and Young, 1977) to determine apparent total tract digestion of DM, OM, CP, NDF, ADF, and starch, which is calculated as follows: 100 − 100 × ([AIA concentration in feed/AIA concentration in feces] × [nutrient concentration in feces/nutrient concentration in feed]). When the orts exceeded 5% of the total amount fed daily, the dietary AIA concentration was adjusted for the AIA concentration of the orts.
Laboratory Analyses
Lab analyses were adjusted to a DM basis (100 °C for 4 h) using method 950.01 (AOAC, 1990). Organic matter was determined by subtracting the residue from the ashing process, which was done in a muffle furnace (550 °C for 4 h) following the method 942.05 (AOAC, 2005). Approximately 0.3 g of each diet, fecal and refusal samples were placed into crucibles for N analysis (FP-200, Leco Corporation, St. Joseph, MI) using the official method 992.15 (AOAC, 1995). Neutral and acid detergent fiber concentrations were analyzed in sequence (Ankom 200, Macedon, NY), where the NDF procedure included thermo-stable amylase, sodium sulfite, and an acetone rinse (Van Soest et al., 1991). Starch and ether extract (EE) concentrations in the various samples were analyzed at a commercially certified laboratory (Servi-Tech, Amarillo, TX).
Feeding Behavior
On day 158, feeding behavior was analyzed for a 24-h period. Observations were recorded every 5 min with respect to whether the cattle in each pen were resting, active, eating, ruminating, or drinking (activities noted by pen). Two trained personnel walked behind pens to determine behaviors (each assigned to evaluate half the pens), over the period. Behavior was recorded continuously during a 24-h period. Cattle were accustomed to people walking in the pen alley and thereby not disturbed by the measurements. Activities were assumed to persist for the full 5-min period. Chewing activity was calculated by adding eating and ruminating time. Because of the 24-h period and 5-min intervals, some data points were missing as a result of human error (approximately 5%); hence, all behavior results were expressed as percentage of the 24-h period.
Statistical Analyses
Data were analyzed using the GLIMMIX procedures of SAS (SAS Inst., Inc., Cary, NC) with pen considered the experimental unit in a randomized complete block design. The fixed effects of treatment were evaluated for intake, feeding behavior, apparent total tract nutrient digestibility, growth performance, and carcass characteristics, with block included in the model as a random effect. Least squares mean differences were adjusted with a Tukey’s test, and degree of freedom bias was adjusted using Kenward Rogers method of SAS. Carcass data (USDA Quality Grade and liver scores) were analyzed using a binomial distribution; the link function of GLIMMIX procedure of SAS was used for data analyses of treatment effects, and the inverse-link function was used for reporting of responses with block included in the model as a random effect. Preplanned linear and quadratic effects of yeast inclusion (CTL, LY, and HY) contrasts were evaluated. Significant differences were considered if P ≤ 0.05 and tendencies if P was between 0.05 and 0.10.
RESULTS
Cattle Health
Throughout the course of the study, cattle were treated for both lameness and respiratory issues. Two steers from the CTL treatment and 1 from the LY treatment were removed early in the study because of lameness. Another steer was removed from the CTL treatment as a result of kidney failure. Seven steers were removed from the CTL group, 9 from the LY treatment, and 10 from the HY treatment and were treated for respiratory illness and subsequently removed from the study, which occurred at approximately days 150 to 175 of the feeding period. Of the remaining steers, 1 from the HY, 3 from the LY, and 3 from the CTL group were treated for respiratory issues, recovered, and remained in the experiment and were slaughtered at a local abattoir; their data were included in current analyses.
Growth Performance, Carcass Characteristics, and Feeding Behavior
Average daily gain and G:F did not differ among treatments (Table 3). There was a tendency (P = 0.08) for G:F to be improved quadratically during the periods of days 0 to 105 and days 0 to 183 (P = 0.10) for the LY treatment.
Table 3.
Effects of ABVista yeast (S. cerevisiae) on growth performance of natural-program beef steers fed steam-flaked corn-based finishing diets
| Item | Treatment | SEMa | P-values* | ||||
|---|---|---|---|---|---|---|---|
| CTL | LY | HY | Linear | Quadratic | Contrast | ||
| Initial BW, kg | 342 | 341 | 342 | 7.03 | 0.80 | 0.56 | 0.61 |
| Adj. FSBW, kgb | 574 | 578 | 574 | 12.56 | 1.00 | 0.67 | 0.83 |
| ADG, kg | |||||||
| days 0–35 | 1.57 | 1.67 | 1.66 | 0.062 | 0.25 | 0.43 | 0.17 |
| days 0–70 | 1.44 | 1.49 | 1.44 | 0.047 | 0.97 | 0.37 | 0.63 |
| days 0–105 | 1.46 | 1.51 | 1.49 | 0.039 | 0.54 | 0.35 | 0.32 |
| days 0–140 | 1.34 | 1.34 | 1.36 | 0.039 | 0.81 | 0.76 | 0.96 |
| days 0–183 | 1.32 | 1.35 | 1.29 | 0.041 | 0.60 | 0.37 | 0.99 |
| day 0-end | 1.19 | 1.21 | 1.18 | 0.033 | 0.76 | 0.49 | 0.93 |
| Adj. 0-endc | 1.18 | 1.20 | 1.19 | 0.036 | 0.89 | 0.65 | 0.73 |
| DMI, kg/d | |||||||
| days 0–35 | 7.42 | 7.46 | 7.48 | 0.064 | 0.46 | 0.89 | 0.48 |
| days 0–70 | 7.90 | 7.97 | 7.91 | 0.093 | 0.95 | 0.60 | 0.75 |
| days 0–105 | 8.31 | 8.28 | 8.33 | 0.093 | 0.84 | 0.77 | 0.98 |
| days 0–140 | 8.27 | 8.22 | 8.42 | 0.122 | 0.30 | 0.29 | 0.71 |
| days 0–183 | 8.36 | 8.32 | 8.50 | 0.255 | 0.44 | 0.46 | 0.76 |
| day 0-end | 8.46 | 8.39 | 8.48 | 0.239 | 0.92 | 0.61 | 0.86 |
| G:F | |||||||
| days 0–35 | 0.211 | 0.224 | 0.222 | 0.008 | 0.29 | 0.38 | 0.18 |
| days 0–70 | 0.182 | 0.187 | 0.181 | 0.005 | 0.90 | 0.34 | 0.70 |
| days 0–105 | 0.176 | 0.183 | 0.178 | 0.004 | 0.53 | 0.10 | 0.18 |
| days 0–140 | 0.163 | 0.163 | 0.160 | 0.004 | 0.62 | 0.74 | 0.79 |
| days 0–183 | 0.158 | 0.163 | 0.152 | 0.004 | 0.20 | 0.08 | 0.81 |
| day 0-end | 0.141 | 0.144 | 0.139 | 0.003 | 0.58 | 0.17 | 0.83 |
| Adj. 0-endc | 0.139 | 0.143 | 0.140 | 0.003 | 0.93 | 0.33 | 0.57 |
aStandard error of the mean, n = 12 pens per treatment.
bCarcass-adjusted FSBW was calculated from HCW divided by the average dressing percent across treatments (63.01%) and adjusted by a 4% shrink.
cCarcass-adjusted ADG and G:F were calculated from carcass-adjusted final shrunk BW, initial BW, and days on feed. Days on feed were 183 (n = 12 pens) and 204 (n = 24 pens).
*P-values for the linear, quadratic, and contrast (control vs. the average of the yeast treatments) effects of yeast dose (CTL = 0; LY = 1.33; and HY = 2.66 g of live yeast per steer daily).
Carcass characteristics and quality grade were evaluated at day 183 (12 pens) and day 204 (24 pens) of the trial for the heavy and light blocks, respectively, and are shown in Table 4. Similar to growth performance, no differences were observed (P > 0.27) for HCW (average = 377 kg), dressing percent (average = 63%), yield grade (average = 3.55), KPH (average = 2.02%), marbling score (average = 59, with 60 being a moderate amount of marbling), loin muscle area (average = 88.41 cm2), and 12th rib fat thickness (average = 19 mm). Although no differences were observed in these carcass measurements, the inclusion of yeast in the diet resulted in some differences in carcass quality. There were no differences (P = 0.11) in the amount of Prime carcasses among treatments; however, the proportion of Premium Choice carcasses increased linearly (P < 0.01) with increasing levels of yeast in the diet.
Table 4.
Effects of ABVista yeast (S. cerevisiae) in natural-program beef steers fed steam-flaked corn-based diets on carcass measurements
| Item | Treatment | SEMa | P-values* | ||||
|---|---|---|---|---|---|---|---|
| CTL | LY | HY | Linear | Quadratic | Contrast | ||
| HCW, kg | 376 | 379 | 376 | 5.3 | 0.99 | 0.67 | 0.83 |
| Dressing percentb | 62.9 | 62.8 | 63.3 | 0.31 | 0.33 | 0.46 | 0.63 |
| 12th rib fat, mm | 19.8 | 18.2 | 19.1 | 0.91 | 0.59 | 0.27 | 0.47 |
| LM area, cm2 | 87.8 | 89.0 | 88.4 | 1.45 | 0.79 | 0.59 | 0.83 |
| Marbling scorec | 59.1 | 57.9 | 60.7 | 2.01 | 0.59 | 0.45 | 0.93 |
| KPH, % | 2.0 | 2.0 | 2.0 | 0.02 | 0.80 | 0.89 | 0.77 |
| Calculated yield grade | 3.7 | 3.4 | 3.6 | 0.13 | 0.56 | 0.30 | 0.31 |
| Quality Graded | |||||||
| Prime, % | 27.7 | 11.2 | 18.9 | 7.71 | 0.37 | 0.15 | 0.11 |
| Premium Choice, % | 33.3 | 68.8 | 70.5 | 8.12 | < 0.01 | 0.12 | < 0.01 |
| Choice, % | 33.8 | 11.3 | 8.7 | 11.63 | 0.05 | 0.45 | 0.03 |
| Liver scoree | |||||||
| Total nonabscessedf, % | 97.6 | 97.7 | 95.5 | 3.27 | 0.52 | 0.70 | 0.74 |
| Total noncondemnedg,% | 81.6 | 86.7 | 81.6 | 6.37 | 0.99 | 0.47 | 0.71 |
aStandard error of the mean, n = 12 pens per treatment.
bDressing percent was calculated using nonshrunk final BW/HCW.
c30 = sight; 40 = small; 50 = modest; 60 = moderate; 70 = slightly abundant.
dQuality grade, as determined by USDA personnel.
eBecause of the limited number of abscesses, the general linear mixed model did not converge; thus, nonabscessed and noncondemned proportions were used.
fExcludes A+, A, and A− abscessed livers.
gExcludes A+, A, A− abscessed livers, and livers with flukes.
*P-values for the linear, quadratic, and contrast (control vs. the average of the yeast treatments) effects of yeast dose (CTL = 0; LY = 1.33; and HY = 2.66 g of live yeast per steer daily).
Liver score data are shown in Table 4. There were very few carcasses with liver abscesses and no major numerical differences among treatments in the various categories of abscessed severity.
Feeding behavior data are shown in Table 5. No differences were noted among treatments in terms of ruminating (P = 0.28), eating (P = 0.51), drinking (P = 0.70), resting (P = 0.48), and chewing (P = 0.56) activities (a combination of rumination and eating times). Cattle spent on average 8% to 9% of their time eating, 10% to 12% ruminating, and 78% to 79% resting.
Table 5.
Effects of ABVista yeast (S. cerevisiae) in natural-program steers fed steam-flaked corn-based finishing diets on feeding behavior on day 158 of the finishing period
| Item | Treatment | SEMa | P-values* | ||||
|---|---|---|---|---|---|---|---|
| CTL | LY | HY | Linear | Quadratic | Contrast | ||
| Time, % of 24 h | |||||||
| Eating | 8.5 | 8.3 | 9.0 | 0.65 | 0.51 | 0.55 | 0.78 |
| Ruminating | 11.5 | 11.7 | 10.3 | 0.96 | 0.28 | 0.43 | 0.58 |
| Chewing | 20.2 | 20.4 | 19.4 | 1.12 | 0.56 | 0.63 | 0.80 |
| Resting | 79.3 | 79.2 | 78.0 | 1.59 | 0.48 | 0.73 | 0.66 |
| Drinking | 0.3 | 0.3 | 0.3 | 0.06 | 0.70 | 0.68 | 0.60 |
| Active | 0.5 | 0.4 | 0.4 | 0.12 | 0.02 | 0.79 | 0.03 |
aStandard error of the mean, n = 12 pens per treatment.
*P-values for the linear, quadratic, and contrast (control vs. the average of the yeast treatments) effects of yeast dose (CTL = 0; LY = 1.33; and HY = 2.66 g of live yeast per steer daily).
Apparent Total Tract Nutrient Digestibility
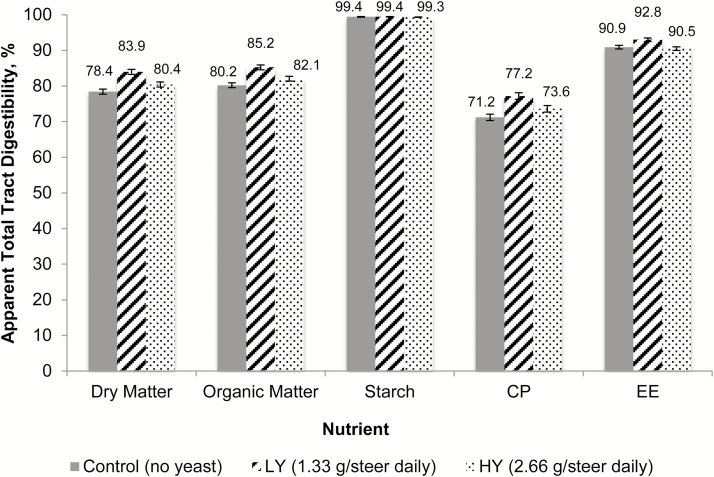
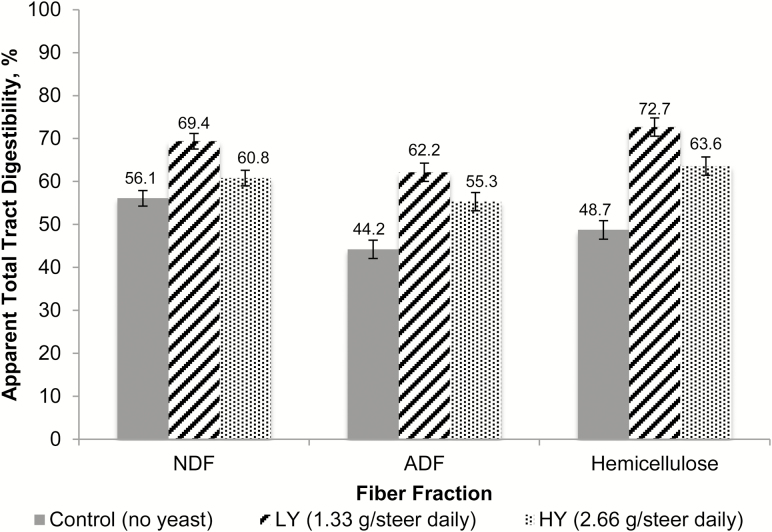
Apparent total tract digestibility data are summarized in Figures 1 and 2. Except for starch (P = 0.55), all nutrients showed a quadratic increase in digestibility (P < 0.01) for the LY treatment. Although the LY cattle had greater apparent total tract nutrient digestibility compared with HY and CTL, the HY cattle also had greater total apparent tract digestibility of all nutrients except for starch and EE compared with the CTL treatment. For the fiber fractions, there was a quadratic response in NDF with greater values for the LY treatment vs. CTL and HY treatments (P < 0.01) and an increase (P < 0.01) for both yeast treatments compared with the CTL treatment.
Figure 1.
Effects of ABVista yeast (S. cerevisiae) on apparent total tract nutrient digestibility of DM, OM, starch, CP, and EE in natural-program beef steers fed steam-flaked corn-based finishing diets. Except for starch (P = 0.55; SEM = 0.06; n = 12), all nutrients showed a quadratic increase (P < 0.01; SEM = 0.70; n = 12) for the LY treatment over CTL and HY treatments. Although the LY had greater apparent total tract nutrient digestibility than both HY and CTL, the HY treatment also had greater digestibility of all nutrients except for starch and EE vs. the CTL treatment (P < 0.01; SEM = 0.49; n = 12 pens per treatment).
Figure 2.
Effects of ABVista yeast (S. cerevisiae) on apparent total tract digestibility of dietary fiber components in natural-program beef steers fed steam-flaked corn-based finishing diets. There was a quadratic response for NDF, with greater digestibility for the LY treatment vs. the CTL and HY treatments (P < 0.01; SEM = 1.808; n = 12), and a substantial increase (P < 0.01) for ADF and hemicellulose with either yeast treatment compared with the CTL treatment. (P < 0.01; SEM = 1.808; n = 12).
DISCUSSION
The objective of current study was to evaluate levels of yeast and its effect on the growth performance, carcass characteristics, apparent total tract digestibility, and feeding behavior of steers fed steam-flaked corn-based finishing diets. The inclusion of yeast in the diet improved apparent total tract nutrient digestibility of the steers, but this effect did not translate to increased growth performance. Although nonsignificant, small increases in ADG were noted for the LY treatment during all periods of the study; a larger response might have been expected given the increase in nutrient digestibility. Digestibility of starch was not affected, however, so the lack of differences in ADG could reflect the lack of change in the digestibility of this major energy component of the diet. Other studies observed no improvement in growth performance or DMI when yeast was included in the diet of cattle that were under stressful conditions (Phillips and VonTungeln, 1985; and Cole et al., 1992).
The increased digestibility of nutrients in the current trial, specifically the fiber fractions, is consistent with the results of other studies. In a review by McAllister et al. (2011), it was reported that yeast improved fiber digestion by increasing cellulolytic bacterial growth within the rumen. Similarly, in the present study, NDF and ADF digestibility (Figure 2) was improved by moderate inclusions of yeast in the diet by 15.2% and 20.2%, respectively. As explained by Jouany et al. (1998), yeast potentially provides an improved environment for cellulolytic bacteria because it possibly decreases the oxygen load in the rumen, improving the environment for obligate anaerobic microbes to grow and digest fiber fractions.
The increased CP digestibility noted with yeast supplementation in the present study is consistent with the results of experiments conducted by Erasmus et al. (1992) and Dawson and Hopkins (1991), which noted improved ammonia uptake with yeast inclusion in the diet. Improved CP digestibility might have resulted from improved microbial efficiency, with ammonia uptake to grow microbial protein and support microbial metabolic activity (Erasmus et al., 1992). This improvement could induce greater microbial protein production, ultimately increasing the protein and thereby amino acids available to the animal to use in production responses. Nonetheless, because of potential contamination of fecal matter with microbial nitrogen from hindgut fermentation, assessment of apparent digestibility of CP in ruminants must be interpreted cautiously. Hindgut microbial attachment to fiber particles can be potentially diminished if greater fiber digestibility occurred for a specific treatment, leading to less contamination of microbial nitrogen recovery in feces. Such an effect for a given treatment could artificially inflate the apparent CP digestibility.
In the current study, cattle on the LY treatment had greater apparent total tract nutrient digestibility than those on the HY treatment. Yoon and Stern (1995) noted that increasing yeast in the diet from 1% to 2% (DM basis) did not improve DMI compared with a control diet. In addition, similar to present results, Zinn et al. (1999) did not observe a growth response to yeast supplementation in feedlot steers. In contrast, Phillips and VonTungeln (1985) noted that yeast in high-concentrate diets improved ADG by 0.10 kg/d in feeder calves. Moloney and Drennan (1994) reported improved growth performance with supplementation of yeast with high-concentrate diets vs. high-roughage diets fed to beef cattle. The LY treatment might have provided the threshold dose for improved performance associated with yeast in the ruminal environment and increasing beyond that dose might have limited benefit with a diet relatively high in starch and energy like the steam-flaked corn-based fed in the current experiment. Yoon and Stern (1995) reported that increasing the inclusion of yeast in the diet of dairy calves from 1% to 2% did not increase the DMI (6.2 vs. 6.4 kg/d), but both yeast treatments were greater than the control diet at 5.6 kg/d. This was similar to the current experiment, where there seemed to be a threshold at which the dietary level of yeast was the most efficient. In a dairy study conducted by Kung et al. (1997), cattle were fed diets including a S. cerevisiae product at 0, 10, and 20 g inclusion per animal daily. They observed that cattle in the control group produced 36.4 kg of milk per day, and milk production was 39.3 and 38.0 kg/d from the 10 and 20 g of yeast per day treatments, respectively, reflecting the idea that a threshold dose might exist at which additional yeast in the diet was not effective.
The lack of differences among the treatments in feeding behavior measurements suggests little effect of yeast on behavior, at least under the conditions of the present study. We hypothesized that increased digestibility resulting from feeding yeast might have resulted from increased chewing and thereby greater particle size reduction and increased buffering via saliva flow. Nonetheless, the lack of behavioral changes does not support this hypothesis. Feed additives such as monensin were not used in current study. In a review by González et al. (2012), it was noted that monensin decreases meal size and frequency of meals, thereby decreasing the starch load within the rumen and increasing average ruminal pH. Lack of feeding behavior differences in the present study suggests that yeast does not have the same modifying effects on feeding behavior of cattle as monensin and would not be a substitute for monensin in feedlot diets from the perspective of alterations in meal size and frequency.
Carcass quality characteristics were affected by treatment (P < 0.01), with increasing levels of yeast in terms of the proportion of Premium Choice carcasses. Swyers et al. (2014) observed an improvement in carcass quality of beef steers with the inclusion of S. cerevisiae fermentation product at 2.8 g/animal daily in a steam-flaked corn-based finishing diet compared with a nonyeast control diet. Other studies that compared the inclusion of yeast with control reported no differences in carcass characteristics between yeast and control treatments (Zerby et al., 2011). Swyers et al. (2014) hypothesized the inclusion of yeast coupled with the improved quality grade of steers and suggested that cattle fed a yeast product were finished at a lower final BW than monensin-fed steers, resulting in fewer days on feed. In the current study, similar 12th rib fat thickness and marbling were observed among treatments, but as noted above, differences in quality were only detected for the proportion of carcasses grading Premium Choice. It is important to note that more than 90% of the cattle in the 3 treatments graded Choice of greater; thus, the practical significance of changes within the Choice grade given the relatively small numbers of cattle used in the present study is open to question.
Although the effect of yeast on growth performance has been inconsistent in the literature, it is evident from our results and other reports in the literature that yeast can positively affect digestion. The beneficial effect of the LY treatment had on apparent total tract nutrient digestibility of fiber suggests that additional research might be beneficial to understand how yeast supplementation might affect the digestion of low-quality roughages typically used during the adaptation phase of the feedlot phase.
As noted by Wileman et al. (2009), natural-program steers do not perform on par with steers fed in conventional feeding programs. Nonetheless, with the increased consumer demand for beef from natural programs, information on yeast and its mode of action would be beneficial. Results of the present study suggest that in terms of its effects on nutrient digestibility, live yeast is a potentially beneficial product in natural beef programs.
CONCLUSIONS
Live yeast (S. cerevisiae) improved overall apparent total tract nutrient digestibility, particularly of the fiber components of the diet, without changing feeding behavior. Nonetheless, growth and feed efficiency of natural-program steers fed steam-flaked corn-based finishing diets was not affected by yeast supplementation.
Footnotes
Supported by funds provided by ABVista Feed Ingredients, United Kingdom.
LITERATURE CITED
- AOAC 1990. Official methods of analysis. 15th ed Association of Official Analytical Chemists, Arlington, VA. [Google Scholar]
- AOAC 1995. Official methods of analysis. 16th ed Association of Official Analytical Chemists, Washington (DC). [Google Scholar]
- AOAC 2005. Official methods of analysis. 18th ed Association of Official Analytical Chemists, Gathersburg (MD). [Google Scholar]
- Brink D. R., Lowry S. R., Stock R. A., and Parrott J. C.. 1990. Severity of liver abscesses and efficiency of feed utilization of feedlot cattle. J. Anim. Sci. 68:1201–1207. doi.10.2527/1990.6851201x [DOI] [PubMed] [Google Scholar]
- Cole N. A., Purdy C. W., and Hutcheson D. P.. 1992. Influence of yeast culture on feeder calves and lambs. J. Anim. Sci. 70:1682–1690. doi:10.2527/1992.7061682x [DOI] [PubMed] [Google Scholar]
- Dawson K. A., and Hopkins D. M.. 1991. Differential effects of live yeast on the cellulolytic activities of anaerobic ruminal bacteria. J. Anim. Sci. 69(Suppl. 1):531. (Abstract)2016183 [Google Scholar]
- Desnoyers M., Giger-Reverdin S., Bertin G., Duvaux-Ponter C., and Sauvant D.. 2009. Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk production of ruminants. J. Dairy Sci. 92:1620–1632. doi. 10.3168/jds.2008-1414 [DOI] [PubMed] [Google Scholar]
- Erasmus L. J., Botha P. M., and Kistner A.. 1992. Effect of yeast culture supplement on production, rumen fermentation, and duodenal nitrogen flow in dairy cows. J. Dairy Sci. 75:3056–3065. doi.10.3168/jds.S0022-0302(92)78069-2 [DOI] [PubMed] [Google Scholar]
- González L. A., Manteca X., Calsamiglia S., Schwartzkopf-Genswein K. S., and Ferret A.. 2012. Ruminal acidosis in feedlot cattle: interplay between feed ingredients, rumen function and feeding behavior (a review). Anim. Feed Sci. Technol. 172:66–79. doi.10.1016/j.anifeedsci.2011.12.009 [Google Scholar]
- Jouany J. P., Mathieu F., Senaud J., Bohatier J., Bertin G., and Mercier M.. 1998. The effect of Saccharomyces cerevisiae and Asperigillus oryzae on the digestion of the cell wall fraction of a mixed diet in defaunated and refaunated sheep rumen. Reprod. Nutr. Dev. 38: 410–416. [DOI] [PubMed] [Google Scholar]
- Knight A., and Warland R.. 2004. The relationship between sociodemographics and concern about food safety issues. J. Consum. Aff. 28:107–120. [Google Scholar]
- Kung L., Kreck E. M., Tung R. S., Hession A. O., Sheperd A. C., Cohen M. A., Swain H. E., and Leedle J. A.. 1997. Effects of a live yeast culture and enzymes on in vitro ruminal fermentation and milk production of dairy cows. J. Dairy Sci. 80:2045–2051. doi.10.3168/jds.S0022-0302(97)76149-6 [DOI] [PubMed] [Google Scholar]
- McAllister T. A., Beauchemin K. A., Alazzeh A. Y., Baah J., Teather R. M., and Stanford K.. 2011. Review: the use of direct fed microbials to mitigate pathogens and enhance production in cattle. Can. J. Anim. Sci. 91:193–211. doi.10.4141/cjas10047 [Google Scholar]
- Moloney A. P., and Drennan M. J.. 1994. Effects of yeast culture on growth of beef cattle fed on grass silage plus barley-based concentrates. Irish J. Agric. Res. 32:125–132. [Google Scholar]
- NRC 2000. Nutrient requirements of beef cattle. 7th ed Washington, DC:Natl. Acad. Press. doi:10.17226/9791. [Google Scholar]
- Phillips W. A., and VonTungeln D. L.. 1985. The effect of yeast culture on the post stress performance of feeder calves. Nutr. Rep. Int. 32:287–294. [Google Scholar]
- Sparling E., Grannis J. L., and Thilmany D. D.. 2002. Regional demand for natural beef products: urban vs. rural willingness to pay and target consumers. Paper presented at the Annual Meeting of the Western Agricultural Economics Association; July 28 to 31, 2002; Long Beach (CA) Available from http://ageconsearch.umn.edu/bitstream/36544/1/sp02sp01.pdf [Google Scholar]
- Swyers K. L., Wagner J. J., Dorton K. L., and Archibeque S. L.. 2014. Evaluation of Saccharomyces cerevisiae fermentation product as an alternative to monensin on growth performance cost of gain, and carcass characteristics of heavyweight yearling beef steers. J. Anim. Sci. 92:2538–2545. doi.10.2527/jas.2013–7559 [DOI] [PubMed] [Google Scholar]
- USDA-APHIS 2013. Feedlot 2011: part III: trends in health and management practices on U.S. feedlots, 1994–2011. USDA Animal and Plant Health Inspection Service; Available from http://www.aphis.usda.gov/animal_health/nahms/feedlot/downloads/feedlot2011/Feed11_dr_Part%2oIII.pdf [Google Scholar]
- Vasconcelos J. T., and Galyean M. L.. 2008. Technical note: do dietary net energy values calculated from performance data offer increased sensitivity for detecting treatment differences?J. Anim. Sci. 86:2756–2760. doi:10.2527/jas.2008-1057 [DOI] [PubMed] [Google Scholar]
- Van Keulen J., and Young B. A.. 1977. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci. 44:282–287. doi: 10.2527/jas1977.442282x [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. 1991. Methods of dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551–2 [DOI] [PubMed] [Google Scholar]
- Wiedmeier R. D., Arambel M. J., and Walters J. L.. 1987. Effect of yeast culture and Aspergillus oryzae fermentation extract on ruminal characteristics and nutrient digestibility. J. Dairy Sci. 70:2063–2068. doi 10.3168/jds.S0022-0302(87)80254-0 [DOI] [PubMed] [Google Scholar]
- Wileman B. W., Thomson D. U., Reinhardt C. D., and Renter D. G.. 2009. Analysis of modern technologies commonly used in beef cattle production: conventional beef production versus nonconventional production using meta-analysis. J. Anim. Sci. 87:3418–3426. doi.10.2527/jas.2009-1778 [DOI] [PubMed] [Google Scholar]
- Yoon I. K., and Stern M. D.. 1995. Influence of direct-fed microbials on ruminal microbial fermentation and performance of ruminants: a review. Asian-Australas. J. Anim. Sci. 8:533–555. doi.10.5713/ajas.1995.553 [Google Scholar]
- Zerby H. N., Bard J. L., Loerch S. C., Kuber P. S., Radunz A. E., and Fluharty F. L.. 2011. Effects of diet and Aspergillus oryzae extract or Saccharomyces cerevisiae on growth and carcass characteristics of lambs and steers fed to meet requirements of natural markets. J. Anim. Sci. 89:2257–2264. doi. 10.2527/jas.2010–3308 [DOI] [PubMed] [Google Scholar]
- Zinn R. A., Alverez E. G., Rogriguez S., and Salinas J.. 1999. Influence of yeast culture on health, performance and digestive function of feedlot steers. Proc. West. Sect. Am. Soc. Anim. Sci. 50:335–338. [Google Scholar]